Theoretical Investigation of C4F7N–CO2 Mixture Decomposition Characteristics Under Extreme Conditions
Abstract
1. Introduction
2. Calculation Models and Methods
3. Results and Discussion
3.1. Pyrolysis
3.2. Electrical Discharge
4. Conclusions
- (1)
- The primary pyrolysis products of C4F7N at moderate temperatures include CFN, CF3, and C2F2, while C3F5, C4F6N, C2F, and CN are also formed in very low amounts. As the temperature increases, additional products such as C2, C2F3, C3F4, C4F7, and C3F6 appear. At very high temperatures (1800 °C), the newly added products include CF, CO, C3F7, C3F2, C3F, C3F3, C3F3N, C3, CF2, and CF2N. Across different temperatures, CF3, C2F2, C4F6N, and CFN are consistently observed, with their concentrations increasing with rising temperature.
- (2)
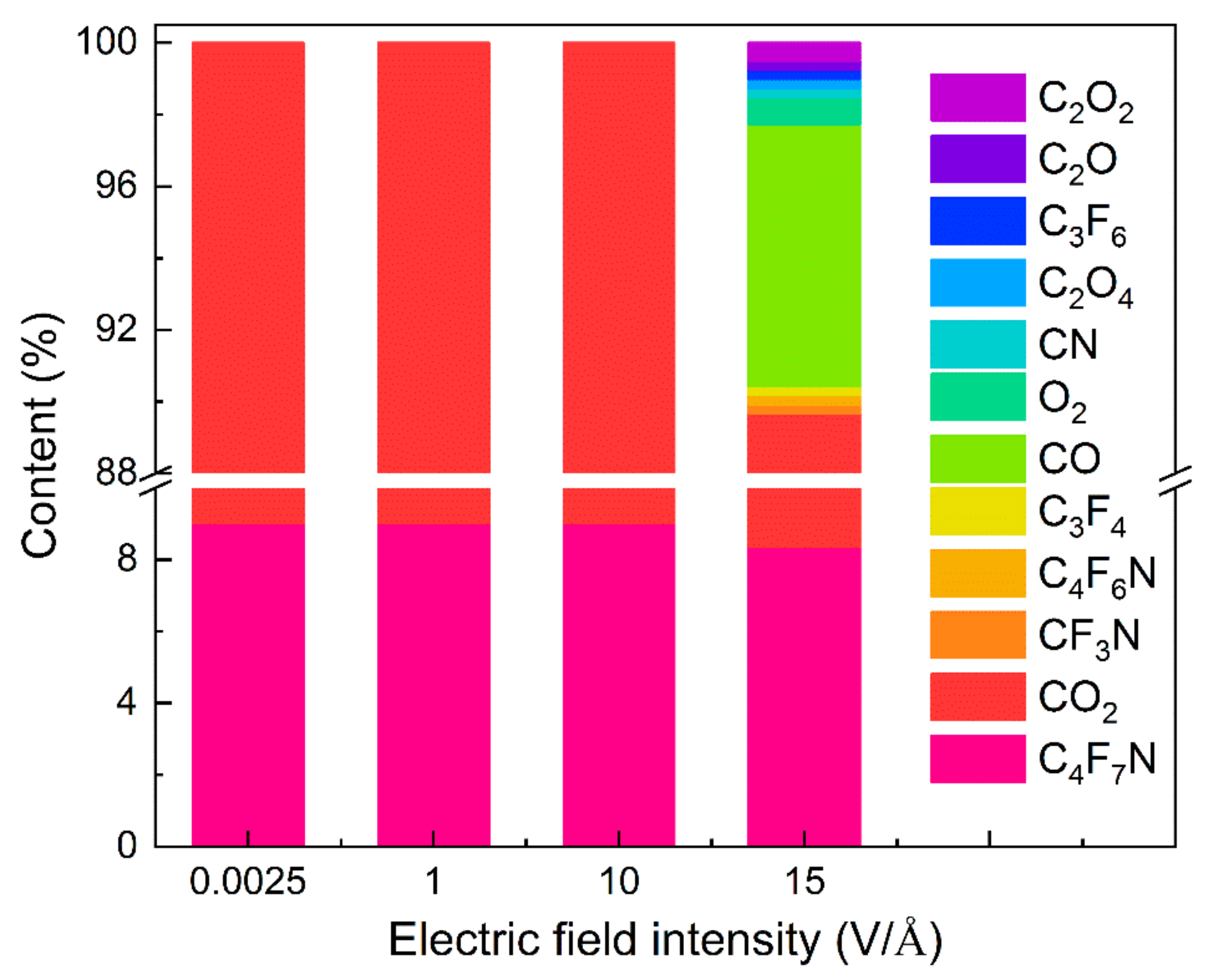
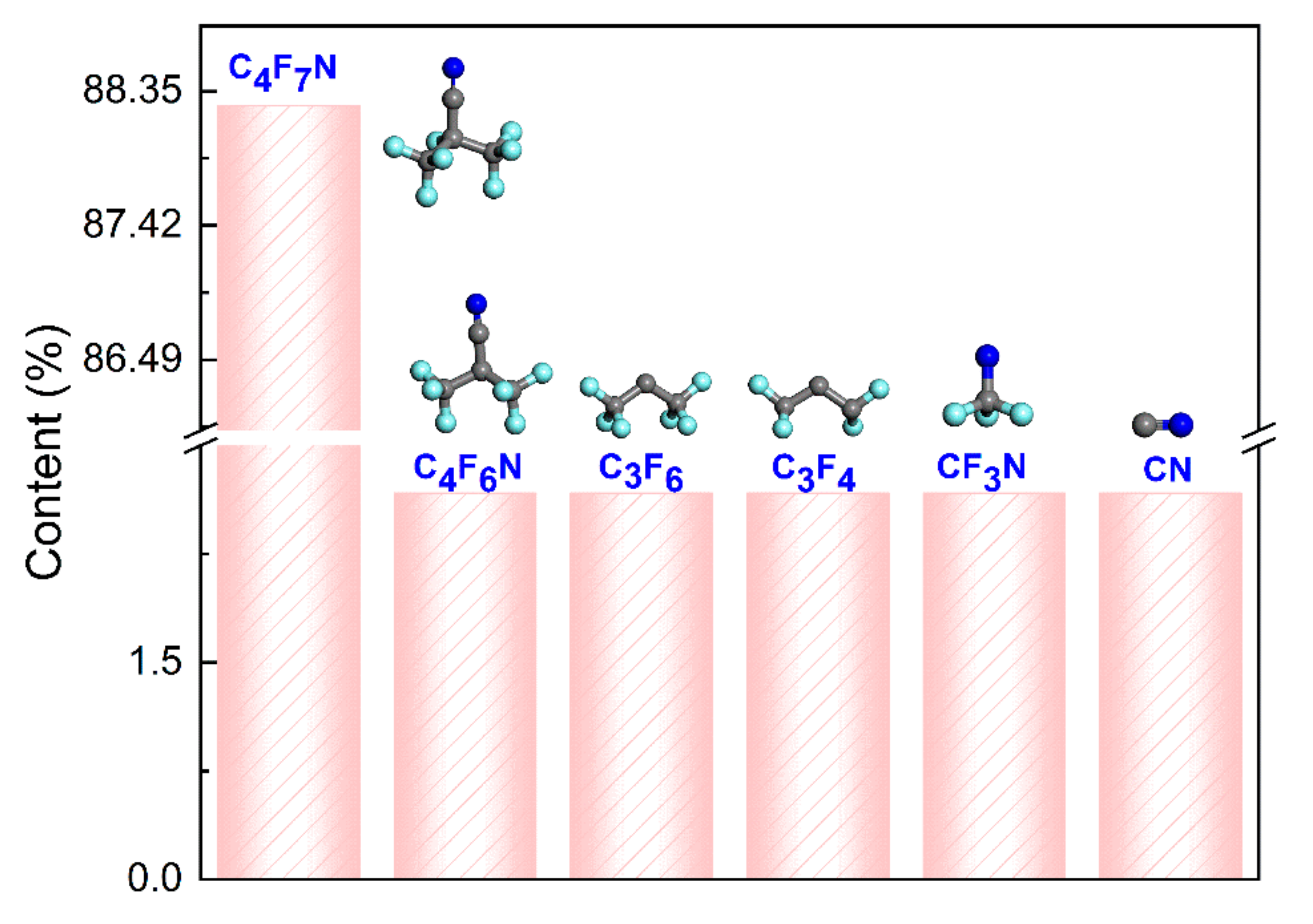
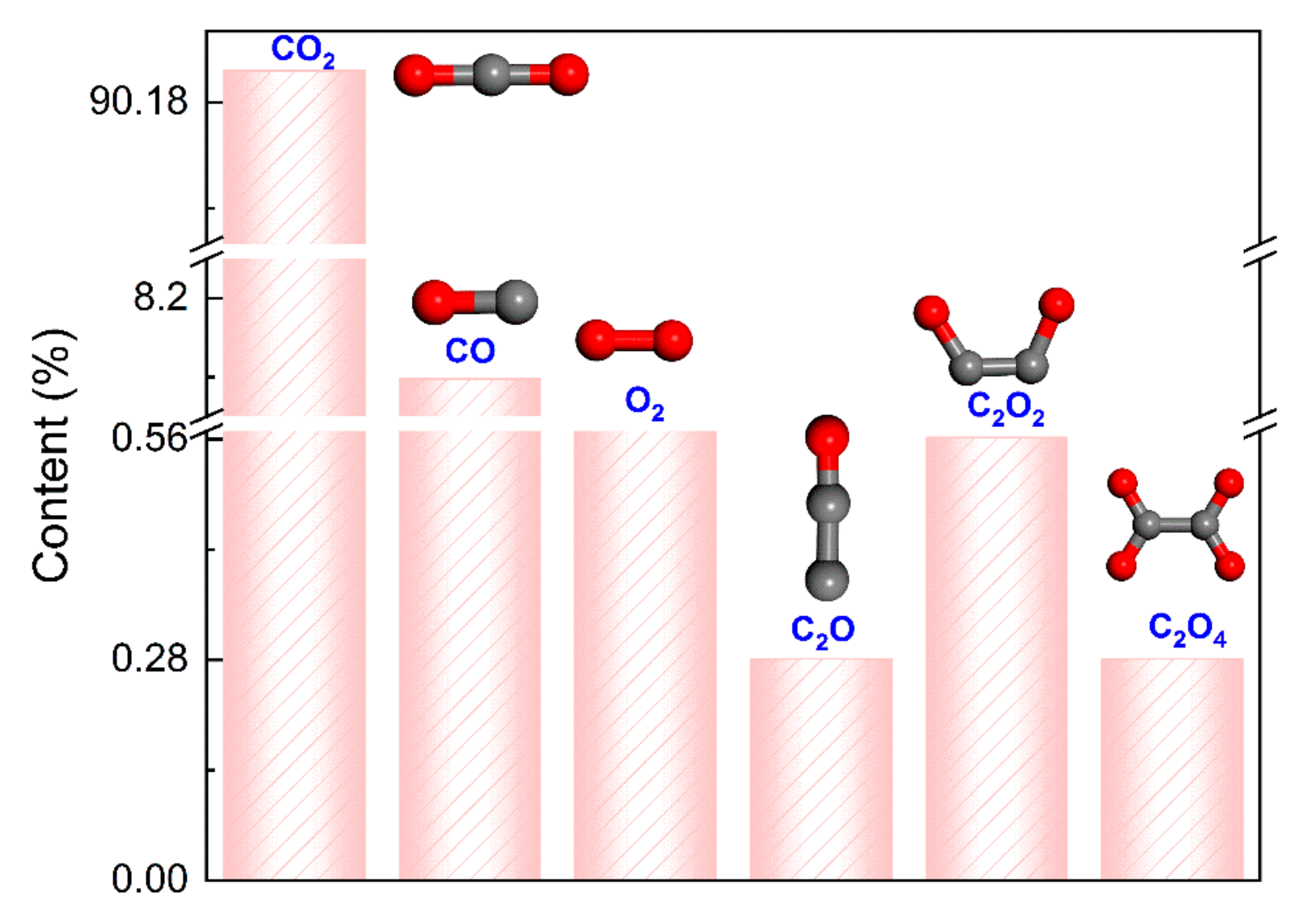
- The decomposition products of electrical discharge include C4F6N, C3F6, C3F4, CF3N, and CN. Among them, C3F6, C3F4, and CF3N were not observed in the pyrolysis, while the decomposition of CO2 is significant under electrical discharge, and the decomposition products include CO, C2O, O2, C2O2, and C2O4. Additionally, increasing the electric field intensity initially increases the number of decomposition components, peaking at 25, primarily from the decomposition of the C4F7N molecule. However, at ultra-high electric field intensities, the number of decomposition products decreases, dominated by small molecules such as O2, C2, C3, and N2.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, M.; Xu, Z.; Zhu, H.; Hwang Goh, H.; Agustiono Kurniawan, T.; Liu, T.; Zhang, D. Integrated demand response modeling and optimization technologies supporting energy internet. Renew. Sustain. Energy Rev. 2024, 203, 114757. [Google Scholar] [CrossRef]
- Faizol, Z.; Zubir, F.; Saman, N.M.; Ahmad, M.H.; Rahim, M.K.A.; Ayop, O.; Jusoh, M.; Majid, H.A.; Yusoff, Z. Detection Method of Partial Discharge on Transformer and Gas-Insulated Switchgear: A Review. Appl. Sci. 2023, 13, 9605. [Google Scholar] [CrossRef]
- Mazzanti, G.; Stomeo, G.; Mancini, S. State of the art in insulation of gas insulated substations: Main issues, achievements, and trends. IEEE Electr. Insul. Mag. 2016, 32, 18–31. [Google Scholar] [CrossRef]
- Chen, G.; Tu, Y.; Wang, C.; Wang, J.; Yuan, Z.; Ma, G.; Wang, J.; Qi, B.; Li, C. Environment-friendly insulating gases for HVDC gas-insulated transmission lines. CSEE J. Power Energy Syst. 2021, 7, 510–529. [Google Scholar]
- Chachereau, A.; Hösl, A.; Franck, C.M. Electrical insulation properties of the perfluoronitrile C4F7N. J. Phys. D Appl. Phys. 2018, 51, 495201. [Google Scholar] [CrossRef]
- Ovad, T.; Sapunar, M.; Sršeň, Š.; Slavíček, P.; Mašín, Z.; Jones, N.C.; Hoffmann, S.V.; Ranković, M.; Fedor, J. Excitation and fragmentation of the dielectric gas C4F7N: Electrons vs photons. J. Chem. Phys. 2023, 158, 014303. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, J.; Xiao, S.; Xie, B.; Chen, D.; Gao, Y.; Tang, J. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. J. Hazard. Mater. 2019, 368, 653–660. [Google Scholar] [CrossRef]
- Tian, S.; Liu, W.; Ding, J.; Liu, J.; Xu, Z.; Yuan, Z.; Zhang, W.; Rao, X.; Wan, Q.; Li, Y.; et al. Study on subacute inhalation toxicity and offspring teratogenicity of C4F7N: An environmentally friendly insulating gas to replace SF6. J. Clean. Prod. 2023, 387, 135799. [Google Scholar] [CrossRef]
- Ahmed, S.; Irshad, M.; Yoon, W.; Karanwal, N.; Sugiarto, J.R.; Khan, M.K.; Kim, S.K.; Kim, J. Evaluation of MgO as a promoter for the hydrogenation of CO2 to long-chain hydrocarbons over Fe-based catalysts. Appl. Catal. B Environ. 2023, 338, 123052. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, J.; Xie, C.; Shao, X.; Wang, Z.; Chen, D.; Xiao, S. Study on the thermal decomposition characteristics of C4F7N–CO2 mixture as eco-friendly gas-insulating medium. High Volt. 2020, 5, 46–52. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, J.; Fu, M.; Zhuo, R.; Luo, Y.; Chen, D.; Xiao, S. Experimental study on the partial discharge and AC breakdown properties of C4F7N/CO2 mixture. High Volt. 2019, 4, 12–17. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Yang, T.; Deng, Y.; Li, X.; Murphy, A.B. Thermal decomposition characteristics and kinetic analysis of C4F7N/CO2 gas mixture. J. Phys. D Appl. Phys. 2020, 53, 055502. [Google Scholar] [CrossRef]
- Zhang, B.; Li, C.; Xiong, J.; Zhang, Z.; Li, X.; Deng, Y. Decomposition characteristics of C4F7N/CO2 mixture under AC discharge breakdown. AIP Adv. 2019, 9, 115212. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, A.; Wang, X.; Rong, M. Theoretical study of the decomposition mechanism of C4F7N. J. Phys. D Appl. Phys. 2019, 52, 245203. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Huang, Y.; Li, Y.; Cheng, H.; Xiao, S. Detection of decomposition products of C4F7N-CO2 gas mixture based on infrared spectroscopy. Vib. Spectrosc. 2020, 110, 103114. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, K.; Wang, H.; Yuan, S.; Du, J.; Ji, Y.; Fu, D.; Huang, Y.; Cui, G. Decomposition characteristics and influencing mechanisms of C4F7N/CO2 gas with different metal materials. AIP Adv. 2024, 14, 095317. [Google Scholar] [CrossRef]
- Zhao, D.; Yan, J.; He, R.; Geng, Y.; Liu, Z.; Wang, J. Decomposition mechanism of C4F7N/CO2 gas mixture based on molecular dynamics and effect of O2 content. J. Appl. Phys. 2024, 135, 024401. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Ding, W.; Zheng, Z. Physical parameters calculation of C4F7N/CO2 mixture. IET Conf. Proc. 2022, 2021, 412–416. [Google Scholar] [CrossRef]
- Available online: http://lammps.sandia.gov (accessed on 7 August 2019).
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for HydroCarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]





| Product | Temperature | Product | Temperature | ||||
|---|---|---|---|---|---|---|---|
| 1000 °C | 1500 °C | 1800 °C | 1000 °C | 1500 °C | 1800 °C | ||
| CO2 | 90.68 | 90.35 | 86.54 | C4F7 | --- | 0.012 | --- |
| C4F7N | 8.797 | 8.43 | 6.16 | C3F6 | --- | 0.002 | 0.003 |
| CF3 | 0.17 | 0.24 | 1.51 | CF | --- | --- | 0.68 |
| C2F | 0.001 | --- | 0.30 | CO | --- | --- | 0.48 |
| C2F2 | 0.17 | 0.20 | 1.26 | C3F7 | --- | --- | 0.07 |
| C3F5 | 0.002 | --- | 0.001 | C3F2 | --- | --- | 0.05 |
| C4F6N | 0.001 | 0.003 | 0.01 | C3F | --- | --- | 0.02 |
| CN | 0.0005 | --- | 0.47 | C3F3 | --- | --- | 0.005 |
| CFN | 0.175 | 0.47 | 1.97 | C3F3N | --- | --- | 0.002 |
| C2 | --- | 0.25 | 0.48 | C3 | --- | --- | 0.002 |
| C2F3 | --- | 0.02 | 0.002 | CF2 | --- | --- | 0.0005 |
| C3F4 | --- | 0.02 | 0.002 | CF2N | --- | --- | 0.0005 |
| Product | Content (%) | Product | Content (%) | Product | Content (%) |
|---|---|---|---|---|---|
| C4F7N | 6.72 | CF3 | 0.42 | CO2 | 42.40 |
| CF | 3.36 | CF2N | 0.42 | CO | 22.27 |
| C2 | 3.21 | C3F4 | 0.42 | O2 | 11.41 |
| CF2 | 1.26 | C3F | 0.42 | C2O4 | 0.57 |
| C2F3 | 1.26 | C3F7N | 0.42 | ||
| C3F4N | 1.26 | FN | 0.42 | CFO | 0.42 |
| C2F | 0.84 | C4F4 | 0.42 | C4F5N2O | 0.42 |
| CN | 0.79 | C3F6 | 0.37 | FO | 0.02 |
| C3 | 0.42 | C4F6N | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wu, J.; Wei, X.; Bai, X.; Shen, C.; Ding, D.; Zheng, B. Theoretical Investigation of C4F7N–CO2 Mixture Decomposition Characteristics Under Extreme Conditions. Energies 2025, 18, 591. https://doi.org/10.3390/en18030591
Wu Y, Wu J, Wei X, Bai X, Shen C, Ding D, Zheng B. Theoretical Investigation of C4F7N–CO2 Mixture Decomposition Characteristics Under Extreme Conditions. Energies. 2025; 18(3):591. https://doi.org/10.3390/en18030591
Chicago/Turabian StyleWu, Yuewei, Jian Wu, Xiaolong Wei, Xiaochun Bai, Chen Shen, De Ding, and Bin Zheng. 2025. "Theoretical Investigation of C4F7N–CO2 Mixture Decomposition Characteristics Under Extreme Conditions" Energies 18, no. 3: 591. https://doi.org/10.3390/en18030591
APA StyleWu, Y., Wu, J., Wei, X., Bai, X., Shen, C., Ding, D., & Zheng, B. (2025). Theoretical Investigation of C4F7N–CO2 Mixture Decomposition Characteristics Under Extreme Conditions. Energies, 18(3), 591. https://doi.org/10.3390/en18030591





