Solar-Powered Interfacial Evaporation for Simultaneous Photocatalytic Hydrogen Production and Salinity Gradient Power Generation
Abstract
1. Introduction
2. Experiment and Methods
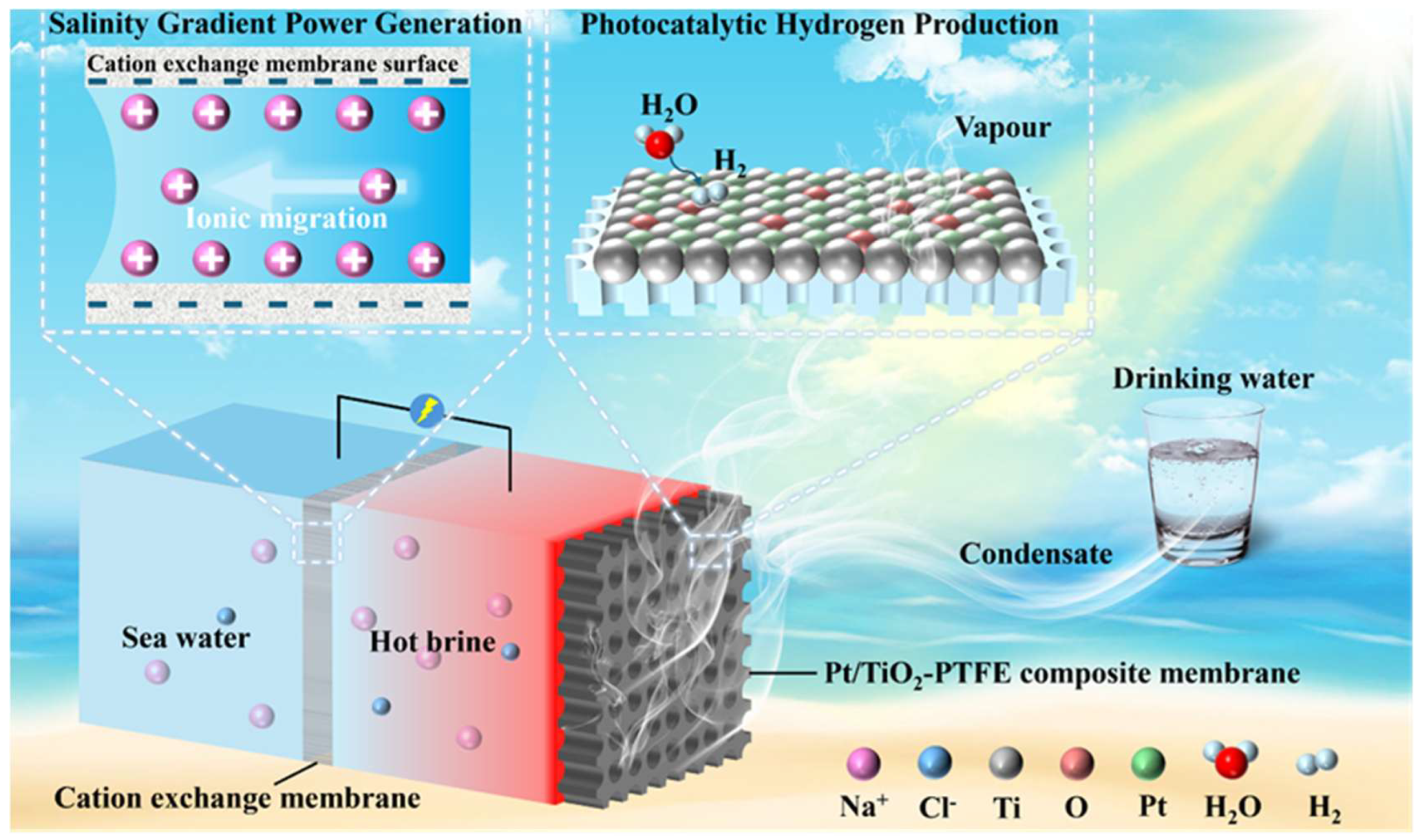
2.1. Design Concept of the SWHE System
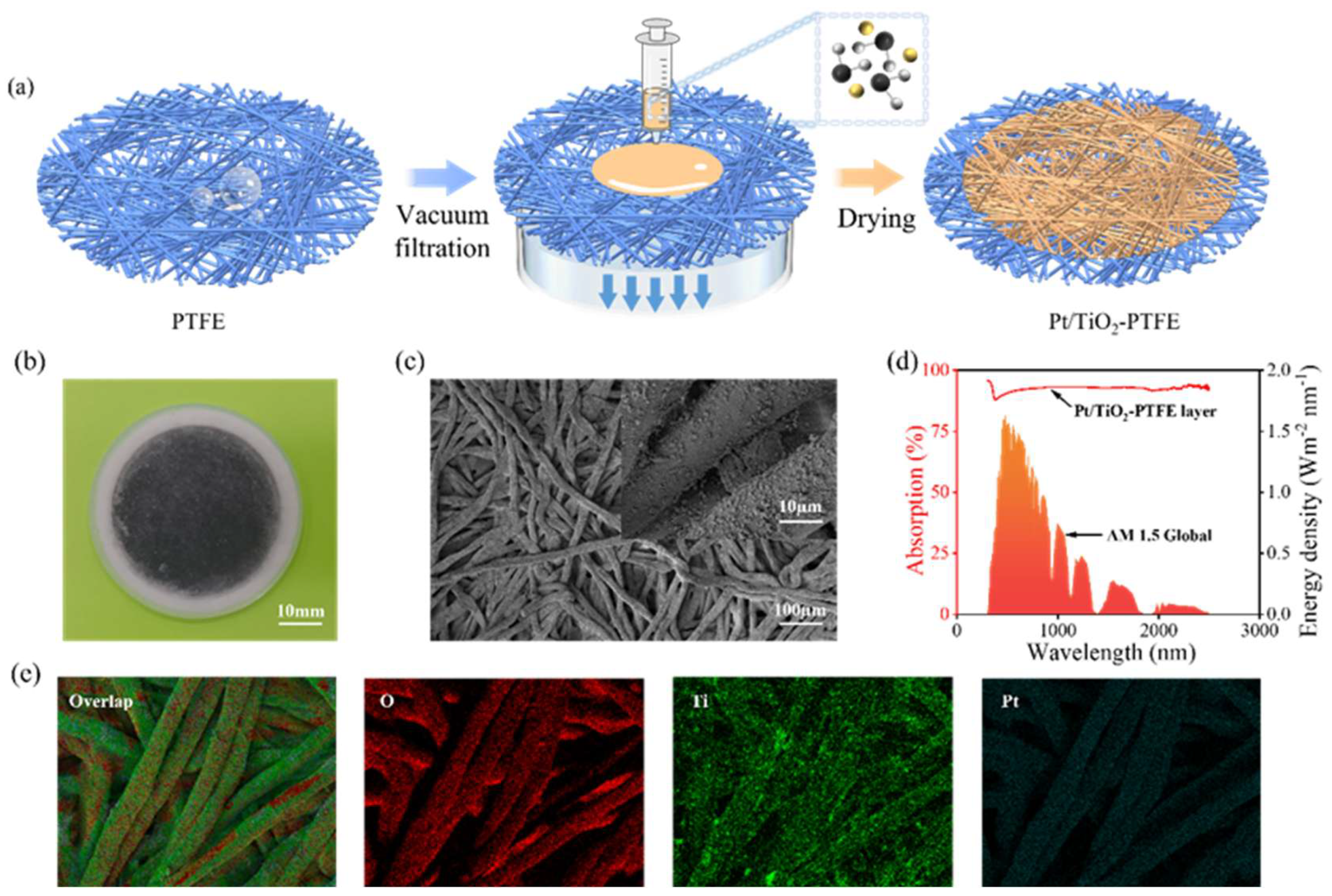
2.2. Material Characterization
3. Results and Discussion
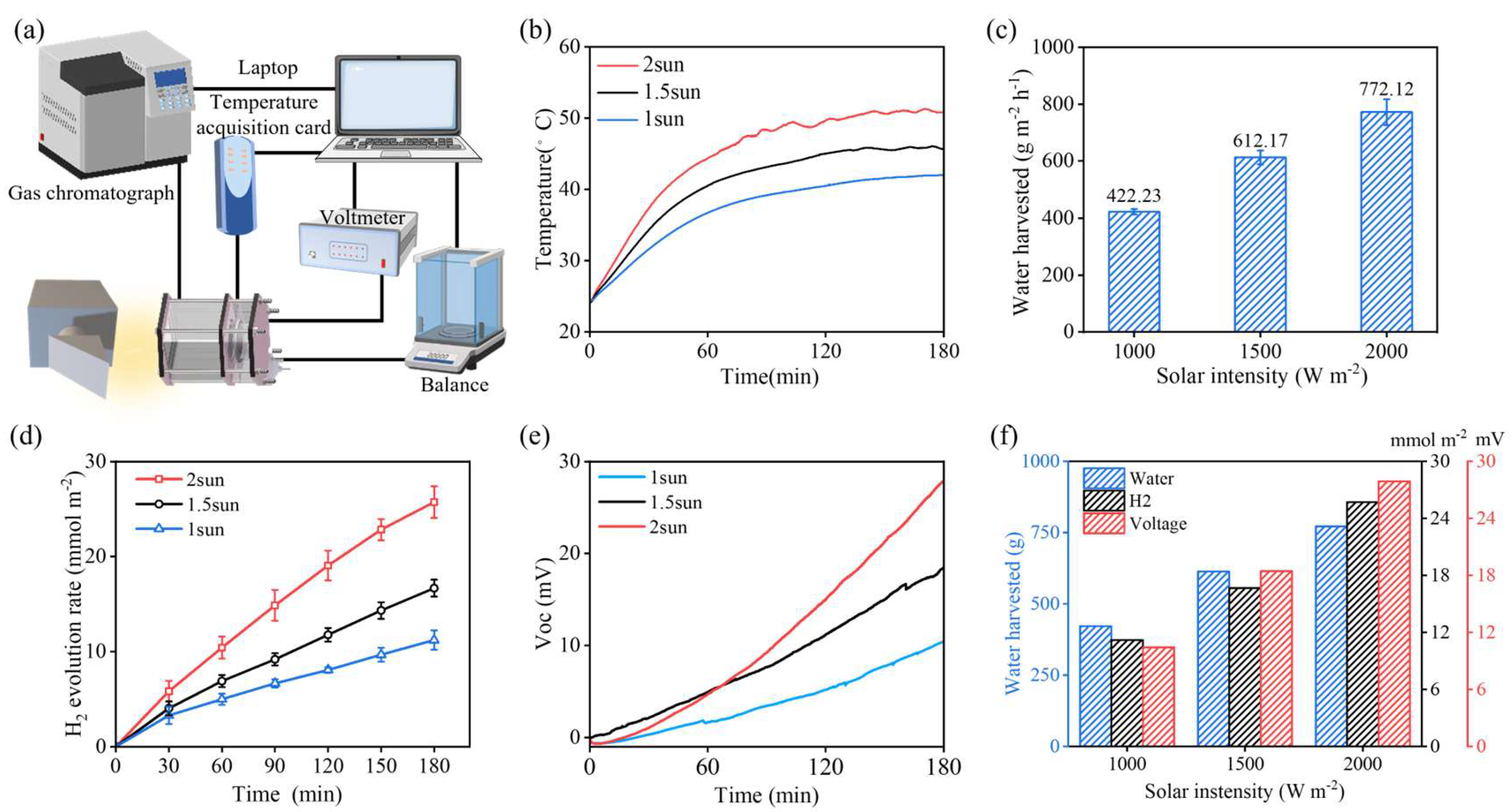
3.1. Evaluation of Basic Performance
3.2. Reliability Testing of the Device
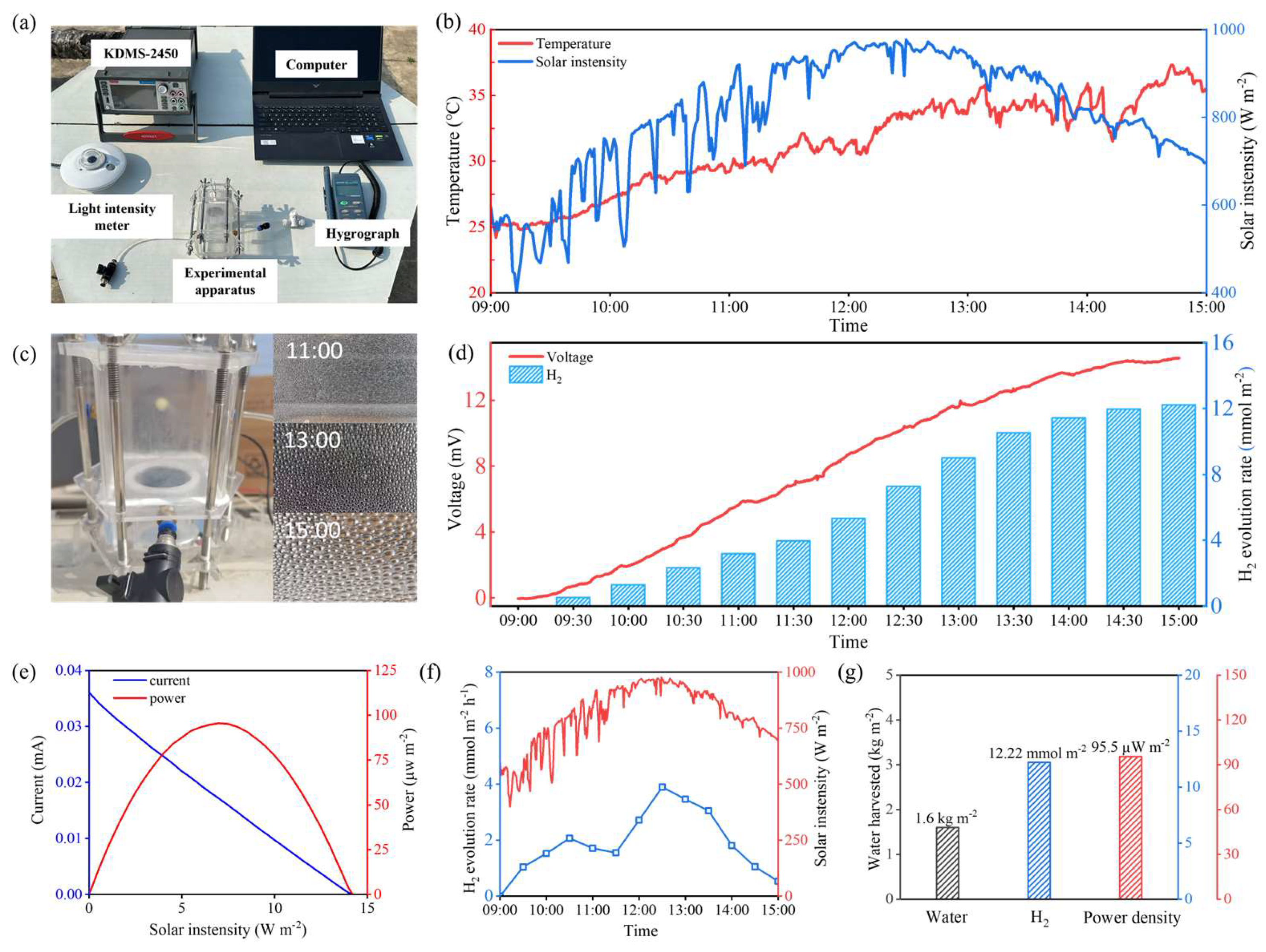
4. Outdoor Performance Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SWHE | Solar-driven water–hydrogen–electricity |
| SIE | Solar-driven interfacial evaporation |
| IPHE | Interface photocatalytic hydrogen evolution |
| RE | Reverse electrodialysis |
| PTFE | Polytetrafluoroethylene |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectrometer |
| XPS | X-ray photoelectron spectroscopy |
References
- Curto, D.; Franzitta, V.; Guercio, A. A review of the water desalination technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Rosa, L.; Sangiorgio, M. Global water gaps under future warming levels. Nat. Commun. 2025, 16, 1192. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, T.; Böhmelt, T. International conflict and cooperation over freshwater resources. Nat. Sustain. 2020, 3, 350–356. [Google Scholar] [CrossRef]
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; van Vliet, M.T.H.; Strokal, M. A triple increase in global river basins with water scarcity due to future pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, Y.; Felfelani, F.; Satoh, Y.; Boulange, J.; Burek, P.; Gädeke, A.; Gerten, D.; Gosling, S.N.; Grillakis, M.; Gudmundsson, L.; et al. Global terrestrial water storage and drought severity under climate change. Nat. Clim. Change 2021, 11, 226–233. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, H.L.; Li, Y.; Tang, Q.; Woolway, R.I.; Merder, J.; Rosa, L.; Michalak, A.M. Decoupling of surface water storage from precipitation in global drylands due to anthropogenic activity. Nat. Water 2025, 3, 80–88. [Google Scholar] [CrossRef]
- He, C.Y.; Liu, Z.F.; Wu, J.G.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Liu, X.H.; Mishra, D.D.; Wang, X.B.; Peng, H.; Hu, C. Towards highly efficient solar-driven interfacial evaporation for desalination. J. Mater. Chem. A 2020, 8, 17907. [Google Scholar] [CrossRef]
- Shen, J.; Cai, Y.C.; Zhang, C.H.; Wei, W.; Chen, C.; Liu, L.; Yang, K.; Ma, Y.; Wang, Y.; Tseng, C.-C.; et al. Fast water transport and molecular sieving through ultrathin ordered conjugated-polymer-framework membranes. Nat. Mater. 2022, 21, 1183–1190. [Google Scholar] [CrossRef]
- Gu, X.S.; Wei, W.F.; Feng, X.Z.; Wang, R.; Yang, S.; Zeng, Z.; Chen, H. Photovoltaic-Driven Battery Deionization System for Efficient and Sustainable Seawater Desalination. Environ. Sci. Technol. 2024, 58, 22992–23003. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Xu, J.W.; Liang, Y.Z.; Luo, X.; Chen, J.; Yang, Z.; He, J.; Chen, Y. Bioinspired Solar-Driven Osmosis for Stable High Flux Desalination. Environ. Sci. Technol. 2024, 58, 3800–3811. [Google Scholar] [CrossRef] [PubMed]
- He, S.X.; Zhang, W.; Gu, Z.L. Ultrafast seawater desalination with phosphorene oxide nanochannel based membrane: Nonmonotonic relationship between oxidation degree and water permeability. Desalination 2023, 563, 116751. [Google Scholar] [CrossRef]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Gabriel, L.; Boriskina, S.; Li, H.; Yang, W.; Zhang, T.; Chen, G. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 2016, 1, 16126. [Google Scholar] [CrossRef]
- Yu, Y.L.; Liu, Y.M.; Zhang, X.Z.; Lv, B.; Xu, Y.; Fan, X. Co-enhancing volatile organic compound degradation and steam generation in solar interfacial evaporation by integrating with electro-Fenton. Water. Res. 2025, 277, 123348. [Google Scholar] [CrossRef]
- Li, T.; Wu, M.; Xu, J.X.; Du, R.; Yan, T.; Wang, P.; Bai, Z.; Wang, R.; Wang, S. Simultaneous atmospheric water production and 24-hour power generation enabled by moisture-induced energy harvesting. Nat. Commun. 2022, 13, 6771. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, T.P.; Gao, M.M.; Chan, K.H.; Cheng, Y.; He, J.; Ho, G.W. Controlled heterogeneous water distribution and evaporation towards enhanced photothermal water-electricity-hydrogen production. Nano Energy 2020, 77, 105102. [Google Scholar] [CrossRef]
- Jang, J.H.; Kang, J.H.; Ghule, B.; Kim, H.; Jang, J.-H. An Approach to Enhance PEC Water Splitting Performance through Al: Ti Codoping in Hematite (α-Fe2O3) Photoanode: The Effect of Al3+ as a Codopant. ACS Materials Lett. 2024, 6, 2897–2904. [Google Scholar]
- Li, X.Q.; Min, X.Z.; Zhu, J.; Li, J.; Xu, N.; Zhu, P.; Zhu, B.; Zhu, S. Storage and recycling of interfacial solar steam enthalpy. Joule 2018, 2, 2477–2484. [Google Scholar] [CrossRef]
- Tanaka, H.; Nosoko, T.; Nagata, T. A highly productive basin-type-multiple-effect coupled solar still. Desalination 2000, 130, 279–293. [Google Scholar] [CrossRef]
- Jiang, M.M.; Zheng, R.Q.; Wang, M.J.; Li, X. A MoS2-based evaporator with porous structure and interlayer channels for solar desalination and photocatalytic degradation. Sol. Energy 2024, 279, 112853. [Google Scholar] [CrossRef]
- Ning, J.Q.; Yang, C.L.; Mei, Q.Y.; Huang, L.; Han, K. One-dimensional Fe/C constructed Janus membrane enables highly-efficient and stable solar-driven interfacial evaporation. Adv. Membr. 2024, 4, 100108. [Google Scholar] [CrossRef]
- Mojtaba, E.M.; Lu, Q.; Yang, R.; Lu, Q. All-in-One Hybrid Solar-Driven Interfacial Evaporators for Cogeneration of Clean Water and Electricity. Adv. Funct. Mater. 2025, 35, 2412870. [Google Scholar]
- Li, X.T.; Zhang, J.P. Highly salt-resistant and all-weather solar-driven interfacial evaporators with photothermal and electrothermal effects based on Janus graphene @ silicone sponges. Nano Energy 2021, 81, 105682. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, L.M.; Yang, W.; Tang, C.-Y.; Pu, J.-H.; Zha, X.-J.; Ke, K.; Bao, R.-Y.; Yang, M.-B. All-weather-available, continuous steam generation based on the synergistic photo-thermal and electro-thermal conversion by MXene-based aerogels. Mater. Horiz. 2020, 7, 855–865. [Google Scholar] [CrossRef]
- Zhu, L.L.; Ding, T.P.; Gao, M.M.; Peh, C.K.N.; Ho, G.W. Shape Conformal and Thermal Insulative Organic Solar Absorber Sponge for Photothermal Water Evaporation and Thermoelectric Power Generation. Adv. Energy Mater. 2019, 9, 1900250. [Google Scholar] [CrossRef]
- Wang, W.B.; Shi, Y.S.; Zhang, C.L.; Hong, S.; Shi, L.; Chang, J.; Li, R.; Jin, Y.; Ong, C.; Zhuo, S.; et al. Simultaneous production of fresh water and electricity via multistage solar photovoltaic membrane distillation. Nat. Commun. 2019, 10, 3012. [Google Scholar] [CrossRef]
- Yang, P.H.; Liu, K.; Chen, Q.; Li, J.; Duan, J.; Xue, G.; Xu, Z.; Xie, W.; Zhou, J. Solar-driven simultaneous steam production and electricity generation from salinity. Energy Environ. Sci. 2017, 10, 1923–1927. [Google Scholar] [CrossRef]
- Sun, Z.A.; Han, C.L.; Gao, S.W.; Li, Z.; Jing, M.; Yu, H.; Wang, Z. Achieving efficient power generation by designing bioinspired and multi-layered interfacial evaporator. Nat. Commun. 2022, 13, 5077. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Qin, C.L.; Gui, C.X.; Ren, T.; Huang, L.; Liu, P.; Wei, Q.; Liu, W.; Zhou, Z.; Huang, Y.; Fan, K. Solar-driven hydrogen and water co-generation based on atmospheric water harvesting. Chem. Eng. J. 2025, 504, 158721. [Google Scholar] [CrossRef]
- Kilsgaard, B.S.; Haldrup, S.; Catalano, J.; Bentien, A. High figure of merit for electrokinetic energy conversion in Nafion membranes. J. Power Sources 2014, 247, 235–242. [Google Scholar] [CrossRef]
- Koh, W.H.; Silverman, H.P. Anion transport in thin-channel cation exchange membranes. J. Membr. Sci. 1983, 13, 279–290. [Google Scholar] [CrossRef]
- Hsu, J.P.; Tai, Y.H.; Yeh, L.H.; Tseng, S. Importance of temperature effect on the electrophoretic behavior of charge-regulated particles. Langmuir 2012, 28, 1013–1019. [Google Scholar] [CrossRef]
- Tseng, S.; Li, Y.M.; Lin, C.Y.; Hsu, J.P. Salinity gradient power: Influences of temperature and nanopore size. Nanoscale 2016, 8, 2350–2357. [Google Scholar] [CrossRef]
- Chaule, S.; Khudoyarov, D.; Kim, S.; Yoon, Y.; Jang, J.H. Inverse-L Shaped Evaporator Based on La1−xSrxMnO3 Perovskite with Efficient Salt Collection via Localized Salt Gradient. Adv. Energy Mater. 2025, 15, 2501360. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.J.; Ye, C.W.; Tang, S.C. Achieving Ultrahigh Voltage Over 100 V and Remarkable Freshwater Harvesting Based on Thermodiffusion Enhanced Hydrovoltaic Generator. Adv. Energy Mater. 2024, 14, 2400529. [Google Scholar] [CrossRef]
- Hu, G.X.; Liu, H.J.; Liu, K.K.; Wang, H.Y.; Wen, X.Y.; Liu, L.J.; She, Y.; Feng, L.L.; Niu, R.; Gong, J. All-In-One Carbon Foam Evaporators for Efficient Co-Generation of Freshwater and Electricity. Adv. Funct. Mater. 2025, 35, 2423781. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Yu, X.; Zhang, L.; Wang, Y.; Wang, H.C.; Zhao, H.N.; Chen, W.S.; Li, J.H.; Deng, L.; et al. Photothermal interface with high-adhesive superhydrophobicity to construct vapor splitting module for hydrogen evolution from seawater. Appl. Catal. B Environ. 2024, 346, 123743. [Google Scholar] [CrossRef]
- Pornrungroj, C.; Mohamad Annuar, A.B.; Wang, Q.; Rahaman, M.; Bhattacharjee, S.; Andrei, V.; Reisner, E. Hybrid photothermal-photocatalyst sheets for solar-driven overall water splitting coupled to water purification. Nat. Water. 2023, 1, 952–960. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Ding, G.; Zhang, Y.; He, H.; Yin, X.; Luo, S.; Huang, B.; Huang, L.; Pei, J.; Hu, X. Solar-Powered Interfacial Evaporation for Simultaneous Photocatalytic Hydrogen Production and Salinity Gradient Power Generation. Energies 2025, 18, 6139. https://doi.org/10.3390/en18236139
Gao R, Ding G, Zhang Y, He H, Yin X, Luo S, Huang B, Huang L, Pei J, Hu X. Solar-Powered Interfacial Evaporation for Simultaneous Photocatalytic Hydrogen Production and Salinity Gradient Power Generation. Energies. 2025; 18(23):6139. https://doi.org/10.3390/en18236139
Chicago/Turabian StyleGao, Ruiying, Gaoming Ding, Ying Zhang, Hanhua He, Xinxing Yin, Shan Luo, Baolin Huang, Lu Huang, Junxian Pei, and Xuejiao Hu. 2025. "Solar-Powered Interfacial Evaporation for Simultaneous Photocatalytic Hydrogen Production and Salinity Gradient Power Generation" Energies 18, no. 23: 6139. https://doi.org/10.3390/en18236139
APA StyleGao, R., Ding, G., Zhang, Y., He, H., Yin, X., Luo, S., Huang, B., Huang, L., Pei, J., & Hu, X. (2025). Solar-Powered Interfacial Evaporation for Simultaneous Photocatalytic Hydrogen Production and Salinity Gradient Power Generation. Energies, 18(23), 6139. https://doi.org/10.3390/en18236139






