Abstract
Methanol synthesis can utilize the product gas from biomass gasification and the hydrogen generated from water electrolysis. Biomass gasification, as an upstream process, affects the subsequent hydrogen supplement amount and has a direct relationship with the methanol yield. Fluidized bed oxygen-enriched gasification has a particular advantage for biomass and is expected to utilize the remaining oxygen from water electrolysis. In this study, the effects of operating parameters, including the equivalence ratio ER, temperature T, oxygen percentage OP in oxygen-enriched air, steam-to-wood pellets mass ratio S/W, and fluidization velocity ug, as well as the choice of bed materials, on the volume fractions of the gas products and the gas yield from the fluidized bed oxygen-enriched gasification of wood pellets were investigated. The effects of the generation characteristics of gas products on the hydrogen supplement amount and the methanol yield were also analyzed. The results showed that the volume fraction of H2 reached its peak values of 10.47% and 18.49% at an ER value of 0.28 and a ug value of 0.187 m/s, respectively. The methanol yield reached its peak value of 0.54 kg/kg at a ug value of 0.155 m/s. The volume fraction of H2 increased from 6.13% to 11.74% with an increasing temperature from 650 °C to 850 °C, increased from 5.72% to 10.77% with an increasing OP value from 21% to 35%, and increased from 12.39% to 19.06% with an increasing S/W value from 0.16 to 0.38. The methanol yield could be improved by increasing the ER value, T value, OP value, or S/W value. When the bed materials were changed from quartz sands to dolomite granules, the H2 volume fraction significantly increased and the hydrogen supplement amount required for methanol synthesis reduced.
1. Introduction
As a low-carbon and oxygen-containing fuel, methanol has the advantages of a high combustion efficiency and clean emissions and is recognized as an ideal renewable energy source worldwide. Methanol is liquid at room temperature and atmospheric pressure, which is convenient for storage, transportation, and refueling, and is regarded as an ideal energy choice for automobiles and a potential fuel for future shipping [1]. When methanol is utilized as an automotive fuel, its advantages in terms of environmental friendliness, applicability, and reliability are evident [2]. Numerous renowned automotive manufacturers in China have actively engaged in the research, development, and manufacturing of methanol-powered vehicles [3]. When used as a shipping fuel, methanol has the advantages of mature technology, a high energy density much closer to that seen in fossil fuels, and a relative lack of ecotoxicity [4]. It is useable without significantly displacing the load capacity and is the lowest cost sustainable fuel option at the point of delivery [5]. Longspur Research, a clean energy specialist financial services firm, identified methanol as the best solution available for decarbonizing shipping [5]. The demand for methanol in future shipping is expected to increase significantly. At present, 65% of global methanol production is based on natural gas as a feedstock, and 35% is based on coal [6]. The former is called ‘grey’ methanol, while the latter is called ‘brown’ methanol, which contradicts the development trend of low-carbon and clean energy utilization. Bio-methanol, derived from biomass and renewable-electricity-based methanol, may offer a pathway to a genuine lower carbon solution. Bio-methanol is typically produced through the gasification of biomass to produce syngas and then converting and distilling the syngas [7]. Renewable-electricity-based methanol is produced by combining green hydrogen, derived from renewable energy through electrolysis, and CO2 (or CO), derived from carbon capture or gasification [8]. Syngas, which is the feedstock for methanol synthesis, can be produced from biomass through gasification. The molar ratio of H2 to CO, as well as the molar ratio of H2 to CO2, in the syngas from biomass gasification generally fails to meet the requirements of the methanol synthesis reaction. In this case, green hydrogen from renewable energy through electrolysis can provide a source for supplementing the hydrogen amount required for methanol synthesis.
In recent years, the production of methanol using syngas from biomass gasification as a raw material has attracted more and more attention. In this context, researchers have investigated various green methanol production systems to improve the methanol yield and the optimize energy efficiency in methanol production. Liu et al. [9] proposed a sorption-enhanced biomass gasification coupled with carbon utilization to produce tunable syngas for methanol synthesis. In their study, Aspen Plus software was used to simulate a biomass gasification process based on sorption-enhanced H2 production coupled with carbon utilization for CO production. Their study found that when the CO2 fraction was 0.6, the H2/CO ratio in the syngas approximated 2, and the methanol yield reached 702.50 kg per ton of biomass briquette fuel, making it suitable to be used for methanol synthesis. The exergy efficiency of this system was about 64%, with the main exergy losses concentrated in the sorption enhanced water gas shift unit, gasification unit, power generation unit, and methanol synthesis unit.
Im-orb et al. [10] performed technical and sustainability analyses on methanol production via the integrated biomass pyrolysis, gasification, and methanol synthesis (IBPGM) process using rice straw as feedstock and performed a feasibility study of using captured CO2 as a gasifying agent. In their study, a model of the IBPGM process was constructed using the Custom Modeler Module in the Aspen Engineering Package. Their study indicated that the production rate of methanol and the syngas yield both increased with the CO2 recycle fraction. In addition, their energy analysis indicated that the IBPGM process was an exothermic process, the energy consumption of the gasifier increased as the CO2 recycle fraction increased, and thermal self-sufficiency was attained when the recycle fraction reached 0.76. Puig-Gamero et al. [11] used Aspen Plus software to simulate methanol synthesis from syngas obtained through pine gasification. In their study, three integrated processes were simulated, including the gasification process with tar reformation, syngas cleaning using pressure swing adsorption, and methanol synthesis, and effects of the gasification temperature and the steam-to-biomass (S/B) mass ratio on the syngas composition, tar yield, and methanol production were evaluated. According to the best results in syngas composition and methanol production, a temperature of 900 °C and a S/B mass ratio of 0.9 were selected as the best calculated operational conditions, and the H2/CO molar ratio of 2.41 was obtained, being closed to the syngas specification needed for the methanol synthesis.
Biomass gasification can be carried out in different types of gasifiers to produce syngas for methanol synthesis. Albarelli et al. [12] evaluated a sugarcane biorefinery producing methanol via the gasification of sugarcane lignocellulosic residues and methanol synthesis. In their study, an entrained flow (EF) gasifier and a circulating fluidized bed (CFB) gasifier were analyzed and compared for syngas production. Their research findings indicated that the methanol produced based by the CFB process is cheaper than in the EF case since the investments in gasification, syngas cleaning, and fuel synthesis are considerably lower. However, the heat demand for methanol production in the CFB case is higher due to the heat requirement for the catalytic tar removal.
Biomass gasification coupled with hydrogen production from water electrolysis for methanol synthesis is one of the most promising pathways for large-scale and low-carbon fuel production. Liu et al. [13] established a model of methanol production from red-mud-enhanced biomass gasification coupled with solid oxide electrolysis cells (SOEC) using Aspen Plus and analyzed the economic feasibility of producing syngas from red-mud-enhanced biomass gasification for methanol synthesis. In their study, hydrogen was supplemented by the water electrolysis of SOEC to adjust the H/C ratio at the inlet for methanol synthesis. The simulation results showed that the red mud increased the methanol yield, with an increase of 23.9%, and the economic analysis indicated that the red mud could reduce the methanol production cost.
Biomass gasification, as an upstream process for methanol synthesis, not only affects the subsequent hydrogen supplement amount but also has a direct relationship with the methanol yield and the methanol production cost. Generally, the H2/CO ratio of the syngas obtained through biomass gasification is challenging to align with the requirements of methanol synthesis, and the proportion of the H2 in the syngas should be increased as much as possible to save the amount of hydrogen supplement. Many studies have focused on the direct production of hydrogen-rich syngas through biomass gasification, which can increase H2 production by adding steam [14,15,16] or catalysis [17] to the gasifier to facilitate the steam reforming. Shayan et al. [18] simulated the gasification process, employing the thermodynamic equilibrium model, and compared and analyzed the hydrogen production from biomass gasification using four different gasifying agents. The simulation results showed that the order of the four gasifying agents according to the H2 mole fraction is as follows: steam > oxygen > oxygen-enriched air > air. Gao et al. [19] found that the H2 volume in the syngas and the total syngas yield gradually increased with the increasing water vapor concentration, but when the water vapor concentration reached 15%, the tar conversion efficiency and the effective gas yield reach their peaks.
Given that the increase in H2 production in syngas through the use of steam as a gasifying agent is limited, it is possible to simultaneously add bed materials that have a catalytic effect on steam reforming, thereby further improving H2 production and syngas quality. Gao et al. [19] evaluated the reactivity of two different bed materials in fluidized bed experiments of biomass gasification. It was found that compared to quartz sand samples, the hematite samples showed a significantly higher H2 volume and CO volume in syngas, syngas yield and carbon conversion efficiency. They pointed out that the main component of hematite was Fe2O3, which underwent the migration of lattice oxygen during the gasification to promote gasification reactions and form a porous structure that absorbed tar molecules. Conversely, the O atoms in the SiO2 were difficult to remove from the lattice, resulting in the low catalytic activity of the quartz sand samples [20]. The addition of CaO-based sorbents, such as calcined dolomite and calcined limestone, during gasification can break the thermodynamic equilibrium constraint and enhance hydrogen production. Among these sorbents or catalysts, calcined limestone proved to be most effective at absorbing CO2 [21], while dolomite showed a better performance in catalytic activity on tar destruction [22]. Zhang et al. [21] investigated the steam gasification of wet biomass with different kinds of CaO-based sorbents in a fixed-bed experiment system. Their experimental results indicated that the sorbent prepared by wet mixing, with a limestone/dolomite mass ratio of one, demonstrated higher activity toward hydrogen production. Pang et al. [23] conducted experiments on the steam gasification of biochar using Fe2O3/CaO as in situ catalysts. It was found that under the combination of two catalysts, the addition of CaO improved the gasification intensity of biochar at a lower temperature and steam flow rate, while the addition of Fe2O3 improved the hydrogen production yield.
In addition, besides iron, transition metals, especially nickel catalysts, have attracted widespread attention in biomass gasification for hydrogen production due to their relatively low metal cost and high redox activity. Jin et al. [24] developed Ni-based catalysts with Ca added and studied the effect of the calcium addition on the catalysts during biomass gasification for hydrogen production. Their experimental results indicated that the hydrogen yield per mole of nickel increased from 50 g mol−1 of Ni to 80 g mol−1 of Ni when the catalyst changed from 0.1 mol% of Ca to 0.8 mol% of Ca, and the 0.1Ca catalyst produced a much lower hydrogen yield compared to the other Ca-added catalysts. They attributed this phenomenon to the much lower content of small NiO particles in the 0.1Ca catalyst.
The use of oxygen-enriched air or oxygen as a gasifying agent can reduce oxygen waste by utilizing the remaining oxygen produced by water electrolysis when utilizing the hydrogen obtained from water electrolysis. Oxygen-enriched air gasification and oxygen gasification utilize oxygen as a gasifying agent, which can reduce the dilution of syngas by nitrogen in the air, increase the heating value of the product gas, and also lead to an increase in carbon-based compounds such as CO and CO2 in the product gas [25]. Using a mixture of oxygen and steam as a gasifying agent, the heat required for the endothermic gasification process can be provided by partial fuel oxidation, unlike using only steam as a gasifying agent, which requires allothermal dual fluidized bed gasification [26]. In addition, oxygen is an effective reactant for improving the gas yield, char conversion rate, and tar reformation. Cao et al. [27] used Aspen Plus software to simulate the oxygen-enriched gasification of five types of biomasses and validated the simulation results with experimental results. They studied the effects of temperature, the equivalence ratio, and oxygen enrichment on the product gas yield, the lower heating value of the product gas, and the tar yield. The results showed that using oxygen-enriched air was favorable for the product gas quality and tar cracking but lowered the gas yield. Cao [28] also conducted parametric studies on biomass gasification by using air, oxygen-enriched air, air/steam, and oxygen-enriched air/steam as gasification agents. They studied the effects of the equivalence ratio, temperature, the oxygen percentage in the air (OP), and the steam/biomass ratio on the gasification process. It was found that the increase in the OP led to an increase in the H2 production, char conversion rate, and tar cracking, but the product gas yield showed an opposite trend due to the decrease in N2 dilution. When oxygen-enriched air and steam were used as gasification agents, the maximum H2 content was achieved due to their two advantages of reducing N2 dilution and promoting the water–gas shift reaction.
Sebastiani et al. [29] developed a one-dimensional, non-isothermal model of a bubbling fluidized bed reactor to simulate the fluidized bed gasification of biomass and plastic waste using oxygen and steam as gasification agents and validated the simulation results with data from a pilot-scale gasifier. Their research results indicated that at the same equivalence ratio, steam–oxygen gasification showed some advantages compared to air gasification, with higher CO and H2 contents and lower tar and CO2 contents. Steam–oxygen gasification also has a significant impact on hydrocarbons reforming, thereby reducing CH4 and light hydrocarbons.
The oxygen-enriched gasification of biomass coupled with hydrogen production through water electrolysis can not only utilize the hydrogen obtained from water electrolysis to supplement the syngas required for methanol synthesis but also utilize the remaining oxygen from water electrolysis as a gasification agent to improve the quality of the product gas in gasification. The fluidized bed gasifier design has a low operating temperature and excellent heat and mass transfer [30,31,32], which is especially suitable for biomass gasification [33], thus is conducive to combining biomass gasification with methanol synthesis. At present, there are few experimental studies on the distribution of gas products from fluidized bed oxygen-enriched gasification with the aim of utilizing the product gas to synthesize methanol. In this study, the effects of the equivalence ratio, ER; gasifier temperature, T; oxygen percentage, OP, in the oxygen-enriched air; steam-to-wood-fuel mass ratio, S/W; fluidization velocity, ug; and bed material type on the distribution and yield of gas products from the oxygen-enriched gasification of fluidized beds were investigated by using a bubbling fluidized bed gasifier. The effects of operating parameters and bed material types on the hydrogen supplement amount, methanol yield, and oxygen consumption were also analyzed.
2. Experimental Section
2.1. Materials
Wood pellets were used as biomass raw materials for oxygen-enriched gasification, which were crushed and screened into granules with particle sizes of 600–830 μm. Their proximate analysis and ultimate analysis results are shown in Table 1. As cheap and the most readily available granular solids, quartz sands were used as bed materials, with a true density of 2470 kg/m3 and particle sizes of 270–425 μm.

Table 1.
Proximate analysis and ultimate analysis results of wood pellets.
2.2. Experimental Apparatus
The oxygen-enriched gasification experiment was carried out in a bubbling fluidized bed experimental apparatus, which consisted of a gasification-agent-conveying part, a biomass-feeding part, a fluidized bed gasifier, a filtration part for the product gas, and a detection and analysis part for product gas, as shown in Figure 1. The gasification-agent-conveying part was used for the transportation of air, oxygen, and steam. The Roots blower (ZLS-32L, manufactured by Zhenglin Machine Ltd. in Zhoushan city of China) was used to provide air, the high-pressure oxygen cylinder was used to store and supply oxygen, the steam generator (SG-3020, produced by Yike Process Control Technology Ltd. in Suzhou city of China) was used to realize the evaporation of water stably and accurately, and the gasification agent mixture was preheated by the preheater. The flow rate of the Roots blower was 30 m3/h, and its exhaust pressure was 29.4 kPa. When the required air flow rate was lower, a diverter valve was used to discharge excess air. The liquid evaporation capacity of the steam generator was 0.5–20 mL/min, the outlet temperature could be adjusted between 100 and 200 °C, and the electric heat tracing was set in the outlet pipeline to control the steam temperature in the pipeline and prevent steam condensation. The response time of the steam generator was less than 10 s, and a peristaltic pump was used to accurately adjust the mass flow of water entering the evaporator, with an accuracy of ±2%. The volume flowrates of air and oxygen were regulated by two rotameters. The LZB-10 rotameter or the LZB-4WB rotameter (both produced by Yuyao Automation Instrument Factory in China) was used for air: their measuring ranges were, respectively, 0.16–1.6 m3/h and 0.3–3 L/min, and the measuring accuracy of both was ±2.5%. The measuring range of the LZB-4WB rotameter for oxygen was 0.3–3 L/min, and its measuring accuracy was ±2.5%. The oxygen provided by the oxygen cylinder entered the steam generator through the carrier gas inlet and was mixed with a certain amount of steam generated.
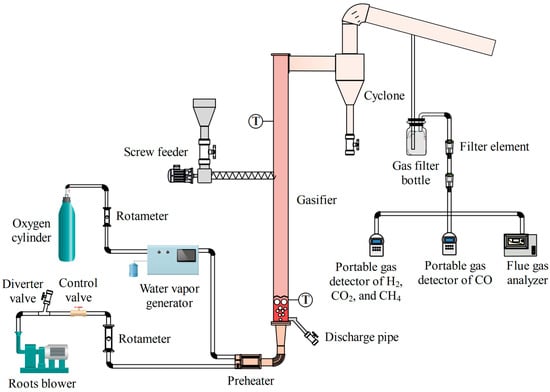
Figure 1.
Schematic diagram of oxygen-enriched gasification experimental apparatus of bubbling fluidized bed.
The biomass feeding part was composed of a hopper and a screw feeder, and the feeding capacity of the screw feeder for wood pellets was 0–0.59 kg/h. The fluidized bed gasifier was composed of a gas distribution plate, a reactor, a horizontal flue, a cyclone separator, and an exhaust pipe. The cross-section of the vertical reactor had an inner diameter of 0.05 m and a height of 1.1 m. The external wall of the vertical reactor was adjacent to the 12 kW ceramic heater, and the outer layer was wrapped with a glass wool insulation layer with a thickness of 0.2 m. A K-type thermocouple was installed at a height of 0.1 m above the gas distribution plate to measure the temperature in the gasifier. Another thermocouple was installed at a height of 0.8 m to control the temperature in the gasifier. The product gas sampling pipeline was arranged at the outlet of the exhaust pipe. The condensed water, fly ash, and condensed tar from the product gas were filtered by the filtration part for product gas. The product gas filtration part consisted of a gas filter bottle filled with absorbent cotton and a filter element.
The detection and analysis part for the product gas consisted of two portable gas detectors and one flue gas analyzer. The MS400-3K gas detector (produced by SAACOO Instruments Ltd. in Chongqing of China) was used to measure the volume fraction of H2, CO2, and CH4 in the product gas. The MS400-CO-K gas detector(produced by SAACOO Instruments Ltd. in Chongqing of China) was used to measure the volume fraction of CO in the product gas. The flue gas analyzer, with model KANE9506 (produced by China Technical Service Center of KANE International Ltd. in Beijing of China), was mainly used to measure the volume fraction of O2 in the product gas. The resolution of the two portable gas detectors was 0.01%VOL, and the accuracy was ≤±3%F.S. The measurement range of CO was 0–50%VOL, the measurement range of CO2 was 0–100%VOL, the measurement range of H2 was 0–40%VOL, and the measurement range of CH4 was 0–20%VOL. The resolution of flue gas analyzer was 0.01%VOL, its accuracy was −0.1%F.S–0.2%F.S, and the measurement range of O2 was 0–25%VOL.
2.3. Experimental Methods
Before the gasification experiment, the gasifier was heated to the required temperature. After the temperature in the gasifier was evenly distributed, the wood pellets and gasification agent were separately delivered to the gasifier. Under the control temperature of 400 °C set for the preheater, the air, oxygen, and steam (if it existed) were separately conveyed at their constant volumetric flow rates and were preheated before being fed into the gasifier. During the experiment, after the outlet temperature and the gas production of the steam generator reached stable levels, the oxygen was supplied into the steam generator to mix with the steam. The mixture of oxygen and steam was then mixed with the conveying air in the preheater. Under stable conditions, the experiment of the oxygen-enriched gasification of wood pellets in a fluidized bed was carried out, and the volume fractions of the gas products were measured by the gas detectors and the flue gas analyzer. Under the stable condition, volume fractions of the product gas components were measured and recorded. Three parallel experiments were carried out for the gasification process under each stable condition. After the experiment, based on the data recorded by the gas detectors and the flue gas analyzer, the average volume fractions of the relevant gas components under each of the stable gasification conditions were calculated.
The feed rate of wood pellets was adjusted under the determined ER value and S/W ratio, allowing for the changes in the volumetric flow rates of oxygen and water vapor. The volumetric flow rate of oxygen was controlled through the valve at the outlet of the oxygen cylinder. The flow rate of the water vapor generated by the water vapor generator was controlled by adjusting the inlet flow rate of the peristaltic pump. The air flow rate was controlled through the valve installed in the air supply pipeline. The volumetric flow rates of oxygen and air were measured by the rotameters installed in their respective pipelines. The mass flow rate of the water vapor was determined by the flow rate setting function of the water vapor generator. Under the constant cross-sectional area in the gasifier, the fluidization velocity was adjusted by controlling the total volumetric flow rate of the gasifying agent. During the experiment, the apparent gas velocity of the gasification agent in the gasifier was controlled to be higher than the minimum fluidization velocity of the bed materials to ensure that the gasification was in a bubbling fluidized bed. The calculating formula for the minimum fluidization velocity umf is
where dp is the average particle size (m), ρp is the true density of the particles (kg/m3), ρg is the density of the gasification agent (kg/m3), g is the gravitational acceleration (m/s2), and µ is the dynamic viscosity of the gas (Pa·s). The maximum particle size of 425 μm of the quartz sands used was substituted into Equation (1), and the minimum fluidization velocity at 800 °C was calculated to be 0.060 m/s, with OP = 35% as an example. In the experiments, the fluidization velocity ranged from 0.122 to 0.187 m/s.
2.4. Relationship Between Feed Rate and Rotational Speed of Screw Feeder
In order to determine the relationship between the feed rate and the rotation speed of the screw feeder, the feeding rates of the screw feeder, corresponding to different rotation speeds of the motor, were measured at the gasifier temperature of 800 °C. Wood pellets of 20.0 g were weighed with an electronic balance before each measuring experiment and then were poured into the hopper above the feeder, and the timing was synchronized until the wood pellets disappeared instantaneously at the bottom of the hopper (screw feeder inlet). The feed rates, corresponding to the rotation speeds of the motor, were then calculated. At the same rotation speed as the motor, the feed rate test was repeated three times. The average value was taken as the feed rate at each rotation speed. Scatter plots of feed rates at different motor rotation speeds were drawn, and the relational expression between the feed speed, qm, and the rotational speed, ω, of the screw feeder was fitted, as depicted in Figure 2. The R-squared (R2) value of the linear fitting was 0.96, indicating that the linear regression model had a high degree of fitting and strong predictive ability.
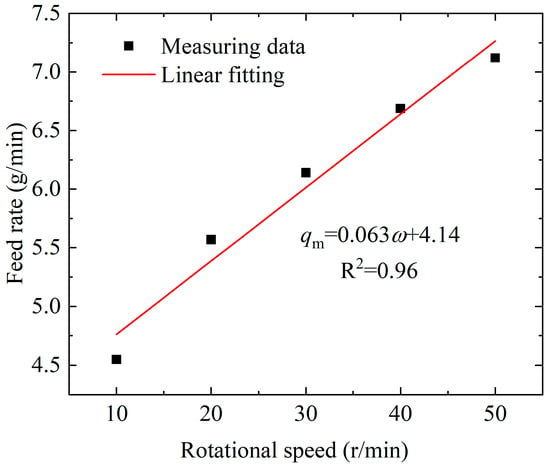
Figure 2.
The relationship between the feed rate and rotational speed of the screw feeder.
2.5. Data Analysis
In the case of utilizing the product gas from biomass gasification for methanol synthesis, the quality of the product gas was evaluated based on the H2/CO ratio; the H2/CO2 ratio; the hydrogen supplement amount, ; the methanol yield, YM; and the oxygen consumption, . Additionally, the syngas production could be evaluated by the product gas yield, Yg.
Based on the conservation of nitrogen, the volume (in the standard state) of the product gas from the gasification per the unit mass of wood pellets could be obtained, i.e., the product gas yield, Yg (m3/kg):
where Vair is the volume flow rate of air before entering the preheater (m3/h); Nar is the mass percentage of nitrogen in the wood pellets (%); XH2, XCO, XCO2, and XCH4 are, respectively, the volume fractions of H2, CO, CO2, and CH4 in the product gas (%vol); and qm is the feed rate of the wood pellets (kg/h).
When analyzing the effects of the equivalence ratio, the equivalence ratio, ER, is determined by
where is the volumetric flow rate of oxygen at the outlet of the oxygen cylinder (L/min) and represents the theoretical air volume required for complete combustion per the unit mass of wood pellets (m3/kg); based on the results of the elemental analysis, = 0.746 m3/kg.
When analyzing the effects of the oxygen percentage in the oxygen-enriched air, the volume percentage of oxygen, OP, is defined by
In order to improve the reaction efficiency of methanol synthesis and the methanol yield, in an ideal methanol synthesis case, the syngas for methanol synthesis satisfies the following specification [34], as shown in Relational Expression (5). This molar ratio is widely used to express the syngas quality.
The amount of H2 in the product gas from biomass gasification is typically lower than that required for methanol synthesis. According to Equation (5), the calculation equation of theoretical hydrogen supplement amount (m3/kg) is derived as follows.
The amount of oxygen consumed per kilogram of wood pellets during gasification, (m3/kg), can be calculated using the following relational expression:
Methanol is produced through the synthesis of CO and H2 or the synthesis of CO2 and H2, as represented by the reactions in Equations (8) and (9):
The theoretical methanol yield, YM (kg/kg), of methanol synthesis using the product gas from the gasification per kilogram of wood pellets, in the case of the hydrogen supplement, can be calculated by Equation (10):
3. Results and Discussions
3.1. Effects of Equivalence Ratio
The equivalence ratio, ER, is defined as the ratio of the actual amount of oxygen supplied to the theoretical amount of oxygen required for the complete combustion of the fuel [35]. It serves as a crucial control parameter in the gasification process. A higher ER value indicates an increased supply of oxygen for the gasification process, thereby releasing more heat by oxidation reactions [36]. An appropriate ER value facilitates the progression of the gasification process. Gasification experiments were conducted at different ER values to investigate the effects of the ER value on the volume fractions and the yield of gas products and methanol synthesis. The total flow rate of the gasification agent was maintained at 3.66 L/min, with a superficial gas velocity, ug, of 0.13 m/s and an oxygen percentage, OP, in oxygen-enriched air set at 35%. At a gasifier temperature of 800 °C, the ER value was changed by adjusting the feed rate of the wood pellets and maintaining a constant oxygen mass flow rate.
Figure 3 illustrates the variations in the volume fractions of the gas products with the changing ER values. As the ER value increased from 0.22 to 0.36, the volume fractions of CO and CH4 decreased from 25.32% and 5.78% to 17.55% and 3.87%, respectively, while the volume fraction of CO2 increased from 16.57% to 21.20%. The volume fraction of H2 initially increased, then decreased, reaching its peak value of 10.47% at ER = 0.28. At lower ER values, a larger number of wood pellets were fed into the gasifier with relatively less oxygen, resulting in pyrolysis being the dominant process. This led to higher volume fractions of CO and CH4 in the product gas. As the ER value increased from 0.22 to 0.36, more O2 participated in the oxidation reactions (Reactions (11)–(16)) in the gasifier. Consequently, CO and CH₄ were partially consumed, producing additional CO2 and releasing heat. This promoted the forward water–gas reaction (Reaction (17)) and reverse Boudouard reaction (Reaction (18)), generating more H2 and CO. The formation of CO also drove the forward water–gas shift reaction (Reaction (19)), leading to the production of more CO2 and H2. Overall, by increasing the ER value, more CO was oxidized to produce CO2. As a result, there was a decreasing trend in the volume fraction of CO. However, when the ER value reached 0.28, an equilibrium was reached between the oxidation of H2 and the reactions that produced H2 (e.g., the forward water–gas reaction and steam-reforming reactions), resulting in the peak volume fraction of H2. As the ER value continued to increase, the oxidation of H2 became dominant, resulting in a reduction in the volume fraction of H2 in the product gas. Both the H2/CO ratio and the H2/CO2 ratio initially increased with the ER value, then decreased, reaching their respective maximum values of 0.52 and 0.54 at an ER value of 0.28.
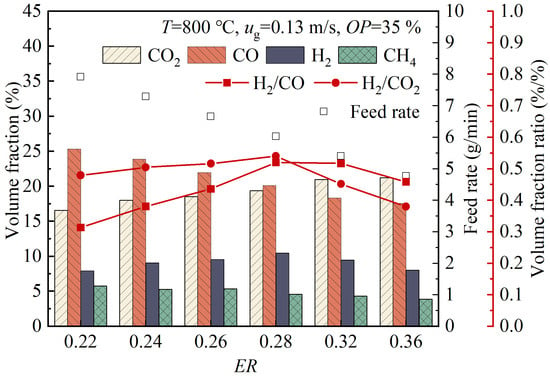
Figure 3.
Variations of volume fractions of gas products with ER value.
Figure 4 shows the effects of the ER value changes on the product gas yield of oxygen-enriched gasification and the hydrogen supplement amount, methanol yield, and oxygen consumption when the product gas was used for methanol synthesis. As the ER value increased from 0.22 to 0.36, the product gas yield, hydrogen supplement amount, oxygen consumption, and methanol yield all showed an upward trend, increasing from 0.69 m3/kg, 0.68 m3/kg, 0.15 m3/kg, and 0.44 kg/kg to 1.02 m3/kg, 0.99 m3/kg, 0.24 m3/kg, and 0.61 kg/kg, respectively. An increase in the ER value meant that the oxygen supply per the unit mass of wood pellets was increased, which not only promoted oxidation reactions, providing more heat, but also facilitated tar cracking, resulting in an increased gas yield. The value was positively correlated with the ER value and changed solely in response to variations in the ER value. According to Equation (6), the value was positively correlated with the volume fractions of CO and CO2 in the product gas, as well as the product gas yield. As the ER value increased, both the volume fraction of CO2 and the product gas yield exhibited significant growth, thereby leading to an increase in the value. According to Equation (10), the YM value was also positively correlated with the volume fractions of CO and CO2, as well as the product gas yield. Therefore, an increase in the ER value resulted in a higher methanol yield. The methanol yield at an ER value of 0.36 was slightly lower than the 0.7025 kg/kg value in the methanol synthesis from the syngas produced by biomass gasification based on CaO sorption-enhanced H2 production coupled with carbon utilization for producing high purity CO, which was reported by Liu et al. [9], but much higher than the 0.32 kg/kg value in the methanol synthesis at a pressure of 55 atm and a lower temperature of 220 °C, which was reported by Puig-Gamero et al. [11] as the methanol production at optimal operation conditions. In order to improve the potentiality and economic viability of the product gas being used for methanol synthesis, it was crucial not only to increase the theoretical methanol yield but also to increase the H2/CO ratio and the H2/CO2 ratio in the product gas as much as possible. This strategy helped to reduce the costs of hydrogen supplementation in methanol synthesis.
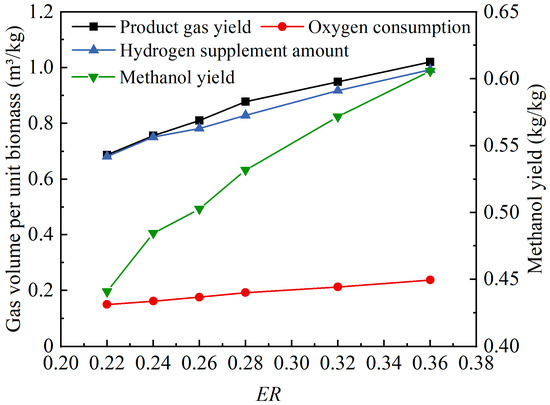
Figure 4.
Effects of ER value changes on product gas yield and methanol synthesis.
3.2. Effects of Gasifier Temperature
An experimental study on the oxygen-enriched gasification of wood pellets was carried out using a bubbling fluidized bed gasifier under ug = 0.13 m/s, ER = 0.28, OP = 35%, and qm = 6.03 g/min, in order to investigate the effects of the gasifier temperature on the product gas composition and methanol synthesis. The gasifier temperature varied in the range of 650–850 °C.
Figure 5 illustrates the variations in the volume fractions of the gas products with the changing gasifier temperature, T. As the T value increased from 650 °C to 850 °C, the volume fractions of H2 and CO in the product gas increased from 6.13% and 14.12% to 11.74% and 20.84%, respectively, while the volume fractions of CO2 and CH₄ decreased from 23.89% and 5.56% to 18.84% and 4.17%, respectively. The increase in temperature led to an increase in the pyrolysis reaction rate of the wood pellets, as well as an increase in the gasification reaction rate and gasification intensity, thus accelerating the release of volatile substances in the gasifier and the secondary reactions of pyrolysis products. Furthermore, the increase in temperature promoted the tar cracking (Reaction (20)) and tar reforming reactions (Reactions (21) and (22)), thereby producing more small-molecule gases. From the perspective of chemical equilibrium, increasing the temperature favored endothermic reactions, driving the forward water–gas reaction (Reaction (17)) and reverse Boudouard reaction (Reaction (18)). As a result, the production of CO and H2 increased, while the production of CO2 decreased. Moreover, the higher temperature promoted the reverse water–gas shift reaction (Reaction (19)), the hydrogasification reaction (Reaction (23)) and the methanation reactions (Reactions (24)–(26)), thereby reducing the volume fractions of CO2 and CH₄ in the product gas. The H2/CO ratio and the H2/CO2 ratio increased with the rising temperature, suggesting that under oxygen-enriched conditions, higher temperatures are beneficial for increasing the ratio of hydrogen to carbon oxide in the product gas [37].
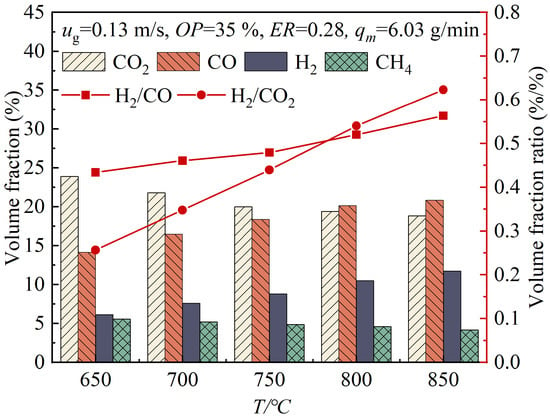
Figure 5.
Variations of volume fractions of gas products with changing gasifier temperatures.
Figure 6 shows the effects of the gasifier temperature changes on the product gas yield of oxygen-enriched gasification and the hydrogen supplement amount and the methanol yield when the product gas was used for methanol synthesis. As the temperature increased from 650 °C to 850 °C, the product gas yield, Yg, and methanol yield, YM, both showed an upward trend, increasing from 0.85 m3/kg and 0.46 kg/kg to 0.96 m3/kg and 0.55 kg/kg, respectively. The value initially decreased and then increased with the rising temperature, with relatively small variations. The increase in temperature enhanced the pyrolysis of the wood pellets, the gasification of small-molecule gases and coke, as well as the cracking and reforming of large-molecular tar compounds, resulting in an increase in the Yg value. The rise in temperature significantly increased the volume fraction of CO and the product gas yield, thereby improving the methanol yield when the product gas was used for methanol synthesis. As the temperature increased within the range of 650–750 °C, the significant decrease in the volume fraction of CO2 and the notable increase in the volume fraction of H2 led to a reduced requirement for hydrogen supplementation in the methanol synthesis. When the temperature increased in the range of 750–850 °C, the slow reduction in the volume fraction of CO2, along with the significant increases in the volume fractions of CO and H2, led to an increased requirement for hydrogen supplementation in the methanol synthesis.
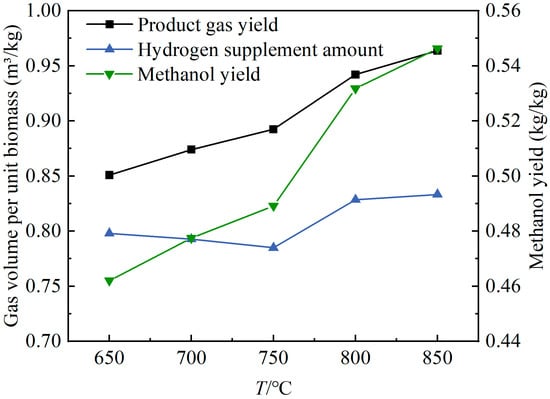
Figure 6.
Effects of gasifier temperature changes on product gas yield and methanol synthesis.
3.3. The Effects of the Oxygen Percentage in the Oxygen-Enriched Air
When air is one gasification medium, the nitrogen in it will dilute the product gas. Increasing the oxygen percentage in the oxygen-enriched air is beneficial for improving the quality of the product gas [38]. When using the hydrogen obtained from water electrolysis, the remaining oxygen from the water electrolysis can be utilized for oxygen-enriched gasification, thereby reducing the large amount of energy spent in separating the oxygen from the air. In order to ensure that the wood pellets have sufficient residence time in the gasifier when fed at the middle height of the gasifier, an experimental study on the oxygen-enriched gasification of wood pellets in a fluidized bed was conducted under the conditions of ug = 0.13 m/s, T = 800 °C, and ER = 0.22. This study aimed to investigate the effects of the oxygen percentage in the oxygen-enriched air on the volume fractions and the yield of the product gas and their effects on methanol synthesis. The oxygen percentage, OP, in the oxygen-enriched air varied in the range of 21% to 35%.
Figure 7 shows the variations in the volume fractions of the gas products with the changes in the oxygen percentage, OP, in the oxygen-enriched air. As the OP value increased from 21% to 35%, the volume fraction of H2 in the product gas rose from 5.72% to 10.77%, the volume fraction of CO increased from 12.51% to 21.48%, the volume fraction of CO2 increased from 13.16% to 20.04%, and the volume fraction of CH4 increased from 2.96% to 6.38%. This phenomenon reflected that, under the constant fluidization velocity, an increase in the OP value led to a decrease in the volumetric flow rate of nitrogen, resulting in an increase in the volume fractions of other gas components in the product gas and the improvement in the product gas quality. As the OP value increased, there was no significant change in the H2/CO ratio, while the H2/CO2 ratio slightly increased, indicating that the increase in the OP had no significant effect on these two ratios.
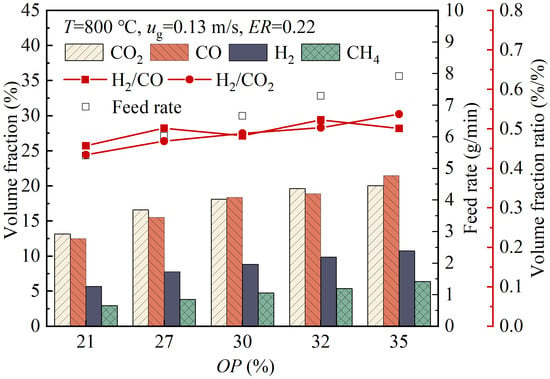
Figure 7.
Variations in the volume fractions of gas products with the changes in the oxygen percentage in the oxygen-enriched air.
Figure 8 shows the effects of the changes in the oxygen percentage in the oxygen-enriched air on the product gas yield and their effects on the hydrogen supplement amount and the methanol yield in methanol synthesis. As the OP value increased from 21% to 35%, the product gas yield decreased from 0.90 m3/kg to 0.80 m3/kg, the hydrogen supplement amount increased from 0.53 m3/kg to 0.74 m3/kg, and the methanol yield increased from 0.33 kg/kg to 0.48 kg/kg. As the OP value increased, the feed rate of the wood pellets increased proportionally, while the amount of bed materials in the dense phase of the gasifier remained unchanged. This led to a weakening of the mixing and heat transfer between the bed material particles and the wood pellets under the constant fluidization velocity, resulting in a certain amount of decrease in the product gas yield. As the OP value increased from 21% to 35%, the volume fractions of CO and CO2 both increased significantly. Despite the slight decrease in the product gas yield, a greater hydrogen supplement amount was still required to convert CO and CO2 into methanol. In the case where the volume fractions of CO and CO2 both increased significantly and the product gas yield slightly decreased, the theoretical methanol yield would have a substantial increase. Overall, the increase in the OP value during the oxygen-enriched gasification in a fluidized bed was beneficial for utilizing the product gas to synthesize methanol.
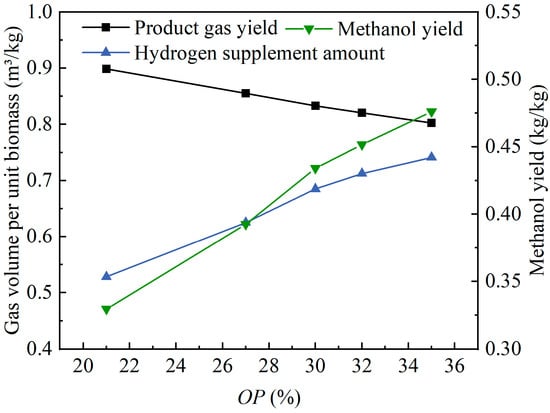
Figure 8.
The effects of the changes in the oxygen percentage in the oxygen-enriched air on the product gas yield and methanol synthesis.
3.4. Effects of S/W Value
When steam reacts with char and CO separately to produce H2, as shown in Equations (17) and (19), the tar reforming reaction reduces tar, as shown in Equation (22). Adding a certain amount of steam to the gasifying agent is beneficial for improving the quality of the product gas. Under T = 800 °C, ug = 0.13 m/s, OP = 35%, and ER = 0.16, the feed rate, qm, was adjusted and the effects of changes in the steam-to-wood-pellet mass ratio, S/W, on product gas compositions and methanol synthesis were experimentally studied at different S/W values. During the experiment, when the S/W value increased from 0.16 to 0.38, the feed rate qm decreased from 7.92 g/min to 5.40 g/min, and the steam mass flow rate increased from 1.37 g/min to 2.22 g/min.
Figure 9 shows the variations in the volume fractions of gas products with the changing S/W value. As the S/W value increased from 0.16 to 0.38, the volume fractions of H2 and CO2 in the product gas increased from 12.39% and 24.51% to 19.06% and 30.18%, respectively, the volume fraction of CO decreased from 25.95% to 17.30%, while the volume fraction of CH₄ did not change significantly. In the case of an increase in the steam mass flow rate relative to the mass flow rate of the wood pellets, both the water–gas Reaction (17) and the water–gas shift Reaction (19) were promoted. Both reactions improved the production of H2, with the latter also promoting the conversion of CO to CO2. In addition, the increase in the S/W value also facilitated the steam reforming of tar (Reaction (22)), producing CO and H2. The content of CO in the product gas was affected by two factors. On the one hand, the increase in the S/W value promoted the water–gas shift reaction, which consumed more CO. On the other hand, both the water–gas reaction and tar reformation produced CO. When the first factor had a greater impact, the volume fraction of CO in the product gas decreased as the S/W value increased. As the S/W value increased from 0.16 to 0.38, the H2/CO ratio increased significantly from 0.48 to 1.10, while the H2/CO2 ratio also increased from 0.51 to 0.63. This indicated that the increase in the S/W value was beneficial for the production of hydrogen-rich syngas. However, if the amount of steam introduced into the gasifier was too large, it could also have some negative effects [39]. These effects included an increase in the superficial gas velocity in the gasifier, which resulted in a shortened residence time of the wood pellets, thereby reducing the gas yield [40], or a significant drop in the gasifier temperature [41], which led to a slowdown in the reaction rates. Therefore, when investigating the effects of changes in the S/W value, the fluidization velocity was kept constant, and the variation range of S/W value was limited.
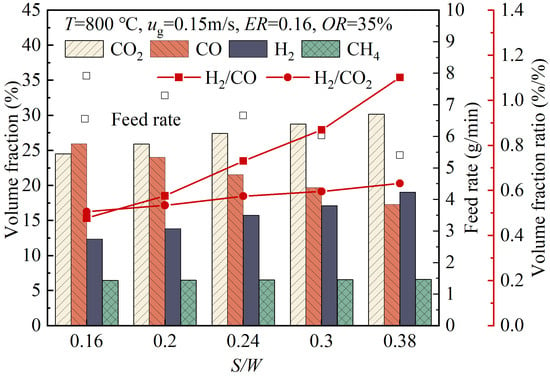
Figure 9.
The variations of the volume fractions of gas products with changes in the S/W value.
Figure 10 shows the effects of the S/W value changes on the product gas yield of oxygen-enriched gasification and the hydrogen supplement amount and the methanol yield in methanol synthesis. As the S/W value increased, the product gas yield increased from 0.79 m3/kg to 0.90 m3/kg, the hydrogen supplement amount increased from 0.89 m3/kg to 0.96 m3/kg, and the methanol yield increased from 0.57 kg/kg to 0.61 kg/kg. When steam was added to the gasification agent, the theoretical methanol yield significantly increased. Moreover, with the increase in the S/W value, the methanol yield further increased. Although the product gas yield was not high, the addition of steam to the gasification agent improved the product gas quality, resulting in a relatively high methanol yield. Furthermore, the increase in the S/W value also contributed to an increase in the product gas yield. However, with a higher methanol yield, the theoretical hydrogen supplement amount was also relatively high. Nonetheless, as the S/W value increased, the rise in the hydrogen supplement amount was not significant.
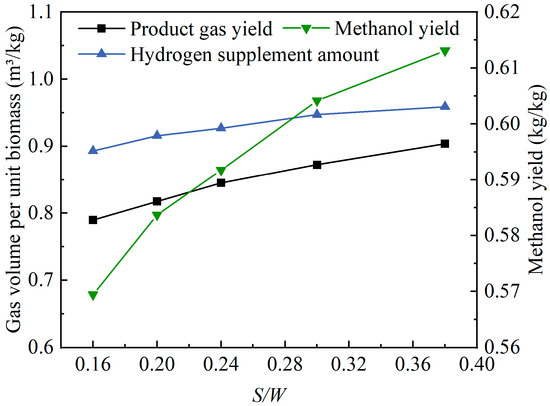
Figure 10.
Effects of S/W value changes on product gas yield and methanol synthesis.
3.5. Effects of Fluidization Velocity
The fluidization velocity in a fluidized bed gasifier determines the gas–solid mixing and flow conditions of particles, thereby affecting the gasification efficiency and carbon conversion of fuel particles. An appropriate fluidization velocity can cause sufficient mixing and effective heat transfer between fuel particles and bed materials and provide adequate residence time for the fuel particles in the gasifier [42]. An experimental study was conducted to investigate the effects of changes in the fluidization velocity, ug, on the volume fractions and the yield of gas products and their effects on methanol synthesis under the conditions of T = 800 °C, ER = 0.16, OP = 35%, and S/W = 0.3.
Figure 11 shows the variations in the volume fractions of gas products with the fluidization velocity, ug. As the ug value increased from 0.122 m/s to 0.203 m/s, the volume fraction of CO increased from 16.33% to 28.02%, the volume fractions of H2 and CO2 both initially increased and then decreased, while the volume fraction of CH₄ showed a slight increase. As the ug value increased within the range of 0.122 m/s to 0.187 m/s, the volume fraction of H2 increased from 13.12% to 18.49%. When the ug value reached 0.187 m/s, further increasing the ug value caused a decrease in the volume fraction of H2. As the ug value increased within the range of 0.122 to 0.155 m/s, the volume fraction of CO2 increased from 20.09% to 26.76%. When the ug value reached 0.155 m/s, further increasing the ug value resulted in a noticeable decrease in the volume fraction of CO2. Increasing the fluidization velocity improved the gas–solid flow conditions and particle mixing and enhanced the heat and mass transfer, thereby promoting pyrolysis and gasification and facilitating the generation of gas products. However, once the ug value reached 0.187 m/s, as the ug value further increased, the gas might have carried some unreacted wood pellets out of the gasifier, resulting in a decrease in the volume fraction of H2 in the product gas. As the ug value increased within the range of 0.155 to 0.203 m/s, the volume fraction of CO2 decreased. This was due to the enhanced mixing of char particles and CO2, which promoted the reverse Boudouard reaction (18), thereby reducing the volume fraction of CO2. Therefore, as the ug value increased, the H2/CO2 ratio showed an overall upward trend. The variation trend in the H2/CO ratio with the changing ug value indicated that there was an optimal fluidization velocity at which the H2/CO ratio reached its maximum value.
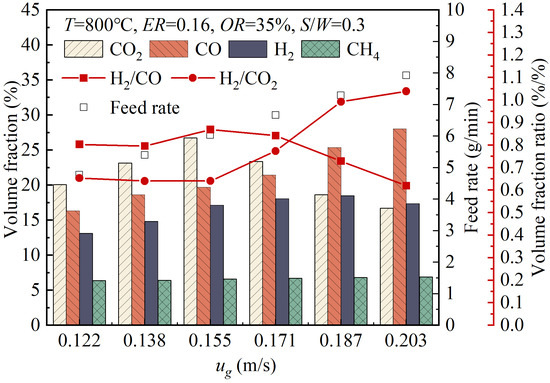
Figure 11.
Variations of volume fractions of gas products with changes in the fluidization velocity.
Figure 12 shows the effects of the ug value changes on the product gas yield of oxygen-enriched gasification and the hydrogen supplement amount and the methanol yield in methanol synthesis. With the increase in the ug value, the product gas yield, hydrogen supplement amount, and methanol yield all showed a trend of first increasing and then decreasing. The maximum values of the three evaluation indices were achieved at ug = 0.155 m/s, which were 0.81 m3/kg, 0.83 m3/kg, and 0.54 kg/kg, respectively. This could be attributed to the fact that at ug = 0.155 m/s, the volume fraction of CO2 in the product gas reached its maximum value, while the volume fraction of CO also had a certain level. Consequently, the theoretical methanol yield reached its maximum value. At this fluidization velocity, the residence time of the wood pellets in the gasifier was sufficient, allowing for good mixing and heat transfer with the bed materials. Additionally, there was adequate contact between the wood pellets and the gasifying agent, resulting in a higher product gas yield. As the ug value increased within the range of 0.155–0.203 m/s, the volume fraction of CO2 decreased significantly, resulting in a corresponding decrease in the theoretical hydrogen supplement amount required for methanol synthesis.
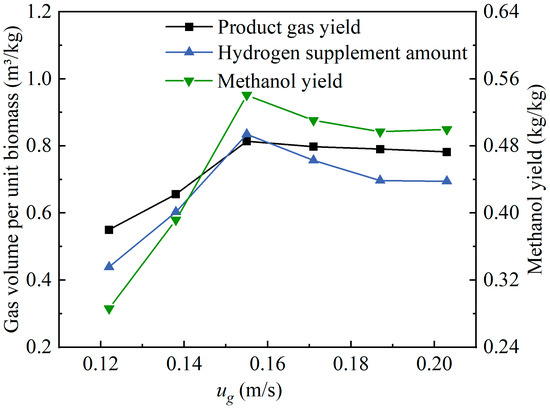
Figure 12.
Effects of fluidization velocity changes on product gas yield and methanol synthesis.
3.6. Effects of Bed Materials
For fluidized bed biomass gasification, quartz sands or other granular solids are used as bed materials. Unlike coal, biomass has little ash, so quartz sands are normally used as inert bed materials. Dolomite granules are alternative bed materials that are very effective for the catalytic removal of tar and are also inexpensive and widely available. At temperatures above 800 °C, dolomite granules undergo calcination and decompose into a mixture of calcium oxide and magnesium oxide, releasing carbon dioxide. Calcined dolomite is significantly more effective than raw dolomite. To obtain a higher H2/CO ratio in the syngas, the effects of various process parameters were comprehensively considered. Under the conditions of T = 850 °C, ER = 0.28, OR = 35%, ug = 0.187 m/s, and S/W = 0.3, the effects of the choice of bed materials on the volume fractions and the yield of gas products and the effects on methanol synthesis were investigated. Dolomite granules and quartz sands were used as bed materials.
Figure 13 shows a comparison of the volume fractions of gas products when dolomite granules or quartz sands were separately used as bed materials. When dolomite granules were used as bed materials, the volume fraction of H2 in the product gas reached 24.85%, which was significantly higher than the H2 volume fraction of 17.24% obtained when quartz sands were used as bed materials. However, when dolomite granules were used, the volume fractions of CO, CO2, and CH₄ in the product gas were lower than the values obtained when quartz sands were used. The dolomite granules in the fluidized bed gasifier acted as catalysts for the tar cracking Reaction (20) and the tar reforming Reactions (21) and (22), thereby enhancing the production of H2 and CO. The calcined dolomite granules in the gasifier significantly absorbed CO2, thereby reducing its volume fraction and promoting the forward water–gas shift Reaction (19), which led to the consumption of a substantial amount of CO. Under selected gasification conditions, although using dolomite promoted the tar cracking and tar reforming reactions, which were conducive to the increase in the CO volume fraction, the water–gas shift reaction played a leading role, resulting in the decrease in the CO volume fraction. The decrease in the CO2 volume fraction was attributed to the absorption of CO2 by the calcined dolomite. Therefore, as shown in Figure 13, when the bed materials changed from quartz sands to dolomite granules, the volume fractions of CO and CO2 both decreased, while the volume fraction of H2 significantly increased. Comparing the volume fractions of CH4 corresponding to dolomite granules and quartz sands, it can be found that dolomite was not very effective in catalyzing methane reforming.
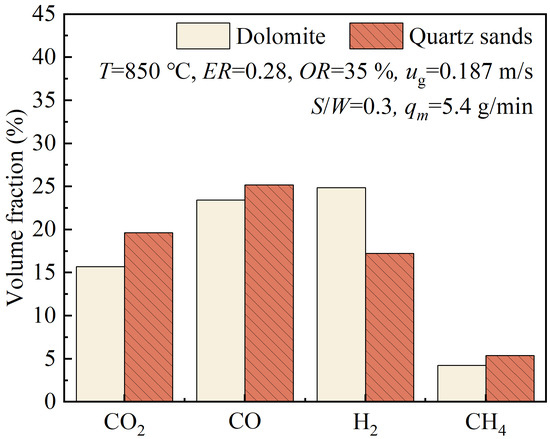
Figure 13.
Volume fractions of gas products when separately using dolomite granules or quartz sands as bed materials.
Table 2 lists the gas yield rate, Yg; H2/CO ratio; H2/CO2 ratio; and hydrogen supplement amount, , required for methanol synthesis and the theoretical methanol yield, YM, when using dolomite granules or quartz sands as bed materials individually. The experimental results showed that using dolomite granules as bed materials significantly increased the H2/CO ratio and the H2/CO2 ratio, which was beneficial for the production of hydrogen-rich syngas. However, it had little effect on the improvement in the gas yield and significantly reduced the hydrogen supplement amount required for methanol synthesis. Nevertheless, due to the reduction in the volume fractions of CO and CO2 in the product gas, using dolomite granules as bed materials led to a certain degree of decrease in the theoretical methanol yield.

Table 2.
Product gas yield and product gas quality when separately using dolomite granules or quartz sands as bed materials and their effects on methanol synthesis.
4. Conclusions
The effects of the equivalence ratio, ER; gasifier temperature, T; oxygen volume percentage, OP, in the oxygen-enriched air; steam-to-wood-pellet mass ratio, S/W; fluidization velocity, ug; and types of bed materials on the volume fractions of gas products and the product gas yield from the oxygen-enriched gasification of wood pellets were investigated in a bubbling fluidized bed gasifier. The effects of the generation characteristics of gas products on methanol synthesis were also analyzed.
As the ER value increased, more CO was oxidized to produce CO2, resulting in a decreasing trend in the volume fraction of CO. The volume fraction of H2 initially increased and then decreased with the increase in the ER value, as the increase in oxygen led to an increase in the amount of H2 consumed in the oxidation reaction. The product gas yield, hydrogen supplement amount, oxygen consumption, and methanol yield all showed an upward trend when increasing the ER value.
Increasing the temperature favored endothermic reactions, driving the forward water–gas reaction and the reverse Boudouard reaction. As a result, the production of CO and H2 increased, while CO2 formation decreased. Moreover, the higher temperature promoted the reverse water–gas shift reaction and the methanation reactions, thereby reducing the volume fractions of CO2 in the product gas. As the temperature increased from 650 °C to 850 °C, the product gas yield and the methanol yield both showed an upward trend.
Under the constant fluidization velocity, an increase in the OP value led to a decrease in the volumetric flow rate of nitrogen, resulting in an increase in the volume fractions of other gas components in the product gas and an improvement in the product gas quality. As the OP value increased, the product gas yield decreased, while the hydrogen supplement amount and the methanol yield both increased.
When the S/W value increased, the water–gas reaction and the water–gas shift reaction were both promoted. Both reactions enhanced the production of H2, with the latter also promoting the conversion of CO to CO2. In addition, the increase in the S/W value also facilitated the steam reforming of tar, producing CO and H2. The variation trends in the H2/CO ratio and the H2/CO2 ratio indicated that the increase in the S/W value was beneficial for the production of hydrogen-rich syngas. If steam was added to the gasification agent, the theoretical methanol yield would significantly increase when the product gas was used for methanol synthesis.
Increasing the fluidization velocity improved the gas–solid flow conditions and particle mixing, facilitating the generation of gas products. However, an excessive ug value would result in a decrease in the volume fraction of H2 due to the gas carrying some unreacted wood pellets out of the gasifier. As the ug value increased, the volume fraction of CO2 would reduce due to the enhanced mixing of carbon particles and CO2, which promoted the reverse Boudouard reaction. The product gas yield, hydrogen supplement amount, and methanol yield all showed a trend of first increasing and then decreasing with the increase in the ug value.
When dolomite granules were used as bed materials, the volume fraction of H2 was significantly higher than that obtained when quartz sands were used. The dolomite granules acted as catalysts for the tar cracking and tar reforming reactions, thereby enhancing the production of H2 and CO. The calcined dolomite granules absorbed CO2, thereby reducing its volume fraction and promoting the forward water–gas shift reaction, which led to the consumption of CO. Using dolomite granules as bed materials significantly increased the H2/CO ratio and the H2/CO2 ratio and reduced the hydrogen supplement amount required for methanol synthesis.
Author Contributions
Conceptualization, H.J.; methodology, H.J. and T.Z.; validation, X.Z. and T.G.; formal analysis, X.Z. and T.G.; investigation, X.Z. and T.G.; resources, X.Z., T.G., H.J. and M.Z.; data curation, X.Z.; writing—original draft preparation, X.Z. and H.J.; writing—review and editing, H.J.; visualization, X.Z. and H.J.; supervision, H.J., M.Z., H.Y. and T.Z.; project administration, H.J.; funding acquisition, H.J., M.Z. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2023YFB4104301-3), the International Science and Technology Cooperation Program of Qingdao City (Grant No. 24-1-6-ghgg-9-hz), and the Huaneng Group Science and Technology Research Project (Grant No. HNKJ23-H71).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Horvath, S.; Fasihi, M.; Breyer, C. Techno-economic analysis of a decarbonized shipping sector: Technology suggestions for a fleet in 2030 and 2040. Energy Convers. Manag. 2018, 164, 230–241. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.G.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Li, C.; Jia, T.; Wang, H.; Wang, X.; Negnevitsky, M.; Hu, Y.-j.; Zhao, G.; Wang, L. Assessing the prospect of deploying green methanol vehicles in China from energy, environmental and economic perspectives. Energy 2023, 263, 125967. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Turnock, S.R.; Hudson, D.A. Route to zero emission shipping: Hydrogen, ammonia or methanol? Int. J. Hydrogen Energy 2021, 46, 28282–28297. [Google Scholar] [CrossRef]
- Methanol and Shipping; Longspur Research: London, UK, 2023.
- Galimova, T.; Fasihi, M.; Bogdanov, D.; Lopez, G.; Breyer, C. Analysis of green e-methanol supply costs: Domestic production in Europe versus imports via pipeline and sea shipping. Renew. Energy 2025, 241, 122336. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Azancot, L.; González-Castaño, M.; Ruíz-López, E.; Pastor-Pérez, L.; Durán-Olivencia, F.J.; Ye, R.; Chong, K.; Blanco-Sánchez, P.H.; Wu, Z.; et al. Biomass gasification, catalytic technologies and energy integration for production of circular methanol: New horizons for industry decarbonisation. J. Environ. Sci. 2024, 140, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, S.; Gu, Y.; Chen, Q.; Tang, Z. Prospective life cycle environmental impact assessment of renewable energy-based methanol production system: A case study in China. J. Clean. Prod. 2023, 425, 139002. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Y.; Ma, X.; Tang, J.; Yue, W. Biomass gasification based on sorption-enhanced hydrogen production coupled with carbon utilization to produce tunable syngas for methanol synthesis. Energy Convers. Manag. 2024, 309, 118428. [Google Scholar] [CrossRef]
- Im-orb, K.; Arpornwichanop, A. Process and sustainability analyses of the integrated biomass pyrolysis, gasification, and methanol synthesis process for methanol production. Energy 2020, 193, 116788. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.L.; Sánchez, P.; Sanchez-Silva, L. Three integrated process simulation using aspen plus®: Pine gasification, syngas cleaning and methanol synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Albarelli, J.Q.; Onorati, S.; Caliandro, P.; Peduzzi, E.; Meireles, M.A.A.; Marechal, F.; Ensinas, A.V. Multi-objective optimization of a sugarcane biorefinery for integrated ethanol and methanol production. Energy 2017, 138, 1281–1290. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Y.; Ma, X.; Tang, J.; Yue, W.; Chen, W.; Sun, Z.; Deng, J. Red mud enhanced biomass gasification to produce syngas: Mechanism, simulation and economic evaluation. Chem. Eng. J. 2024, 499, 156208. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Miranda, M.; Neves, D.; Varela, F.; Santos, J. Effect of gasification agent on co-gasification of rice production wastes mixtures. Fuel 2016, 180, 407–416. [Google Scholar] [CrossRef]
- Shen, Y. Biomass pretreatment for steam gasification toward H2-rich syngas production—An overview. Int. J. Hydrogen Energy 2024, 66, 90–102. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Li, Y.; Williams, P.T. Catalytic steam reforming of waste tire pyrolysis volatiles using a tire char catalyst for high yield hydrogen-rich syngas. Fuel Process. Technol. 2024, 265, 108150. [Google Scholar] [CrossRef]
- Shayan, E.; Zare, V.; Mirzaee, I. Hydrogen production from biomass gasification; a theoretical comparison of using different gasification agents. Energy Convers. Manag. 2018, 159, 30–41. [Google Scholar] [CrossRef]
- Gao, N.; Zhu, K.; Fang, S.; Deng, L.; Lin, Y.; Huang, Z.; Li, J.; Huang, H. A Numerical Simulation and Experimental Study of Fluidization Characteristics of a Bubbling Fluidized Bed in Biomass Gasification. Energies 2024, 17, 2302. [Google Scholar] [CrossRef]
- Lu, Q.; Yuan, S.; Liu, C.; Zhang, T.; Xie, X.; Deng, X.; He, R. A Fe-Ca/SiO2 catalyst for efficient production of light aromatics from catalytic pyrolysis of biomass. Fuel 2020, 279, 118500. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Yang, Z.; Yan, Y.; Pu, G.; Guo, M. Hydrogen-rich gas production from wet biomass steam gasification with CaO/MgO. Int. J. Hydrogen Energy 2015, 40, 8816–8823. [Google Scholar] [CrossRef]
- Varjani, S. Efficient removal of tar employing dolomite catalyst in gasification: Challenges and opportunities. Sci. Total Environ. 2022, 836, 155721. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yang, C.; Wu, Y.; Chen, Y.; Li, H. Study on counter-flow steam gasification characteristics of biochar with Fe2O3/CaO in-situ catalysis in fixed bed. Appl. Energy 2022, 326, 120046. [Google Scholar] [CrossRef]
- Jin, F.; Sun, H.; Wu, C.; Ling, H.; Jiang, Y.; Williams, P.T.; Huang, J. Effect of calcium addition on Mg-AlOx supported Ni catalysts for hydrogen production from pyrolysis-gasification of biomass. Catal. Today 2018, 309, 2–10. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.G.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Schmid, M.; Beirow, M.; Schweitzer, D.; Waizmann, G.; Spörl, R.; Scheffknecht, G. Product gas composition for steam-oxygen fluidized bed gasification of dried sewage sludge, straw pellets and wood pellets and the influence of limestone as bed material. Biomass Bioenergy 2018, 117, 71–77. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Du, J.; Chen, J. Oxygen-enriched air gasification of biomass materials for high-quality syngas production. Energy Convers. Manag. 2019, 199, 111628. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Y.; Du, J. Parametric study on biomass gasification by using air, oxygen-enriched air, air/steam and oxygen-enriched air/steam agents: An ASPEN plus modeling. Int. J. Hydrogen Energy 2024, 73, 265–273. [Google Scholar] [CrossRef]
- Sebastiani, A.; Parrillo, F.; Ardolino, F.; Arena, U.; Iannello, S.; Materazzi, M. Modelling of oxygen-steam gasification of waste feedstock in industrial fluidized bed reactors. Chem. Eng. J. 2025, 506, 159763. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Cao, J.; Liu, C.; Dong, L.; Xu, L.; Zha, J. Experimental study of biomass gasification with oxygen-enriched air in fluidized bed gasifier. Sci. Total Environ. 2018, 626, 423–433. [Google Scholar] [CrossRef]
- Fu, Z.; Aghdam, N.C.; Nekoeian, S.; He, J.; Cheng, L.; Liu, S.; Zhang, L.; Chao, J.; Wei, X.; Wang, R.; et al. Hot syngas cleanup for pilot two-stage fluidized bed steam-oxygen biomass gasification plant. Bioresour. Technol. 2025, 418, 131876. [Google Scholar] [CrossRef]
- Adil, A.; Prasad, B.; Rao, L. Methanol generation from bio-syngas: Experimental analysis and modeling studies. Environ. Dev. Sustain. 2024, 26, 21503–21527. [Google Scholar] [CrossRef]
- Upadhyay, D.S.; Sakhiya, A.K.; Panchal, K.; Patel, A.H.; Patel, R.N. Effect of equivalence ratio on the performance of the downdraft gasifier—An experimental and modelling approach. Energy 2019, 168, 833–846. [Google Scholar] [CrossRef]
- Samimi, F.; Marzoughi, T.; Rahimpour, M.R. Energy and exergy analysis and optimization of biomass gasification process for hydrogen production (based on air, steam and air/steam gasifying agents). Int. J. Hydrogen Energy 2020, 45, 33185–33197. [Google Scholar] [CrossRef]
- Inayat, M.; Sulaiman, S.A.; Kurnia, J.C.; Naz, M.Y. Catalytic and noncatalytic gasification of wood–coconut shell blend under different operating conditions. Environ. Prog. Sustain. Energy 2019, 38, 688–698. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F. Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass. Energies 2021, 14, 2675. [Google Scholar] [CrossRef]
- Hejazi, B. Heat integration and waste minimization of biomass steam gasification in a bubbling fluidized bed reactor. Biomass Bioenergy 2022, 159, 106409. [Google Scholar] [CrossRef]
- Hoang, A.T.; Huang, Z.; Nižetić, S.; Pandey, A.; Nguyen, X.P.; Luque, R.; Ong, H.C.; Said, Z.; Le, T.H.; Pham, V.V. Characteristics of hydrogen production from steam gasification of plant-originated lignocellulosic biomass and its prospects in Vietnam. Int. J. Hydrogen Energy 2022, 47, 4394–4425. [Google Scholar] [CrossRef]
- Chen, A.; Hu, R.; Han, R.; Wei, X.; Tian, Z.; Chen, L. The production of hydrogen-rich gas from sludge steam gasification catalyzed by Ni-based sludge char prepared with mechanical ball-milling. J. Energy Inst. 2021, 99, 21–30. [Google Scholar] [CrossRef]
- Müller, F.J.; Fuchs, J.; Fanjul Cuesta, M.; Oblanca Gutiérrez, A.; Pratschner, S.; Müller, S.; Winter, F. CO2 conversion to CO by fluidized bed biomass gasification: Analysis of operational parameters. J. CO2 Util. 2024, 81, 102706. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).