A Novel Integrated Biorefinery for the Valorization of Residual Cardoon Biomass: Overview of Technologies and Process Simulation
Abstract
1. Introduction
2. Inulin Extraction and FDCA Production
2.1. Cardoon Root Processing
2.2. Inulin Exctraction
2.3. Inulin Hydrolysis, HMF, and FDCA Production
3. Syngas Fermentation and Ethanol Production
3.1. Cardoon Lignocellulosic Residues’ Gasification
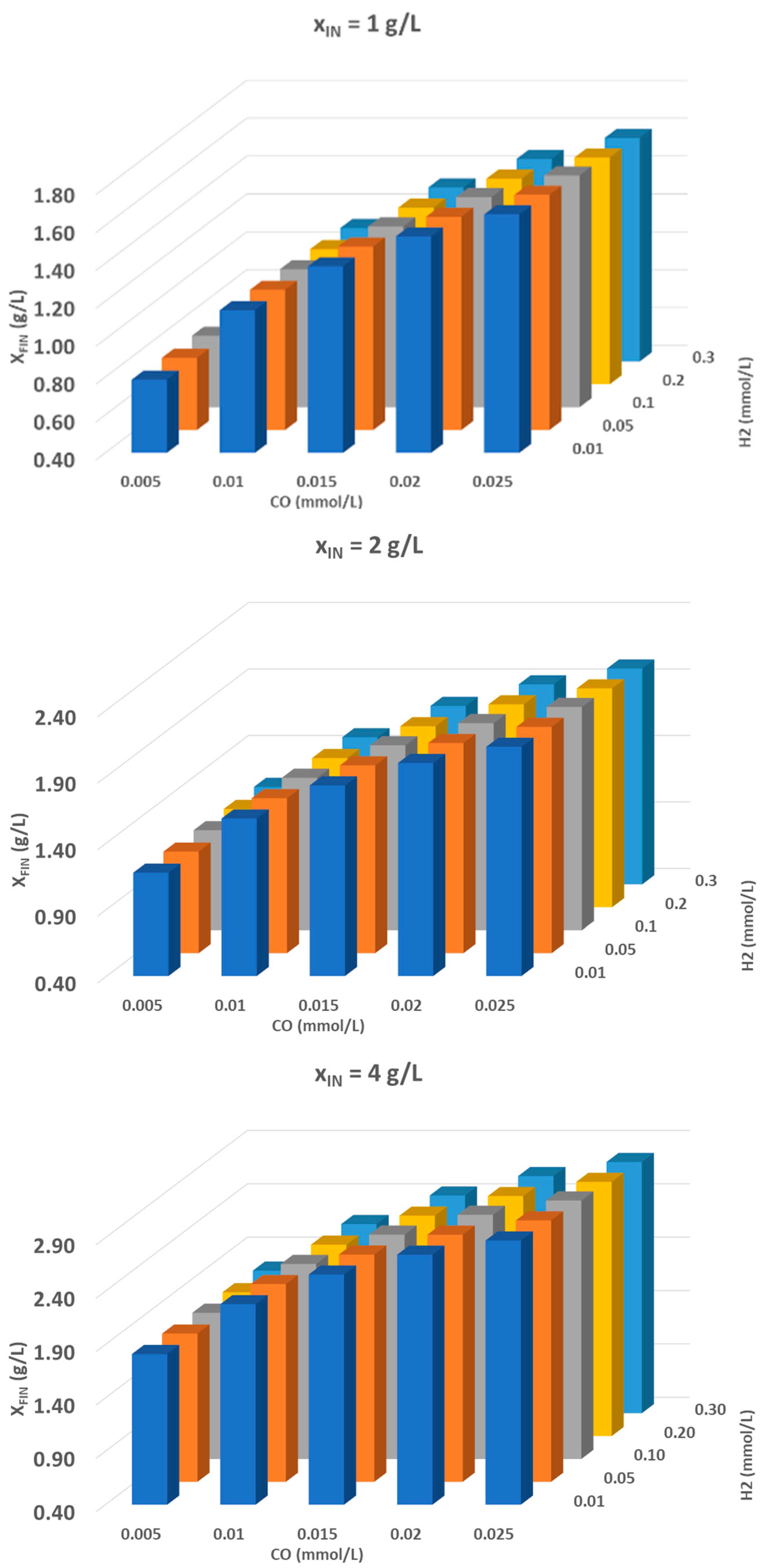
3.2. Syngas Fermentation
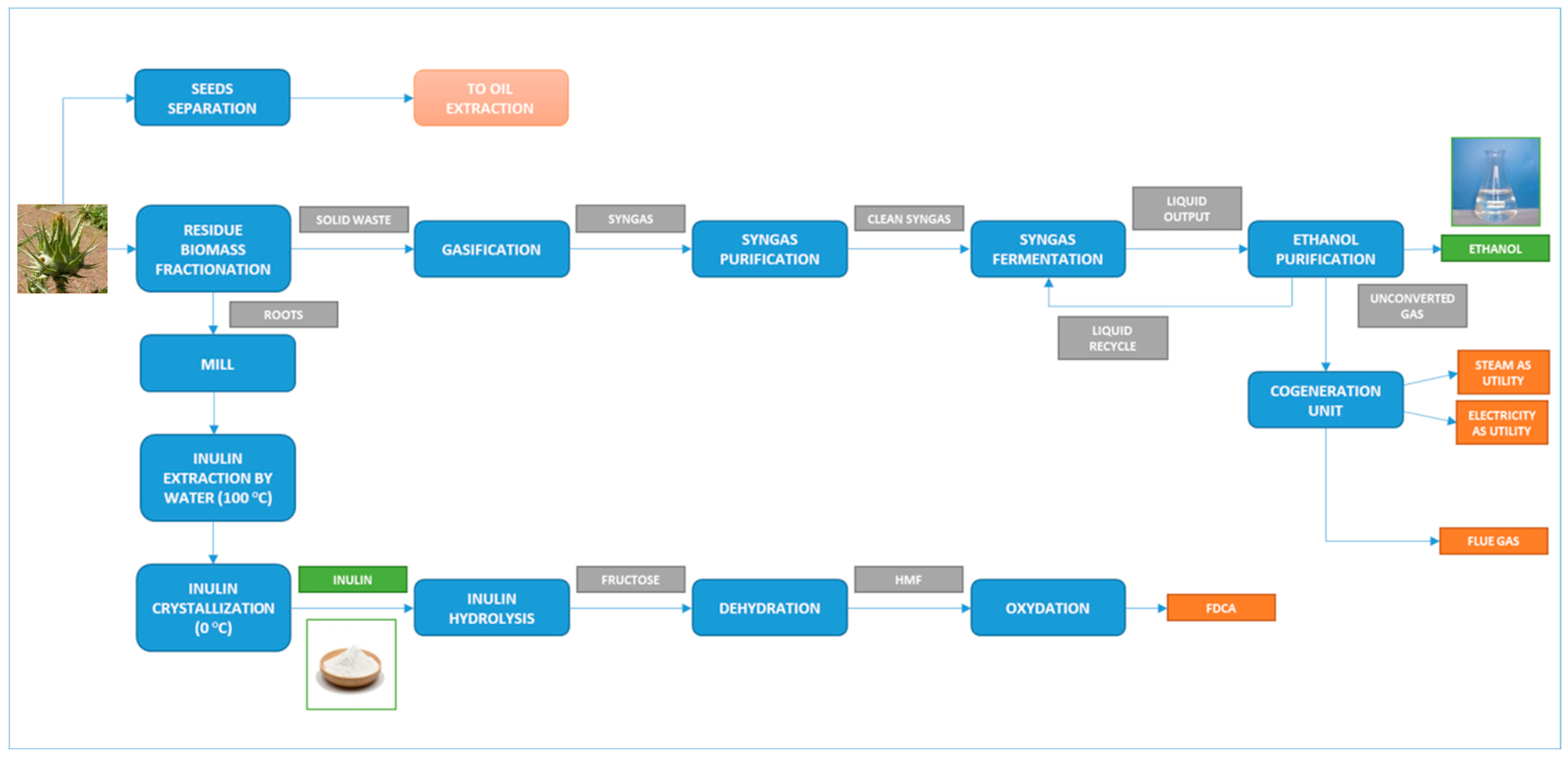
4. Novel Integrated Biorefinery Flowsheet for the Exploitation of Lignocellulosic Cardoon Biomass
4.1. Methodology and Process Description
- -
- Residual roots are valorized first through high-added-value compound extraction processes, and then the solid residues after extraction are used for a thermochemical process, following a cascade valorization approach.
- -
- Typical second-generation sugar platforms cannot convert a very high percentage of the raw material, as they primarily utilize the sugar content (mainly cellulose), while lignin and other solid residues must be valorized through thermochemical processes. In contrast, this flowsheet directly converts all solids to syngas with very high yields (about 200% in mass, if gasification is performed with air).
- -
- The syngas is converted using novel biotechnological processes (syngas fermentation), and the off gases after fermentation are used as fuel for gas turbines and in a heat recovery and steam generation section, resulting in very high energy efficiencies compared to solid-to-energy systems.
- -
- Mass integration is achieved from the residual solids after extraction to the gasification section (cascade approach).
- -
- Energy integration is possible using the cogeneration section, which produces electricity for all of the plant’s power needs. Additionally, it supports heat integration due to the high heat consumption of ethanol distillation columns and the medium temperature requirements of the inulin extraction and conversion sections.
4.2. Process Simulation
4.3. Environmental and Economic Feasibility Analysis
- -
- CO2 savings related to carbon dioxide binding from cardoon growth;
- -
- CO2 emissions during the cultivation phase, which includes cultivation and harvesting of residual thistle biomass in the field;
- -
- Transportation, involving the movement of biomass collected from the field to the biorefinery plant;
- -
- The biorefinery phase or the transformation phase of lignocellulosic residue into FDCA and ethanol, also considering the impact of the solvent (fresh water, while GVL was considered recycled), reactant (enzymes) consumption, and wastewater treatment;
- -
- Direct CO2 emissions from flue gases;
- -
- The impact/savings of bioproduct end use in relation to fossil-based alternative carbon emissions.
4.4. Results
- -
- CO2 savings by cardoon growth: −192.6 ktCO2eq;
- -
- CO2 emissions for cultivation, harvesting, and transportation: 34.6 ktCO2eq;
- -
- Freshwater and wastewater treatment emissions: 40.7 ktCO2eq;
- -
- Direct CO2 emissions from flue gases: 123.8 ktCO2;
- -
- Green electricity production savings: −18.1 ktCO2eq;
- -
- Ethanol emissions (including end use emissions by combustion): 4.9 ktCO2eq;
- -
- Savings from the FDCA replacing fossil-based alternatives: −33.3 ktCO2eq.
Discussion
5. Conclusions
6. Future Directions
- -
- Investment cost analysis for inulin’s conversion to FDCA, considering the costs and performances of catalysts/biocatalysts.
- -
- Identification and optimization of critical steps in the process, such as effective tar removal and optimization of the CO/H2 mixture to maximize microbial growth.
- -
- Assessment of the realistic environmental impacts of each process stream and the target products considered.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic Biorefineries: The Current State of Challenges and Strategies for Efficient Commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Giuliano, A.; Poletto, M.; Barletta, D. Process Optimization of a Multi-Product Biorefinery: The Effect of Biomass Seasonality. Chem. Eng. Res. Des. 2016, 107, 236–252. [Google Scholar] [CrossRef]
- D’Avino, L.; Di Bene, C.; Farina, R.; Razza, F. Introduction of Cardoon (Cynara cardunculus L.) in a Rainfed Rotation to Improve Soil Organic Carbon Stock in Marginal Lands. Agronomy 2020, 10, 946. [Google Scholar] [CrossRef]
- Barros Lovate Temporim, R.; Cavalaglio, G.; Petrozzi, A.; Coccia, V.; Iodice, P.; Nicolini, A.; Cotana, F. Life Cycle Assessment and Energy Balance of a Polygeneration Plant Fed with Lignocellulosic Biomass of Cynara cardunculus L. Energies 2022, 15, 2397. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a Biomass and Multi-Purpose Crop: A Review of 30 Years of Research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- De Bari, I.; Giuliano, A.; Petrone, M.T.; Stoppiello, G.; Fatta, V.; Giardi, C.; Razza, F.; Novelli, A. From Cardoon Lignocellulosic Biomass to Bio-1,4 Butanediol: An Integrated Biorefinery Model. Processes 2020, 8, 1585. [Google Scholar] [CrossRef]
- Christodoulou, C.; Tsekos, C.; Tsalidis, G.; Fantini, M.; Panopoulos, K.D.; de Jong, W.; Kakaras, E. Attempts on Cardoon Gasification in Two Different Circulating Fluidized Beds. Case Stud. Therm. Eng. 2014, 4, 42–52. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Adhya, T.K.; Pattnaik, R.; Mishra, S. A Critical Review on Prospects and Challenges in Production of Biomethanol from Lignocellulose Biomass. Biomass Conv. Bioref. 2022, 12, 1835–1849. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C. Process Simulation and Environmental Aspects of Dimethyl Ether Production from Digestate-Derived Syngas. Int. J. Environ. Res. Public Health 2021, 18, 807. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Martin, M.E.; Tappel, R.C.; Heijstra, B.D. Gas Fermentation—A Flexible Platform for Commercial Scale Production of Low-Carbon-Fuels and Chemicals from Waste and Renewable Feedstocks. Front. Microbiol. 2016, 7, 694. [Google Scholar] [CrossRef] [PubMed]
- Almeida Benalcázar, E.; Noorman, H.; Maciel Filho, R.; Posada, J.A. Decarbonizing Ethanol Production via Gas Fermentation: Impact of the CO/H2/CO2 Mix Source on Greenhouse Gas Emissions and Production Costs. Comput. Chem. Eng. 2022, 159, 107670. [Google Scholar] [CrossRef]
- Pari, L.; Alfano, V.; Stefanoni, W.; Latterini, F.; Liuzzi, F.; De Bari, I.; Valerio, V.; Ciancolini, A. Inulin Content in Chipped and Whole Roots of Cardoon after Six Months Storage under Natural Conditions. Sustainability 2021, 13, 3902. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Seasonal Dynamics of Biomass, Inulin, and Water-Soluble Sugars in Roots of Cynara cardunculus L. Field Crops Res. 2010, 116, 147–153. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Patanè, C.; Melilli, M.G. Multiple Utilisation of the Plant in Cynara cardunculus L. Var. Sylvestris Lam.: Inulin Yield. Acta Hortic. 2005, 681, 475–482. [Google Scholar] [CrossRef]
- De Jong, E.; Visser, H.A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring FDCA and PEF to the Market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef] [PubMed]
- Haas, V.; Wenger, J.; Ranacher, L.; Guigo, N.; Sousa, A.F.; Stern, T. Developing Future Visions for Bio-Plastics Substituting PET—A Backcasting Approach. Sustain. Prod. Consum. 2022, 31, 370–383. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; Da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Tabak, B.M.; E Silva, I.B.D.R.; Quintino, D.D.; Silva, T.C. Fuel Prices Connectedness across Brazilian Capitals: The Case of Ethanol and Gasoline. Renew. Sustain. Energy Rev. 2025, 210, 115148. [Google Scholar] [CrossRef]
- Alfano, V.; Stefanoni, W.; Latterini, F.; Liuzzi, F.; De Bari, I.; Viola, E.; Ciancolini, A.; Pari, L. Inulin Content in Chipped Roots of Cardoon Stored at Different Initial Moisture Contents After Six-Month Storage. Front. Energy Res. 2022, 10, 834443. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Cynara cardunculus L., a Potential Source of Inulin in the Mediterranean Environment: Screening of Genetic Variability. Aust. J. Agric. Res. 2004, 55, 693–698. [Google Scholar] [CrossRef]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Bals, O.; Grimi, N.; Vorobiev, E. Recent Insights for the Green Recovery of Inulin from Plant Food Materials Using Non-Conventional Extraction Technologies: A Review. Innov. Food Sci. Emerg. Technol. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- US Patent for Process for Obtaining Inulin from Roots of the Cardoon Plant Patent (Patent # 10,723,810 Issued July 28, 2020)—Justia Patents Search. Available online: https://patents.justia.com/patent/10723810 (accessed on 18 July 2022).
- Kim, W.Y.; Byun, S.M. Hydrolysis of Inulin from Jerusalem Artichoke Helianthus Tuberosus by Inulinase Ec 3.2.1.7 Immobilized on Aminoethyl Cellulose. Enzym. Microb. Technol. 1982, 4, 239–244. [Google Scholar] [CrossRef]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent Advances in Catalytic Conversion of Biomass to 2,5-Furandicarboxylic Acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xie, X.; Huang, C.; Yang, S. Dehydration of Fructose, Sucrose and Inulin to 5-Hydroxymethylfurfural over Yeast-Derived Carbonaceous Microspheres at Low Temperatures. RSC Adv. 2019, 9, 9041–9048. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J. Steam Gasification of Cynara cardunculus L.: Influence of Variables. Fuel Process. Technol. 2002, 75, 27–43. [Google Scholar] [CrossRef]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Bitou, P.; Kanellis, T.; Manara, P.; Stavropoulos, G. Investigating Cynara C. Biomass Gasification Producer Gas Suitability for CHP, Second Generation Biofuels, and H2 Production. Ind. Crops Prod. 2014, 61, 308–316. [Google Scholar] [CrossRef]
- Frantzi, D.; Zabaniotou, A. Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model. Energies 2021, 14, 7366. [Google Scholar] [CrossRef]
- Serrano, D.; Kwapinska, M.; Horvat, A.; Sánchez-delgado, S.; Leahy, J.J. Cynara cardunculus L. Gasification in a Bubbling Fluidized Bed: The Effect of Magnesite and Olivine on Product Gas, Tar and Gasification Performance. Fuel 2016, 173, 247–259. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Liu, H.; Zhang, X. Life Cycle Assessment and Economic Analysis of Methanol Production from Coke Oven Gas Compared with Coal and Natural Gas Routes. J. Clean. Prod. 2018, 185, 299–308. [Google Scholar] [CrossRef]
- Sivalingam, V.; Dinamarca, C. High Pressure Moving Bed Biofilm Reactor for Syngas Fermentation. Chem. Eng. Trans. 2021, 86, 1483–1488. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Gavala, H.N.; Skiadas, I.V. Reactor Systems for Syngas Fermentation Processes: A Review. Chem. Eng. J. 2018, 348, 732–744. [Google Scholar] [CrossRef]
- Pardo-planas, O.; Atiyeh, H.K.; Phillips, J.R.; Aichele, C.P.; Mohammad, S. Bioresource Technology Process Simulation of Ethanol Production from Biomass Gasi Fi Cation and Syngas Fermentation. Bioresour. Technol. 2017, 245, 925–932. [Google Scholar] [CrossRef]
- Almeida Benalcázar, E.; Noorman, H.; Maciel Filho, R.; Posada, J.A. Modeling Ethanol Production through Gas Fermentation: A Biothermodynamics and Mass Transfer-Based Hybrid Model for Microbial Growth in a Large-Scale Bubble Column Bioreactor. Biotechnol. Biofuels 2020, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.; Yasin, M.; Kang, H.; Lee, Y.; Woo, G. Bioresource Technology Bubble Coalescence Suppression Driven Carbon Monoxide (CO)-Water Mass Transfer Increase by Electrolyte Addition in a Hollow Fi Ber Membrane Bioreactor (HFMBR) for Microbial CO Conversion to Ethanol. Bioresour. Technol. 2018, 263, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Integrated with Syngas Fermentation. Fermentation 2021, 7, 139. [Google Scholar] [CrossRef]
- Medeiros, E.M.; Posada, J.A.; Noorman, H.; Filho, R.M. Dynamic Modeling of Syngas Fermentation in a Continuous Stirred-tank Reactor: Multi-response Parameter Estimation and Process Optimization. Biotechnol. Bioeng. 2019, 116, 2473–2487. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Albergo, R.; De Bari, I. Kinetics Analysis of the Syngas Fermentation to Produce Acetic Acid from Cardoon Residual Biomass. Chem. Eng. Trans. 2022, 92, 91–96. [Google Scholar] [CrossRef]
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; ISBN 978-92-5-105521-2. [Google Scholar]
- Sillitti, C.; Melilli, M.G.; Padalino, L.; Bognanni, R.; Tringali, S.; Conte, A.; Raccuia, S.A. Healthy Pasta Production Using Inulin from Cardoon: First Results of Sensory Evaluation. In Proceedings of the IX International Symposium on Artichoke, Cardoon and Their Wild Relatives, La Plata, Argentina, 29 September–2 October 2015; pp. 407–412. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Genovese, C.; Leonardi, C.; Bognanni, R.; Platania, C.; Calderaro, P. Fructose Production by Cynara cardunculus Inulin Hydrolysis. In Proceedings of the IX International Symposium on Artichoke, Cardoon and Their Wild Relatives, La Plata, Argentina, 29 September–2 October 2015; pp. 309–314. [Google Scholar] [CrossRef]
- Campo, I.; Alegr, I.; Zazpe, M.; Echeverr, M.; Echeverr, I. Diluted Acid Hydrolysis Pretreatment of Agri-Food Wastes for Bioethanol Production. Ind. Crops Prod. 2006, 24, 214–221. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward Biomass-Derived Renewable Plastics: Production of 2,5-Furandicarboxylic Acid from Fructose. Sci. Adv. 2018, 4, eaap9722. [Google Scholar] [CrossRef] [PubMed]
- Devarapalli, M.; Lewis, R.; Atiyeh, H. Continuous Ethanol Production from Synthesis Gas by Clostridium Ragsdalei in a Trickle-Bed Reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef]
- Wainaina, S.; Horváth, I.S.; Taherzadeh, M.J. Bioresource Technology Biochemicals from Food Waste and Recalcitrant Biomass via Syngas Fermentation: A Review. Bioresour. Technol. 2018, 248, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Devarapalli, M.; Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Bioresource Technology Ethanol Production during Semi-Continuous Syngas Fermentation in a Trickle Bed Reactor Using Clostridium Ragsdalei. Bioresour. Technol. 2016, 209, 56–65. [Google Scholar] [CrossRef]
- Rodrigues Gurgel da Silva, A.; Giuliano, A.; Errico, M.; Rong, B.G.; Barletta, D. Economic Value and Environmental Impact Analysis of Lignocellulosic Ethanol Production: Assessment of Different Pretreatment Processes. Clean Technol. Environ. Policy 2019, 21, 637–654. [Google Scholar] [CrossRef]
- Castellini, M.; Ubertini, S.; Barletta, D.; Baffo, I.; Buzzini, P.; Barbanera, M. Techno-Economic Analysis of Biodiesel Production from Microbial Oil Using Cardoon Stalks as Carbon Source. Energies 2021, 14, 1473. [Google Scholar] [CrossRef]
- Galanopoulos, C.; Giuliano, A.; Barletta, D.; Zondervan, E. An Integrated Methodology for the Economic and Environmental Assessment of a Biorefinery Supply Chain. Chem. Eng. Res. Des. 2020, 160, 199–215. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Bao, J. Cost Evaluation of Cellulase Enzyme for Industrial-Scale Cellulosic Ethanol Production Based on Rigorous Aspen Plus Modeling. Bioprocess Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef]
- Dessbesell, L.; Souzanchi, S.; Venkateswara Rao, K.T.; Carrillo, A.A.; Bekker, D.; Hall, K.A.; Lawrence, K.M.; Tait, C.L.J.; Xu, C. (Charles) Production of 2,5-Furandicarboxylic Acid (FDCA) from Starch, Glucose, or High-Fructose Corn Syrup: Techno-Economic Analysis. Biofuels Bioprod. Biorefining 2019, 13, 1234–1245. [Google Scholar] [CrossRef]
- Espada, J.J.; Villalobos, H.; Rodríguez, R. Environmental Assessment of Different Technologies for Bioethanol Production from Cynara cardunculus: A Life Cycle Assessment Study. Biomass Bioenergy 2021, 144, 105910. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.C.; Mendes, J.A.S.; Gravitis, J.; Evtuguin, D.V.; Xavier, A.M.R.B. Enzymatic Saccharification and Bioethanol Production from Cynara cardunculus Pretreated by Steam Explosion. Bioresour. Technol. 2015, 186, 309–315. [Google Scholar] [CrossRef]
- Pesce, G.R.; Negri, M.; Bacenetti, J.; Mauromicale, G. The Biomethane, Silage and Biomass Yield Obtainable from Three Accessions of Cynara cardunculus. Ind. Crops Prod. 2017, 103, 233–239. [Google Scholar] [CrossRef]
- Kennes, D.; Abubackar, N.; Diaz, M.; Veiga, C.; Kennes, C. Bioethanol Production from Biomass: Carbohydrate vs Syngas Fermentation. J. Chem. Technol. Biotechnol. 2015, 91, 304–317. [Google Scholar] [CrossRef]

| Compound | g/kgROOTS |
|---|---|
| inulin | 333.37 |
| sucrose | 26.27 |
| glucose | 0.962 |
| fructose | 9.62 |
| other solid | 629.78 |
| Operation | T (°C) | P (bar) | Catalytic System | Yield w/w |
|---|---|---|---|---|
| Inulin leaching | 70 | 1.013 | --- | 34.7% (inulin and sugars/rootsDM) |
| Inulin hydrolysis | 40.2 | 2.013 | Endoinulinase on aminoethyl cellulose | 90% (fructose/inulin) |
| HMF production | 179.9 | 20 | FDCA | 51% HMF/fructose |
| FDCA production | 110 | 40 | Pt/C | 91% FDCA/HMF |
| Gasifier technology | Bubbling fluidized bed |
| Gasifier process conditions | Air at 320 °C, gasifier at 800 °C, ER 0.2, bed material: olivine |
| Cleaning treatment | Cyclones, hot filter, tar trap |
| Compound | %volDRY (yi) |
| CO | 17.0 |
| H2 | 16.7 |
| CH4 | 5.0 |
| CO2 | 16.9 |
| N2 | 44.4 |
| Process condition | |
| Pressure (bar) | 1.025 |
| Temperature (°C) | 37 |
| Impact Parameter | CO2eq Emissions (−If Savings) | Cost (−If Gaining) |
|---|---|---|
| CO2 captured from air [49] | −1.493 kgCO2/kg | - |
| Cultivation and harvesting [6,50] | 1.94 kgCO2eq/kg | EUR 40/t |
| Residual biomass transport [6,51] | 0.07 kgCO2eq/kg | EUR 15/t |
| Enzymes [52] | - | EUR 5/kg |
| Fresh water [49] | 6.52 kgCO2eq/t | EUR 0.4/t |
| Wastewater treatment [49] | 500 kgCO2eq/t | EUR 10/t |
| Green electricity [49] | −668 kgCO2eq/MWhe | −EUR 50/MWhe |
| Ethanol [49] | 0.348 kgCO2eq/kg | −EUR 0.75/kg |
| FDCA [16,53] | −2 kgCO2eq/kg | −EUR 2/kg |
| Raw Materials | In (t/y) | Out (t/y) |
|---|---|---|
| Wild cardoon roots (dry matter) | 78,980 | - |
| Inulin | 26,330 | 1667 |
| Sucrose | 2075 | 105.2 |
| Glucose | 76 | 3.8 |
| Fructose | 760 | 38.5 |
| Other solid | 49,740 | 49,740 |
| 5-HMF | 0 | 0.01240 |
| FDCA | 0 | 16,635 |
| Utilities | MW | |
| Electricity demand | 1.23 | |
| Heating demand | 50 |
| t/h | Clean Syngas | Gas Output | To DIST | Unconverted Syngas | Flue Gas | Pure Ethanol |
|---|---|---|---|---|---|---|
| H2O | 2.2 | 1.1 | 32.1 | - | 4.1 | - |
| Ethanol | - | 0.8 | 1.8 | 0.1 | - | 1.8 |
| Acetic Acid | - | - | 0.4 | - | - | - |
| O2 | - | - | - | - | 6.0 | - |
| N2 | 14.0 | 14.0 | - | 14.0 | 52.6 | - |
| CO | 5.4 | 0.5 | - | 0.5 | - | - |
| CO2 | 8.4 | 12.3 | - | 12.3 | 16.5 | - |
| CH4 | 0.9 | 0.9 | - | 0.9 | - | - |
| H2 | 0.4 | 0.2 | - | 0.2 | - | - |
| Total | 29.1 | 29.8 | 34.3 | 28 | 79.2 | 1.8 |
| Beer Column | Rectifying Column | |
|---|---|---|
| Reflux ratio | 3.2 | 6.0 |
| Distillate/feed ratio | 0.06 | 0.55 |
| Reboiler heat (MWt) | 7.4 | 3.5 |
| Condenser heat (MWt) | 4.0 | 3.0 |
| Work | Final Product | Mass Yield (%) | Energy Efficiency (%) 3 |
|---|---|---|---|
| Present | Ethanol + FDCA | 14.0 1 + 21.1 2 | 22.7 4 |
| Temporim et al. [4] | Electricity | - | 18.0 |
| Pesce et al. [56] | Methane | 12.8 | 40.1 |
| De Bari et al. [6] | BDO | 13.3 | - |
| Espada et al. [54] | Ethanol | 12.9 | 21.0 |
| Fernandes et al. [55] | Ethanol | 10.1 | 16.4 |
| Castellini et al. [50] | Biodiesel | 4.1 | 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatta, V.; Giuliano, A.; Petrone, M.T.; Nanna, F.; Villone, A.; Barisano, D.; Albergo, R.; Liuzzi, F.; Barletta, D.; De Bari, I. A Novel Integrated Biorefinery for the Valorization of Residual Cardoon Biomass: Overview of Technologies and Process Simulation. Energies 2025, 18, 973. https://doi.org/10.3390/en18040973
Fatta V, Giuliano A, Petrone MT, Nanna F, Villone A, Barisano D, Albergo R, Liuzzi F, Barletta D, De Bari I. A Novel Integrated Biorefinery for the Valorization of Residual Cardoon Biomass: Overview of Technologies and Process Simulation. Energies. 2025; 18(4):973. https://doi.org/10.3390/en18040973
Chicago/Turabian StyleFatta, Vittoria, Aristide Giuliano, Maria Teresa Petrone, Francesco Nanna, Antonio Villone, Donatella Barisano, Roberto Albergo, Federico Liuzzi, Diego Barletta, and Isabella De Bari. 2025. "A Novel Integrated Biorefinery for the Valorization of Residual Cardoon Biomass: Overview of Technologies and Process Simulation" Energies 18, no. 4: 973. https://doi.org/10.3390/en18040973
APA StyleFatta, V., Giuliano, A., Petrone, M. T., Nanna, F., Villone, A., Barisano, D., Albergo, R., Liuzzi, F., Barletta, D., & De Bari, I. (2025). A Novel Integrated Biorefinery for the Valorization of Residual Cardoon Biomass: Overview of Technologies and Process Simulation. Energies, 18(4), 973. https://doi.org/10.3390/en18040973









