An Investigation into the Insertion of a Solid Mandrel into a Commercial Cylindrical Li-Ion Cell for Improved Thermal Performance
Abstract
1. Introduction
2. Experimental Method
2.1. Definition of Cell Concept
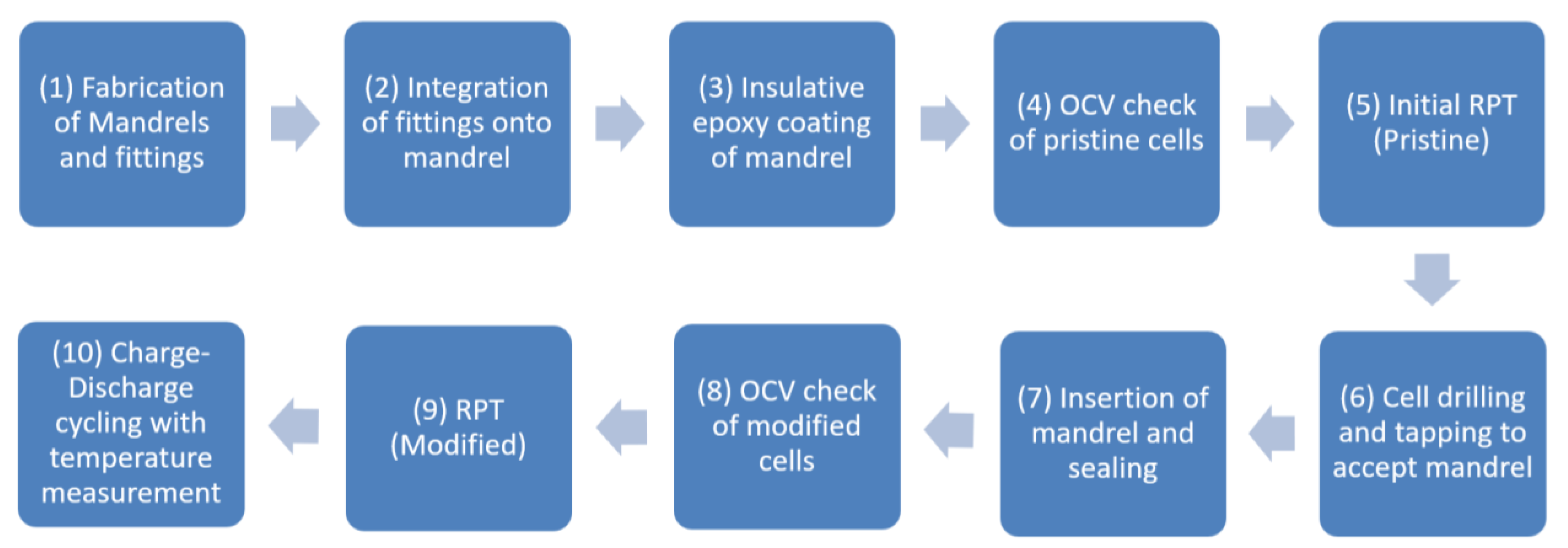
2.2. Cell Modification Process
- (1)
- Mandrel fabrication was conducted, which involved cutting the mandrels to 60 mm length and filing down one end of the mandrel to approx. 1 mm in diameter.
- (2)
- The mandrels were then secured into a hollowed M2.5 screw with epoxy and dried in a vacuum oven at 50 °C for 5 h.
- (3)
- The mandrel was coated in a thin layer of MG Chemicals 4225 epoxy and dried in a vacuum oven at 60 °C for 7 h to ensure full electrical insulation during insertion and operation of the cell.
- (4–5)
- The pristine LGM50 cells were subjected to Open Circuit Voltage (OCV) and Reference Performance Tests (RPT) to verify functionality. A full description of this experimental process is provided in Section 2.3.
- (6–7)
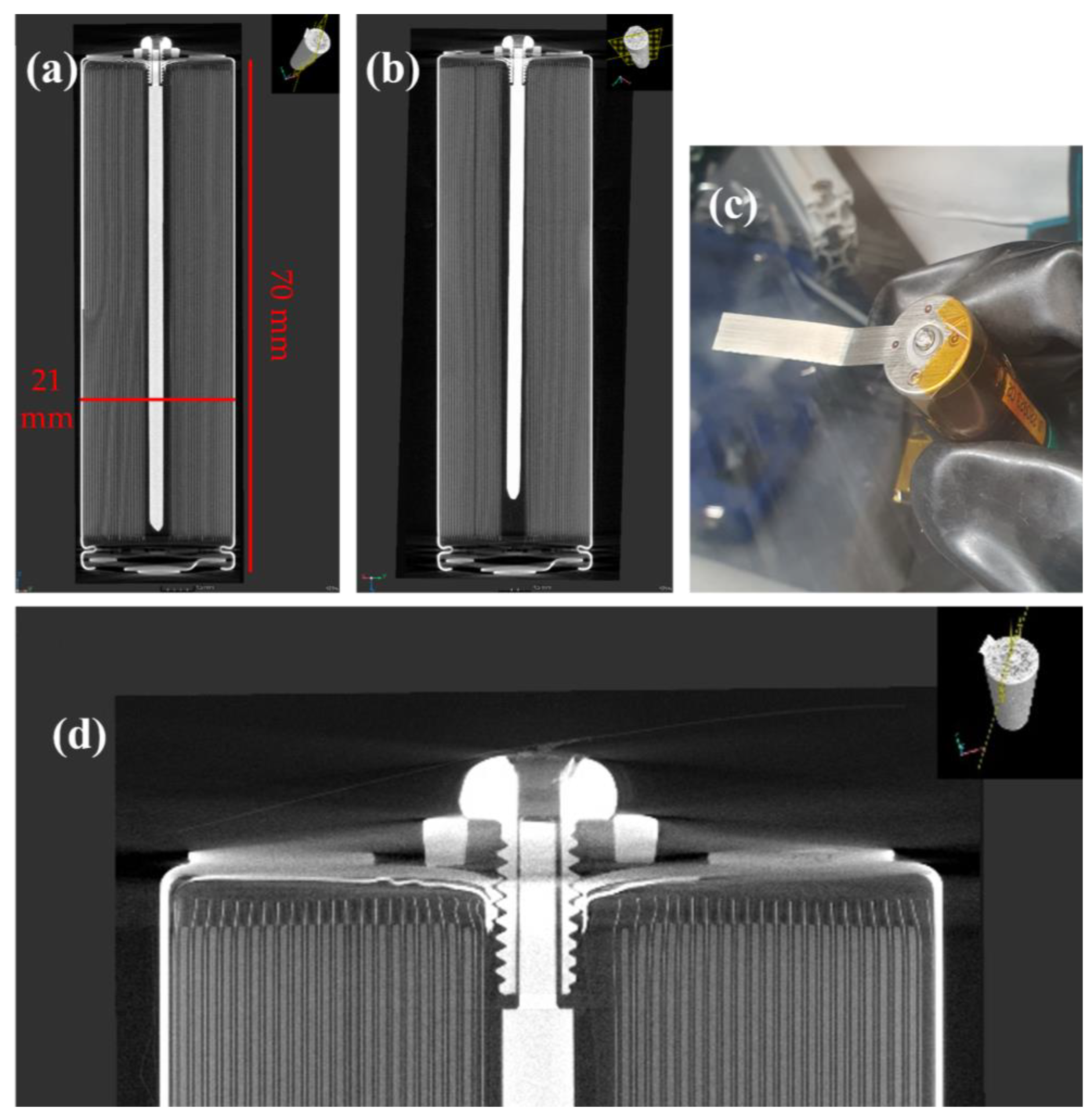
- The cells had a 2 mm hole drilled through the base, then they were tapped using an M2.5 thread, and the screw and mandrel unit were inserted with a rubber sealing washer to ensure a gas-tight seal. X-ray tomography images in Figure 2a,b show the modified cell, whilst the base of the cell can be seen in Figure 2c,d.
- (8–9)
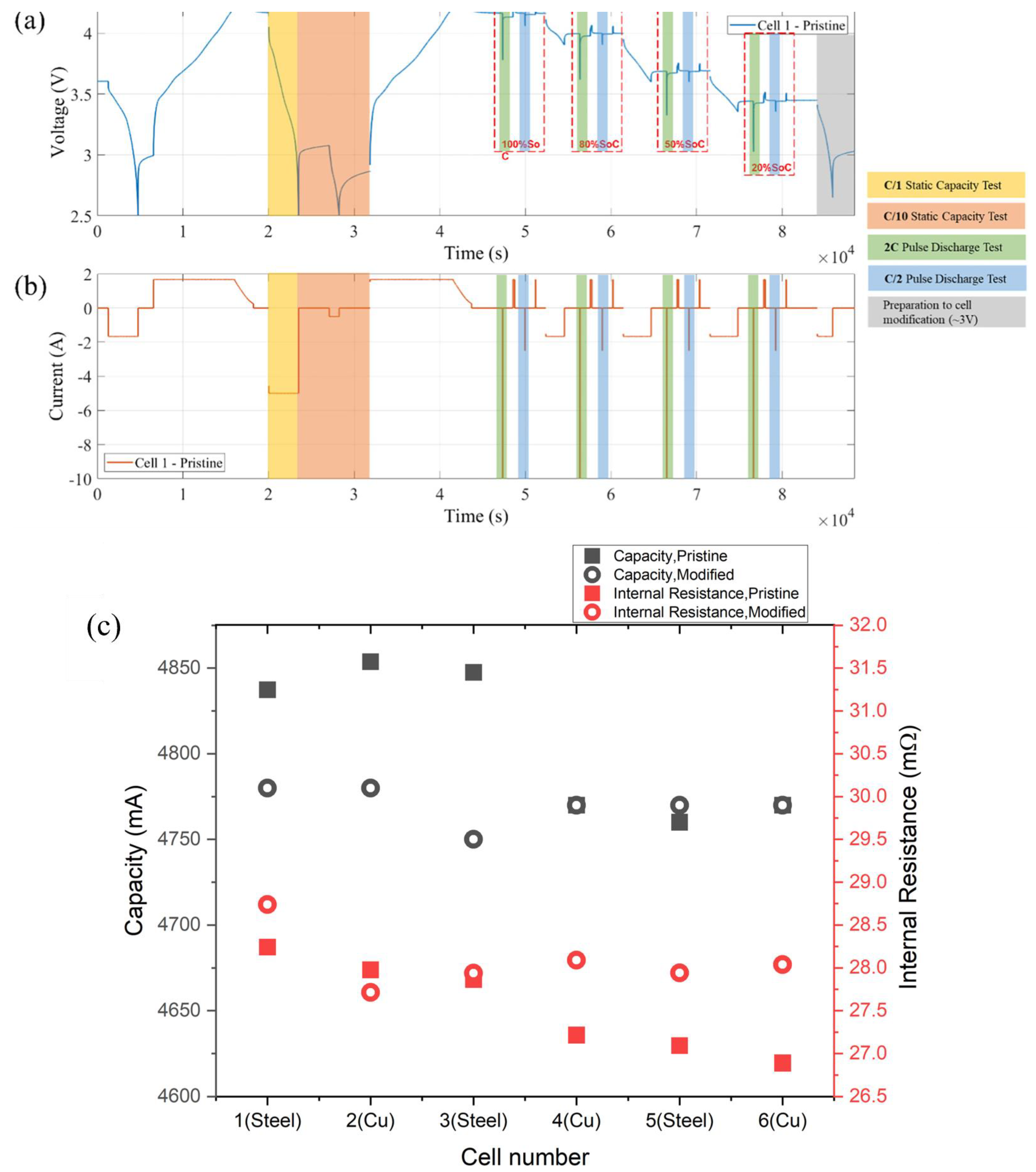
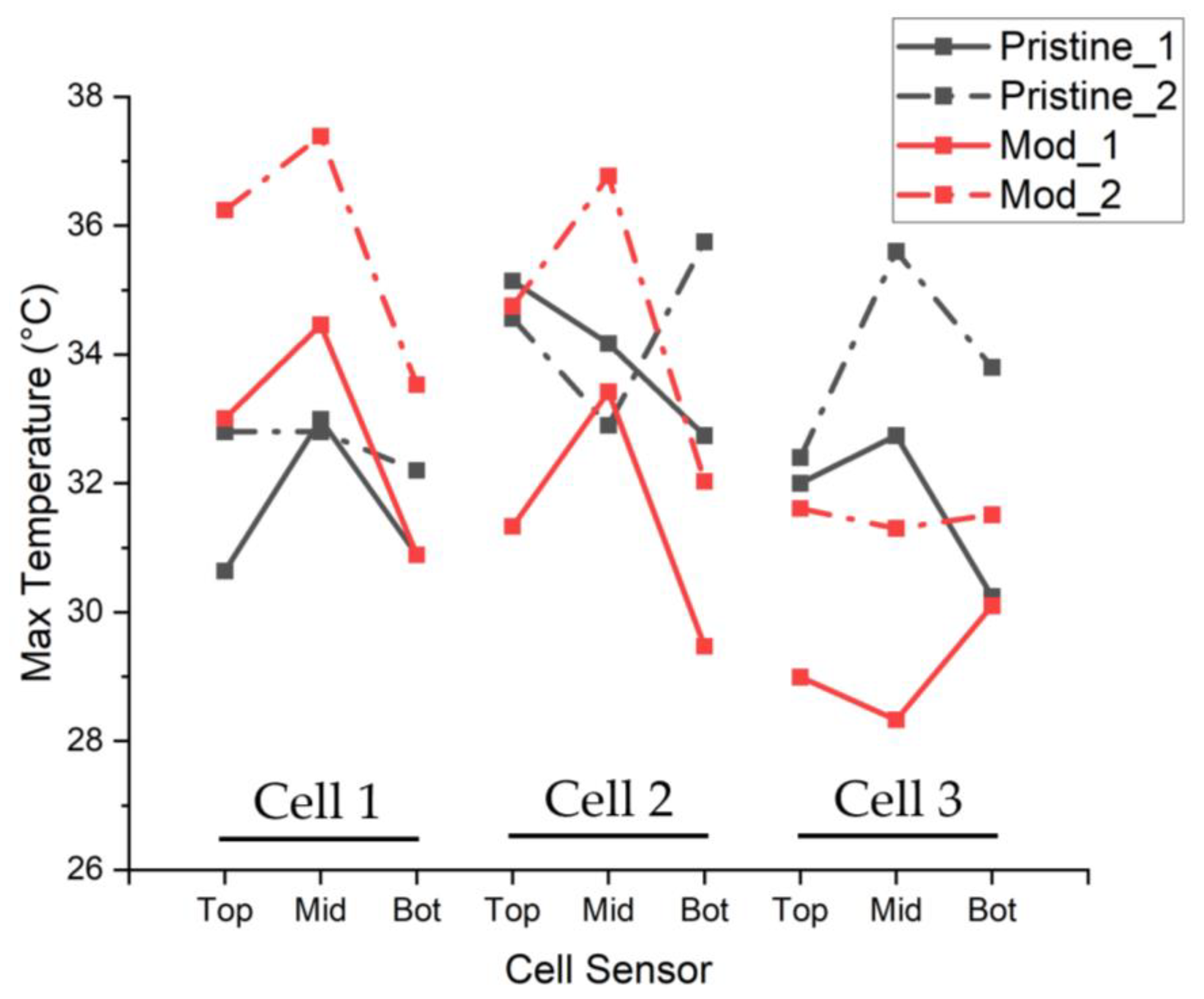
- For modified cells, the same OCV and RPT were conducted to ensure the cells were not adversely affected by the inclusion of the solid mandrel, with results shown in Figure 3c.
- (10)
- Thermal characterisation of the cells was undertaken using the test procedure in Section 2.4.
2.3. Reference Performance Tests and Functionality Verification
2.4. Experimental Setup and Test Procedure
3. Results
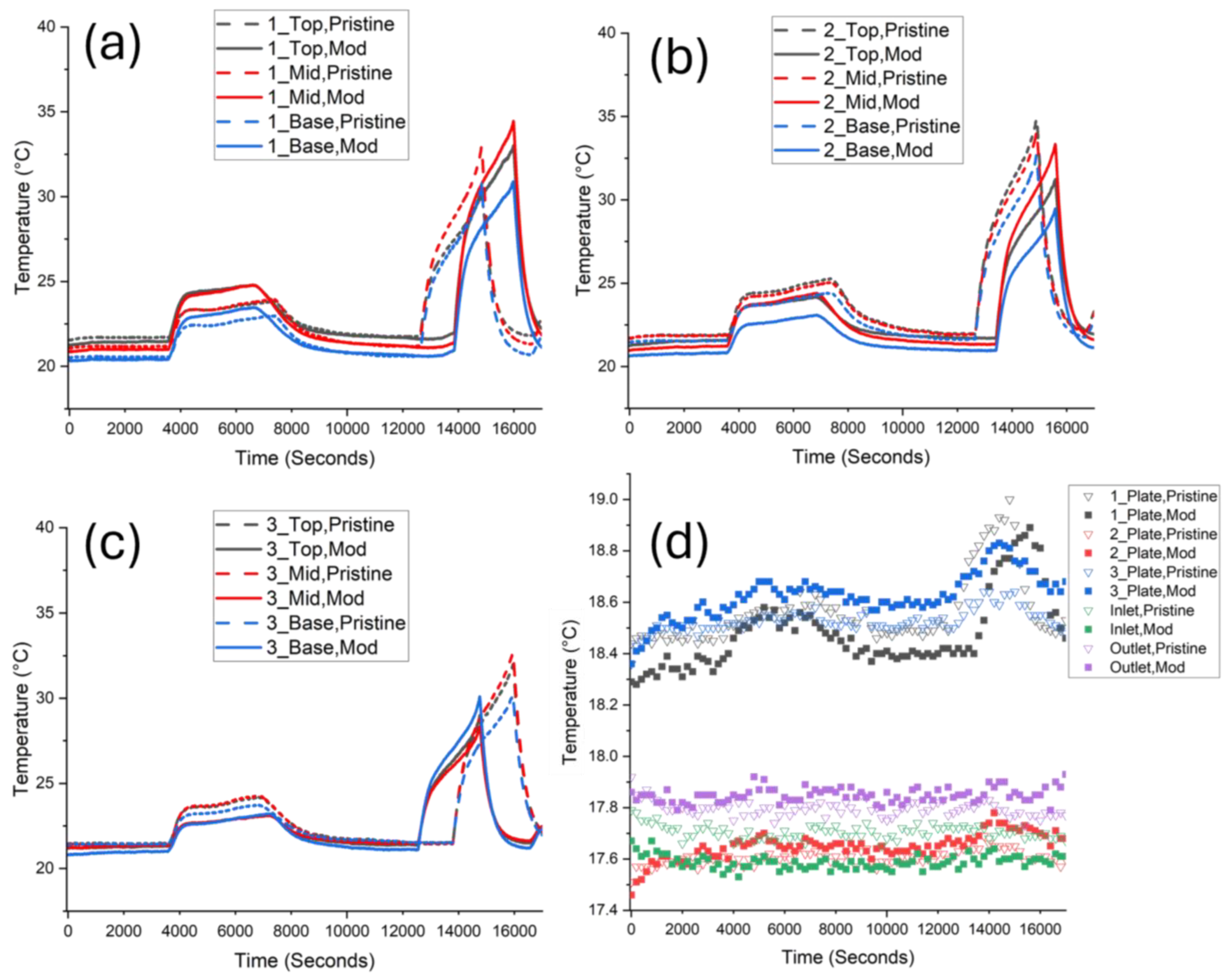
3.1. Pristine Reference Cell
3.2. Modified Reference Cell
4. Simulation
4.1. Model Parameters and Test Cycle
4.2. Idealised Cell Results
4.3. Validation of Experimental Cell Modifications
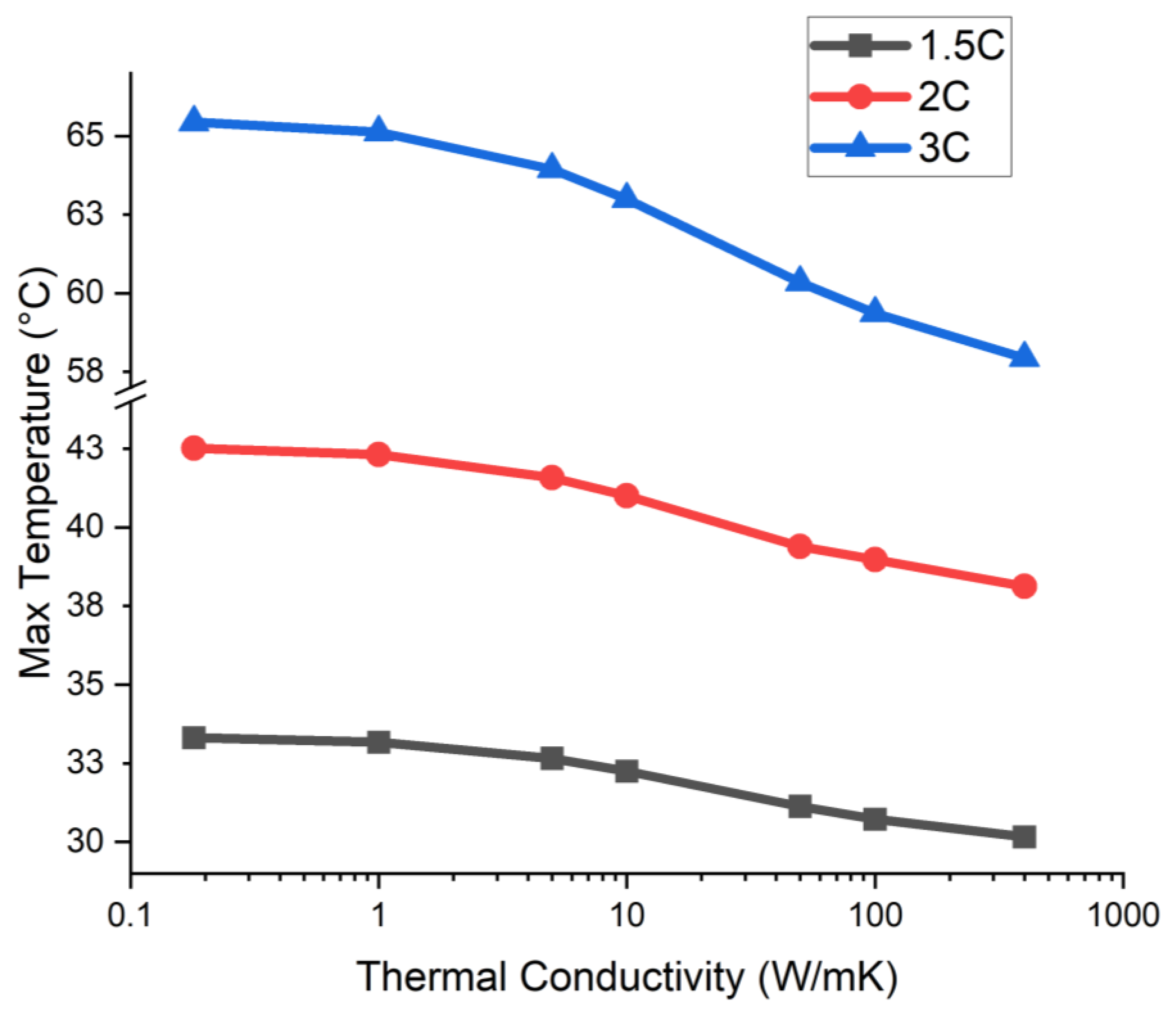
4.4. Variation of Mandrel Base Thermal Conductivity
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baazouzi, S.; Feistel, N.; Wanner, J.; Landwehr, I.; Fill, A.; Birke, K.P. Design, Properties, and Manufacturing of Cylindrical Li-Ion Battery Cells—A Generic Overview. Batteries 2023, 9, 309. [Google Scholar] [CrossRef]
- Yan, H.; Marr, K.C.; Ezekoye, O.A. Towards Fire Forensic Characteristics of Failed Cylindrical Format Lithium–Ion Cells and Batteries. Fire Technol. 2021, 57, 1723–1752. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Tranter, T.G.; Timms, R.; Shearing, P.R.; Brett, D.J.L. Communication—Prediction of Thermal Issues for Larger Format 4680 Cylindrical Cells and Their Mitigation with Enhanced Current Collection. J. Electrochem. Soc. 2020, 167, 160544. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Drake, S.; Wetz, D.; Ostanek, J.; Miller, S.; Heinzel, J.; Jain, A. Measurement of anisotropic thermophysical properties of cylindrical Li-ion cells. J. Power Sources 2014, 252, 298–304. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Bohlen, O.; Roscher, M.A.; Bäker, B. Current density and state of charge inhomogeneities in Li-ion battery cells with LiFePO4 as cathode material due to temperature gradients. J. Power Sources 2011, 196, 4769–4778. [Google Scholar] [CrossRef]
- Nguyen, T.; Deng, J.; Robert, B.; Chen, W.; Siegmund, T. Experimental investigation on cooling of prismatic battery cells through cell integrated features. Energy 2022, 244, 122580. [Google Scholar] [CrossRef]
- Kleiner, J.; Lambauer, F.; Singh, R.; Komsiyska, L.; Hinterberger, M.; Endisch, C. Experimental study of cell integrated heat pipe cooling with a lithium-ion cell emulator. J. Energy Storage 2022, 56, 105808. [Google Scholar] [CrossRef]
- Tran, T.-H.; Harmand, S.; Sahut, B. Experimental investigation on heat pipe cooling for Hybrid Electric Vehicle and Electric Vehicle lithium-ion battery. J. Power Sources 2014, 265, 262–272. [Google Scholar] [CrossRef]
- Mo, X.; Hu, X.; Tang, J.; Tian, H. A comprehensive investigation on thermal management of large-capacity pouch cell using micro heat pipe array. Int. J. Energy Res. 2019, 43, 7444–7458. [Google Scholar] [CrossRef]
- Li, S.; Kirkaldy, N.; Zhang, C.; Gopalakrishnan, K.; Amietszajew, T.; Diaz, L.B.; Barreras, J.V.; Shams, M.; Hua, X.; Patel, Y.; et al. Optimal cell tab design and cooling strategy for cylindrical lithium-ion batteries. J. Power Sources 2021, 492, 229594. [Google Scholar] [CrossRef]
- Sievers, M.; Sievers, U.; Mao, S.S. Thermal modelling of new Li-ion cell design modifications. Forsch. Ingenieurwesen 2010, 74, 215–231. [Google Scholar] [CrossRef][Green Version]
- Shah, K.; McKee, C.; Chalise, D.; Jain, A. Experimental and numerical investigation of core cooling of Li-ion cells using heat pipes. Energy 2016, 113, 852–860. [Google Scholar] [CrossRef]
- Worwood, D.; Kellner, Q.; Wojtala, M.; Widanage, W.; McGlen, R.; Greenwood, D.; Marco, J. A new approach to the internal thermal management of cylindrical battery cells for automotive applications. J. Power Sources 2017, 346, 151–166. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Luo, Y. The thermal performance of a novel internal cooling method for the electric vehicle battery: An experimental study. Appl. Therm. Eng. 2019, 161, 114102. [Google Scholar] [CrossRef]
- Waldmann, T.; Scurtu, R.-G.; Richter, K.; Wohlfahrt-Mehrens, M. 18650 vs. 21700 Li-ion cells—A direct comparison of electrochemical, thermal, and geometrical properties. J. Power Sources 2020, 472, 228614. [Google Scholar] [CrossRef]
- Anthony, D.; Wong, D.; Wetz, D.; Jain, A. Improved Thermal Performance of a Li-Ion Cell through Heat Pipe Insertion. J. Electrochem. Soc. 2017, 164, 961–967. [Google Scholar] [CrossRef]
- Gulsoy, B.; Vincent, T.; Sansom, J.; Marco, J. In-situ temperature monitoring of a lithium-ion battery using an embedded thermocouple for smart battery applications. J. Energy Storage 2022, 54, 105260. [Google Scholar] [CrossRef]
- Vincent, T.A.; Gulsoy, B.; Sansom, J.E.; Marco, J. In-situ instrumentation of cells and power line communication data acquisition towards smart cell development. J. Energy Storage 2022, 50, 104218. [Google Scholar] [CrossRef]
- Chen, S.; Wan, C.; Wang, Y. Thermal analysis of lithium-ion batteries. J. Power Sources 2005, 140, 111–124. [Google Scholar] [CrossRef]
- Droese, D.; Luc, P.-M.; Otto, M.; Schlösser, A.E.K.; Evans, D.; Kowal, J. Comparison and Teardown of Na- and Li-Ion 18650 Cells. SSRN 2024. [Google Scholar] [CrossRef]
- Sarmadian, A.; Widanage, W.D.; Shollock, B.; Restuccia, F. Experimentally-verified thermal-electrochemical simulations of a cylindrical battery using physics-based, simplified and generalised lumped models. J. Energy Storage 2023, 70, 107910. [Google Scholar] [CrossRef]
- Özdemir, T.; Amini, A.; Ekici, Ö.; Köksal, M. Experimental Assessment of the Lumped Lithium Ion Battery Model at Different Operating Conditions. Heat Transf. Eng. 2021, 43, 314–325. [Google Scholar] [CrossRef]
- Ireland, J.C.; Marco, J.; McGlen, R. The effect of increasing the thermal conductivity of a cylindrical cell housing on thermal performance. In Proceedings of the 2024 IEEE Vehicle Power and Propulsion Conference (VPPC), Washington DC, USA, 7–10 October 2024; pp. 1–6. [Google Scholar]
- Sarmadian, A.; Yu, Y.; Marco, J.; Shollock, B.; Restuccia, F. An Experimentally-Verified Thermal-Electrochemical Simulation Model of a 21700 Cell Using a Lumped Semi-Empirical Battery Model. In Proceedings of the 16th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Virtual Conference, 8–10 August 2022; pp. 128–133. [Google Scholar]
- Fleckenstein, M.; Fischer, S.; Bohlen, O.; Bäker, B. Thermal Impedance Spectroscopy—A method for the thermal characterization of high power battery cells. J. Power Sources 2013, 223, 259–267. [Google Scholar] [CrossRef]
- Maleki, H.; Al Hallaj, S.; Selman, J.R.; Dinwiddie, R.B.; Wang, H. Thermal Properties of Lithium-Ion Battery and Components. J. Electrochem. Soc. 1999, 146, 947–954. [Google Scholar] [CrossRef]
- Böger, T.; Bernges, T.; Li, Y.; Canepa, P.; Zeier, W.G. Thermal Conductivities of Lithium-Ion-Conducting Solid Electrolytes. ACS Appl. Energy Mater. 2023, 6, 10704–10712. [Google Scholar] [CrossRef]





| Capacity (mAh) | Internal Resistance (mΩ) | |||||
|---|---|---|---|---|---|---|
| Cell ID | Pristine | Modified | Change | Pristine | Modified | Change |
| 1 | 4837 | 4780 | −1.2% | 28.24 | 28.74 | 1.8% |
| 2 | 4853 | 4780 | −1.5% | 27.98 | 27.71 | −0.9% |
| 3 | 4847 | 4750 | −2.0% | 27.86 | 27.94 | 0.3% |
| 4 | 4770 | 4770 | 0.0% | 27.21 | 28.09 | 3.2% |
| 5 | 4760 | 4770 | 0.2% | 27.09 | 27.94 | 3.1% |
| 6 | 4770 | 4770 | 0.0% | 26.89 | 28.04 | 4.3% |
| 7 | 4800 | 27.17 | ||||
| 8 | 4820 | 27.14 | ||||
| 9 | 4780 | 27.47 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ireland, J.; Marco, J.; Dinh, T.; McGlen, R.; Lynn, K. An Investigation into the Insertion of a Solid Mandrel into a Commercial Cylindrical Li-Ion Cell for Improved Thermal Performance. Energies 2025, 18, 1825. https://doi.org/10.3390/en18071825
Ireland J, Marco J, Dinh T, McGlen R, Lynn K. An Investigation into the Insertion of a Solid Mandrel into a Commercial Cylindrical Li-Ion Cell for Improved Thermal Performance. Energies. 2025; 18(7):1825. https://doi.org/10.3390/en18071825
Chicago/Turabian StyleIreland, Joshua, James Marco, Truong Dinh, Ryan McGlen, and Kevin Lynn. 2025. "An Investigation into the Insertion of a Solid Mandrel into a Commercial Cylindrical Li-Ion Cell for Improved Thermal Performance" Energies 18, no. 7: 1825. https://doi.org/10.3390/en18071825
APA StyleIreland, J., Marco, J., Dinh, T., McGlen, R., & Lynn, K. (2025). An Investigation into the Insertion of a Solid Mandrel into a Commercial Cylindrical Li-Ion Cell for Improved Thermal Performance. Energies, 18(7), 1825. https://doi.org/10.3390/en18071825









