Synergistic Effects of Dimethyl Ether and LSW in a CO2 WAG Process for Enhanced Oil Recovery and CO2 Sequestration
Abstract
1. Introduction
2. Numerical Modeling and Simulation
2.1. Geochemical Reaction
2.2. Fluid Modeling
| Parameters | Fluid Model | Experimental Data |
|---|---|---|
| Saturation pressure (psi) | 694 | 713 |
| Oil density (lb/ft3) | 50.3 | 50.3 |
| Oil viscosity (cP) | 1.76 | 1.76 |
| Formation volume factor (ft3/ft3) | 1.11 | 1.12 |
| Oil gravity (°API) | 35 | 31 |
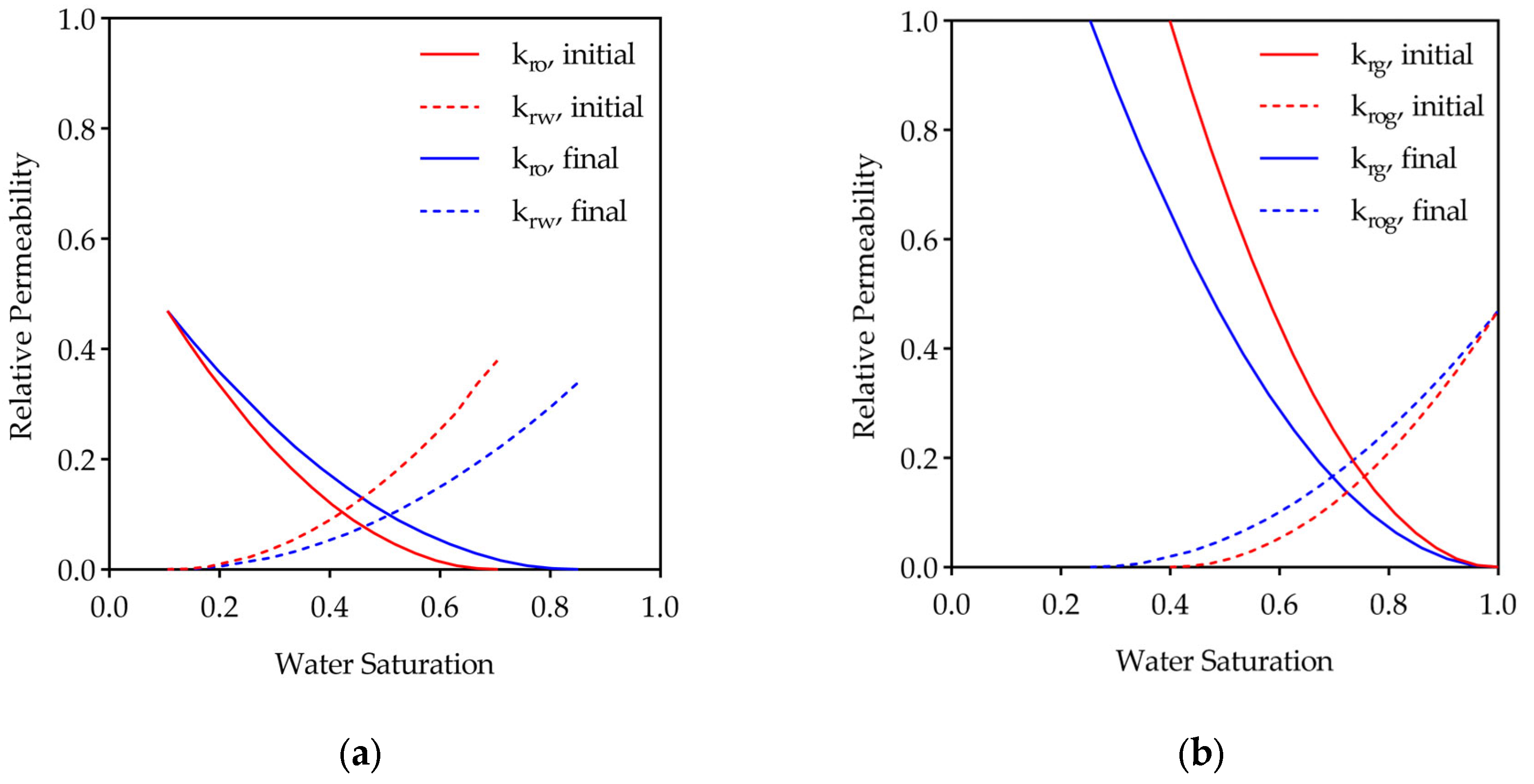
2.3. Low-Salinity Water Injection
2.4. Reservoir Modeling
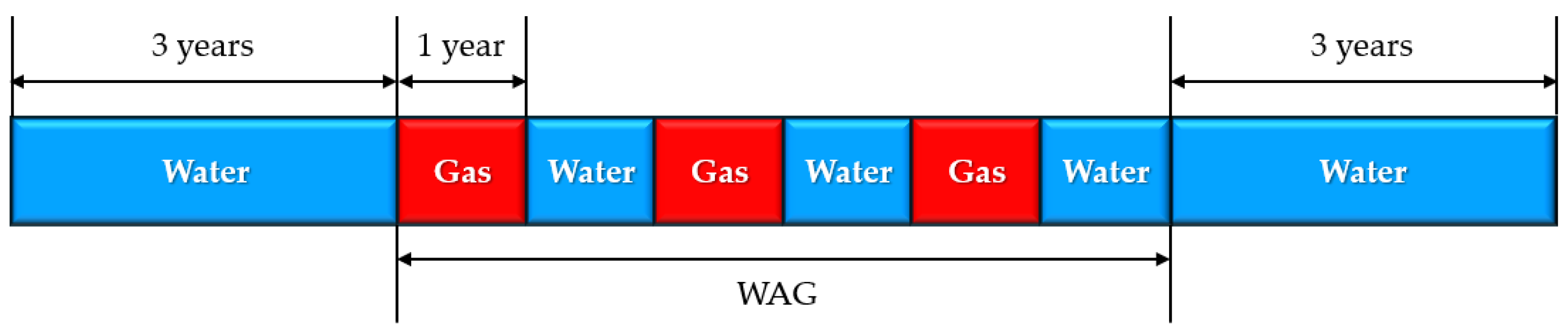
2.5. Injection Design
3. Results and Discussion
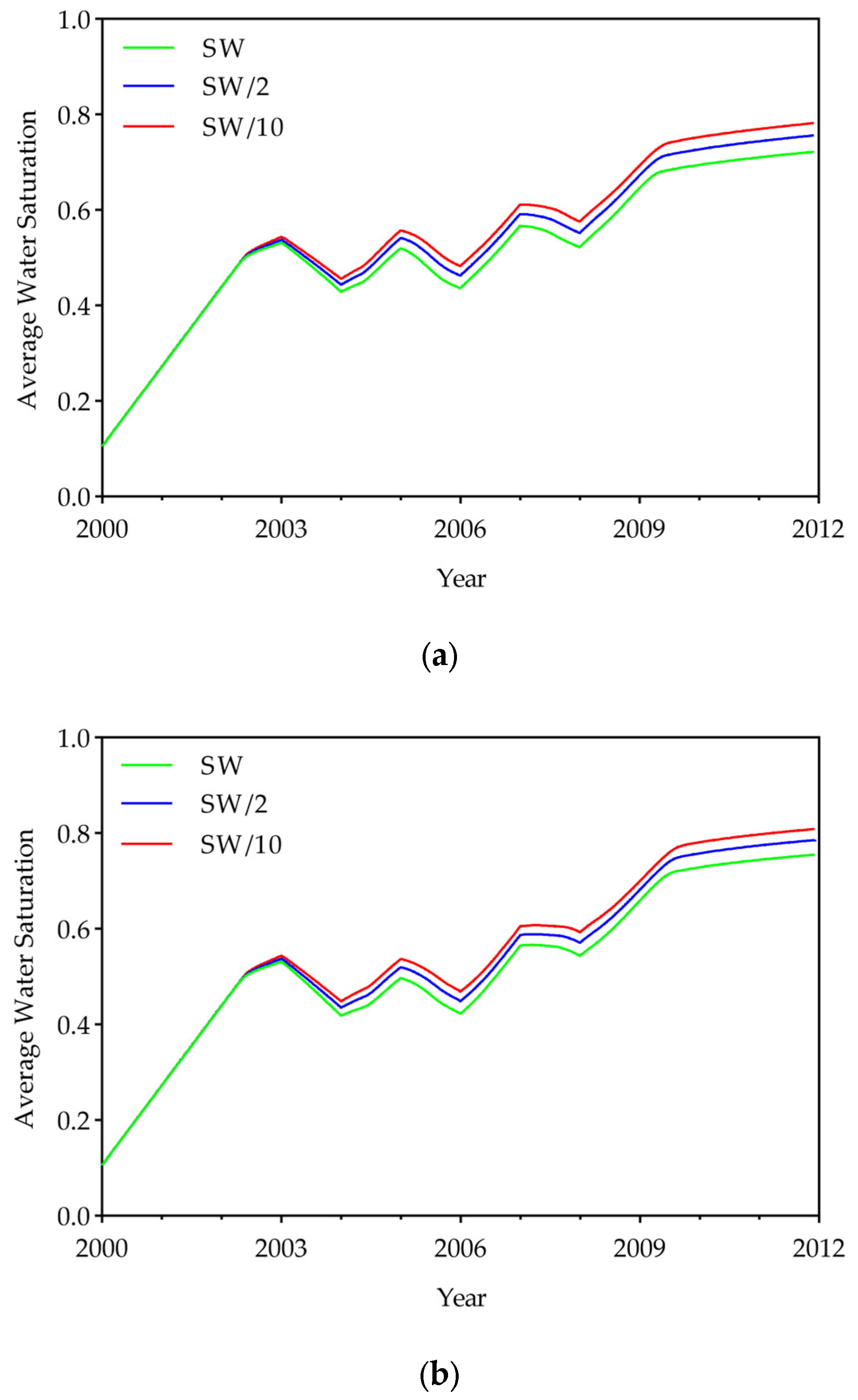
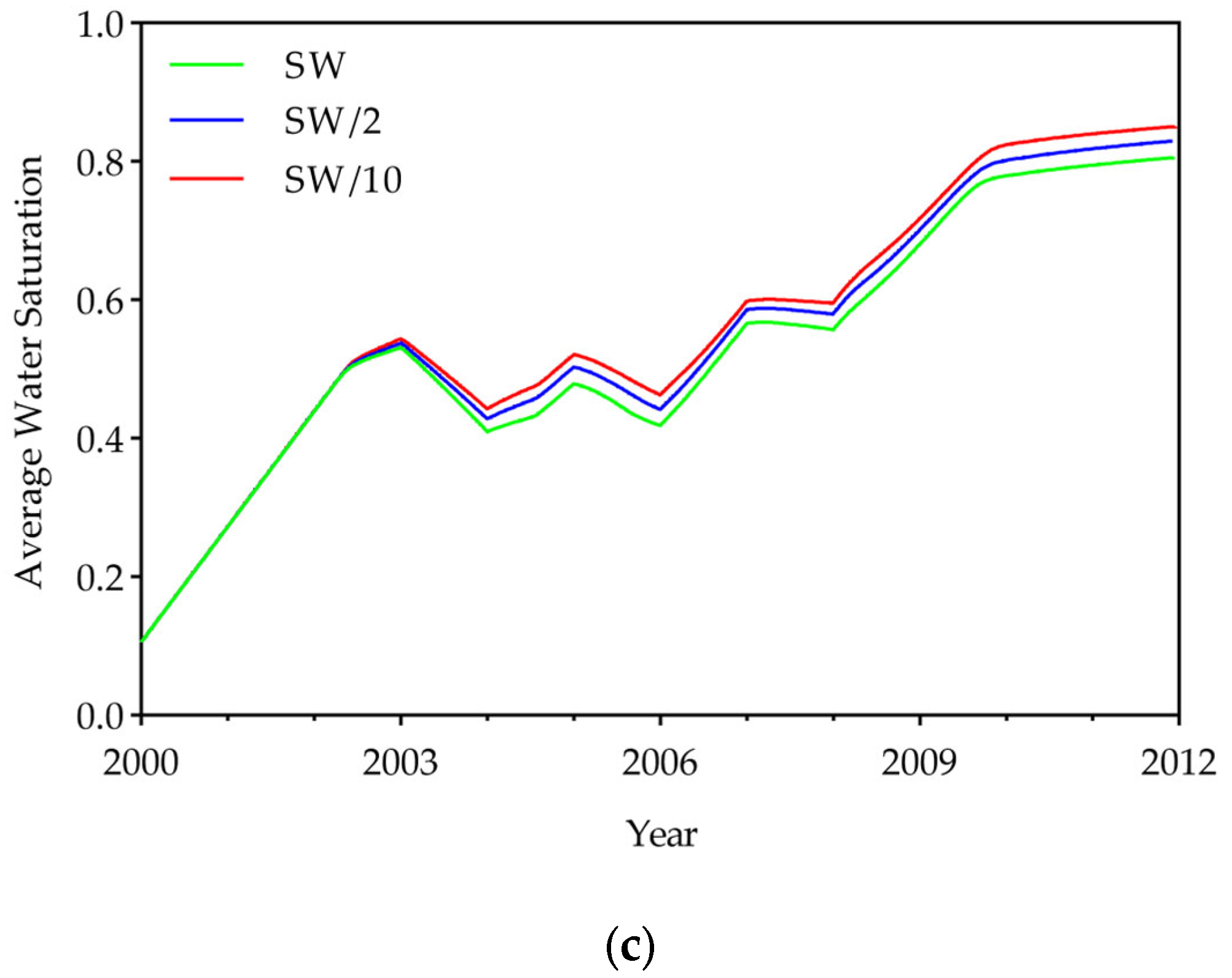
3.1. Effect of Low-Salinity Water
3.2. Evaluation of the Incremental Recovery Factor
3.2.1. Sweep Efficiency
3.2.2. Displacement Efficiency
3.2.3. Oil Recovery
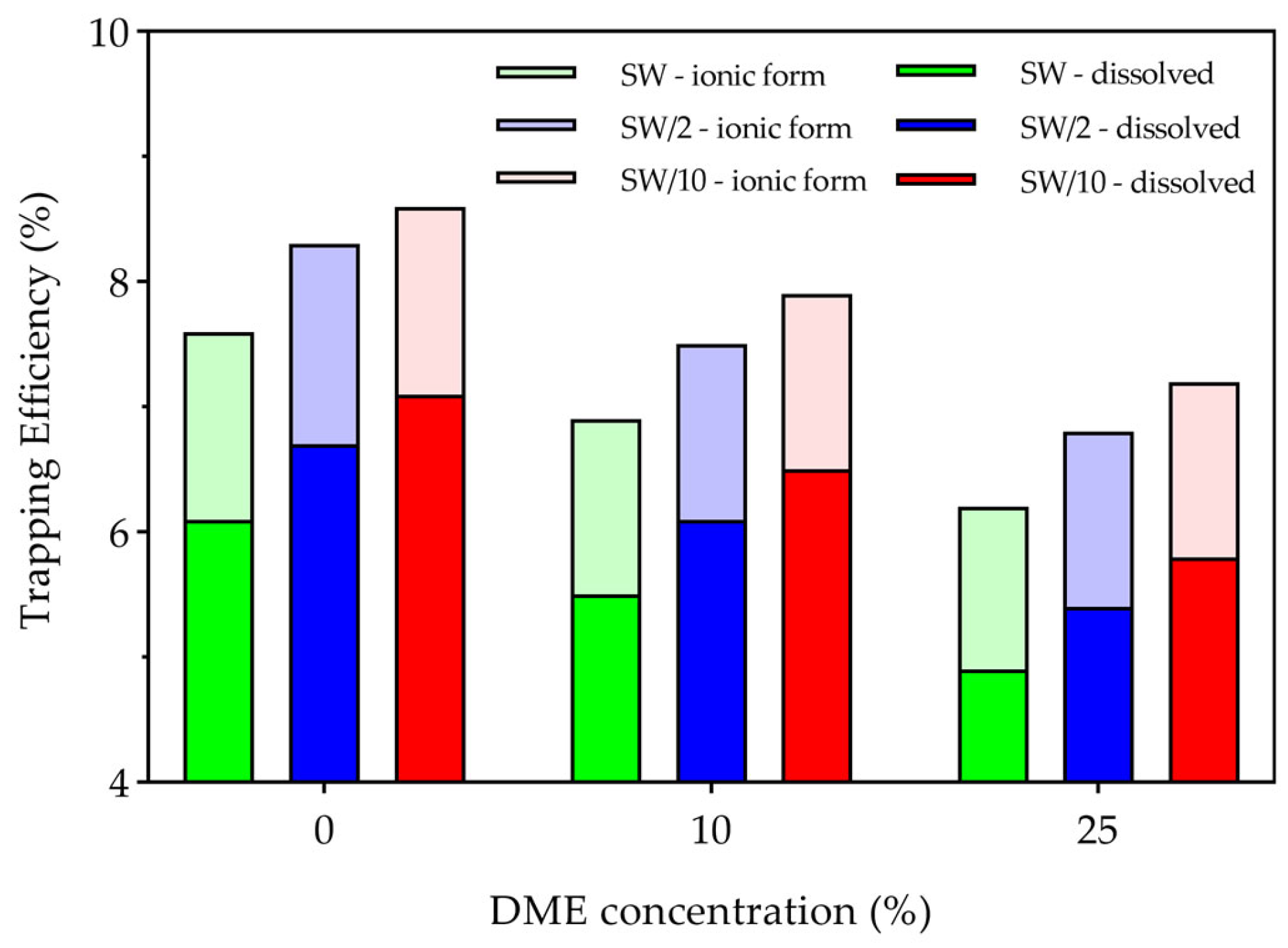
3.3. CO2 Storage
3.3.1. Structural Trapping
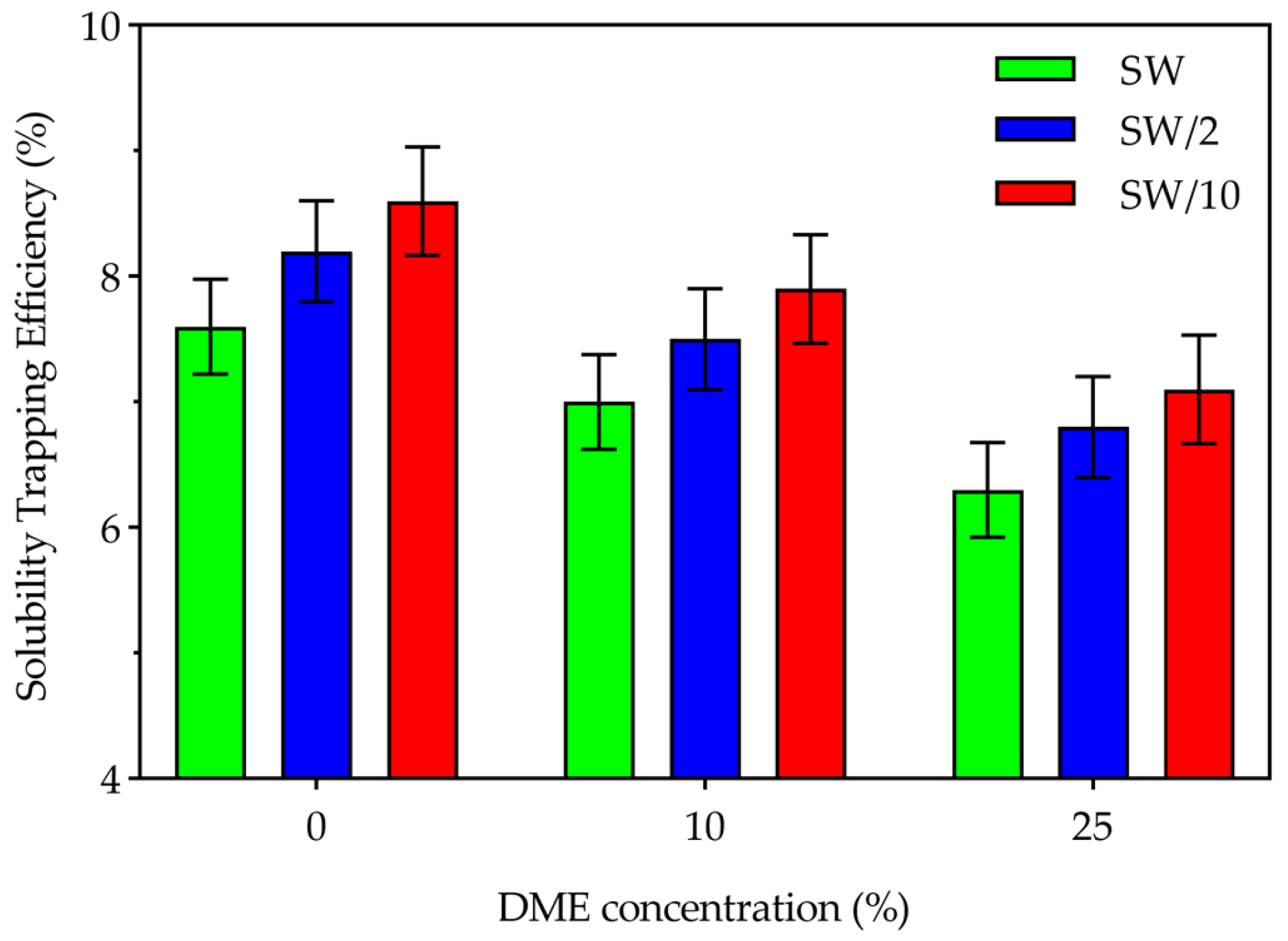
3.3.2. Solubility Trapping
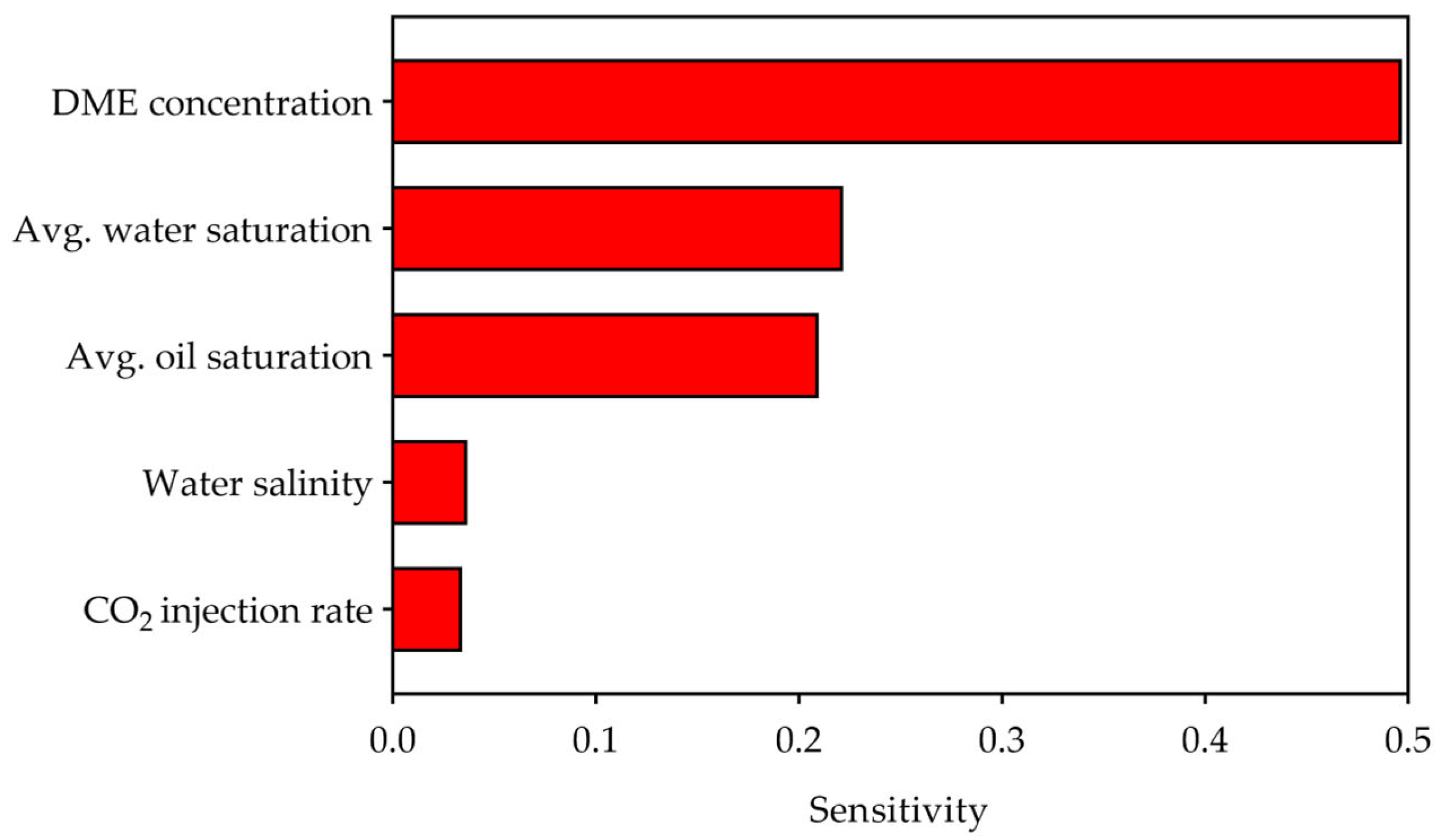
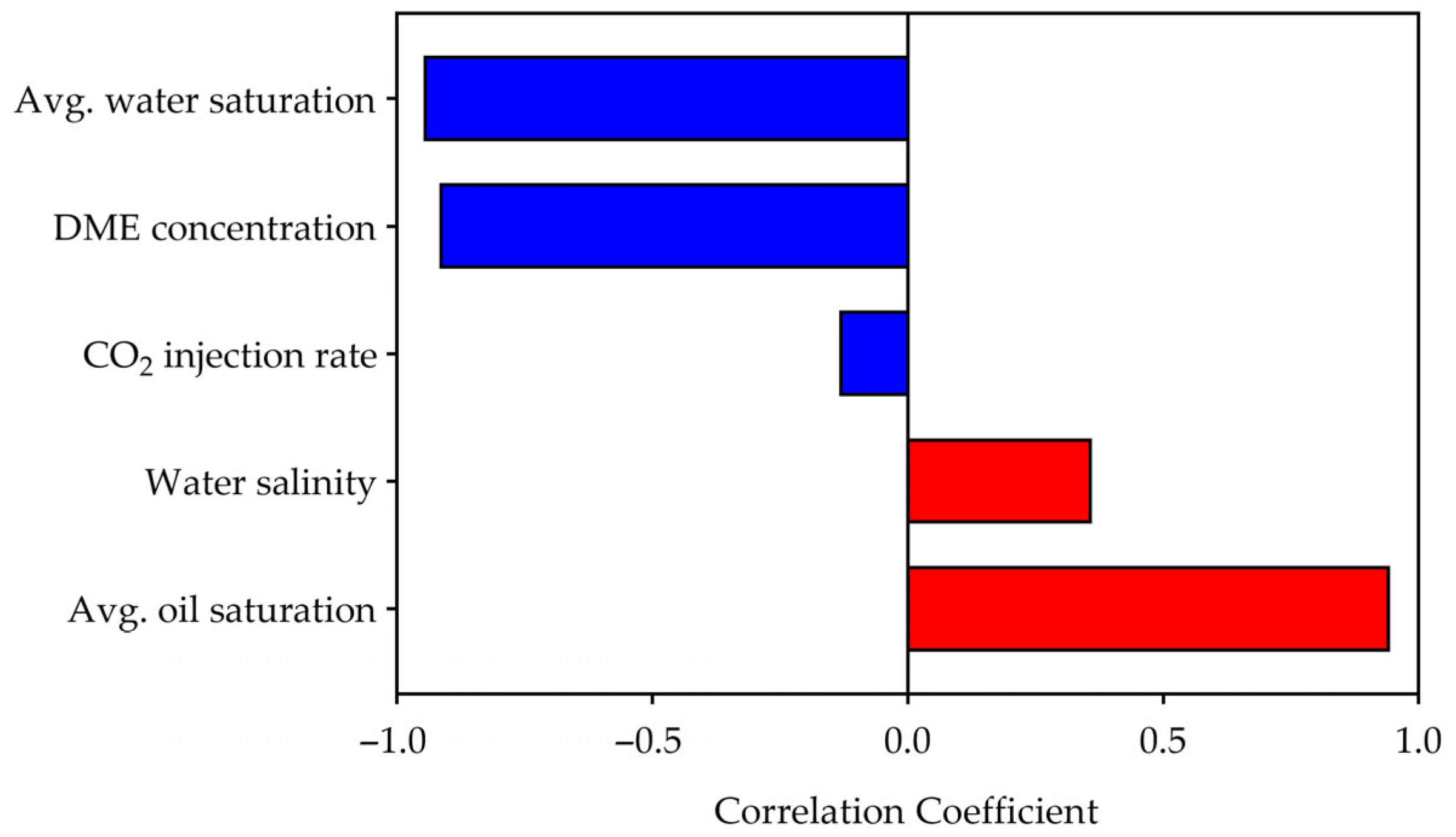
3.3.3. Sensitivity Analysis
4. Conclusions
- (1)
- Simulations showed that DME addition substantially improved volumetric sweep, increasing the swept area by 5–8% depending on concentration, while LSW effects were negligible (<1%). The improvement in sweep efficiency was accompanied by changes in the displacement front. The slope of the swept front gradually steepened with increasing DME concentration. The slope increased from 2.4 for the 0% DME case to 4–6 for the 10% DME case and 6–14 for the 25% DME case, with the slope being steeper in lower-salinity water. This steeper front and delayed breakthrough date suggest that DME improved sweep efficiency by reducing CO2 mobility and mitigating gravity override. These results demonstrate that DME injection effectively addresses the primary limitation of CO2-EOR—poor sweep efficiency—making it a promising strategy for enhanced oil recovery.
- (2)
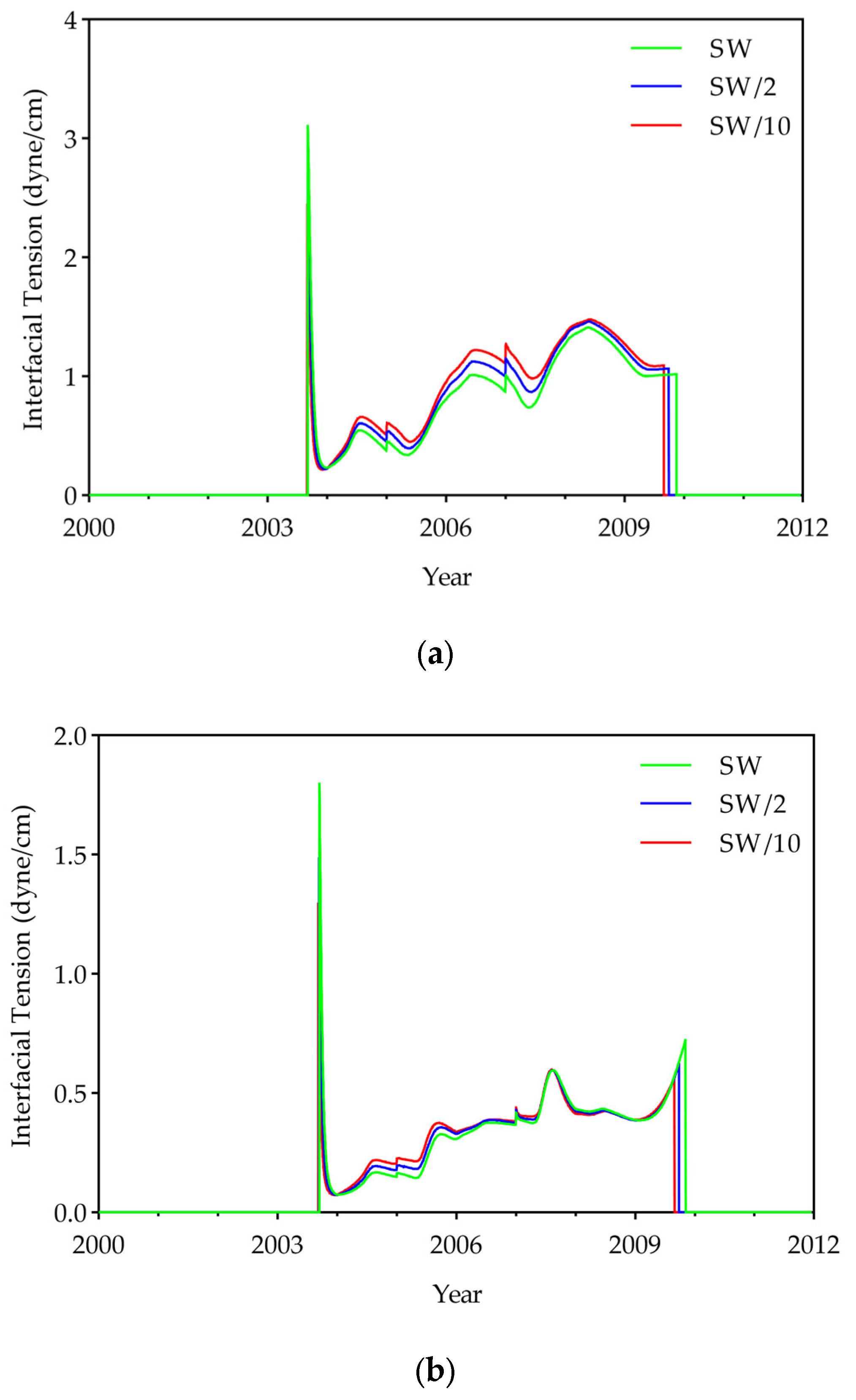
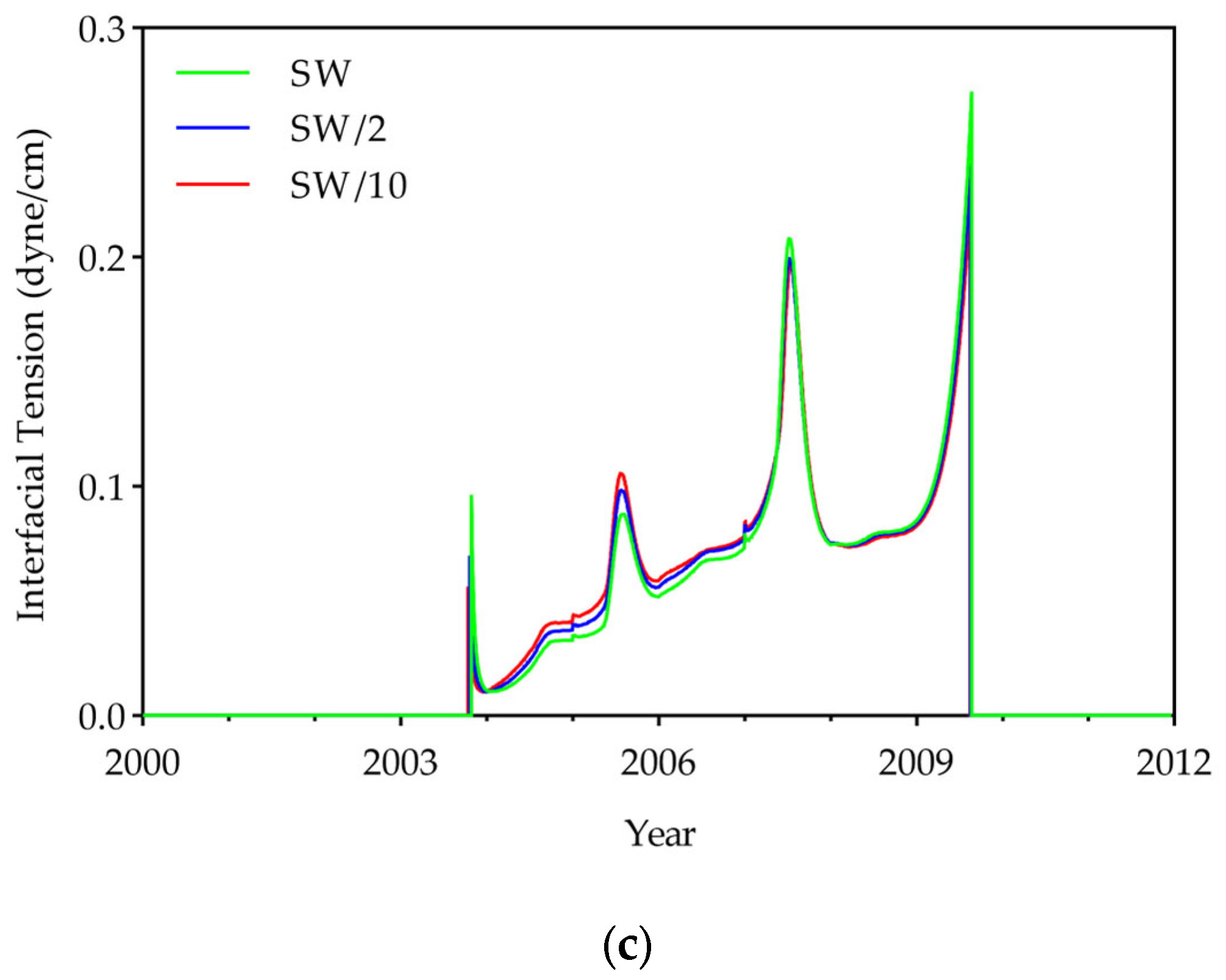
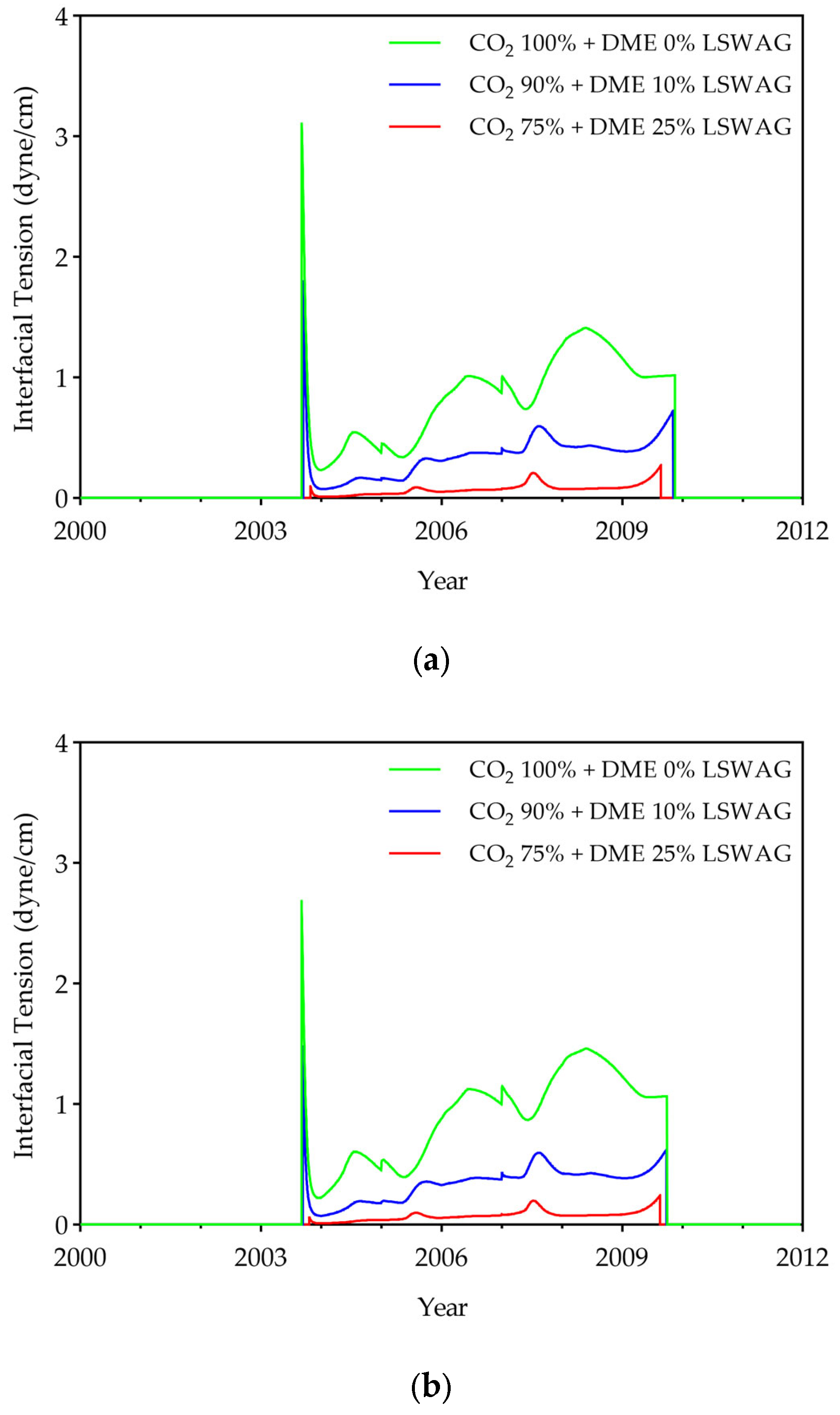
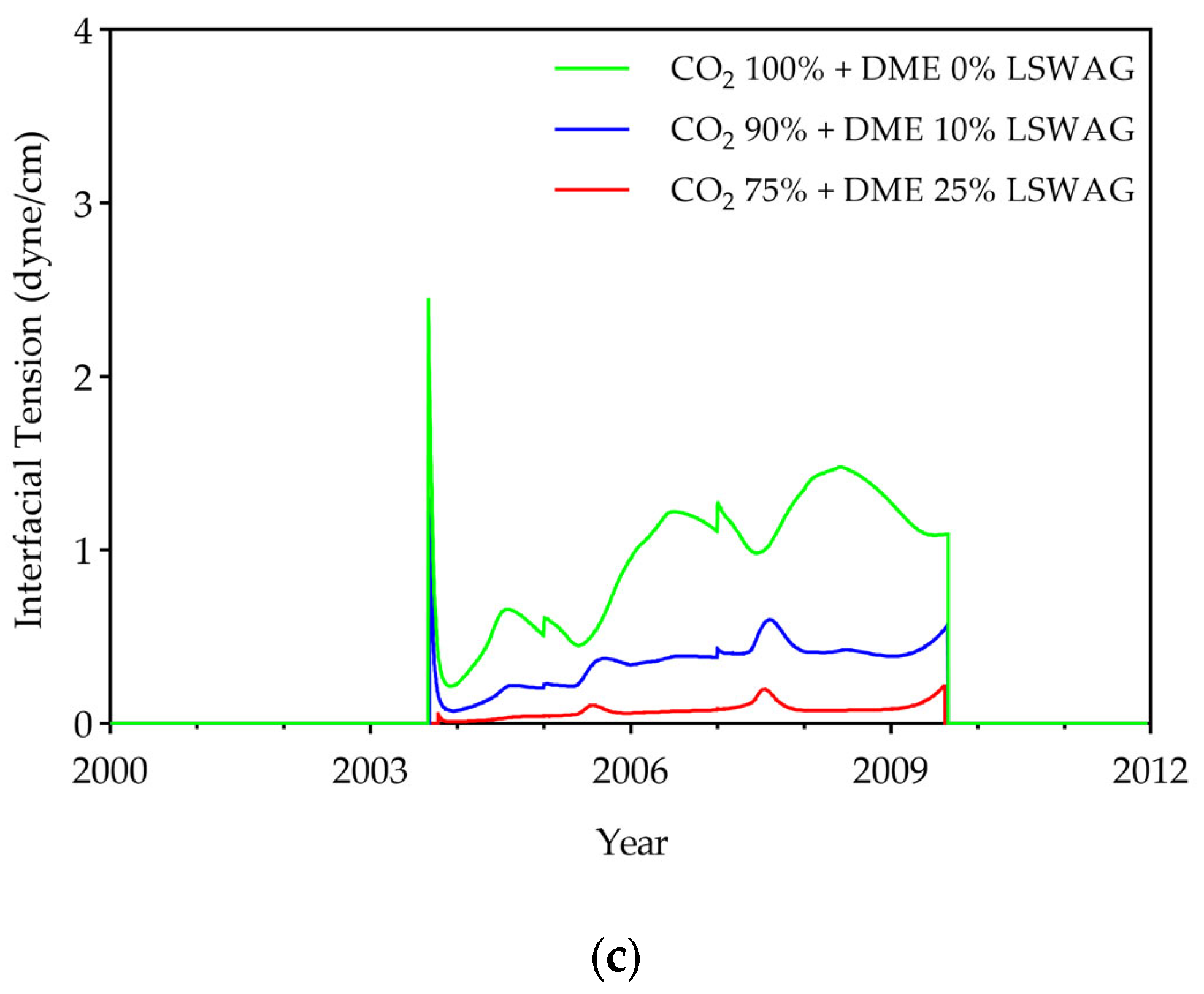
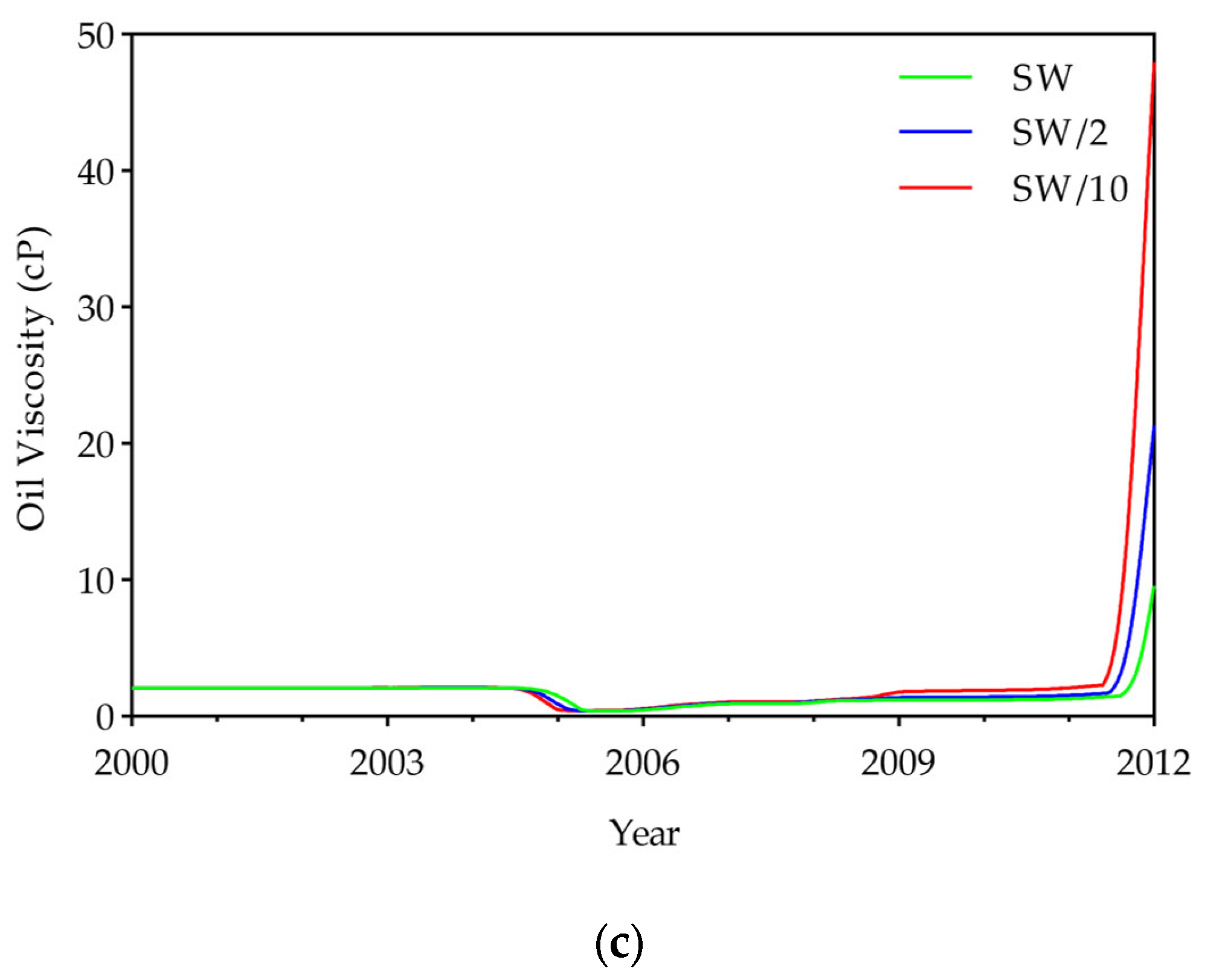
- To evaluate displacement efficiency, IFT, oil viscosity, and the displacement efficiency of hydrocarbon components were investigated. Results showed that DME concentration dominates IFT reduction (up to 95% at 25% DME), while LSW effects were relatively small (<7%). DME also substantially reduced oil viscosity from 2.1 cP to a minimum of 0.38 cP, with LSW accelerating this reduction through enhanced mobilization. Compositional analysis revealed that both DME and LSW significantly enhanced the displacement of hydrocarbon components (C6+), with their combined application yielding the most extensive extraction. These findings demonstrate that DME in low salinity conditions improves displacement efficiency.
- (3)
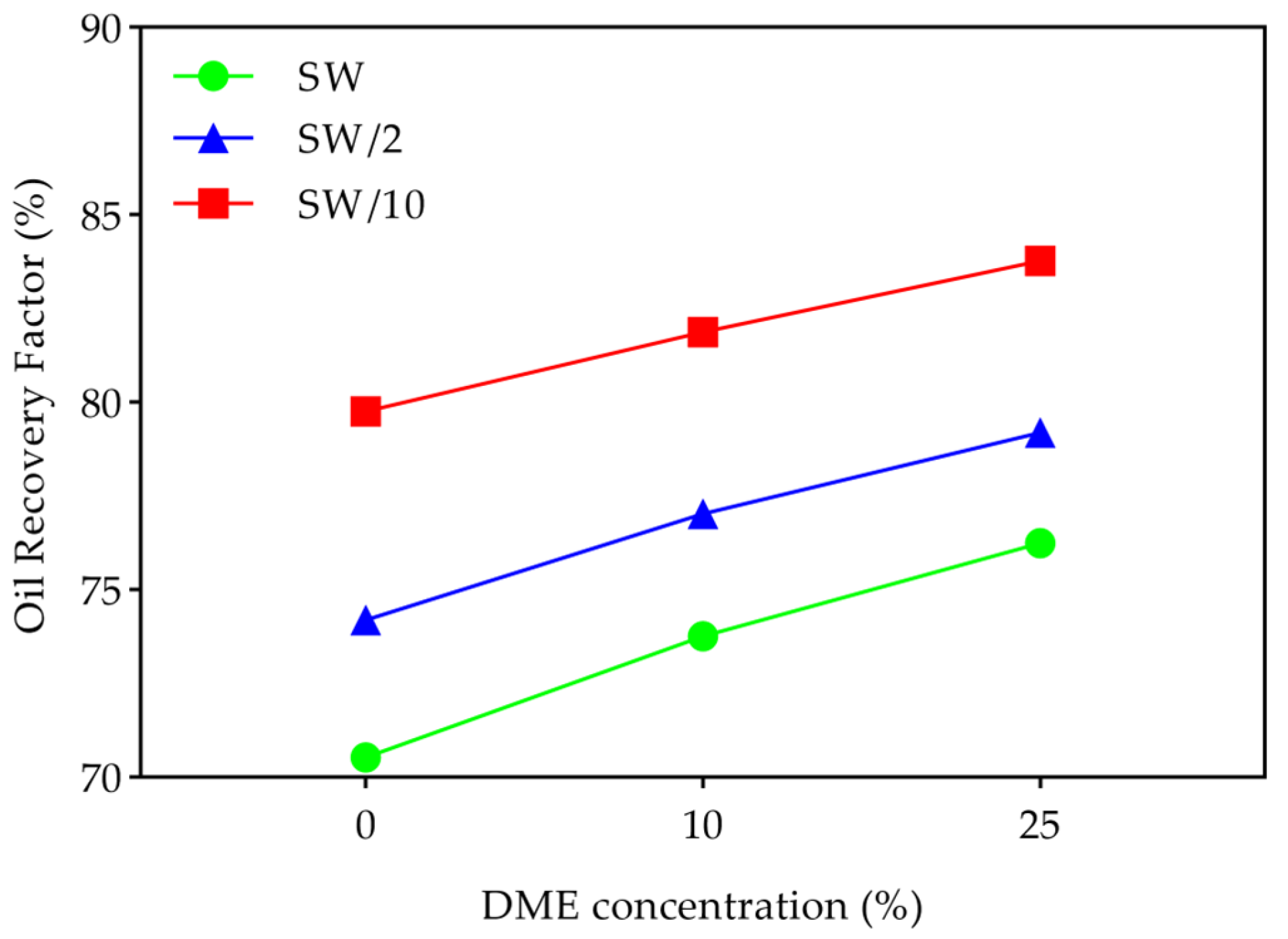
- Oil recovery increased from 70.5% (in the 0% DME + SW case) to 83.8% (in the 25% DME + SW/10 case), demonstrating the significant advantages of using DME and LSW together. Both factors contributed through distinct mechanisms—DME enhanced sweep and displacement efficiency, while LSW promoted wettability alteration. However, their interaction showed asymmetric patterns due to salting-out effects. The benefits of DME dominated in high-salinity conditions, while LSW’s benefits were strongest at low DME concentrations. Equivalent recoveries achieved through different combinations highlight optimization opportunities.
- (4)
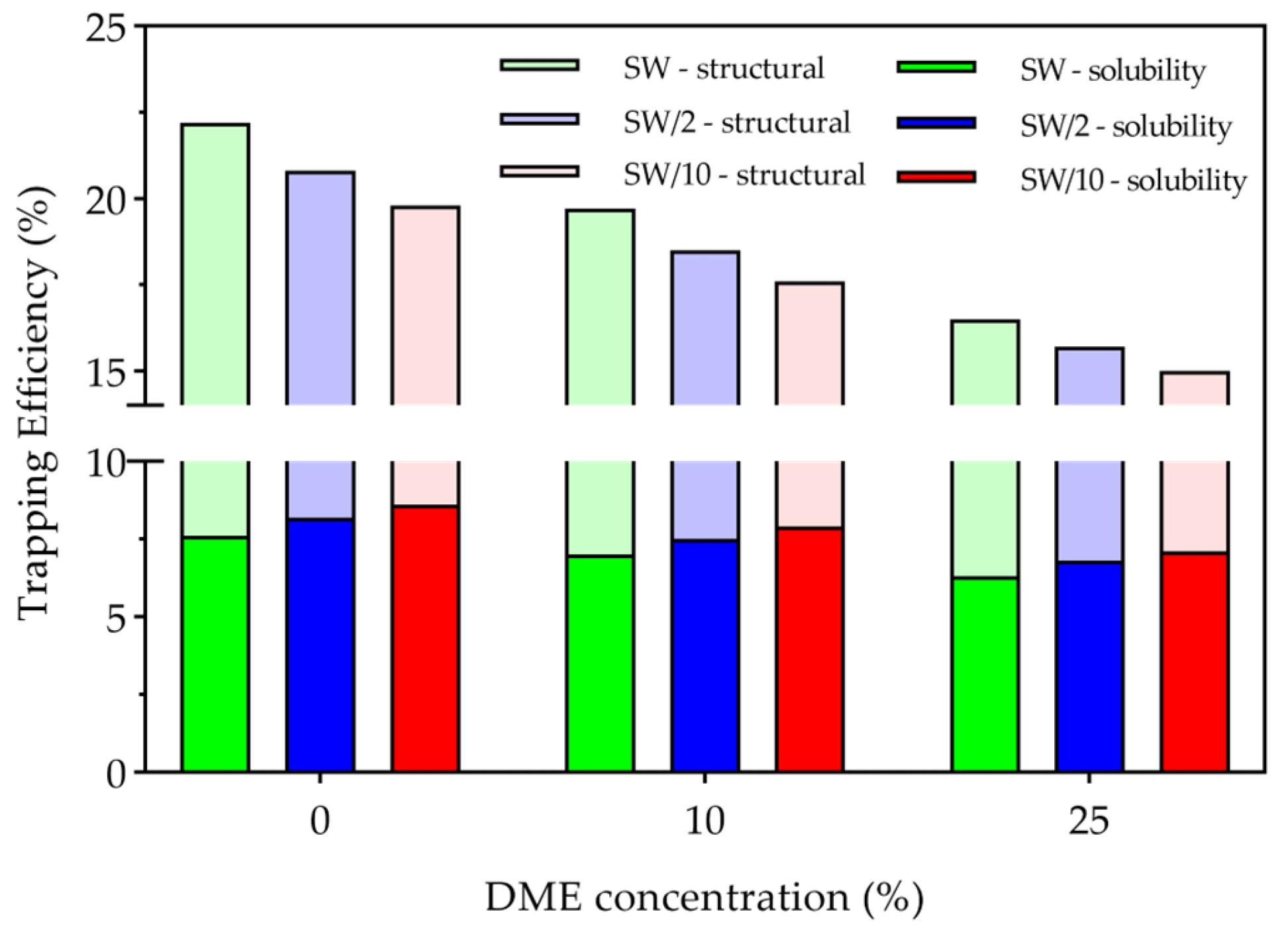
- Structural trapping, which constituted the majority of stored CO2, was enhanced in higher salinity water and lower DME concentrations through three mechanisms: first, higher absolute amounts of CO2 at lower DME concentrations; second, reduced CO2 dissolution in the aqueous phase due to stronger salting-out effects at higher salinity water; and third, less efficient displacement of structurally trapped CO2 during the three years of waterflooding after the WAG cycle. Conversely, solubility trapping decreased with increasing DME concentrations due to reduced CO2 partial pressures, though this effect was partially offset by enhanced CO2 solubility at lower salinity.
- (5)
- Correlation analysis for CO2 storage efficiency revealed that DME concentration is the dominant controlling factor, showing a strong negative correlation (r = −0.92) with storage efficiency. Machine learning-based sensitivity analysis confirmed DME as the most influential parameter (sensitivity = 0.50), followed by water saturation (0.22) and oil saturation (0.21), while water salinity (0.04) and CO2 injection rate (0.03) had minimal influence. Storage efficiency decreased with increasing DME concentration but increased with higher water salinity, driven by competing trapping mechanisms.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| CMG | Computer Modeling Group |
| DME | Dimethyl Ether |
| EOR | Enhanced Oil Recovery |
| EOS | Equation Of State |
| IFT | Interfacial Tension |
| LSW | Low-Salinity Water |
| LSWI | Low-Salinity Water Injection |
| LSWAG | Low-Salinity Water-Alternating Gas |
| PR | Peng–Robinson |
| PV | Pore Volume |
| SW | Seawater |
| SW/2 | Twice-diluted Seawater |
| SW/10 | Ten-times-diluted Seawater |
| WAG | Water-Alternating Gas |
| Symbol | |
| EOS model parameters | |
| Dimensionless parameter | |
| Reaction | |
| Temperature-related parameters | |
| Fugacity | |
| Henry’s constant | |
| Henry’s constant at reference pressure | |
| Component | |
| Ion | |
| Equilibrium constant value | |
| Relative permeability | |
| Activity coefficient | |
| Molality | |
| Total component number in the aqueous phase | |
| The number of soluble hydrocarbon components | |
| Pressure | |
| Reference pressure | |
| Activity product | |
| Universal gas constant | |
| Temperature | |
| Molar volume | |
| Stoichiometric coefficient | |
| Partial molar volume | |
| Mole fraction |
References
- Campbell, B.T.; Orr, F.M. Flow visualization for CO2/crude-oil displacements. SPE J. 1985, 25, 665–678. [Google Scholar] [CrossRef]
- Hustad, O.S.; Holt, T. Gravity Stable Displacement of Oil by Hydrocarbon Gas After Waterflooding. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–24 April 1992. [Google Scholar]
- Afzali, S.; Rezaei, N.; Zendehboudi, S. A comprehensive review on enhanced oil recovery by water alternating gas (WAG) injection. Fuel 2018, 227, 218–246. [Google Scholar] [CrossRef]
- Nygård, J.I.; Andersen, P.Ø. Simulation of immiscible water-alternating-gas injection in a stratified reservoir: Performance characterization using a new dimensionless number. SPE J. 2020, 25, 1711–1728. [Google Scholar] [CrossRef]
- Sheng, J.J. Critical review of low-salinity waterflooding. J. Pet. Sci. Eng. 2014, 120, 216–224. [Google Scholar] [CrossRef]
- Ma, S.; James, L.A. Literature review of hybrid CO2 low salinity water-alternating-gas injection and investigation on hysteresis effect. Energies 2022, 15, 7891. [Google Scholar] [CrossRef]
- Behera, U.S.; Sangwai, J.S.; Baskaran, D.; Byun, H.-S. A comprehensive review on low salinity water injection for enhanced oil recovery: Fundamental insights, laboratory and field studies, and economic aspects. Energy Fuels 2024, 39, 72–103. [Google Scholar] [CrossRef]
- Fang, P.; Zhang, Q.; Zhou, C.; Yang, Z.; Yu, H.; Du, M.; Chen, X.; Song, Y.; Wang, S.; Gao, Y. Chemical-assisted CO2 water-alternating-gas injection for enhanced sweep efficiency in CO2-EOR. Molecules 2024, 29, 3978. [Google Scholar] [CrossRef]
- Parsons, C.; Chernetsky, A.; Eikmans, D.; Te Riele, P.; Boersma, D.; Sersic, I.; Broos, R. Introducing a Novel Enhanced Oil Recovery Technology. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Gao, Y.; Jia, J.; Tian, F.; Liang, Y.; Wang, D.; Wang, X.; Tsuji, T. Interfacial activity and phase behavior of dimethyl ether in decane/CO2–water systems: A molecular dynamics study. Chem. Eng. Sci. 2025, 314, 121826. [Google Scholar] [CrossRef]
- Cho, J.; Kim, T.H.; Lee, K.S. Compositional modeling and simulation of dimethyl ether (DME)-enhanced waterflood to investigate oil mobility improvement. Pet. Sci. 2018, 15, 297–304. [Google Scholar] [CrossRef]
- Kong, S.; Feng, G.; Liu, Y.; Li, K. Potential of dimethyl ether as an additive in CO2 for shale oil recovery. Fuel 2021, 296, 120643. [Google Scholar] [CrossRef]
- Huang, X.; Tian, Z.; Zuo, X.; Li, X.; Yang, W.; Lu, J. The microscopic pore crude oil production characteristics and influencing factors by DME-assisted CO2 injection in shale oil reservoirs. Fuel 2023, 331, 125843. [Google Scholar] [CrossRef]
- Wang, C.; Su, Y.; Wang, W.; Li, L.; Hao, Y. Experimental study on ethane-dimethyl ether assisted CO2 displacement to improve crude oil recovery in tight reservoirs. Energy Fuels 2024, 38, 12546–12554. [Google Scholar] [CrossRef]
- Choi, Y.J.; Seo, K.; Lee, K.S. Techno-economical optimization of water-alternating-CO2/dimethyl ether process for enhanced oil recovery. Pet. Sci. Technol. 2024, 42, 4913–4931. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lee, H.S.; Jeong, M.S.; Cho, J.; Lee, K.S. Compositional modeling of dimethyl ether-CO2 mixed solvent for enhanced oil recovery. Appl. Sci. 2021, 11, 406. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zou, R.; Zou, R.; Huang, L.; Liu, Y.; Lei, H. Molecular dynamics investigation of DME assisted CO2 injection to enhance shale oil recovery in inorganic nanopores. J. Mol. Liq. 2023, 385, 122389. [Google Scholar] [CrossRef]
- Choi, Y.; Seo, K.; Lee, K.S. Compositional Modelling of Enhanced Oil Recovery Using Dimethyl Ether-Enhanced Carbon Dioxide Water-Alternating-Gas Process. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 10–12 October 2023. [Google Scholar]
- Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. A Comprehensive Geochemical-Based Approach at Modeling and Interpreting Brine Dilution in Carbonate Reservoirs. In Proceedings of the SPE Reservoir Simulation Conference, Montgomery, TX, USA, 20–22 February 2017. [Google Scholar]
- Helgeson, H.C.; Brown, T.H.; Leeper, R.H. Handbook of Theoretical Activity Diagrams Depicting Chemical Equilibria in Geologic Systems: Involving an Aqueous Phase at one Atm and 0 °C to 300 °C; Freeman, Cooper: San Francisco, CA, USA, 1969. [Google Scholar]
- Srivastava, R.K.; Huang, S.S.; Dong, M. Laboratory investigation of Weyburn CO2 miscible flooding. J. Can. Pet. Technol. 2000, 39, PETSOC-00-02-04. [Google Scholar] [CrossRef]
- Ganjdanesh, R.; Rezaveisi, M.; Pope, G.A.; Sepehrnoori, K. Treatment of condensate and water blocks in hydraulic-fractured shale-gas/condensate reservoirs. SPE J. 2016, 21, 665–674. [Google Scholar] [CrossRef]
- Li, Y.K.; Nghiem, L.X. Phase equilibria of oil, gas and water/brine mixtures from a cubic equation of state and Henry’s law. Can. J. Chem. Eng. 1986, 64, 486–496. [Google Scholar] [CrossRef]
- Morrow, N.R.; Buckley, J. Wettability and Oil Recovery by Imbibition and Viscous Displacement from Fractured and Heterogeneous Carbonates; Univ. of Wyoming: Laramie, WY, USA, 2006. [Google Scholar]
- Yousef, A.A.; Al-Saleh, S.; Al-Kaabi, A.; Al-Jawfi, M. Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs. SPE Reserv. Eval. Eng. 2011, 14, 578–593. [Google Scholar] [CrossRef]
- Tang, G.; Morrow, N.R. Injection of dilute brine and crude oil/brine/rock interactions. In Geophysical Monograph Series; Raats, P.A.C., Smiles, D., Warrick, A.W., Eds.; American Geophysical Union: Washington, DC, USA, 2002; Volume 129, pp. 171–179. [Google Scholar]
- Kakati, A.; Sangwai, J.S. Effect of monovalent and divalent salts on the interfacial tension of pure hydrocarbon-brine systems relevant for low salinity water flooding. J. Pet. Sci. Eng. 2017, 157, 1106–1114. [Google Scholar] [CrossRef]
- Madadizadeh, A.; Aghdam, M.A.; Sadeghein, A.; Hosseini, S.; Riahi, S.; Saviz, S. Ionic optimization of engineered water for enhanced oil recovery in carbonate reservoirs: A case study. Sci. Rep. 2025, 15, 15665. [Google Scholar] [CrossRef]
- Mohammadi, A.; Chahardowli, M.; Simjoo, M. The effect of diethyl ether on interfacial tension and oil swelling in crude oil-brine system. J. Pet. Explor. Prod. Technol. 2025, 15, 83. [Google Scholar] [CrossRef]
- Deshmukh, A.; Wilson, A.D.; Lienhard, J.H. Electrically powered high-salinity brine separation using dimethyl ether. Ind. Eng. Chem. Res. 2024, 63, 8341–8356. [Google Scholar] [CrossRef]
- Salam, A.H.; Nazar, M.; Kamal, M.S.; Mahboob, A.; Hussain, S.M.S.; Aljawad, M.S.; Raza, A.; Patil, S. A perspective on ionic liquids as multifunctional agents in enhanced oil recovery and CO2 sequestration in carbonate formations. Carbon Capture Sci. Technol. 2025, 15, 100443. [Google Scholar] [CrossRef]


| Hydrocarbon Solubility | |
| (R1) | |
| (R2) | |
| Aqueous reactions | |
| (R3) | |
| (R4) | |
| (R5) | |
| (R6) | |
| (R7) | |
| (R8) | |
| (R9) | |
| (R10) | |
| Mineral reactions | |
| (R11) | |
| (R12) | |
| (R13) | |
| Ion exchange reactions | |
| (R14) | |
| (R15) | |
| Component | Composition | Critical Pressure (psi) | Critical Temperature (°F) | Acentric Factor | Molecular Weight |
|---|---|---|---|---|---|
| N2 | 0.0207 | 492.31 | −232.51 | 0.040 | 28.013 |
| CO2 | 0.0074 | 1,069.87 | 87.89 | 0.225 | 44.010 |
| H2S | 0.0012 | 1,296.18 | 212.09 | 0.100 | 34.080 |
| C1 | 0.0749 | 667.20 | −116.59 | 0.008 | 16.043 |
| C2 | 0.0422 | 708.34 | 90.05 | 0.098 | 30.070 |
| C3 | 0.0785 | 615.76 | 205.97 | 0.152 | 44.097 |
| DME | 0.0000 | 789.39 | 260.84 | 0.200 | 46.070 |
| IC4 | 0.0158 | 529.05 | 274.91 | 0.176 | 58.124 |
| NC4 | 0.0497 | 551.10 | 305.69 | 0.193 | 58.124 |
| IC5 | 0.0201 | 490.84 | 369.05 | 0.227 | 72.151 |
| NC5 | 0.0258 | 489.38 | 385.61 | 0.251 | 72.151 |
| C6– 9 | 0.2156 | 437.95 | 541.79 | 0.331 | 102.500 |
| C10–17 | 0.2202 | 292.61 | 786.46 | 0.584 | 184.000 |
| C18–27 | 0.1027 | 192.46 | 995.50 | 0.893 | 306.200 |
| C28+ | 0.1252 | 167.53 | 1188.30 | 1.100 | 565.613 |
| N2 | CO2 | H2S | C1 | C2 | C3 | DME | IC4 | NC4 | IC5 | NC5 | C6–9 | C10–17 | C18–27 | C28+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N2 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| CO2 | 0.00 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| H2S | 0.13 | 0.14 | 0.00 | - | - | - | - | - | - | - | - | - | - | - | - |
| C1 | 0.25 | 0.11 | 0.07 | 0.00 | - | - | - | - | - | - | - | - | - | - | - |
| C2 | 0.01 | 0.13 | 0.09 | 0.00 | 0.00 | - | - | - | - | - | - | - | - | - | - |
| C3 | 0.09 | 0.13 | 0.08 | 0.01 | 0.00 | 0.00 | - | - | - | - | - | - | - | - | - |
| DME | 0.10 | 0.00 | 0.00 | 0.29 | 0.25 | 0.25 | 0.00 | - | - | - | - | - | - | - | - |
| IC4 | 0.10 | 0.12 | 0.08 | 0.02 | 0.01 | 0.00 | 0.25 | 0.00 | - | - | - | - | - | - | - |
| NC4 | 0.10 | 0.12 | 0.08 | 0.02 | 0.01 | 0.00 | 0.25 | 0.00 | 0.00 | - | - | - | - | - | - |
| IC5 | 0.10 | 0.12 | 0.07 | 0.02 | 0.01 | 0.00 | 0.25 | 0.00 | 0.00 | 0.00 | - | - | - | - | - |
| NC5 | 0.10 | 0.12 | 0.07 | 0.02 | 0.01 | 0.00 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | - | - | - | - |
| C6–9 | 0.11 | 0.12 | 0.05 | 0.03 | 0.02 | 0.01 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - | - | - |
| C10–17 | 0.11 | 0.12 | 0.05 | 0.06 | 0.04 | 0.02 | 0.08 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | - | - |
| C18–27 | 0.11 | 0.12 | 0.05 | 0.09 | 0.06 | 0.05 | 0.08 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.00 | 0.00 | - |
| C28+ | 0.11 | 0.12 | 0.05 | 0.12 | 0.09 | 0.07 | 0.08 | 0.05 | 0.05 | 0.05 | 0.05 | 0.03 | 0.01 | 0.00 | 0.00 |
| Ion Component | Na+ | Ca2+ | Mg2+ | SO42− | HCO3− | Cl− | |
|---|---|---|---|---|---|---|---|
| Brine Type | |||||||
| Seawater (SW) | 18,300 | 650 | 2110 | 4290 | 120 | 32,200 | |
| Twice-Diluted Seawater (SW/2) | 9150 | 325 | 1055 | 2145 | 60 | 16,100 | |
| Ten-times-Diluted Seawater (SW/10) | 1830 | 65 | 211 | 429 | 12 | 3220 | |
| Property | Values |
|---|---|
| Grids | 52 × 1 × 20 |
| Size of each grid (ft) | 10 × 10 × 5 |
| Depth (ft) | 4000 |
| Initial pressure (psi) | 2000 |
| Temperature (°F) | 145 |
| Porosity | 0.251 |
| Mineral volume fraction | Calcite 64%, Dolomite 10%, Anhydrite 2% |
| Permeability (I/J/K directions, mD) | 39.6/39.6/3.96 |
| 0.8948 | |
| 0.1052 | |
| Pore volume (ft3) | 130,520 |
| Injected Solvent | Brine |
|---|---|
| CO2 100% | Seawater (SW) |
| CO2 90% + DME 10% | Twice-diluted seawater (SW/2) |
| CO2 75% + DME 25% | Ten-times-diluted seawater (SW/10) |
| Solvent | Brine | Breakthrough Date | Swept Area (ft3) |
|---|---|---|---|
| CO2 100% | SW | 12 May 2004 | 271,500 |
| SW/2 | 13 May 2004 | 272,000 | |
| SW/10 | 14 May 2004 | 273,500 | |
| CO2 90% + DME 10% | SW | 8 June 2004 | 284,000 |
| SW/2 | 14 June 2004 | 285,500 | |
| SW/10 | 17 June 2004 | 287,500 | |
| CO2 75% + DME 25% | SW | 23 July 2004 | 294,500 |
| SW/2 | 30 July 2004 | 292,000 | |
| SW/10 | 4 August 2004 | 296,500 |
| Solvent | Brine | C1 | C2 | C3 | IC4 | NC4 | IC5 | NC5 | C6–9 | C10–17 | C18–27 | C28+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 100% | SW | 89.44 | 88.05 | 86.73 | 85.72 | 85.12 | 83.64 | 83.15 | 78.47 | 71.84 | 70.63 | 70.51 |
| SW/2 | 91.45 | 90.41 | 89.38 | 88.55 | 88.04 | 86.73 | 86.28 | 81.73 | 75.16 | 73.92 | 73.79 | |
| SW/10 | 93.05 | 92.25 | 91.44 | 90.76 | 90.34 | 89.17 | 88.75 | 84.25 | 77.69 | 76.42 | 76.30 | |
| CO2 90% + DME 10% | SW | 92.38 | 91.66 | 91.00 | 90.49 | 90.19 | 89.34 | 89.04 | 85.22 | 77.15 | 73.60 | 72.99 |
| SW/2 | 93.98 | 93.43 | 92.93 | 92.53 | 92.31 | 91.64 | 91.40 | 87.98 | 80.02 | 76.45 | 75.84 | |
| SW/10 | 95.34 | 94.92 | 94.54 | 94.23 | 94.06 | 93.54 | 93.35 | 90.25 | 82.21 | 78.61 | 77.99 | |
| CO2 75% + DME 25% | SW | 94.03 | 93.70 | 93.38 | 93.13 | 93.01 | 92.62 | 92.50 | 90.95 | 85.89 | 78.84 | 76.44 |
| SW/2 | 95.20 | 94.97 | 94.76 | 94.60 | 94.52 | 94.26 | 94.19 | 93.03 | 88.19 | 81.11 | 78.71 | |
| SW/10 | 96.82 | 96.58 | 96.37 | 96.22 | 96.15 | 95.93 | 95.86 | 94.92 | 90.20 | 83.07 | 80.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, Y.; Kim, B.; Liu, Q.; Wang, L.; Lee, K.S. Synergistic Effects of Dimethyl Ether and LSW in a CO2 WAG Process for Enhanced Oil Recovery and CO2 Sequestration. Energies 2025, 18, 6104. https://doi.org/10.3390/en18236104
Seong Y, Kim B, Liu Q, Wang L, Lee KS. Synergistic Effects of Dimethyl Ether and LSW in a CO2 WAG Process for Enhanced Oil Recovery and CO2 Sequestration. Energies. 2025; 18(23):6104. https://doi.org/10.3390/en18236104
Chicago/Turabian StyleSeong, Yongho, Bomi Kim, Qingquan Liu, Liang Wang, and Kun Sang Lee. 2025. "Synergistic Effects of Dimethyl Ether and LSW in a CO2 WAG Process for Enhanced Oil Recovery and CO2 Sequestration" Energies 18, no. 23: 6104. https://doi.org/10.3390/en18236104
APA StyleSeong, Y., Kim, B., Liu, Q., Wang, L., & Lee, K. S. (2025). Synergistic Effects of Dimethyl Ether and LSW in a CO2 WAG Process for Enhanced Oil Recovery and CO2 Sequestration. Energies, 18(23), 6104. https://doi.org/10.3390/en18236104







