Porous LiFePO4 Cathode Synthesized via Spray Drying for Enhanced Electrochemical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterization
2.2. Electrochemical Characterization
2.3. Fabrication of the RS-LFP Composite
2.4. Preparation of the Cathodes and Test Cell
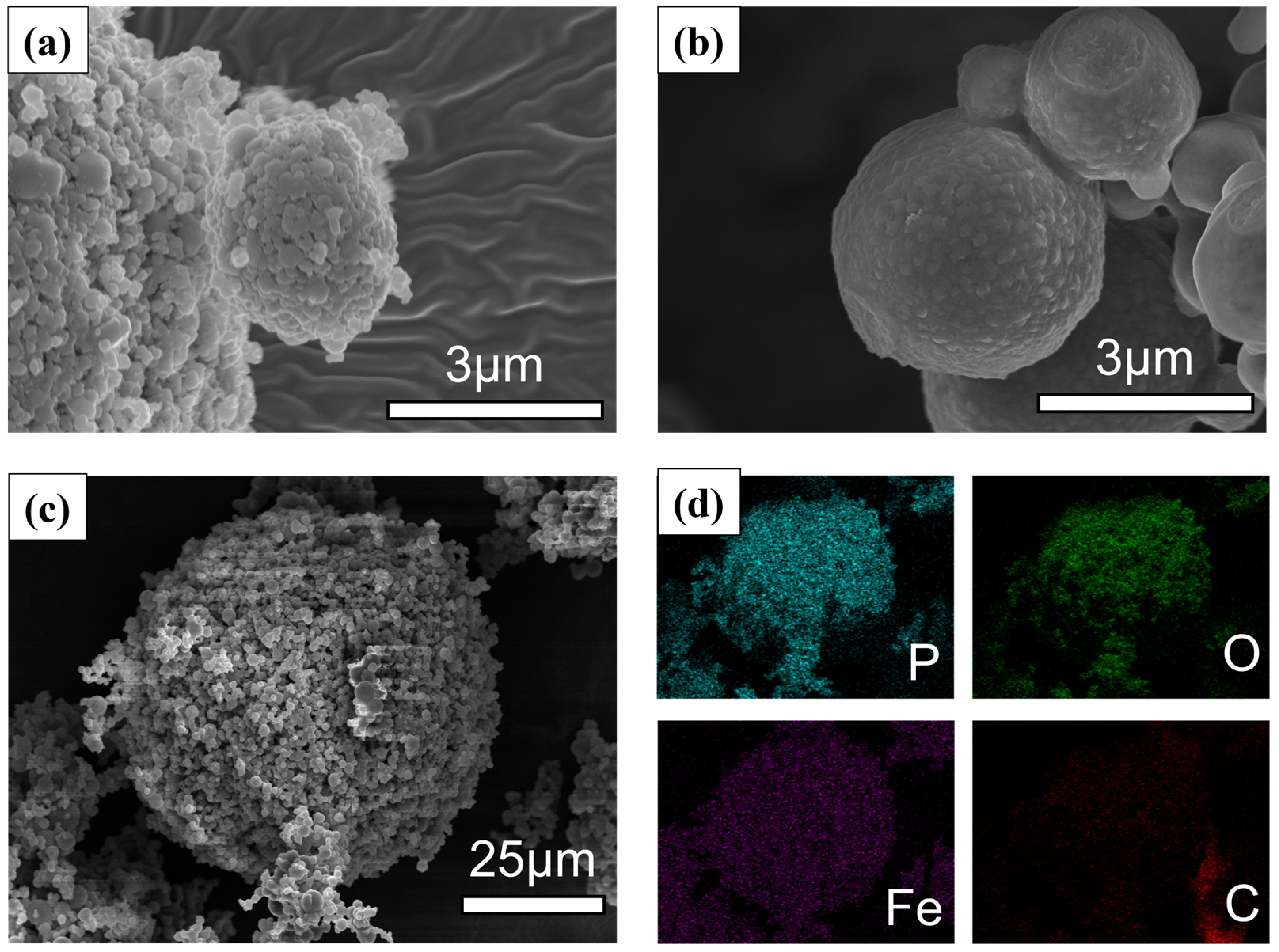
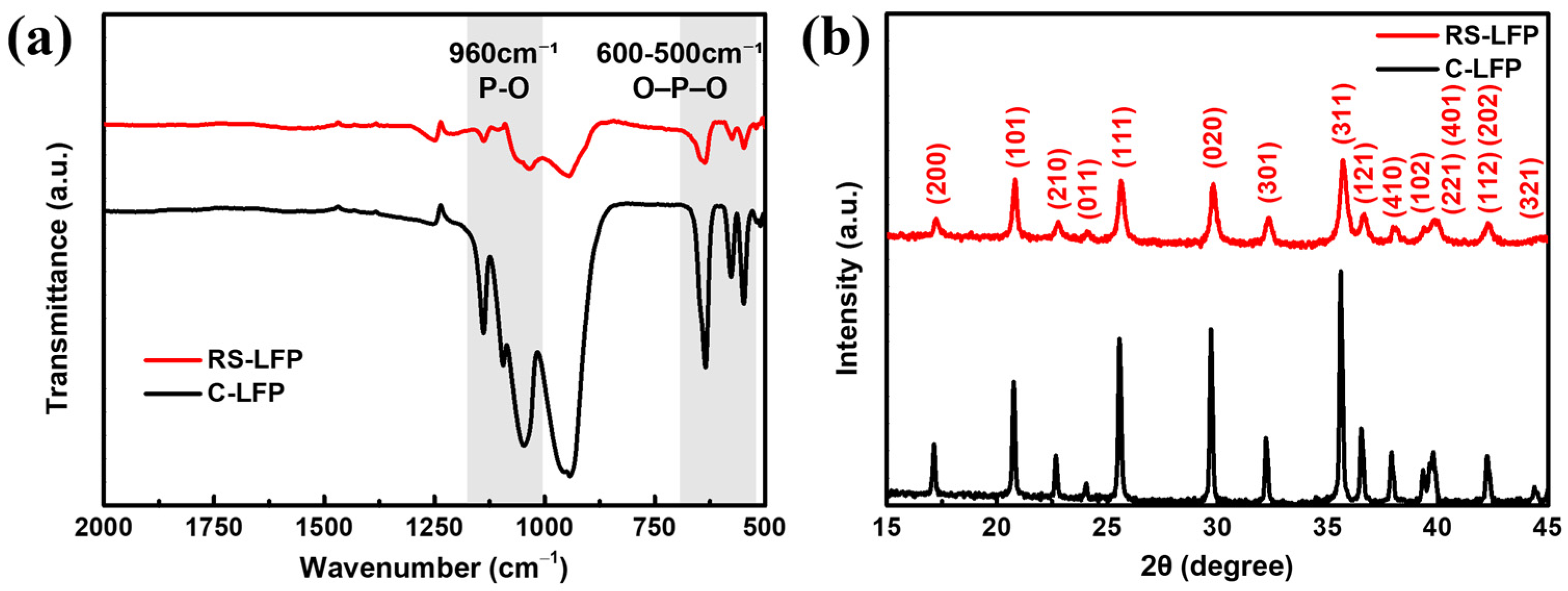
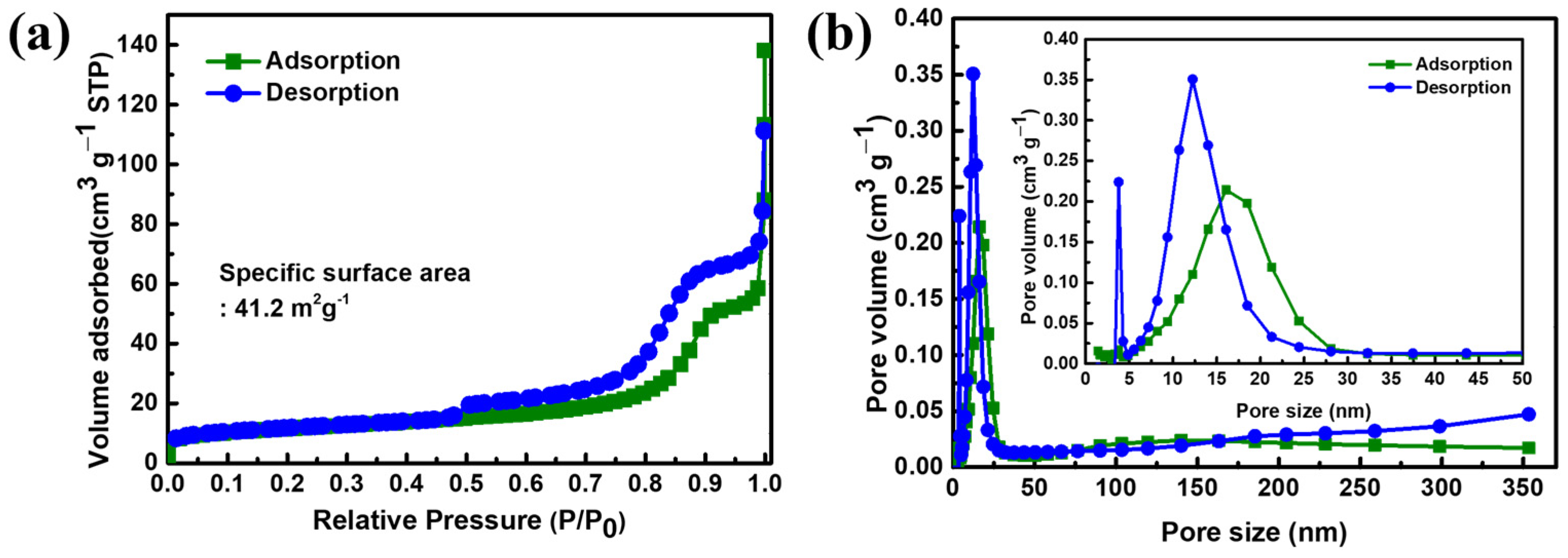
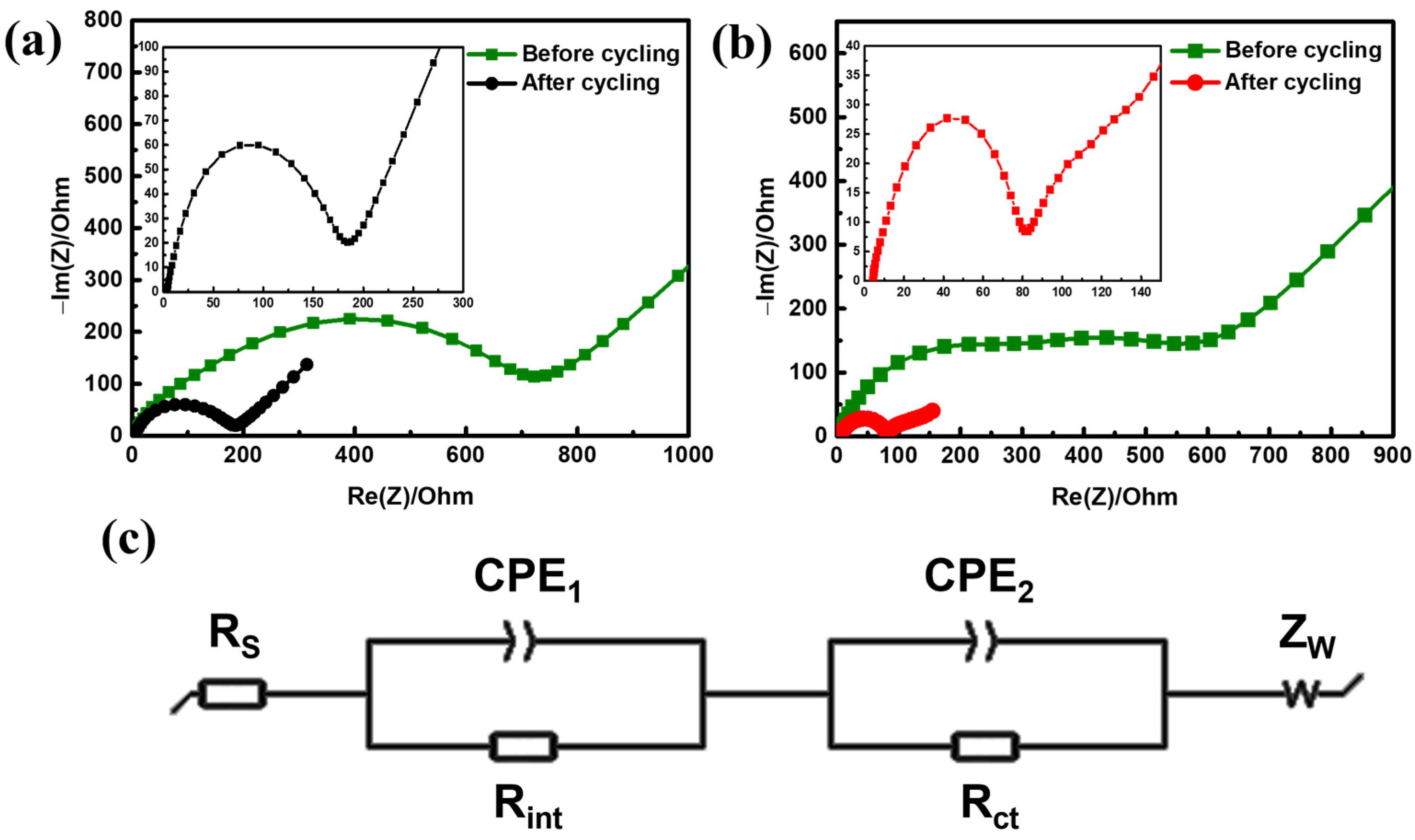
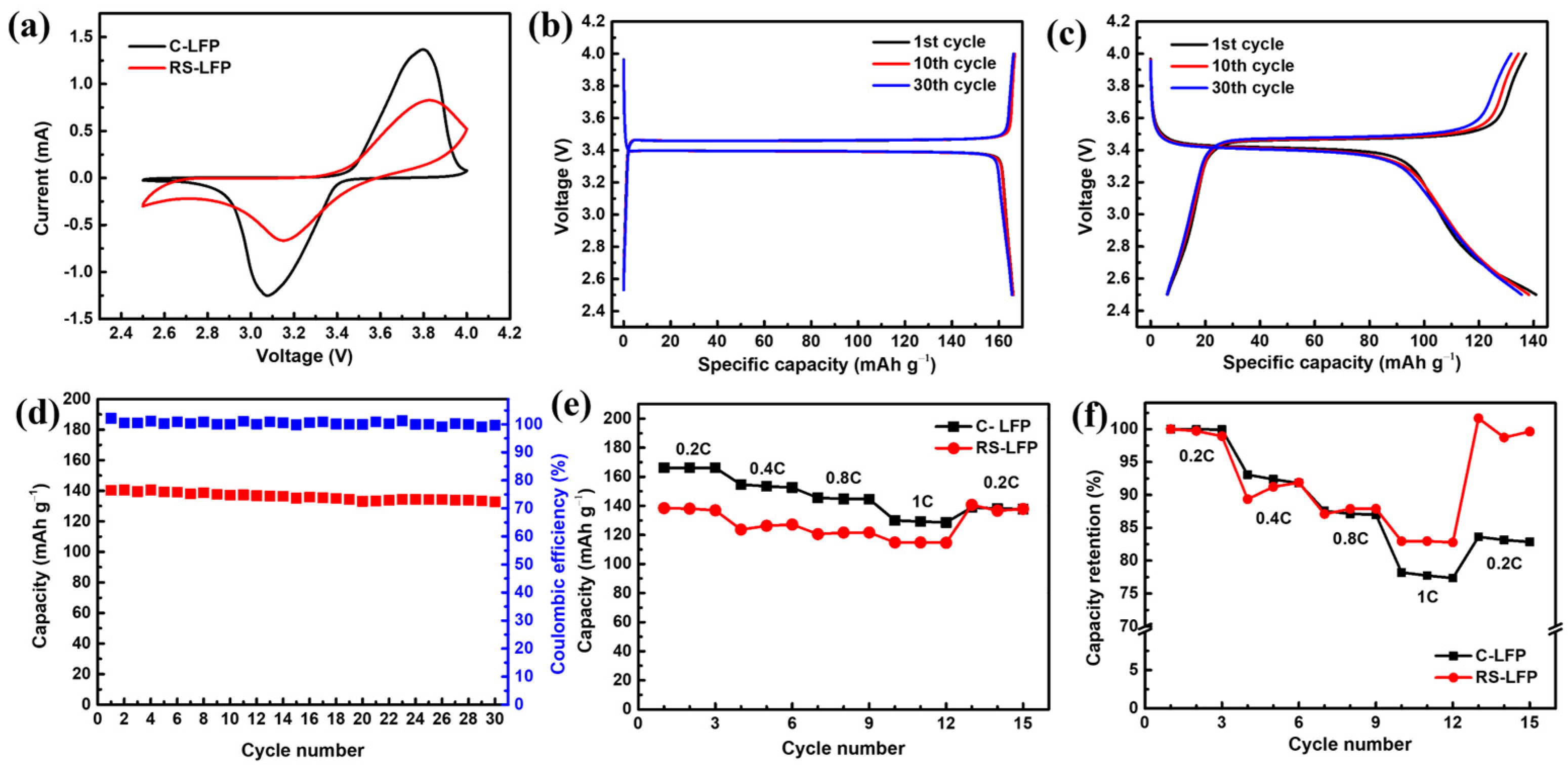
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.; Haque, R.; Jahirul, M.; Rasul, M.G.; Fattah, I.; Hassan, N.; Mofijur, M. Advancing energy storage: The future trajectory of lithium-ion battery technologies. J. Energy Storage 2025, 120, 116511. [Google Scholar] [CrossRef]
- Yang, J.; Guan, N.; Xu, C.; Si, L.; Wen, B.; Yuan, J.; Yang, H.; Zhong, H.; Lin, X.; Wu, Y. Synthesis and modification of LiFePO4 lithium-ion battery cathodes: A mini review. CrystEngComm 2024, 26, 3441–3454. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Mei, W.; Jiang, L.; Jia, Z.; Cheng, Z.; Sun, J.; Wang, Q. Understanding of thermal runaway mechanism of LiFePO4 battery in-depth by three-level analysis. Appl. Energy 2023, 336, 120695. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, F.; Fang, Z.; Gu, N.; Chen, B.; Yang, N.; Jia, Y. A comparative study of overcharge thermal runaway force-electrical-thermal characteristics and safety assessment of lithium batteries with different cathode materials. Appl. Therm. Eng. 2024, 256, 124092. [Google Scholar] [CrossRef]
- Quan, J.; Zhao, S.; Song, D.; Wang, T.; He, W.; Li, G. Comparative life cycle assessment of LFP and NCM batteries including the secondary use and different recycling technologies. Sci. Total Environ. 2022, 819, 153105. [Google Scholar] [CrossRef]
- Visone, B.; Senneca, O.; Prosini, P.; Apicella, B. Recovery of LiFePO4 cathodes: Criticalities and prospect towards a long-term eco-friendly solution. J. Power Sources Adv. 2025, 31, 100168. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Wang, X.; Chen, L.; Cao, G.; Wang, J.; Zhang, H.; He, X. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2022, 2, 125–137. [Google Scholar] [CrossRef]
- Na, S.; Park, C.; Park, K. LiFePO4 (LFP) coating and blending on Li1.05 (Ni0.88Co0.08Mn0.04)O2 (Ni-rich NCM): A strategy for reduced gas generation. J. Alloys Compd. 2025, 1031, 180771. [Google Scholar] [CrossRef]
- Xia, D.; Huang, W.; Shi, C.; Promi, A.; Hou, D.; Sun, C.; Hwang, S.; Kwon, G.; Huang, H.; Lin, F. Stable-Cycling Sustainable Na-Ion Batteries with Olivine Iron Phosphate Cathode in an Ether Electrolyte. ACS Sustain. Chem. Eng. 2025, 13, 241–250. [Google Scholar] [CrossRef]
- Jiao, L.; Li, Z.; Zhu, Y.; Wei, Z.; Liang, Y.; Wang, X.; Cui, Y.; Zhang, Z.; He, M.; Song, B. Enhanced electrical conductivity and lithium ion diffusion rate of LiFePO4 by Fe site and P site doping. AIP Adv. 2023, 13, 075306. [Google Scholar] [CrossRef]
- Kim, C.; Yang, Y.; Ha, D.; Kim, D.H.; Kim, H. Crystal alignment of a LiFePO4 cathode material for lithium ion batteries using its magnetic properties. RSC Adv. 2019, 9, 31936–31942. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tse, J.S. Li ion diffusion mechanisms in LiFePO4: An ab initio molecular dynamics study. J. Phys. Chem. A 2011, 115, 13045–13049. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, K.; Sultanov, F.; Mentbayeva, A.; Umirov, N.; Bakenov, Z.; Tatykayev, B. Advances in multi-element doping of LiFePO4 cathode material for capacity enhancement in Li-ion batteries. J. Power Sources 2024, 624, 235531. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, X.; Gridley, B.; Golden, W.; Ji, Y.; Johnson, S.; Lu, D.; Lin, F.; Liu, J.; Liu, Y. From Mining to Manufacturing: Scientific Challenges and Opportunities behind Battery Production. Chem. Rev. 2025, 125, 6397–6431. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, X.; Xiang, H.; Xie, J.; Li, J.; Zhou, Y. Mechanism for hydrothermal synthesis of LiFePO4 platelets as cathode material for lithium-ion batteries. J. Phys. Chem. C 2010, 114, 16806–16812. [Google Scholar] [CrossRef]
- Lan, T.; Guo, X.; Li, D.; Chen, Y. Preparation of LiFePO4 powders by ultrasonic spray drying method and their memory effect. Materials 2021, 14, 3193. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, L.; Zhao, S.; Hai, C.; Chen, T.; Zhang, J.; Zhao, Y.; Sun, Y.; Dong, S.; He, X. Cost-effective and efficient preparation of FePO4 electrodes from spent LiFePO4 batteries for enhanced lithium extraction from salt lakes. Mater. Today Energy 2025, 50, 101848. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.; Han, S.; Lee, J.; Lee, H.; Kwon, J.; Lee, J.; Seo, J.; Kim, P.J.; Song, T. Low-Resistance LiFePO4 Thick Film Electrode Processed with Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries. Small Sci. 2024, 4, 2300302. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, Z.; Li, L.; Li, Y. Unleash the Capacity Potential of LiFePO4 through Rocking-Chair Coordination Chemistry. Adv. Funct. Mater. 2022, 32, 2108692. [Google Scholar] [CrossRef]
- Pan, X.; Liang, Q.; Zhu, B.; He, R.; Jiang, S.; Ren, Y.; Lu, M.; Zhang, W.; Zhuang, S. Accelerating charge-transfer kinetics in LiFePO4 cathode through a construction of multifunctional layer for advanced lithium-ion batteries. Appl. Energy 2025, 382, 125229. [Google Scholar] [CrossRef]
- Rigamonti, M.G.; Chavalle, M.; Li, H.; Antitomaso, P.; Hadidi, L.; Stucchi, M.; Galli, F.; Khan, H.; Dolle, M.; Boffito, D.C. LiFePO4 spray drying scale-up and carbon-cage for improved cyclability. J. Power Sources 2020, 462, 228103. [Google Scholar] [CrossRef]
- Chen, C.; Luo, C.; Jin, Y.; Li, J.; Zhao, Q.; Yang, W. Short-process spray-drying synthesis of lithium iron phosphate@ carbon composite for lithium-ion batteries. ACS Sustain. Chem. Eng. 2024, 12, 14077–14086. [Google Scholar] [CrossRef]
- Styuf, E.A.; Othman, M.; Savina, A.A.; Komayko, A.I.; Filimonov, D.S.; Nikitina, V.A.; Abakumov, A.M. The microwave-assisted spray drying synthesis: Application to the LiFePO4/C cathode material for Li-ion batteries. J. Power Sources 2025, 642, 236951. [Google Scholar] [CrossRef]
- Rajasekar, K.; Raja, B. Heat and mass transfer characteristics during spray drying of Na2Fe0.6Mn0.4PO4F/C cathode material for Na-ion batteries. Appl. Therm. Eng. 2023, 221, 119838. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, S.; Shi, H.; Hu, H. Synthesis of FePO4·xH2O for fabricating submicrometer structured LiFePO4/C by a co-precipitation method. Ceram. Int. 2014, 40, 2685–2690. [Google Scholar] [CrossRef]
- Chairunnisa, A. Synthesis of LiFePO4 (lithium iron phosphate) with several methods: A review. RHAZES Green Appl. Chem. 2020, 10, 49–81. [Google Scholar]
- Lim, J.; Seo, S.; Kim, C. Synthesis of Iron Phosphate Via Coprecipitation Method for LiFePO4 Cathode. Korean J. Mater. Res. 2024, 34, 482–490. [Google Scholar] [CrossRef]
- Chen, L.; Feng, W.; Pu, Z.; Wang, X.; Su, W.; Li, M.; Song, C.; Shi, Z.; Zheng, Y. Effects of citric acid on the preparation of a LiFePO4@C cathode material assisted by biomineralization. Int. J. Electrochem. Sci. 2019, 14, 8048–8057. [Google Scholar] [CrossRef]
- Xie, G.; Zhu, H.-J.; Liu, X.-M.; Yang, H. A core–shell LiFePO4/C nanocomposite prepared via a sol–gel method assisted by citric acid. J. Alloys Compd. 2013, 574, 155–160. [Google Scholar] [CrossRef]
- Rostami, H.; Valio, J.; Tynjälä, P.; Lassi, U.; Suominen, P. Life cycle of LiFePO4 batteries: Production, recycling, and market trends. ChemPhysChem 2024, 25, e202400459. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Faizan, M.; Ali, B.; Bhuyan, M.M.; Bari, G.A.R.; Nam, K.-W. Review on the Polymeric and Chelate Gel Precursor for Li-Ion Battery Cathode Material Synthesis. Gels 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, J.; Yang, Y.; Song, G. Preparation and characterization of mesoporous LiFePO4/C microsphere by spray drying assisted template method. J. Power Sources 2009, 189, 794–797. [Google Scholar] [CrossRef]
- Liang, F.; Yao, Y.; Dai, Y.; Yang, B.; Ma, W.; Watanabe, T. Preparation of porous structure LiFePO4/C composite by template method for lithium-ion batteries. Solid State Ion. 2012, 214, 31–36. [Google Scholar] [CrossRef]
- Deng, H.; Jin, S.; Zhan, L.; Qiao, W.; Ling, L. Nest-like LiFePO4/C architectures for high performance lithium ion batteries. Electrochim. Acta 2012, 78, 633–637. [Google Scholar] [CrossRef]
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling particle formation: From single droplet drying to spray drying and electrospraying. Pharmaceutics 2020, 12, 625. [Google Scholar] [CrossRef]
- Sloth, J.; Jørgensen, K.; Bach, P.; Jensen, A.D.; Kiil, S.; Dam-Johansen, K. Spray drying of suspensions for pharma and bio products: Drying kinetics and morphology. Ind. Eng. Chem. Res. 2009, 48, 3657–3664. [Google Scholar] [CrossRef]
- Finotello, G. Droplet Collision Dynamics in a Spray Dryer: Experiments and Simulations. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2019. [Google Scholar]
- Liu, L.; Deng, Q.-F.; Ma, T.-Y.; Lin, X.-Z.; Hou, X.-X.; Liu, Y.-P.; Yuan, Z.-Y. Ordered mesoporous carbons: Citric acid-catalyzed synthesis, nitrogen doping and CO2 capture. J. Mater. Chem. 2011, 21, 16001–16009. [Google Scholar] [CrossRef]
- Pavlenko, V.; Żółtowska, S.; Haruna, A.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Yang, L.H.; Takeuchi, M.; Chen, Y.; Ng, N.L. Characterization of thermal decomposition of oxygenated organic compounds in FIGAERO-CIMS. Aerosol Sci. Technol. 2021, 55, 1321–1342. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Li, C.; Kong, L.; Zhang, S.; Hu, X. In-situ decomposition of citric acid for eliminating coking in steam reforming of acetic acid through enhanced gasification of coke precursors. J. Environ. Chem. Eng. 2024, 12, 114332. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Miao, Z.; Huang, K.; Liao, Y.; Xu, X.; Meng, J.; Li, Z.; Huang, Y. Hierarchical electrode architecture enabling ultrahigh-capacity LiFePO4 cathodes with low tortuosity. ACS Appl. Mater. Interfaces 2023, 15, 26824–26833. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, M.; Cao, Y.; Ai, X.; Yang, H. Template-free hydrothermal synthesis of nanoembossed mesoporous LiFePO4 microspheres for high-performance lithium-ion batteries. J. Phys. Chem. C 2010, 114, 3477–3482. [Google Scholar] [CrossRef]
- Ait Salah, A.; Jozwiak, P.; Zaghib, K.; Garbarczyk, J.; Gendron, F.; Mauger, A.; Julien, C. FTIR features of lithium-iron phosphates as electrode materials for rechargeable lithium batteries. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 1007–1013. [Google Scholar] [CrossRef]
- Abhilash, K.; Selvin, P.C.; Nalini, B.; Xia, H.; Adams, S.; Reddy, M. Electrochemical analysis of the carbon-encapsulated lithium iron phosphate nanochains and their high-temperature conductivity profiles. ACS Omega 2018, 3, 6446–6455. [Google Scholar] [CrossRef]
- Miao, C.; Bai, P.; Jiang, Q.; Sun, S.; Wang, X. A novel synthesis and characterization of LiFePO4 and LiFePO4/C as a cathode material for lithium-ion battery. J. Power Sources 2014, 246, 232–238. [Google Scholar] [CrossRef]
- Lin, Q.; Su, K.; Huang, Y.; He, Y.; Zhang, J.; Yang, X.; Xu, H. Molecular Crystal Structure Simulations and Structure-Magnetic Properties of LiFePO4 Composite Particles Optimized by La. Molecules 2024, 29, 3933. [Google Scholar] [CrossRef]
- Burba, C.M.; Frech, R. Raman and FTIR spectroscopic study of LixFePO4 (0 ≤ x ≤ 1). J. Electrochem. Soc. 2004, 151, A1032. [Google Scholar] [CrossRef]
- Benoy, S.M.; Singh, S.; Pandey, M. Characterization of nanocarbon based electrode material derived from anthracite coal. Mater. Res. Express 2020, 6, 125624. [Google Scholar] [CrossRef]
- Julien, C.M.; Zaghib, K.; Mauger, A.; Groult, H. Enhanced electrochemical properties of LiFePO4 as positive electrode of Li-ion batteries for HEV application. Adv. Chem. Eng. Sci. 2012, 2, 321–329. [Google Scholar] [CrossRef]
- Ait-Salah, A.; Zaghib, K.; Mauger, A.; Gendron, F.; Julien, C. Magnetic studies of the carbothermal effect on LiFePO4. Phys. Status Solidi A 2006, 203, R1–R3. [Google Scholar] [CrossRef]
- Kroff, M.; Hevia, S.A.; O’Shea, J.N.; Muro, I.G.d.; Palomares, V.; Rojo, T.; Del Río, R. Lithium iron phosphate/carbon (LFP/C) Composite using nanocellulose as a reducing agent and carbon source. Polymers 2023, 15, 2628. [Google Scholar] [CrossRef]
- Lin, L.; Wen, Y.; Guo, Y.; Xiao, D. X-ray diffraction study of LiFePO4 synthesized by hydrothermal method. RSC Adv. 2013, 3, 14652–14660. [Google Scholar] [CrossRef]
- Naik, A.; Zhou, J.; Gao, C.; Wang, L. Microwave Assisted Solid State Synthesis of LiFePO4/C Using Two Different Carbon Sources. Int. J. Electrochem. Sci. 2014, 9, 6124–6133. [Google Scholar] [CrossRef]
- Guo, B.; Ruan, H.; Zheng, C.; Fei, H.; Wei, M. Hierarchical LiFePO4 with a controllable growth of the (010) facet for lithium-ion batteries. Sci. Rep. 2013, 3, 2788. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, Z.; Pang, W.; Tong, G.; Liang, F. High electrochemical performance of highly (020) preferred orientation LiFePO4 for lithium ion battery. J. Power Sources 2025, 644, 237131. [Google Scholar] [CrossRef]
- Shiraishi, K.; Dokko, K.; Kanamura, K. Formation of impurities on phospho-olivine LiFePO4 during hydrothermal synthesis. J. Power Sources 2005, 146, 555–558. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.-C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224. [Google Scholar] [CrossRef]
- Hsu, K.-F.; Tsay, S.-Y.; Hwang, B.-J. Synthesis and characterization of nano-sized LiFePO4 cathode materials prepared by a citric acid-based sol–gel route. J. Mater. Chem. 2004, 14, 2690–2695. [Google Scholar] [CrossRef]
- Cabán-Huertas, Z.; Ayyad, O.; Dubal, D.P.; Gómez-Romero, P. Aqueous synthesis of LiFePO4 with fractal granularity. Sci. Rep. 2016, 6, 27024. [Google Scholar] [CrossRef]
- Yim, C.-H.; Baranova, E.A.; Abu-Lebdeh, Y.; Davidson, I. Highly ordered LiFePO4 cathode material for Li-ion batteries templated by surfactant-modified polystyrene colloidal crystals. J. Power Sources 2012, 205, 414–419. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.-Z.; Jin, J.; Liu, J.; Li, Y.; Wang, H.-E.; Chen, L.-H.; Wang, B.-J.; Su, B.-L. Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability. Sci. Rep. 2016, 6, 25942. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H. Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv. Mater. 2010, 22, 4944–4948. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Sun, D.; Tang, Y.; He, K.; Ren, Y.; Liu, S.; Wang, H. Long-lived aqueous rechargeable lithium batteries using mesoporous LiTi2 (PO4)3@C anode. Sci. Rep. 2015, 5, 17452. [Google Scholar] [CrossRef]
- Dai, F.; Yi, R.; Yang, H.; Zhao, Y.; Luo, L.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, C.; Zhang, S. Minimized volume expansion in hierarchical porous silicon upon lithiation. ACS Appl. Mater. Interfaces 2019, 11, 13257–13263. [Google Scholar] [CrossRef]
- Meng, Z.; Ma, X.; Azhari, L.; Hou, J.; Wang, Y. Morphology controlled performance of ternary layered oxide cathodes. Commun. Mater. 2023, 4, 90. [Google Scholar] [CrossRef]
- Kim, J.; Lesmana, L.A.; Aziz, M. Impact analysis of particle sphericity on the properties of porous materials via particle packing method for hydrogen fuel and electrolysis cells. Comput. Chem. Eng. 2025, 192, 108907. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. Npj Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Müller, M.; Schneider, L.; Bohn, N.; Binder, J.R.; Bauer, W. Effect of nanostructured and open-porous particle morphology on electrode processing and electrochemical performance of Li-ion batteries. ACS Appl. Energy Mater. 2021, 4, 1993–2003. [Google Scholar] [CrossRef]
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of functional binders for high-specific-energy lithium-ion batteries: From molecular structure to electrode properties. Ind. Chem. Mater. 2024, 2, 191–225. [Google Scholar] [CrossRef]
- Chang, J.H.; Pin, M.W.; Kim, I.; Kim, S.; Kim, S.; Moon, S.; Cho, J.; Choi, S.; Heo, B.; Chandio, Z.A. Binder migration: Frequently observed yet overlooked phenomena in electrode processing for lithium-ion batteries. J. Energy Storage 2024, 83, 110729. [Google Scholar] [CrossRef]
- Wang, S.-H.; Yue, J.; Dong, W.; Zuo, T.-T.; Li, J.-Y.; Liu, X.; Zhang, X.-D.; Liu, L.; Shi, J.-L.; Yin, Y.-X. Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat. Commun. 2019, 10, 4930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Youn, J.W.; Park, G.H.; Kim, M.; Kang, S.K.; Jang, D.; Kim, W.B. Surface modification with F-doped carbon layer coating on natural graphite anode for improving interface compatibility and electrochemical performance of lithium-ion capacitors. ACS Appl. Electron. Mater. 2023, 5, 4344–4353. [Google Scholar] [CrossRef]
- Zabara, M.A.; Katırcı, G.k.; Ülgüt, B. Operando investigations of the interfacial electrochemical kinetics of metallic lithium anodes via temperature-dependent electrochemical impedance spectroscopy. J. Phys. Chem. C 2022, 126, 10968–10976. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Shimizu, R.; Momma, T.; Osaka, T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J. Power Sources 2019, 409, 139–147. [Google Scholar] [CrossRef]
- Peng, Y.; Zeng, L.; Dai, S.; Liu, F.; Rao, X.; Zhang, Y. LiFePO4/C twin microspheres as cathode materials with enhanced electrochemical performance. RSC Adv. 2023, 13, 6983–6992. [Google Scholar] [CrossRef]
- Ma, Z.-P.; Shao, G.-J.; Wang, W.; Zhang, C.-Y. Preparation and Study on Electrochemical Performance of High-Rate LiFePO4/C Cathode Material. Asian J. Chem. 2013, 25, 3579. [Google Scholar] [CrossRef]
- Syed, M.A.; Salehabadi, M.; Obrovac, M. High energy density large particle LiFePO4. Chem. Mater. 2024, 36, 803–814. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Y.; Zhao, X.; Zhi, X.; Liang, G. Effect of particle size and purity on the low temperature electrochemical performance of LiFePO4/C cathode material. J. Alloys Compd. 2016, 683, 123–132. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, M.; Liu, F.; Yang, J.; Feng, T. Variation of carbon coatings on the electrochemical performance of LiFePO4 cathodes for lithium ionic batteries. RSC Adv. 2017, 7, 44296–44302. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Safari, M.; Delacourt, C. Aging of a commercial graphite/LiFePO4 cell. J. Electrochem. Soc. 2011, 158, A1123. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Z.; Li, J. Hierarchical carbon-coated LiFePO4 nanoplate microspheres with high electrochemical performance for Li-ion batteries. Adv. Mater. 2011, 23, 1126–1129. [Google Scholar] [CrossRef]
- Zaghib, K.; Dubé, J.; Dallaire, A.; Galoustov, K.; Guerfi, A.; Ramanathan, M.; Benmayza, A.; Prakash, J.; Mauger, A.; Julien, C. Enhanced thermal safety and high power performance of carbon-coated LiFePO4 olivine cathode for Li-ion batteries. J. Power Sources 2012, 219, 36–44. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ahn, S. Porous LiFePO4 Cathode Synthesized via Spray Drying for Enhanced Electrochemical Performance. Energies 2025, 18, 6228. https://doi.org/10.3390/en18236228
Kim J, Ahn S. Porous LiFePO4 Cathode Synthesized via Spray Drying for Enhanced Electrochemical Performance. Energies. 2025; 18(23):6228. https://doi.org/10.3390/en18236228
Chicago/Turabian StyleKim, Jimin, and Seongki Ahn. 2025. "Porous LiFePO4 Cathode Synthesized via Spray Drying for Enhanced Electrochemical Performance" Energies 18, no. 23: 6228. https://doi.org/10.3390/en18236228
APA StyleKim, J., & Ahn, S. (2025). Porous LiFePO4 Cathode Synthesized via Spray Drying for Enhanced Electrochemical Performance. Energies, 18(23), 6228. https://doi.org/10.3390/en18236228




