Gas Production and Storage Using Hydrates Through the Replacement of Multicomponent Gases: A Critical Review
Abstract
1. Introduction
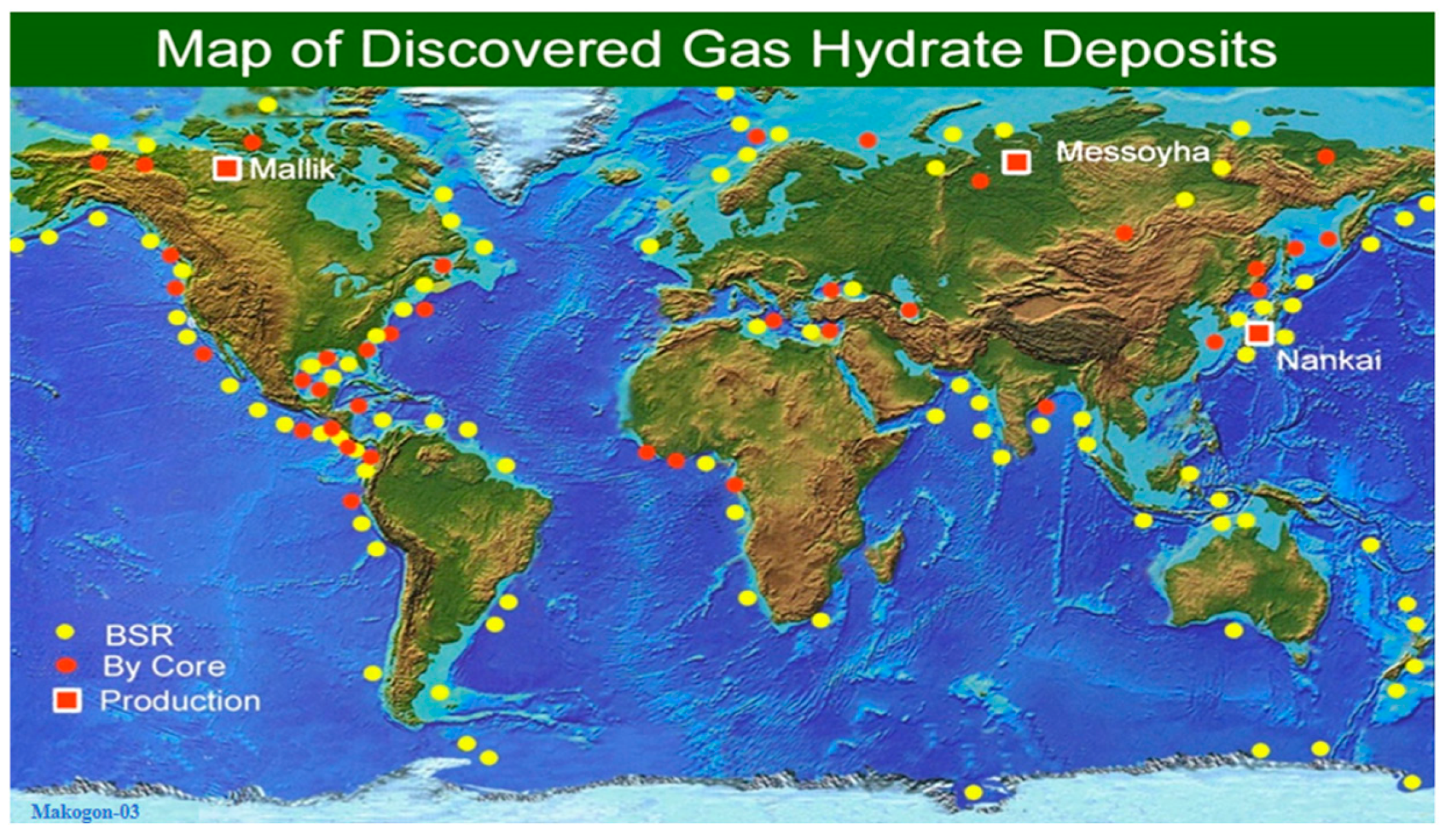
1.1. Natural Gas Hydrate
1.2. Natural Gas Hydrate Extraction
1.2.1. Traditional Natural Gas Hydrate Extraction Methods
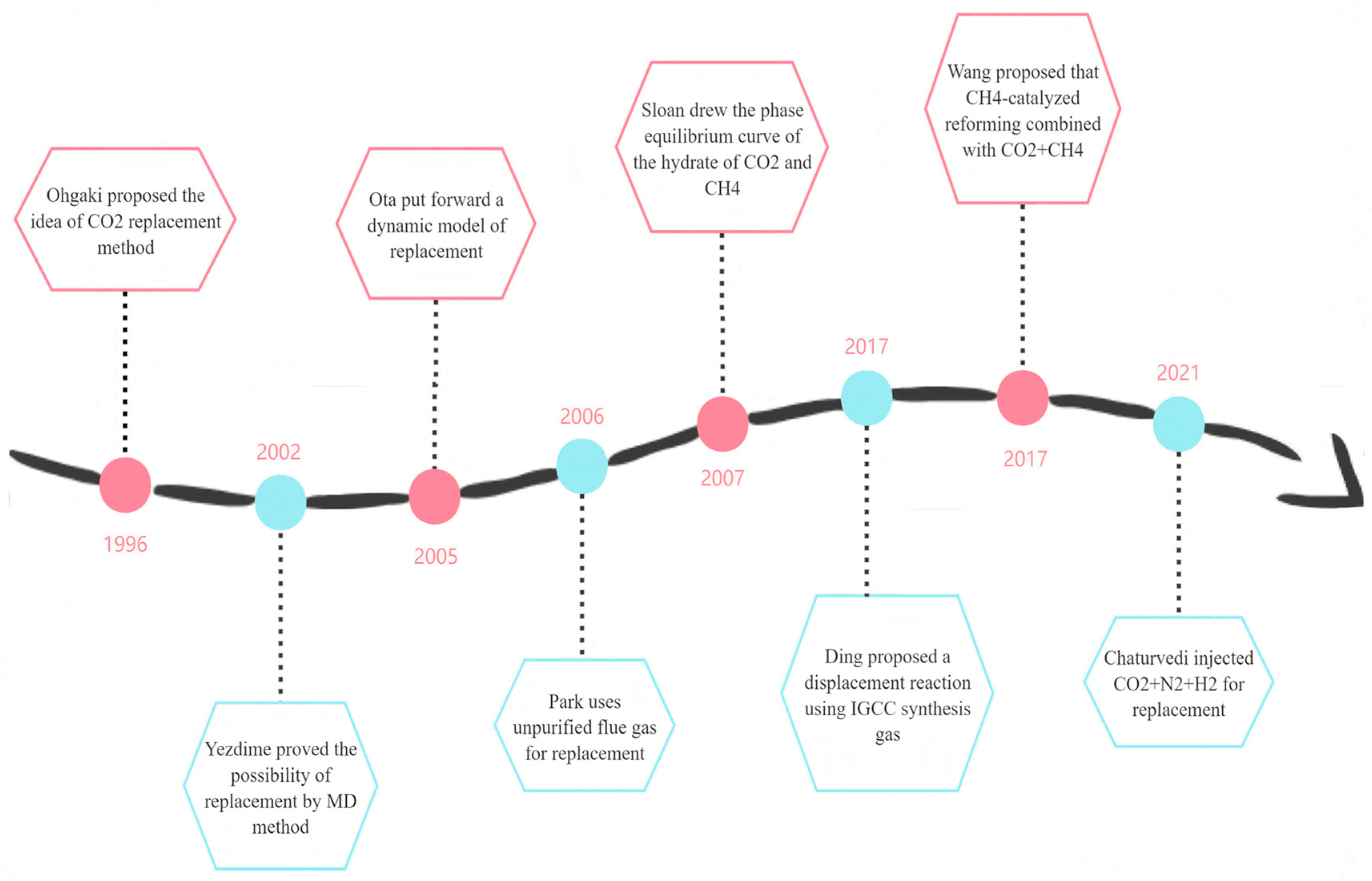
1.2.2. CO2 and CH4 Displacement Method
2. Structure and Properties of Hydrates
2.1. Crystal Structure of Hydrates
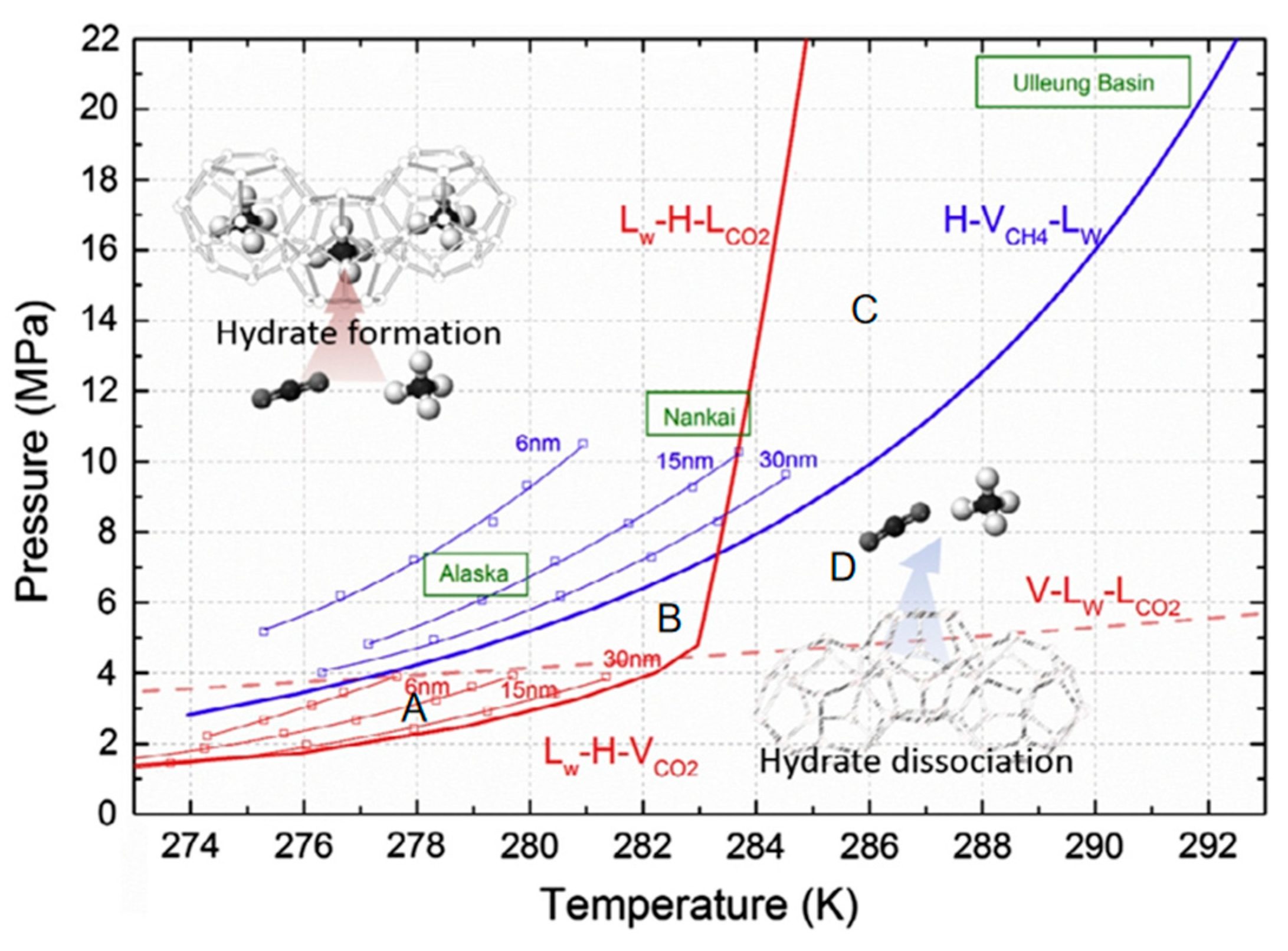
2.2. Thermodynamic Study
2.2.1. Thermodynamic Characteristics of Replacement Reactions
2.2.2. Stability of Hydrates and Gas Distribution Behavior
2.3. Kinetic Studies
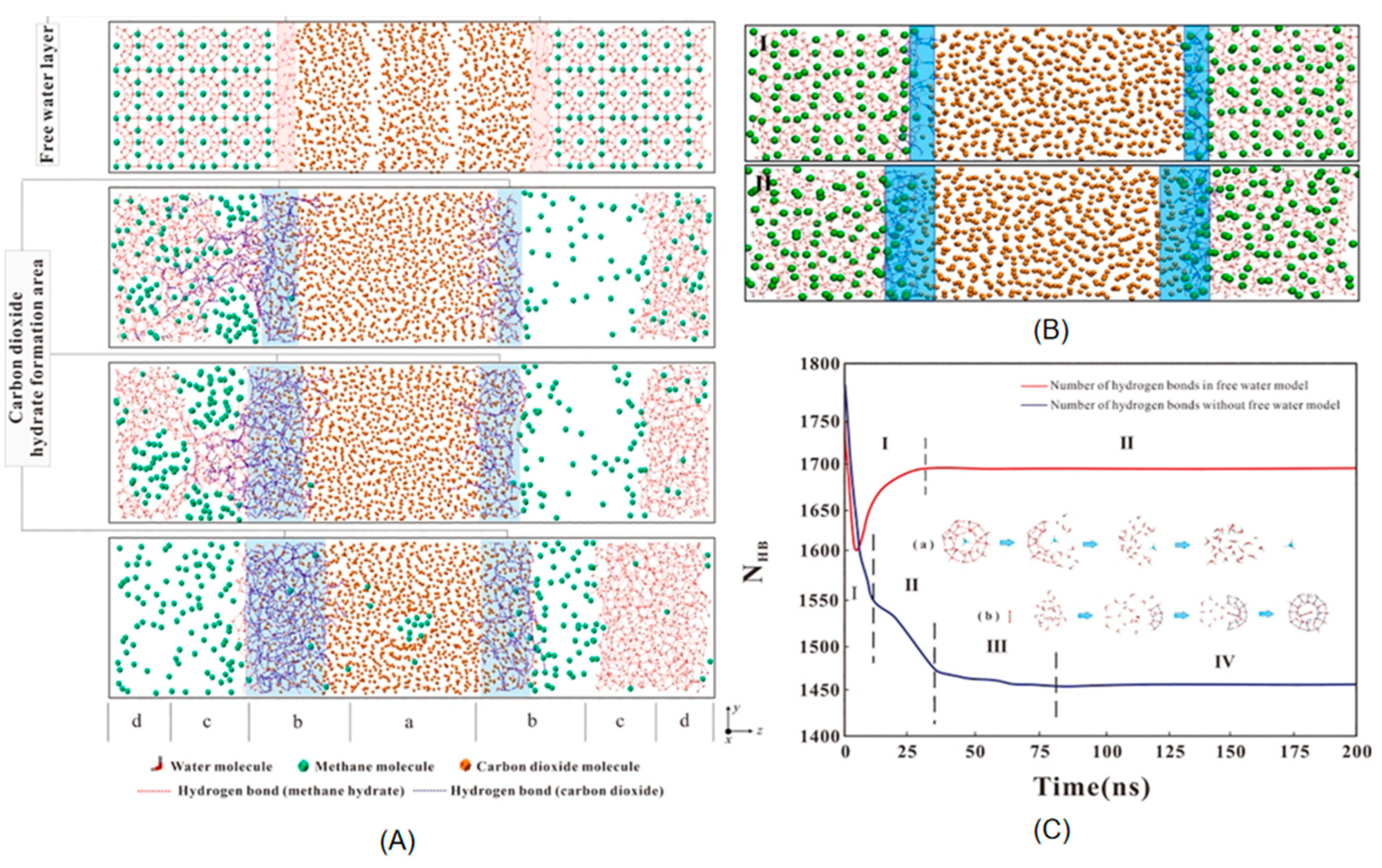
2.3.1. Gas Diffusion and Exchange Rate
2.3.2. Reaction Mechanisms in the Displacement Process
2.3.3. Kinetic Characteristics of the Displacement Reaction
3. Combined Enhancement of Displacement Method and Traditional Methods
3.1. Combined Extraction of Displacement Method and Depressurization Method
3.2. Combined Extraction of Displacement and Thermal Stimulation Methods
3.3. Combined Extraction of Displacement and Chemical Inhibitor Methods
4. Multicomponent Gas Displacement Process
4.1. CO2-H2 Mixture Replacement to Improve CH4 Recovery Rate
4.2. CO2 and N2 Mixture Displacement to Improve CH4 Recovery Rate
4.3. The Displacement of CH4 Recovery Is Improved by Using CO2 Mixed with N2 and H2
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Y.-S.; Zhang, X.; Liu, J.-W.; Lee, Y.; Li, X.S. Natural gas hydrate resources and hydrate technologies: A review and analysis of the associated energy and global warming challenges. Energy Environ. Sci. 2021, 14, 5611–5668. [Google Scholar] [CrossRef]
- Musakaev, N.; Khasanov, M.; Borodin, S.L. The mathematical model of the gas hydrate deposit development in permafrost. Int. J. Heat Mass Transf. 2018, 118, 455–461. [Google Scholar] [CrossRef]
- Wei, N.; Pei, J.; Li, H.; Zhou, S.; Zhao, J.; Kvamme, B.; Coffin, R.B.; Zhang, L.; Zhang, Y.; Xue, J.; et al. Classification of natural gas hydrate resources: Review, application and prospect. Gas Sci. Eng. 2024, 124, 205269. [Google Scholar] [CrossRef]
- Ma, K.; Li, D.; Liang, D. Reservoir stimulation technologies for natural gas hydrate: Research progress, challenges, and perspectives. Energy Fuels. 2023, 37, 10112–10133. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Sun, H.; Chen, B.; Zhu, Z.; Zhang, L.; Yang, M.; Song, Y. Research development in the traditional methods and water flow erosion for natural gas hydrate production: A review. Energy Technol. 2023, 11, 2201011. [Google Scholar] [CrossRef]
- Ji, C.; Ahmadi, G.; Smith, D.H. Natural gas production from hydrate decomposition by depressurization. Chem. Eng. Sci. 2001, 56, 5801–5814. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J. Thermal Stimulation Based Methane Production from Hydrate Bearing Quartz Sediment. Ind. Eng. Chem. Res. 2013, 52, 6571–6581. [Google Scholar] [CrossRef]
- Farhadian, A.; Shadloo, A.; Zhao, X.; Pavelyev, R.S.; Peyvandi, K.; Qiu, Z.; Varfolomeev, M.A. Challenges and advantages of using environmentally friendly kinetic gas hydrate inhibitors for flow assurance application: A comprehensive review. Fuel 2023, 336, 127055. [Google Scholar] [CrossRef]
- Li, X.-S.; Yang, B.; Zhang, Y.; Li, G.; Duan, L.-P.; Wang, Y.; Chen, Z.-Y.; Huang, N.-S.; Wu, H.-J. Experimental investigation into gas production from methane hydrate in sediment by depressurization in a novel pilot-scale hydrate simulator. Appl. Energy 2012, 93, 722–732. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-S.; Chen, Z.-Y.; Wang, Y.; Ruan, X.-K. Effect of Hydrate Saturation on the Methane Hydrate Dissociation by Depressurization in Sediments in a Cubic Hydrate Simulator. Ind. Eng. Chem. Res. 2015, 54, 2627–2637. [Google Scholar] [CrossRef]
- Wan, K.; Wang, Y.; Li, X.-S.; Zhang, L.-H.; Meng, T. Pilot-scale experimental study on natural gas hydrate decomposition with innovation depressurization modes. Appl. Energy 2024, 373, 123921. [Google Scholar] [CrossRef]
- Cranganu, C. In-situ thermal stimulation of gas hydrates. J. Pet. Sci. Eng. 2009, 65, 76–80. [Google Scholar] [CrossRef]
- Yamada, H.; Chen, L.; Lacaille, G.; Shoji, E.; Okajima, J.; Komiya, A.; Maruyama, S. Experimental Study of Methane Hydrate Dissociation and Gas Production Behaviors under Depressurization. Int. J. Mech. Eng. Robot. Res. 2017, 6, 140–146. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Du, Q.; Liu, Y.; Ji, Y.; Bai, Y. Numerical modeling of gas production from methane hydrate deposits using low-frequency electrical heating assisted depressurization method. Fuel 2021, 290, 120075. [Google Scholar] [CrossRef]
- Liao, B.; Wang, J.; Sun, J.; Lv, K.; Liu, L.; Wang, Q.; Wang, R.; Lv, X.; Wang, Y.; Chen, Z. Microscopic insights into synergism effect of different hydrate inhibitors on methane hydrate formation: Experiments and molecular dynamics simulations. Fuel 2023, 340, 127488. [Google Scholar] [CrossRef]
- Yagasaki, T.; Matsumoto, M.; Tanaka, H. Effects of thermodynamic inhibitors on the dissociation of methane hydrate: A molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 32347–32357. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Sun, N.; Han, S.; Wang, X.; Su, Q.; Li, Y.; He, J.; Yu, X.; Du, S. Hydrate technologies for CO2 capture and sequestration: Status and perspectives. Chem. Rev. 2024, 124, 10363–10385. [Google Scholar] [CrossRef]
- Yan, P.; Luan, H.; Jiang, Y.; Liang, W.; Liu, M.; Chen, H. Influence of depressurization mode on natural gas hydrate production characteristics: One-dimensional experimental study. Geoenergy Sci. Eng. 2024, 234, 212671. [Google Scholar] [CrossRef]
- Lee, H.; Seo, Y.; Seo, Y.T.; Moudrakovski, I.L.; Ripmeester, J.A. Recovering methane from solid methane hydrate with carbon dioxide. Angew. Chem. Vol. 2003, 115, 5202–5205. [Google Scholar] [CrossRef]
- Wang, Y.; Lang, X.; Fan, S.; Wang, S.; Yu, C.; Li, G. Review on Enhanced Technology of Natural Gas Hydrate Recovery by Carbon Dioxide Replacement. Energy Fuels 2021, 35, 3659–3674. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by carbon dioxide from gas hydrates—Phase equilibria for CO2-CH4 mixed hydrate system. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Yezdimer, E.M.; Cummings, P.T.; Chialvo, A.A. Determination of the Gibbs free energy of gas replacement in SI clathrate hydrates by molecular simulation. J. Phys. Chem. A 2002, 106, 7982–7987. [Google Scholar] [CrossRef]
- Ota, M.; Abe, Y.; Watanabe, M.; Smith, R.L.; Inomata, H. Methane recovery from methane hydrate using pressurized CO2. Fluid Phase Equilibria 2005, 228–229, 553–559. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.-Y.; Lee, J.-W.; Huh, D.-G.; Park, K.-P.; Lee, J.; Lee, H. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. Proc. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef]
- Sloan Jr, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ding, Y.-L.; Xu, C.-G.; Yu, Y.-S.; Li, X.-S. Methane recovery from natural gas hydrate with simulated IGCC syngas. Energy 2017, 120, 192–198. [Google Scholar] [CrossRef]
- Wang, X.-H.; Sun, Y.-F.; Wang, Y.-F.; Li, N.; Sun, C.-Y.; Chen, G.-J.; Liu, B.; Yang, L.-Y. Gas production from hydrates by CH4-CO2/H2 replacement. Appl. Energy 2017, 188, 305–314. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Sinha, A.; Nair, V.C.; Sharma, T. Enhanced carbon dioxide sequestration by direct injection of flue gas doped with hydrogen into hydrate reservoir: Possibility of natural gas production. Energy 2021, 227, 120521. [Google Scholar] [CrossRef]
- Wei, W.-N.; Li, B.; Gan, Q.; Li, Y.-L. Research progress of natural gas hydrate exploitation with CO2 replacement: A review. Fuel 2022, 312, 122873. [Google Scholar] [CrossRef]
- Arora, A.; Cameotra, S.S.; Balomajumder, C. Techniques for exploitation of gas hydrate (clathrates) an untapped resource of methane gas. Energy Fuels 2015, 7, 108–111. [Google Scholar]
- Lo, H.; Lee, M.-T.; Lin, S.-T. Water vacancy driven diffusion in clathrate hydrates: Molecular dynamics simulation study. J. Phys. Chem. C 2017, 121, 8280–8289. [Google Scholar] [CrossRef]
- Peters, B.; Zimmermann, N.E.; Beckham, G.T.; Tester, J.W.; Trout, B.L. Path sampling calculation of methane diffusivity in natural gas hydrates from a water-vacancy assisted mechanism. J. Am. Chem. Soc. 2008, 130, 17342–17350. [Google Scholar] [CrossRef] [PubMed]
- Schicks, J.M. Thermodynamic properties and phase equilibria characteristics of natural gas hydrates. In Advances in Natural Gas: Formation, Processing, and Applications. Volume 3: Natural Gas Hydrates; Elsevier: Amsterdam, The Netherlands, 2024; pp. 65–86. [Google Scholar]
- Anderson, G.K. Enthalpy of dissociation and hydration number of carbon dioxide hydrate from the Clapeyron equation. J. Chem. Thermodyn. 2003, 35, 1171–1183. [Google Scholar] [CrossRef]
- Ma, Z.W.; Zhang, P.; Bao, H.S.; Deng, S. Review of fundamental properties of CO2 hydrates and CO2 capture and separation using hydration method. Renew. Sustain. Energy Rev. 2016, 53, 1273–1302. [Google Scholar] [CrossRef]
- Hachikubo, A.; Miura, T.; Yamada, K.; Sakagami, H.; Takahashi, N.; Hyakutake, K.; Abe, K.; Shoji, H. Phase equilibrium and comparison of formation speeds of CH4 and CO2 hydrate below the ice point. In Proceedings of the 5th International Conference on Gas Hydrates, Trondheim, Norway, 13–16 June 2005. [Google Scholar]
- Goel, N. In situ methane hydrate dissociation with carbon dioxide sequestration: Current knowledge and issues. J. Pet. Sci. Eng. 2006, 51, 169–184. [Google Scholar] [CrossRef]
- Circone, S.; Stern, L.A.; Kirby, S.H.; Durham, W.B.; Chakoumakos, B.C.; Rawn, C.J.; Rondinone, A.J.; Ishii, Y. CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate. J. Phys. Chem. B 2003, 107, 5529–5539. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H.; Vandeginste, V.; Chen, S.; Gan, H.; Wang, M.; Yu, Z. Thermodynamic and kinetic affinity of CO2 relative to CH4 and their pressure, temperature and pore structure sensitivity in the competitive adsorption system in shale gas reservoirs. Energy 2023, 277, 127591. [Google Scholar] [CrossRef]
- Yin, Z.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
- Koh, D.-Y.; Kang, H.; Lee, J.-W.; Park, Y.; Kim, S.-J.; Lee, J.; Lee, J.Y.; Lee, H. Energy-efficient natural gas hydrate production using gas exchange. Appl. Energy 2016, 162, 114–130. [Google Scholar] [CrossRef]
- Sloan, E.; Koh, C.A. Estimation techniques for phase Equilibria of natural gas hydrates. In Clathrate Hydrates of Natural Gases; Taylor & Francis Group: London, UK, 2008; pp. 320–523. [Google Scholar]
- Zhao, J.; Xu, K.; Song, Y.; Liu, W.; Lam, W.; Liu, Y.; Xue, K.; Zhu, Y.; Yu, X.; Li, Q. A Review on Research on Replacement of CH4 in Natural Gas Hydrates by Use of CO2. Energies 2012, 5, 399–419. [Google Scholar] [CrossRef]
- Speight, J.G. Natural Gas: A Basic Handbook; Gulf Professional Publishing: Houston, TX, USA, 2018. [Google Scholar]
- Liu, T.; Wu, P.; Chen, Z.; Li, Y. Review on carbon dioxide replacement of natural gas hydrate: Research progress and perspectives. Energy Fuels 2022, 36, 7321–7336. [Google Scholar] [CrossRef]
- Ota, M.; Morohashi, K.; Abe, Y.; Watanabe, M.; Smith Jr, R.L.; Inomata, H. Replacement of CH4 in the hydrate by use of liquid CO2. Energy Convers. Manag. 2005, 46, 1680–1691. [Google Scholar] [CrossRef]
- Bai, D.; Zhang, X.; Chen, G.; Wang, W. Replacement mechanism of methane hydrate with carbon dioxide from microsecond molecular dynamics simulations. Energy Environ. Sci. 2012, 5, 7033–7041. [Google Scholar] [CrossRef]
- Nakano, S.; Moritoki, M.; Ohgaki, K. High-pressure phase equilibrium and Raman microprobe spectroscopic studies on the CO2 hydrate system. J. Chem. Eng. Data 1998, 43, 807–810. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, L.-J.; Chen, Y.-P.; Lin, S.-T. Growth of structure I carbon dioxide hydrate from molecular dynamics simulations. J. Phys. Chem. C 2011, 115, 7504–7515. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Yang, K.; Wu, S.; Chen, Q.; Bian, J. Hydrate-based CO2 sequestration technology: Feasibilities, mechanisms, influencing factors, and applications. J. Pet. Sci. Eng. 2022, 219, 111121. [Google Scholar] [CrossRef]
- Uchida, T.; Ikeda, I.Y.; Takeya, S.; Kamata, Y.; Ohmura, R.; Nagao, J.; Zatsepina, O.Y.; Buffett, B.A. Kinetics and stability of CH4–CO2 mixed gas hydrates during formation and long-term storage. ChemPhysChem 2005, 6, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, L.; Zheng, Y. Enhanced CO2 sequestration based on hydrate technology with pressure oscillation in porous medium using NMR. Energy 2022, 252, 124082. [Google Scholar] [CrossRef]
- Farahani, M.V.; Guo, X.; Zhang, L.; Yang, M.; Hassanpouryouzband, A.; Zhao, J.; Yang, J.; Song, Y.; Tohidi, B. Effect of thermal formation/dissociation cycles on the kinetics of formation and pore-scale distribution of methane hydrates in porous media: A magnetic resonance imaging study. Sust. Energy Fuels 2021, 5, 1567–1583. [Google Scholar] [CrossRef]
- Wallmann, K.; Riedel, M.; Hong, W.; Patton, H.; Hubbard, A.; Pape, T.; Hsu, C.; Schmidt, C.; Johnson, J.; Torres, M. Gas hydrate dissociation off Svalbard induced by isostatic rebound rather than global warming. Nat. Commun. 2018, 9, 83. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Song, Y.; Zhu, Z.; Yang, L.; Tian, Y.; Wang, J.; Yang, M.; Zhang, Y. Experimental study on a novel way of methane hydrates recovery: Combining CO2 replacement and depressurization. Energy Procedia 2014, 61, 75–79. [Google Scholar] [CrossRef]
- Lee, Y.; Deusner, C.; Kossel, E.; Choi, W.; Seo, Y.; Haeckel, M. Influence of CH4 hydrate exploitation using depressurization and replacement methods on mechanical strength of hydrate-bearing sediment. Appl. Energy 2020, 277, 115569. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, L.; Wang, X.; Liu, K.; Sun, B. Experimental investigation of the behavior of methane gas hydrates during depressurization-assisted CO2 replacement. J. Nat. Gas Sci. Eng. 2019, 61, 284–292. [Google Scholar] [CrossRef]
- Yang, J.; Okwananke, A.; Tohidi, B.; Chuvilin, E.; Maerle, K.; Istomin, V.; Bukhanov, B.; Cheremisin, A. Flue gas injection into gas hydrate reservoirs for methane recovery and carbon dioxide sequestration. Energy Convers. Manag. 2017, 136, 431–438. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Li, S.; Jiang, S. CO2/N2 injection into CH4 + C3H8 hydrates for gas recovery and CO2 sequestration. Chem. Eng. J. 2019, 375, 121973. [Google Scholar] [CrossRef]
- Defu, M.; Yingxia, Q.I. Experimental study on the replacement of methane hydrate by CO2 with thermal excitation. Energy 2017, 33, 13–18. [Google Scholar]
- Zhang, L.; Yang, L.; Wang, J.; Zhao, J.; Dong, H.; Yang, M.; Liu, Y.; Song, Y. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate. Chem. Eng. J. 2017, 308, 40–49. [Google Scholar] [CrossRef]
- Tupsakhare, S.S.; Castaldi, M.J. Efficiency enhancements in methane recovery from natural gas hydrates using injection of CO2/N2 gas mixture simulating in-situ combustion. Appl. Energy 2019, 236, 825–836. [Google Scholar] [CrossRef]
- Fan, S.; Yu, W.; Yu, C.; Wang, Y.; Lang, X.; Wang, S.; Li, G.; Huang, H. Investigation of enhanced exploitation of natural gas hydrate and CO2 sequestration combined gradual heat stimulation with CO2 replacement in sediments. J. Nat. Gas Sci. Eng. 2022, 104, 104686. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Eslamimanesh, A.; Richon, D. Semi-clathrate hydrate phase equilibrium measurements for the CO2+H2/CH4+tetra-n-butylammonium bromide aqueous solution system. Chem. Eng. Sci. 2013, 94, 284–290. [Google Scholar] [CrossRef]
- Khlebnikov, V.; Antonov, S.; Mishin, A.; Bakulin, D.; Khamidullina, I.; Liang, M.; Vinokurov, V.; Gushchin, P.A. A new method for the replacement of CH4 with CO2 in natural gas hydrate production. Nat. Gas Ind. B 2016, 3, 445–451. [Google Scholar] [CrossRef]
- Heydari, A.; Peyvandi, K. Study of biosurfactant effects on methane recovery from gas hydrate by CO2 replacement and depressurization. Fuel 2020, 272, 117681. [Google Scholar] [CrossRef]
- Choi, W.; Mok, J.; Lee, J.; Lee, Y.; Lee, J.; Sum, A.K.; Seo, Y. Effective CH4 production and novel CO2 storage through depressurization-assisted replacement in natural gas hydrate-bearing sediment. Appl. Energy 2022, 326, 119971. [Google Scholar] [CrossRef]
- Adibi, N.; Mohammadi, M.; Ehsani, M.R.; Khanmohammadian, E. Experimental investigation of using combined CH4/CO2 replacement and thermal stimulation methods for methane production from gas hydrate in the presence of SiO2 and ZnO nanoparticles. J. Nat. Gas Sci. Eng. 2020, 84, 103690. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Lee, J.; Lee, H.; Seo, Y. Experimental verification of methane–carbon dioxide replacement in natural gas hydrates using a differential scanning calorimeter. Environ. Sci. Technol. 2013, 47, 13184–13190. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Wang, Y.-F.; Zhong, J.-R.; Li, W.-Z.; Li, R.; Cao, B.-J.; Kan, J.-Y.; Sun, C.-Y.; Chen, G.-J. Gas hydrate exploitation using CO2/H2 mixture gas by semi-continuous injection-production mode. Appl. Energy 2019, 240, 215–225. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yin, S.; Guanyu, H.; Li, J.; Wu, Q. Research progress of the kinetics on natural gas hydrate replacement by CO2-containing mixed gas: A review. J. Nat. Gas Sci. Eng. 2022, 108, 104837. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhong, J.-R.; Li, R.; Zhu, T.; Cao, X.-Y.; Chen, G.-J.; Wang, X.-H.; Yang, L.-Y.; Sun, C.-Y. Natural gas hydrate exploitation by CO2/H2 continuous Injection-Production mode. Appl. Energy 2018, 226, 10–21. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Deng, C.; Liu, B.; Sun, C.; Chen, G.; El-Halwagi, M. Process modeling and energy efficiency analysis of natural gas hydrate production by CH4-CO2/H2 replacement coupling steam methane reforming. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 47, pp. 131–136. [Google Scholar] [CrossRef]
- Xu, C.-G.; Cai, J.; Yu, Y.-S.; Chen, Z.-Y.; Li, X.-S. Research on micro-mechanism and efficiency of CH4 exploitation via CH4-CO2 replacement from natural gas hydrates. Fuel 2018, 216, 255–265. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, T.; Zhu, Y.; Sun, C.; Chen, G.; Feng, J. H2 promotes the premature replacement of CH4–CO2 hydrate even when the CH4 gas-phase pressure exceeds the phase equilibrium pressure of CH4 hydrate. Renew. Sustain. Energy Rev. 2024, 200, 114582. [Google Scholar] [CrossRef]
- Sun, S.; Hao, Y.; Zhao, J. Analysis of gas source for the replacement of CH4 with CO2 in gas hydrate production from the perspective of dissociation enthalpy. J. Chem. Eng. Data 2018, 63, 684–690. [Google Scholar] [CrossRef]
- Pandey, J.S.; Solms, N. Hydrate stability and methane recovery from gas hydrate through CH4–CO2 replacement in different mass transfer scenarios. Energies 2019, 12, 2309. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.; Lee, J.; Lee, H.; Seo, Y. CH4 recovery and CO2 sequestration using flue gas in natural gas hydrates as revealed by a micro-differential scanning calorimeter. Appl. Energy 2015, 150, 120–127. [Google Scholar] [CrossRef]
- Niu, M.; Wu, G.; Yin, Z.; Sun, Y.; Liu, K.; Chen, D. Effectiveness of CO2-N2 injection for synergistic CH4 recovery and CO2 sequestration at marine gas hydrates condition. Chem. Eng. J. 2021, 420, 129615. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, S.; Liang, D.; Du, J. Replacement of methane from quartz sand-bearing hydrate with carbon dioxide-in-water emulsion. Energy Fuels 2008, 22, 1759–1764. [Google Scholar] [CrossRef]
- Seo, Y.-j.; Park, S.; Kang, H.; Ahn, Y.-H.; Lim, D.; Kim, S.-J.; Lee, J.; Lee, J.Y.; Ahn, T.; Seo, Y. Isostructural and cage-specific replacement occurring in sII hydrate with external CO2/N2 gas and its implications for natural gas production and CO2 storage. Appl. Energy 2016, 178, 579–586. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Sharma, T.; Kumar, G.S. Experimental investigation for comparative effectiveness of CO2+N2 and CO2+N2+H2 on integrated methane production and carbon storage from natural hydrate media. J. Environ. Chem. Eng. 2023, 11, 109388. [Google Scholar] [CrossRef]

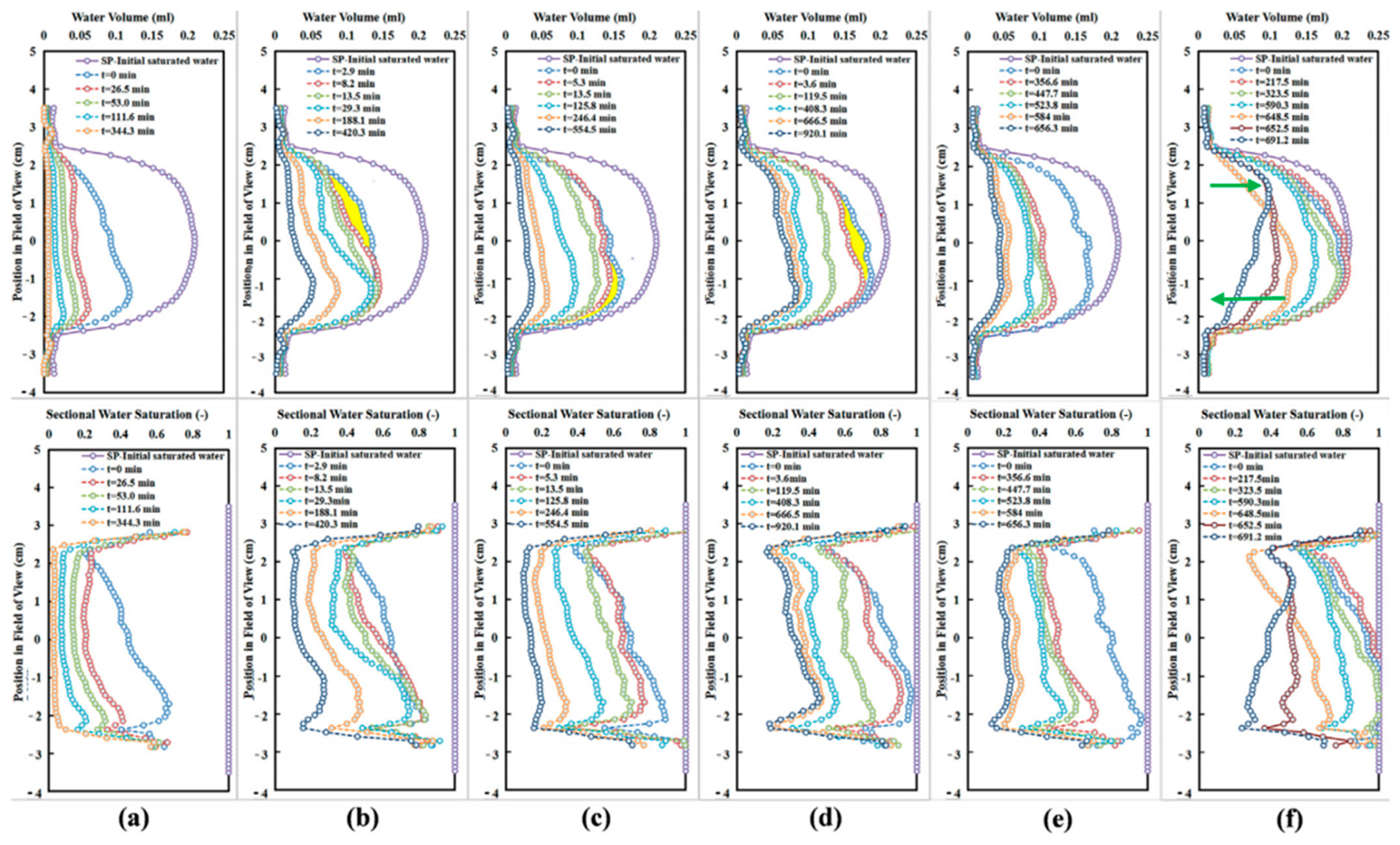
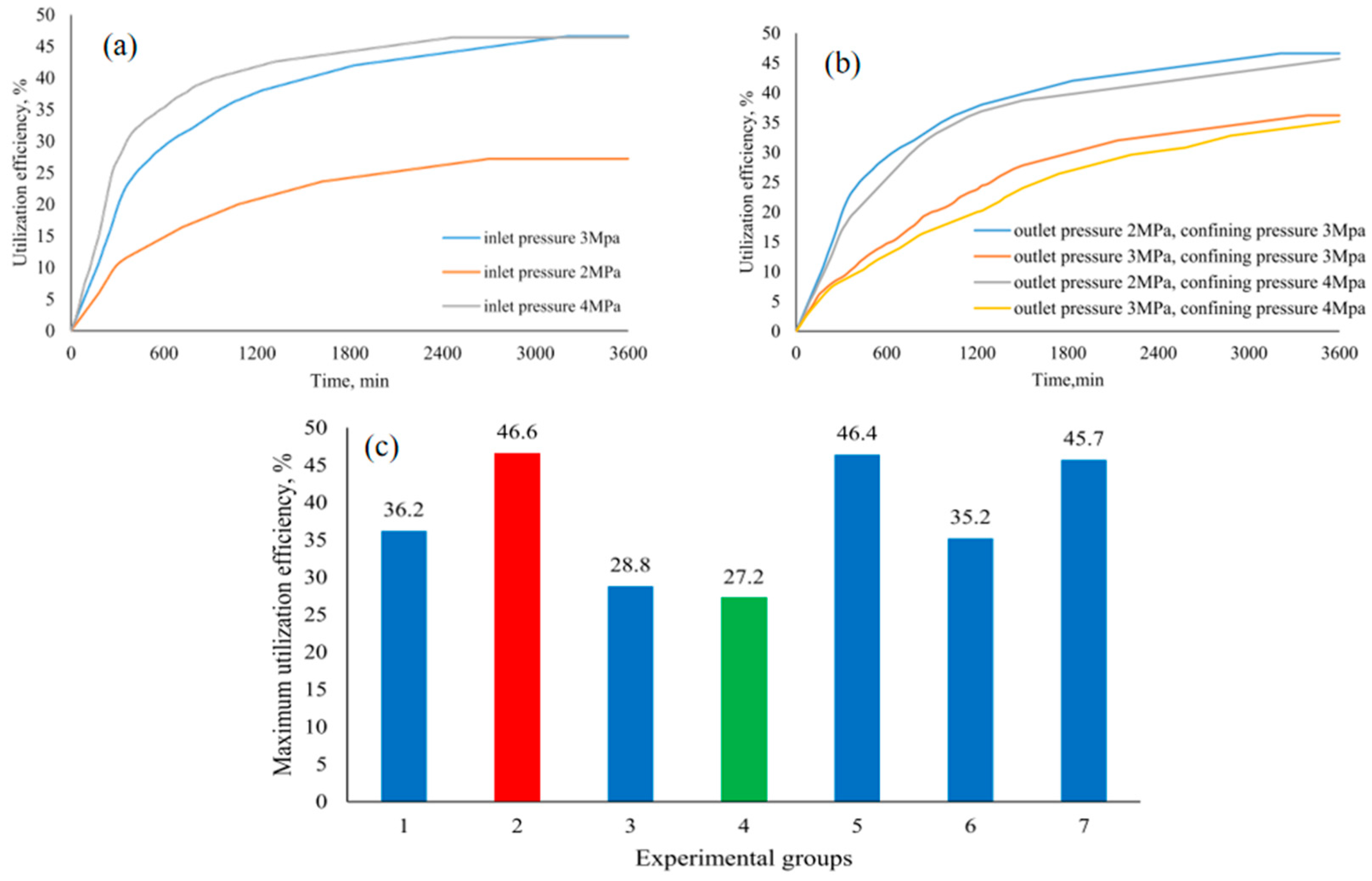
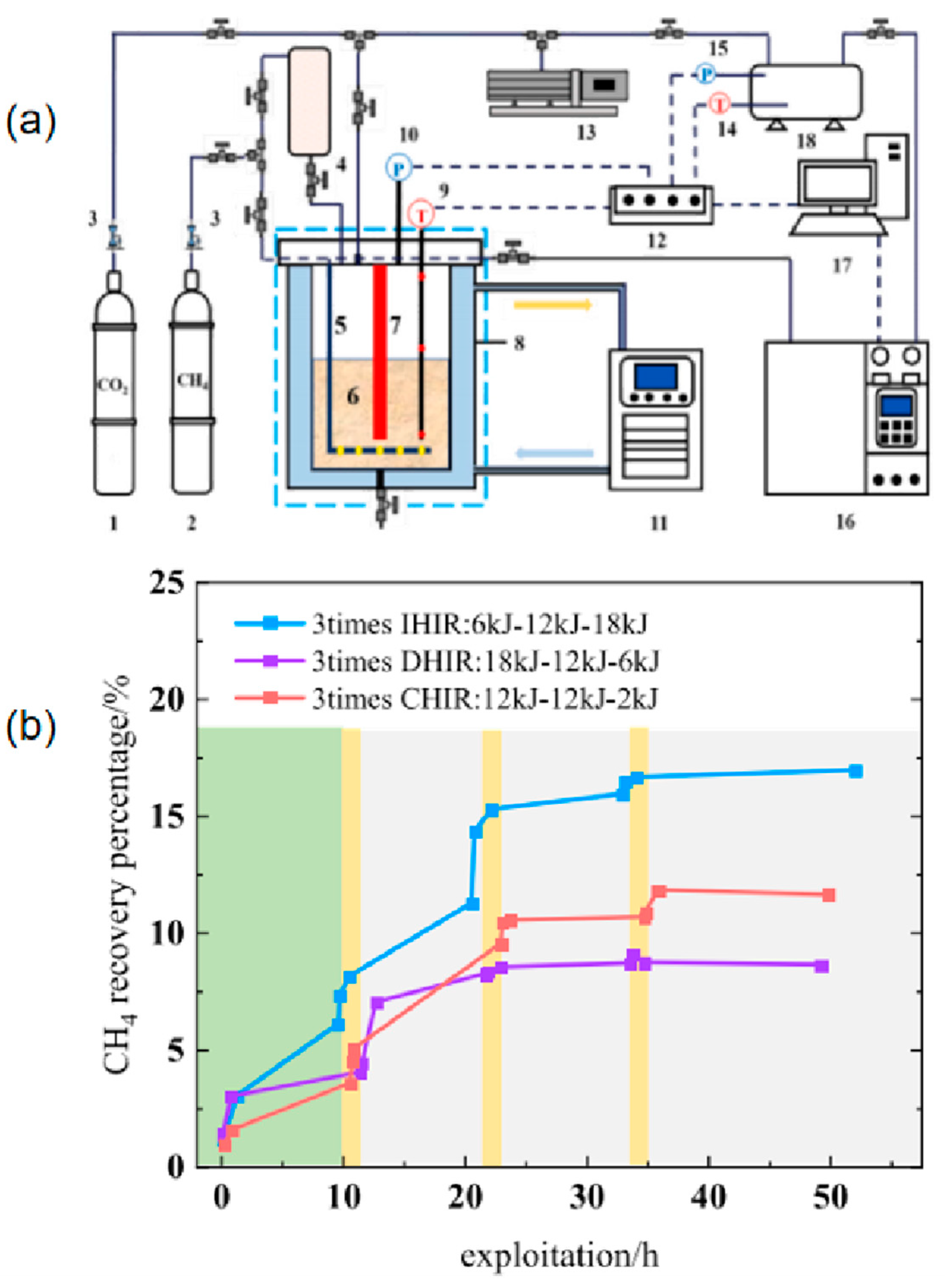
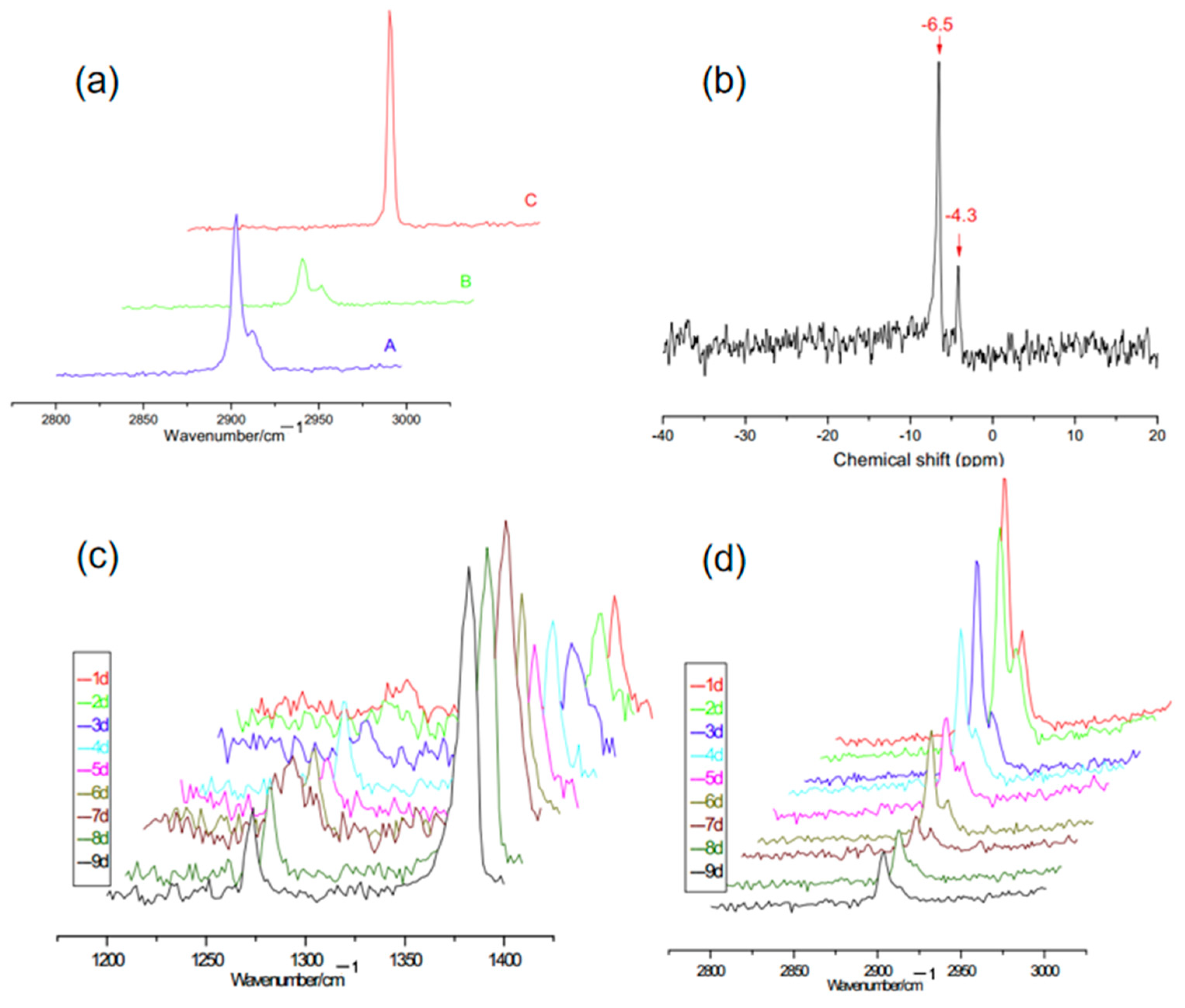

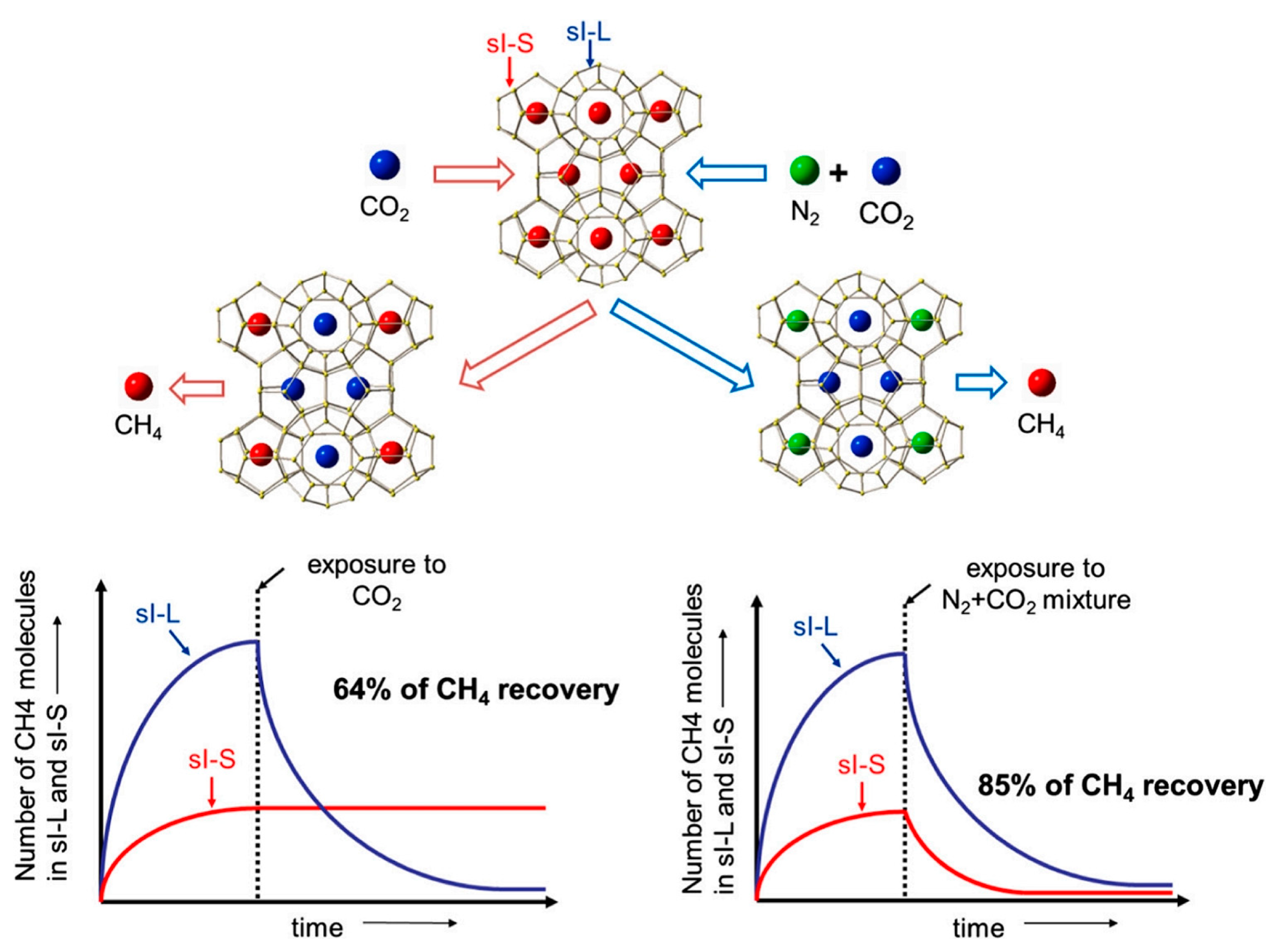
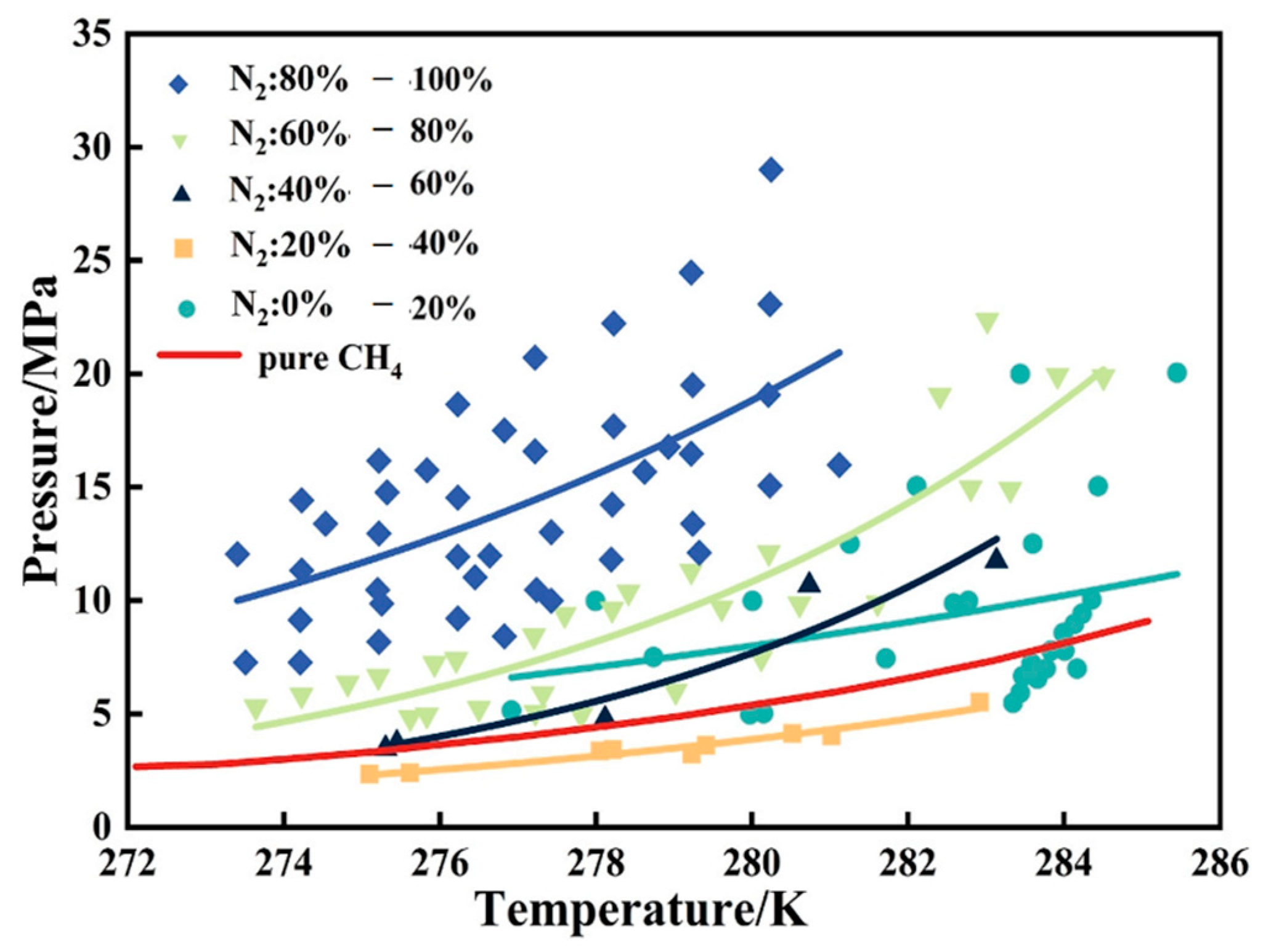
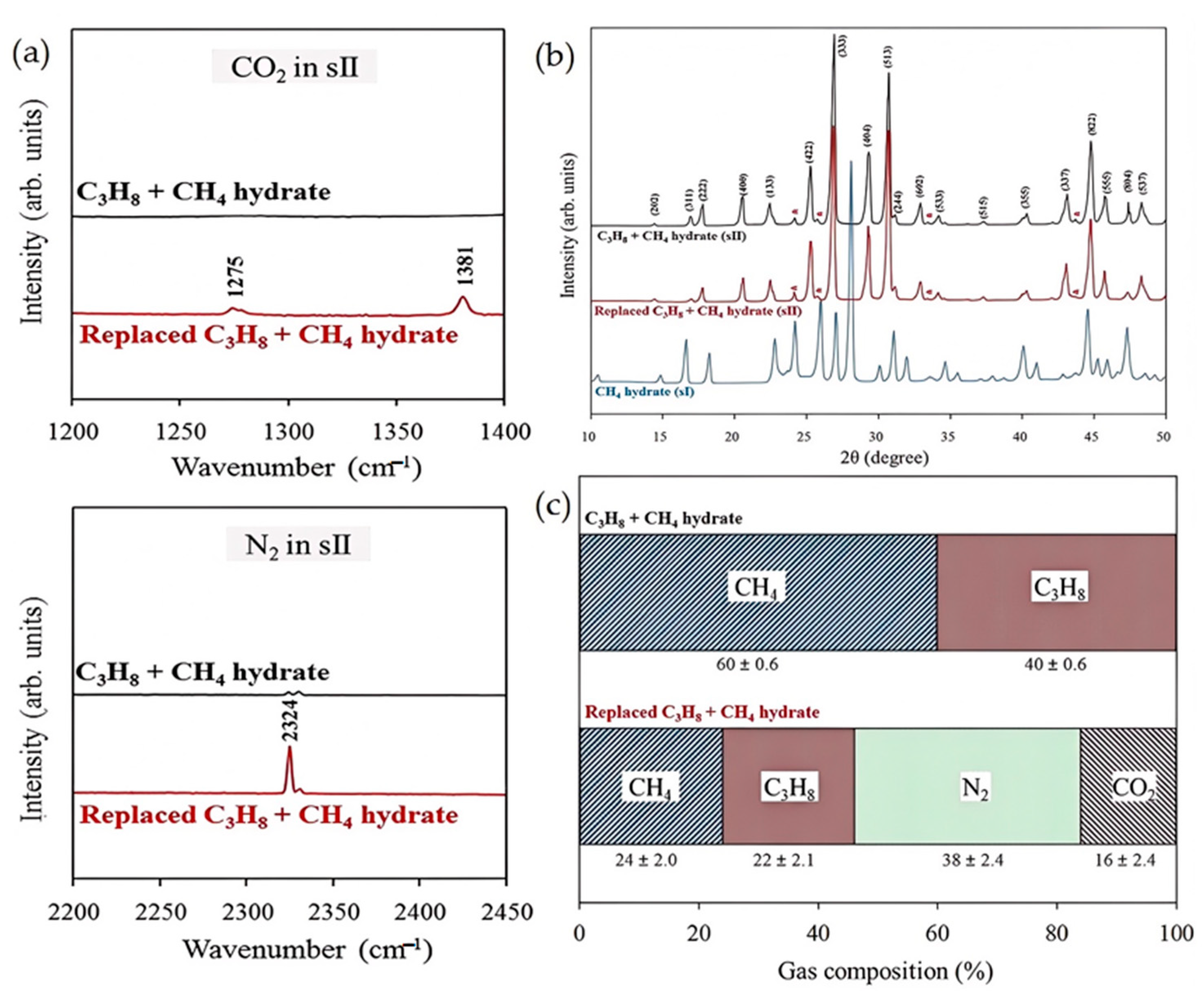
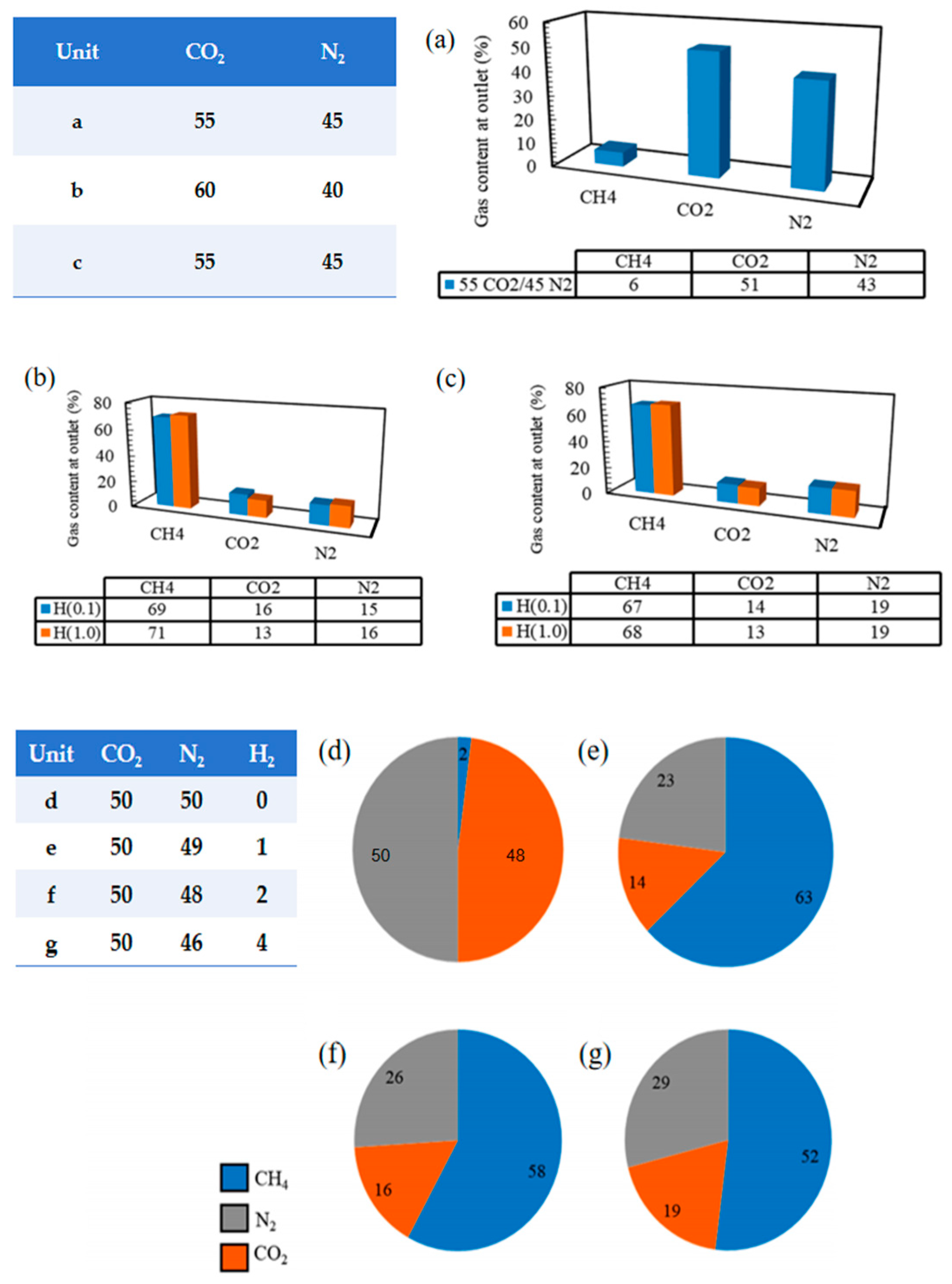

| Method | Advantages | Disadvantages |
|---|---|---|
| Depressurization Method | Technologically mature; simple to operate; no chemical additives; no negative impact on the environment | Requires prolonged depressurization, leading to increased extraction costs; relatively low extraction rate; rapid pressure changes may cause well leakage or collapse |
| Thermal Stimulation Method | Increase the decomposition rate of hydrates; applicable to low-temperature areas | High energy consumption; damages wellbore and reservoir; using thermal fluids may require treatment of the injected water; otherwise, it could introduce environmental contaminants |
| Chemical Inhibitor Injection Method | Low energy demand; adaptable to various reservoir conditions | High chemical costs; requires precise control of injection volume |
| Method | Advantages | Disadvantages |
|---|---|---|
| Thermal fluid injection method | Increase recovery rate; widely applicable; high heat transfer efficiency | High energy consumption; significant heat loss; complex equipment maintenance |
| Electric heating method | High energy efficiency; precise control; minimal equipment requirements | High equipment cost; high power consumption; poor adaptability |
| Geothermal heating method | Low energy consumption; minimal heat loss; wide applicability | Slow heating effect; limited improvement in yield; less effective than high-temperature heating |
| Inhibitor | Type | Advantages | Disadvantages |
|---|---|---|---|
| Thermodynamic Hydrate Inhibitors | Alcohols and electrolytes | Effectively reduce the formation temperature of hydrates, thereby preventing their formation | It usually requires a high dosage, leading to increased costs and environmental impact |
| Kinetic Hydrate Inhibitors | Polymeric compound | Effectively inhibit hydrate formation at a lower dosage, suitable for long-term flow assurance | In some cases, it may not be stable enough, and its effectiveness varies with different gas compositions |
| Anti-agglomerants | Compounds with various chemical structures | Prevent the agglomeration of hydrate particles, thereby reducing the risk of blockage | It needs to be used in combination with other inhibitors to enhance effectiveness |
| Dual-function Hydrate Inhibitors | Amino acids, ionic liquids, and nanoparticles | Combining the advantages of thermodynamic and kinetic inhibition provides a more comprehensive inhibitory effect | Relatively novel, and it requires further research to determine its long-term effects and cost-effectiveness |
| Extraction Method Combination | Advantages | Disadvantages | Suitable Geological Conditions | Key Factors Affecting Extraction |
|---|---|---|---|---|
| Displacement Method Combined with Pressure Reduction Method | Increase extraction rate; sustainable gas release; reduce the risk of hydrate re-crystallization | High cost of gas injection; extraction efficiency limited by reservoir characteristics | Higher bottom pressure, better permeability, and porosity | Reservoir temperature and pressure; gas injection rate and gas selection |
| Displacement Method Combined with Thermal Stimulation Method | Thermal energy promotes hydrate dissociation, enhancing displacement effectiveness | Thermal stimulation method may cause potential damage to the reservoir; high energy consumption and relatively high cost | The hydrate layer at a lower temperature (0 °C to 10 °C) is relatively thick and evenly distributed | Reservoir temperature and thermal response characteristics; heat injection methods and temperature control; thermal stability and structure of the reservoir |
| Displacement Method Combined with Chemical Inhibitor Method | Improve the long-term stability of natural gas production; prevent hydrate recrystallization | Inhibitors may increase environmental risks; chemical inhibitors are expensive and could negatively impact extraction costs | Mid- to high-saturation hydrate reservoirs under low-temperature and high-pressure conditions | Selection and injection concentration of chemical inhibitors; cost and environmental friendliness of inhibitors; synergistic effect of inhibitors and displacement gases |
| Method | Experimental Conditions | Advantages | CH4 Recovery Rate | Literature Source |
|---|---|---|---|---|
| Combined Pressure Reduction and Substitution Method | Experiments conducted using a customized high-pressure flow-through apparatus at different methane hydrate dissociation levels (0%, 20%, 40%, 60%, 80%, 100%) | The mechanical properties of methane hydrate-bearing sediments were considered to provide a basis for economically safe extraction; experimental studies were conducted to investigate the effects of various factors on mechanical properties and methane recovery rate | 35.4–63.3% | Lee et al. [57] |
| Pressure Reduction-Assisted CO2 Substitution Method | Design of a one-dimensional experimental setup to simulate the interface between horizontal wells, investigating the impact of different pressures (inlet pressure, outlet pressure) on CO2 substitution behavior. The experimental temperature is 275 K, and the methane hydrate saturation is 32% | By combining the advantages of CO2 substitution and pressure reduction, production efficiency is improved, and risks are reduced; the impact of pressure parameters on natural gas extraction was studied, providing theoretical support for further research and application | 27.2–46.6% | Chen et al. [58] |
| Pressure Reduction-Assisted CO2 Substitution Method | Study of the depressurization-assisted CO2 substitution process by varying initial hydrate dissociation ratio (0%, 50%, 100%), substitution period (1, 4, 7 days), and CO2 injection flow rate | The issues of weakened geomechanical strength of methane hydrate-bearing sediments caused by pressure reduction alone and the slow production rate during substitution were addressed; methane production and CO2 sequestration efficiency were improved | Through depressurization-assisted substitution, the amount of CO2 stored in the sediment can be greater than the amount of CH4 produced, with approximately 92% of the initial methane being replaced by CO2 | Choi et al. [68] |
| Combined CH4/CO2 Substitution and Thermal Stimulation Method | Experiments conducted under different methane hydrate saturations, substitution zones, and freezing point conditions | The diffusion rate of CO2 was increased through thermal stimulation, overcoming the diffusion limitation in the CO2 substitution process alone; the methane substitution percentage, CO2 storage efficiency, and energy efficiency under different conditions were analyzed and discussed | 64.63% | Zhang et al. [62] |
| Combined CH4/CO2 Substitution and Thermal Stimulation Method | Experiments conducted in a large-scale hydrate vessel (LSHV) with heating rates of 20, 50, and 100 W | The effect of temperature on N2 capture was studied, and it was found that N2 is selectively captured in hydrate cages at temperatures below 12 °C | At a heating rate of 100 W, the mole number of methane during thermal stimulation is 8.5; during thermal stimulation with CO2 substitution, it is 16; and during thermal stimulation with CO2 + N2 substitution, it is 20 | Tupsakhare et al. [63] |
| Combined Thermal Stimulation and CH4/CO2 Substitution Method with Nanoparticle Addition | Experiments conducted in a high-pressure stainless steel reactor under different pressures (40 bar, 45 bar) and temperatures (5.5 °C, 8 °C, 10 °C) | Without the need for vacuum extraction, this method can effectively increase methane recovery and CO2 storage efficiency; the optimal experimental conditions (45 bar and 8 °C) were determined | The recovery rate of CH4 increased from 19.8% to 51.9% | Adibi et al. [69] |
| Inhibitor-Assisted Substitution Method (Using Methanol Solution) | Using an automated core flooding system to simulate and monitor fluid flow and studying the effects of different inhibitors on CH4 hydrate dissociation and CO2 substitution by varying the injected fluid | The CO2 substitution method and thermodynamic hydrate inhibitor technology were combined to replace the simple CH4 hydrate substitution process | Under the experimental conditions, the methane recovery rate exceeds 92% | Khlebnikov et al. [66] |
| Bio-Surfactant-Assisted Method (Using Rhamnolipid) | Studying the effect of different concentrations of rhamnolipids on the kinetics of methane hydrate formation and comparing it with the chemical surfactant SDS | Compared to the chemical surfactant SDS, it significantly reduces the induction time and total time; improves gas consumption and increases the kinetic growth rate of the hydrate; it can enhance the substitution rate and CO2 storage capacity | Injecting rhamnolipid increased the substitution percentage by approximately 72.6% | Heydari et al. [67] |
| Mixed Gas Ratio (CO2/H2) | Hydrate Medium | Temperature | Pressure | CH4 Recovery Rate | Literature Source |
|---|---|---|---|---|---|
| 0.72/0.28 | Sandstone + brine | 275.6 K | 5.0 MPa | 28.0% | Wang et al. [28] |
| 0.55/0.45 | 47.0% | ||||
| 0.36/0.64 | 25.0% | ||||
| 0.18/0.82 | 70.0% | Xu et al. [75] | |||
| 0.4/0.6 | Pure water | 274.0 K | 4.5 MPa | 78% | |
| 0.601/0.399 | 274.2 K | 6.0 MPa | 32% | Sun et al. [73] | |
| 0.74/0.26 | Quartzite + brine | 276.0 K | 3.6 MPa | 41.4~52.4% | |
| 0.74/0.26 | Quartz sand + brine | 276.0 K | 3.7 MPa | 30.0~50.0% | |
| 0.4/0.6 | Quartz sand + brine | 276.0 K | 3.7 MPa | 40.0~75.0% | Sun et al. [73] |
| 0.22/0.78 | 276.0 K | 3.7 MPa | 12.0~88.0% |
| Gas Mixture Ratio (CO2/N2) | Hydrate Medium | Temperature | Pressure | CH4 Recovery Rate | Literature Source |
|---|---|---|---|---|---|
| 0.1/0.9 | Porous silica + water | 274.0 K | 11.5/14.6/18.6 MPa | 77%/80%/79% | Lee et al. [79] |
| 0.2/0.8 | 13.7 MPa | 80% | |||
| 0.6/0.4 | Pure water | 4.5 MPa | 73.4% | Xu et al. [75] | |
| 0.146/0.854 | Silica sand + water | 273.3 K | 4.2 MPa | 53.3% | Yang et al. [59] |
| 0.28/0.72 | Pure water + SDS solution | 284.3K | 9.0 MPa | 13.2% | Niu et al. [80] |
| 0.5/0.5 | Pure water | 273.9 K | 5.0/6.7 MPa | 8.3%/17.7% | Zhou et al. [81] |
| 0.75/0.25 | 274.0 K | 2.6/3.2/3.5 MPa | 9.5%/12.6%/17.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhao, X.; Wang, S.; Jiang, L.; Dong, H.; Lv, P. Gas Production and Storage Using Hydrates Through the Replacement of Multicomponent Gases: A Critical Review. Energies 2025, 18, 975. https://doi.org/10.3390/en18040975
Zhu Z, Zhao X, Wang S, Jiang L, Dong H, Lv P. Gas Production and Storage Using Hydrates Through the Replacement of Multicomponent Gases: A Critical Review. Energies. 2025; 18(4):975. https://doi.org/10.3390/en18040975
Chicago/Turabian StyleZhu, Zhiyuan, Xiaoya Zhao, Sijia Wang, Lanlan Jiang, Hongsheng Dong, and Pengfei Lv. 2025. "Gas Production and Storage Using Hydrates Through the Replacement of Multicomponent Gases: A Critical Review" Energies 18, no. 4: 975. https://doi.org/10.3390/en18040975
APA StyleZhu, Z., Zhao, X., Wang, S., Jiang, L., Dong, H., & Lv, P. (2025). Gas Production and Storage Using Hydrates Through the Replacement of Multicomponent Gases: A Critical Review. Energies, 18(4), 975. https://doi.org/10.3390/en18040975