Study on Pore Structure of Tectonically Deformed Coals by Carbon Dioxide Adsorption and Nitrogen Adsorption Methods
Abstract
1. Introduction
2. Samples and Experimental Methods
2.1. Geological Background and Sample Selection
2.2. Methods
2.2.1. Low-Temperature N2 Adsorption
2.2.2. CO2 Adsorption Experiment
3. Results
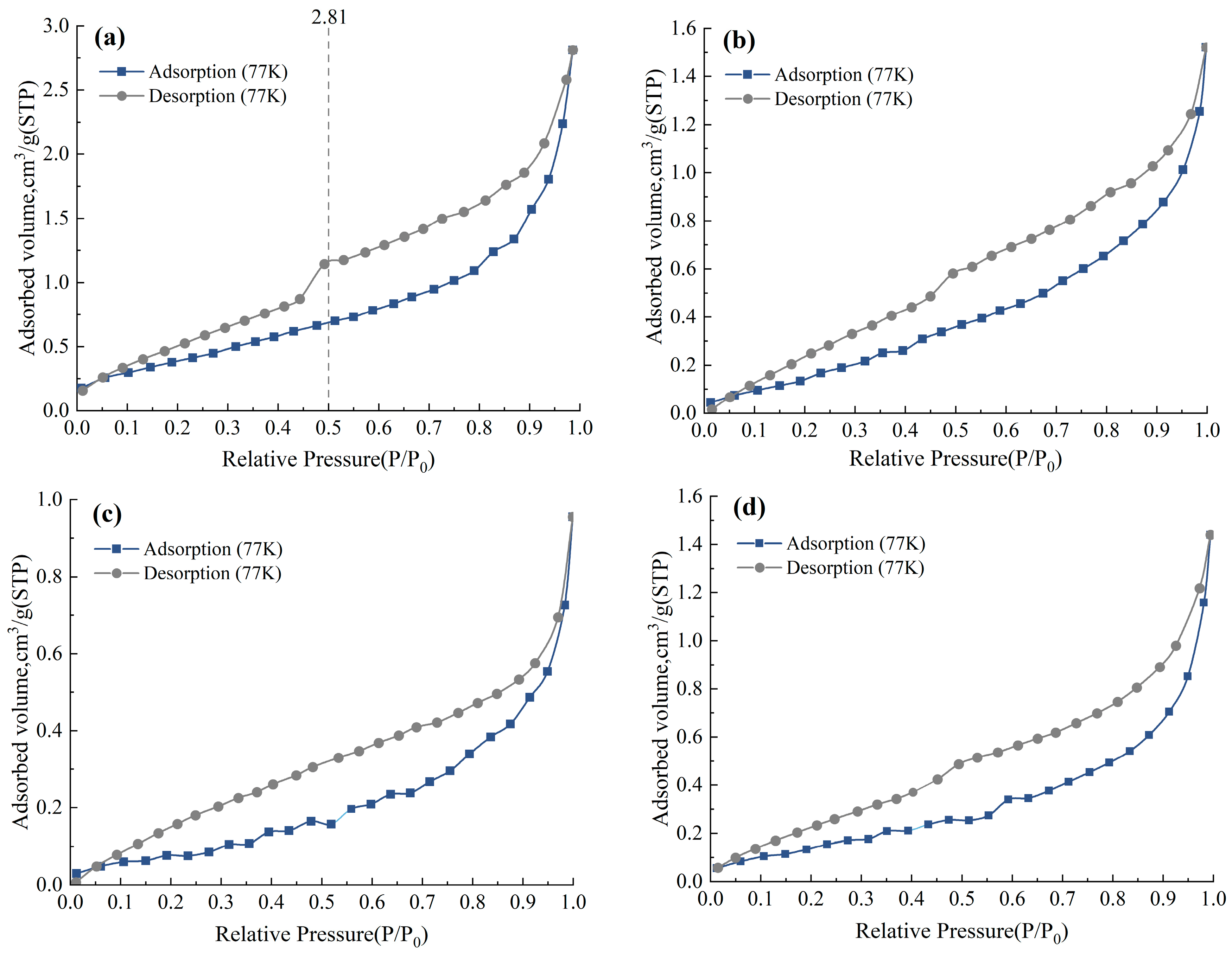
3.1. Adsorption and Desorption Characteristics
3.1.1. Mesoporous Adsorption and Desorption Characteristics
3.1.2. Microporous Adsorption Characteristics
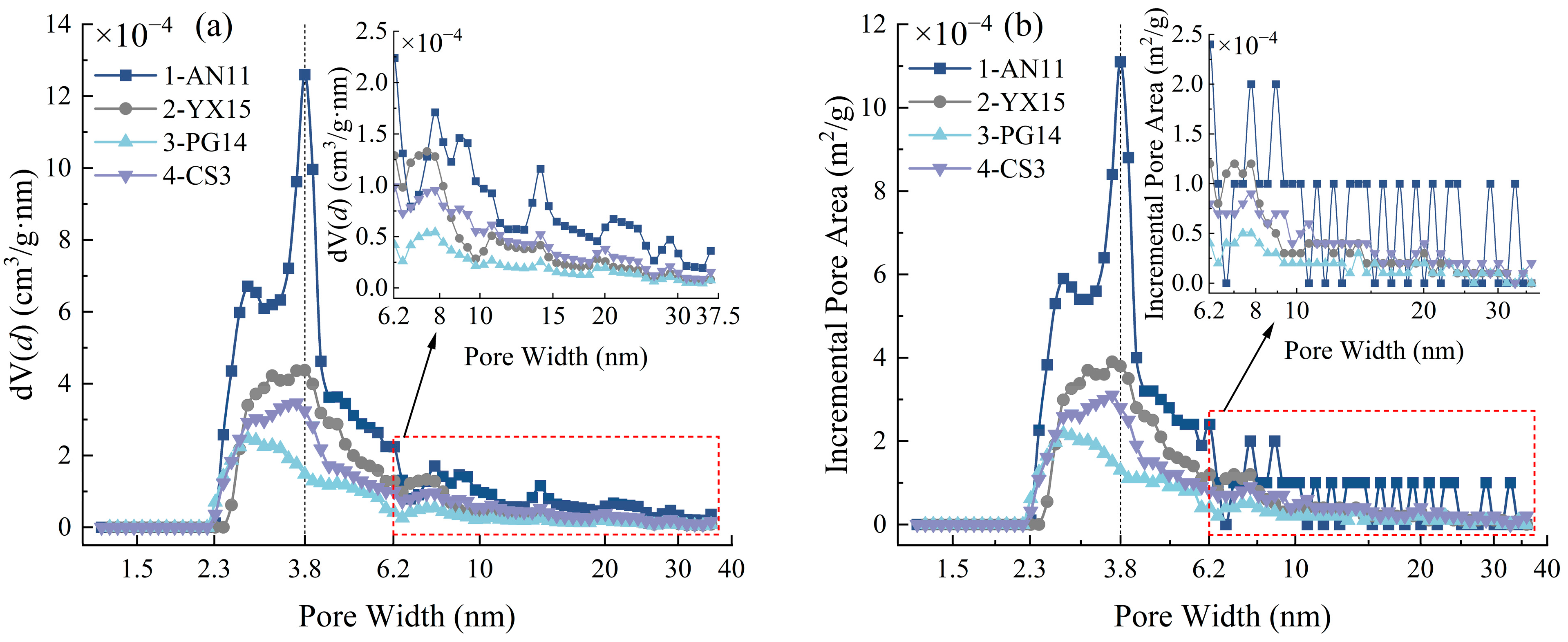
3.2. Pore Distribution
4. Discussion
4.1. Study on the Evolution Characteristics and Formation Mechanism of Pore Structures in Tectonic Coals
4.2. Fractal Characteristics of Pores
5. Conclusions
- (1)
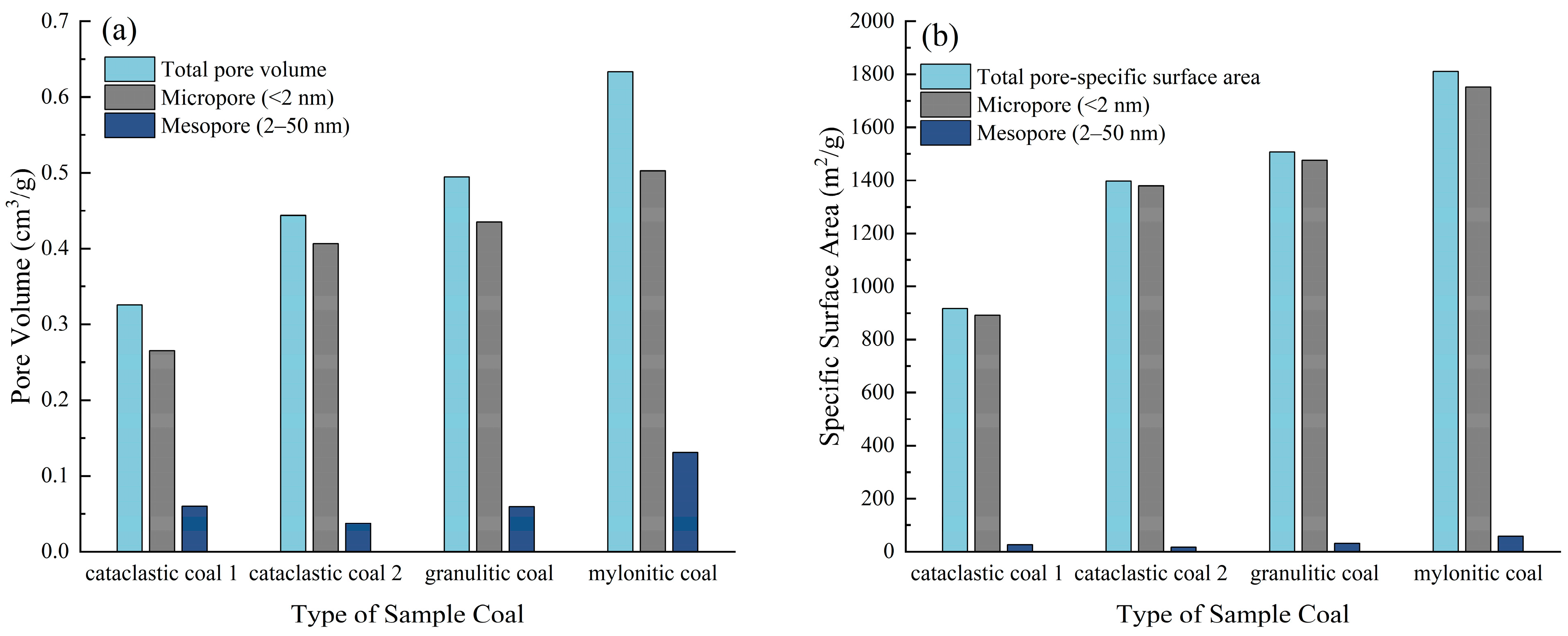
- The adsorption of N2 and CO2 gases by the four tectonically deformed coal samples is primarily physical adsorption. The low-temperature N2 adsorption–desorption isotherms of these samples all fall under the H3 type according to the IUPAC classification. The degree of structural deformation in coal has a significant impact on its pore characteristics. As the degree of deformation increases, both the pore volume and specific surface area of the coal continue to grow, with the increase in micropore volume and specific surface area being slightly slower than that of mesopores. This is closely related to the destruction and reorganization of the pore structure under tectonic stress.
- (2)
- The degree of structural deformation in coal affects the pore fractal dimension, and a positive correlation exists between the two when the relative pressure is low (P/P0 < 0.5). At a relative pressure of P/P0 < 0.5, as the fractal dimension increases, the pore volume, specific surface area, and gas adsorption capacity of coal gradually increase, while the average pore size of the coal pores gradually decreases with the increase in the fractal dimension.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, S.; Wang, C.F.; Liu, Q.Q.; Zhang, Y.L.; Liu, J.; Sa, Z.Y.; Wang, L. Numerical assessment of the energy instability of gas outburst of deformed and normal coal combinations during mining. Process Saf. Environ. Prot. 2019, 132, 351–366. [Google Scholar] [CrossRef]
- Ju, Y.; Li, X. New research progress on the ultrastructure of tectonically deformed coals. Prog. Nat. Sci. Mater. Int. 2009, 19, 1455–1466. [Google Scholar] [CrossRef]
- Lu, S.; Li, L.; Cheng, Y.; Sa, Z.; Zhang, Y.; Yang, N. Mechanical failure mechanisms and forms of normal and deformed coal combination containing gas: Model development and analysis. Eng. Fail. Anal. 2017, 80, 241–252. [Google Scholar] [CrossRef]
- Pan, J.; Wang, S.; Ju, Y.; Hou, Q.; Niu, Q.; Wang, K.; Li, M.; Shi, X. Quantitative study of the macromolecular structures of tectonically deformed coal using high-resolution transmission electron microscopy. J. Nat. Gas Sci. Eng. 2015, 27, 1852–1862. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, G.G.X.; Jiang, B.; Rudolph, V.; Dou, X.; Lit, M. Experimental Study on the Porous Structure and Compressibility of Tectonized Coals. Energy Fuels 2010, 24, 2964–2973. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cheng, Y.P.; Zhang, K.Z.; Hao, C.M.; Wang, L.; Li, W.; Hu, B. Characteristics of microscopic pore structure and fractal dimension of bituminous coal by cyclic gas adsorption/desorption: An experimental study. Fuel 2018, 232, 495–505. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wilcox, J. Effects of Surface Heterogeneity on the Adsorption of CO2 in Microporous Carbons. Environ. Sci. Technol. 2012, 46, 1940–1947. [Google Scholar] [CrossRef]
- Cao, Y.X.; Mitchell, G.D.; Davis, A.; Wang, D.M. Deformation metamorphism of bituminous and anthracite coals from China. Int. J. Coal Geol. 2000, 43, 227–242. [Google Scholar] [CrossRef]
- Godyn, K. Structurally altered hard coal in the areas of tectonic disturbances—An initial attempt at classification. Arch. Min. Sci. 2016, 61, 677–694. [Google Scholar] [CrossRef]
- Ju, Y.W.; Jiang, B.; Hou, Q.L.; Wang, G.L. Relationship between nanoscale deformation of coal structure and metamorphic-deformed environments. Chin. Sci. Bull. 2005, 50, 1784–1795. [Google Scholar] [CrossRef]
- Ju, Y.W.; Jiang, B.; Hou, Q.L.; Tan, Y.J.; Wang, G.L.; Xiao, W.J. Behavior and mechanism of the adsorption/desorption of tectonically deformed coals. Chin. Sci. Bull. 2009, 54, 88–94. [Google Scholar] [CrossRef]
- Pan, J.N.; Hou, Q.L.; Ju, Y.W.; Bai, H.L.; Zhao, Y.Q. Coalbed methane sorption related to coal deformation structures at different temperatures and pressures. Fuel 2012, 102, 760–765. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.; Roberts, M.J.; Sakurovs, R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, S.D.; Wu, J.Q.; Li, Z.; Wang, X.Y. Full-Scale Pore Structure Characterization and Its Impact on Methane Adsorption Capacity and Seepage Capability: Differences between Shallow and Deep Coal from the Tiefa Basin in Northeastern China. Fractal Fract. 2024, 8, 48. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Clarkson, C.; Bustin, R. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Wang, T.; Deng, Z.; Hu, H.Y.; Ding, R.; Tian, F.H.; Zhang, T.; Ma, Z.R.; Wang, D.X. Pore structure of deep coal of different ranks and its effect on coalbed methane adsorption. Int. J. Hydrogen Energy 2024, 59, 144–158. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Tang, D.; Tang, S.; Huang, W. Fractal characterization of adsorption-pores of coals from North China: An investigation on CH4 adsorption capacity of coals. Int. J. Coal Geol. 2008, 73, 27–42. [Google Scholar] [CrossRef]
- Huang, H.Z.; Sun, Y.T.; Chang, X.T.; Wu, Z.Q.; Li, M.; Qu, S.L. Experimental Investigation of Pore Characteristics and Permeability in Coal-Measure Sandstones in Jixi Basin, China. Energ. 2022, 15, 5898. [Google Scholar] [CrossRef]
- Nakagawa, T.; Komaki, I.; Sakawa, M.; Nishikawa, K. Small angle X-ray scattering study on change of fractal property of Witbank coal with heat treatment. Fuel 2000, 79, 1341–1346. [Google Scholar] [CrossRef]
- Rexer, T.; Mathia, E.; Aplin, A.; Thomas, K. High-pressure methane adsorption and characterization of pores in Posidonia shales and isolated kerogens. Abstr. Pap. Am. Chem. Soc. 2019, 258, 2886–2901. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Che, Y.; Tang, D.; Tang, S.; Huang, W. Non-destructive characterization of coal samples from China using microfocus X-ray computed tomography. Int. J. Coal Geol. 2009, 80, 113–123. [Google Scholar] [CrossRef]
- Yin, T.; Liu, D.; Cai, Y.; Zhou, Y.; Yao, Y. Size distribution and fractal characteristics of coal pores through nuclear magnetic resonance cryoporometry. Energy Fuels 2017, 31, 7746–7757. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, G.; Dong, Y.; Danesh, N.N.; Chen, Z.; Zhang, T. Comparison of low-field NMR and microfocus X-ray computed tomography in fractal characterization of pores in artificial cores. Fuel 2017, 210, 217–226. [Google Scholar] [CrossRef]
- Zhou, S.D.; Liu, D.M.; Cai, Y.D.; Yao, Y.B.; Che, Y.; Liu, Z.H. Multi-scale fractal characterizations of lignite, subbituminous and high-volatile bituminous coals pores by mercury intrusion porosimetry. J. Nat. Gas Sci. Eng. 2017, 44, 338–350. [Google Scholar] [CrossRef]
- Mangi, H.N.; Yan, D.T.; Hameed, N.; Ashraf, U.; Rajper, R.H. Pore structure characteristics and fractal dimension analysis of low rank coal in the Lower Indus Basin, SE Pakistan. J. Nat. Gas Sci. Eng. 2020, 77, 103231. [Google Scholar] [CrossRef]
- Paul, N.; Mohammad, A.; Keka, O. Integrated fractal description of nanopore structure and its effect on CH4 adsorption on Jharia coals, India. Fuel 2018, 232, 190–204. [Google Scholar]
- Pfeifer, P.; Wu, Y.J.; Cole, M.W.; Krim, J. Multilayer adsorption on a fractally rough surface. Phys. Rev. Lett. 1989, 62, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Song, B.B.; Zhai, X.W.; Ma, T.; Wang, B.; Hao, L.; Zhou, Y.J. Effect of water immersion on pore structure of bituminous coal with different metamorphic degrees. Energy 2023, 274, 127449. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Li, H.J.; Zeng, Q.; Kang, J.H.; Cheng, G.; Cheng, J.W.; Wang, S.C. A Comparative Investigation of the Adsorption Characteristics of CO2, O2 and N2 in Different Ranks of Coal. Sustainability 2023, 15, 8075. [Google Scholar] [CrossRef]
- Hong, L.; Wang, W.J.; Gao, D.M.; Liu, W.T. Critical pore size for micropore filling in coal samples with different rank coals. PLoS ONE 2022, 17, e0264225. [Google Scholar] [CrossRef] [PubMed]
- Deinert, M.R.; Parlange, J.Y. Effect of pore structure on capillary condensation in a porous medium. Phys. Rev. E 2009, 79, 021202. [Google Scholar] [CrossRef]
- Qi, L.L.; Tang, X.; Wang, Z.F.; Peng, X.S. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Wang, L.; Long, Z. Evolutions of CO2 Adsorption and Nanopore Development Characteristics during Coal Structure Deformation. Appl. Sci. 2020, 10, 4997. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.F.; Song, X.X. Multifractal analysis of Hg pore size distributions of tectonically deformed coals. Int. J. Coal Geol. 2015, 144, 138–152. [Google Scholar] [CrossRef]
- Yao, H.F.; Kang, Z.Q.; Li, W. Deformation and reservoir properties of tectonically deformed coals. Pet. Explor. Dev. 2014, 41, 460–467. [Google Scholar] [CrossRef]
- Cao, D.Y.; Li, X.M.; Zhang, S.R. Influence of tectonic stress on coalification: Stress degradation mechanism and stress polycondensation mechanism. Sci. China Ser. D Earth Sci. 2007, 50, 43–54. [Google Scholar] [CrossRef]
- Ju, Y.; Luxbacher, K.; Li, X.; Wang, G.; Yan, Z.; Wei, M.; Yu, L. Micro-structural evolution and their effects on physical properties in different types of tectonically deformed coals. Int. J. Coal Sci. Technol. 2014, 1, 364–375. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xing, Y.W.; Shi, K.Y.; Wang, S.W.; Xia, Y.C.; Li, J.H.; Gui, X.H. Chemical Structure Characteristics and Model Construction of Coal with Three Kinds of Coalification Degrees. ACS Omega 2023, 9, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, R.; Wu, J.; Sun, J. Fractal Analysis of Coal Pore Structure Based on Computed Tomography and Fluid Intrusions. Fractal Fract. 2023, 7, 439. [Google Scholar] [CrossRef]
- Li, Z.B.; Ren, T.; Li, X.C.; Qiao, M.; Yang, X.H.; Tan, L.H.; Nie, B.S. Multi-scale pore fractal characteristics of differently ranked coal and its impact on gas adsorption. Int. J. Min. Sci. Technol. 2023, 33, 389–401. [Google Scholar] [CrossRef]
- Zhang, J.J.; Hu, Y.B. Comparative Evaluation of Pore Structure Heterogeneity in Low-Permeability Tight Sandstones Using Different Fractal Models Based on NMR Technology: A Case Study of Benxi Formation in the Central Ordos Basin. Energy Fuels 2020, 34, 13924–13942. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, S.H.; Wang, S.D.; Tong, Y.Z.; Li, Z.; Wu, J.Q. Fractal and Multifractal Characteristics on Pore Structure of Coal-Based Sedimentary Rocks Using Nuclear Magnetic Resonance. SPE J. 2024, 29, 2624–2637. [Google Scholar] [CrossRef]
- Zhang, H.M.; Guo, L.; Wu, Z.Y.; Ma, J.B. Pore-throat structure, fractal characteristics and permeability prediction of tight sandstone: The Yanchang Formation, Southeast Ordos Basin. Sci. Rep. 2024, 14, 27913. [Google Scholar] [CrossRef]





| Sample No. | Basin | Mining Area | Sampling Depth (m) | Visible Coal Thickness/(m) | Tectonically Deformed Coal Types |
|---|---|---|---|---|---|
| 1-AN11 | Hegang | Xing’an | 765 | 1.5 | mylonitic coal |
| 2-YX15 | Yixin | 600 | 1.5 | granulitic coal | |
| 3-PG14 | Jixi | Pinggang | 900 | 1.6 | cataclastic coal |
| 4-CS3 | Chengshan | 720 | 1.5 | cataclastic coal |
| Sample No. | Sample Type | Low-Pressure Segment | High-Pressure Segment | ||||
|---|---|---|---|---|---|---|---|
| A1 | D1 | R2 | A2 | D2 | R2 | ||
| 1-AN11 | mylonitic coal | −0.716 | 2.284 | 0.999 | −0.371 | 2.629 | 0.991 |
| 2-YX15 | granulitic coal | −1.169 | 1.831 | 0.998 | −0.252 | 2.748 | 0.893 |
| 3-PG14 | cataclastic coal | −0.923 | 2.077 | 0.984 | −0.275 | 2.725 | 0.858 |
| 4-CS3 | cataclastic coal | −0.865 | 2.135 | 0.995 | −0.366 | 2.634 | 0.957 |
| Sample No. | Sample Type | Pore Area | Pore Volume | Average Pore Width | Adsorption Volume | Fractal Dimension (D1) |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | (cm3/g) | |||
| 1-AN11 | mylonitic coal | 1.525 | 0.004300 | 11.4098 | 2.819 | 2.284 |
| 2-YX15 | granulitic coal | 0.754 | 0.002354 | 12.4841 | 1.522 | 1.831 |
| 3-PG14 | cataclastic coal | 0.288 | 0.001478 | 20.5316 | 0.955 | 2.077 |
| 4-CS3 | cataclastic coal | 0.610 | 0.002228 | 14.6002 | 1.440 | 2.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Huang, H.; Zhou, W.; Sun, L.; Huang, Z. Study on Pore Structure of Tectonically Deformed Coals by Carbon Dioxide Adsorption and Nitrogen Adsorption Methods. Energies 2025, 18, 887. https://doi.org/10.3390/en18040887
Zhang J, Huang H, Zhou W, Sun L, Huang Z. Study on Pore Structure of Tectonically Deformed Coals by Carbon Dioxide Adsorption and Nitrogen Adsorption Methods. Energies. 2025; 18(4):887. https://doi.org/10.3390/en18040887
Chicago/Turabian StyleZhang, Jinbo, Huazhou Huang, Wenbing Zhou, Lin Sun, and Zaixing Huang. 2025. "Study on Pore Structure of Tectonically Deformed Coals by Carbon Dioxide Adsorption and Nitrogen Adsorption Methods" Energies 18, no. 4: 887. https://doi.org/10.3390/en18040887
APA StyleZhang, J., Huang, H., Zhou, W., Sun, L., & Huang, Z. (2025). Study on Pore Structure of Tectonically Deformed Coals by Carbon Dioxide Adsorption and Nitrogen Adsorption Methods. Energies, 18(4), 887. https://doi.org/10.3390/en18040887







