A Universal Highly Concentrated Electrolyte for Improved Cycling Stability in Li(Ni1-x-yMnxCoy)O2-NMC-Based Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Cathodes Preparation
2.2. Electrolyte Preparation
2.3. Half-Cell Assembly, Electrochemical Testing, and Electrolyte Properties
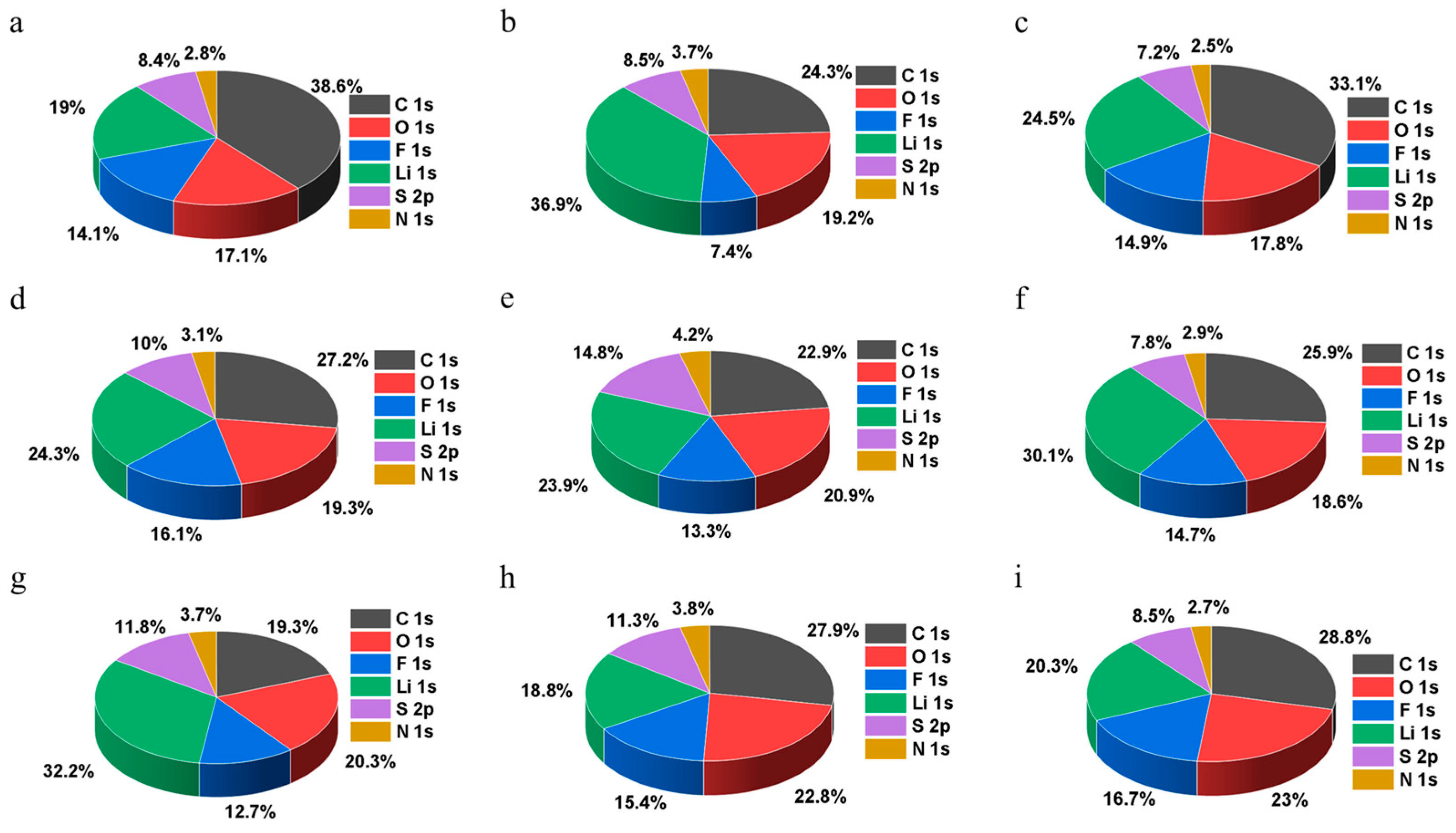
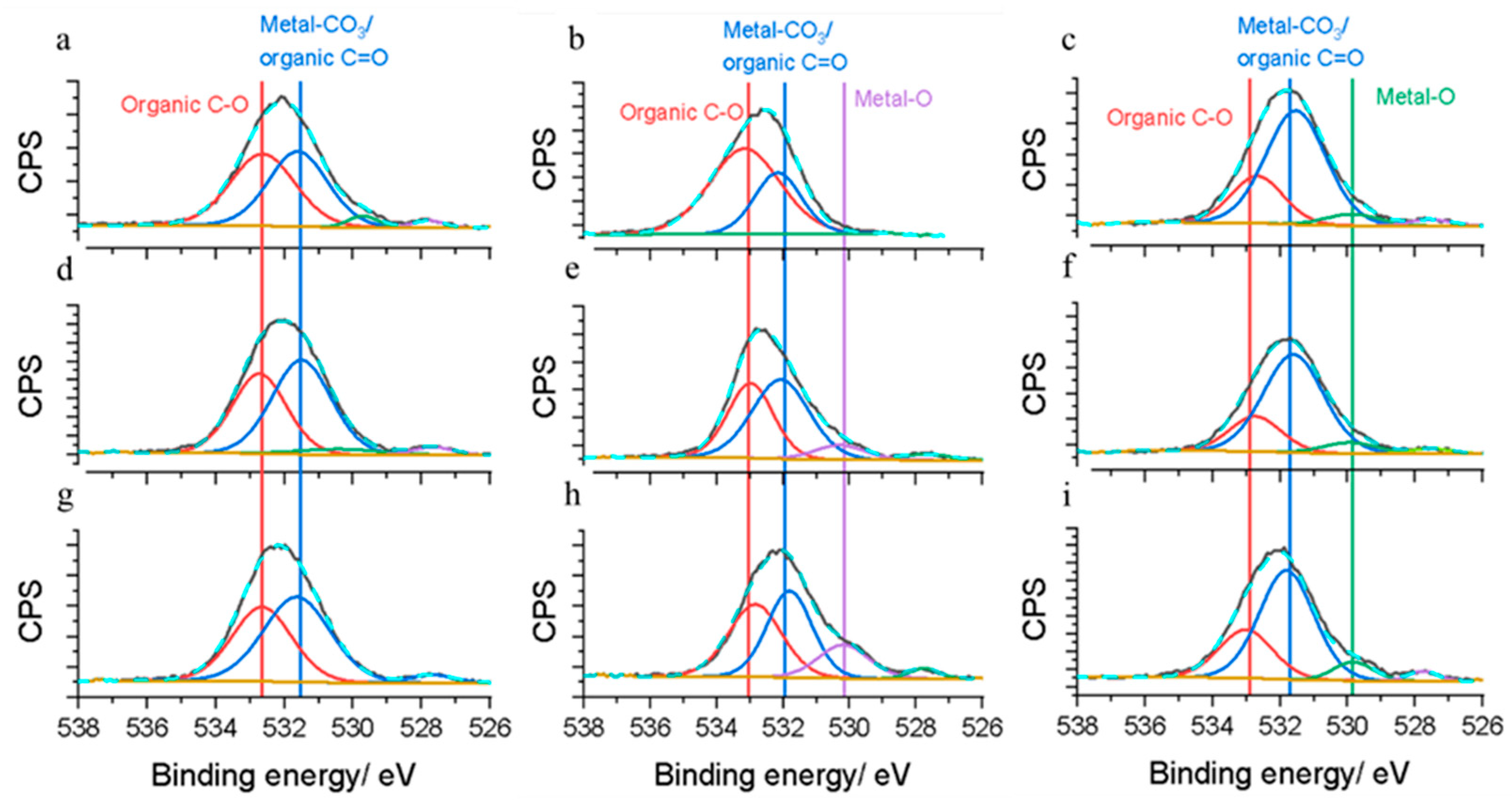
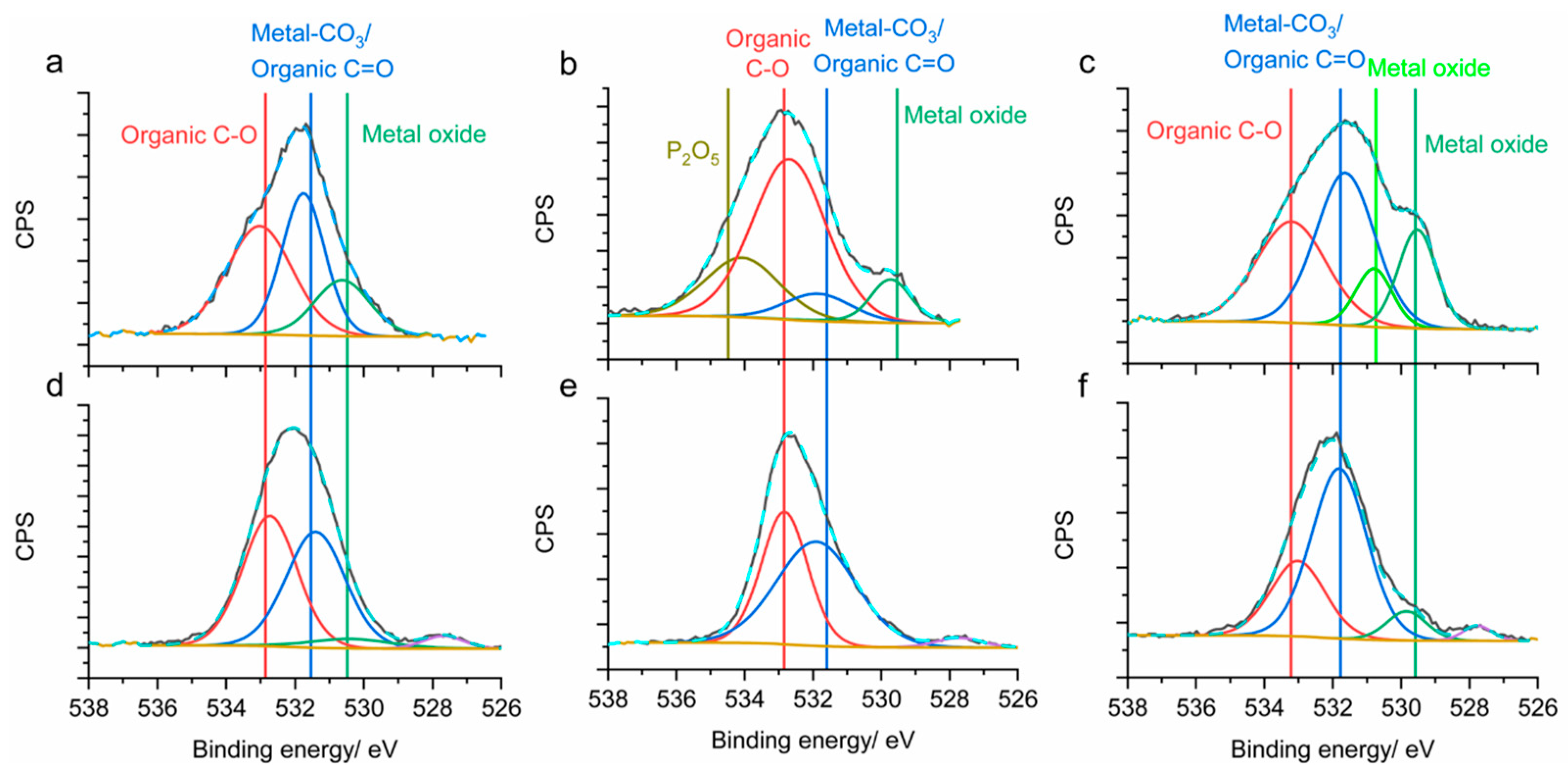
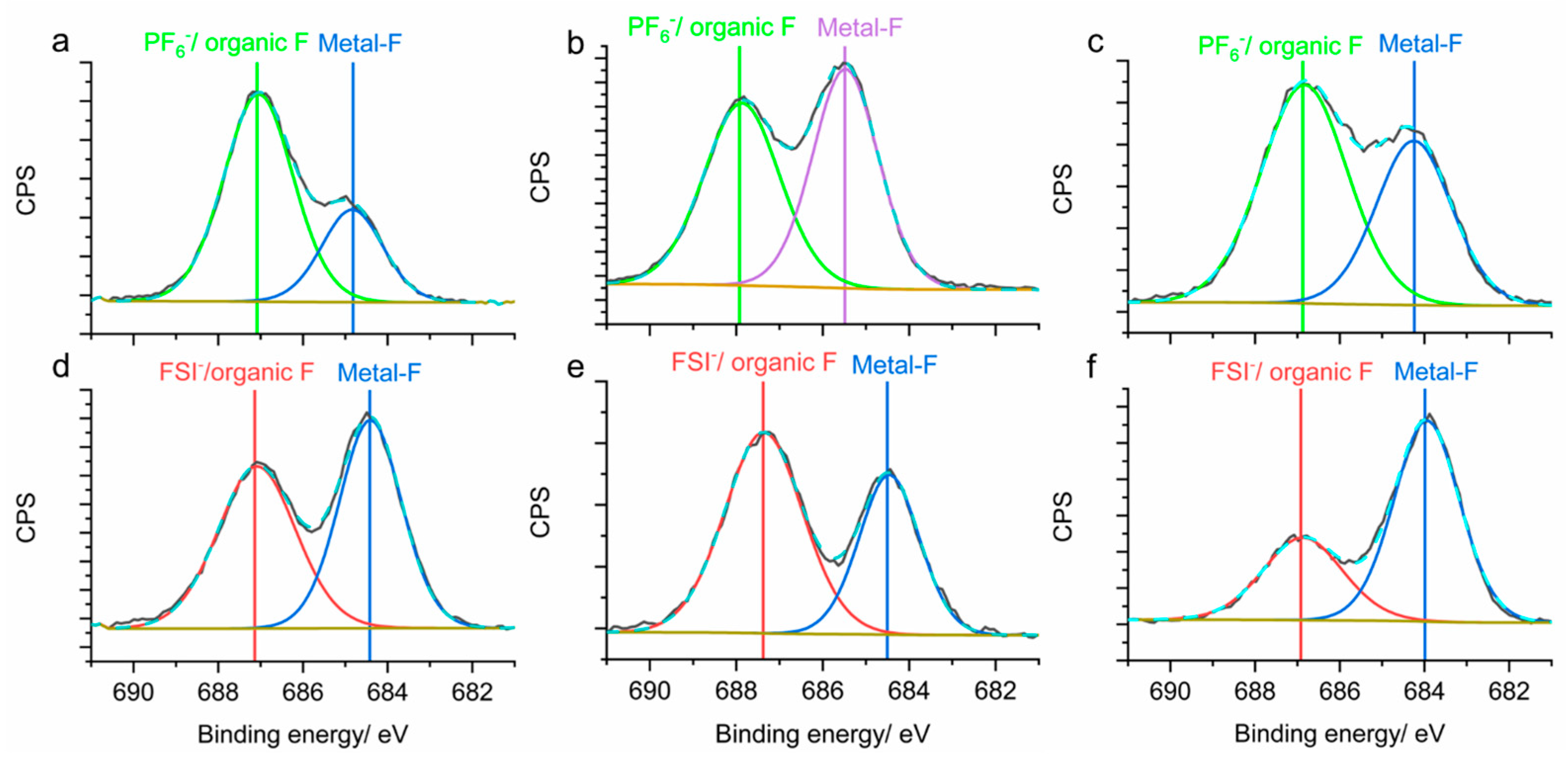
2.4. Characterization Techniques
3. Results and Discussion
3.1. Understanding the Effect of LiFSI Concentration
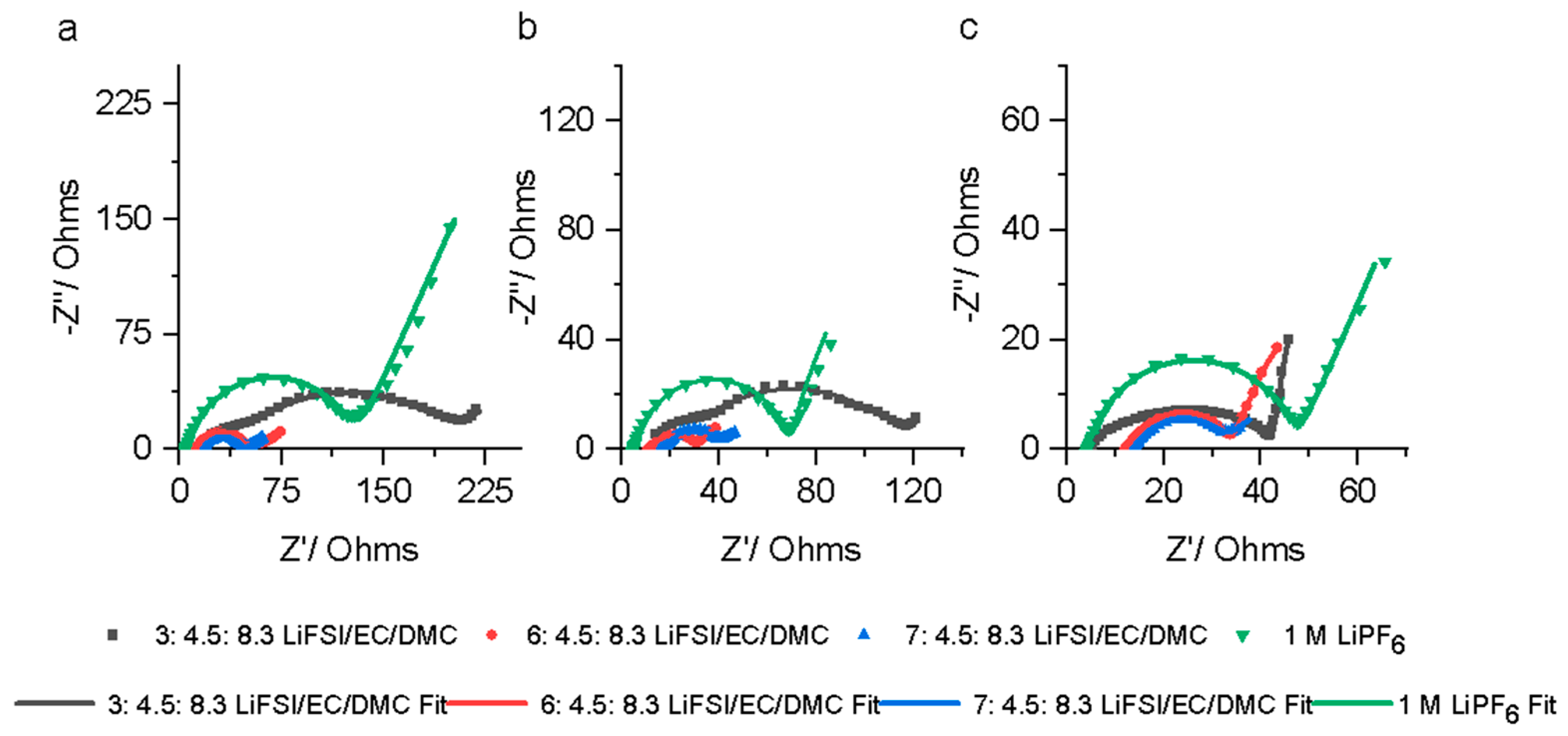
3.2. Properties of LiFSI/EC/DMC and 1 M LiPF6 Electrolytes
3.3. Optimal LiFSI Electrolyte Versus State-of-the-Art LiPF6 Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Electrification. Available online: https://www.iea.org/energy-system/electricity/electrification (accessed on 24 March 2024).
- What Is Electrification? Available online: https://www.energy.gov/electricity-insights/what-electrification (accessed on 24 March 2024).
- Beyond Batteries: Most Efficient Energy Storage 2023. Available online: https://solar.huawei.com/za/blog/za/2023/most-efficient-energy-storage#:~:text=Green%20hydrogen%2C%20also%20known%20as,energy%20sources%20like%20solar%20power (accessed on 24 March 2024).
- Ahmed, S.; Ali, A.; Asif, M.; Shim, J.; Park, G. Exploring innovative trends and advancements in rechargeable zinc-air batteries. Inorg. Chem. Commun. 2024, 170, 113288. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- What Are Sodium-Ion Batteries and How Do They Compare to Lithium-Ion Batteries? Available online: https://www.driveelectrictn.org/what-are-sodium-ion-batteries-and-how-do-they-compare-to-lithium-ion-batteries/#:~:text=Lithium%2Dion%20batteries%20have%20faced,storage%20and%20household%20energy%20systems (accessed on 11 February 2025).
- Huang, X.; Jing, H.; Yang, M.; Lu, H.; Xue, F.; Zhao, J.; Cheng, X.; Zhang, H.; Fu, Y. Comparative study on thermal and gas characteristics of 26700 sodium-ion and lithium-ion batteries. J. Power Sources 2025, 631, 236270. [Google Scholar] [CrossRef]
- Sodium-Ion Batteries: A Sustainable Energy Solution for 2024. Available online: https://www.monolithai.com/blog/sodium-ion-battery-news#:~:text=As%20one%20of%20the%20most,just%2020%20ppm%20for%20lithium.&text=Given%20that%20sodium%20is%20both,(source) (accessed on 11 February 2025).
- Building Electrification Gains Momentum with Heat Pump Adoption. Available online: https://www.facilitiesnet.com/hvac/article/Building-Electrification-Gains-Momentum-with-Heat-Pump-Adoption--20057 (accessed on 24 March 2024).
- [Battery101] Cathode Materials That Determine the Power of Li-ion Battery. Available online: https://news.samsungsdi.com/global/articleView?seq=178 (accessed on 30 July 2024).
- Liu, D.W.; Oh, P.; Liu, D.X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, P.D.Y.; Cho, P.D.J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Liu, M.; Vatamanu, J.; Chen, X.; Xing, L.; Xu, K.; Li, W. Hydrolysis of LiPF6-Containing Electrolyte at High Voltage. ACS Energy Lett. 2021, 6, 2096–2102. [Google Scholar] [CrossRef]
- Guo, K.; Qi, S.; Wang, H.; Huang, J.; Wu, M.; Yang, Y.; Li, X.; Ren, Y.; Ma, J. High-Voltage Electrolyte Chemistry for Lithium Batteries. Small Sci. 2022, 2, 2100107. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Battery Electrolyte Solutions. Available online: https://www.targray.com/li-ion-battery/electrolyte#:~:text=The%20Role%20of%20Electrolyte%20in%20Lithium%2Dion%20Batteries&text=The%20most%20commonly%20used%20electrolyte,LiPF6%20in%20an%20organic%20solution (accessed on 24 March 2024).
- Han, H.-B.; Zhou, S.-S.; Zhang, D.-J.; Feng, S.-W.; Li, L.-F.; Liu, K.; Feng, W.-F.; Nie, J.; Li, H.; Huang, X.-J.; et al. Lithium bis(fluorosulfonyl)imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties. J. Power Sources 2011, 196, 3623–3632. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Shilina, Y.; Luski, S.; Aurbach, D.; Halalay, I.C. Acid-Scavenging Separators: A Novel Route for Improving Li-Ion Batteries’ Durability. ACS Energy Lett. 2017, 2, 2388–2393. [Google Scholar] [CrossRef]
- Han, J.Y.; Jung, S. Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6. Batteries 2022, 8, 61. [Google Scholar] [CrossRef]
- Shchurov, N.I.; Dedov, S.I.; Malozyomov, B.V.; Shtang, A.A.; Martyushev, N.V.; Klyuev, R.V.; Andriashin, S.N. Degradation of Lithium-Ion Batteries in an Electric Transport Complex. Energies 2021, 14, 8072. [Google Scholar] [CrossRef]
- Aktekin, B.; Hernández, G.; Younesi, R.; Brandell, D.; Edström, K. Concentrated LiFSI–Ethylene Carbonate Electrolytes and Their Compatibility with High-Capacity and High-Voltage Electrodes. ACS Appl. Energy Mater. 2022, 5, 585–595. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R Rep. 2018, 134, 1–21. [Google Scholar] [CrossRef]
- Overview of Lithium Salts in Li Ion Battery Electrolyte. Available online: https://www.dnkpower.com/lithium-salts-in-li-ion-battery-electrolyte/ (accessed on 24 March 2024).
- Liu, W.; Li, J.; Li, W.; Xu, H.; Zhang, C.; Qiu, X. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 2020, 11, 3629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-N.; Li, Q.; Wang, Y.; Zheng, J.; Yu, X.; Li, H. Dynamic evolution of cathode electrolyte interphase (CEI) on high voltage LiCoO2 cathode and its interaction with Li anode. Energy Storage Mater. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, F.; Liu, X.; Yang, S.; Ma, J. Formation and modification of cathode electrolyte interphase: A mini review. Electrochem. Commun. 2021, 122, 106870. [Google Scholar] [CrossRef]
- Giffin, G.A. The role of concentration in electrolyte solutions for non-aqueous lithium-based batteries. Nat. Commun. 2022, 13, 5250. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q.; Fang, M.; Ko, S.; Yamada, Y.; Yamada, A. Concentrated Electrolytes Widen the Operating Temperature Range of Lithium-Ion Batteries. Adv. Sci. 2021, 8, 2101646. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K.; et al. Highly Fluorinated Interphases Enable High-Voltage Li-Metal Batteries. Chem 2018, 4, 174–185. [Google Scholar] [CrossRef]
- Piao, N.; Ji, X.; Xu, H.; Fan, X.; Chen, L.; Liu, S.; Garaga, M.N.; Greenbaum, S.G.; Wang, L.; Wang, C.; et al. Countersolvent Electrolytes for Lithium-Metal Batteries. Adv. Energy Mater. 2020, 10, 1903568. [Google Scholar] [CrossRef]
- Table of Elements. Available online: https://www.thermofisher.com/sg/en/home/materials-science/learning-center/periodic-table.html (accessed on 15 March 2024).
- National Institute of Standards and Technology. NIST X-Ray Photoelectron Spectroscopy Database (SRD 20), Version 5.0. 2024. Available online: https://srdata.nist.gov/xps/SpectraIdentifier (accessed on 15 March 2024).
- Guo, J.; Guo, Q.; Liu, J.; Wang, H. The Polarization and Heat Generation Characteristics of Lithium-Ion Battery with Electric–Thermal Coupled Modeling. Batteries 2023, 9, 529. [Google Scholar] [CrossRef]
- Kim, D.-H.; Hwang, S.; Cho, J.-J.; Yu, S.; Kim, S.; Jeon, J.; Ahn, K.H.; Lee, C.; Song, H.-K.; Lee, H. Towards Fast Operation of Lithium Batteries: Ion Activity as the Factor To Determine the Concentration Polarization. ACS Energy Lett. 2019, 4, 1265–1270. [Google Scholar] [CrossRef]
- Biologic. Internal Resistance Series. Part I: What Is Internal Resistance in a Battery? Available online: https://www.biologic.net/topics/internal-resistance-series-part-i-what-is-internal-resistance-in-a-battery/ (accessed on 24 March 2024).
- Why Is It Important to Measure Battery’s Internal Resistance? Available online: https://www.hioki.com/sg-en/learning/electricity/internal-resistance.html#:~:text=Internal%20resistance%20is%20one%20of,a%20small%20amount%20of%20current (accessed on 24 March 2024).
- Jow, T.R.; Delp, S.A.; Allen, J.L.; Jones, J.-P.; Smart, M.C. Factors Limiting Li+ Charge Transfer Kinetics in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A361. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Xie, J.-D.; Patra, J.; Rath, P.C.; Liu, W.-J.; Su, C.-Y.; Lee, S.-W.; Tseng, C.-J.; Gandomi, Y.A.; Chang, J.-K. Highly concentrated carbonate electrolyte for Li-ion batteries with lithium metal and graphite anodes. J. Power Sources 2020, 450, 227657. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Shimizu, R.; Momma, T.; Osaka, T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J. Power Sources 2019, 409, 139–147. [Google Scholar] [CrossRef]
- Wu, H.-C.; Wu, H.-C.; Lee, E.; Wu, N.-L. High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries. Electrochem. Commun. 2010, 12, 488–491. [Google Scholar] [CrossRef]
- BU-802a: How Does Rising Internal Resistance Affect Performance? Available online: https://batteryuniversity.com/article/bu-802a-how-does-rising-internal-resistance-affect-performance#:~:text=A%20battery%20with%20low%20internal%20resistance%20delivers%20high%20current%20on,last%20for%20a%20few%20seconds (accessed on 24 March 2024).
- Yua, X.; Manthiram, A. Electrode–electrolyte interfaces in lithium-based batteries. Energy Environ. Sci. 2018, 11, 527–543. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, C.; Wang, K.; Han, X.; Li, J.; Sui, M.; Yan, P. Deciphering the critical effect of cathode-electrolyte interphase by revealing its dynamic evolution. J. Energy Chem. 2023, 81, 192–199. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, B.; Jin, F.; Chen, Y.; Jiang, Y.; Bao, C.; Tian, J.; Wang, J.; Xu, R.; Li, Y.; et al. Modified cathode-electrolyte interphase toward high-performance batteries. Cell Rep. Phys. Sci. 2022, 3, 101197. [Google Scholar] [CrossRef]
- Zhang, R.; Bao, J.; Wang, Y.; Sun, C.-F. Concentrated electrolytes stabilize bismuth–potassium batteries. Chem. Sci. 2018, 9, 6193–6198. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, W.; Zhang, Q.; Yang, G.; Zheng, J.; Tang, W.; Xu, Q.; Lai, C.; Yang, J.; Peng, C. Armoring LiNi1/3Co1/3Mn1/3O2 Cathode with Reliable Fluorinated Organic–Inorganic Hybrid Interphase Layer toward Durable High Rate Battery. Adv. Funct. Mater. 2020, 30, 2000396. [Google Scholar] [CrossRef]
- Liu, T.; Dai, A.; Lu, J.; Yuan, Y.; Xiao, Y.; Yu, L.; Li, M.; Gim, J.; Ma, L.; Liu, J.; et al. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 2019, 10, 4721. [Google Scholar] [CrossRef]
- Kim, T.; Ono, L.K.; Qi, Y. Understanding the active formation of a cathode–electrolyte interphase (CEI) layer with energy level band bending for lithium-ion batteries. J. Mater. Chem. A 2023, 11, 221–231. [Google Scholar] [CrossRef]
- Cherkashinin, G.; Motzko, M.; Schulz, N.; Späth, T.; Jaegermann, W. Electron Spectroscopy Study of Li[Ni,Co,Mn]O2/Electrolyte Interface: Electronic Structure, Interface Composition, and Device Implications. Chem. Mater. 2015, 27, 2875–2887. [Google Scholar] [CrossRef]
- Takahashi, I.; Kiuchi, H.; Ohma, A.; Fukunaga, T.; Matsubara, E. Cathode Electrolyte Interphase Formation and Electrolyte Oxidation Mechanism for Ni-Rich Cathode Materials. J. Phys. Chem. C 2020, 124, 9243–9248. [Google Scholar] [CrossRef]
- Gu, W.; Borodin, O.; Zdyrko, B.; Lin, H.-T.; Kim, H.; Nitta, N.; Huang, J.; Magasinski, A.; Milicev, Z.; Berdichevsky, G.; et al. Conversion cathodes: Lithium–iron fluoride battery with in situ surface protection. Adv. Funct. Mater. 2016, 26, 1490. [Google Scholar] [CrossRef]
- Cho, S.-J.; Yu, D.-E.; Pollard, T.P.; Moon, H.; Jang, M.; Borodin, O.; Lee, S.-Y. Nonflammable Lithium Metal Full Cells with Ultra-high Energy Density Based on Coordinated Carbonate Electrolytes. iScience 2020, 23, 100844. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yim, T. Sustainable cathode-electrolyte interphases of nickel-rich layered oxides in-situ functionalized by lithium fluoride abundant layers for lithium-ion batteries. J. Energy Storage 2024, 99, 113264. [Google Scholar] [CrossRef]
- Chae, B.-J.; Yim, T. Effect of surface modification using a sulfate-based surfactant on the electrochemical performance of Ni-rich cathode materials. Mater. Chem. Phys. 2018, 214, 66–72. [Google Scholar] [CrossRef]
- Bauer, I.; Thieme, S.; Brückner, J.; Althues, H.; Kaskel, S. Reduced polysulfide shuttle in lithium–sulfur batteries using Nafion-based separators. J. Power Sources 2014, 251, 417–422. [Google Scholar] [CrossRef]
- Borah, R.; Hughson, F.R.; Johnston, J.; Nann, T. On battery materials and methods. Mater. Today Adv. 2020, 6, 100046. [Google Scholar] [CrossRef]
- Galek, P.; Slesinski, A.; Fic, K.; Menzel, J. Peculiar role of the electrolyte viscosity in the electrochemical capacitor performance. J. Mater. Chem. A 2021, 9, 8644–8654. [Google Scholar] [CrossRef]
- Xu, J. Critical Review on cathode–electrolyte Interphase Toward High-Voltage Cathodes for Li-Ion Batteries. Nano Micro Lett. 2022, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Bolloju, S.; Chiou, C.-Y.; Vikramaditya, T.; Lee, J.-T. (Pentafluorophenyl)diphenylphosphine as a dual-functional electrolyte additive for LiNi0.5Mn1.5O4 cathodes in high-voltage lithium-ion batteries. Electrochim. Acta 2019, 299, 663–671. [Google Scholar] [CrossRef]
- Minato, T.; Abe, T. Surface and interface sciences of Li-ion batteries: -Research progress in electrode–electrolyte interface. Prog. Surf. Sci. 2017, 92, 240–280. [Google Scholar] [CrossRef]
- Yana, C.; Yuan, H.; Park, H.S.; Huang, J.-Q. Perspective on the critical role of interface for advanced batteries. J. Energy Chem. 2020, 47, 217–220. [Google Scholar] [CrossRef]
- Borodin, O.; Self, J.; Persson, K.A.; Wang, C.; Xu, K. Uncharted Waters: Super-Concentrated Electrolytes. Joule 2020, 4, 69–100. [Google Scholar] [CrossRef]
- Jiang, D.L.-L.; Yan, D.C.; Yao, Y.-X.; Cai, D.W.; Huang, P.J.-Q.; Zhang, P.Q. Inhibiting Solvent Co-Intercalation in a Graphite Anode by a Localized High-Concentration Electrolyte in Fast-Charging Batteries. Angew. Chem. Int. Ed. 2020, 60, 3402–3406. [Google Scholar] [CrossRef]
- Bai, P.; Ji, X.; Zhang, J.; Zhang, W.; Hou, S.; Su, H.; Li, M.; Deng, T.; Cao, L.; Liu, S.; et al. Formation of LiF-rich Cathode-Electrolyte Interphase by Electrolyte Reduction. Angew. Chem. Int. Ed. 2022, 61, e202202731. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Allouche, J.; Lindgren, F.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Nanosilicon Electrodes for Lithium-Ion Batteries: Interfacial Mechanisms Studied by Hard and Soft X-ray Photoelectron Spectroscopy. Chem. Mater. 2012, 24, 1107–1115. [Google Scholar] [CrossRef]
- Tanaka, S.; Taniguchi, M.; Tanigawa, H. XPS and UPS studies on electronic structure of Li2O. J. Nucl. Mater. 2000, 283–287, 1405–1408. [Google Scholar] [CrossRef]
- Lowe, J.S.; Siegel, D.J. Modeling the Interface between Lithium Metal and Its Native Oxide. ACS Appl. Mater. Interfaces 2020, 12, 46015–46026. [Google Scholar] [CrossRef]
- Shen, C.; Yan, H.; Gu, J.; Gao, Y.; Yang, J.; Xie, K. Li2O-Reinforced Solid Electrolyte Interphase on Three-Dimensional Sponges for Dendrite-Free Lithium Deposition. Front. Chem. 2018, 6, 422220. [Google Scholar] [CrossRef]
- Pietrowski, M.; Zieliński, M.; Alwin, E.; Suchora, A.; Gawarecka, J. Synthesis and characterization of MgF2–CoF2 binary fluorides. Influence of the treatment atmosphere and temperature on the structure and surface properties. RSC Adv. 2019, 9, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Liu, X.-Y.; Qiana, W.-Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries. Energy Environ. Sci. 2014, 7, 347–353. [Google Scholar]
- Cheng, X.-B.; Yang, S.-J.; Liu, Z.; Guo, J.-X.; Jiang, F.-N.; Jiang, F.; Xiong, X.; Tang, W.-B.; Yuan, H.; Huang, J.-Q.; et al. Electrochemically and Thermally Stable Inorganics–Rich Solid Electrolyte Interphase for Robust Lithium Metal Batteries. Adv. Mater. 2024, 36, 2307370. [Google Scholar] [CrossRef]
- Borodin, O.; Ren, X.; Vatamanu, J.; Cresce, A.v.W.; Knap, J.; Xu, K. Modeling Insight into Battery Electrolyte Electrochemical Stability and Interfacial Structure. Acc. Chem. Res. 2017, 50, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.S.; Danner, D.T.; Hein, D.S.; Hoffmann, D.A.; Prifling, B.; Schmidt, P.D.V.; Latz, P.D.A.; Wohlfahrt-Mehrens, D.M. Influence of the Electrolyte Salt Concentration on the Rate Capability of Ultra-Thick NCM 622 Electrodes. Batter. Supercaps 2020, 3, 1172–1182. [Google Scholar] [CrossRef]






| 1 M LiPF6 EC/DMC | 6:4.5:8.3 LiFSI/EC/DMC | |||||
|---|---|---|---|---|---|---|
| Cathode cell | NMC 111 | NMC 622 | NMC 811 | NMC 111 | NMC 622 | NMC 811 |
| Initial discharge capacity (mAh/g) | 185.0 | 190.1 | 200.4 | 191.2 | 190.8 | 195.5 |
| 200th cycles discharge capacity (mAh/g) | 111.5 | 117.9 | 87.4 | 157.8 | 164.1 | 171.0 |
| Capacity retention (%) | 60.3 | 62.0 | 43.6 | 82.5 | 86.0 | 87.5 |
| Electrolyte | NMC 111 | NMC 622 | NMC 811 |
|---|---|---|---|
| 3:4.5:8.3 LiFSI/EC/DMC | 0.37 | 0.31 | 0.45 |
| 6:4.5:8.3 LiFSI/EC/DMC | 0.59 | 0.58 | 0.57 |
| 7:4.5:8.3 LiFSI/EC/DMC | 0.66 | 0.55 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.J.N.; Cai, Y.; Srinivasan, M. A Universal Highly Concentrated Electrolyte for Improved Cycling Stability in Li(Ni1-x-yMnxCoy)O2-NMC-Based Batteries. Energies 2025, 18, 974. https://doi.org/10.3390/en18040974
Lim JJN, Cai Y, Srinivasan M. A Universal Highly Concentrated Electrolyte for Improved Cycling Stability in Li(Ni1-x-yMnxCoy)O2-NMC-Based Batteries. Energies. 2025; 18(4):974. https://doi.org/10.3390/en18040974
Chicago/Turabian StyleLim, Jun Ji Nicholas, Yi Cai, and Madhavi Srinivasan. 2025. "A Universal Highly Concentrated Electrolyte for Improved Cycling Stability in Li(Ni1-x-yMnxCoy)O2-NMC-Based Batteries" Energies 18, no. 4: 974. https://doi.org/10.3390/en18040974
APA StyleLim, J. J. N., Cai, Y., & Srinivasan, M. (2025). A Universal Highly Concentrated Electrolyte for Improved Cycling Stability in Li(Ni1-x-yMnxCoy)O2-NMC-Based Batteries. Energies, 18(4), 974. https://doi.org/10.3390/en18040974






