Design and Performance Studies on Series of Tetrazole-Based Ultra-High-Energy Density High-Nitrogen Heterocyclic Power Systems
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
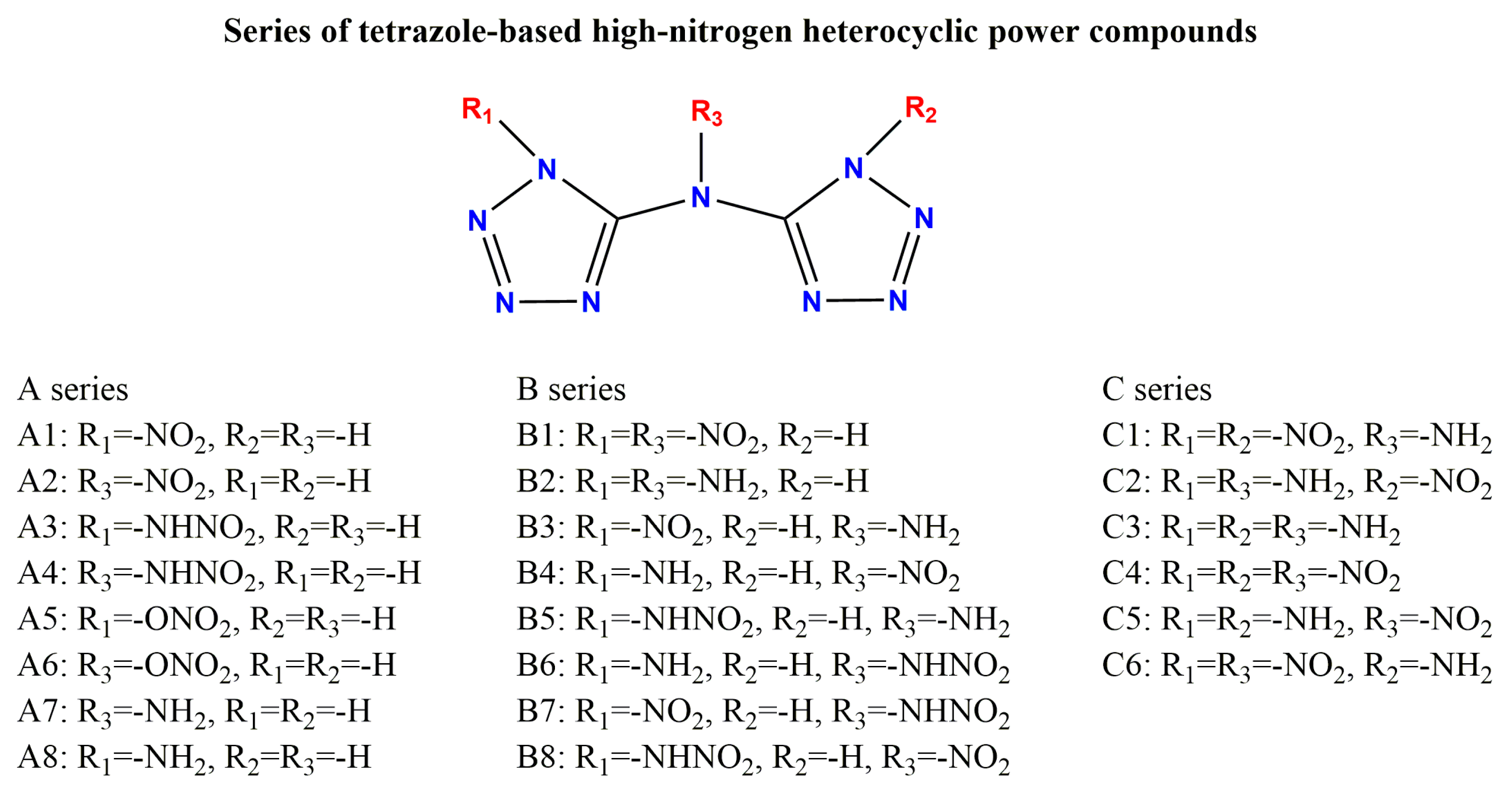
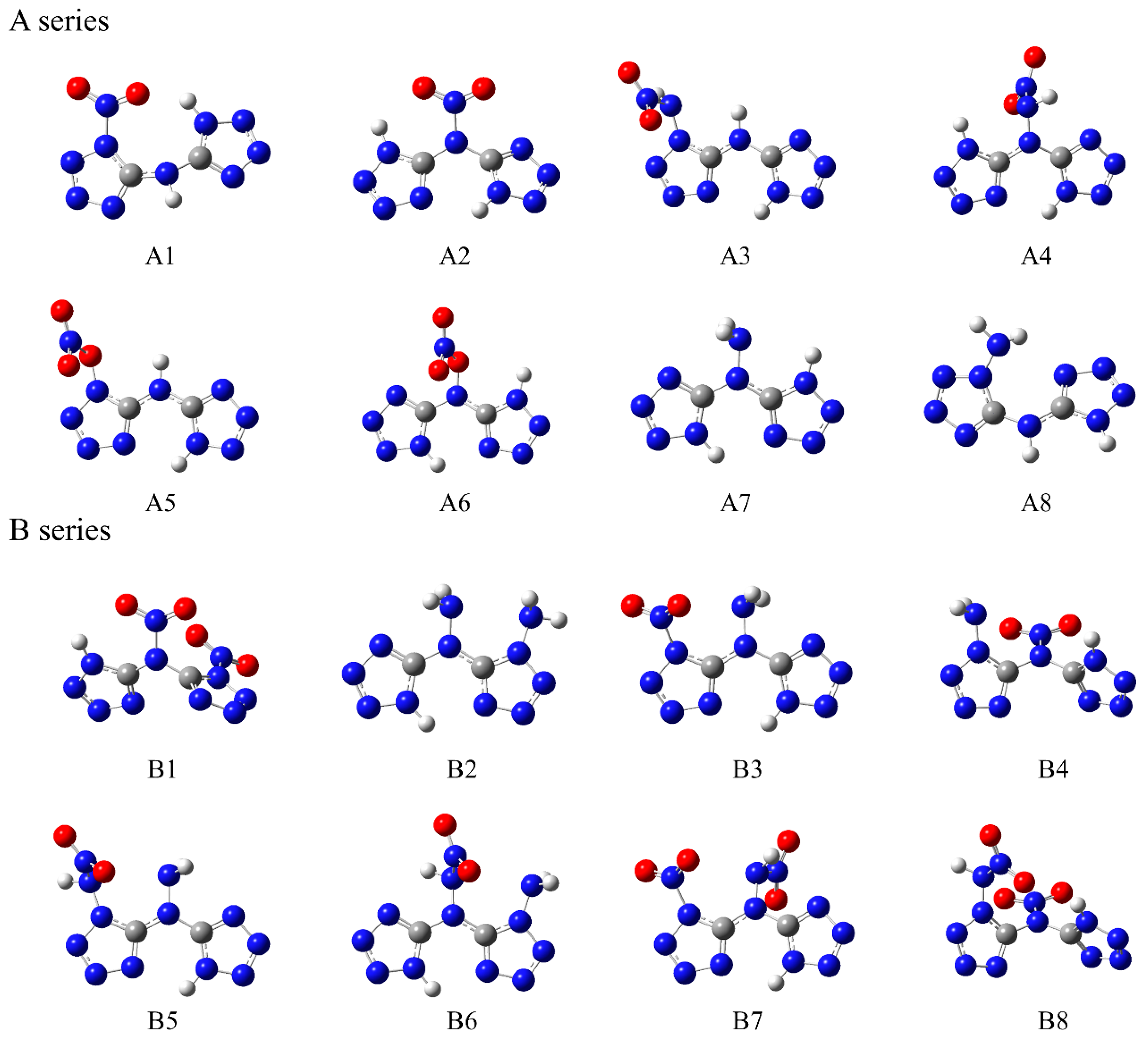
3.1. Design Mentality
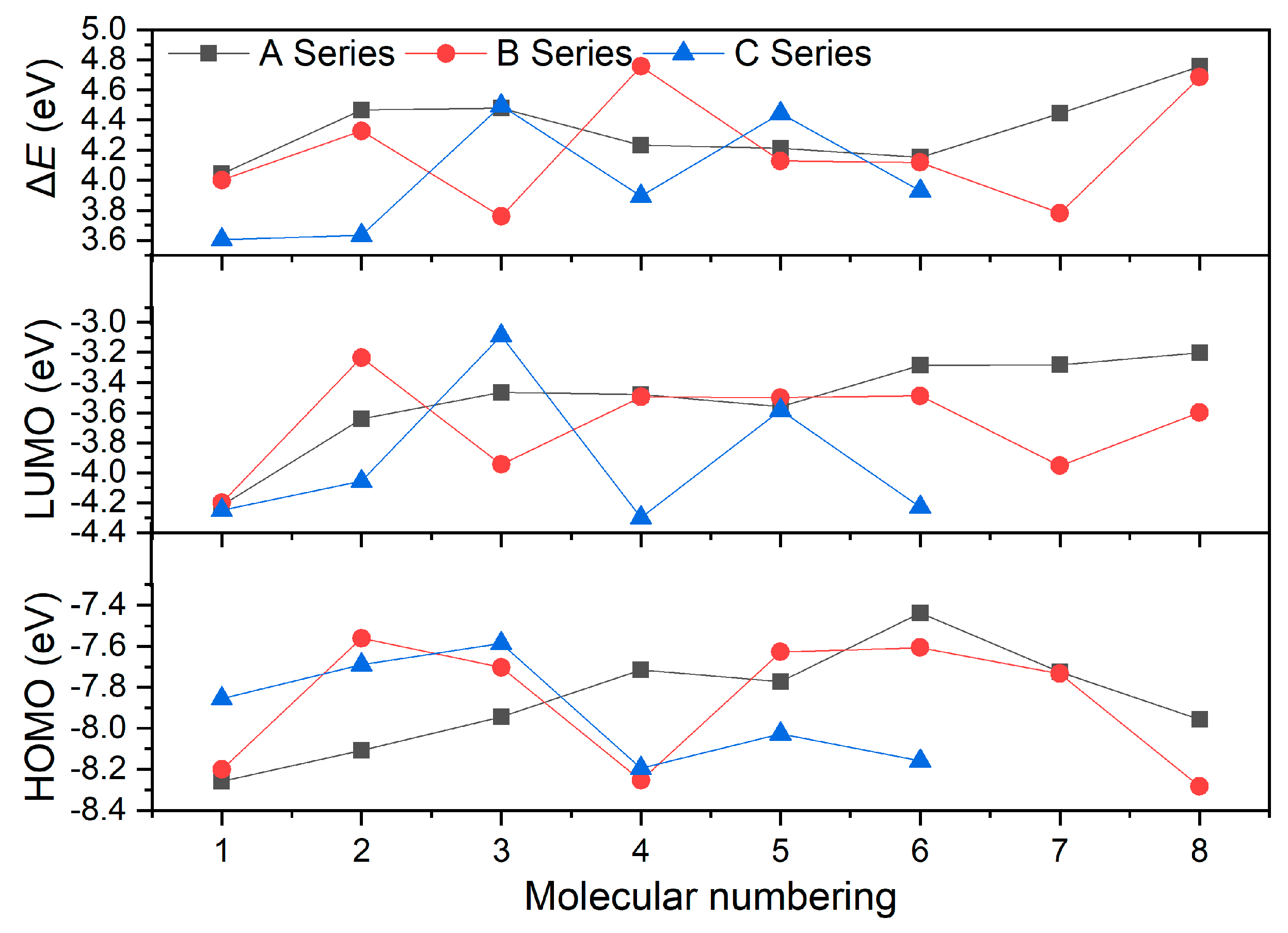
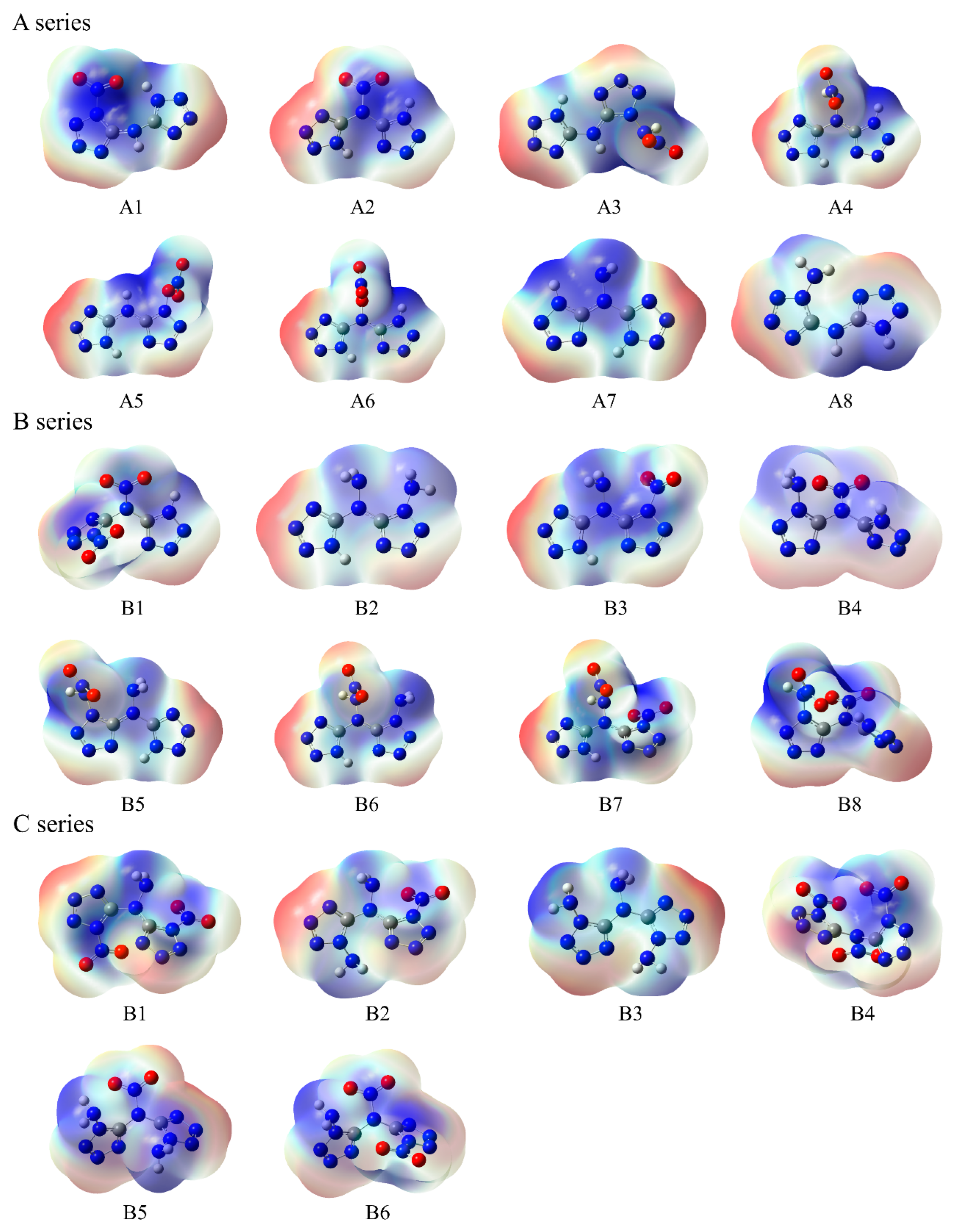
3.2. Electronic Features
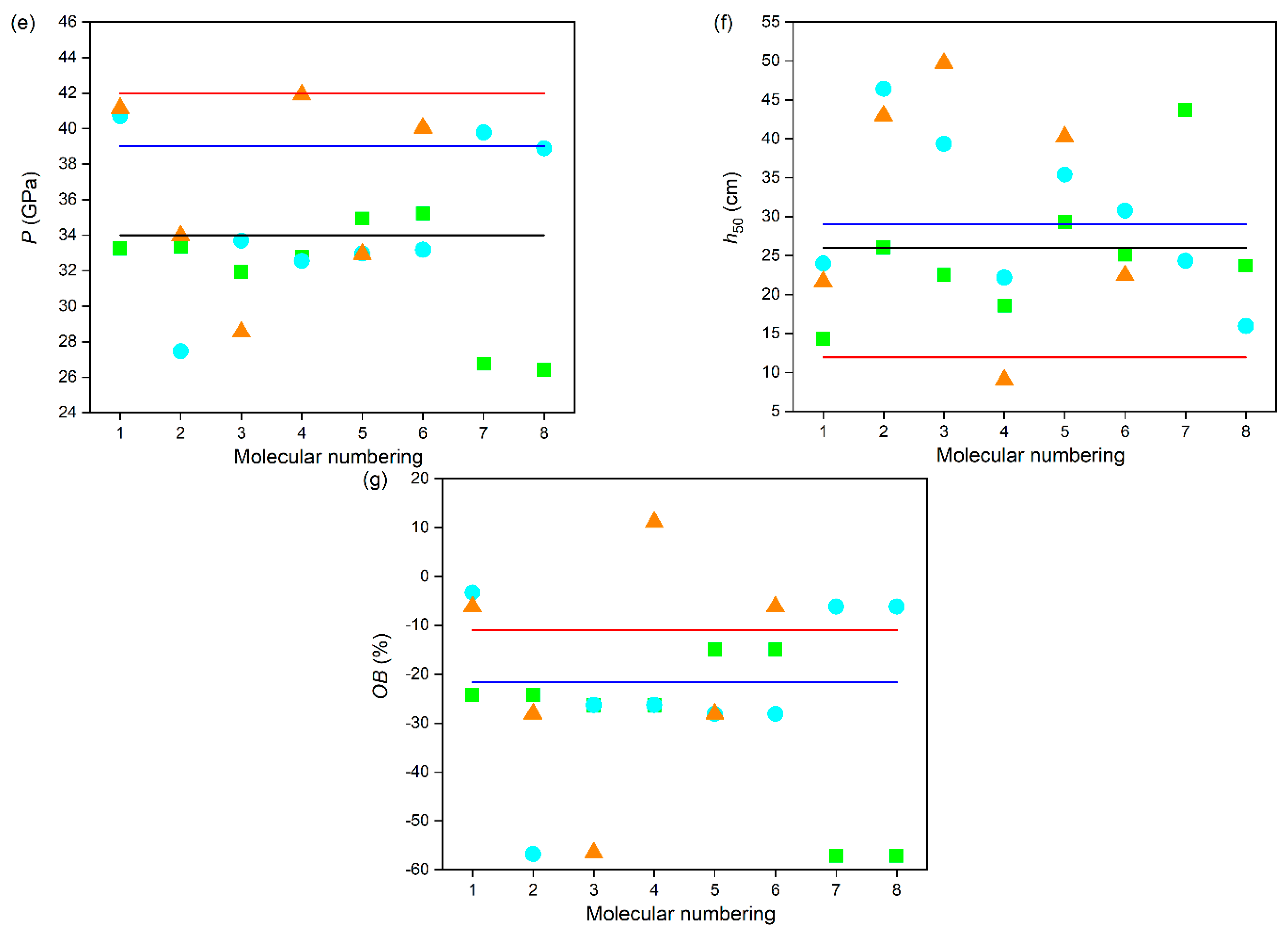
3.3. Energy Characteristics
3.4. Safety Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.A.; et al. 3D Asymmetric Bilayer Garnet-hybridized High-energy-density Lithium-sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Aghmadi, A.; Mohammed, O.A. Energy Storage Systems: Technologies and High-Power Applications. Batteries 2024, 10, 10040141. [Google Scholar] [CrossRef]
- Ali, Z.M.; Calasan, M.; Aleem, S.H.E.A.; Jurado, F.; Gandoman, F.H. Applications of Energy Storage Systems in Enhancing Energy Management and Access in Microgrids: A Review. Energies 2023, 16, 5930. [Google Scholar] [CrossRef]
- Aghmadi, A.; Hussein, H.; Polara, K.H.; Mohammed, O.A. Comprehensive Review of Architecture, Communication, and Cybersecurity in Networked Microgrid Systems. Inventions 2023, 8, 84. [Google Scholar] [CrossRef]
- Kandari, R.; Neeraj, N.; Micallef, A. Review on Recent Strategies for Integrating Energy Storage Systems in Microgrids. Energies 2022, 16, 317. [Google Scholar] [CrossRef]
- Aghmadi, A.; Ali, O.; Mohammed, O.A. Enhancing DC Microgrid Stability under Pulsed Load Conditions through Hybrid Energy Storage Control Strategy. In Proceedings of the 2023 IEEE Industry Applications Society Annual Meeting (IAS), Nashville, TN, USA, 29 October–2 November 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Faisal, M.; Hannan, M.A.; Ker, P.J.; Hussain, A.; Mansor, M.B.; Blaabjerg, F. Review of Energy Storage System Technologies in Microgrid Applications: Issues and Challenges. IEEE Access 2018, 6, 35143–35164. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Zhang, Q.; Shreeve, J.M. Growing Catenated Nitrogen Atom Chains. Angew. Chem. Int. Ed. 2013, 52, 8792–9794. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Qi, C.; Zhao, X.; Zhang, J.; Zhang, S.; Pang, S. 3D Energetic Metal-Organic Frameworks: Synthesis and Properties of High Energy Materials. Angew. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Petermayer, C.; Piercey, D.G.; Jo, S. 1,3-Bis(nitroimido)-1,2,3-triazolate Anion, the N-Nitroimide Moiety, and the Strategy of Alternating Positive and Negative Charges in the Design of Energetic Materials. J. Am. Chem. Soc. 2012, 134, 20827–20836. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, J.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.N.M. Taming of 3,4-Di(nitramino)furazan. J. Am. Chem. Soc. 2015, 137, 15984–15987. [Google Scholar] [CrossRef] [PubMed]
- Chavez, D.E.; Parrish, D.A.; Mitchell, L.; Imler, G.H. Azido and Tetrazolo 1,2,4,5-Tetrazine N-Oxides. Angew. Chem. Int. Ed. 2017, 56, 3575–3578. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Martin, F.A.; Stierstorfer, J. C2N14: An Energetic and Highly Sensitive Binary Azidotetrazole. Angew. Chem. Int. Ed. 2011, 50, 4227–4229. [Google Scholar] [CrossRef]
- Stierstorfer, J.; Klapötke, T.M.; Hammerl, A.; Chapman, R.D. 5-Azido-1H-tetrazole-Improved Synthesis, Crystal Structure and Sensitivity Data. Z. Anorg. Allg Chem. 2008, 634, 1051–1057. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Piercey, D.G. 1,1′-Azobis(tetrazole): A Highly Energetic Nitrogen-Rich Compound with a N10 Chain. Inorg. Chem. 2011, 50, 2732–2734. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.K.; Gandhe, B.R.; Rao, A.S. Environmentally Compatible Next Generation Green Energetic Materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, G.X.; Gong, X.D. Theoretical Studies on 2-(5-Amino-3-nitro-1,2,4-triazolyl)-3,5-dinitropyridine (PRAN) and Its Derivatives. J. Phys. Org. Chem. 2013, 26, 30–36. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, P.; Kumar, D.; Imler, G.H.; Parrish, D.A.; Shreeve, J.N.M. Bis-(3-nitro-1-(trinitromethyl)-1H-1,2,4-triazol-5-yl)methanone: An Applicable and Very Dense Green Oxidizer. J. Am. Chem. Soc. 2019, 141, 19581–19584. [Google Scholar] [CrossRef]
- Yang, H.; Huang, L.; Xu, M.; Tang, Y.; Wang, B.; Cheng, G. Strategy for Extending the Nitrogen Chain: The Bis-(1,2,3-triazole) Formation Reaction from Tosylhydrazones and N-Amino Azole. J. Org. Chem. 2019, 84, 10629–10634. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, G.; Li, J.; Zhang, Z.; Lu, H.; Liao, L.; Fan, G.; Nie, F. Pyrazol-triazole energetic hybrid with high thermal stability and decreased sensitivity: Facile synthesis, characterization and promising performance. Chem. Eng. J. 2020, 379, 31734–31736. [Google Scholar] [CrossRef]
- Benz, M.; Klapötke, T.M.; Stierstorfer, J. Combining Performance with Thermal Stability: Synthesis and Characterization of 5-(3,5-Dinitro-1H-pyrazol-4-yl)-1H-tetrazole and its Energetic Derivatives. Z. Anorg. Allg Chem. 2020, 646, 1380–1388. [Google Scholar] [CrossRef]
- Gettings, M.L.; Thoenen, M.T.; Byrd, E.F.; Sabatini, J.J.; Zeller, M.; Piercey, D.G. Tetrazole Azasydnone (C2N7O2H) And Its Salts: High-Performing Zwitterionic Energetic Materials Containing A Unique Explosophore. Chemistry 2020, 26, 14530–14535. [Google Scholar] [CrossRef]
- Shlomovich, A.; Pechersky, T.; Cohen, A.; Yan, Q.L.; Kosa, M.; Petrutik, N.; Tal, N.; Aizikovich, A.; Gozin, M. Energetic isomers of 1,2,4,5-tetrazine-bis-1,2,4- triazoles with low toxicity. Dalton Tran. 2017, 46, 5994–6002. [Google Scholar] [CrossRef]
- Fei, T.; Du, Y.; Chen, P.; He, C.; Pang, S. N-Fluoro functionalization of heterocyclic azoles: A new strategy towards insensitive high energy density materials. New J. Chem. 2018, 42, 16244–16257. [Google Scholar] [CrossRef]
- Johnson, E.C.; Sabatini, J.J.; Chavez, D.E.; Wells, L.A.; Banning, J.E.; Sausa, R.C.; Byrd, E.F.; Orlicki, J.A. Bis-(Nitroxymethylisoxazolyl) Furoxan: A Promising Standalone Melt-Castable Explosive. Chempluschem 2020, 85, 237–239. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, H.; Imler, G.H.; Parrish, D.A.; Cheng, G.; Shreeve, J.N.M. Derivatives of 3,6-Bis(3-aminofurazan-4-ylamino)-1,2,4,5- tetrazine: Excellent Energetic Properties with Lower Sensitivities. ACS Appl. Mater. Interfaces 2020, 12, 31522–31531. [Google Scholar] [CrossRef]
- Thottempudi, V.; Gao, H.X.; Shreeve, J.M. Trinitromethyl-Substituted 5-Nitro- or 3-Azo-1,2,4-triazoles: Synthesis, Characterization, and Energetic Properties. J. Am. Chem. Soc. 2011, 133, 6464–6471. [Google Scholar] [CrossRef]
- Hahre, W.J.; Radom, L.; Schleyer, P.V.R.; Pole, J.A. Ab Initio Molecular Orbital Theory; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 09; Gaussian Inc.: Wallingford, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Joo, Y.H.; Shreeve, J.M. High-density Energetic Mono-or Bis (oxy) -5-nitroiminotetrazoles. Angew. Chem. Int. Ed. 2010, 49, 7320–7323. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Jacobs, S.J. A Simple Method for Calculating Detonation Properties of C-H-N-O Explosives. J. Chem. Phys. 1968, 48, 23–35. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Some Perspectives on Estimating Detonation Properties of C, H, N, O Compounds. Cent. Eur. J. Energ. Mat. 2011, 8, 209–220. [Google Scholar] [CrossRef]
- Pospíšil, M.; Vavra, P.; Concha, M.C.; Murray, J.S.; Politzer, P. A possible crystal volume factor in the impact sensitivities of some energetic compounds. J. Mol. Model. 2010, 16, 895–901. [Google Scholar] [CrossRef] [PubMed]



| Compounds | HOMO (eV) | LUMO (eV) | ΔE (eV) |
|---|---|---|---|
| A1 | −8.26 | −4.22 | 4.04 |
| A2 | −8.11 | −3.64 | 4.47 |
| A3 | −7.94 | −3.46 | 4.48 |
| A4 | −7.71 | −3.48 | 4.23 |
| A5 | −7.77 | −3.56 | 4.21 |
| A6 | −7.44 | −3.29 | 4.15 |
| A7 | −7.73 | −3.28 | 4.44 |
| A8 | −7.96 | −3.20 | 4.76 |
| B1 | −8.20 | −4.20 | 4.00 |
| B2 | −7.56 | −3.23 | 4.33 |
| B3 | −7.70 | −3.94 | 3.76 |
| B4 | −8.25 | −3.50 | 4.76 |
| B5 | −7.63 | −3.50 | 4.13 |
| B6 | −7.61 | −3.49 | 4.12 |
| B7 | −7.73 | −3.95 | 3.78 |
| B8 | −8.28 | −3.60 | 4.68 |
| C1 | −7.85 | −4.25 | 3.61 |
| C2 | −7.69 | −4.06 | 3.63 |
| C3 | −7.59 | −3.09 | 4.50 |
| C4 | −8.19 | −4.30 | 3.89 |
| C5 | −8.03 | −3.58 | 4.44 |
| C6 | −8.16 | −4.23 | 3.93 |
| Compounds | ρ (g·cm−3) | ΔfH298K(s) (kJ·mol−1) | Q (cal·g−1) | D (km·s−1) | P (GPa) | h50 (cm) | OB (%) |
|---|---|---|---|---|---|---|---|
| A1 | 1.78 | 816.32 | 1513.98 | 8.68 | 33.25 | 14.35 | −24.23 |
| A2 | 1.80 | 783.77 | 1474.72 | 8.67 | 33.36 | 26.06 | −24.23 |
| A3 | 1.76 | 816.36 | 1432.64 | 8.54 | 31.93 | 22.50 | −26.28 |
| A4 | 1.77 | 855.88 | 1476.97 | 8.64 | 32.77 | 18.60 | −26.28 |
| A5 | 1.80 | 764.83 | 1563.01 | 8.88 | 34.93 | 29.36 | −14.95 |
| A6 | 1.80 | 767.14 | 1565.58 | 8.90 | 35.20 | 25.13 | −14.95 |
| A7 | 1.66 | 759.56 | 1079.80 | 7.96 | 26.76 | 43.69 | −57.10 |
| A8 | 1.66 | 747.10 | 1062.07 | 7.91 | 26.40 | 23.70 | −57.10 |
| B1 | 1.88 | 968.38 | 1747.96 | 9.46 | 40.72 | 24.00 | −3.29 |
| B2 | 1.63 | 890.23 | 1161.79 | 8.11 | 27.47 | 46.40 | −56.79 |
| B3 | 1.77 | 938.02 | 1569.09 | 8.76 | 33.70 | 39.37 | −26.28 |
| B4 | 1.75 | 892.20 | 1517.70 | 8.64 | 32.55 | 22.18 | −26.28 |
| B5 | 1.75 | 978.94 | 1532.30 | 8.70 | 32.96 | 35.41 | −28.05 |
| B6 | 1.75 | 987.86 | 1541.64 | 8.72 | 33.17 | 30.77 | −28.05 |
| B7 | 1.85 | 1025.57 | 1720.10 | 9.39 | 39.77 | 24.32 | −6.20 |
| B8 | 1.84 | 992.68 | 1689.65 | 9.30 | 38.89 | 15.96 | −6.20 |
| C1 | 1.86 | 1142.27 | 1828.17 | 9.54 | 41.15 | 21.69 | −6.20 |
| C2 | 1.75 | 1070.46 | 1628.17 | 8.83 | 33.96 | 42.97 | −28.05 |
| C3 | 1.62 | 1016.52 | 1226.08 | 8.29 | 28.56 | 49.74 | −56.52 |
| C4 | 1.95 | 1156.10 | 1611.98 | 9.50 | 41.91 | 9.04 | 11.11 |
| C5 | 1.74 | 1015.43 | 1570.53 | 8.71 | 32.93 | 40.27 | −28.05 |
| C6 | 1.85 | 1080.24 | 1770.73 | 9.43 | 40.04 | 22.45 | −6.20 |
| RDX | 1.82 | 79.00 | 1501 | 8.70 | 34.00 | 26.00 | −21.62 |
| HMX | 1.91 | 102.41 | 1498 | 9.10 | 39.00 | 29.00 | −21.62 |
| CL-20 | 2.04 | 377.04 | 1567 | 9.40 | 42.00 | 11.94 | −10.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yu, Q. Design and Performance Studies on Series of Tetrazole-Based Ultra-High-Energy Density High-Nitrogen Heterocyclic Power Systems. Energies 2025, 18, 1609. https://doi.org/10.3390/en18071609
Li Y, Yu Q. Design and Performance Studies on Series of Tetrazole-Based Ultra-High-Energy Density High-Nitrogen Heterocyclic Power Systems. Energies. 2025; 18(7):1609. https://doi.org/10.3390/en18071609
Chicago/Turabian StyleLi, Yunqiu, and Qiyao Yu. 2025. "Design and Performance Studies on Series of Tetrazole-Based Ultra-High-Energy Density High-Nitrogen Heterocyclic Power Systems" Energies 18, no. 7: 1609. https://doi.org/10.3390/en18071609
APA StyleLi, Y., & Yu, Q. (2025). Design and Performance Studies on Series of Tetrazole-Based Ultra-High-Energy Density High-Nitrogen Heterocyclic Power Systems. Energies, 18(7), 1609. https://doi.org/10.3390/en18071609







