Fertilizer-Derived Low-Cost Culture Medium for Microalgae and Biofuel Production from Hydrothermal Liquefaction
Abstract
1. Introduction
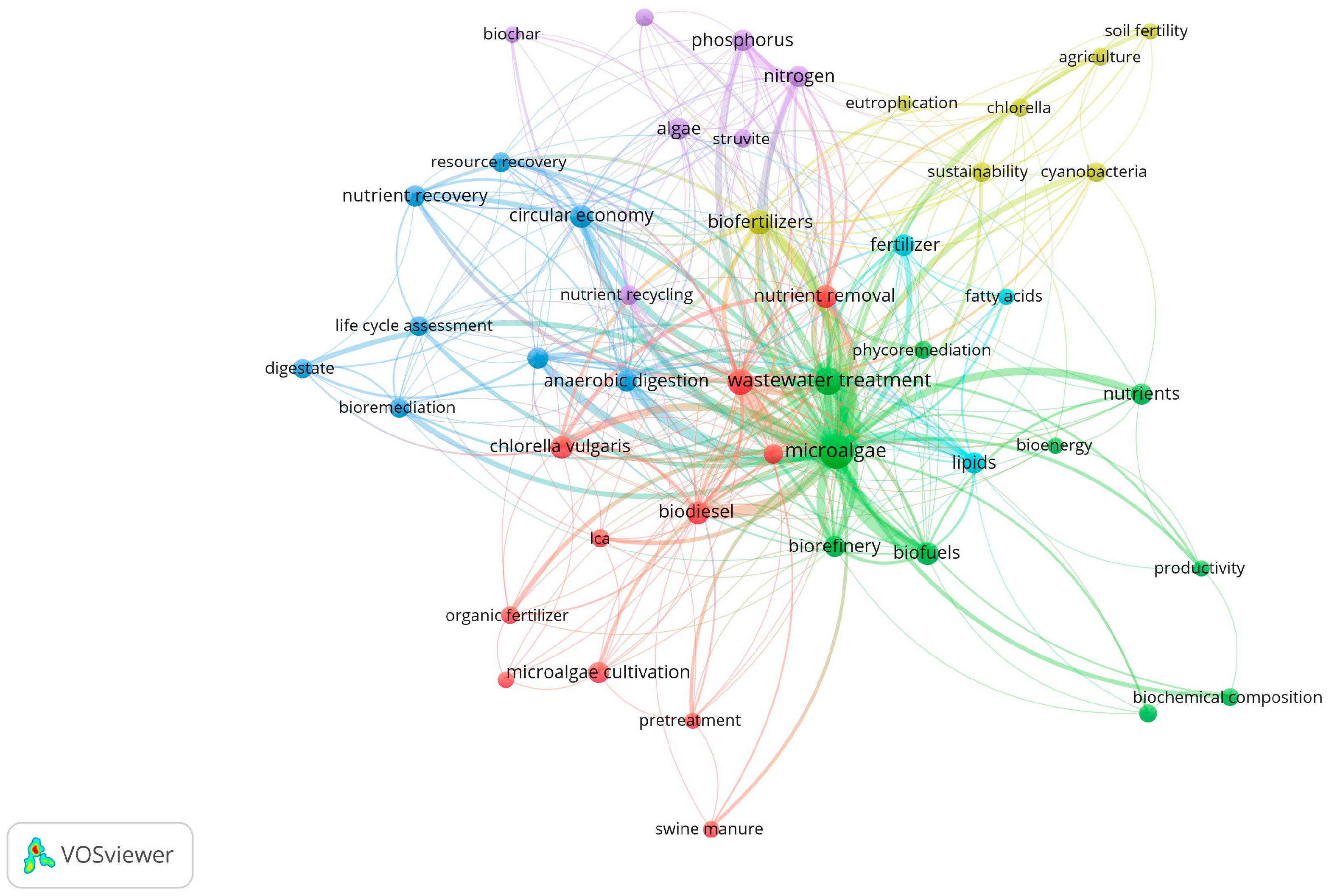
2. Bibliometric Analysis of the Use of Fertilizers as Microalgae Culture Media
3. Materials and Methods
3.1. Growth Conditions
3.2. Proximal Biomass Analysis
3.3. Production of Biocrude Through HTL
3.4. Experiment Design
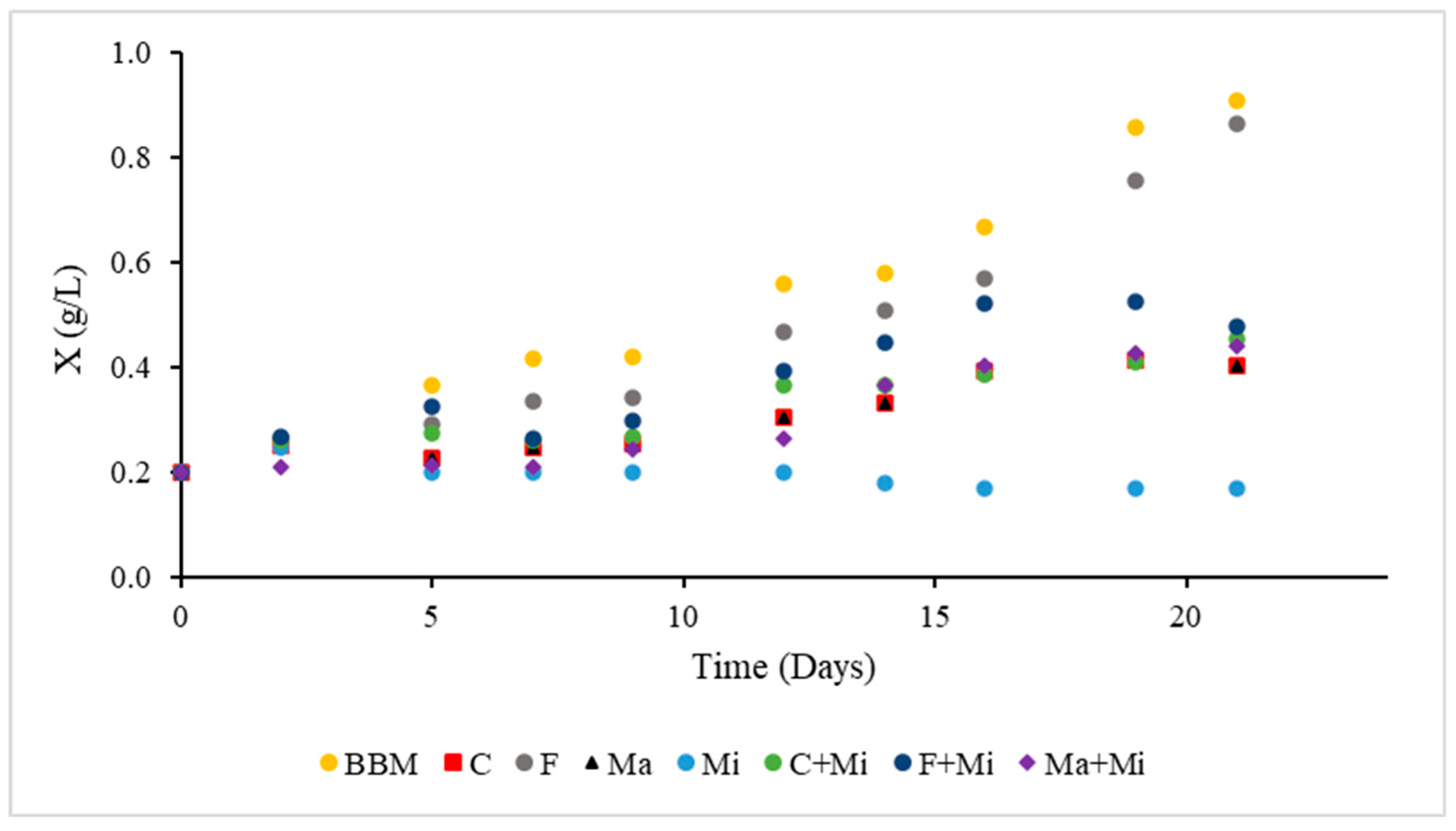
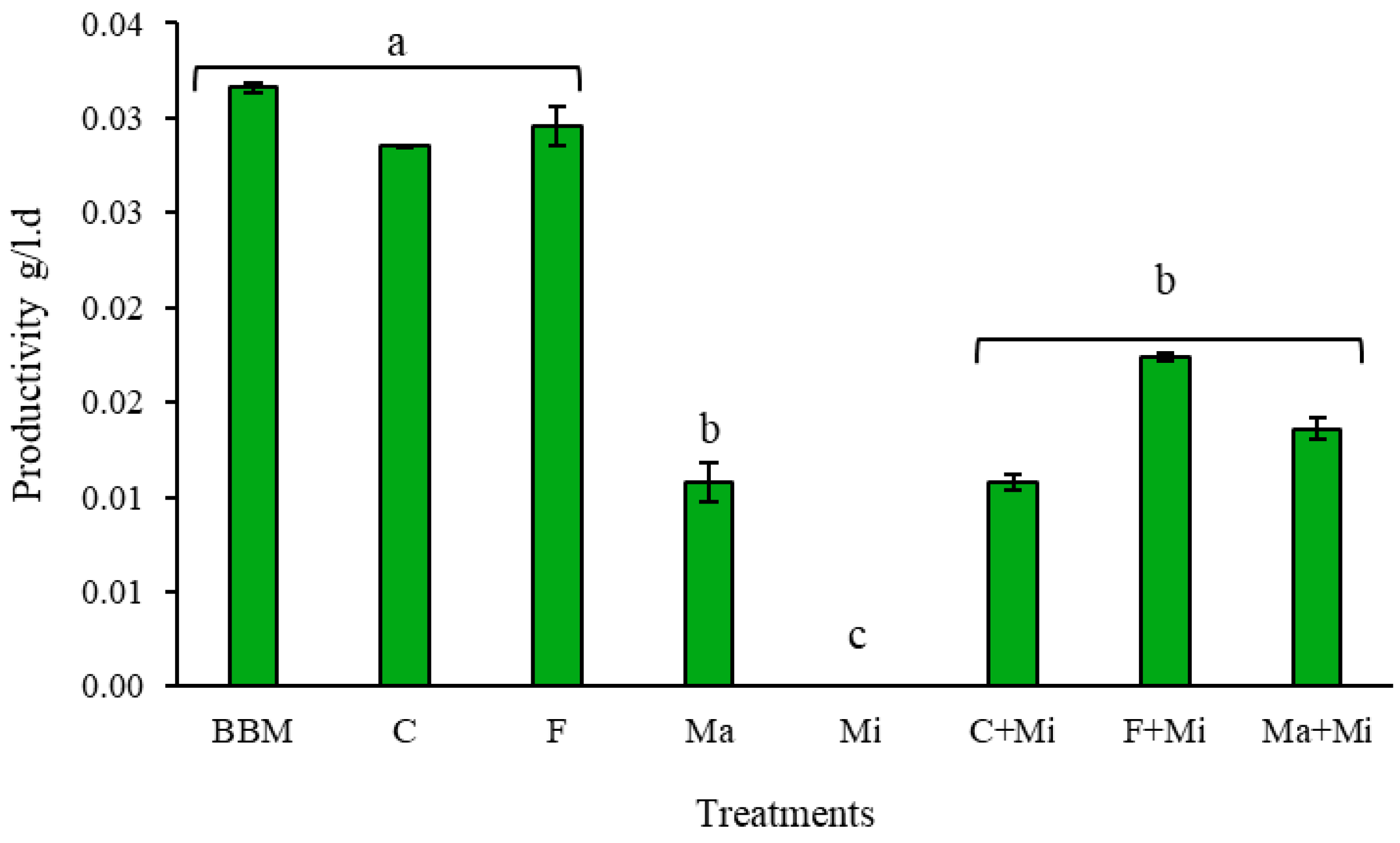
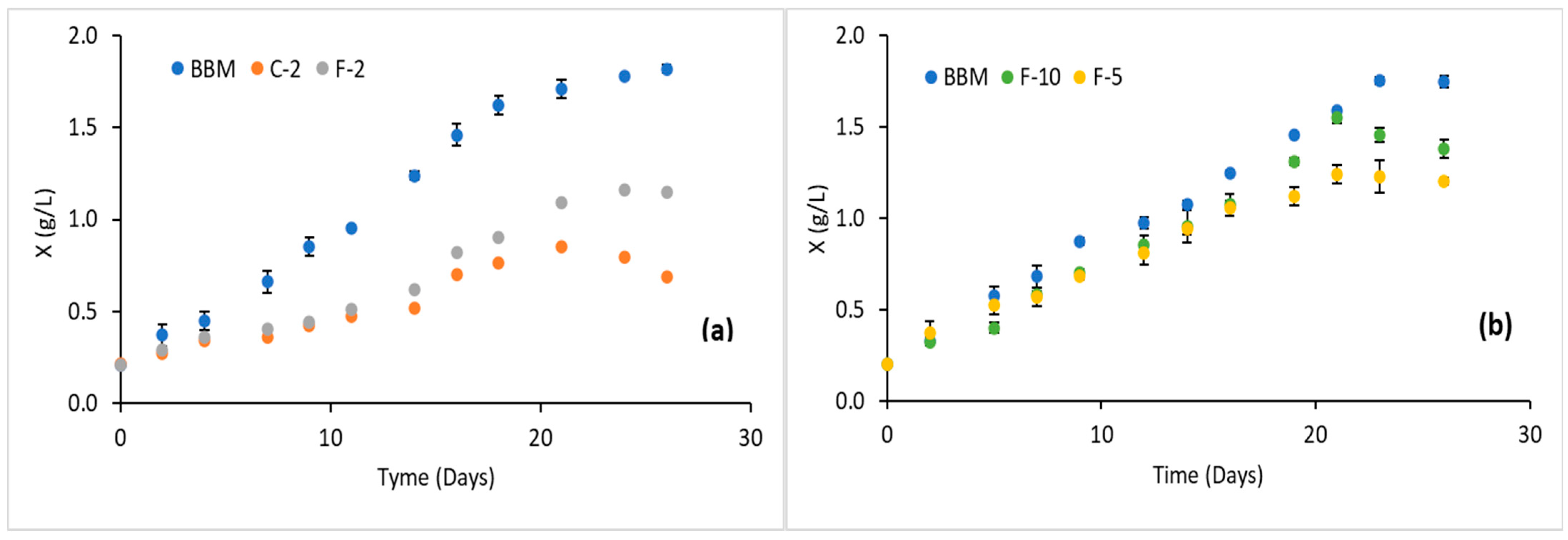
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTL | Hydrothermal liquefaction |
| PBR | Cylindrical photobioreactors |
| BBM | Bold’s Basal Medium |
| HHV | Higher Heating Value |
| AOAC | Association of Official Agricultural Chemists |
| ANOVA | Analysis of variance |
| LSD | Least Significant Difference |
References
- Brasil, B.S.A.F.; Silva, F.C.P.; Siqueira, F.G. Microalgae Biorefineries: The Brazilian Scenario in Perspective. New Biotechnol. 2017, 39, 90–98. [Google Scholar] [CrossRef]
- Díaz-Pérez, M.; Moreno, J.M.M.; García, J.J.H.; Callejón-Ferre, Á.J. Application of Microalgae in Cauliflower Fertilisation. Sci. Hortic. 2024, 337, 113468. [Google Scholar] [CrossRef]
- Miranda, A.M.; Ocampo, D.; Vargas, G.J.; Ríos, L.A.; Sáez, A.A. Nitrogen Content Reduction on Scenedesmus Obliquus Biomass Used to Produce Biocrude by Hydrothermal Liquefaction. Fuel 2021, 305, 121592. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Chew, K.W.; Ting, H.-Y.; Koji, I.; Show, P.L. Digitalised Prediction of Blue Pigment Content from Spirulina Platensis: Next-Generation Microalgae Bio-Molecule Detection. Algal Res. 2024, 83, 103642. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Wan Razali, W.A.; Manan, H.; Hersi, M.A.; Ishak, S.D.; Cheah, W.; Chan, D.J.C.; Sonne, C.; Show, P.L.; Lam, S.S. Recent Advances on Microalgae Cultivation for Simultaneous Biomass Production and Removal of Wastewater Pollutants to Achieve Circular Economy. Bioresour. Technol. 2022, 364, 128085. [Google Scholar] [CrossRef] [PubMed]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Costs Analysis of Microalgae Production. In Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444641922. [Google Scholar]
- Carvalho, J.C.D.; Sydney, E.B.; Ferrari, L.; Tessari, A.; Soccol, C.R. Chapter 2—Culture Media for Mass Production of Microalgae. In Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444641922. [Google Scholar]
- An, S.M.; Cho, K.; Kang, N.S.; Kim, E.S.; Ki, H.; Choi, G.; An, H.S.; Go, G.M. Development of a Cost-Effective Medium Suitable for the Growth and Fucoxanthin Production of the Microalgae Odontella Aurita Using Jeju Lava Seawater and Agricultural Fertilizers. Biomass Bioenergy 2024, 188, 107310. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients From Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Murprayana, R.; Stella, M.; Pukan, H.; Prastiwi, T.; Widjaja, A.; Wirawasista, H. The Effects of UV-C and HNO2 Mutagen, PH and the Use of Commercial Fertilizers on the Growth of Microalgae Botryoc Brauniioccus. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012095. [Google Scholar] [CrossRef]
- Banerjee, A.; Guria, C.; Maiti, S.K. Fertilizer Assisted Optimal Cultivation of Microalgae Using Response Surface Method and Genetic Algorithm for Biofuel Feedstock. Energy 2016, 115, 1272–1290. [Google Scholar] [CrossRef]
- Nordio, R.; Viviano, E.; Sánchez-Zurano, A.; Hernández, J.G.; Rodríguez-Miranda, E.; Guzmán, J.L.; Acién, G. Influence of PH and Dissolved Oxygen Control Strategies on the Performance of Pilot-Scale Microalgae Raceways Using Fertilizer or Wastewater as the Nutrient Source. J. Environ. Manag. 2023, 345, 118899. [Google Scholar] [CrossRef]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. Microalgae-Based Nitrogen Bioremediation. Algal Res. 2020, 46, 101775. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Huang, Y.; Xia, A.; Zhu, X.; Zhu, X.; Liao, Q. Synergistic Treatment of Digested Wastewater with High Ammonia Nitrogen Concentration Using Straw and Microalgae. Bioresour. Technol. 2024, 412, 131406. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, P.; Addy, M.; Zhang, R.; Deng, X.; Ma, Y.; Cheng, Y.; Hussain, F.; Chen, C.; Liu, Y.; et al. Carbon-Dependent Alleviation of Ammonia Toxicity for Algae Cultivation and Associated Mechanisms Exploration. Bioresour. Technol. 2018, 249, 99–107. [Google Scholar] [CrossRef]
- Dyhrman, S.T. The Physiology of Microalgae; Springer: Berlin/Heidelberg, Germany, 2016; Volume 2008. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Shah, A.A.; Sharma, K.; Haider, M.S.; Toor, S.S.; Rosendahl, L.A.; Pedersen, T.H.; Castello, D. The Role of Catalysts in Biomass Hydrothermal Liquefaction and Biocrude Upgrading. Processes 2022, 10, 207. [Google Scholar] [CrossRef]
- Baouchi, A.; Boulif, R. Agriculture Fertilizer-Based Media for Cultivation of Marine Microalgae Destined for Biodiesel Production. J. Energy Manag. Technol. 2020, 4, 49–56. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Roncaratti, L.F.; Possa, G.C.; Garcia, L.C.; Cançado, L.J.; Williams, T.C.R.; dos Santos Alves Figueiredo Brasil, B. A Low-Cost Approach for Chlorella Sorokiniana Production through Combined Use of Urea, Ammonia and Nitrate Based Fertilizers. Bioresour. Technol. Rep. 2020, 9, 100354. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sirohi, R.; Lichtfouse, E.; Chen, W.H.; Len, C.; Show, P.L.; Le, A.T.; Nguyen, X.P.; Hoang, A.T. Microalgae Bio-Oil Production by Pyrolysis and Hydrothermal Liquefaction: Mechanism and Characteristics. Bioresour. Technol. 2023, 376, 128860. [Google Scholar] [CrossRef]
- Melo-Espinosa, E.A.; Bellettre, J.; Tarlet, D.; Montillet, A.; Piloto-Rodríguez, R.; Verhelst, S. Experimental Investigation of Emulsified Fuels Produced with a Micro-Channel Emulsifier: Puffing and Micro-Explosion Analyses. Fuel 2018, 219, 320–330. [Google Scholar] [CrossRef]
- Tang, C.; Dai, D.; Li, S.; Qv, M.; Liu, D.; Li, Z.; Huang, L.Z.; Zhu, L. Responses of Microalgae under Different Physiological Phases to Struvite as a Buffering Nutrient Source for Biomass and Lipid Production. Bioresour. Technol. 2023, 384, 129352. [Google Scholar] [CrossRef]
- Moed, N.M.; Lee, D.J.; Chang, J.S. Struvite as Alternative Nutrient Source for Cultivation of Microalgae Chlorella Vulgaris. J. Taiwan Inst. Chem. Eng. 2015, 56, 73–76. [Google Scholar] [CrossRef]
- Guleria, A.; Chakma, S. A Bibliometric and Visual Analysis of Contaminant Transport Modeling in the Groundwater System: Current Trends, Hotspots, and Future Directions. Environ. Sci. Pollut. Res. 2023, 30, 32032–32051. [Google Scholar] [CrossRef]
- Ocampo, E.; Beltrán, V.V.; Gómez, E.A.; Ríos, L.A.; Ocampo, D. Hydrothermal Liquefaction Process: Review and Trends. Curr. Res. Green Sustain. Chem. 2023, 7, 100382. [Google Scholar] [CrossRef]
- Miranda, A.M.; Ossa, E.A.; Vargas, G.J.; Sáez, A.A. Efecto de Las Bajas Concentraciones de Nitratos y Fosfatos Sobre La Acumulación de Astaxantina En Haematococcus Pluvialis UTEX 2505. Inf. Tecnol. 2019, 30, 23–32. [Google Scholar] [CrossRef]
- Ocampo Echeverri, D.; Rios, L.A.; Gómez Mejía, E.A.; Vargas Betancur, G.J. Solvothermal Liquefaction Process from Biomass for Biocrude Production. U.S. Patent 11814586B2, 14 November 2023. Available online: https://patents.google.com/patent/US11814586B2/en (accessed on 10 September 2025).
- Ríos, L.A.; Vargas, G.J.; Ocampo, D.; Elkin, A.G. Effects of the Use of Acetone as Co-Solvent on the Financial Viability of Bio-Crude Production by Hydrothermal Liquefaction of CO2 Captured by Microalgae. J. CO2 Util. 2024, 89, 102960. [Google Scholar] [CrossRef]
- ASTM D240; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D5185; Standard Test Method for Multielement Determination of Used and Unused Lubricating Oils and Base Oils by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). ASTM International: West Conshohocken, PA, USA, 2018.
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the Bio—Prospective of Microalgae by Different Light Systems and Photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Prihantini, N.B.; Prihantini, N.B.; Rakhmayanti, N.; Handayani, S. Biomass Production of Indonesian Indigenous Leptolyngbya Strain on NPK Fertilizer Medium and Its Potential as a Source of Biofuel. Evergreen 2020, 7, 593–601. [Google Scholar] [CrossRef]
- Fikri, R.; Hidayati, N.A. Effect of Commercial NPK Fertilizer on Growth and Biomass of Navicula Sp. and Nannochloropsis Sp. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012060. [Google Scholar] [CrossRef]
- Koley, S.; Mathimani, T.; Bagchi, S.K.; Sonkar, S.; Mallick, N. Microalgal Biodiesel Production at Outdoor Open and Polyhouse Raceway Pond Cultivations: A Case Study with Scenedesmus Accuminatus Using Low-Cost Farm Fertilizer Medium. Biomass Bioenergy 2019, 120, 156–165. [Google Scholar] [CrossRef]
- Koley, S.; Sonkar, S.; Kumar, S.; Patnaik, R.; Mallick, N. Development of a Low-Cost Cultivation Medium for Simultaneous Production of Biodiesel and Bio-Crude from the Chlorophycean Microalga Tetradesmus Obliquus: A Renewable Energy Prospective. J. Clean. Prod. 2022, 364, 132658. [Google Scholar] [CrossRef]
- Abdel-baset, A.; Matter, I.A.; Ali, M.A. Enhanced Scenedesmus Obliquus Cultivation in Plastic-Type Flat Panel Photobioreactor for Biodiesel Production. Sustainability 2024, 16, 3148. [Google Scholar] [CrossRef]
- Mandal, S.H.M.; Hurin, J.O.B.S.; Froymson, R.E.A.E.; Athews, T.E.J.M. Functional Divergence in Nitrogen Uptake Rates Explains Diversity—Productivity Relationship in Microalgal Communities. Ecosphere 2018, 9, e02228. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Hurd, C.L.; Beardall, J.; Hepburn, C.D. Restricted Use of Nitrate and a Strong Preference for Ammonium Reflects the Nitrogen Ecophysiology of a Light-Limited Red Alga. J. Phycol. 2014, 51, 277–287. [Google Scholar] [CrossRef]
- Lachmann, S.C.; Mettler-Altmann, T.; Wacker, A.; Spijkerman, E. Nitrate or Ammonium: Influences of Nitrogen Source on the Physiology of a Green Alga. Ecol. Evol. 2019, 9, 1070–1082. [Google Scholar] [CrossRef]
- Praveen, P.; Guo, Y.; Kang, H.; Lefebvre, C.; Loh, K. Enhancing Microalgae Cultivation in Anaerobic Digestate through Nitrification. Chem. Eng. J. 2018, 354, 905–912. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Glass, A.D.M.; Siddiqi, M.Y. Inhibition of Nitrate Uptake by Ammonium in Barley. Analysis of Component Fluxes. Plant Physiol. 1999, 120, 283–291. [Google Scholar] [CrossRef]
- L’Helguen, S.; Maguer, J.-F.; Caradec, J. Inhibition Kinetics of Nitrate Uptake by Ammonium in Size-Fractionated Oceanic Phytoplankton Communities: Implications for New Production and f-Ratio Estimates. J. Plankton Res. 2008, 30, 1179–1188. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of Ammonium Nitrogen on Microalgal Growth, Biochemical Composition and Photosynthetic Performance in Mixotrophic Cultivation. Bioresour. Technol. 2018, 273, 368–376. [Google Scholar] [CrossRef]
- Metin, U.; Altınba, M. Evaluating Ammonia Toxicity and Growth Kinetics of Four Different Microalgae Species. Microorganisms 2024, 12, 1542. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Ren, H.; Wang, Z.; Ouyang, Y.; Wang, S.; Hussain, J.; Zeb, I.; Kong, Y.; Liu, S.; et al. Identification of Potassium Transport Proteins in Algae and Determination of Their Role under Salt and Saline-Alkaline Stress. Algal Res. 2023, 69, 102923. [Google Scholar] [CrossRef]
- Ho, B.; In, Y.; Park, K.; Kim, Y.; Keun, H.; Suk, O.; Choo, K.; Bo, Y. Incidence of Nonunion after Surgery of Distal Femoral Fractures Using Contemporary Fixation Device: A Meta-Analysis. Arch. Orthop. Trauma. Surg. 2020, 141, 225–233. [Google Scholar] [CrossRef]
- Obeid, F.; Van, T.C.; Brown, R.; Rainey, T. Nitrogen and Sulphur in Algal Biocrude: A Review of the HTL Process, Upgrading, Engine Performance and Emissions. Energy Convers. Manag. 2019, 181, 105–119. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Hu, T.; Nugroho, Y.K.; Yin, Z.; Hu, D.; Chu, R.; Mo, F.; Liu, C.; Hiltunen, E. Effects of Nitrogen Source Heterogeneity on Nutrient Removal and Biodiesel Production of Mono- and Mix-Cultured Microalgae. Energy Convers. Manag. 2019, 201, 112144. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for High-Value Compounds and Biofuels Production: A Review with Focus on Cultivation under Stress Conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Ogden, M.; Hoefgen, R.; Roessner, U.; Persson, S.; Khan, G.A. Feeding the Walls: How Does Nutrient Availability Regulate Cell Wall Composition? Int. J. Mol. Sci. 2018, 19, 2691. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Rofidi, I. Commercial Fertilizer as Cheaper Alternative Culture Medium for Microalgal Growth (Chlorella Sp.). Ph.D. Thesis, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia, 2017. [Google Scholar]
- Lananan, F.; Jusoh, A.; Ali, N.; Lam, S.S.; Endut, A. Effect of Conway Medium and f/2 Medium on the Growth of Six Genera of South China Sea Marine Microalgae. Bioresour. Technol. 2013, 141, 75–82. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships Derived from Physical Properties of Vegetable Oil and Biodiesel Fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Mosqueira, M.d.L.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Cruz Clavel-López, J.; Aburto, J. Transportation of Heavy and Extra-Heavy Crude Oil by Pipeline: A Review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Feng, S.; Yuan, Z.; Leitch, M.; Xu, C.C. Hydrothermal Liquefaction of Barks into Bio-Crude—Effects of Species and Ash Content/Composition. Fuel 2014, 116, 214–220. [Google Scholar] [CrossRef]

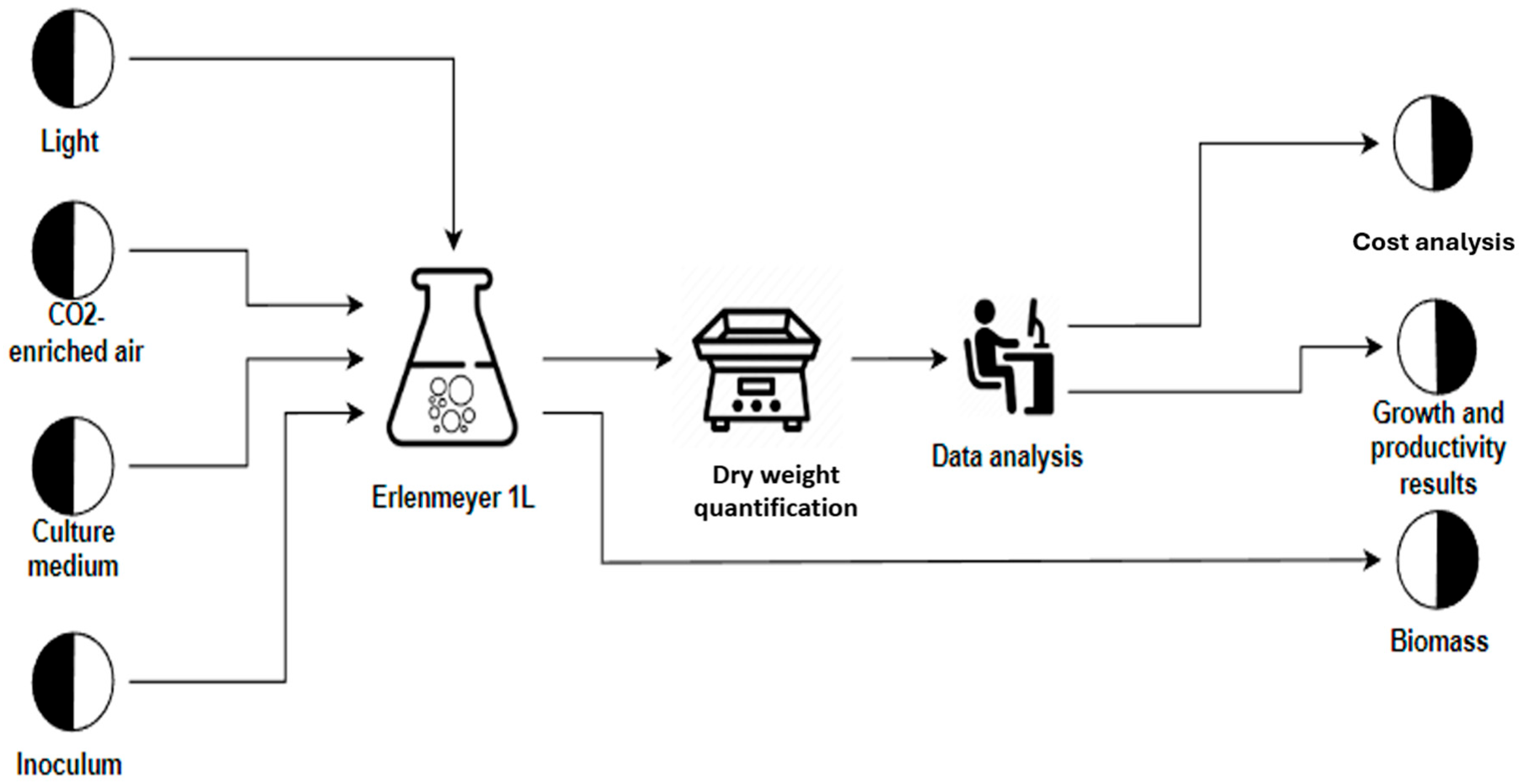
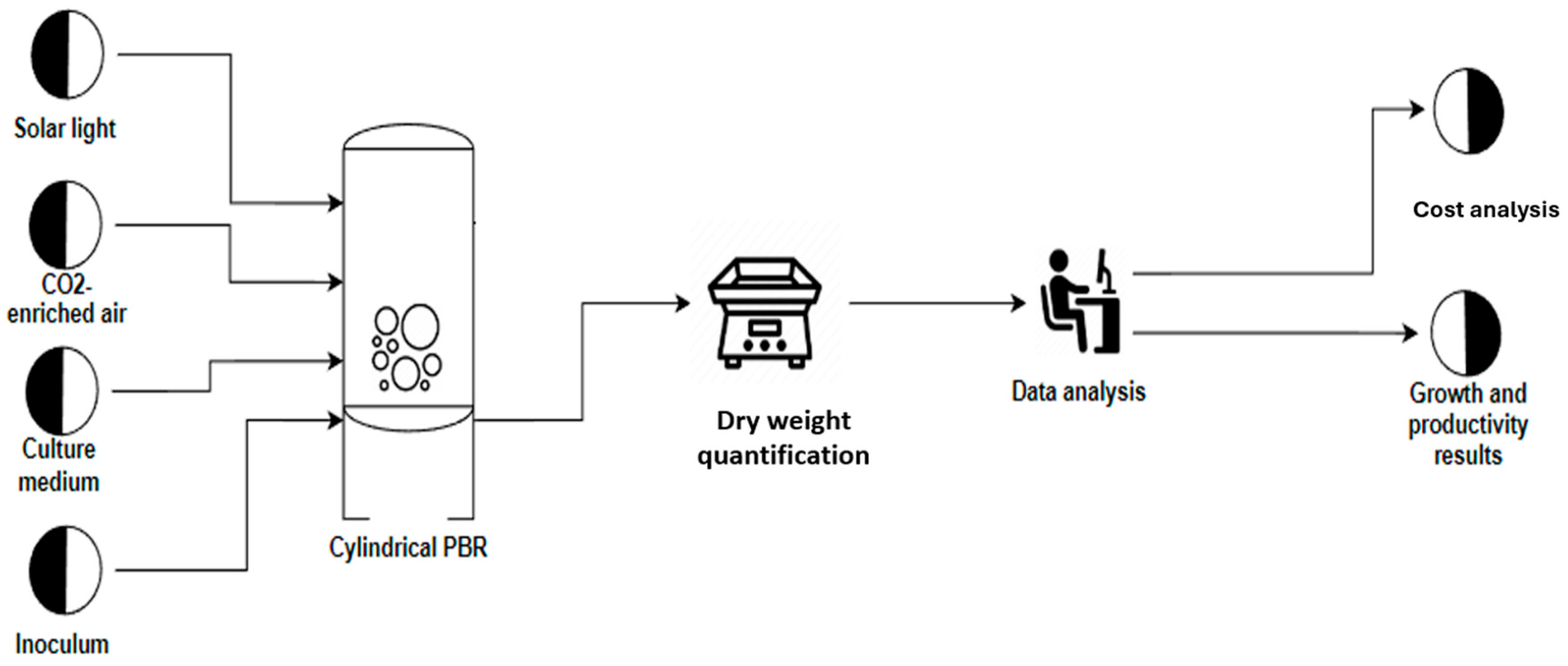
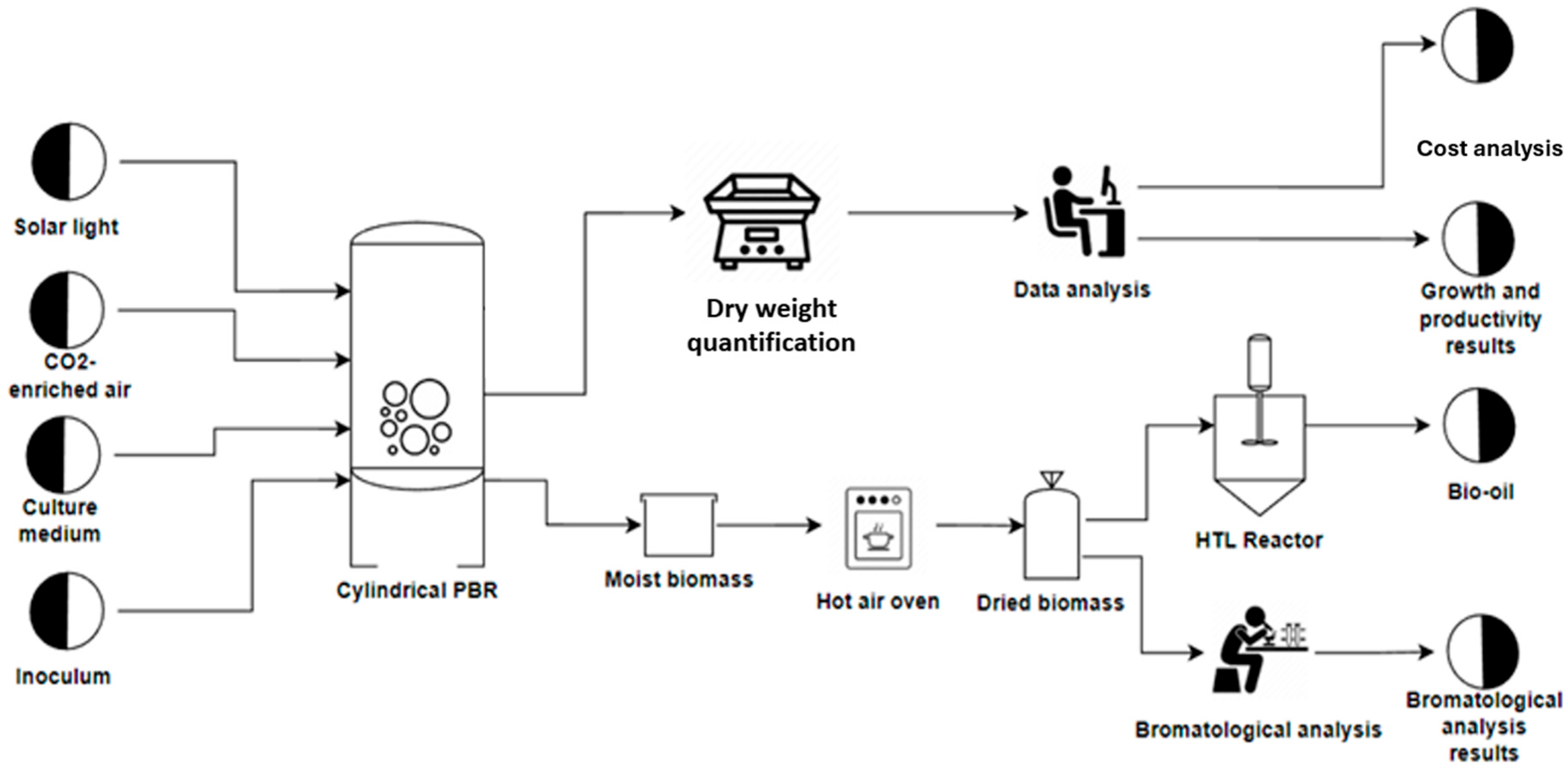

| Fertilizer Name | Nitrogen Species (g/L) | Composition | Function | Color | |||
|---|---|---|---|---|---|---|---|
| N (g/L) | P (g/L) | K (g/L) | Other (g/L) | ||||
| Florilizer® | NH4: 128.8 | 100.0 | 87.4 | 83.0 | O: 129.7 | Promotes flowering and fruit formation | Pale pink |
| Crecilizer® | NH4: 257.6 | 200.0 | 43.7 | 83.0 | O: 73.3 | Promotes plant growth and development | Pale green |
| AcuaLeaf macro® | NH4: 14.5 | 11.3 | 1.6 | 50.0 | O: 42.0 | Promotes plant development | Pale Blue |
| AcuaLeaf micros® | Not reported | Not reported | Not reported | 2.1 | Fe: 3.0 Mn: 0.12 Zn: 0.015 Mo: 0.009 Cu: 0.0001 Co: 0.0001 Mg: 0.8 B: 0.1 Ni: 0.0001 | Helps prevent and correct iron deficiencies in plants, supporting vital functions such as chlorophyll synthesis and photosynthesis | Burgundy |
| Treatment | Amount Added in mL/L of Each Fertilizer | |||
|---|---|---|---|---|
| C-Crecilizer® | F-Florilizer® | Ma-AcuaLeaf Macros® | Mi-AcuaLeaf Micro® | |
| C | 1.0 | |||
| F | 1.5 | |||
| Ma | 14.0 | |||
| Mi | 5.0 | |||
| C + Mi | 1.0 | 5.0 | ||
| F + Mi | 1.5 | 5.0 | ||
| Ma + Mi | 14.0 | 5.0 | ||
| Phase | Treatment | Amount Added in mL/L of Each Fertilizer | N Composition in g/L of Fertilizer | ||
|---|---|---|---|---|---|
| Crecilizer® | Florilizer® | Crecilizer® | Florilizer® | ||
| II | F-2 | 0.0 | 1.5 | 0.00 | 0.04 |
| C-2 | 1.0 | 0.0 | 0.04 | 0.00 | |
| III | F-5 | 0.0 | 5.0 | 0.00 | 0.50 |
| F-10 | 0.0 | 10.0 | 0.00 | 1.00 | |
| Sample | Ashes (%) | Protein (%) | Fiber (%) | Fats (%) | Carbohydrates (%) |
|---|---|---|---|---|---|
| F-5 | 24.08 | 50.05 | 0.98 | 3.32 | 21.57 |
| F-10 | 24.96 | 60.05 | 1.66 | 4.73 | 8.60 |
| BBM | 12.10 | 40.50 | 15.40 | 3.20 | 28.80 |
| Sample | % Yield Bio Crude | % Nitrogen | HHV MJ/Kg | °API | Density g/mL |
|---|---|---|---|---|---|
| F-5 | 34.2 | 5.58 | 35.1 | 11.96 | 0.986 |
| F-10 | 36.1 | 6.28 | 34.3 | 11.80 | 0.987 |
| BBM | 35.2 | 4.61 | 34.8 | 12.30 | 0.984 |
| Compound | % Area | |||
|---|---|---|---|---|
| F-5 | F-10 | BBM | ||
| Ketones | 2-Acetonylcyclopentanone | 2.56 | 2.15 | 2.41 |
| 2,3-Dimethyl-2-cyclopenten-1-one | 1.12 | 1.15 | 1.13 | |
| Aromatics | Cyclo(L-leucyl-L-phenylalanyl) | 1.06 | 1.05 | 1.10 |
| Morphinan-6-ol,4,5-epoxy-N-methyl | 1.30 | 1.36 | 1.38 | |
| Phenol,3,5-dimethoxy | 1.15 | 1.08 | 1.07 | |
| Pyridines | 1,2,5-Oxadiazole | 2.11 | 2.15 | 2.10 |
| 2-Ethyl-1,3,4-trimethyl-3-pyrazole | 1.83 | 1.94 | 1.80 | |
| 3,6-Diisopropylpiperazine-2,5-dione | 1.21 | 1.35 | 1.15 | |
| Pyridine,2,4,6-trimethyl | 1.44 | 1.58 | 1.32 | |
| Pyrimidine,4,6-dimethyl | 1.51 | 1.87 | 1.48 | |
| Pyrrolo[1,2-a]pyrazine | 3.12 | 3.25 | 2.94 | |
| Furans | 1,3-Dioxolane,2-(3,4-dihydroxy) | 1.56 | 1.76 | 1.81 |
| Furan,2-methyl-5-(methylthio) | 6.21 | 6.11 | 6.51 | |
| Quinoleines | 1H-Indole,3-methyl | 1.19 | 1.25 | 1.01 |
| 2,5-Piperazinedione,3-(phenylme) | 2.31 | 2.45 | 2.02 | |
| 2,5-Piperazinedione,3,6-bis(2-methyl) | 8.26 | 9.01 | 7.15 | |
| Alkanes | 1-Heptadecanol | 2.80 | 2.91 | 2.82 |
| (Z)-3-Methyl-2-decene | 4.25 | 3.85 | 4.30 | |
| (E)-8-Methyl-4-decene | 4.25 | 3.88 | 4.28 | |
| 5-Fluoro-m-xylene | 3.15 | 2.99 | 3.01 | |
| (Z,Z)-9,12-Octadecadienoic acid | 3.25 | 3.2 | 3.22 | |
| 9-Hexadecenoic acid | 6.28 | 6.12 | 6.32 | |
| Dodecane | 1.10 | 1.08 | 1.12 | |
| Heptadecane | 3.58 | 3.48 | 3.61 | |
| Hexadecane | 4.25 | 4.31 | 4.25 | |
| Pentadecane | 2.54 | 2.38 | 2.61 | |
| Phytol | 20.15 | 19.28 | 19.98 | |
| 7-Methyltridecane | 1.89 | 1.96 | 1.83 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, A.M.; Hernandez-Tenorio, F.; Vargas, G.J.; Ocampo, D.; Sáez, A.A. Fertilizer-Derived Low-Cost Culture Medium for Microalgae and Biofuel Production from Hydrothermal Liquefaction. Energies 2025, 18, 6559. https://doi.org/10.3390/en18246559
Miranda AM, Hernandez-Tenorio F, Vargas GJ, Ocampo D, Sáez AA. Fertilizer-Derived Low-Cost Culture Medium for Microalgae and Biofuel Production from Hydrothermal Liquefaction. Energies. 2025; 18(24):6559. https://doi.org/10.3390/en18246559
Chicago/Turabian StyleMiranda, Alejandra M., Fabian Hernandez-Tenorio, Gabriel J. Vargas, David Ocampo, and Alex A. Sáez. 2025. "Fertilizer-Derived Low-Cost Culture Medium for Microalgae and Biofuel Production from Hydrothermal Liquefaction" Energies 18, no. 24: 6559. https://doi.org/10.3390/en18246559
APA StyleMiranda, A. M., Hernandez-Tenorio, F., Vargas, G. J., Ocampo, D., & Sáez, A. A. (2025). Fertilizer-Derived Low-Cost Culture Medium for Microalgae and Biofuel Production from Hydrothermal Liquefaction. Energies, 18(24), 6559. https://doi.org/10.3390/en18246559






