Abstract
An exergy analysis of maize cob gasification in a concentric tube fixed-bed reactor was conducted to define the relationship between biomass in the combustion and gasification zones. Biomass exergy was estimated based on its elemental composition, and syngas was treated as an ideal gas. The results show a linear correlation between temperature and the mass ratio in both the combustion and gasification zones. The optimum exergy efficiency was 68.2% at a mass ratio of 2. Most irreversibilities were found in the combustion zone, with 42.9 kJ/kg destroyed, compared to 33.7 kJ/kg in the gasification zone. It is concluded that the allothermal gasification of biomass in two-zone gasifiers with concentric tubes improves the syngas LHV, demonstrating good reactor performance.
1. Introduction
Biomass is one of the most widely available renewable energy sources globally [1]. Due to its renewable nature and lower carbon footprint, biomass serves as a suitable alternative to fossil fuels [2,3]. Additionally, the proper management of biomass waste is crucial to prevent the pollution of water bodies and the atmosphere, and valorization strategies have emerged as effective solutions [4,5,6].
The agroindustrial processing of maize, one of the world’s most significant crops, generates between 1500 and 5400 million tons of waste annually [7]. Typically, these wastes are either incinerated or discarded, contributing to air pollution and greenhouse gas emissions. Therefore, it is essential to explore valorization strategies for agroindustrial waste, with energy recovery being a promising approach to sustainably reduce carbon emissions [8]. In particular, the department of Córdoba, Colombia, produces approximately 44,000 tons of maize cobs annually [9].
The energy valorization of maize cobs has been widely studied, highlighting their favorable properties for thermochemical processes. However, their application is limited due to challenges such as low energy and mass density, heterogeneous size and shape, and high moisture content [10]. Gasification offers a viable solution for the energy valorization of maize cobs, providing higher energy conversion efficiencies compared to combustion and pyrolysis [11]. Biomass gasification plays a crucial role in the transition toward cleaner energy systems to meet the growing global energy demand [12].
In the gasification process, both steam and oxygen are commonly used as gasifying agents. Oxygen facilitates partial oxidation, generating the heat necessary for the endothermic reactions within the gasifier, helping to maintain the temperature required for efficient gasification. Steam, on the other hand, is typically introduced to enhance hydrogen production, particularly when the objective is to produce syngas with a high hydrogen content. The literature emphasizes the importance of selecting the appropriate gasifying agent based on the desired output but does not prescribe a strict preference for either oxygen or steam [13,14].
The air-to-biomass ratio is critical in combustion processes for ensuring efficiency and minimizing emissions. In both studies, the role of the excess air (EA) ratio is thoroughly discussed, particularly in the context of biomass and coal co-combustion. For instance, in the case of lignite coal and woodchip co-combustion, optimal excess air ratios were identified for various biomass proportions [13,14]:
With 10% woodchips, the optimal EA ratio was 1.18.
With 30% woodchips, the optimal EA ratio increased to 1.32.
With 50% woodchips, the EA ratio further increased to 1.41.
The ratio of combustion air to biomass is essential because too much air can lower the combustion temperature and lead to higher CO emissions, while too little air can result in incomplete combustion. These studies demonstrate the need for an optimal EA ratio to reduce emissions, such as NOx and CO, while maintaining efficient combustion [13,14].
Integrating biomass gasification into power generation systems requires optimizing the syngas composition to increase its calorific value [15]. Various approaches have been proposed to improve the heating value of syngas, including fluidized bed gasification [16], catalytic tar cracking [7], the use of steam as a gasifying agent [4], plasma torch gasification [17], selective CO2 absorption [18], and allothermal gasification [19]. While steam gasification can produce syngas with a higher hydrogen content, it has certain drawbacks, such as low particle reactivity and reduced flow temperature due to highly endothermic reactions [20]. Plasma gasification, meanwhile, demands substantial amounts of electricity to generate thermal plasma, making it expensive [21]. Similarly, selective CO2 absorption incurs high investment and operational costs, primarily due to low efficiency, high solvent circulation rates, elevated pressures in the absorber, and the large diameter of the reactor [22].
In contrast, allothermal gasification generates syngas with a higher calorific value by reducing nitrogen dilution, thereby offering economic benefits through the use of low-cost heat sources [23]. Allothermal gasification utilizes an external heat source [24] and can be implemented in concentric tube reactors, where a hot flow through the annulus supplies the necessary heat for gasification in the inner tube [19].
A fluidodynamic study of allothermal gasification in two decoupled fluidized bed reactors, where the combustion zone is separated from other gasification stages, highlighted the strong dependence of process parameters on the circulation factor [25]. In this configuration, the fraction of carbon monoxide, hydrogen, and tars in the syngas increased due to reduced nitrogen dilution, which was explained by the lower gasifying agent-to-biomass ratio. Additionally, using a fluidized bed reactor with concentric tubes for the allothermal gasification of biomass with steam as the gasifying agent resulted in lower overall efficiency compared to autothermal gasification [26]. Similarly, concentric tube reactors with bubbling fluidized beds in both chambers, using steam as the gasifying agent, were employed to gasify hazelnut shells with LPG to supply the necessary heat [27]. This approach produced high-quality syngas, although it required four hours to reach the operating temperature in the reactor.
Furthermore, a comparative analysis of allothermal gasification using oxygen as the gasifying agent for biomass and coal in entrained-flow concentric tube reactors revealed higher gas quality and efficiency for coal compared to biomass [28]. A numerical simulation of the allothermal gasification of four lignocellulosic biomasses abundant in Pakistan, using oxygen as the gasifying agent in a concentric tube fluidized bed reactor, demonstrated that the highest efficiency and gas quality were achieved with oxygen–fuel ratios between 0.3 and 0.4 [29]. A similar reactor geometry was used to preheat the gasifying agent by injecting it at multiple points in the concentric tube reactor [30].
Overall, most studies on the allothermal gasification of biomass focus on fluidized beds, while there is limited discussion of fixed-bed reactors. In fixed-bed systems, the limited contact between the biomass and the gasifying agent means that the process is highly influenced by biomass characteristics, gasification temperature, and the gasifying agent-to-biomass ratio [31,32,33].
Therefore, in this study, the fixed-bed gasification reactor is evaluated to identify the optimum process conditions. To this end, exergy assessment is employed. The exergy method has been widely used to evaluate the efficiency of bioenergy systems, addressing the limitations of traditional energy assessment approaches [34]. Exergy assessment allows for identifying the benefits and inefficiencies of the gasification process and optimizing it accordingly [35,36,37]. However, to the best of the authors’ knowledge, there is no discussion in the specialized literature regarding the exergy efficiency of the allothermal gasification of maize cobs in concentric tube reactors. Therefore, this study aims to assess the exergy efficiency of biomass gasification in a novel allothermal gasification reactor.
2. Materials and Methods
2.1. Raw Material Preparation
The maize cobs used as raw material in the gasification process were collected from local crops and stored under ambient conditions prior to preparation. Before use in the experiments, the cobs were crushed to an average particle size of 5–10 mm to ensure adequate homogeneity and facilitate heat and mass transfer within the gasification reactor. Subsequently, the bulk density of the biomass was determined, which was approximately 250 kg/m3.
To ensure consistency in composition, the moisture content of the cobs was measured by drying them at 105 °C for 24 h in a laboratory oven, resulting in a moisture content of 8% by weight. This step was necessary to standardize the gasification conditions and minimize variations due to moisture. The prepared biomass was stored in a dry environment until it was used in the allothermal gasification experiments.
2.2. Characteristics of Maize Cob
The elemental composition of maize cobs typically includes a higher oxygen content relative to carbon, which can affect energy yield during gasification. Studies indicate that maize cobs have a carbon content ranging from 39.3% to 51.8%, an oxygen content between 41.6% and 48.3%, and a hydrogen content between 5.0% and 6.2% [38]. This higher proportion of oxygen improves thermal reactivity, which can facilitate the gasification process, but also leads to a lower overall energy value compared to denser biomass sources such as wood [39].
In contrast, woody biomass generally has a higher carbon-to-oxygen ratio, resulting in a higher calorific value and better gasification efficiency [40]. For instance, hardwoods can have carbon contents above 50%, making them more energy-dense than maize cobs [41,42].
The proximate analysis of maize cobs shows a significant ash content, which can be problematic for gasification due to potential fouling and sintering issues. Maize cobs typically have an ash content that can exceed 6%, which is considered undesirable for efficient gasification [39]. In comparison, nut shells and some types of straw may have lower ash contents, making them more favorable for gasification applications [43].
Maize cob is a lignocellulosic biomass residue derived from maize processing in the agroindustry. Table 1 presents the elemental and proximate analysis of maize cob. For the determination of elemental composition, a CHNS-O elemental analyzer (model EMA 502, VELP Scientifica, Usmate Velate, Italy was used, allowing for the measurement of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S), while oxygen (O) was calculated by difference. The proximate analysis was performed following ASTM standards: ASTM D3173 for moisture determination [44], ASTM D3175 for volatile matter [45], ASTM D3174 for ash [46], and ASTM D3172 for fixed carbon by calculation through difference [47].

Table 1.
Elemental and proximal analysis of maize cob [9].
The composition shows a high percentage of oxygen (O), which promotes oxidation processes during gasification, while the high content of volatile materials indicates a lower energy demand for the thermal degradation of biomass. Additionally, the low moisture content is optimal for gasification.
The properties listed in Table 1—ultimate analysis (C, H, N, O, and S) and proximate analysis (residual moisture, volatile matter, fixed carbon, ash, and higher heating value (HHV))—are critical for biomass conversion as they directly influence the thermochemical performance during processes such as gasification, combustion, and pyrolysis.
From the ultimate analysis:
Carbon (C) and hydrogen (H) are essential for the energy content of biomass. Higher concentrations of these elements result in higher HHVs, which represent the amount of energy released during combustion. As noted in studies, C and H are oxidized during combustion, contributing to energy release [48,49]. Nitrogen (N) and sulfur (S) are important because they contribute to NOx and SOx emissions during combustion. Sulfur can also influence ash-related issues, such as corrosion and slagging [48,49]. Oxygen (O), calculated by difference, helps estimate the overall composition and combustion efficiency. Biomasses with higher oxygen content tend to have lower HHVs, reducing their energy efficiency [48]. Sulfur (S) levels are particularly important for predicting ash-related issues like corrosion in boilers and furnaces, especially in systems handling agricultural residues [48,49].
From the proximate analysis:
High moisture content negatively impacts energy efficiency during combustion and gasification, as additional energy is required to evaporate the water [48]. Volatile matter is directly associated with how easily biomass releases gasses during pyrolysis or gasification. Biomasses with higher volatile content tend to have more efficient conversion processes [48,49]. Fixed carbon represents the portion of the biomass that contributes to char formation, which is important for processes like gasification. A higher fixed carbon content enhances char production, which can be further processed or utilized in other applications [49]. High ash content, common in agricultural residues, can cause operational issues such as slagging, fouling, and corrosion in conversion systems. The composition of ash also influences melting behavior and can lower the efficiency of the conversion process [48,49]. The high heating value (HHV) is one of the most important metrics for assessing the energy potential of biomass. It is directly related to carbon, hydrogen, and oxygen content and helps evaluate the suitability of a particular biomass feedstock for energy production [48,49]. Ultimate and proximate analyses are essential for determining the conversion efficiency, emission levels, and ash-related challenges in biomass systems. High carbon and hydrogen contents, along with low ash content, are desirable for achieving high energy output. However, the presence of elements such as sulfur and nitrogen, and high moisture can introduce challenges in emission control and system efficiency. These analyses help optimize feedstock for processes like combustion and gasification [48,49].
2.3. Concentric Tube Gasification Reactor
Figure 1 represents a concentric tube gasification reactor. This figure, the red arrows on the left and right indicate the movement direction of biomass and volatiles, respectively. The reactor consists of two concentric tubes, with the inner tube serving as the biomass combustion zone to produce the necessary heat for gasification, while biomass gasification occurs in the annular space. Six thermocouples were installed as shown in this figure—three in the gasification zone (i.e., TG1, TG2, and TG3) and three in the combustion zone (i.e., TC1, TC2, and TC3). The temperatures were measured using K-type thermocouples with an accuracy of ±1.5 °C and an Omega TC-08 data acquisition module, with a sampling rate of 10 readings per second and a measurement range of −270 to 1820 °C. To report data only under steady-state conditions, the reactor was ignited, and measurements were delayed for a few minutes until temperature fluctuations were less than ±2 °C. Once this stability was achieved, the temperature data were averaged to minimize the impact of small residual fluctuations. This approach ensured that the measurements reflected stable process conditions, which is crucial for the accuracy of the results.
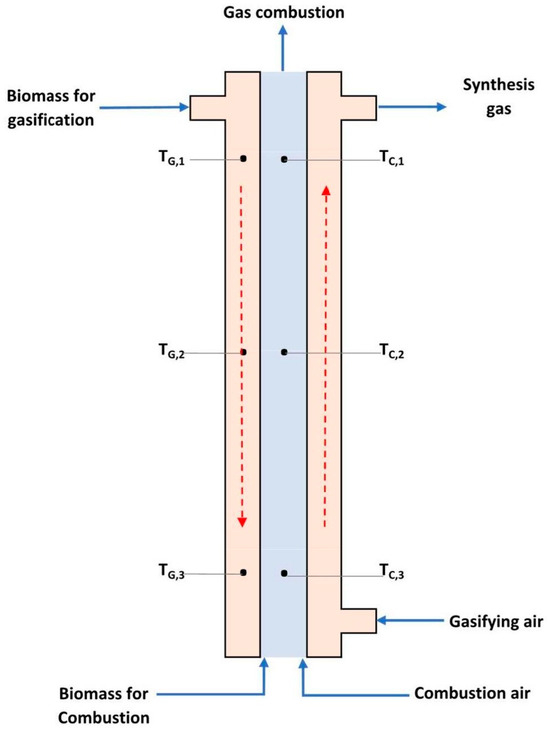
Figure 1.
Concentric tubes gasification reactor.
This reactor differs from conventional updraft or downdraft gasifiers, which have a single reaction zone for pyrolysis, combustion, and reduction processes. Conventional gasifiers burn some pyrolysis products to generate the necessary heat for the process. During gasification, a significant portion of the fuel is oxidized to carbon dioxide, carbon monoxide, and water vapor, thereby reducing the calorific value of the produced gas. By separating the combustion and gasification processes, the volume of thermally degraded gas is reduced, resulting in syngas with a higher calorific value.
In this reactor, air is injected for complete combustion in the central tube at a ratio of 4:1 relative to biomass, and a small amount of air is introduced into the annulus to ensure the thermal degradation of oils produced during pyrolysis. The physical characteristics of the gasification system are detailed in the Supplementary Materials section.
2.4. Experimental Evaluation
To evaluate the gasification process, four experimental tests were conducted. The biomass was fed every 10 min to the combustion and gasification zones. The feeding rate is presented in Table 2. Combustion and gasification temperatures were measured during the experiments using thermocouples installed in the reactor, allowing for the determination of average temperatures for both combustion and gasification.

Table 2.
Experimental tests of the gasification process.
The composition of the syngas was determined using a SIGMA model 606 gas chromatograph with a TCD detector for online gas measurements, which directly measured the concentrations of hydrogen, oxygen, carbon monoxide, nitrogen, and methane. The concentration of carbon dioxide was determined by difference. Additionally, the calorific value of the syngas was calculated based on its composition, as described in [50]:
Each experimental configuration was repeated three times to ensure the reliability of the results.
2.5. Exergy Assessment
The exergy assessment is based on the following considerations:
- The system operates under stationary conditions.
- The reference state is 298 K and 100 kPa.
- Syngas is considered to be tar-free.
- Ashes from combustion have zero exergy.
- Biochar from gasification is assumed to have the same chemical exergy as carbon (404,589 kJ/kmol).
- Syngas and combustion gasses are considered ideal gasses.
The total exergy of a flow is the sum of its chemical exergy and its physical exergy [51]:
where the subscript i refers to the i-est flow in the system. The physical exergy is calculated as described in [52]:
where the subscripts 0 refers to the reference thermodynamic state. Moreover, the chemical exergy of syngas and combustion gasses is calculated as described in [53]:
where the subscript k refers to the chemical of the k-est component in the gas, y is the mole fraction of the component, R is the universal gas constant, and is the chemical exergy in the reference state. Table 3 shows the chemical exergy for the components of syngas and combustion gasses.

Table 3.
Chemical exergy of the compounds considered [54].
The chemical exergy of maize cob is calculated as described in [55]:
where is the chemical exergy of the biomass fuel and is a dimensionless factor that depends on the chemical composition of the biomass [56]:
where H is the share of hydrogen, C is the share of carbon, and O is the share of oxygen in the biomass. The exergy of the heat transfer between the combustion and gasification zones is calculated as described in [57]:
where is the exergy of heat, is the heat transfer temperature, represents the enthalpy of combustion gasses right after combustion, and represents the enthalpy of combustion gasses at the reactor exit.
The exergy destruction (χ) in the combustion and gasification zones is calculated from the exergy balance:
Exergy balance (combustion zone).
Exergy balance (gasification zone).
where and are the biomass mass flow in the combustion and gasification zones, the subscript com refers to combustion gasses, and the subscript gas refers to syngas. The combustion zone transforms biomass chemical exergy into combustion gas and heat exergies, while the gasification zone uses the heat transfer from the combustion zone for the endothermic reaction of gasification that transforms biomass into syngas. The exergy efficiency , based on the exergy balance, is calculated as the ratio of syngas exergy to the sum of biomass combustion and biomass gasification exergies [56].
In order to reduce the number of controlled variables in the experiments, the amount of biomass in gasification was adimentionalized, thus defining the proportion of biomass for combustion and gasification, which was calculated as follows:
3. Results
The experimental results and the exergy calculations are presented and discussed in this section.
3.1. Experimental Results
Figure 2 shows the relationship between the mass ratio and the average gasification temperature during the experiments.
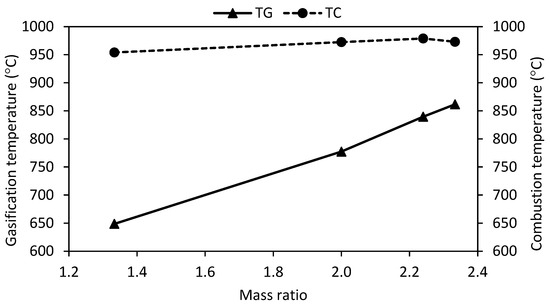
Figure 2.
Effect of mass ratio on gasification temperature.
The results show that increasing the mass ratio leads to higher gasification temperatures and a more complete combustion of the tars and biochar generated during pyrolysis. This trend indicates that the gasification process is dominated by the kinetic regime rather than heat transfer. This suggests that the heat from combustion is primarily used for biomass pyrolysis, while secondary thermal degradations are driven by the combustion of tars and biochar.
Figure 3 presents the syngas composition and the calculated lower calorific value for the different experimental tests.
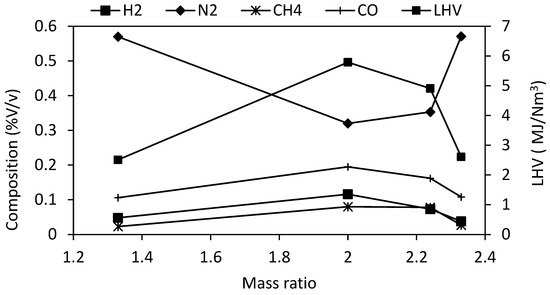
Figure 3.
Composition and lower heating value of syngas.
The results show that the highest LHV of syngas is obtained for a mass ratio of 2, because of the lower share of N2 and the higher shares of H2, CH4, and CO in syngas contrasted to lower or higher ratios.
Using a downdraft gasifier for the gasification of maize cob produced syngas with 3.5 MJ/Nm3 LHV [58], which is 65% lower than the experimental results in test 2. Moreover, using an inverted draft gasifier and maize cob yielded syngas with 5.6 MJ/Nm3 LHV [59]. Furthermore, using an inverted draft gasifier and maize cob yielded syngas with 5.32 MJ/Nm3 LHV [60]. These results are 3 to 9% lower than those obtained in experimental test 2. Overall, these results show the advantages of allothermal gasification compared to conventional reactors. Figure 4 shows the temperature profiles for experimental test 2.
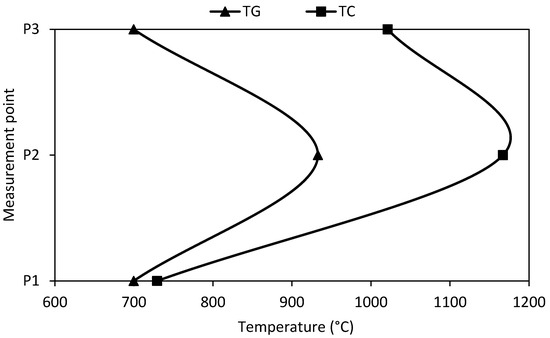
Figure 4.
Optimum temperature profile.
The results show that the highest heat transfer process occurs in the devolatilization zone (P2), where the temperature differences are the highest. Afterward, the process still demands significant amounts of heat for the reforming reactions.
3.2. Exergy Assessment Results
Figure 5 shows the variation of the exergy efficiency with the mass ratio.
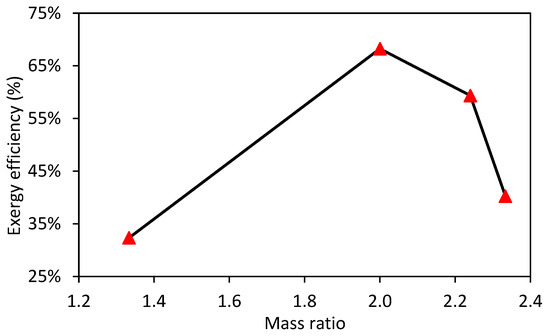
Figure 5.
Variation of exergy efficiency with the mass ratio.
The results show that the exergy efficiency varies from 33% to 68.2% in the experiments. Similarly, the highest efficiency is obtained for a mass ratio of 2 that coincides with the higher HHV of syngas. These results indicate that the optimum combustion mass ratio is around 2, which should be the object of further investigation.
For the gasification of pine wood chip waste in a fixed-bed reactor, exergy efficiency values of around 66% have been reported [61]. Similarly, Mountouris et al. [62] report exergy efficiencies between 65% and 70% for the gasification of municipal solid waste in a plasma torch, attributed to the higher temperatures in plasma gasification that promote reforming reactions, producing high-exergy products. Simulation studies with hybrid cross/updraft gasifiers report even higher exergy efficiency (81%) with municipal solid waste, which can be explained by the higher carbon conversion efficiency and significant tar reforming achieved in this type of reactor [63]. Figure 6 shows the relationship between exergy destruction and mass ratio during gasification.
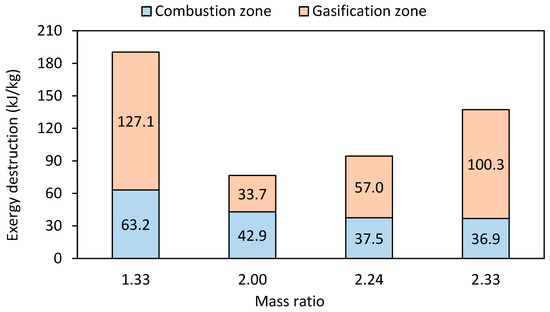
Figure 6.
Exergy destruction in the reactor by mass ratio in the gasification zone.
As expected, the results show the lowest exergy destruction in the reactor for a mass ratio of 2. For higher and lower mass ratios, the exergy destruction in the gasification zone increases. For mass ratios higher than 2, the reactor operates at higher temperatures, resulting in the higher thermal degradation of the fuel species. The result is a significant increase in exergy destruction in the gasification zone, which increases with mass ratio. On the other hand, for mass ratios below 2, lower gasification temperatures inhibit the oxidation and gasification of coal and the cracking of tars, which also results in higher exergy destruction rates in the gasification zone. In addition, the exergy destruction in the combustion zone decreases with higher ratio, and this is due to the higher heat demand in the gasification zone.
4. Discussion
Autothermal gasification traditionally uses air as the gasifying agent, serving two key functions. First, it supports the complete combustion of biomass to provide the necessary heat for the process. Second, it participates in the partial oxidation reactions of char, tar, and biomass, primarily generating carbon monoxide. For this purpose, equivalence ratios between 0.3 and 0.4 are commonly used, resulting in lower LHV values because air, which contains 79% nitrogen, dilutes the syngas and reduces its LHV. In contrast, the reactor used in this study reduces N2 by limiting the air used in gasification to 50% of that used in conventional gasifiers.
The results show that this type of reactor not only produces syngas with a higher LHV but also achieves similar efficiencies to conventional reactors. The presence of high-energy chemical species enhances process efficiency, demonstrating the technical feasibility of this reactor type.
The exergy analysis reveals a direct correlation between exergy efficiency and process irreversibilities. The highest exergy efficiency corresponds to the mass ratio that generates the least exergy destruction. This, in turn, aligns with the mass ratio that favors the formation of hydrogen and carbon monoxide, the most valuable species in the process.
Additionally, it was observed that the temperature in the two zones decreases rapidly in the upper part of the reactor, which could promote tar formation and limit homogeneous reforming reactions. Therefore, alternative approaches must be explored to maintain a consistent temperature throughout the reactor.
The fixed-bed concentric tube reactor produces gas with a higher calorific value than conventional reactors and demonstrates high energy efficiency. However, scaling up for industrial applications may present challenges, suggesting that this reactor’s potential lies in small-scale gasification.
5. Conclusions
An exergetic analysis was conducted to evaluate biomass gasification in a novel concentric tube allothermal reactor, where biomass is gasified in the inner tube, and the required heat is supplied through the annular zone. The results showed that the highest calorific value, 5.6 MJ/Nm3, was achieved at a biomass ratio of 2.0 between the gasification and combustion zones. This value is 30% higher than what is typically reported for maize cob gasification in inverted downdraft gasifiers, which can be attributed to the reduced dilution effect of nitrogen in the combustible species.
The optimal exergetic efficiency of the process was calculated at 68.2%, comparable to results from studies on conventional gasifiers. Therefore, it is concluded that although allothermal gasification leads to lower cold gas efficiencies than conventional reactors, the exergetic performance is similar in both cases, as allothermal gasification promotes the formation of high-value combustible species.
Further research is recommended to study exergy destruction along the reactor and the reforming of tars produced during the process. Additionally, it was found that exergy destruction in the combustion zone was significantly higher than in the gasification zone at the optimal mass ratio. Consequently, concentric tube allothermal reactors should be implemented for small-scale biomass gasification due to the high energy quality of the product gas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en18030606/s1, Figure S1: Gasification unit, Figure S2: Photograph of the gasification equipment, Table S1: Gasifier parameters.
Author Contributions
Conceptualization, J.D.R.-J. and J.M.M.; methodology, A.S.G. and J.D.R.-J.; validation, A.S.G. and J.D.R.-J.; formal analysis, A.S.G. and A.B.S.; investigation, J.D.R.-J., J.M.M., and A.F.J.; resources, J.M.M.; writing—original draft preparation J.D.R.-J. and A.F.J.; writing—review and editing, A.S.G., A.B.S. and J.M.M.; supervision, A.B.S.; project administration, J.M.M.; funding acquisition, J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technology, and Innovation of Colombia through the project BPIN 2021000100282.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. In addition, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors express their gratitude to the Ministry of Science, Technology and Innovation of Colombia for the funding provided within the framework of the project “IMPLEMENTATION OF THE RESIDUAL BIOMASS GASIFICATION PROCESS FOR ELECTRICITY GENERATION AND POTENTIAL REDUCTION OF EMISSIONS DERIVED FROM INADEQUATE WASTE DISPOSAL IN THE BATATA VILLAGE, TIERRALTA - CÓRDOBA” with code BPIN 2021000100282. We also thank the University of Cordoba for funding under the program to support and improve research group indicators and approve the internal call for grants for the year 2023” according to minute N°. FI-02-23 of 2024.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mehrpooya, M.; Khalili, M.; Sharifzadeh, M.M.M. Model Development and Energy and Exergy Analysis of the Biomass Gasification Process (Based on the Various Biomass Sources). Renew. Sustain. Energy Rev. 2018, 91, 869–887. [Google Scholar] [CrossRef]
- Karamarkovic, R.; Karamarkovic, V. Energy and Exergy Analysis of Biomass Gasification at Different Temperatures. Energy 2010, 35, 537–549. [Google Scholar] [CrossRef]
- Zhao, Z.; Andre Situmorang, Y.; An, P.; Yang, J.; Hao, X.; Rizkiana, J.; Abudula, A.; Guan, G. A Biomass-Based Small-Scale Power Generation System with Energy/Exergy Recuperation. Energy Convers. Manag. 2021, 227, 113623. [Google Scholar] [CrossRef]
- Pacioni, T.R.; Soares, D.; Di Domenico, M.; Rosa, M.F.; de Fátima Peralta Muniz Moreira, R.; José, H.J. Bio-Syngas Production from Agro-Industrial Biomass Residues by Steam Gasification. Waste Manag. 2016, 58, 221–229. [Google Scholar] [CrossRef]
- Sattar, A.; Leeke, G.A.; Hornung, A.; Wood, J. Steam Gasification of Rapeseed, Wood, Sewage Sludge and Miscanthus Biochars for the Production of a Hydrogen-Rich Syngas. Biomass Bioenergy 2014, 69, 276–286. [Google Scholar] [CrossRef]
- Sohni, S.; Norulaini, N.A.N.; Hashim, R.; Khan, S.B.; Fadhullah, W.; Mohd Omar, A.K. Physicochemical Characterization of Malaysian Crop and Agro-Industrial Biomass Residues as Renewable Energy Resources. Ind. Crops Prod. 2018, 111, 642–650. [Google Scholar] [CrossRef]
- Gómez-Vásquez, R.D.; Castiblanco, E.A.; Zapata Benabithe, Z.; Bula Silvera, A.J.; Camargo-Trillos, D.A. CaCO3 and Air/Steam Effect on the Gasification and Biohydrogen Performance of Corn Cob as Received: Application in the Colombian Caribbean Region. Biomass Bioenergy 2021, 153, 106207. [Google Scholar] [CrossRef]
- Arenas Castiblanco, E.; Montoya, J.H.; Rincón, G.V.; Zapata-Benabithe, Z.; Gómez-Vásquez, R.; Camargo-Trillos, D.A. A New Approach to Obtain Kinetic Parameters of Corn Cob Pyrolysis Catalyzed with CaO and CaCO3. Heliyon 2022, 8, e10195. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Rhenals-Julio, J.; Avila, A.; Durando, E. Análise Exergoeconômica Da Gasificação de Sabugo de Milho Integrado Em Um Sistema de Geração de Energia: Estudo de Caso Na Colômbia. Rev. Virtual De Quim. 2021, 13, 919–925. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of Non-Woody Biomass: A Literature Review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass Gasification for Sustainable Energy Production: A Review Int. J. Hydrog. Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Ayol, A.; Tezer Yurdakos, O.; Gurgen, A. Investigation of Municipal Sludge Gasification Potential: Gasification Characteristics of Dried Sludge in a Pilot-Scale Downdraft Fixed Bed Gasifier. Int. J. Hydrogen Energy 2019, 44, 17397–17410. [Google Scholar] [CrossRef]
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef]
- Varol, M.; Atimtay, A.T.; Olgun, H.; Atakül, H. Emission Characteristics of Co-Combustion of a Low Calorie and High Sulfur–Lignite Coal and Woodchips in a Circulating Fluidized Bed Combustor: Part 1. Effect of Excess Air Ratio. Fuel 2014, 117, 792–800. [Google Scholar] [CrossRef]
- Piekarczyk, W.; Czarnowska, L.; Ptasiński, K.; Stanek, W. Thermodynamic Evaluation of Biomass-to-Biofuels Production Systems. Energy 2013, 62, 95–104. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jeong, Y.-S.; Kim, J.-S. Bubbling Fluidized Bed Biomass Gasification Using a Two-Stage Process at 600 °C: A Way to Avoid Bed Agglomeration. Energy 2022, 250, 123882. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Zhang, Y.; Li, T.; Wei, X. Performance Investigation of the Gasification for the Kitchen Waste Powder in a Direct Current Plasma Reactor. J. Energy Inst. 2022, 100, 170–176. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Haridasan, N.; Muraleedharan, C.; Arun, P. Allothermal Air–Steam Gasification of Biomass with CO2 (Carbon Dioxide) Sorption: Performance Prediction Based on a Chemical Kinetic Model. Energy 2014, 69, 399–408. [Google Scholar] [CrossRef]
- Barco-Burgos, J.; Carles-Bruno, J.; Eicker, U.; Saldana-Robles, A.L.; Alcántar-Camarena, V. Hydrogen-Rich Syngas Production from Palm Kernel Shells (PKS) Biomass on a Downdraft Allothermal Gasifier Using Steam as a Gasifying Agent. Energy Convers. Manag. 2021, 245, 114592. [Google Scholar] [CrossRef]
- Umeki, K.; Yamamoto, K.; Namioka, T.; Yoshikawa, K. High Temperature Steam-Only Gasification of Woody Biomass. Appl. Energy 2010, 87, 791–798. [Google Scholar] [CrossRef]
- Byun, Y.; Cho, M.; Hwang, S.-M.; Chung, J.; Byun, Y.; Cho, M.; Hwang, S.-M.; Chung, J. Thermal Plasma Gasification of Municipal Solid Waste (MSW). Gasif. Pract. Appl. 2012, 1, 183–210. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The Removal of CO2 and N2 from Natural Gas: A Review of Conventional and Emerging Process Technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Mayerhofer, M.; Mitsakis, P.; Meng, X.; De Jong, W.; Spliethoff, H.; Gaderer, M. Influence of Pressure, Temperature and Steam on Tar and Gas in Allothermal Fluidized Bed Gasification. Fuel 2012, 99, 204–209. [Google Scholar] [CrossRef]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Göransson, K.; Söderlind, U.; Zhang, W. Experimental Test on a Novel Dual Fluidised Bed Biomass Gasifier for Synthetic Fuel Production. Fuel 2011, 90, 1340–1349. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Q.; Qi, F.; Xiao, B.; Liu, S.; Hu, Z.; He, P. Allothermal Gasification of Biomass Using Micron Size Biomass as External Heat Source. Bioresour. Technol. 2012, 107, 471–475. [Google Scholar] [CrossRef]
- Di Carlo, A.; Savuto, E.; Foscolo, P.U.; Papa, A.A.; Tacconi, A.; Del Zotto, L.; Aydin, B.; Bocci, E. Preliminary Results of Biomass Gasification Obtained at Pilot Scale with an Innovative 100 KWth Dual Bubbling Fluidized Bed Gasifier. Energies 2022, 15, 4369. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.J.; Hung, C.I.; Shen, C.H.; Hsu, H.W. A Comparison of Gasification Phenomena among Raw Biomass, Torrefied Biomass and Coal in an Entrained-Flow Reactor. Appl. Energy 2013, 112, 421–430. [Google Scholar] [CrossRef]
- Maitlo, G.; Unar, I.N.; Mahar, R.B.; Brohi, K.M. Numerical Simulation of Lignocellulosic Biomass Gasification in Concentric Tube Entrained Flow Gasifier through Computational Fluid Dynamics. Energy Explor. Exploit. 2019, 37, 1073–1097. [Google Scholar] [CrossRef]
- Sazali, S.N.; Al-attab, K.A.; Zainal, Z.A. Gasification Enhancement and Tar Reduction Using Air Fogging System in a Double Walled Downdraft Biomass Gasifier. Energy 2019, 186, 115901. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Song, Y.; Shan, Y.; Wang, C.; Wang, G. Biomass Steam Gasification for Hydrogen-Rich Gas Production in a Decoupled Dual Loop Gasification System. Fuel Process. Technol. 2017, 165, 54–61. [Google Scholar] [CrossRef]
- Jahromi, R.; Rezaei, M.; Hashem Samadi, S.; Jahromi, H. Biomass Gasification in a Downdraft Fixed-Bed Gasifier: Optimization of Operating Conditions. Chem. Eng. Sci. 2021, 231, 116249. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. From Coal to Biomass Gasification: Comparison of Thermodynamic Efficiency. Energy 2007, 32, 1248–1259. [Google Scholar] [CrossRef]
- Shahbeig, H.; Shafizadeh, A.; Rosen, M.A.; Sels, B.F. Exergy Sustainability Analysis of Biomass Gasification: A Critical Review. Biofuel Res. J. 2022, 9, 1592–1607. [Google Scholar] [CrossRef]
- Caglar, B.; Tavsanci, D.; Biyik, E. Multiparameter-Based Product, Energy and Exergy Optimizations for Biomass Gasification. Fuel 2021, 303, 121208. [Google Scholar] [CrossRef]
- Rashidi, H.; Khorshidi, J. Exergy Analysis and Multiobjective Optimization of a Biomass Gasification Based Multigeneration System. Int. J. Hydrogen Energy 2018, 43, 2631–2644. [Google Scholar] [CrossRef]
- Samimi, F.; Marzoughi, T.; Rahimpour, M.R. Energy and Exergy Analysis and Optimization of Biomass Gasification Process for Hydrogen Production (Based on Air, Steam and Air/Steam Gasifying Agents). Int. J. Hydrogen Energy 2020, 45, 33185–33197. [Google Scholar] [CrossRef]
- Manzini, F.; Islas-Samperio, J.M.; Grande-Acosta, G.K. Exploring Corn Cob Gasification as a Low-Carbon Technology in the Corn Flour Industry in Mexico. Energies 2024, 17, 2256. [Google Scholar] [CrossRef]
- Anukam, A.I.; Goso, B.P.; Okoh, O.O.; Mamphweli, S.N. Studies on Characterization of Corn Cob for Application in a Gasification Process for Energy Production. J. Chem. 2017, 2017, 6478389. [Google Scholar] [CrossRef]
- Saito, Y.; Sakuragi, K.; Shoji, T.; Otaka, M. Expedient Prediction of the Fuel Properties of Carbonized Woody Biomass Based on Hue Angle. Energies 2018, 11, 1191. [Google Scholar] [CrossRef]
- Antwi-Boasiako, C.; Acheampong, B.B. Strength Properties and Calorific Values of Sawdust-Briquettes as Wood-Residue Energy Generation Source from Tropical Hardwoods of Different Densities. Biomass Bioenergy 2016, 85, 144–152. [Google Scholar] [CrossRef]
- Djurdjevic, M.; Papuga, S. Torrefaction: Process Parameters and Reactor Design. Period. Polytech. Chem. Eng. 2023, 67, 416–426. [Google Scholar] [CrossRef]
- Bakisgan, C.; Dumanli, A.G.; Yürüm, Y. Trace Elements in Turkish Biomass Fuels: Ashes of Wheat Straw, Olive Bagasse and Hazelnut Shell. Fuel 2009, 88, 1842–1851. [Google Scholar] [CrossRef]
- ASTM ASTM D3173; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM ASTM D3175; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM ASTM D3174; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM ASTM D3172; Standard Test Method for Fixed Carbon in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2017.
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal Properties and Combustion-related Problems Prediction of Agricultural Crop Residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Motta, I.L.; Marchesan, A.N.; Maciel Filho, R.; Wolf Maciel, M.R. Correlating Biomass Properties, Gasification Performance, and Syngas Applications of Brazilian Feedstocks via Simulation and Multivariate Analysis. Ind. Crops Prod. 2022, 181, 114808. [Google Scholar] [CrossRef]
- Gomez, R.D.; Palacio, M.; Arango, J.F.; Avila, A.E.; Mendoza, J.M. Evaluation of the Energy Generation Potential by an Experimental Characterization of Residual Biomass Blends from Córdoba, Colombia in a Downdraft Gasifier. Waste Manag. 2021, 120, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, R.; Mojaver, P.; Azdast, T.; Khalilarya, S.; Chitsaz, A.; Rosen, M.A. Decision Analysis for Plastic Waste Gasification Considering Energy, Exergy, and Environmental Criteria Using TOPSIS and Grey Relational Analysis. Process Saf. Environ. Prot. 2023, 174, 414–423. [Google Scholar] [CrossRef]
- Mu, R.; Liu, M.; Yan, J. Advanced Exergy Analysis on Supercritical Water Gasification of Coal Compared with Conventional O2-H2O and Chemical Looping Coal Gasification. Fuel Process. Technol. 2023, 245, 107742. [Google Scholar] [CrossRef]
- Mojaver, M.; Azdast, T.; Hasanzadeh, R. Assessments of Key Features and Taguchi Analysis on Hydrogen Rich Syngas Production via Gasification of Polyethylene, Polypropylene, Polycarbonate and Polyethylene Terephthalate Wastes. Int. J. Hydrogen Energy 2021, 46, 29846–29857. [Google Scholar] [CrossRef]
- Bejan, A. Advanced Engineering Thermodynamics; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781119052098. [Google Scholar]
- Costa, V.A.F.; Tarelho, L.A.C.; Sobrinho, A. Mass, Energy and Exergy Analysis of a Biomass Boiler: A Portuguese Representative Case of the Pulp and Paper Industry. Appl. Therm. Eng. 2019, 152, 350–361. [Google Scholar] [CrossRef]
- Ayub, Y.; Zhou, J.; Ren, J.; Wang, Y.; Shen, W.; He, C.; Dong, L.; Toniolo, S. Plasma Gasification Based Monetization of Poultry Litter: System Optimization and Comprehensive 5E (Energy, Exergy, Emergy, Economic, and Environmental) Analysis. Energy Convers. Manag. 2023, 282, 116878. [Google Scholar] [CrossRef]
- Panopoulos, K.D.; Fryda, L.; Karl, J.; Poulou, S.; Kakaras, E. High Temperature Solid Oxide Fuel Cell Integrated with Novel Allothermal Biomass Gasification: Part II: Exergy Analysis. J. Power Sources 2006, 159, 586–594. [Google Scholar] [CrossRef]
- Arango, J.; Gonzalez, L. Evaluación Del Potencial Energético De La Gasificación En Lecho Fijo Para Cuatro Biomasas Residuales En El Departamento De Córdoba, Universidad Pontificia Bolivariana: Montería-Colombia, 2016.
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of Agricultural Residues in a Demonstrative Plant: Corn Cobs. Bioresour. Technol. 2015, 173, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Martillo Aseffe, J.A.; Martínez González, A.; Jaén, R.L.; Silva Lora, E.E. The Corn Cob Gasification-Based Renewable Energy Recovery in the Life Cycle Environmental Performance of Seed-Corn Supply Chain: An Ecuadorian Case Study. Renew. Energy 2021, 163, 1523–1535. [Google Scholar] [CrossRef]
- Martínez González, A.; Lesme Jaén, R.; Silva Lora, E.E. Thermodynamic Assessment of the Integrated Gasification-Power Plant Operating in the Sawmill Industry: An Energy and Exergy Analysis. Renew. Energy 2020, 147, 1151–1163. [Google Scholar] [CrossRef]
- Mountouris, A.; Voutsas, E.; Tassios, D. Solid Waste Plasma Gasification: Equilibrium Model Development and Exergy Analysis. Energy Convers. Manag. 2006, 47, 1723–1737. [Google Scholar] [CrossRef]
- Moshi, R.E.; Kivevele, T.T.; Jande, Y.A.C. The Exergy Analysis for the Air Gasification in a Hybrid Fixed Bed Gasifier. Curr. Appl. Sci. Technol. 2021, 18–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).