The Complete Chain Management of Organochlorine in Crude Oil: Sources, Detection, Removal, and Low-Carbon Risk Control Strategies
Abstract
1. Introduction
1.1. Industrial Challenge and Research Significance
1.2. Concept Definition and Terminology
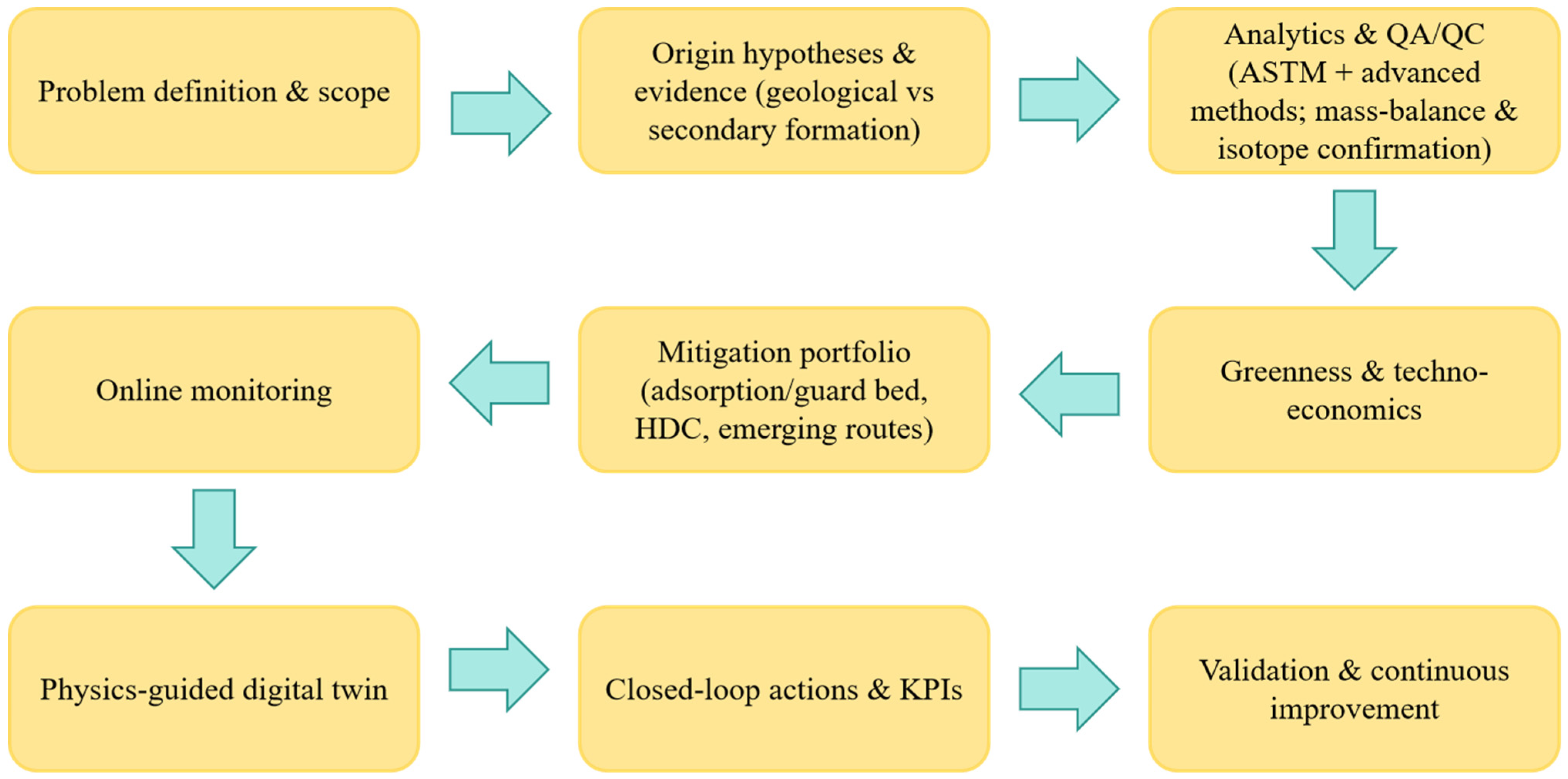
1.3. Scope, Structure, and Contributions of This Review
1.4. Methodology for Literature Selection and Appraisal
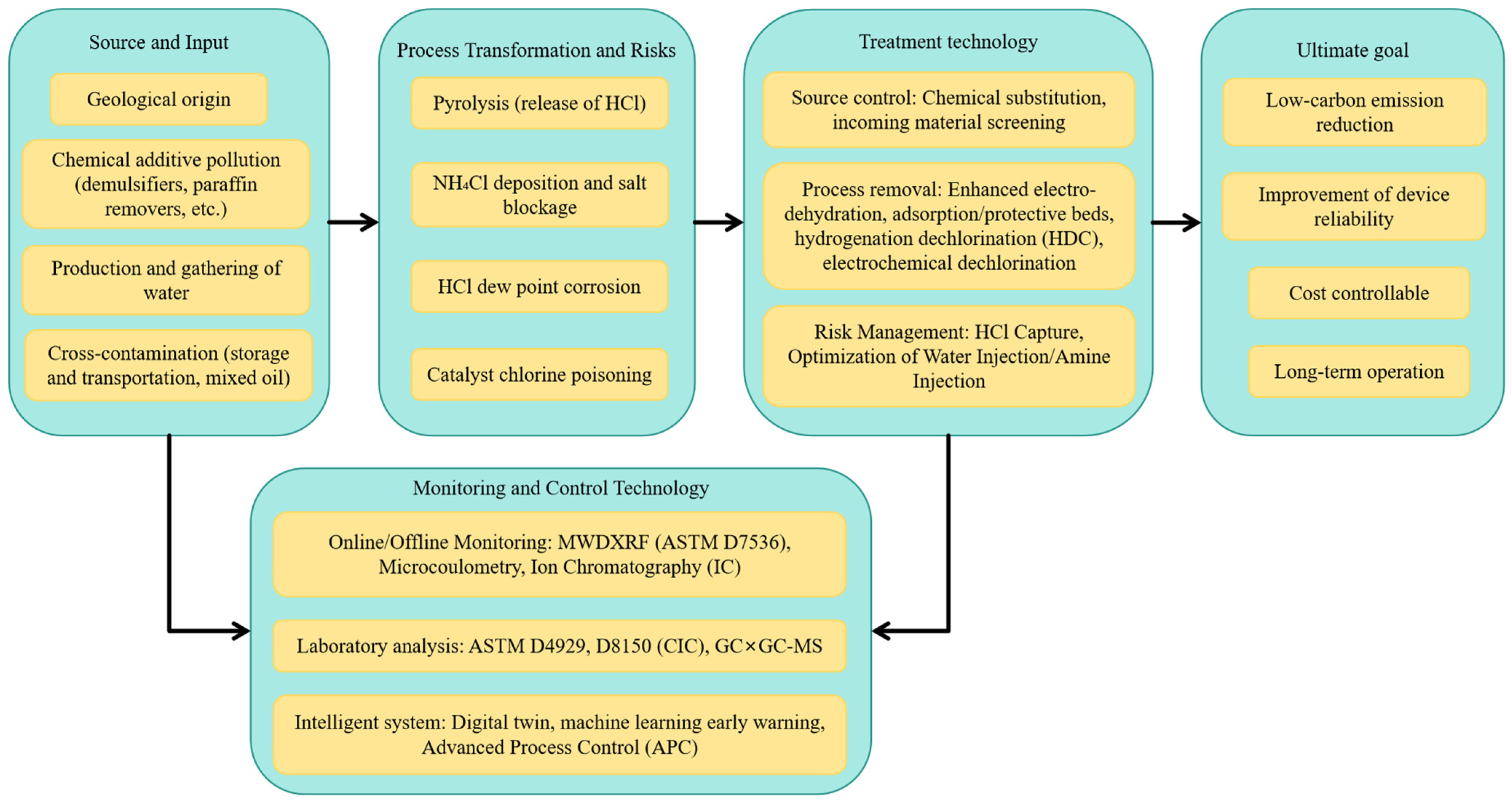
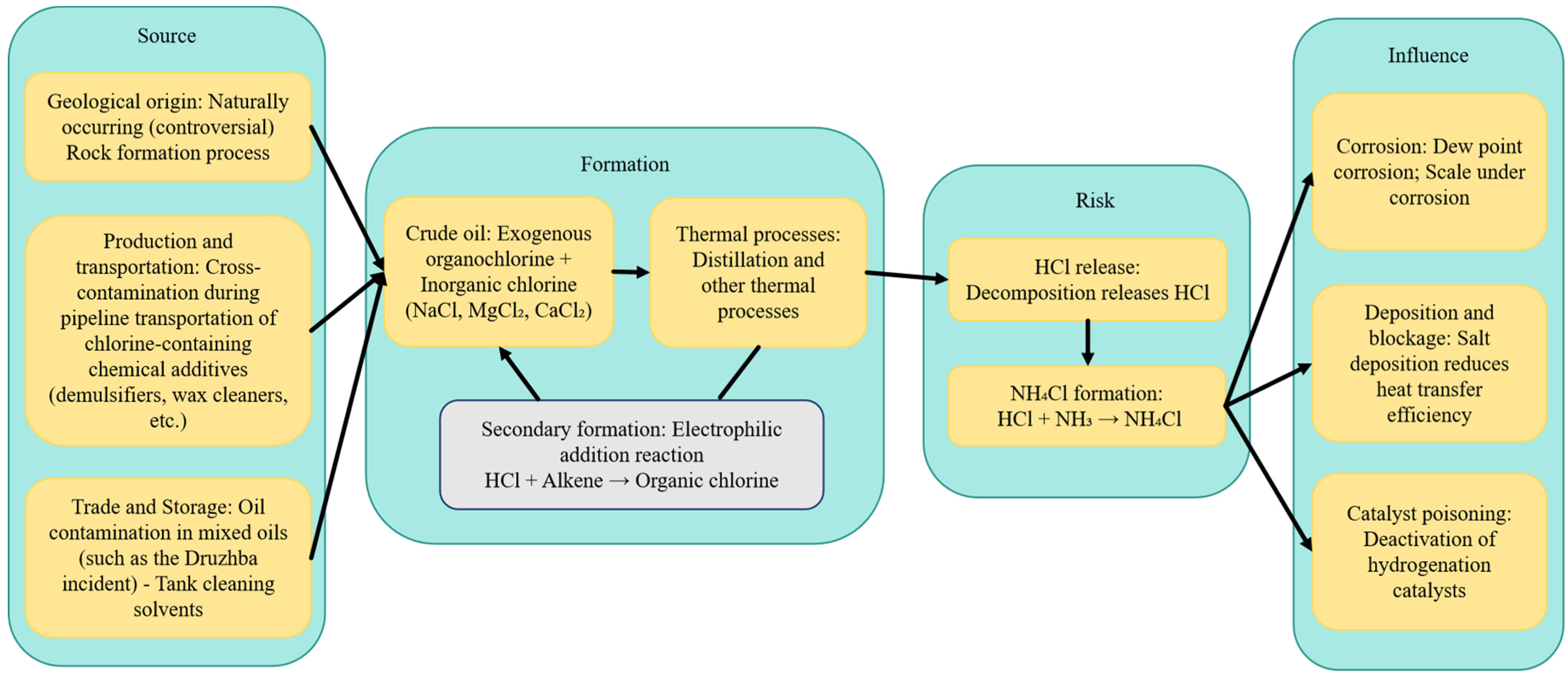
2. Sources and Formation Mechanisms of Organic Chlorine in Crude Oil
2.1. Geological and Genetic Sources: Controversies and New Insights
2.2. Introduction During Production and Gathering
2.3. Cross-Contamination in the Pre-Refining Sector (Storage, Transportation, and Trading)
2.4. Chemical Speciation and Distribution Characteristics
2.5. Process and Environmental Risk Pathways
3. Analytical Methods and Quality Control
3.1. Overview of Total and Organic Chlorine Determination Methods
3.2. Key Analytical Techniques and Advances
3.2.1. Combustion-Microcoulometry and Combustion-Ion Chromatography (CIC)
3.2.2. High-Temperature Pyrolysis-Coulometry and Portable Analysis
3.2.3. AOX/EOX-like Systems and Online Combustion–Extraction Coupling
3.3. Speciation and Molecular Fingerprinting Analysis
3.3.1. GC-MS and Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry (GC × GC-TOFMS)
3.3.2. High-Resolution Mass Spectrometry and Chlorine Isotope Analysis
3.4. Sample Preparation and Matrix Effect Control
3.5. Method Validation and Quality Control
3.5.1. Analytical Performance Parameters
3.5.2. Matrix Tolerance, Throughput, and Cost
3.5.3. Reference Materials and Inter-Laboratory Comparison
3.6. Online Analysis and Process Monitoring
3.6.1. Online Total Chlorine Analysis at Desalting and Process Nodes
3.6.2. Corrosion and Deposition Risk Correlation Monitoring
3.7. Green Analysis and ESG Considerations
3.7.1. Method Greenness Assessment
3.7.2. Treatment of Chlorinated By-Products
4. Removal and Control Technologies and Engineering Applications
4.1. Source Control and Pre-Refining Management
4.2. Physicochemical Removal Technologies
4.2.1. Enhanced Electrostatic Desalting and Multi-Process Coupling
4.2.2. Adsorption and Ion Exchange
4.2.3. Membrane Separation and Integrated Processes
4.3. Catalytic and Electrochemical Dechlorination
4.3.1. Hydrodechlorination (HDC)
4.3.2. Process Coupling and Guard Beds
4.3.3. Electrochemical and Photochemical Dechlorination
4.3.4. Phase-Transfer Catalysis (PTC) Dechlorination
4.4. Biological and Hybrid Treatment Strategies
4.5. Scale-Up and Performance Assessment
5. Risk, Standards, and Regulatory Framework
5.1. Quality Indicators and Internal Control Limits
5.2. Corrosion Mechanisms and Safety Impacts
5.3. Compliance Requirements and Trade Practices
6. Data-Driven Source Apportionment and Process Optimization
6.1. Fingerprint Analysis and Multivariate Statistical Methods
6.2. Application of Machine Learning in Spectral Deconvolution and Concentration Prediction
6.3. Digital Twin and Online Control Loop
7. Research Gaps and Future Directions
7.1. Challenges in Chlorine Analysis of Highly Complex Matrices
7.2. Method Comparability and Metrological Traceability
7.3. Low-Carbon and Anti-Poisoning Dechlorination Technologies
7.4. Data Sharing and Algorithm Interpretability
7.5. Evolution of Process Modeling and Control
8. Conclusions and Outlook
8.1. Conclusions
8.2. Limitations
8.3. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AED | Atomic emission detector (element-selective GC detection). |
| AGREE | Analytical GREEnness metric. |
| AGREEprep | Greenness metric for sample preparation. |
| AOX | Adsorbable organic halogens; halogenated organics retained on activated carbon and quantified after combustion. |
| APC | Advanced process control. |
| ASTM D4929 | Organic chlorides in light petroleum distillates (Procedures A/B/C: titration/microcoulometry/XRF). |
| ASTM D5808 | High-temperature pyrolysis microcoulometry; total chlorine determination. |
| ASTM D8150 | Combustion-ion chromatography; total organic halogens in light fractions. |
| Bland–Altman | Agreement analysis for two measurement methods (bias and limits of agreement). |
| CAPEX | Capital expenditure (equipment and installation). |
| CDU | Crude distillation unit. |
| CIC | Combustion-ion chromatography; ASTM D8150 for total organic halogens in light fractions. |
| CRM | Certified reference material (ISO 17034). |
| CSIA-Cl | Compound-specific chlorine isotope analysis. |
| DESs | Deep eutectic solvents. |
| Dew point/Salt point | Onset of acid/water dew or ammonium-salt deposition; predicted by thermodynamic models. |
| DMI | Direct matrix introduction (chromatography inlet). |
| ECH/ECHD | Electrocatalytic (hydro)dehalogenation. |
| ELCD | Electrolytic conductivity detector (halogen-selective) for GC. |
| EOX | Extractable organic halogens; solvent-extractable halogenated organics quantified after combustion. |
| FT-ICR MS | Fourier transform ion cyclotron resonance mass spectrometry. |
| GAC | Green analytical chemistry (greenness). |
| GAPI | Green analytical procedure index. |
| GC × GC-MS | Comprehensive two-dimensional gas chromatography–mass spectrometry (often TOFMS). |
| GC-MS | Gas chromatography–mass spectrometry. |
| GPC | Gel permeation chromatography. |
| HCl | Hydrogen chloride. |
| HDC | Hydrodechlorination (catalytic C-Cl cleavage with H2). |
| HS-SPME | Headspace solid-phase microextraction. |
| ILs | Ionic liquids. |
| Inorganic Cl− | Inorganic chloride ion. |
| KPI | Key performance indicator. |
| LLE | Liquid–liquid extraction. |
| LOD | Limit of detection. |
| LOQ | Limit of quantitation. |
| Mass-balance closure | Reconciliation of Cl-in vs. Cl-out across phases/units. |
| MD | Membrane distillation. |
| MSE | Multi-scale electrolyte thermodynamic model for ion properties and dew/salt-point prediction. |
| MVI | Minimum viable implementation (auditable closed-loop deployment bundle). |
| MWDXRF | Monochromatic wavelength dispersive X-ray fluorescence; ASTM D7536 for total chlorine. |
| NF | Nanofiltration (membrane). |
| NHT | Naphtha hydrotreating unit. |
| OPEX | Operating expenditure (consumables, energy, labor). |
| Orbitrap MS | High-resolution Orbitrap mass spectrometry. |
| Org-Cl | Organically bound chlorine in crude oil. |
| Passing–Bablok | Non-parametric regression for method comparison/equivalence assessment. |
| ppm (as Cl) | Parts per million expressed as chlorine. |
| Precision/Recall | Classification metrics used for ML model evaluation (PPV and sensitivity). |
| PT/PTP | Proficiency testing/proficiency testing program. |
| PTC | Phase-transfer catalysis (biphasic nucleophilic substitution). |
| QA/QC | Quality assurance/quality control. |
| QACs | Quaternary ammonium compounds (biocides/corrosion control). |
| RI | Retention index (chromatography). |
| RO | Reverse osmosis (membrane). |
| SPE | Solid-phase extraction. |
| Total Cl | Total chlorine (organic + inorganic) in a sample. |
| TOX | Total organic halogens; AOX/EOX-like or combustion-based total halogen content. |
| TRL | Technology readiness level (ISO 16290/EU-H2020 mapping). |
| XSD | Halogen-specific detector for GC. |
References
- Mitra, S.; Sulakhe, S.; Shown, B.; Mandal, S.; Das, A.K. Organic Chlorides in Petroleum Crude Oil: Challenges for Refinery and Mitigations. ChemBioEng Rev. 2022, 9, 319–332. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion Challenges in Petroleum Refinery Operations: Sources, Mechanisms, Mitigation, and Future Outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Chis, T.; Sterpu, A.E.; Săpunaru, O.V. The Effect of Corrosion on Crude Oil Distillation Plants. ChemEngineering 2022, 6, 41. [Google Scholar] [CrossRef]
- Li, R.; Huang, H.; Wang, X.; Wang, Y. Effect of Ammonium Salt on Corrosion of Pipelines and Components in a Crude Oil Distillation Column: Electrochemical and AIMD Studies. Corros. Sci. 2022, 203, 110362. [Google Scholar] [CrossRef]
- Spaan, K.M.; Yuan, B.; Plassmann, M.M.; Benskin, J.P.; de Wit, C.A. Characterizing the Organohalogen Iceberg: Extractable, Multihalogen Mass Balance Determination in Municipal Wastewater Treatment Plant Sludge. Environ. Sci. Technol. 2023, 57, 9309–9320. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Li, X.; Zhu, J. Distribution and Identification of Chlorides in Distillates from YS Crude Oil. Energy Fuels 2015, 29, 1391–1396. [Google Scholar] [CrossRef]
- Pagliano, E.; Gajdosechova, Z.; Lopez-Linares, F.; Mester, Z. Conversion of Inorganic Chlorides into Organochlorine Compounds during Crude Oil Distillation: Myth or Reality? Energy Fuels 2021, 35, 894–897. [Google Scholar] [CrossRef]
- Hassanshahi, N.; Hu, G.; Li, J. Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules 2020, 25, 4915. [Google Scholar] [CrossRef] [PubMed]
- ASTM D8150-22; Standard Test Method for Determination of Organic Chloride Content in Crude Oil by Distillation Followed by Detection Using Combustion Ion Chromatography. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM D4929-24; Standard Test Method for Determination of Organic Chloride Content in Crude Oil. ASTM International: West Conshohocken, PA, USA, 2025. [CrossRef]
- Souchon, V.; Maleval, M.; Chainet, F.; Lienemann, C.-P. Chlorine Speciation in Complex Hydrocarbon Matrices Using GC-ICP-MS/MS. J. Anal. At. Spectrom. 2023, 38, 1634–1642. [Google Scholar] [CrossRef]
- Melder, J.; Zinsmeister, J.; Grein, T.; Jürgens, S.; Köhler, M.; Oßwald, P. Comprehensive Two-Dimensional Gas Chromatography: A Universal Method for Composition-Based Prediction of Emission Characteristics of Complex Fuels. Energy Fuels 2023, 37, 4580–4595. [Google Scholar] [CrossRef]
- ASTM D5808-23; Standard Test Method for Determining Chloride in Aromatic Hydrocarbons and Related Chemicals by Microcoulometry. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Horst, A.; Renpenning, J.; Richnow, H.-H.; Gehre, M. Compound Specific Stable Chlorine Isotopic Analysis of Volatile Aliphatic Compounds Using Gas Chromatography Hyphenated with Multiple Collector Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2017, 89, 9131–9138. [Google Scholar] [CrossRef]
- ASTM D7536-24; Standard Test Method for Chlorine in Aromatics by Monochromatic Wavelength Dispersive X-Ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2024. [CrossRef]
- Zimmermann, J.; Halloran, L.J.S.; Hunkeler, D. Tracking Chlorinated Contaminants in the Subsurface Using Compound-Specific Chlorine Isotope Analysis: A Review of Principles, Current Challenges and Applications. Chemosphere 2020, 244, 125476. [Google Scholar] [CrossRef]
- Kowalewska, Z.; Brzezińska, K.; Zieliński, J.; Pilarczyk, J. Method Development for Determination of Organic Fluorine in Gasoline and Its Components Using High-Resolution Continuum Source Flame Molecular Absorption Spectrometry with Gallium Fluoride as a Target Molecule. Talanta 2021, 227, 122205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Tan, X.; Li, J.; Croué, J.-P.; Chen, B. Insights into Adsorbable Organic Halogen Analysis: Two Overlooked Factors Impacting Water Quality Assessment. Sci. Total Environ. 2024, 928, 172429. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Gong, X.; He, M.; Wang, X.; Zhao, R.; Hao, Z.; Liu, J. Total Organic Halogen (TOX) Analysis in Waters: A Short Review. Environ. Pollut. Bioavailab. 2023, 35, 2203350. [Google Scholar] [CrossRef]
- Gröger, T.M.; Käfer, U.; Zimmermann, R. Gas Chromatography in Combination with Fast High-Resolution Time-of-Flight Mass Spectrometry: Technical Overview and Perspectives for Data Visualization. TrAC Trends Anal. Chem. 2020, 122, 115677. [Google Scholar] [CrossRef]
- Andersson, A.; Ashiq, M.J.; Shoeb, M.; Karlsson, S.; Bastviken, D.; Kylin, H. Evaluating Gas Chromatography with a Halogen-Specific Detector for the Determination of Disinfection by-Products in Drinking Water. Env. Sci. Pollut. Res. 2019, 26, 7305–7314. [Google Scholar] [CrossRef]
- Nicolas, M.; Theurillat, X.; Redeuil, K.; Nagy, K. Monochromatic Wavelength Dispersive X-Ray Fluorescence as a Tool for Monitoring the Source of Chlorine in the Formation of Monochloropropane-Diol Esters during Vegetable Oil Refining. J. Agric. Food Res. 2022, 8, 100297. [Google Scholar] [CrossRef]
- Ajaero, C.; Vander Meulen, I.; Heshka, N.E.; Xin, Q.; McMartin, D.W.; Peru, K.M.; Chen, H.; McKenna, A.M.; Reed, K.; Headley, J.V. Evaluations of Weathering of Polar and Nonpolar Petroleum Components in a Simulated Freshwater–Oil Spill by Orbitrap and Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2024, 38, 6753–6763. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Pudenzi, M.A.; Santos, J.M.; Angolini, C.F.F.; Pereira, R.C.L.; Rocha, Y.S.; Denisov, E.; Damoc, E.; Makarov, A.; Eberlin, M.N. Petroleomics via Orbitrap Mass Spectrometry with Resolving Power above 1,000,000 at m/z 200. RSC Adv. 2018, 8, 6183–6191. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Gui, J.; Li, X.; Qi, G. Compound-Specific Chlorine Isotope Analysis of Organochlorine Pesticides by Gas Chromatography-Negative Chemical Ionization Mass Spectrometry. J. Anal. Methods Chem. 2021, 2021, 8874679. [Google Scholar] [CrossRef] [PubMed]
- Ponsin, V.; Torrentó, C.; Lihl, C.; Elsner, M.; Hunkeler, D. Compound-Specific Chlorine Isotope Analysis of the Herbicides Atrazine, Acetochlor, and Metolachlor. Anal. Chem. 2019, 91, 14290–14298. [Google Scholar] [CrossRef]
- Vinyes-Nadal, M.; Masbou, J.; Kümmel, S.; Gehre, M.; Imfeld, G.; Otero, N.; Torrentó, C. Novel Extraction Methods and Compound-Specific Isotope Analysis of Methoxychlor in Environmental Water and Aquifer Slurry Samples. Sci. Total Environ. 2024, 931, 172858. [Google Scholar] [CrossRef]
- ASTM D5854-25; Standard Practice for Mixing and Handling of Liquid Samples of Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2025. [CrossRef]
- ASTM D4057-22; Standard Practice for Manual Sampling of Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Marguí, E.; Eichert, D.; Jablan, J.; Bilo, F.; Depero, L.E.; Pejović-Milić, A.; Gross, A.; Stosnach, H.; Kubala-Kukuś, A.; Banaś, D.; et al. An Overview of the Applications of Total Reflection X-Ray Fluorescence Spectrometry in Food, Cosmetics, and Pharmaceutical Research. J. Anal. At. Spectrom. 2024, 39, 1700–1719. [Google Scholar] [CrossRef]
- Bulsink, P.; de Miguel Mercader, F.; Sandström, L.; van de Beld, B.; Preto, F.; Zacher, A.; Oasmaa, A.; Dahmen, N.; Funke, A.; Bronson, B. Results of the International Energy Agency Bioenergy Round Robin on the Analysis of Heteroatoms in Biomass Liquefaction Oils. Energy Fuels 2020, 34, 11123–11133. [Google Scholar] [CrossRef]
- Holkem, A.P.; Agostini, G.; Costa, A.B.; Barin, J.S.; Mello, P.A. A Simple and Green Analytical Alternative for Chloride Determination in High-Salt-Content Crude Oil: Combining Miniaturized Extraction with Portable Colorimetric Analysis. Processes 2024, 12, 2425. [Google Scholar] [CrossRef]
- ISO 17034:2016; General Requirements for the Competence of Reference Material Producers. International Organization for Standardization (ISO): Beijing, China, 2016.
- D4327-17R25; Standard Test Method for Anions in Water by Suppressed Ion Chromatography. ASTM International: West Conshohocken, PA, USA, 2025. [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Adv. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Romero, A.; Moreno, I.; Escudero, L.; Yuste, R.; Pizarro, P.; Moreno, J.M.; Serrano, D.P. Dechlorination of a Real Plastic Waste Pyrolysis Oil by Adsorption with Zeolites. J. Environ. Chem. Eng. 2024, 12, 112638. [Google Scholar] [CrossRef]
- Yin, L.; Yu, L.; Guo, Y.; Wang, C.; Ge, Y.; Zheng, X.; Zhang, N.; You, J.; Zhang, Y.; Shi, M. Green Analytical Chemistry Metrics for Evaluating the Greenness of Analytical Procedures. J. Pharm. Anal. 2024, 14, 101013. [Google Scholar] [CrossRef]
- Hammad, S.F.; Hamid, M.A.A.; Adly, L.; Elagamy, S.H. Comprehensive Review of Greenness, Whiteness, and Blueness Assessments of Analytical Methods. Green Anal. Chem. 2025, 12, 100209. [Google Scholar] [CrossRef]
- Husain, A.; Adewunmi, A.A.; Kamal, M.S.; Mahmoud, M.; Al-Harthi, M.A. Demulsification of Heavy Petroleum Emulsion Using Pyridinium Ionic Liquids with Distinct Anion Branching. Energy Fuels 2021, 35, 16527–16533. [Google Scholar] [CrossRef]
- Tang, L.; Wang, T.; Xu, Y.; He, X.; Yan, A.; Zhang, Z.; Li, Y.; Chen, G. Research and Application Progress of Crude Oil Demulsification Technology. Processes 2024, 12, 2292. [Google Scholar] [CrossRef]
- Low, J.Y.; Khe, C.S.; Usman, F.; Hassan, Y.M.; Lai, C.W.; You, K.Y.; Lim, J.W.; Khoo, K.S. Review on Demulsification Techniques for Oil/Water Emulsion: Comparison of Recyclable and Irretrievable Approaches. Environ. Res. 2024, 243, 117840. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, R.; Sand, W.; Mathivanan, K.; Zhang, Y.; Wang, N.; Duan, J.; Hou, B. Comprehensive Review on the Use of Biocides in Microbiologically Influenced Corrosion. Microorganisms 2023, 11, 2194. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Jasra, R.V. Sorption of HCl from an Aromatic Hydrocarbon Mixture Using Modified Molecular Sieve Zeolite 13X. ACS Omega 2021, 6, 28742–28751. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Argaez, M.A.; Abreú-López, D.; Gracia-Fadrique, J.; Dutta, A. Numerical Study of Electrostatic Desalting: A Detailed Parametric Study. Processes 2022, 10, 2118. [Google Scholar] [CrossRef]
- Kooti, G.; Dabir, B.; Taherdangkoo, R.; Butscher, C. Modelling Droplet Size Distribution in Inline Electrostatic Coalescers for Improved Crude Oil Processing. Sci. Rep. 2023, 13, 20209. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Sun, Z.; Wang, X.; Chen, Q.; Liu, W.; Qi, Z.; Wei, L.; Li, B. Molecular Dynamics Study of Droplet Electrocoalescence in the Oil Phase and the Gas Phase. Sep. Purif. Technol. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Ranaee, E.; Ghorbani, H.; Keshavarzian, S.; Ghazaeipour Abarghoei, P.; Riva, M.; Inzoli, F.; Guadagnini, A. Analysis of the Performance of a Crude-Oil Desalting System Based on Historical Data. Fuel 2021, 291, 120046. [Google Scholar] [CrossRef]
- Liu, S.; Otero, J.A.; Martin-Martinez, M.; Rodriguez-Franco, D.; Rodriguez, J.J.; Gómez-Sainero, L.M. Understanding Hydrodechlorination of Chloromethanes. Past and Future of the Technology. Catalysts 2020, 10, 1462. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Wang, Z. Electrocatalytic Hydro-Dehalogenation of Halogenated Organic Pollutants from Wastewater: A Critical Review. Water Res. 2023, 234, 119810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, R.; Zhang, G.; Dong, L.; Zhang, D.; Wang, G.; Li, T. Zn-Modified Hβ Zeolites Used in the Adsorptive Removal of Organic Chloride from Model Naphtha. ACS Omega 2020, 5, 11987–11997. [Google Scholar] [CrossRef]
- Huang, B.; Sun, Q.; Bing, L.; Han, D.; Wang, G.; Wang, F. Adsorptive Removal of Organochlorine from Oils by Modified Activated Carbon: Effect of Surface Groups and Kinetic and Thermodynamic Studies. Ind. Eng. Chem. Res. 2024, 63, 10955–10964. [Google Scholar] [CrossRef]
- Sharma, R.; Segato, T.; Delplancke, M.-P.; Terryn, H.; Baron, G.V.; Denayer, J.F.M.; Cousin-Saint-Remi, J. Hydrogen Chloride Removal from Hydrogen Gas by Adsorption on Hydrated Ion-Exchanged Zeolites. Chem. Eng. J. 2020, 381, 122512. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Tarach, K.A.; Góra-Marek, K. Top-down Engineering of Zeolite Porosity. Chem. Soc. Rev. 2025, 54, 7484–7560. [Google Scholar] [CrossRef] [PubMed]
- Dadari, S.; Rahimi, M.; Zinadini, S. Crude Oil Desalter Effluent Treatment Using High Flux Synthetic Nanocomposite NF Membrane-Optimization by Response Surface Methodology. Desalination 2016, 377, 34–46. [Google Scholar] [CrossRef]
- Norouzbahari, S.; Roostaazad, R.; Hesampour, M. Crude Oil Desalter Effluent Treatment by a Hybrid UF/RO Membrane Separation Process. Desalination 2009, 238, 174–182. [Google Scholar] [CrossRef]
- Huang, J.; Ran, X.; Sun, L.; Bi, H.; Wu, X. Recent Advances in Membrane Technologies Applied in Oil–Water Separation. Discov. Nano 2024, 19, 66. [Google Scholar] [CrossRef]
- Li, S.; Dong, R.; Musteata, V.-E.; Kim, J.; Rangnekar, N.D.; Johnson, J.R.; Marshall, B.D.; Chisca, S.; Xu, J.; Hoy, S.; et al. Hydrophobic Polyamide Nanofilms Provide Rapid Transport for Crude Oil Separation. Science 2022, 377, 1555–1561. [Google Scholar] [CrossRef]
- Jeon, J.; Park, Y.; Hwang, Y. Catalytic Hydrodechlorination of 4-Chlorophenol by Palladium-Based Catalyst Supported on Alumina and Graphene Materials. Nanomaterials 2023, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Potylitsyna, A.R.; Kadtsyna, A.S.; Chesnokov, V.V.; Nalivaiko, A.Y.; Gromov, A.A.; Buyanov, R.A. Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst. Catalysts 2020, 10, 1446. [Google Scholar] [CrossRef]
- Mahy, J.G.; Delbeuck, T.; Tran, K.Y.; Heinrichs, B.; Lambert, S.D. Green Chemistry for the Transformation of Chlorinated Wastes: Catalytic Hydrodechlorination on Pd-Ni and Pd-Fe Bimetallic Catalysts Supported on SiO2. Gels 2023, 9, 275. [Google Scholar] [CrossRef]
- Beil, S.B.; Bonnet, S.; Casadevall, C.; Detz, R.J.; Eisenreich, F.; Glover, S.D.; Kerzig, C.; Næsborg, L.; Pullen, S.; Storch, G.; et al. Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop. JACS Au 2024, 4, 2746–2766. [Google Scholar] [CrossRef]
- Niu, H.; Zhao, D.; Xie, G.; Yuan, Y.; Zhang, W.; Zhang, C.; Li, C.; Cui, L. Deep Dechlorination of Model Oil by Reactive Adsorption on Porous Oxides. Fuel 2021, 304, 121410. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, L.; Cai, B.; Zhao, P.; Ding, M.; Wang, C.; Qu, C. Recent Developments in Electrochemical Reduction for Remediation of Chlorinated Hydrocarbons Contaminated Groundwater. J. Environ. Chem. Eng. 2025, 13, 115125. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, X.; Tian, K.; Tan, D.; Song, X.; Wang, P.; Jiang, Q.; Lu, J. Electrochemical Reduction and Oxidation of Chlorinated Aromatic Compounds Enhanced by the Fe-ZSM-5 Catalyst: Kinetics and Mechanisms. ACS Omega 2022, 7, 33500–33510. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, G.; Song, Z.; He, Z.; Zhong, A.; Cui, M. Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid. Catalysts 2024, 14, 424. [Google Scholar] [CrossRef]
- Hu, B.; Wu, B.; Du, Y.; Zhu, J. The Removal of Organochlorine Compounds from Model Oil by Nucleophilic Substitution. ACS Omega 2025, 10, 24089–24096. [Google Scholar] [CrossRef]
- Milani, M.; Mazzanti, M.; Stevanin, C.; Chenet, T.; Magnacca, G.; Pasti, L.; Molinari, A. CdS-Based Hydrothermal Photocatalysts for Complete Reductive Dehalogenation of a Chlorinated Propionic Acid in Water by Visible Light. Nanomaterials 2024, 14, 579. [Google Scholar] [CrossRef]
- Čičáková, C.; Tóth, R.; Horváthová, H.; Jurkovič, Ľ.; Špirová, V.; Drábik, A.; Kravchenko, D. Integrated Method of Electrochemical Dechlorination of Chlorinated Aliphatic Hydrocarbons in Combination with Groundwater Pumping in Highly Contaminated Groundwater–Field Application. Electrochim. Acta 2025, 525, 146125. [Google Scholar] [CrossRef]
- ISO 16290:2013; Space Systems—Definition of the Technology Readiness Levels (TRLs) and Their Criteria of Assessment. BSI British Standards Institution: Reston, VA, USA, 2013.
- Pereira Nicacio, J.A.; Castro Oliveira, F.; Rosa Dumont, M. Failure Analysis and Electrochemical Testing of Ammonium Chloride Corrosion in a Heat Exchanger in a Diesel Hydrotreating Unit of a Petroleum Refinery. Eng. Fail. Anal. 2024, 156, 107758. [Google Scholar] [CrossRef]
- Jin, H.; Yin, H.; Xu, S.; Chen, W.; Liu, X.; Jiang, Y. Experimental Study on the Deposition Characteristics of Ammonium Chloride Particles in a Hydrogenation Air Cooler. Powder Technol. 2025, 452, 120533. [Google Scholar] [CrossRef]
- Li, R.; Jin, H.; Sun, J.; Liu, X.; Wang, C.; Zhang, L. Numerical and Experimental Study on Corrosion Mechanism of the Water-Oil Two-Phase Flow in the Atmospheric Tower Top System. Eng. Fail. Anal. 2025, 167, 108981. [Google Scholar] [CrossRef]
- Sudol, P.E.; Pierce, K.M.; Prebihalo, S.E.; Skogerboe, K.J.; Wright, B.W.; Synovec, R.E. Development of Gas Chromatographic Pattern Recognition and Classification Tools for Compliance and Forensic Analyses of Fuels: A Review. Anal. Chim. Acta 2020, 1132, 157–186. [Google Scholar] [CrossRef]
- Santos, M.C.; Coutinho, D.M.; Torres, C.L.; Barra, T.A.; Cardoso, V.G.K.; Silva, R.V.S.; Dubois, D.S.; Lopes, J.P.; Neto, F.R.A.; Azevedo, D.A. Pixel-Based Chemometric Analysis of Pre-Salt Crude Oils: Advancing GC×GC-TOFMS for Reservoir Characterization. Energy Fuels 2025, 39, 7204–7213. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, D.; Sun, J. Application of GC-IMS Coupled with Chemometric Analysis for the Classification and Authentication of Geographical Indication Agricultural Products and Food. Front. Nutr. 2023, 10, 1247695. [Google Scholar] [CrossRef] [PubMed]
- Devers, J.; Pattison, D.I.; Hansen, A.B.; Christensen, J.H. Comprehensive Two-Dimensional Gas Chromatography as a Tool for Targeted and Non-Targeted Analysis of Contaminants of Emerging Concern in Wastewater. Talanta 2025, 282, 127032. [Google Scholar] [CrossRef] [PubMed]
- Kaspi, O.; Avissar, Y.Y.; Grafit, A.; Chibel, R.; Girshevitz, O.; Senderowitz, H. Machine Learning-Based Identification of Petroleum Distillates and Gasoline Traces Using Measured and Synthetic GC Spectra from Collected Samples. Mol. Inf. 2025, 44, e202400371. [Google Scholar] [CrossRef]
- Arey, J.S.; Martin Aparicio, A.; Vaiopoulou, E.; Forbes, S.; Lyon, D. Modeling the GCxGC Elution Patterns of a Hydrocarbon Structure Library To Innovate Environmental Risk Assessments of Petroleum Substances. Environ. Sci. Technol. 2022, 56, 17913–17923. [Google Scholar] [CrossRef]
- Zweigle, J.; Tisler, S.; Bevilacqua, M.; Tomasi, G.; Nielsen, N.J.; Gawlitta, N.; Lübeck, J.S.; Smilde, A.K.; Christensen, J.H. Prioritization Strategies for Non-Target Screening in Environmental Samples by Chromatography—High-Resolution Mass Spectrometry: A Tutorial. J. Chromatogr. A 2025, 1751, 465944. [Google Scholar] [CrossRef]
- Badea, S.-L.; Niculescu, V.-C.; Popescu (Stegarus), D.-I.; Geana, E.-I.; Ciucure, C.-T.; Botoran, O.-R.; Ionete, R.-E. Recent Progresses in Compound Specific Isotope Analysis of Halogenated Persistent Organic Pollutants. Assessing the Transformation of Halogenated Persistent Organic Pollutants at Contaminated Sites. Sci. Total Environ. 2023, 899, 165344. [Google Scholar] [CrossRef] [PubMed]
- Shelor, C.P.; Warren, C.; Odinaka, C.V.; Dumre, K. Comprehensive Review of Combustion Ion Chromatography for the Analysis of Total, Adsorbable, and Extractable Organic Fluorine. J. Sep. Sci. 2024, 47, 2400235. [Google Scholar] [CrossRef] [PubMed]
- Katona, R.; Krójer, A.; Locskai, R.; Bátor, G.; Kovács, T. Comparison of Analytical Methods for Measuring Chloride Content in Crude Oil. Appl. Radiat. Isot. 2021, 170, 109594. [Google Scholar] [CrossRef] [PubMed]
- Mitländer, K.; Henseler, J.; Rullo, F.; Nathrath, P.; Geißelbrecht, M.; Wasserscheid, P.; Schühle, P. Continuous (Hydro-)Dechlorination of Aromatic Chloride Compounds in Benzyltoluene. Int. J. Hydrog. Energy 2025, 128, 674–683. [Google Scholar] [CrossRef]
- Gkatziouras, C.; Solakidou, M.; Louloudi, M. Formic Acid Dehydrogenation over a Recyclable and Self-Reconstructing Fe/Activated Carbon Catalyst. Energy Fuels 2024, 38, 17914–17926. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, J.; Wang, P.; Jin, H.; Zheng, Y.; Qiao, S.-Z. Recent Advances in Electrocatalytic Hydrogenation Reactions on Copper-Based Catalysts. Adv. Mater. 2024, 36, 2307913. [Google Scholar] [CrossRef]
- Weggler, B.A.; Dubois, L.M.; Gawlitta, N.; Gröger, T.; Moncur, J.; Mondello, L.; Reichenbach, S.; Tranchida, P.; Zhao, Z.; Zimmermann, R.; et al. A Unique Data Analysis Framework and Open Source Benchmark Data Set for the Analysis of Comprehensive Two-Dimensional Gas Chromatography Software. J. Chromatogr. A 2021, 1635, 461721. [Google Scholar] [CrossRef]
- Moreira de Oliveira, A.; Teixeira, C.A.; Castiblanco, J.E.B.; Medeiros Júnior, Í.; Hantao, L.W. Multiparameter Modeling for 10 Crude Oil Properties Using Comprehensive Two-Dimensional Gas Chromatography and Pixel-Based Chemometrics. Energy Fuels 2025, 39, 12882–12892. [Google Scholar] [CrossRef]
- Morales-Medina, G.; Berbesí, E. Analysis of the Environmental Impact of Fuel Hydrotreating through Life Cycle Assessment and Process Data. Energy Convers. Manag. 2025, 345, 120333. [Google Scholar] [CrossRef]
- Ilyushin, Y.; Nosova, V.; Krauze, A. Application of Systems Analysis Methods to Construct a Virtual Model of the Field. Energies 2025, 18, 1012. [Google Scholar] [CrossRef]

| Procedure | GAPI (Pictogram Rationale) | AGREE (0–1) | AGREEprep (0–1) | Main Penalties/Credits |
|---|---|---|---|---|
| D7536 MWDXRF | minimal sample, no solvents | 0.65–0.75 | 0.70–0.80 | + non-destructive, + short time; − calibration standards |
| D8150 CIC | combustion + IC | 0.50–0.60 | 0.45–0.55 | − absorbent waste, − carrier gas purity control |
| D5808 microcoulometry | high-T pyrolysis | 0.55–0.60 | 0.45–0.55 | − high-T energy; + absolute quantification |
| D4929 B/C | wash + microcoul./XRF | 0.45–0.55 | 0.35–0.45 | − washing/solvent use, multi-step handling |
| GC × GC-MS | speciation (broad) | 0.30–0.40 | 0.25–0.35 | − solvent and time intensive; + information richness |
| Method | Target | Typical Range (mg·kg−1) | LOD (mg·kg−1) | LOQ (mg·kg−1) | Accuracy/Recovery | Repeatability (RSD%) | Throughput | Capex/Opex | Key Notes |
|---|---|---|---|---|---|---|---|---|---|
| ASTM D4929 A/B/C (wash + titration/microcoul./XRF) | Organic Cl | ≥1 | ~0.5–1.0 (Proc. B/C) | ~1.0–2.0 | 85–110% (spiked naphtha) | 5–10 | Low–moderate (multi–step) | $$/$ | Arbitration; matrix sensitive; requires strict blanks |
| D8150 CIC (naphtha) | Total organic halogens | 0.5–10 (naphtha) | ~0.2–0.5 | ~0.5–1.5 | 90–110% | 3–8 | Moderate | $$/$$ | High selectivity; strong anti–interference; light fractions |
| D5808 Pyrolysis–microcoulometry | Total Cl | 0.2–25 | 0.2 | 0.7 | 90–105% | 3–6 | High (fast runs) | $$/$$ | Absolute quant.; interpret with D4929 for “organic Cl” |
| D7536 MWDXRF | Total Cl | 0.66–10 | 0.3–0.6 | 0.66–1.0 | CRM–traceable | 2–5 | Very high (5–10 min) | $$$/$ | Non-destructive; ideal at/online monitor |
| GC–MS/GC × GC–MS (+XSD/AED) | Speciation | - | Method–dependent | - | ID/confirmation; semi–quant | - | Low (data-heavy) | $$–$$$/$$ | Source tracing; targeted confirmation |
| Scenario | Scope | Major CAPEX Items | Major OPEX Drivers | Indicative CAPEX Tier | Indicative OPEX Tier | Notes |
|---|---|---|---|---|---|---|
| Bypass adsorption (guard bed) | Side-stream or slipstream; desalter/CDU outlet | Vessels/piping/valving; analyzers; initial adsorbent inventory | Adsorbent procurement/replacement; pressure drop; disposal | $–$$ | $–$$ (load-dependent) | Fast to deploy; sensitive to spikes and heavy fouling |
| Full HDC (dedicated reactor) | Mainstream treatment of naphtha/straight-run cuts | Reactor; catalyst load; heater; H2 supply/compression; G-L separation | H2 consumption; catalyst deactivation/replacement; utilities | $$–$$$ | $$–$$$ (H2-price sensitive) | High removal efficiency; requires off-gas handling and robust QA/QC |
| Hybrid: guard bed + mild HDC | Guard bed upstream; HDC for residuals | Smaller HDC reactor; guard bed vessels; inline analyzers | Reduced H2 vs. full HDC; longer adsorbent intervals | $$ | $$ | Balanced capex/opex; resilient to feed variability |
| Technology Type | Principle and Mechanism | Main Advantages | Main Limitations and Challenges | Typical Efficiency and Conditions | Typical Applications | Technology Readiness Level (TRL) |
|---|---|---|---|---|---|---|
| Guard Bed/Adsorption | Physical or chemical adsorption at selective sites (e.g., molecular sieves, modified activated carbon) | Low energy consumption, simple operation, effective for light fractions | Frequent adsorbent procurement/replacement | ~70–90% (batch tests); adsorption capacity: 1–5 wt% | Slipstream polishing; buffering chloride spikes near desalter/CDU outlets | 7–8 |
| Hydrodechlorination (HDC) | Catalytic C-Cl bond cleavage: R-Cl + H2 → R-H + HCl | High efficiency, thorough dechlorination, widely applied | Catalyst easily poisoned by Cl/HCl; high H2 consumption; requires high temperature and pressure | >95%; 250–400 °C, 3.0–5.0 MPa H2 | Mainstream removal in naphtha/straight-run feeds | RL 6–7 (crude feeds with mixed organochlorines); TRL 8–9 in petrochemical/final-product chloride removal. |
| Electrochemical Dechlorination | Reductive cleavage: R-Cl + 2e− + H+ → R-H + Cl− | Mild conditions, no H2 required, modular design | Slow reaction rate in organic phase; electrode fouling; scale-up difficult | ~80% (lab scale); requires supporting electrolyte | Niche, low-flow polishing after bulk removal; R&D/pilot demonstrations on authentic matrices | 3–4 |
| Photocatalysis | Radical-mediated cleavage via photogenerated electron-hole pairs | Utilizes solar energy; ambient temperature and pressure | Very low throughput; poor catalyst stability; reactor design challenges | Laboratory-scale (model compounds) | Batch/loop polishing of light fractions with UV/visible LED reactors | 2–3 |
| Phase-Transfer Catalysis (PTC) | Transfers anions from aqueous to organic phase for nucleophilic substitution | Mild conditions; high selectivity | Solvent loss; salt waste generation; separation difficulties | Laboratory-scale (model oils) | Small-loop treatment of narrow light cuts to convert alkyl chlorides under mild base + PTC | 2–3 |
| Biological Treatment | Microbial reductive dechlorination or cometabolism | Green, low-energy, mild conditions | Very slow rate; inhibited by oil toxicity; poor mass transfer | Very low efficiency; only suitable for aqueous phase | Biological polishing of refinery wastewater/produced water containing trace dissolved organochlorines (post oil–water separation and equalization) via MBR/MBBR or aerobic biofilm units | 1–2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Liu, W.; Shu, Y.; Chen, Q.; Wei, K. The Complete Chain Management of Organochlorine in Crude Oil: Sources, Detection, Removal, and Low-Carbon Risk Control Strategies. Energies 2025, 18, 6047. https://doi.org/10.3390/en18226047
Chen Z, Liu W, Shu Y, Chen Q, Wei K. The Complete Chain Management of Organochlorine in Crude Oil: Sources, Detection, Removal, and Low-Carbon Risk Control Strategies. Energies. 2025; 18(22):6047. https://doi.org/10.3390/en18226047
Chicago/Turabian StyleChen, Zhihua, Weidong Liu, Yong Shu, Qiang Chen, and Keqiang Wei. 2025. "The Complete Chain Management of Organochlorine in Crude Oil: Sources, Detection, Removal, and Low-Carbon Risk Control Strategies" Energies 18, no. 22: 6047. https://doi.org/10.3390/en18226047
APA StyleChen, Z., Liu, W., Shu, Y., Chen, Q., & Wei, K. (2025). The Complete Chain Management of Organochlorine in Crude Oil: Sources, Detection, Removal, and Low-Carbon Risk Control Strategies. Energies, 18(22), 6047. https://doi.org/10.3390/en18226047






