1. Introduction
In 2000, several nuclear power-promoting countries, including the United States, established the Generation IV International Forum (GIF) to develop fourth-generation nuclear energy technologies collaboratively. These technologies are characterized by superior safety and reliability, economic competitiveness, sustainability, and proliferation resistance. The six candidates proposed by GIF include sodium-cooled fast reactors (SFRs), lead-cooled fast reactors (LFRs), supercritical water-cooled reactors (SCWRs), very high-temperature reactors (VHTRs), molten salt reactors (MSRs), and gas-cooled fast reactors (GFRs) [
1].
The 20th National Congress of CPC report emphasized the active and steady pursuit of carbon peaking and carbon neutrality goals, the in-depth promotion of an energy revolution, and the safe and orderly development of nuclear power to ensure energy security. Fourth-generation nuclear power technologies represent a critical path for the large-scale, long-term sustainable development of nuclear energy and offer significant support for achieving China’s “dual carbon” goals and advancing a new power system. Therefore, the technologies for fourth-generation nuclear power are expected to accelerate development in China.
According to China’s nuclear energy sustainability goals and the three-step strategy, as well as predictions from the fourth-generation nuclear energy systems development roadmap, a lot of nuclear research institutes and universities in China have initiated research into the key technologies and conceptual designs of lead-cooled fast reactors [
2,
3,
4,
5,
6,
7,
8].
The lead-cooled fast reactor (LFR) is a fast neutron reactor that employs molten lead or lead–bismuth eutectic (44.5% lead and 55.5% bismuth, LBE) as the coolant. Both molten lead and LBE are highly promising coolants due to their advantageous properties, which have spurred active research of LFRs worldwide.
Based on extensive comparative analyses conducted in prior studies, the similarities and differences are analyzed comparatively in the present study between lead and LBE in terms of neutron physics, physical and thermal-hydraulic properties, chemical inertness, material corrosion, radioactivity, and the costs due to the resource. Then, the advantages and the challenges are also addressed accordingly. Through comprehensive assessment, this study aims to provide insights into the development of common and specific technologies for lead/LBE coolants and offers references for the coolant selection and conceptual design of future LFRs under varying scenarios.
2. Development History of Lead and LBE as Coolants
In the early development of nuclear power, E. Fermi in the United States and A. I. Leypunsky in the Soviet Union demonstrated the feasibility of using fast reactors for fuel breeding [
9,
10,
11]. In 1951, the Idaho National Laboratory built the first fast reactor, which used a sodium–potassium alloy as its coolant. By 1959, the Soviet Union, under the leadership of A. I. Leypunsky constructed the BR-5 and BOR-60 test reactors, both cooled by sodium. During this period, both the United States and the Soviet Union considered using lead or lead–bismuth alloys as fast reactor coolants. However, the United States ultimately shifted its focus to sodium-cooled fast reactors due to the high corrosion rates of the lead–bismuth alloy at elevated temperatures and its longer doubling time, and lower power density compared to sodium [
9,
12].
In contrast, the Soviet Union (later Russia) conducted extensive experiments to address the material compatibility issues of lead–bismuth reactors, overcoming key technical challenges to develop feasible designs. Between the 1950s and 1980s, Russia’s Institute for Physics and Power Engineering developed lead–bismuth reactors for naval propulsion, eventually constructing fifteen lead–bismuth reactors, including two submarine prototypes (each with two reactors), seven Alfa-class submarines, three land-based prototype reactors, and one backup reactor. These reactors accumulated approximately 80 reactor years of operational experience [
9,
13].
In recent years, growing concerns over nuclear safety have renewed the interest in lead and LBE coolants due to their potential to significantly reduce the likelihood of severe accidents. This has driven the increased research on and development of LFRs [
14]. In the 1990s, Russia resumed the development of civilian fast reactors cooled by lead and LBE. In 2002, the IAEA published a comparative study by Bogolovskaia G. P., analyzing the physical properties of sodium, lead, and lead–bismuth coolants. The report noted that while sodium offered superior neutron and heat transfer properties for fast reactor designs, lead and lead–bismuth coolants posed challenges such as corrosion and toxicity. Furthermore, LFRs with a two-loop design, requiring the secondary supercritical steam generator to be located inside the reactor vessel, needed further validation for operational reliability [
15]. In 2012, the IAEA released another technical report on using sodium, lead, and lead–bismuth as fast reactor coolants, acknowledging the strengths and weaknesses of each option. It noted that multiple countries were considering lead or LBE coolants for fast reactors and accelerator-driven subcritical systems [
10]. By 2023, some scholars assumed sodium-cooled and lead/lead–bismuth-cooled fast reactors as short-term and long-term fast reactor solutions, respectively [
16].
Regarding the choice between lead and lead–bismuth coolants for LFRs, different countries, and research institutes have adopted varying approaches. Russia has used LBEs for Alfa-class submarines, accelerator-driven systems, and some large-scale thermal-hydraulic test facilities at the early stage. Now, it has designed both the lead–bismuth-cooled SVBR series and the lead-cooled BREST series. The SVBR-100 design has been completed, while the BREST-OD-300 achieved its first concrete pour (FCD) in 2021 and is expected to begin operation by 2027, making it the most likely LFR to achieve commercial operation [
17]. Most European Union institutes and companies, as well as Westinghouse, have focused on lead as the coolant for commercial nuclear power, while in China, several institutes and universities have conducted conceptual designs and research on LBE-cooled fast reactors.
In 2005, the GIF established a temporary guidance committee for LFR research and design, adopting a dual-track strategy with molten lead as the primary coolant and LBE as the backup [
1]. The European Union has proposed the ELFR as its reference LFR design and the ALFRED demonstration reactor in Romania, along with the ATHENA research facility, all of which use lead as the coolant [
18]. In Sweden, LeadCold has designed the SEALER, choosing Oskarshamn as the potential location, which also uses lead [
19]. In Belgium, the SCK-CEN research center is developing the MYRRHA accelerator-driven subcritical system, which employs lead–bismuth and begins construction in June 2024 [
20].
In recent years, the Newcleo company in the United Kingdom and the European Union has secured significant investments to advance LFR research and deployment, with all types using lead as the coolant [
21]. In the United States, prominent LFR designs include the SSATR (using lead), G4M (using lead–bismuth), and the Westinghouse LFR (using lead) [
22]. Westinghouse is actively developing its LFR under the UK’s Advanced Modular Reactor (AMR) program, aiming for deployment between 2030 and 2035 [
23].
In China, the Chinese Academy of Sciences has proposed using the CLEAR-I lead–bismuth-cooled reactor as part of its “Future Advanced Nuclear Fission Energy-ADS Transmutation System” strategic project. The China General Nuclear Power Corporation has designed the CLFR series with LBE as the coolant. The China National Nuclear Corporation has conducted material testing and built a megawatt-scale-integrated lead–bismuth test facility. The State Power Investment Corporation has developed a preliminary LBE-cooled concept (BLESS-D) and lead-cooled design (SMILE) [
2,
3,
24,
25,
26,
27].
3. Comparison of the Properties of Lead and LBE
To explore the reasons why different countries and institutes have not reached a consensus on the choice of coolants for LFR, this section will compare and analyze lead and LBE in terms of neutron physics, fundamental physical properties, thermal-hydraulic characteristics, chemical inertness, material corrosion, radioactivity, and the resource which influences the costs. The advantages of different coolant choices and the challenges that need to be addressed will also be discussed.
3.1. Neutron Physics Properties
Neutron physics properties are decisive factors affecting the physical design of reactors, including neutron scattering and absorption cross-sections. Both the neutron scattering and absorption cross-sections of lead and lead–bismuth is relatively small. Accordingly, the following formula can be used to determine the average logarithmic energy loss in elastic scattering:
It can be observed that due to the high atomic numbers of lead (Pb) and bismuth (Bi), neutrons colliding with these heavy nuclei are easily scattered, resulting in minimal loss of energy. Both lead and LBE have relatively small neutron capture cross-sections, as shown in
Figure 1. However, the neutron capture cross-section of bismuth is slightly larger than that of lead.
Overall, the neutron physics properties of lead and LBE are similar. Their relatively low neutron moderation capabilities result in a harder neutron spectrum for LFRs, which ensures a sufficient number of fast neutrons to sustain the fission reaction within the reactor core. During the operation, the reactivity changes are minimal, and the distribution of fuel composition and power density within the core remains stable, promoting reactor safety and long-term operational stability. Additionally, LFRs have the ability to breed fissile isotopes like
239Pu and transmute long-lived fission products and actinides with relatively high transmutation efficiency [
28]. In previous research and development, LFRs were generally not used for plutonium production or extraction [
11], with most reactors designed to have a breeding ratio of around one [
29]. Coupled with a closed fuel cycle, including a subcritical accelerator-driven system, LFRs can also help to dispose of the spent fuel, reducing the difficulty of its management [
1].
3.2. Basic Physical Properties
3.2.1. Common Characteristics
The OECD has organized a compilation of studies on the basic physical properties of lead and LBE, including boiling points, densities, specific temperatures, and other characteristics, with the recommended values shown in
Table 1 [
30]. The SCK-CEN Research Center in Belgium has also compiled a database of the basic physical properties of liquid metals as coolants for fourth-generation reactors [
31]. While some values may differ slightly, the general trends remain consistent. The common characteristics of lead and LBE include the following:
- (1)
High Boiling Point:
The boiling points of lead and LBE are 1745 °C and 1670 °C, respectively, which means a difference of over 1000 °C from the melting points for both of them. This large gap between melting and boiling points provides a significant safety margin for the reactor core, keeping it well away from the boiling crisis. Additionally, a primary loop can be designed for operation at atmospheric pressure, thereby eliminating potential issues such as control rods unexpectedly ejected due to high pressure, the loss of coolant pressure, or overheating of the coolant, all of which could worsen heat transfer and increase the risk of positive void reactivity or primary loop overpressure. This greatly enhances the inherent safety of the system.
- (2)
High Density:
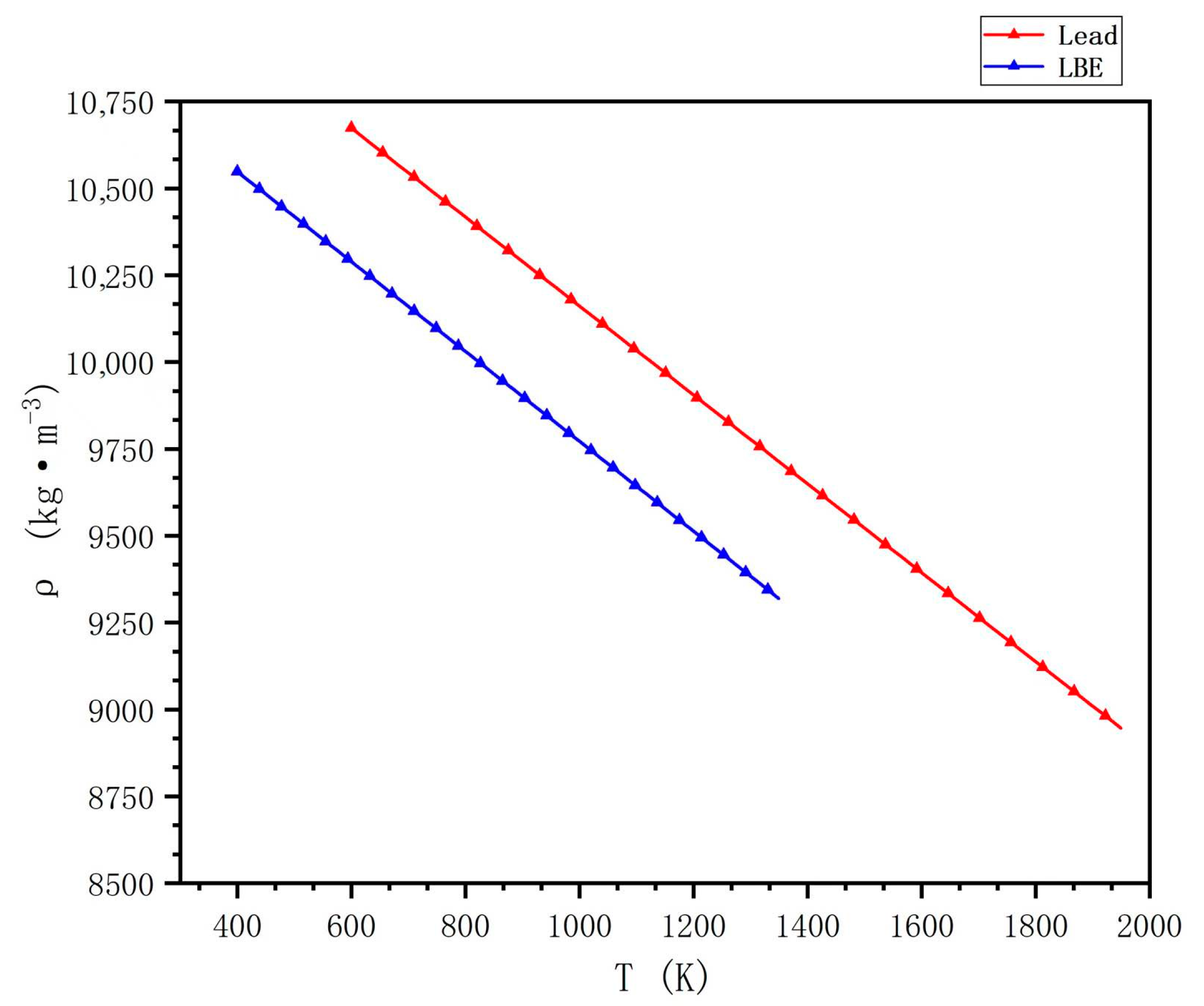
Lead and LBE are typical heavy metals with high densities, both exceeding 10,000 kg/m
3. At 450 °C, the density of liquid lead is approximately 3.5% greater than that of lead–bismuth, as shown in
Figure 2. Therefore, lead and LBE-cooled fast reactors are less suitable for scenarios where the overall weight of the reactor is a critical factor, such as in space applications. At the same time, the high density of the coolant introduces several considerations that need to be taken into consideration. On the one hand, the reactor design must account for reducing flow velocity to avoid high-energy consumption in the coolant circulation. On the other hand, the influence of buoyancy needs to be considered during the placement of reactor components. Structural integrity and seismic resistance also require special attention. However, high density has advantages as well; in the event of a severe accident, such as fuel rod failure or disintegration, molten fuel will not accumulate at the bottom of the reactor vessel, thus preventing issues like the pressure vessel meltdown.
In the temperature range of 601–1900 K, the density of lead largely follows the equation provided in [
31].
The density of lead–bismuth can be derived based on Wiegand’s law by combining the molar mass and density of lead and bismuth. Some scholars also give the experimental data and the fitted formula using the least squares method [
32,
33,
34,
35]. For instance, in the temperature range of 601–1300 K, the density of lead–bismuth conforms to the following formula given by Vitaly [
31].
- (3)
High Thermal Conductivity:
As liquid metals, both lead and LBE possess high thermal conductivity. The thermal conductivity of lead–bismuth can be derived based on Wiedemann–Franz–Lorenz’s law or the fitting of experimental data [
32,
34,
36]. In the temperature range of 601–1300 K, the thermal conductivity of lead largely follows the equation provided in [
31].
In the temperature range of 403–1100 K, the thermal conductivity of lead–bismuth conforms to the following formula.
As shown in
Figure 3, it can be observed that within the reactor’s operating temperature range, the thermal conductivity of both lead and LBE increases with temperature. Additionally, lead has higher thermal conductivity than LBE.
Furthermore, the thermal conductivity
also contributes to the thermal diffusivity α using the following formula [
1]:
where
is the density, and
is the specific heat.
The thermal diffusivity characterizes the heat propagation ability. Both lead and LBE have relatively large thermal diffusivity, and excellent heat transfer properties allow for the effective conduction of heat generated within the reactor core. This characteristic is particularly important for the design of high-power density fast reactor cores, as it contributes to the miniaturization of the core, system, and equipment.
- (4)
Low Kinematic Viscosity:
Although the dynamic viscosity of lead and LBE is not small, their high-density results in a low kinematic viscosity, meaning that the internal friction is relatively low and the flowability is better [
25]. This good flowability helps to maintain uniform heat flow distribution in the coolant circulation system.
In the temperature range of 601–1470 K, the dynamic viscosity of lead largely follows the equation provided in [
31].
In the temperature range of 545–1300 K, the dynamic viscosity of lead–bismuth conforms to the following formula.
As shown in
Figure 4, as the temperature rises, the kinetic energy of metal molecules increases, promoting molecular thermal motion and increasing liquid dynamics while reducing dynamic viscosity.
3.2.2. Differences
From
Table 1, it can also be observed that there are several notable differences between lead and LBE:
- (1)
Different Melting Points:
The melting point of lead (327 °C) is significantly higher than that of LBE (125 °C). It should be noted that the eutectic composition of the LBE compared in this article is fixed at 45.5 wt% Pb + 55.5 wt% Bi, which shows the lowest melting point in the phase diagram of the Pb-Bi system [
37]. Therefore, choosing lead as a coolant presents a higher risk of solidification, and special technical considerations and measures need to be taken for the reactor startup, operation, shutdown, fuel replacement, and maintenance. Overall, this makes the design and operation more complex. Additionally, the core inlet and outlet temperatures of a lead-cooled reactor are generally higher than those of an LBE-cooled reactor. This requires higher steam parameters for the secondary loop or the use of advanced energy conversion systems such as supercritical carbon dioxide Brayton cycles. Although these systems can improve power generation efficiency, they may also increase power consumption and facility costs. On the other hand, the higher melting point of lead allows for better sealing in the event of small leaks or cracks, reducing the risk of coolant loss and enhancing inherent safety.
- (2)
Different Volume Changes due to Melting:
The volume change in lead due to melting (+3.6%) is much larger than that of LBE (~+0.5%). Research on military lead–bismuth reactors in Russia has shown that it is difficult to avoid the issue of the unexpected “freezing” of the coolant. During the re-melting process, lead expands while bismuth contracts. This results in a smaller volume change during the solid–liquid phase transition of LBE, which generates less destructive stress on the reactor structure. In contrast, for lead, in addition to the volume increase, the formation of shrinkage cavities during solidification needs to be addressed. This could cause the volume increase during melting to be misaligned with the volume decrease during solidification, which leads to significant destructive stress on the contacting structure. However, solid LBEs may experience spontaneous volume expansion due to phenomena like lattice recrystallization. Experiments have shown that the volume of solid LBEs increases by about 0.5% over two months [
1].
3.3. Thermal-Hydraulic Properties
The density, dynamic viscosity, thermal conductivity, and specific heat capacity affect the velocity distribution and heat transfer within the fluid, thereby influencing the flow and heat transfer in the reactor. Based on the basic physical properties listed in
Table 1, the following thermal-hydraulic characteristics of lead and LBE can be inferred.
- (1)
High Heat Capacity and Thermal Storage Ability:
As mentioned earlier, lead and LBE have high densities and boiling points, which significantly enhance the heat capacity and thermal storage ability per unit volume in the reactor. This enables the reactor to provide sufficient response times during accident conditions, reducing the risk of melting and maintaining the integrity of the reactor vessel [
24].
- (2)
High Heat Transfer Ability:
It is important to note that the heat transfer mechanism of lead and LBE differs greatly from that of typical fluids such as water. Lead and LBE have a low Prandtl number (Pr), about 1% of water’s number, or even lower. A low Pr number means that the energy exchange dominated by molecular thermal conductivity is much greater than the momentum exchange dominated by molecular viscosity. In other words, heat transfer relies much more on molecular thermal conduction from the heated surface, and the heat transfer mechanism is significantly different from that of fluids like air and water. Especially under turbulent flow conditions, the molecular thermal conduction of lead and LBE is of the same order as turbulent heat conduction. This leads to temperature and velocity field separation, requiring the development of specific turbulent heat transfer relationships. The turbulent Prandtl number model for the numerical simulation of liquid metal flow and heat transfer is proposed based on experimental induction and theoretical derivation [
38], including the Cheng Tak model [
39], Reynolds model [
40], Aoki model [
41], Jischa model [
42], etc.
- (3)
Strong Natural Circulation and Flow Capability:
Lead and LBE have low kinematic viscosities, resulting in stronger flowability. In particular, the Grashof number (
Gr), which represents the ratio of buoyancy to viscous forces in a fluid, is a typical indicator of a fluid’s natural circulation capability. The Gr number is inversely proportional to the square of kinematic viscosity. As a result, the Gr numbers for lead and LBE are higher, and under the same conditions, their natural circulation capabilities are greater than that of sodium [
13].
Overall, lead and LBE have favorable thermal-hydraulic properties, with minimal differences between them. However, compared to sodium, lead and LBEs have slightly lower heat removal capabilities. Therefore, under the same design conditions, the LFR design typically incorporates larger fuel rod spacings (i.e., a larger P/D ratio) to reduce pressure drops and minimize coolant flow velocity and peak fuel cladding temperatures, which increase safety margins.
3.4. Chemical Inertness
Both lead and LBE have relatively stable chemical properties and do not react to air or water. However, in a moist air environment, they can form an oxidation layer on the surface. Additionally, the core and vessel materials do not undergo chemical reactions with coolants such as lead or LBE due to irradiation or heating, which would release hydrogen or other explosive gasses. Even in the case of small leaks or accidents, such as the rupture of steam generator heat transfer tubes, there is no risk of fire or chemical explosions if air or another coolant in the secondary loop (such as water or CO2) enters the primary loop. This allows for the use of a two-loop design without the need for a separate isolation loop, as required in sodium-cooled fast reactors.
3.5. Material Corrosion
Lead and LBE are corrosive to the reactor structural materials. The rate of corrosion increases with temperature, and when temperatures exceed 500–600 °C, the accelerated corrosion of materials occurs. This is one of the key engineering challenges affecting the deployment of LFRs. The corrosion primarily stems from the dissolution, oxidation, and erosion of metal components of the structural materials in the liquid lead or LBE. Elements from the structural materials dissolve into the liquid lead or LBE, causing the material to gradually lose its composition, become thinner, and reduce in mechanical strength, eventually causing failure or breakage. When lead or LBE is flowing at high speeds, it causes further erosion of the structural materials. The primary factors influencing the corrosion rate include the composition of the structural material, the temperature, exposure time, coolant flow rate, oxygen content, and so on.
The saturation concentration (
Cs, wt%) of different elements in the molten lead and LBE follows the Arrhenius equation [
15]:
where A and B are constants determined experimentally.
The solubility or corrosion resistance of different elements in lead and LBE is shown in
Table 2 and
Table 3.
It can be observed that, under the same conditions, the corrosion of LBE is more severe than that of lead. Taking iron as an example, the solubility of iron at 700 °C in pure lead is similar to that in LBE at 572 °C under anoxic conditions [
1]. This is the primary reason why the core outlet temperature of reactors using lead as a coolant can be higher than that of LBE-cooled reactors, and it may also be a key factor behind the choice of lead as a coolant by many international institutes.
At present, adding oxygen is the main technology adopted to deal with the corrosion problem, which helps to form a protective oxide film on the surface of the material, isolate the material and coolant, and play a role in corrosion prevention. The solubility and diffusion ability of oxygen in the coolant system determines the effectiveness and implementation difficulties of the oxygen control measures [
43]. As shown in
Table 4, oxygen solubility in LBE is slightly higher under the same temperature. For the diffusion coefficient, Gromov gives the following experimental data fitted formula [
44]:
where
R is the ideal gas constant.
Under the typical operation temperature of the LBE-cooled reactor, which may be lower than the lead-cooled reactor [
9,
17], the oxygen solubility is lower, but the diffusion coefficient is higher. However, taking the whole system into consideration, substantial differences are not expected.
The anti-corrosion technology of LFR materials is a key technology that needs continuous breakthroughs, including studies on the performance of main materials, surface treatment engineering, coolant thermodynamics, and the chemical service environment. In fact, some new material has also been developed and tested. For example, the elements that are prone to form dense and stable oxides in high-temperature liquid lead/LBE environments are selected as the basis for new material composition. Then, new alloys that can reduce material dissolution corrosion in a wider range of oxygen concentrations are developed through a series of performance selections and evaluation tests in SPICRI [
45,
46].
Currently, a maintenance temperature of around 500 °C can be supported with the long-term reliable service of LFR materials [
47]. However, there is still a large margin of about 1200 °C for the boiling points of lead and LBE, and hence, room for technological optimization and improvement.
3.6. Radioactivity and Containment
LBE-cooled fast reactors face the issue of the production of
210Po (Polonium-210). This is because the natural isotope of bismuth,
209Bi, undergoes the following neutron capture reaction, resulting in the formation of
210Po [
15].
210Po is an alpha-emitting radioactive isotope with a half-life of 138 days, and it is one of the most toxic elements known to mankind. It primarily causes harm to the human body through alpha radiation. 210Po is volatile at the operating temperatures of LFRs, and a portion of it may migrate into the cover gas, forming radioactive aerosols. If the cover gas or coolant leaks, it could lead to contamination. If inhaled, 210Po poses significant radiological risks. Therefore, a monitoring and treatment system for 210Po leakage must be implemented in the operation and maintenance of LFRs.
Lead, as a coolant, also presents the issue of
210Po contamination. But in this case,
210Po is produced in a two-step process: first,
208Pb captures a neutron to form
209Bi, and then
209Bi captures a neutron to form
210Po [
15]. As a result, the production rate of
210Po is much lower—up to 1000 times or lower than that of LBE. However, the migration rate of
210Po is still affected by the reactor operating temperature, the cover gas solvent, and temperature conditions. For example, the higher the operating temperature, the higher the migration rate. An IAEA report notes that the equilibrium activity of Po in LBE-cooled reactors is approximately 10 Ci/kg, while the activity in pure lead-cooled reactors is expected to be 5 × 10
−4 Ci/kg [
10].
At the same time, both lead and LBE are effective gamma-ray shielding materials and can adsorb and suppress fission products, especially some volatile fission products. Typically, iodine, cesium, and actinides are the primary contributors to radiological risks following an accident. At 600 °C, lead can form compounds with iodine and cesium, and upon solidification, it can encapsulate these radioactive products, thereby reducing the release of radioactive materials and minimizing their migration. This physical process can eliminate the risk of large-scale radioactive releases [
1].
3.7. Resource Costs
The resource availability, yielding, and purity of the coolant directly affect the cost, which, in turn, influences the overall cost and economic viability of a nuclear power plant. Lead is relatively abundant in the Earth’s crust, with a content of approximately 4 × 10
−3 [
15], making it a low-cost material. In contrast, bismuth is a scarce resource, with an Earth crust concentration of only 2 × 10
−5 [
15]. Bismuth ore deposits are rare and extracting bismuth from bismuth-containing ore is a complex, multi-stage process. Most bismuth is obtained through the reprocessing of polymetallic copper ores and the refining stages of lead production. Currently, bismuth costs about 10 times more than lead, making LBE more expensive.
An article published by Toshinsky GI in 2020 noted that LBEs are not yet suitable for large-scale nuclear power applications, according to proven resources. However, it also mentioned that Russia had conducted a technical and economic survey of bismuth resources and identified some potential reserves [
26]. Nevertheless, when considering large-scale applications, cost-effectiveness must be comprehensively evaluated, and LBEs do not offer a clear advantage. Additionally, the composition and quality control of both lead and LBEs may impact their long-term usability and performance, especially in reactor environments that require specific material properties.
4. Discussion and Summary
The properties of the coolant directly influence the overall design of the reactor, including parameters such as the material selection, fuel rod, fuel assembly configuration design, and so on. These factors subsequently affect the reactor’s safety, reliability, economy, sustainability, and proliferation resistance. Therefore, understanding the characteristics of lead and LBE is crucial for optimizing the design of LFRs to better meet the requirements of fourth-generation nuclear technology. Based on the comparisons made, both lead and lead–bismuth eutectics (LBEs) have their own unique advantages and disadvantages as coolants for fast reactors.
Overall, both lead and LBE exhibit excellent neutron physics and thermal-hydraulic properties, along with chemical inertness, which contributes to the high nuclear fuel utilization, inherent safety, and simplified design of LFRs. However, they also present significant challenges, such as severe material corrosion under high-temperature flow conditions and the production of the highly toxic α-emitting radioactive isotope 210Po, which must be addressed for further engineering development.
Lead shows several notable advantages. It is relatively abundant in the Earth’s crust, which leads to lower costs, making it economically favorable for large-scale nuclear energy applications. At the same temperature, lead exhibits less corrosiveness to materials compared to LBE. This property helps to prolong the service life of the reactor’s structural materials and reduces the maintenance cost. Under the same irradiation conditions, lead produces a much lower amount of the toxic radioactive isotope than LBE. This significantly decreases the risk of radioactive pollution, which is beneficial for the safe operation of the reactor and its environmental friendliness. Moreover, the relatively high melting point of lead can enhance the sealing performance of the reactor in certain situations, reducing the risk of coolant leakage and improving inherent safety.
However, lead also has some drawbacks. Its high melting point necessitates special technical measures during reactor startup, operation, shutdown, fuel replacement, and maintenance to prevent solidification, thereby increasing the complexity of the design and operation. Simultaneously, the higher melting point results in the generally higher inlet and outlet temperatures of the lead-cooled reactor core. This demands higher steam parameters for the secondary loop or the adoption of advanced energy conversion systems such as the supercritical carbon dioxide Brayton cycle. Although these can improve power generation efficiency, they may also increase power consumption and facility costs.
LBE has its own merits. The lower melting point of LBE allows for a wider operating temperature range when the core outlet temperature is low. This effectively avoids the problem of accelerated material corrosion at high temperatures (i.e., higher than 550 °C), reduces the insulation requirements, and makes the operation and maintenance more convenient and flexible. Under current conditions, LBE is more conducive to achieving engineering and miniaturization, making it suitable for experimental facilities and small reactors with special applications.
Nevertheless, LBE also faces many challenges. It is more corrosive to the reactor’s structural materials, especially when the temperature exceeds 550 °C and the corrosion rate increases significantly. This imposes higher requirements on material selection and the reactor’s structural design. LBE-cooled fast reactors produce toxic elements during operation. 210Po is an alpha-emitting radioactive isotope with a half-life of 138 days and is highly toxic. It is volatile and can form radioactive aerosols. In the case of leakage, it may cause serious pollution. Therefore, a dedicated monitoring and treatment system is required during the operation and maintenance of LBE-cooled reactors. In addition, bismuth is a scarce resource with a crustal content of only 2 × 10−5, and its extraction process is complex, resulting in a higher cost of LBE, which is about 10 times that of lead. Thus, LBE does not have an obvious advantage in cost-effectiveness within large-scale applications.
It should be emphasized that there is no clear boundary of lead and bismuth content in LBEs. As mentioned before, in this paper, LBE refers to the alloy with 45.5 wt% Pb + 55.5 wt% Bi, which shows the lowest melting point. However, it is not certain that alloys with such a composition ratio have the best comprehensive performance. It is found that Bismuth concentration severely affects the liquid metal embrittlement (LME) of a Fe-10Cr-4Al ferritic steel [
48,
49]. And if the bismuth content is as low as 10%, the melting point of the alloy can be lowered from 327 °C of lead to 250 °C [
43]. As a result, the proportion of bismuth in the alloy is worth studying in order to achieve optimal comprehensive performance or compatibility with current technology and materials. Such research is particularly suitable for using artificial intelligence methods, i.e., big data or big models, in which other components can also be considered. Bruno used Machine Learning-Assisted Canonical Sampling (MLACS) to calculate the thermodynamic quantities of molten lead, bismuth, and their eutectic from melting to boiling temperatures [
50].
In conclusion, the characteristics of lead and LBE determine their applicability in different application scenarios. When choosing a coolant, and more precisely, choosing the proportion of bismuth, multiple factors such as specific reactor design requirements, economic considerations, safety standards, material options, and resource conditions need to be weighed to optimize the design of lead-cooled fast reactors.