Research of CO2-Responsive Surfactants for Enhanced Oil Recovery: Review and Outlook
Abstract
1. Introduction
2. Structure, Classification and Synthesis of CO2-Responsive Surfactants
2.1. Structure of CO2-Responsive Surfactant Molecules
2.2. Response Mechanism of CO2-Eesponsive Surfactants
2.3. Classification of CO2-Responsive Surfactants
2.3.1. Amidinoyl Responsive Surfactants
2.3.2. Guanidine-Based Responsive Surfactants
2.3.3. Amine-Based Responsive Surfactants
2.4. Synthesis of CO2-Responsive Surfactants
2.4.1. Synthesis of Amidine Surfactant
Direct Synthesis
Indirect Synthesis
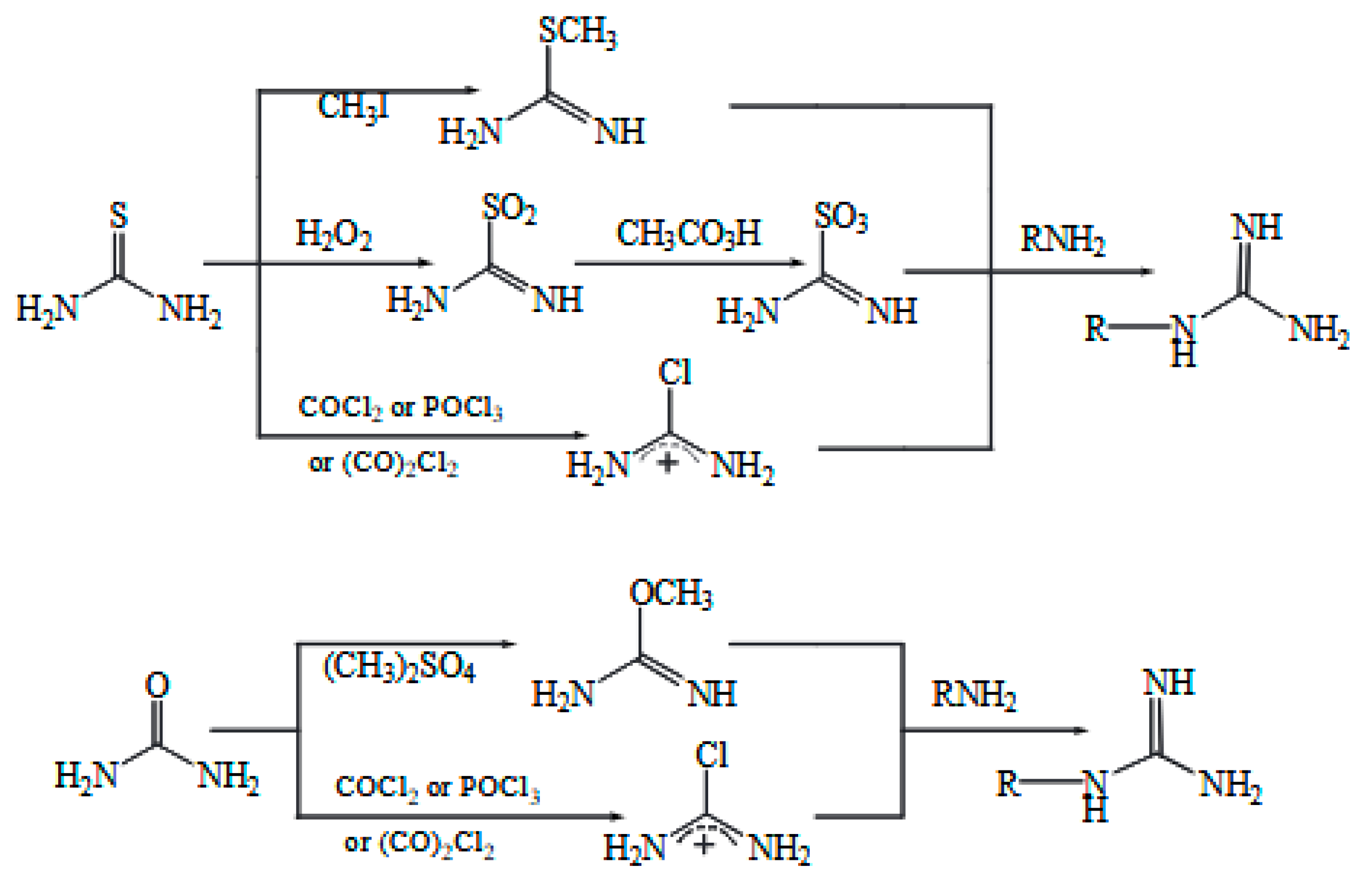
2.4.2. Preparation of Guanidine Surfactants
2.4.3. Preparation of Amine-Based Surfactants
3. CO2-Responsive Surfactants in Enhanced Oil Recovery Technology
3.1. Application in CO2 Foam Flooding for Oil Recovery
3.2. Application in Emulsion and Microemulsion forOil Recovery
3.3. Application in Hydrogel Flooding for Oil Recovery
4. Effect of CO2-Responsive Surfactants on Enhancing Oil Recovery
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H. New Progress of CO2 Flooding and Storage Technology. Pet. Geol. Recovery Effic. 2023, 30, 18–26. [Google Scholar] [CrossRef]
- Hill, L.B.; Li, X.; Wei, N. CO2-EOR in China: A Comparative Review. Int. J. Greenh. Gas Control 2020, 103, 103173. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. A Review of Recent Developments in CO2 Mobility Control in Enhanced Oil Recovery. Petroleum 2022, 8, 291–317. [Google Scholar] [CrossRef]
- Yuan, S.; Ma, D.; Li, J.; Zhou, T.; Ji, Z.; Han, H. Progress and Prospects of Carbon Dioxide Capture, EOR-Utilization and Storage Industrialization. Pet. Explor. Dev. 2022, 49, 955–962. [Google Scholar] [CrossRef]
- Perera, M.; Gamage, R.; Rathnaweera, T.; Ranathunga, A.; Koay, A.; Choi, X. A Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, 9, 481. [Google Scholar] [CrossRef]
- Lu, J.; Liyanage, P.J.; Solairaj, S.; Adkins, S.; Arachchilage, G.P.; Kim, D.H.; Britton, C.; Weerasooriya, U.; Pope, G.A. New Surfactant Developments for Chemical Enhanced Oil Recovery. J. Pet. Sci. Eng. 2014, 120, 94–101. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The Use of Surfactants in Enhanced Oil Recovery: A Review of Recent Advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Dey, S.; Mandal, A. Characterizations of Surfactant Synthesized from Palm Oil and Its Application in Enhanced Oil Recovery. J. Taiwan Inst. Chem. Eng. 2017, 81, 343–355. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; Gamal El-Din, M.; Kemppi, T.; et al. Stabilization Mechanism and Chemical Demulsification of Water-in-Oil and Oil-in-Water Emulsions in Petroleum Industry: A Review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Chen, W.; Liu, B.; Zhang, X. A Comprehensive Review of Emulsion and Its Field Application for Enhanced Oil Recovery. Energy Sci. Eng. 2019, 7, 1046–1058. [Google Scholar] [CrossRef]
- Jia, J.; Xu, Z.; Zhang, B.; Wang, P.; Kong, W.; Xu, Z.; Xiong, W.; Jia, Y.; Cao, J. Microemulsion Oil Displacement Effect of Fracture Reservoirs. E3S Web Conf. 2020, 145, 2059. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A. Microemulsions: A Novel Approach to Enhanced Oil Recovery: A Review. J. Pet. Explor. Prod. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef]
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L.J. Foam Assisted CO2-EOR: A Review of Concept, Challenges, and Future Prospects. J. Pet. Sci. Eng. 2014, 120, 202–215. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wei, Y.; Dang, F.; Li, M. Investigation of Oil-Based CO2 Foam EOR and Carbon Mitigation in a 2D Visualization Physical Model: Effects of Different Injection Strategies. Energy 2024, 313, 133800. [Google Scholar] [CrossRef]
- Bai, Y.; Pu, W.; Jin, X.; Shen, C.; Ren, H. Review of the Micro and Macro Mechanisms of Gel-Based Plugging Agents for Enhancing Oil Recovery of Unconventional Water Flooding Oil Reservoirs. J. Mol. Liq. 2024, 399, 124318. [Google Scholar] [CrossRef]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable Surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Schmittel, M.; Lal, M.; Graf, K.; Jeschke, G.; Suske, I.; Salbeck, J. N,N′-Dimethyl-2,3-Dialkylpyrazinium Salts as Redox-Switchable Surfactants? Redox, Spectral, EPR and Surfactant Properties. Chem. Commun. 2005, 45, 5650–5652. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.P.; Eastoe, J. Stimuli-Responsive Surfactants. Soft Matter 2013, 9, 2365. [Google Scholar] [CrossRef]
- Minkenberg, C.B.; Florusse, L.; Eelkema, R.; Koper, G.J.M.; Van Esch, J.H. Triggered Self-Assembly of Simple Dynamic Covalent Surfactants. J. Am. Chem. Soc. 2009, 131, 11274–11275. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Irvin, K.; Thayumanavan, S. Tunable Disassembly of Micelles Using a Redox Trigger. Langmuir 2007, 23, 7916–7919. [Google Scholar] [CrossRef]
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The Role of New Energy in Carbon Neutral. Pet. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Zou, C.; Wu, S.; Yang, Z.; Pan, S.; Wang, G.; Jiang, X.; Guan, M.; Yu, C.; Yu, Z.; Shen, Y. Progress, Challenge and Significance of Building a Carbon Industry System in the Context of Carbon Neutrality Strategy. Pet. Explor. Dev. 2023, 50, 210–228. [Google Scholar] [CrossRef]
- Scott, L.M.; Robert, T.; Harjani, J.R.; Jessop, P.G. Designing the Head Group of CO2-Triggered Switchable Surfactants. RSC Adv. 2012, 2, 4925. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Li, Y.; Zhi, L. Synthesis and Properties of Dicephalic Cationic Surfactants Containing a Quaternary Ammonium and a Guanidine Group. J. Surfactants Deterg. 2013, 16, 71–76. [Google Scholar] [CrossRef]
- Su, X.; Jessop, P.G.; Cunningham, M.F. Switchable Surfactants at the Polystyrene–Water Interface: Effect of Molecular Structure. Green Mater. 2014, 2, 69–81. [Google Scholar] [CrossRef]
- Fowler, C.I.; Jessop, P.G.; Cunningham, M.F. Aryl Amidine and Tertiary Amine Switchable Surfactants and Their Application in the Emulsion Polymerization of Methyl Methacrylate. Macromolecules 2012, 45, 2955–2962. [Google Scholar] [CrossRef]
- Björneholm, O.; Öhrwall, G.; De Brito, A.N.; Ågren, H.; Carravetta, V. Superficial Tale of Two Functional Groups: On the Surface Propensity of Aqueous Carboxylic Acids, Alkyl Amines, and Amino Acids. Acc. Chem. Res. 2022, 55, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Chiappisi, L. Polyoxyethylene Alkyl Ether Carboxylic Acids: An Overview of a Neglected Class of Surfactants with Multiresponsive Properties. Adv. Colloid Interface Sci. 2017, 250, 79–94. [Google Scholar] [CrossRef]
- Lee, N.-M.; Lee, B.-H. Thermodynamics on the Micellization of Various Pure and Mixed Surfactants: Effects of Head- and Tail-Groups. J. Chem. Thermodyn. 2016, 95, 15–20. [Google Scholar] [CrossRef]
- Butler, C.S.G.; Kelleppan-Meaney, V.T.; Williams, A.P.; Giles, L.W.; Vidallon, M.L.P.; Sokolova, A.; De Campo, L.; Tuck, K.L.; Tabor, R.F. Influence of Tail Group Length, Amide Functionality and Added Salt Ion Identity on the Behaviour of Betaine Surfactants. J. Colloid Interface Sci. 2024, 653, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.C.; Morgado, P.; Filipe, E.J.M. Towards Compartmentalized Micelles: Mixed Perfluorinated and Hydrogenated Ionic Surfactants in Aqueous Solution. J. Colloid Interface Sci. 2024, 654, 906–914. [Google Scholar] [CrossRef]
- Li, X.; Turánek, J.; Knötigová, P.; Kudláčková, H.; Mašek, J.; Parkin, S.; Rankin, S.E.; Knutson, B.L.; Lehmler, H.-J. Hydrophobic Tail Length, Degree of Fluorination and Headgroup Stereochemistry Are Determinants of the Biocompatibility of (Fluorinated) Carbohydrate Surfactants. Colloids Surf. B 2009, 73, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.S.; Adewunmi, A.A.; Mahboob, A.; Murtaza, M.; Zhou, X.; Kamal, M.S. Fluorinated Surfactants: A Review on Recent Progress on Synthesis and Oilfield Applications. Adv. Colloid Interface Sci. 2022, 303, 102634. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shao, Y.; Wang, W.; Zhu, L.; Liu, H.; Yang, S. Fluorinated Polyhedral Oligomeric Silsesquioxanes End-Capped Poly(Ethylene Oxide) Giant Surfactants: Precise Synthesis and Interfacial Behaviors. Polymer 2020, 186, 122055. [Google Scholar] [CrossRef]
- Guo, S.; Guo, Y.; Huang, M.; Qian, L.; Su, Z.; Chen, Q.-Y.; Wu, C.; Liu, C. Synthesis, Surface Activity, and Foamability of Two Short-Chain Fluorinated Sulfonate Surfactants with Ether Bonds. Langmuir 2023, 39, 14519–14527. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Shinde, S.B.; Paso, K.G.; Sjöblom, J.; Kumar, L. Chemical Additives as Flow Improvers for Waxy Crude Oil and Model Oil: A Critical Review Analyzing Structure–Efficacy Relationships. Energy Fuels 2022, 36, 3372–3393. [Google Scholar] [CrossRef]
- Quan, H.; Chen, L.; Huang, Z.; Wang, J.; Zheng, C. The Effect of a Kind of Hyperbranched Polyester with Different Carbon Length on Flowability for Crude Oil. Fuel 2018, 214, 356–362. [Google Scholar] [CrossRef]
- Czajka, A.; Hazell, G.; Eastoe, J. Surfactants at the Design Limit. Langmuir 2015, 31, 8205–8217. [Google Scholar] [CrossRef] [PubMed]
- Benedix, R.R.; Botsch, S.; Preisig, N.; Kovalchuk, V.; Jessop, P.G.; Stubenrauch, C. Influence of a CO2 -Switchable Additive on the Surface and Foaming Properties of a Cationic Non-Switchable Surfactant. Soft Matter 2023, 19, 2941–2948. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Reversible Nonpolar-to-Polar Solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, Z.; Zhang, G.; Zhang, H.; Zou, Y.; Hao, X.; Ye, Z. Synthesis and Characterization of a Series of Amidine-Based Switch Surfactants: Toward Environmental Surface-Active Materials. J. Dispers. Sci. Technol. 2024, 1–6. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Sun, J.; Yi, X.; Chang, Z.; Hou, Q. Promotion of Salinity to Protonation of Amidine-Based CO2 -Switchable Surfactant. J. Phys. Conf. Ser. 2023, 2587, 12010. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z. Synthesis and Properties of Double-Chain Single-Head Acetamidinium Bicarbonate Switchable Surfactants. J. Dispers. Sci. Technol. 2019, 40, 355–360. [Google Scholar] [CrossRef]
- Qiao, W.; Zheng, Z.; Shi, Q. Synthesis and Properties of a Series of CO2 Switchable Surfactants with Imidazoline Group. J. Surfactants Deterg. 2012, 15, 533–539. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, Y.; Wang, Z.; Wu, Q.; Wang, F.; Ni, C.; Zheng, X.; Xu, Y.; Zhao, Y. Temperature Sensitivity of CO2-Triggered Switchable Surfactants with Acetamidine Group. J. Pet. Sci. Eng. 2020, 186, 106677. [Google Scholar] [CrossRef]
- Harjani, J.R.; Liang, C.; Jessop, P.G. A Synthesis of Acetamidines. J. Org. Chem. 2011, 76, 1683–1691. [Google Scholar] [CrossRef]
- Hegh, D.Y.; Mackay, S.M.; Tan, E.W. CO2 -Triggered Release from Switchable Surfactant Impregnated Liposomes. RSC Adv. 2014, 4, 31771–31774. [Google Scholar] [CrossRef]
- Debas, M.; Freire, R.V.M.; Salentinig, S. Supramolecular Design of CO2-Responsive Lipid Nanomaterials. J. Colloid Interface Sci. 2023, 637, 513–521. [Google Scholar] [CrossRef]
- Pauer, W. (Ed.) Polymer Reaction Engineering of Dispersed Systems: Volume II; Advances in Polymer Science; Springer International Publishing: Cham, Switzerland, 2018; Volume 281, ISBN 978-3-319-96435-5. [Google Scholar]
- Miyake, M.; Oyama, N. Effect of Amidoalkyl Group as Spacer on Aggregation Properties of Guanidine-Type Surfactants. J. Colloid Interface Sci. 2009, 330, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Yamada, K.; Oyama, N. Self-Assembling of Guanidine-Type Surfactant. Langmuir 2008, 24, 8527–8532. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Hatano, K.; Kamio, K.; Miyake, M.; Tamura, T.; Hayakawa, K. Cooperative Binding and the Conformation of Poly(L -Glutamic Acid) in Guanidinium Salts with an Alkanoylamidoalkyl Group. J. Phys. Chem. B 2003, 107, 8218–8222. [Google Scholar] [CrossRef]
- Mehta, S.K.; Bhasin, K.K.; Chauhan, R.; Dham, S. Effect of Temperature on Critical Micelle Concentration and Thermodynamic Behavior of Dodecyldimethylethylammonium Bromide and Dodecyltrimethylammonium Chloride in Aqueous Media. Colloids Surf. A Physicochem. Eng. Asp. 2005, 255, 153–157. [Google Scholar] [CrossRef]
- Šarac, B.; Bešter-Rogač, M. Temperature and Salt-Induced Micellization of Dodecyltrimethylammonium Chloride in Aqueous Solution: A Thermodynamic Study. J. Colloid Interface Sci. 2009, 338, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.A.; Patoary, M.-O.-F.; Rashid, M.M.; Molla, M.R.; Rub, M.A. Physico-Chemical Investigation of Mixed Micelle Formation between Tetradecyltrimethylammonium Bromide and Dodecyltrimethylammonium Chloride in Water and Aqueous Solutions of Sodium Chloride. J. Solut. Chem. 2017, 46, 682–703. [Google Scholar] [CrossRef]
- Marcolongo, J.P.; Mirenda, M. Thermodynamics of Sodium Dodecyl Sulfate (SDS) Micellization: An Undergraduate Laboratory Experiment. J. Chem. Educ. 2011, 88, 629–633. [Google Scholar] [CrossRef]
- Domínguez, H. Structural Transition of the Sodium Dodecyl Sulfate (SDS) Surfactant Induced by Changes in Surfactant Concentrations. J. Phys. Chem. B 2011, 115, 12422–12428. [Google Scholar] [CrossRef]
- Khodaparast, S.; Sharratt, W.N.; Tyagi, G.; Dalgliesh, R.M.; Robles, E.S.J.; Cabral, J.T. Pure and Mixed Aqueous Micellar Solutions of Sodium Dodecyl Sulfate (SDS) and Dimethyldodecyl Amine Oxide (DDAO): Role of Temperature and Composition. J. Colloid Interface Sci. 2021, 582, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Long, J.; He, S. Reversible Solubilization of Typical Polycyclic Aromatic Hydrocarbons (PAH) by a Gas Switchable Surfactant. J. Surfactants Deterg. 2015, 18, 1–7. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Wang, C. Mechanism and Properties of Recycled Pigment Foam Dyeing Controlled by Alkylguanidine-Type Switchable Surfactant. J. Text. Res. 2015, 36, 71–76. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, J.; Wang, Y.; Ding, X. Performance Studies of Dodecyltetramethylguanidine CO2 Switch Surfactants. Deterg. Cosmet. 2009, 32, 18–22. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Jessop, P.G.; Thomas, C.A.; Eckert, C.A.; Liotta, C.L. The Reaction of 1,8-Diazabicyclo[5.4.0]Undec-7-Ene (DBU) with Carbon Dioxide. J. Org. Chem. 2005, 70, 5335–5338. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Q.; Li, Y. Self-Aggregation and Antimicrobial Activity of Alkylguanidium Salts. Colloids Surf. A 2012, 393, 11–16. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Li, Y. Effect of Temperature and Added Counter Ions on Micelle Formation of Guanidine Surfactants. Tenside Surfactants Deterg. 2012, 49, 390–393. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Li, Y.; Zhi, L. Biological Behaviors of Guanidine-based Cationic Surfactants. J. Surfactants Deterg. 2014, 17, 459–464. [Google Scholar] [CrossRef]
- Shakil Hussain, S.M.; Kamal, M.S.; Sultan, A.S. Amido-amine-based Cationic Gemini Surfactants: Thermal and Interfacial Properties and Interactions with Cationic Polyacrylamide. J. Surfactants Deterg. 2017, 20, 47–55. [Google Scholar] [CrossRef]
- Pichetwanit, P.; Kungsanant, S.; Supap, T. Effects of Surfactant Type and Structure on Properties of Amines for Carbon Dioxide Capture. Colloids Surf. A 2021, 622, 126602. [Google Scholar] [CrossRef]
- Cho, J.; Shin, H.; Jeong, N. Amine-based Cationic Surfactants: Synthesis and Utilization of Their Physical Properties to Prepare Liposomes. J. Chem. Technol. Biotechnol. 2019, 94, 2318–2326. [Google Scholar] [CrossRef]
- Diz, M.; Manresa, A.; Pinazo, A.; Erra, P.; Infante, M.R. Synthesis, Surface Active Properties and Antimicrobial Activity of New Bis Quaternary Ammonium Compounds. J. Chem. Soc. Perkin Trans. 1994, 2, 1871. [Google Scholar] [CrossRef]
- Ghumare, A.K.; Pawar, B.V.; Bhagwat, S.S. Synthesis and Antibacterial Activity of Novel Amido-amine-based Cationic Gemini Surfactants. J. Surfactants Deterg. 2013, 16, 85–93. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary Ammonium Compounds (QACs): A Review on Occurrence, Fate and Toxicity in the Environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Arnold, W.A.; Blum, A.; Branyan, J.; Bruton, T.A.; Carignan, C.C.; Cortopassi, G.; Datta, S.; DeWitt, J.; Doherty, A.-C.; Halden, R.U.; et al. Quaternary Ammonium Compounds: A Chemical Class of Emerging Concern. Environ. Sci. Technol. 2023, 57, 7645–7665. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, C.; Lv, Z.; Liang, Y.; Xie, Y.; Wang, C.; Wan, S.; Leng, X.; Hu, M.; Zheng, G. High-Resolution Mass Spectrometry Screening of Quaternary Ammonium Compounds (QACs) in Dust from Homes and Various Microenvironments in South China. Environ. Sci. Technol. 2024, 58, 3182–3193. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, H.; Chen, H.; Xia, Q.; Huang, D.; Meng, L.; Liu, X. Synthesis and Surface Properties of N,N-dimethyl-N-dodecyl Polyoxyethylene Amine-based Surfactants: Amine Oxide, Betaine and Sulfobetaine. J. Surfactants Deterg. 2014, 17, 403–408. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Quan, H.; Su, X. Soybean-Oil-Based CO2-Switchable Surfactants with Multiple Heads. Molecules 2021, 26, 4342. [Google Scholar] [CrossRef]
- Dib, N.; Falcone, R.D.; Acuña, A.; García-Río, L. The Ionic Liquid-Surfactant Bmim-AOT and Nontoxic Lipophilic Solvents as Components of Reverse Micelles Alternative to the Traditional Systems. A Study by 1H NMR Spectroscopy. J. Mol. Liq. 2020, 304, 112762. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W. Study on Osmotic Pressure of Non-Ionic and Ionic Surfactant Solutions in the Micellar and Microemulsion Regions. Fluid Phase Equilib. 2008, 263, 231–235. [Google Scholar] [CrossRef]
- Goloub, T.P.; Pugh, R.J. The Role of the Surfactant Head Group in the Emulsification Process: Binary (Nonionic–Ionic) Surfactant Mixtures. J. Colloid Interface Sci. 2005, 291, 256–262. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Gao, W.; Chen, C. Experimental Investigation on Transport Property and Emulsification Mechanism of Polymeric Surfactants in Porous Media. J. Pet. Sci. Eng. 2020, 186, 106687. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ling, Y.T.Q.; Heng, Y.X. An Environment-Friendly Palm Fatty Acid-Based Polymeric Surfactants for Coating Applications: Physicochemical, Surface Tension and Low-Foaming Properties. J. Polym. Environ. 2019, 27, 2707–2719. [Google Scholar] [CrossRef]
- Lyu, B.; Liu, H.; Li, P.; Gao, D.; Ma, J. Preparation and Properties of Polymeric Surfactants: A Potential Corrosion Inhibitor of Carbon Steel in Acidic Medium. J. Ind. Eng. Chem. 2019, 80, 411–424. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Zhu, L.; Fan, Y.; Wang, Y. Partition and Solubilization of Phospholipid Vesicles by Noncovalently Constructed Oligomeric-like Surfactants. Langmuir 2020, 36, 8733–8744. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and Aquatic Toxicity of New Cleavable Betainate Cationic Oligomeric Surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, J.; Zhao, Y.; Deng, X.; Ni, R.; Zhao, Y.; He, Y. Synthesis and Physicochemical Properties of CO2-Switchable Gemini Surfactants. J. Mol. Liq. 2022, 352, 118642. [Google Scholar] [CrossRef]
- Weintraub, L.; Oles, S.R.; Kalish, N. Convenient General Synthesis of Amidines. J. Org. Chem. 1968, 33, 1679–1681. [Google Scholar] [CrossRef]
- Lange, U.E.W.; Schäfer, B.; Baucke, D.; Buschmann, E.; Mack, H. A New Mild Method for the Synthesis of Amidines. Tetrahedron Lett. 1999, 40, 7067–7070. [Google Scholar] [CrossRef]
- Gielen, H.; Alonso-Alija, C.; Hendrix, M.; Niewöhner, U.; Schauss, D. A Novel Approach to Amidines from Esters. Tetrahedron Lett. 2002, 43, 419–421. [Google Scholar] [CrossRef]
- Watanabe, K.; Kogoshi, N.; Miki, H.; Torisawa, Y. Improved Pinner Reaction with CPME as a Solvent. Synth. Commun. 2009, 39, 2008–2013. [Google Scholar] [CrossRef]
- Mei, P.; Min, H.B.; Lai, L.; Yang, Y.L.; Wu, X.M.; Zheng, Y.C. Advances in the Synthesis and Application of CO2-Switching Surfactants. Oilfield Chem. 2016, 33, 564–570. [Google Scholar] [CrossRef]
- Qiao, W.; Zheng, Z.; Peng, H. Synthesis of Switchable Amphipathic Molecules Triggered by CO2 through Carbonyl-amine Condensation. Eur. J. Lipid Sci. Technol. 2011, 113, 841–847. [Google Scholar] [CrossRef]
- Liu, H.; Yin, H.; Feng, Y. A CO2 -Switchable Amidine Monomer: Synthesis and Characterization. Des. Monomers Polym. 2017, 20, 363–367. [Google Scholar] [CrossRef]
- Kruijer, P.S.; Van Leuffen, P.J.; Herscheid, J.D.M. Synthesis of [11C]Cyanamide, a Versatile Synthon for the Production of 11C-Labelled Radiopharmaceuticals. Appl. Radiat. Isot. 1996, 47, 611–616. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Y.; Yang, J.; Kennedy, J.; Wang, X.; Wang, L. Synthesis, Characterization and Antibacterial Activity of Guanidinylated Chitosan. Carbohydr. Polym. 2007, 67, 66–72. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Tan, C.K.; Chen, J.; Yeung, Y.-Y. N-Bromosuccinimide Initiated One-Pot Synthesis of Imidazoline. Org. Lett. 2011, 13, 2448–2451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, J.; Zhou, J.; Yeung, Y.-Y. N-Bromosuccinimide Promoted One-Pot Synthesis of Guanidine: Scope and Mechanism. Org. Lett. 2011, 13, 5804–5807. [Google Scholar] [CrossRef]
- Kumar, R.; Flodén, N.J.; Whitehurst, W.G.; Gaunt, M.J. A General Carbonyl Alkylative Amination for Tertiary Amine Synthesis. Nature 2020, 581, 415–420. [Google Scholar] [CrossRef]
- Stevenson, P. (Ed.) Foam Engineering: Fundamentals and Applications, 1st ed.; John Wiley & Sons: Chichester, West Sussex, UK, 2012; ISBN 978-0-470-66080-5. [Google Scholar] [CrossRef]
- Enick, R.M.; Olsen, D.; Ammer, J.; Schuller, W. Mobility and Conformance Control for CO2 EOR via Thickeners, Foams, and Gels—A Literature Review of 40 Years of Research and Pilot Tests. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; p. SPE-154122-MS. [Google Scholar] [CrossRef]
- Kokal, S. Crude Oil Emulsions: A State-of-the-Art Review. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September–2 October 2002; p. SPE-77497-MS. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Poon, B.M.; Cui, L.; Ma, K.; Liao, S.Y.; Reddy, P.P.; Worthen, A.J.; Hirasaki, G.J.; Nguyen, Q.P.; et al. Switchable Nonionic to Cationic Ethoxylated Amine Surfactants for CO2 Enhanced Oil Recovery in High-Temperature, High-Salinity Carbonate Reservoirs. SPE J. 2014, 19, 249–259. [Google Scholar] [CrossRef]
- Lu, H.; He, Y.; Huang, Z. Foaming Properties of CO2 -Triggered Surfactants for Switchable Foam Control. J. Dispers. Sci. Technol. 2014, 35, 832–839. [Google Scholar] [CrossRef]
- Wang, J.; Liang, M.; Tian, Q.; Feng, Y.; Yin, H.; Lu, G. CO2-Switchable Foams Stabilized by a Long-Chain Viscoelastic Surfactant. J. Colloid Interface Sci. 2018, 523, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, Z.; Zheng, C.; Lu, H.; Liu, D. Switchable Surfactant-Based CO2 -in-Water Foam Stabilized by Wormlike Micelle. Ind. Eng. Chem. Res. 2018, 57, 13291–13299. [Google Scholar] [CrossRef]
- Zhang, P.; Diao, Y.; Shan, Y.; Pei, S.; Ren, S.; Zhang, L.; Yang, H. Experimental Investigation of Amine-Surfactant CO2 Foam for Smart Mobility Control during CO2 Flooding. J. Pet. Sci. Eng. 2020, 184, 106511. [Google Scholar] [CrossRef]
- Poole, H.; Jessop, P.G.; Stubenrauch, C. Foaming and Defoaming Properties of CO2-switchable Surfactants. J. Surfact Deterg. 2022, 25, 467–475. [Google Scholar] [CrossRef]
- Pei, X.; Wu, J.; Zou, X.; Song, B.; Chen, Z.; Liu, P.; Cui, Z.; Pan, T.; Gu, Y. The Switching Behavior of CO2/N2 Responsive Emulsion Systems Formed by an Amine Functionalized Quaternary Ammonium Surfactant. J. Mol. Liq. 2022, 363, 119915. [Google Scholar] [CrossRef]
- Guan, X.; Liu, D.; Lu, H.; Huang, Z. CO2 Responsive Emulsions: Generation and Potential Applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123919. [Google Scholar] [CrossRef]
- Ceschia, E.; Harjani, J.R.; Liang, C.; Ghoshouni, Z.; Andrea, T.; Brown, R.S.; Jessop, P.G. Switchable Anionic Surfactants for the Remediation of Oil-Contaminated Sand by Soil Washing. RSC Adv. 2014, 4, 4638–4645. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, C.; Liu, X.; Fang, Y.; Feng, Y. CO2 -Responsive Microemulsion: Reversible Switching from an Apparent Single Phase to near-Complete Phase Separation. Green Chem. 2016, 18, 392–396. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Liu, X.; Chen, H.; Fang, Y. Retrieving Oil and Recycling Surfactant in Surfactant-Enhanced Soil Washing. ACS Sustain. Chem. Eng. 2018, 6, 4981–4986. [Google Scholar] [CrossRef]
- Dai, S.; Zhu, P.; Suo, Y.; Lu, H. Controllable CO2 -Responsiveness of an Oil-in-Water Emulsion by Varying the Number of Tertiary Amine Groups or the Position of the Hydroxyl Group of Tertiary Amine. J. Phys. Chem. B 2019, 123, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Suo, Y.; Liu, D.; Zhu, P.; Zhao, J.; Tan, J.; Lu, H. Controllable CO2 -Responsiveness of O/W Emulsions by Varying the Alkane Carbon Number of a Tertiary Amine. Phys. Chem. Chem. Phys. 2018, 20, 11285–11295. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, J.; Wang, D.; Xu, J.; Zeng, H. CO2/N2-Responsive Oil-in-Water Emulsions Using a Novel Switchable Surfactant. J. Colloid Interface Sci. 2020, 571, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, X.; Li, D.; Yin, T.; Zhang, P.; Dong, Z.; Lin, M.; Zhang, J. New Method Based on CO2 -Switchable Wormlike Micelles for Controlling CO2 Breakthrough in a Tight Fractured Oil Reservoir. Energy Fuels 2019, 33, 4806–4815. [Google Scholar] [CrossRef]
- Lu, H.; Ma, Y.; Wang, B.; Huang, Z. The CO2 Stimulus-Responsive Spherical-Worm-like Micelles Transition Based on Anionic Surfactant Sodium Oleate and N, N, N′, N′, N″-Pentamethyldipropylenetriamine. J. Dispers. Sci. Technol. 2017, 38, 1618–1624. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, Z.; Zheng, C. CO2 Responsive Wormlike Micelles Based on Sodium Oleate, Potassium Chloride and N,N-Dimethylethanolamine. J. Dispers. Sci. Technol. 2018, 39, 1606–1612. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, C.; Lu, H. CO2 -Responsive Wormlike Micelles Based on Sodium Oleate and Hydrophobic Tertiary Amines. J. Dispers. Sci. Technol. 2017, 38, 1824–1831. [Google Scholar] [CrossRef]
- Su, X.; Cunningham, M.F.; Jessop, P.G. Switchable Viscosity Triggered by CO2 Using Smart Worm-like Micelles. Chem. Commun. 2013, 49, 2655. [Google Scholar] [CrossRef]
- Lu, H.; Huang, Z.; Yang, L.L.; Dai, S. CO2 -/N2 -Triggered Viscoelastic Fluid with N,N-Dimethyl Oleoaminde-Propylamine and Sodium p-Toluenesulfonate. J. Dispers. Sci. Technol. 2015, 36, 252–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y. CO2-Induced Smart Viscoelastic Fluids Based on Mixtures of Sodium Erucate and Triethylamine. J. Colloid Interface Sci. 2015, 447, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, L.; Wang, B.; Huang, Z. Switchable Spherical-Wormlike Micelle Transition in Sodium Oleate/N-(3-(Dimethylamino)Propyl)-Octanamide Aqueous System Induced by Carbon Dioxide Stimuli and pH Regulation. J. Dispers. Sci. Technol. 2016, 37, 159–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, J.; He, S.; Guo, Z.; Chu, Z.; Dreiss, C.A. CO2-Switchable Wormlike Micelles. Chem. Commun. 2013, 49, 4902. [Google Scholar] [CrossRef]
- Fang, P.; Zhang, Q.; Wu, M.; Zhou, C.; Yang, Z.; Yu, H.; Ji, Z.; Yi, L.; Jiang, W.; Chen, X.; et al. An Intelligent CO2-Responsive Hydrogel for Applications in Enhanced Oil Recovery and CO2 Geological Storage. Sep. Purif. Technol. 2024, 359, 130526. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Sun, D.; Xu, Z. Recent Advances in Switchable Surfactants for Heavy Oil Production: A Review. Energy Geosci. 2024, 5, 100342. [Google Scholar] [CrossRef]
- Liu, Y. The First Carbon Dioxide-Responsive Alkyamidine Surfactant Successful Production in China. China Petroleum News, 5 January 2024. Available online: http://news.cnpc.com.cn/system/2024/01/05/030122187.shtml (accessed on 11 January 2025).
- Du, D.; Pu, W.; Chen, B.; Varfolomeev, M.A.; Liu, R. Experimental Study on EOR Potential of Water-in-Oil Emulsion via CO2/N2 Triggered Wormlike Micelle Solution. Fuel 2021, 288, 119639. [Google Scholar] [CrossRef]


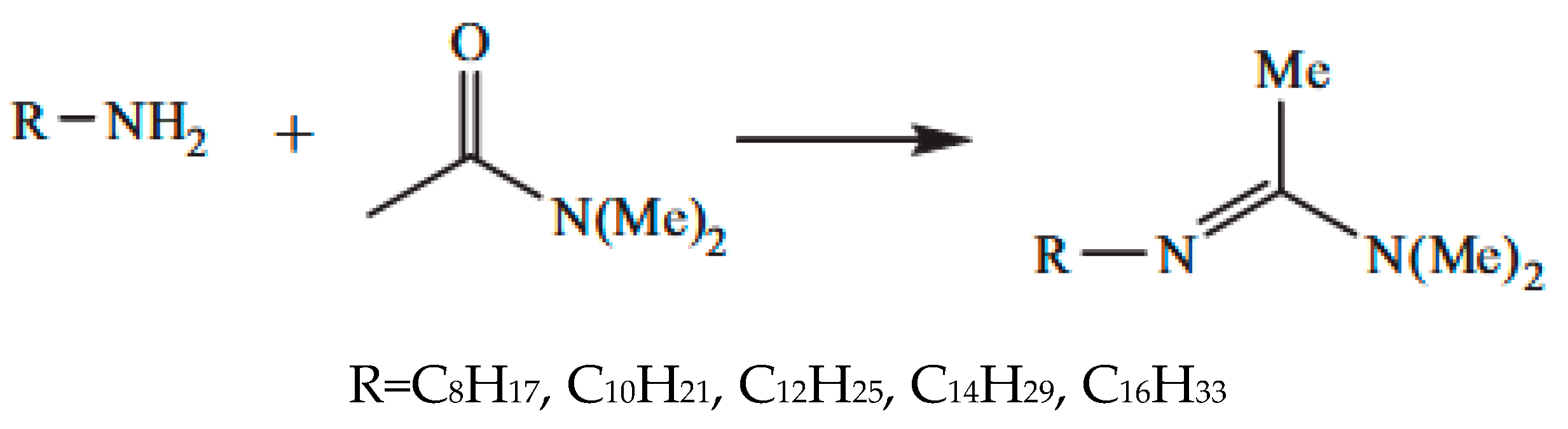



| Name | Structure | CMC (mol/L) | Ref. |
|---|---|---|---|
| DBU | 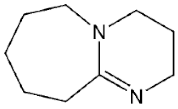 | - | [40] |
| N,N-dimethyl-N′-dodecylamidine | 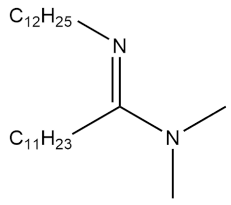 | 5.62 × 10−4 ± 1.27 × 10−6 | [41] |
| C12-DMAA |  | - | [42] |
| N,N′-tetracylamidine | 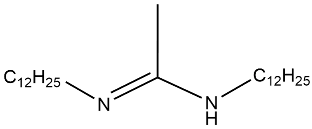 | 8.97 × 10−5 | [43] |
| N′-(4-decylphenyl)-N,N-dimethylacetamidine | 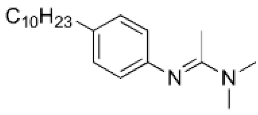 | - | [26] |
| 2-alkyl-1-hydroxyethylimidazolines | 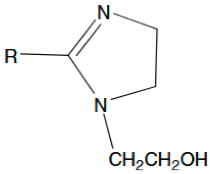 , R=C17H35 , R=C17H35 | 1.35 × 10−4 | [44] |
| C14-DMAA | 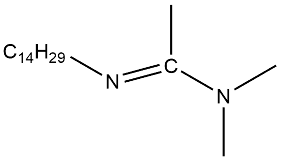 | 2.0 × 10−3 | [45] |
| C16-DMAA | 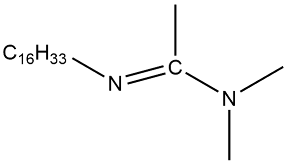 | 1.0 × 10−3 | [45] |
| C18-DMAA | 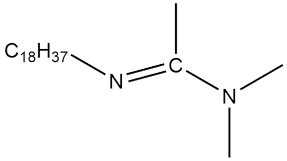 | 7.0 × 10−4 | [45] |
| N,N-Dimethyl-N-(pyridin-4-ylmethyl)acetimidamide | 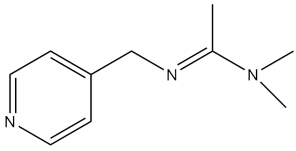 | - | [46] |
| N-Benzyl-N,N-dimethylacetimidamide | 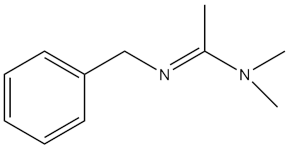 | - | [46] |
| N,N-Dimethyl-N-(thiophen-2-ylmethyl)acetimidamide | 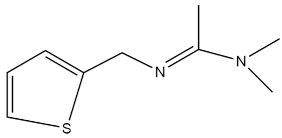 | - | [46] |
| N-(2-(2-Methoxyethoxy)ethyl)-N,N-dimethylacetimidamide |  | - | [46] |
| N-(2-(2-(2-Butoxyethoxy)ethoxy)ethyl)-N,N-dimethylacetimidamide | 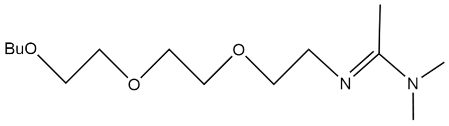 | - | [46] |
| Aryl acetamidine | 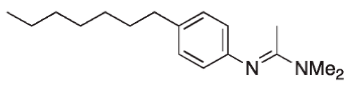 | - | [23] |
| Imidazolines | 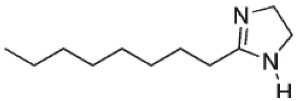 | - | [23] |
| OAm |  | - | [48] |
| Name | Structure | CMC (mol/L) | Ref. |
|---|---|---|---|
| C12G | 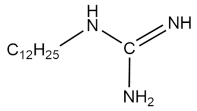 | 6.0 × 10−3 | [51] |
| C12AmG | 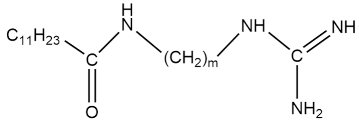 m = 2, 3, 4, 6 m = 2, 3, 4, 6 | m = 2: 8.6 × 10−3 m = 3: 7.2 × 10−3 m = 4: 6.2 × 10−3 m = 6: 3.8 × 10−3 | [50] |
| DTMG |  | 2.8 × 10−4 | [60,61] |
| CnG | 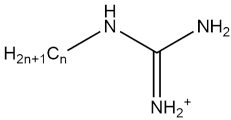 | - | [65] |
| CnMG | 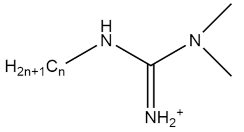 | - | [65] |
| CnGQ |  | - | [65] |
| DMAPA |  | 1.45 × 10−2 | [24] |
| Name | Structure | CMC | Ref. |
|---|---|---|---|
| DABK |  | 4.1 × 10−5 | [69] |
| DABB | 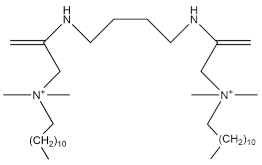 | 3.9 × 10−5 | [69] |
| QACs | 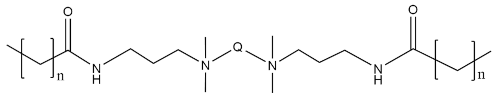 n = 18, Q = 6 n = 18, Q = 6 | 0.46 × 10−3 | [70] |
| C12EO3AO | 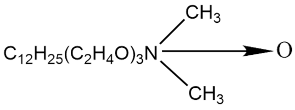 | 1.32 × 10−4 | [75] |
| TEA-12 |  | 1.48 × 10−3 | [68] |
| Oligomeric surfactants |  | 0.2 × 10−3 | [76] |
| N,N′-dimethyl N,N′-bisdodecyl ethylenediamine |  | 1.0 × 10−4 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Xu, Q.; Liu, J.; Du, S.; Luo, W.; Wu, W.; Zou, X.; Liang, S. Research of CO2-Responsive Surfactants for Enhanced Oil Recovery: Review and Outlook. Energies 2025, 18, 574. https://doi.org/10.3390/en18030574
Dong B, Xu Q, Liu J, Du S, Luo W, Wu W, Zou X, Liang S. Research of CO2-Responsive Surfactants for Enhanced Oil Recovery: Review and Outlook. Energies. 2025; 18(3):574. https://doi.org/10.3390/en18030574
Chicago/Turabian StyleDong, Bo, Quan Xu, Jierui Liu, Shuming Du, Wenli Luo, Wei Wu, Xinyuan Zou, and Shisheng Liang. 2025. "Research of CO2-Responsive Surfactants for Enhanced Oil Recovery: Review and Outlook" Energies 18, no. 3: 574. https://doi.org/10.3390/en18030574
APA StyleDong, B., Xu, Q., Liu, J., Du, S., Luo, W., Wu, W., Zou, X., & Liang, S. (2025). Research of CO2-Responsive Surfactants for Enhanced Oil Recovery: Review and Outlook. Energies, 18(3), 574. https://doi.org/10.3390/en18030574






