Unconventional Fossil Energy Carrier Assessment of the Influence of the Gas Permeability Coefficient on the Structure of Porous Materials: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Stand
2.2. Scope and Research Methodology
3. Results and Discussion
3.1. Gas Permeability Mechanisms Based on Models
3.2. The Use of Halloysite in the Form of Nanotubes (HNT) as a Model Material for Testing Gas Permeability
4. Conclusions
- (1)
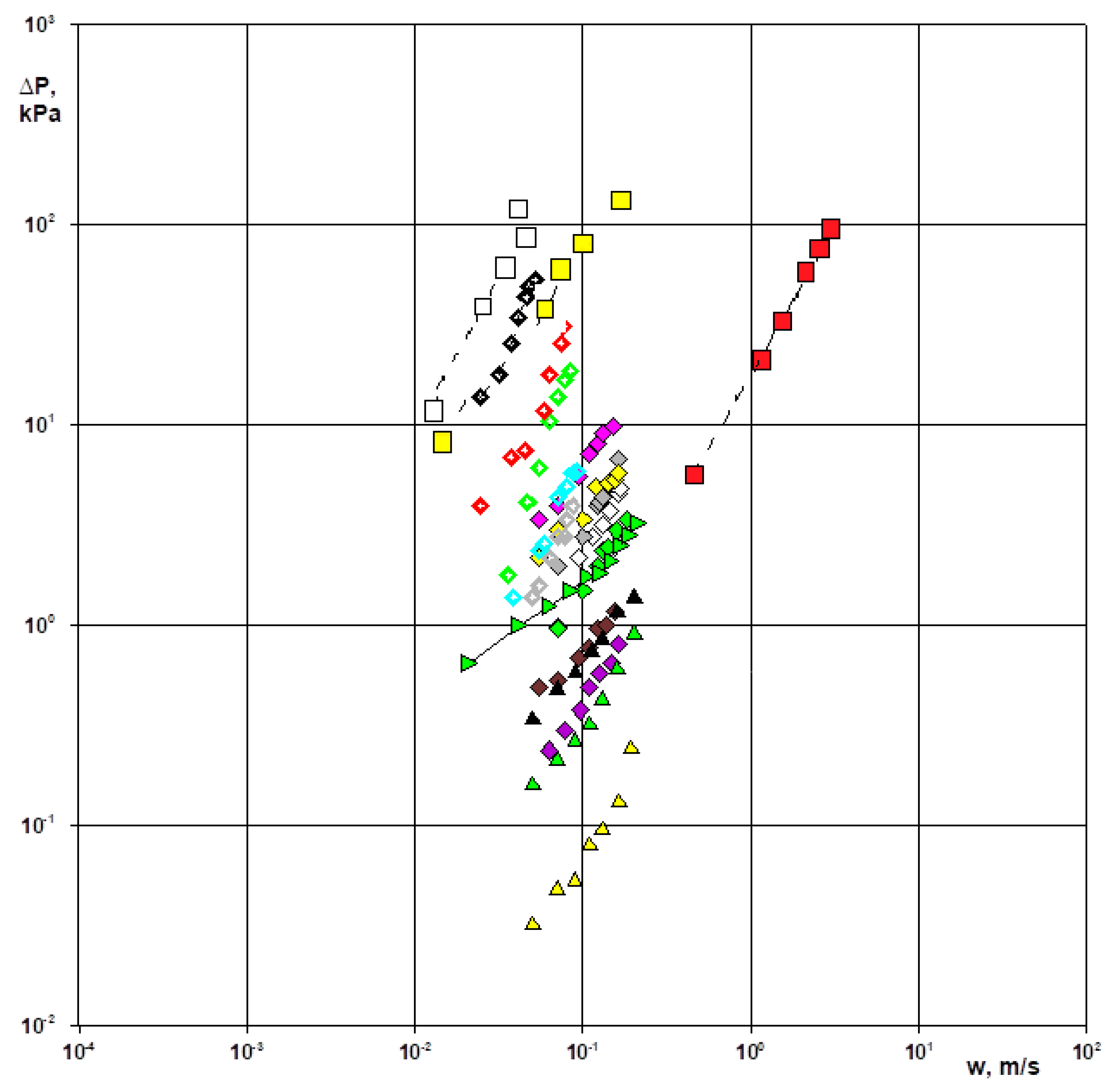
- Appropriate experimental tests of gas permeability through materials have been performed and hydrodynamic phenomena resulting from the pressure gradient of the gas flow have been assessed. The “directed horizontal region” characteristic of skeletal deposits (velocities from 0.01 to 5 m∙s−1, resistances from 0.7 to 150 kPa) was determined. In this region, the in situ char is characterized by the largest number of fractures, while the two pumices distinguish open and closed pores. Additionally, selected granular materials forming the “directed vertical region” (velocities from 0.01 to 0.5 m∙s−1, resistances from 0.005 to 100 kPa) were listed.
- (2)
- The phenomenon of “twin gas permeability” was observed for Kv equal to 10−8, which results in flow resistances for pumice of the order of magnitude from 104 to 105 Pa, with respect to epoxy resin from 106 to 107 Pa. In this way, the gas permeability mechanism for isotropic pumice can be correlated with hydrogen storage for epoxy (polymer).
- (3)
- The results of studies on the hydrodynamics of gas flow through porous skeleton beds and the proven usefulness of the process evaluation of these studies are an innovation for practical use for energy storage.
- (4)
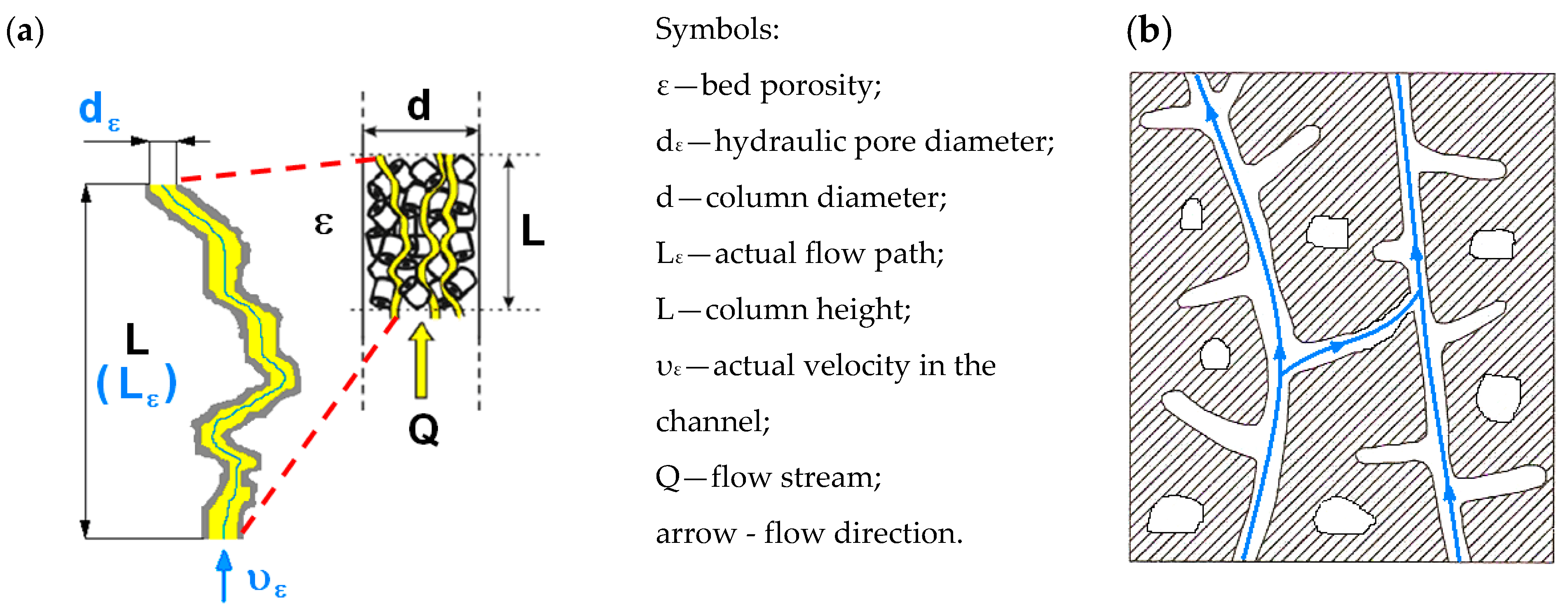
- Numerous studies show that at low pressure gradients, the gas slip effect occurs, which causes an overestimation of the flow velocity compared to Darcy’s law. The characteristics of gas permeability through a porous material are directly related to the values of the pressure gradient that occurs at a given gas stream on a given porous bed. Most of the theoretical considerations resulting from this are based on models of rectilinear channels and on Poissuille’s law (laminar flow), and even more often, on models identifying a porous medium, with a skeleton form with a granular bed, with the same features and flow parameters of the bed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
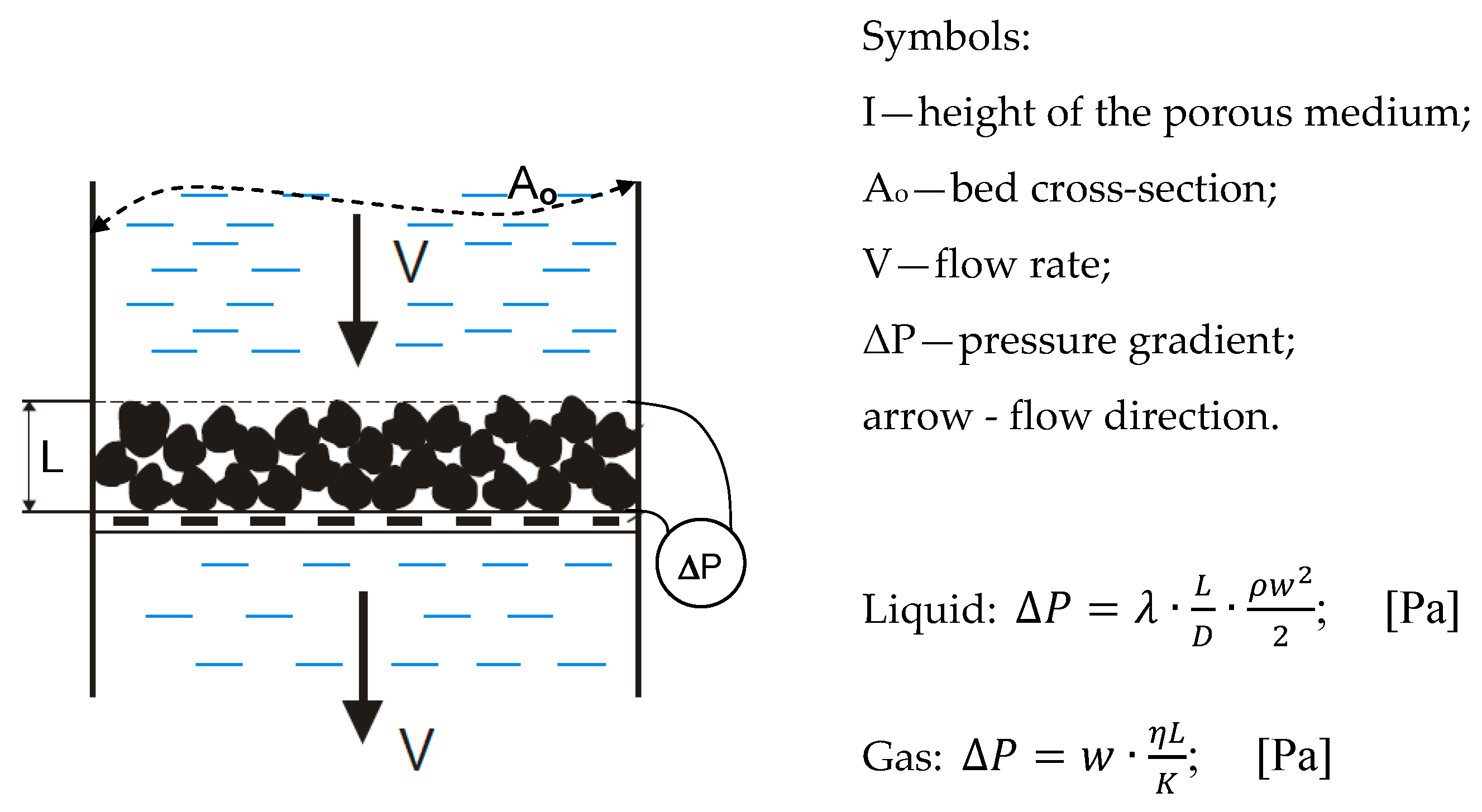
| Ao | bed cross-section | m2 |
| L | height of the porous medium | m |
| K | coefficient of permeability | m2 |
| Q | volumetric flow rate | m3/s |
| ΔP | pressure gradient | Pa |
| g | earth acceleration | m/s2 |
| Δh | pressure height | Pa |
| η | viscosity | Pa∙s |
| ρ | density | kg/m3 |
Appendix A
| m | reservoir porosity (4) |
| Sg | gas saturation; partial velocity (4) |
| kg | permeability of the gas mixture (4) |
| μg | dynamic viscosity (4) |
| p | pressure (4) |
| ΔP | pressure gradient (5) |
| q | flow rate per unit width of the fracture normal to the flow (6) and (7) |
| e | equivalent crack aperture (6) and (7) |
| ν | fluid viscosity (6) and (7) |
| ∂P\∂X | pressure gradient (6) and (7) |
| k | permeability (6) and (7) |
| Ω | correction factor, dimensionless (8) |
| β | dimensionless radius of a sphere, dimensionless (8) |
| kr | relative permeability of gas/liquid (9) |
| QRC | phase is the volumetric flow rate at the network inlet reported at network average pressure (9) |
| A | network cross-sectional (9) |
| L | length of the network (9) |
| v | gas velocity (10) |
| K | permeability tensor (10) |
| μ | viscosity (10) |
| p | pressure (10) |
| ρJ | density (11) |
| vJ | volumetric fluid velocity (11) |
| kJ | rank-2 positive-definite effective permeability tensor (11) |
| μJ | viscosity (11) |
| pJ | pressure of phase J (11) |
| g | gravity vector (11) |
| m | porosity of the membrane (12)–(14) |
| l | thickness (12)–(14) |
| c | local concentrations of the diffusant in the pores and the polymeric phase (12)–(14) |
| a | local concentrations of the diffusant in the pores and the polymeric phase (12)–(14) |
| Di | diffusion coefficients in the pores and the polymeric phase (12)–(14) |
| Da | diffusion coefficients in the pores and the polymeric phase (12)–(14) |
| −dP/dx | pressure gradient (15) |
| μ | absolute viscosity (15) |
| ρ | density (15) |
| υs | superficial fluid velocity (15) |
| K∗ | intrinsic permeability (16) |
| φ | porosity, fraction (16) |
| Re | equivalent hydraulic radius of a channel (16) |
| τh | tortuosity of capillary transport paths, dimensionless (16) |
| φ | fugacity coefficient (17) and (18) |
| bi | parameter in equation of state of component i (17) and (18) |
| bm | parameter in equation of state of mixture (17) and (18) |
| ZL | compressibility factor for liquid phase (17) and (18) |
| ZV | compressibility factor for vapor phase (17) and (18) |
| PL | pressure of liquid phase (17) and (18) |
| PV | pressure of vapor phase (17) and (18) |
| am | parameter in equation of state of mixture (17) and (18) |
| a | parameter in equation of state (17) and (18) |
| Pcap | capillary pressure (19) |
| σ | interfacial tension (19) |
| r | pore radius (19) |
| Kg | gas permeability of a network link (20) |
| Ai | effective surface area of a network link in the direction of flow (20) |
| μg | gas viscosity (20) |
| Li | length of a network link and its equal to the distance between two connected nodes (20) |
| G | pore conductance (21) |
| ge | an effective pore conductance (21) |
| Z | the average pore coordination number (21) |
| acreep | constant micro-scale deformation permeability (22)–(24) |
| θcreep, ref | reference (gas) porosity associated with micro-scale deformation (22)–(24) |
| cr | micro-scale deformation rate (22)–(24) |
| fi | discrete density distribution function (25) and (26) |
| fieq | local equilibrium function (25) and (26) |
| ei | discrete velocity of the particle (25) and (26) |
| cs | lattice sound velocity (25) and (26) |
| τ | relaxation time (25) and (26) |
| ωi | weight coefficient (25) and (26) |
| δt | time step (25) and (26) |
| P | pressure (27) |
| μ | viscosity, (27) |
| k | permeability (27) |
| u | velocity (27) |
| β | non-Darcy coefficient (27) |
| ρ | density (27) |
| Sw | wetting phase saturation (28) and (29) |
| Qtot | total injection rate (28) and (29) |
| ff | fractional flow (28) and (29) |
| A | core cross-sectional area (28) and (29) |
| μi | the phase viscosity (28) and (29) |
| ε | porosity (30)–(33) |
| k | permeability (30)–(33) |
| μ | gas dynamic viscosity (30)–(33) |
| ∆P | pressure gradient resistance flow (34) |
| A | experimental constant (34) |
| Re | Reynolds number (34) |
| n | constant (34) |
| ρ | fluid density (34) |
| w | velocity (34) |
| L | flow path length for the height of the porous bed (34) |
| de | equivalent diameter (34) |
| μ | viscosity (35) and (36) |
| mtotal | total mass flow (35) and (36) |
| ρ | gas density (35) and (36) |
| A | area (35) and (36) |
| p | pressure (35) and (36) |
| Q | stream (37) |
| K | coefficient of permeability (37) |
| A0 | reference layer bed cross-section (37) |
| ρ | density (37) |
| g | Earth acceleration (37) |
| η | viscosity (37) |
| ∆h | pressure height (37) |
| L | height of porous medium (37) |
| w | Darcy velocities (38) and (39) |
| k | permeability matrices (38) and (39) |
| S | saturation (38) and (39) |
| q | volume flux (40) and (41) |
| k | Darcy’s permeability (40) and (41) |
| μ | dynamic viscosity (40) and (41) |
| p | pressure (40) and (41) |
| R | universal gas constant (40) and (41) |
| T | temperature (40) and (41) |
| M | molar mass (40) and (41) |
| AM | membrane area (40) and (41) |
| ρ | density (40) and (41) |
| ka | apparent permeability (42) |
| k∞ | liquid permeability (42) |
| Kn | Knudsen number (42) |
| ρ | density (42) |
| μ | viscosity (42) |
| M | molecular weight (42) |
| R | gas constant (42) |
| T | temperature (42) |
| r | pore size (42) |
| p | pressure (42) |
| γ | “momentum accommodation coefficient” that depends on flow conditions, gas type, and surface roughness (42) |
| κapp | apparent gas permeability (43)–(48) |
| d | pore size or diameter (43)–(48) |
| Ds | surface diffusion of adsorbed gas (43)–(48) |
| pL | Langmuir pressure (43)–(48) |
| M | gas molar mass (43)–(48) |
| Kn | Knudsen number (43)–(48) |
| μg | viscosity of gaseous phase (49) |
| k | intrinsic permeability or absolute permeability of coal, which is assumed to be isotropic (49) |
| ψK | permeability shape factor (50)–(52) |
| d | mean particle size of the porous media (50)–(52) |
| A | Ergun equation coefficient (50)–(52) |
| λ | phase mobility (53)–(56) |
| kr | relative permeability (53)–(56) |
| μ | dynamic fluid viscosity (53)–(56) |
| k | intrinsic permeability (53)–(56) |
| p | phase pressure (53)–(56) |
| g | gravitational acceleration (53)–(56) |
| z | pointing upward (53)–(56) |
| k | formation permeability (57) and (58) |
| μ | fluid viscosity (57) and (58) |
| g | gravitational acceleration (57) and (58) |
| p | reservoir pressure (57) and (58) |
| ϕ | formation porosity (57) and (58) |
| ρ | fluid density (57) and (58) |
| t | time (57) and (58) |
| zg | elevation (57) and (58) |
| Q | mass entering (leaving) a control volume from a sink (source) (57) and (58) |
| k | permeability (59) |
| μ | dynamic viscosity (59) |
| m | mass flow (59) |
| ρ | density of fluid (59) |
| A | cross-sectional area of a wick (59) |
| g | gravitational acceleration (59) |
| θin | inclined angle of platform (59) |
| V | volume flow rate (59) |
| Kapp | apparent permeability (60) |
| q | flow rate (60) |
| μ | viscosity (60) |
| L | pore length (60) |
| A | cross-sectional area (60) |
| P | pressure (60) |
| kg | gas permeability (62) |
| k | permeability (62) |
| β | non-Darcy coefficient (62) |
| ρ | density (62) |
| v | gas flow rate (62) |
| μ | gas viscosity (62) |
| ηf | fluid viscosity (65) and (66) |
| ρf | fluid density (65) and (66) |
| k | total permeability tensor (65) and (66) |
| g | gravity acceleration (65) and (66) |
| Krg | gas relative permeability (67) and (68) |
| Krw | water relative permeability (67) and (68) |
| K∗r | points Kr at irreducible water saturation (Swirr) and residual gas saturation (Sgr) (67) and (68) |
| Sw | normalized water saturation (67) and (68) |
| Kint | intrinsic permeability (70) and (71) |
| Kapp | apparent permeability (67)–(68) |
| U | gas velocity (70) and (71) |
| m | dynamic viscosity (70) and (71) |
| Uavg | average velocity magnitude (70) and (71) |
| L | length of the porous structure (70) and (71) |
| ka | apparent permeability (72) |
| μ | dynamic viscosity (72) |
| Ls | unit sample length (72) |
| Ss | unit sample permeation surface (72) |
| d, di | pore diameter (72) |
| R | ideal gas constant (72) |
| T | temperature (72) |
| M | molar mass (72) |
| α | tangential momentum accommodation coefficient (72) |
| Pm | mean pressure between upstream and downstream (72) |
References
- Lorenzi, A.; Sotgnia, G. Comparative investigation of some characteristic quantities of two-phase cocurrent upward and downward flow. In Proceedings of the Two-Phase Flow and Heat Transfer Symposium-Workshop: Proceedings of Condensed Papers, Fort Lauderdale, FL, USA, 18–20 October 1976. [Google Scholar]
- Sadatomi, M.; Sato, Y.; Saruwatari, S. Two-Phase Flow in Vertical Noncircular Channels. Int. J. Multiph. Flow 1982, 8, 641–655. [Google Scholar] [CrossRef]
- Prakash, A.; Briens, C.L. Porous Gas Distributors in Bubble Columns. Effect of Liquid Presence on Distributor Pressure Drop. Effect of Start-up on Distributor Performance. Can. J. Chem. Eng. 1990, 68, 204. [Google Scholar] [CrossRef]
- Praser, H.M.; Krepper, E.; Lucas, D. Evolution of the two-phase flow in a vertical tube—Decomposition of gas fraction profiles according to bubble size classes using wiremesh sensors. Int. J. Therm. Sci. 2002, 41, 17. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Monoliths in Heterogeneous Catalysis. Catal. Rev. 1994, 36, 179–270. [Google Scholar] [CrossRef]
- Williams, J.L. Monolith structures, materials, properties and uses. Catal. Today 2001, 69, 3. [Google Scholar] [CrossRef]
- Błaszczyk, M. Research Upon Processes of Migration of Petroleum Substances and Their Emulsions in Porous Structures. Ph.D. Thesis, Lodz University of Technology, Faculty of Chemistry, Łódź, Poland, 2014. [Google Scholar]
- Stańczyk, K.; Świądrowski, J.; Kapusta, K.; Howaniec, N.; Cybulski, K.; Rogut, J.; Smolinski, A.; Wiatowski, M.; Kotyrba, A.; Krause, E.; et al. Hydrogen-Oriented Underground Coal Gasification for Europe (HUGE); Final Report; Research Found for Coal and Steel; (EUR 25044 EN 2012); European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Aksielrud, G.A.; Altszuler, M.A. Movement of Mass in Porous Bodies; WNT: Warsaw, Poland, 1987. [Google Scholar]
- Dulniew, G.N.; Nowikow, W.W. Prociessy Pierienosa w Nieodnorodnych Sriedach; Eniergoatomizdat: Moscow, Russia, 1991; p. 248. [Google Scholar]
- Mozhaev, A.P. Chaotic Homogeneous Porous Media. 2. Theory of Dispersion Turbulence: Basic Propositions. J. Eng. Phys. Thermophys. 2002, 75, 371–383. [Google Scholar] [CrossRef]
- Wałowski, G.; Filipczak, G. Hydrodynamics of Gas Flow Through Porous Deposits. Ph.D. Thesis, Opole University of Technology, Opole, Poland, 2015. [Google Scholar]
- Lambe, T.; Whitman, R.V. Soil Mechanics; Arkady Publishing House: Warsaw, Poland, 1978. [Google Scholar]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon; Valmont, V., Ed.; Dalmont: Kraljevica, Croatia, 1856. [Google Scholar]
- Strzelecki, T.; Kostecki, S.; Żak, S. Modeling of Flows Through Porous Media; Lower Silesian Educational Publishing House: Wrocław, Poland, 2008. [Google Scholar]
- Waluk, J. Soil Mechanics Laboratory; Wroclaw University of Technology: Wroclaw, Poland, 1973. [Google Scholar]
- Wałowski, G.; Filipczak, G. Assessment of process conditions associated with hydrodynamics of gas flow through materials with anisotropic internal structure. J. Sustain. Min. 2016, 15, 156–169. [Google Scholar] [CrossRef]
- Wałowski, G.; Filipczak, G.; Krause, E. Hydrodynamics of gas flow in porous carbonisate structures in the aspect of underground coal gasification technology. Young for Technic 2013. In Selected Research and Development Issues in Chemistry and Chemical Technology; Warsaw University of Technology: Płock, Poland, 2013; p. 253. [Google Scholar]
- Palica, M.; Chmiel, K.; Waluś, J. Hydraulics of selected biologically active deposits. In Yearbook of Environmental Protection; Middle-Pomeranian Scientific Society for Environmental Protection: Koszalin, Poland, 1999; Volume 1, p. 85. [Google Scholar]
- Chmiel, K.; Palica, M.; Waluś, J. Hydraulics and biofiltration of triethylamine on the modified EKOSORB – 100 deposit. In Yearbook of Environmental Protection; Middle-Pomeranian Scientific Society for Environmental Protection: Koszalin, Poland, 1999; Volume 1, p. 125. [Google Scholar]
- Łukaszuk, J.; Molenda, M.; Szwed, G. Influence of bulk density of seeds on airflow resistance. Acta Agrophysica 2004, 4, 77–83. [Google Scholar]
- Kusińska, E.; Nadulski, R.; Kobus, Z.; Guz, T. Air flow resistance during aeration of wheat meal. Agric. Eng. 2011, 4, 159. [Google Scholar]
- Khasanov, M.K.; Rafikova, G.R.; Musakaev, N.G. Mathematical model of carbon dioxide injection into a porous reservoir saturated with methane and its gas hydrate. Energies 2020, 13, 440. [Google Scholar] [CrossRef]
- Haghi, A.H.; Talman, S.; Chalaturnyk, R. Consecutive Experimental Determination of Stress-Dependent Fluid Flow Properties of Berea Sandstone and Implications for Two-Phase Flow Modeling. Water Resour. Res. 2019, 56, e2018WR024245. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Xu, J.; Chen, H.; Xie, W.-C. Variation of permeability of natural filled jointed rock under repeated loading and unloading conditions. Eur. J. Environ. Civ. Eng. 2020, 27, 2447–2459. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J.; Li, Q.; Chen, X.; Chen, Z.; Huang, B.; Chen, S. Variation law of coal permeability under cyclic loading and unloading. Therm. Sci. 2019, 23, 1487–1494. [Google Scholar] [CrossRef]
- Ma, L.; Xu, S.; Li, X.; Guo, Q.; Gao, D.; Ding, Y.; Ye, M.; Liu, Z. Particle tracking velocimetry of porous sphere settling under gravity: Preparation of the model porous particle and measurement of drag coefficients. Powder Technol. 2020, 360, 241–252. [Google Scholar] [CrossRef]
- Santos, M.P.P.C.; Carvalho, M.S. Pore network model for retrograde gas flow in porous media. J. Pet. Sci. Eng. 2020, 185, 106635. [Google Scholar] [CrossRef]
- Seitz, G.; Helmig, R.; Class, H. A numerical modeling study on the influence of porosity changes during thermochemical heat storage. Appl. Energy 2020, 259, 114152. [Google Scholar] [CrossRef]
- Yoon, H.C.; Zhou, P.; Kim, J. Robust modeling of hysteretic capillary pressure and relative permeability for two phase flow in porous media. J. Comput. Phys. 2020, 402, 108915. [Google Scholar] [CrossRef]
- Chalykh, A.; Zolotarev, P.; Chalykh, T.; Rubtsov, A.; Zolotova, S. Water vapor permeability through porous polymeric membranes with various hydrophilicity as synthetic and natural barriers. Polymers 2020, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, Z.; Wang, X.; Liu, H.; Ma, Y. Correlations between pore structure parameters and gas permeability of corundum porous materials. J. Am. Ceram. Soc. 2020, 103, 2137–2145. [Google Scholar] [CrossRef]
- Civan, F. Can Moderately Rarefied Gas Transport Through Round and Flat Tight Channels of Fractured Porous Media be Described Accurately? Transp. Porous Media 2020, 132, 157–181. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Wang, Z.; Sarma, H.K.; Zhang, C.; Wu, M. Investigation of CO2/N2 injection in tight oil reservoirs with confinement effect. Energy Sci. Eng. 2020, 8, 1194–1208. [Google Scholar] [CrossRef]
- Rabbani, A.; Mostaghimi, P.; Armstrong, R.T. Pore network extraction using geometrical domain decomposition. Adv. Water Resour. 2019, 123, 70–83. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Liang, F.; Liu, H.-H. Modeling gas relative permeability in shales and tight porous rocks. Fuel 2020, 272, 117686. [Google Scholar] [CrossRef]
- Chittenden, N.; Benbow, S.; Bond, A.; Norris, S. Development of an upscalable HM model for representing advective gas migration through saturated bentonite. Int. J. Rock Mech. Min. Sci. 2020, 133, 104415. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, S.; Li, Y.; Deng, Z.; Feng, H. Pore-scale lattice Boltzmann simulation of gas diffusion–adsorption kinetics considering adsorption-induced diffusivity change. Energies 2020, 13, 4927. [Google Scholar] [CrossRef]
- Zolotukhin, A.B.; Gayubov, A.T. Semi-analytical Approach to Modeling Forchheimer Flow in Porous Media at Meso- and Macroscales. Transp. Porous Media 2021, 136, 715–741. [Google Scholar] [CrossRef]
- Moreno, Z.; Anto-Darkwah, E.; Rabinovich, A. Semi-Analytical Modeling of Rate-Dependent Relative Permeability in Heterogeneous Formations. Water Resour. Res. 2021, 57, e2021WR029710. [Google Scholar] [CrossRef]
- Jannot, Y.; Degiovanni, A.; Moyne, C.; Lasseux, D. New developments of the Gas Research Institute method for the permeability measurement of porous media. Rev. Sci. Instrum. 2021, 92, 065102. [Google Scholar] [CrossRef] [PubMed]
- Wałowski, G. Model of Flow Resistance Coefficient for a Fragment of a Porous Material Deposit with Skeletal Structure. Energies 2021, 14, 3355. [Google Scholar] [CrossRef]
- Wu, K.L.; Chen, Z.X.; Li, X.F. Real gas transport through nanopores of varying cross-section type and shape in shale gas reservoirs. Chem. Eng. J. 2015, 281, 813–825. [Google Scholar] [CrossRef]
- Wałowski, G. Experimental and Numerical Studies of Gas Permeability through Orthogonal Networks for Isotropic Porous Material. Materials 2021, 14, 3832. [Google Scholar] [CrossRef] [PubMed]
- Khoei, A.R.; Amini, D.; Mortazavi, S.M.S. Modeling non-isothermal two-phase fluid flow with phase change in deformable fractured porous media using extended finite element method. Int. J. Numer. Methods Eng. 2021, 122, 4378–4426. [Google Scholar] [CrossRef]
- Mrazík, L.; Kříž, P. Porous Medium Equation in Graphene Oxide Membrane: Nonlinear Dependence of Permeability on Pressure Gradient Explained. Membranes 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Cui, X. Pore pressure dependent gas flow in tight porous media. J. Pet. Sci. Eng. 2021, 205, 108835. [Google Scholar] [CrossRef]
- Afagwu, C.; Abubakar, I.; Kalam, S.; Al-Afnan, S.F.; Awotunde, A.A. Pressure-transient analysis in shale gas reservoirs: A review. J. Nat. Gas Sci. Eng. 2020, 78, 103319. [Google Scholar] [CrossRef]
- Gao, W.; Zagorščak, R.; Thomas, H.R.; An, N. Simulation of underground coal gasification based on a coupled thermal-hydraulic-chemical model. Combust. Theory Model. 2022, 26, 110–134. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, B.; Zhou, Y.; Li, Y.; Ting, L. Experimental verification of permeability and inertial resistance coefficient model in the goaf. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 1–20. [Google Scholar] [CrossRef]
- Becker, B.; Guo, B.; Buntic, I.; Flemisch, B.; Helmig, R. An Adaptive Hybrid Vertical Equilibrium/Full-Dimensional Model for Compositional Multiphase Flow. Water Resour. Res. 2022, 58, e2021WR030990. [Google Scholar] [CrossRef]
- Wang, O.; Moridis, G.J.; Blasingame, T.A. Wavelet Transforms for the Simulation of Flow Processes in Porous Geologic Media. Transp. Porous Media 2023, 146, 771–803. [Google Scholar] [CrossRef]
- Tan, S.-C.; Guo, C.; Jiang, Y.-Y.; Wang, T.; Li, C.-Z. Investigation on permeability of ultra-thin screen wick with free surface using gravity flow and numerical simulation methods. Int. Commun. Heat Mass Transf. 2022, 131, 105879. [Google Scholar] [CrossRef]
- Mehmani, A.; Prodanović, M.; Javadpour, F. Multiscale, Multiphysics Network Modeling of Shale Matrix Gas Flows. Transp. Porous Media 2013, 99, 377–390. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, H.; Jiang, E.; Zhao, N.; Guo, C.; Bai, B. Study on Apparent Permeability Model for Gas Transport in Shale Inorganic Nanopores. Energies 2022, 15, 6301. [Google Scholar] [CrossRef]
- Li, J.; Chen, C. Numerical Simulation of the Non-Darcy Flow Based on Random Fractal Micronetwork Model for Low Permeability Sandstone Gas Reservoirs. Geofluids 2020, 2020, 8884885. [Google Scholar] [CrossRef]
- Li, H.; Lei, H.; Yang, Z.; Wu, J.; Zhang, X.; Shouding, L. A hydro-mechanical-damage fully coupled cohesive phase field model for complicated fracking simulations in poroelastic media. Comput. Methods Appl. Mech. Eng. 2022, 399, 115451. [Google Scholar] [CrossRef]
- Lysyy, M.; Føyen, T.; Birkeland Johannesen, E.; Fernø, M.; Ersland, G. Hydrogen Relative Permeability Hysteresis in Underground Storage. Geophys. Res. Lett. 2022, 49, e2022GL100364. [Google Scholar] [CrossRef]
- Galteland, O.; Rauter, M.T.; Bratvold, M.S.; Trinh, T.T.; Bedeaux, D.; Kjelstrup, S. Local Thermodynamic Description of Isothermal Single-Phase Flow in Simple Porous Media. Transp. Porous Media 2022, 145, 153–173. [Google Scholar] [CrossRef]
- Shariati, V.; Roohi, E.; Ebrahimi, A. Numerical Study of Gas Flow in Super Nanoporous Materials Using the Direct Simulation Monte-Carlo Method. Micromachines 2023, 14, 139. [Google Scholar] [CrossRef]
- Shi, J.; Chun, Q.; Zhang, Y.; Wang, D.; Liu, Z.; Jin, H.; Liu, C.; Liu, G.; Qian, R. Modified random hierarchical bundle model for predicting gas permeability of cement-based materials. Constr. Build. Mater. 2023, 363, 129859. [Google Scholar] [CrossRef]
- Gajda, D.; Lutyński, M. Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage. Energies 2022, 15, 2663. [Google Scholar] [CrossRef]
- Rabbani, A.; Babaei, M.; Javadpour, F. A Triple Pore Network Model (T PNM) for Gas Flow Simulation in Fractured, Micro porous and Meso porous Media. Transp. Porous Media 2020, 132, 707–740. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; Wang, S. An Apparent Gas Permeability Model for Real Gas Flow in Fractured Porous Media with Roughened Surfaces. Polymers 2021, 13, 1937. [Google Scholar] [CrossRef]
- Afagwu, C.; Alafnan, S.; Mahmoud, M.A.; Patil, S. Permeability model for shale and ultra-tight gas formations: Critical insights into the impact of dynamic adsorption. Energy Rep. 2021, 7, 3302–3316. [Google Scholar] [CrossRef]
- Dyrka, I.; Kiersnowski, H.; Wójcicki, A. Geology of Tight Gas Deposits. National Geological Institute—National Research Institute. 2025. Available online: https://www.pgi.gov.pl/psg-1/psg-2/informacja-i-szkolenia/wiadomosci-surowcowe/10869-geologia-zloz-gazu-zamknietego.html (accessed on 1 February 2025).
- Ahmadi, S.M.A.; Mohammadi, T.; Azizi, N. Effect of halloysite nanotubes incorporation on morphology and CO2/CH4 separation performance of Pebax-based membranes. Korean J. Chem. Eng. 2021, 38, 104–113. [Google Scholar] [CrossRef]
- Alakrach, A.M.; Al-Rashdi, A.A.; Al-Omar, M.K.; Jassam, T.M.; Ting, S.S.; Dahham, O.S.; Zulkepli, N.N. Physical and Barrier Properties of Polylactic Acid/Halloysite Nanotubes-Titanium Dioxide Nanocomposites. Mater. Sci. Forum 2021, 1021, 280–289. [Google Scholar] [CrossRef]
- Keskin, B.; Ağtaş, M.; Ormancı-Acar, T.; Türken, T.; İmer, D.Y.; Ünal, S.; Menceloğlu, Y.Z.; Uçar-Demir, T.; Koyuncu, İ. Halloysite nanotube blended nanocomposite ultrafiltration membranes for reactive dye removal. Water Sci. Technol. 2021, 83, 271–283. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Caruso, M.R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Hydroxypropyl Cellulose Films Filled with Halloysite Nanotubes/Wax Hybrid Microspheres. Ind. Eng. Chem. Res. 2021, 60, 1656–1665. [Google Scholar] [CrossRef]
- AbdulKadir, W.A.F.W.; Ahmad, A.L.; Seng, O.B. Carnauba Wax/Halloysite Nanotube with Improved Anti-Wetting and Permeability of Hydrophobic PVDF Membrane via DCMD. Membranes 2021, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Zeng, Z.; Du, S.; Liu, E. Superoleophobic graphene oxide/halloysite nanotube composite membranes for oil-water separation. Mater. Chem. Phys. 2021, 263, 124347. [Google Scholar] [CrossRef]
- Ahmadi, S.M.A.; Mohammadi, T.; Azizi, N. Superior Pebax-1657/amine-modified halloysite nanotubes mixed-matrix membranes to improve the CO2/CH4 separation efficiency. J. Appl. Polym. Sci. 2021, 138, e50749. [Google Scholar] [CrossRef]
- Garcia-Escobar, F.; Bonilla-Rios, J.; Espinoza-Martinez, A.B.; Cerda-Hurtado, P. Halloysite silanization in polyethylene terephthalate composites for bottling and packaging applications. J. Mater. Sci. 2021, 56, 16376–16386. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; D’Amico, F.; Tammaro, L.; Gorrasi, G. Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection. Nanomaterials 2022, 12, 1265. [Google Scholar] [CrossRef]
- Kaze, C.R.; Adesina, A.; Lecomte-Nana, G.L.; Assaedi, H.; Alomayri, T.; Kamseu, E.; Melo, U.C. Physico-mechanical and microstructural properties of geopolymer binders synthesized with metakaolin and meta-halloysite as precursors. Clean. Mater. 2022, 4, 100070. [Google Scholar] [CrossRef]
- Wang, B.; He, H.; Li, Y.; Liu, C.; Bai, J.; Zhou, X.; Li, J.; Wang, Y. Fabrication of green poly (L-lactic acid) hybrid membrane through incorporation of functionalized natural halloysite nanotubes. J. Chem. Technol. Biotechnol. 2022, 97, 1676–1683. [Google Scholar] [CrossRef]
- Boro, U.; Priyadarsini, A.; Moholkar, V.S. Synthesis and characterization of poly(lactic acid)/clove essential oil/alkali-treated halloysite nanotubes composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 216, 927–939. [Google Scholar] [CrossRef]
- Shahabi, N.; Soleimani, S.; Ghorbani, M. Investigating functional properties of halloysite nanotubes and propolis used in reinforced composite film based on soy protein/basil seed gum for food packaging application. Int. J. Biol. Macromol. 2023, 231, 123350. [Google Scholar] [CrossRef]
- Wałowski, G. Development of Biogas and Biorafinery Systems in Polish Rural Communities. J. Water Land Dev. 2021, 49, 156–168. [Google Scholar] [CrossRef]
- Wałowski, G. Assessment of polydisperse substrate flow in a fermentor for computational fluid dynamics modelling. J. Water Land Dev. 2022, 1–7. [Google Scholar] [CrossRef]
- Bade, S.O.; Taiwo, K.; Ndulue, U.F.; Tomomewo, O.S.; Oni, B.A. A review of underground hydrogen storage systems: Current status, modeling approaches, challenges, and future prospective. Int. J. Hydrog. Energy 2024, 80, 449. [Google Scholar] [CrossRef]
- Dodangoda, C.; Ranjith, P.G.; Haque, A. Unlocking the potential of underground hydrogen storage for clean energy solutions. Geomech. Geophys. Geo Energy Geo Resour. 2024, 10, 130. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, Y.; Ouyang, J.; Yang, H. Halloysite nanotubes as hydrogen storage materials. Phys. Chem. Miner. 2014, 41, 323. [Google Scholar] [CrossRef]
- Jin, J.; Fu, L.; Yang, H.; Ouyang, J. Carbon hybridized halloysite nanotubes for high-performance hydrogen storage capacities. Sci. Rep. 2015, 5, 12429. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Assemi, S.; Asgar, H.; Gadikota, G.; Tran, T.; Nguyen, W.; McLennan, J.D.; Miller, J.D. Characterization of Natural Consolidated Halloysite Nanotube Structures. Minerals 2021, 11, 1308. [Google Scholar] [CrossRef]
- Zou, Q.; Chen, Z.; Zhan, J.; Chen, C.; Gao, S.; Kong, F.; Xia, X. Morphological evolution and flow conduction characteristics of fracture channels in fractured sandstone under cyclic loading and unloading. Int. J. Min. Sci. Technol. 2023, 33, 1527–1540. [Google Scholar] [CrossRef]
- Zou, Q.; Li, Q.; Liu, T.; Li, X.; Liang, Y. Peak strength property of the pre-cracked similar material: Implications for the application of hydraulic slotting in ECBM. J. Nat. Gas Sci. Eng. 2017, 37, 106–115. [Google Scholar] [CrossRef]
- Zhang, L.; Kan, Z.; Zhang, C.; Tang, J. Experimental study of coal fow characteristics under mining disturbance in China. Int. J. Coal Sci. Technol. 2022, 9, 66. [Google Scholar] [CrossRef]
- Cheng, Z.; Pan, H.; Zou, Q.; Li, Z.; Chen, L.; Cao, J.; Zhang, K.; Cui, Y. Gas Flow Characteristics and Optimization of Gas Drainage Borehole Layout in Protective Coal Seam Mining: A Case Study from the Shaqu Coal Mine, Shanxi Province, China. Nat. Resour. Res. 2021, 30, 1481–1493. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The Crack Propagation Behaviour of CO2 Fracturing Fluid in Unconventional Low Permeability Reservoirs: Factor Analysis and Mechanism Revelation. Processes 2025, 13, 159. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wu, J.; Li, X.; Li, H.; Cheng, Y. Wellhead Stability During Development Process of Hydrate Reservoir in the Northern South China Sea: Evolution and Mechanism. Processes 2025, 13, 40. [Google Scholar] [CrossRef]
- Wałowski, G. Wałbrzych from the Poor Mineshafts to… Underground Gasification of Hard Coal; Catholic Action of the Archdiocese of Lublin: Lublin, Poland, 2009; ISBN 978-83-927527-8-3. Available online: https://bractwotrojka.pl/pl/p/Walbrzych-od-biedaszybow-do...-Podziemne-zgazowanie-wegla-kamiennego-Grzegorz-Walkowski/2797 (accessed on 1 February 2025).
- Wałowski, G. Nowa Ruda: From Poor Mineshafts to… Underground Hydrogenation of Hard Coal; Poligraf Publishing House: Brzezia Łąka, Poland, 2021; ISBN 978-83-62752-68-3. Available online: https://www.ksiazki24h.pl/wiecej.php?id=tt-549 (accessed on 1 February 2025).
- Javadpour, F. Nanopores and apparent permeability of gas flow in mudrocks (shales and siltstone). J. Can. Pet. Technol. 2009, 48, 16. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Jensen, J.L.; Blasingame, T.A. Reservoir engineering for unconventional gas reservoirs: What Do We.Have to Consider? 2011, p. SPE-145080-MS. Available online: https://www.researchgate.net/profile/Sohail-Khan-23/post/How_to_calculate_the_fluid_flow_in_unconventional_shale_reservoirs/attachment/59d6232f79197b807798183c/AS%3A305909538983942%401449945925552/download/SPE_145080_%28Clarkson%29_Res_Eng_Uncon_Reservoirs.pdf (accessed on 1 February 2025).
- Wang, M.; Yu, Q. A method to determine the permeability of shales by using the dynamic process data of methane adsorption. Eng. Geol. 2019, 253, 111–122. [Google Scholar] [CrossRef]
- Yao, J.; Sun, H.; Fan, D.-Y.; Wang, C.-C.; Sun, Z.-X. Numerical simulation of gas transport mechanisms in tight shale gas reservoirs. Pet. Sci. 2013, 10, 528–537. [Google Scholar] [CrossRef]
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale gas flow in shale gas sediments. J. Can. Pet. Technol. 2007, 46, PETSOC-07-10-06. [Google Scholar] [CrossRef]
- Wałowski, G. Assessment of gas permeability coefficient of porous materials. J. Sustain. Min. 2017, 16, 55–65. [Google Scholar] [CrossRef]
- Wałowski, G. Gas Permeability Model for Porous Materials from Underground Coal Gasification Technology. Energies 2021, 14, 4462. [Google Scholar] [CrossRef]
- Wałowski, G. The method to assess the gas flow of a porous bed product derived from underground coal gasification technology. Energy 2020, 199, 117456. [Google Scholar] [CrossRef]
- Wałowski, G. Assessment of coke quality related to of effective permeability coefficient and anisotropy coefficient. Fuel 2019, 236, 82–91. [Google Scholar] [CrossRef]
- Jarosz, Z.; Kapłan, M.; Klimek, K.; Anders, D.; Dybek, B.; Herkowiak, M.; Hołaj-Krzak, J.T.; Syrotyuk, S.; Korobka, S.; Syrotyuk, H.; et al. Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy. Energies 2024, 17, 2524. [Google Scholar] [CrossRef]

| Characteristics of the Deposit | ||||||
|---|---|---|---|---|---|---|
| Type Material | Height of the Deposit L, m | Diameter of the Column (Deposit) D, m | Grain Replacement Diameter (Pores) de, m | Sample Cross-Section A, m2 | Sample Porosity (Deposit) ε | Gas Velosity wo, m/s |
| acidic peat [19] | 0.45 | 0.175 | 0.0240 | 0.47 | 0.07–0.16 | |
| bark from deciduous trees [19] | 0.65 | 0.07–0.18 | ||||
| peat for mushroom production [19] | 0.75 | 0.07–0.16 | ||||
| wheat straw [19] | 0.93 | 0.05–0.16 | ||||
| wood chips [19] | 0.68 | 0.05–0.15 | ||||
| compost soil [19] | 0.62 | 0.05–0.15 | ||||
| stalks from heather [19] | 0.93 | 0.06–0.16 | ||||
| Ecosorb-100 [20] | 0.45 | 0.175 | 0.0005 | 0.0240 | 0.62 | 0.05–0.08 |
| 0.0008 | 0.51 | 0.03–0.09 | ||||
| 0.0021 | 0.30 | 0.03–0.08 | ||||
| 0.0029 | 0.22 | 0.02–0.07 | ||||
| 0.0167 | 0.07 | 0.02–0.05 | ||||
| rape [21] | 0.95 | 0.196 | 0.00186 | 0.0121 | 0.40 | 0.05–0.20 |
| wheat [21] | 0.00410 | 0.0142 | 0.47 | 0.05–0.25 | ||
| corn [21] | 0.00785 | 0.0130 | 0.43 | 0.05–0.25 | ||
| wheat meal [22] | 10 | 0.196 | 0.004 | 0.0302 | - | 0.021–0.21 |
| karbonizat (coal char) in situ (Wałowski—own research) | 0.02 | - | 0.000068 | 0.0004 | 0.45 | 0.03–0.11 |
| natural pumice (Wałowski—own research) | - | 0.63 | 0.01–0.06 | |||
| synthetic pumice (Wałowski—own research) | - | 0.88 | 0.02–0.18 | |||
| Model Authors | Material | Flow | Model | Equation |
|---|---|---|---|---|
| Darcy [23] | porous tank | CO2 CH4 CH4 gas hydrate | (4) | |
| Darcy [24] | Berea sandstone | two-phase fluid | (5) | |
| Darcy–Waite [25] | cohesive rock | fluid | (6) | |
| Darcy [26] | cohesive rock | fluid | (7) | |
| Darcy–Brinkman [27] | particles of a porous ball | fluid | (8) | |
| Darcy [28] | pore networks in porous media | throttle reverse | (9) | |
| Darcy [29] | porous medium | numeric | (10) | |
| Darcy [30] | porous medium | two-phase | (11) | |
| Zolotareva–Dubininina [31] | porous polymer membranes | steam | (12) (13) (14) | |
| Darcy–Forchheimer [32] | corundum porous materials | gas | (15) | |
| Carman [33] | round and flat, tight channels of fractured porous media | gas | (16) | |
| Soave’a–Redlicha–Kwonga [34] | porous structures with oil deposits | CO2 N2 | (17) (18) | |
| Younga–Laplace’a [34] | porous structures with oil deposits | CO2 N2 | (19) | |
| Darcy [35] | triple pore networks (T-PNM) in fractured, microporous and mesoporous media | gas | (20) | |
| Effective-medium approximation [36] | shales and porous rocks | gas | (21) | |
| Hydro-mechanical [37] | saturated bentonite | gas | (22) (23) (24) | |
| Network Boltzmanna [38] | Boltzmann networks on the pore scale | fluid | (25) (26) | |
| Forchheimer [39] | porous media on the meso and macro scale | fluid | (27) | |
| Type (extended) Darcy [40] | heterogeneous formations | fluid | (28) (29) | |
| Darcy [41] | porous medium | fluid | (30) (31) (32) (33) | |
| Darcy–Weisbach [42] | a fragment of a deposit of porous material with a skeletal structure | fluid | (34) | |
| Darcy [43] | cracked porous media with rough surfaces | gas | (35) (36) | |
| Darcy [44] | orthogonal networks of isotropic porous material | gas | (37) | |
| Darcy [45] | cracked porous media | two-phase fluid | (38) (39) | |
| Darcy–PDE [46] | porous medium in a graphene oxide membrane | gas | (40) (41) | |
| Beskok– Karniadakis [47] | porous medium | gas | (42) | |
| Wasaki–Akkutlu [48] | shale and ultratight formation | CH4 | (43) | |
| Geng [48] | shale and ultratight formation | CH4 | (44) | |
| Cai [48] | shale and ultratight formation | CH4 | (45) | |
| Yanfeng [48] | shale and ultratight formation | CH4 | (46) | |
| Wu [48] | shale and ultratight formation | CH4 | (47) (48) | |
| Darcy [49] | coal | synthesis gas | (49) | |
| Liu [50] | porous medium, goafs | fluid | (50) (51) (52) | |
| Darcy [51] | porous medium | multiphase | (53) (54) (55) (56) | |
| Darcy [52] | porous geological media | fluid | (57) | |
| Buckley’a–Leveretta [52] | porous geological media | fluid | (58) | |
| Darcy [53] | ultra-thin sieve wick with a free surface | numeric, fluid | (59) | |
| Darcy [54] | slate matrix | gas | (60) | |
| Javadpoura [55] | inorganic shale nanopores | gas | (61) | |
| Forchheimer [56] | sandstone with low permeability | numeric, gas | (62) | |
| Forchheimer–Zolotukhina–Gaybova [39] | porous medium | liquid | (63) | |
| Zolotukhina–Gaybova [39] | porous medium | liquid | (64) | |
| Darcy [57] | poroelastic center | fluid | (65) | |
| Darcy–Poiseuille’a [57] | poroelastic center | fluid | (66) | |
| LET [58] | underground warehouse | H2 | (67) (68) | |
| Darcy [59] | porous medium | single-phase | (69) | |
| DSMC [60] | supernanoporous medium | numeric, gas | (70) (71) | |
| Modified RHBM [61] | cement-based | gas | (72) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hołaj-Krzak, J.T.; Dybek, B.; Szymenderski, J.; Koniuszy, A.; Wałowski, G. Unconventional Fossil Energy Carrier Assessment of the Influence of the Gas Permeability Coefficient on the Structure of Porous Materials: A Review. Energies 2025, 18, 870. https://doi.org/10.3390/en18040870
Hołaj-Krzak JT, Dybek B, Szymenderski J, Koniuszy A, Wałowski G. Unconventional Fossil Energy Carrier Assessment of the Influence of the Gas Permeability Coefficient on the Structure of Porous Materials: A Review. Energies. 2025; 18(4):870. https://doi.org/10.3390/en18040870
Chicago/Turabian StyleHołaj-Krzak, Jakub T., Barbara Dybek, Jan Szymenderski, Adam Koniuszy, and Grzegorz Wałowski. 2025. "Unconventional Fossil Energy Carrier Assessment of the Influence of the Gas Permeability Coefficient on the Structure of Porous Materials: A Review" Energies 18, no. 4: 870. https://doi.org/10.3390/en18040870
APA StyleHołaj-Krzak, J. T., Dybek, B., Szymenderski, J., Koniuszy, A., & Wałowski, G. (2025). Unconventional Fossil Energy Carrier Assessment of the Influence of the Gas Permeability Coefficient on the Structure of Porous Materials: A Review. Energies, 18(4), 870. https://doi.org/10.3390/en18040870








