Ammonia—A Fuel of the Future? Economies of Production and Control of NOx Emissions via Oscillating NH3 Combustion for Process Heat Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Gaseous Fuels
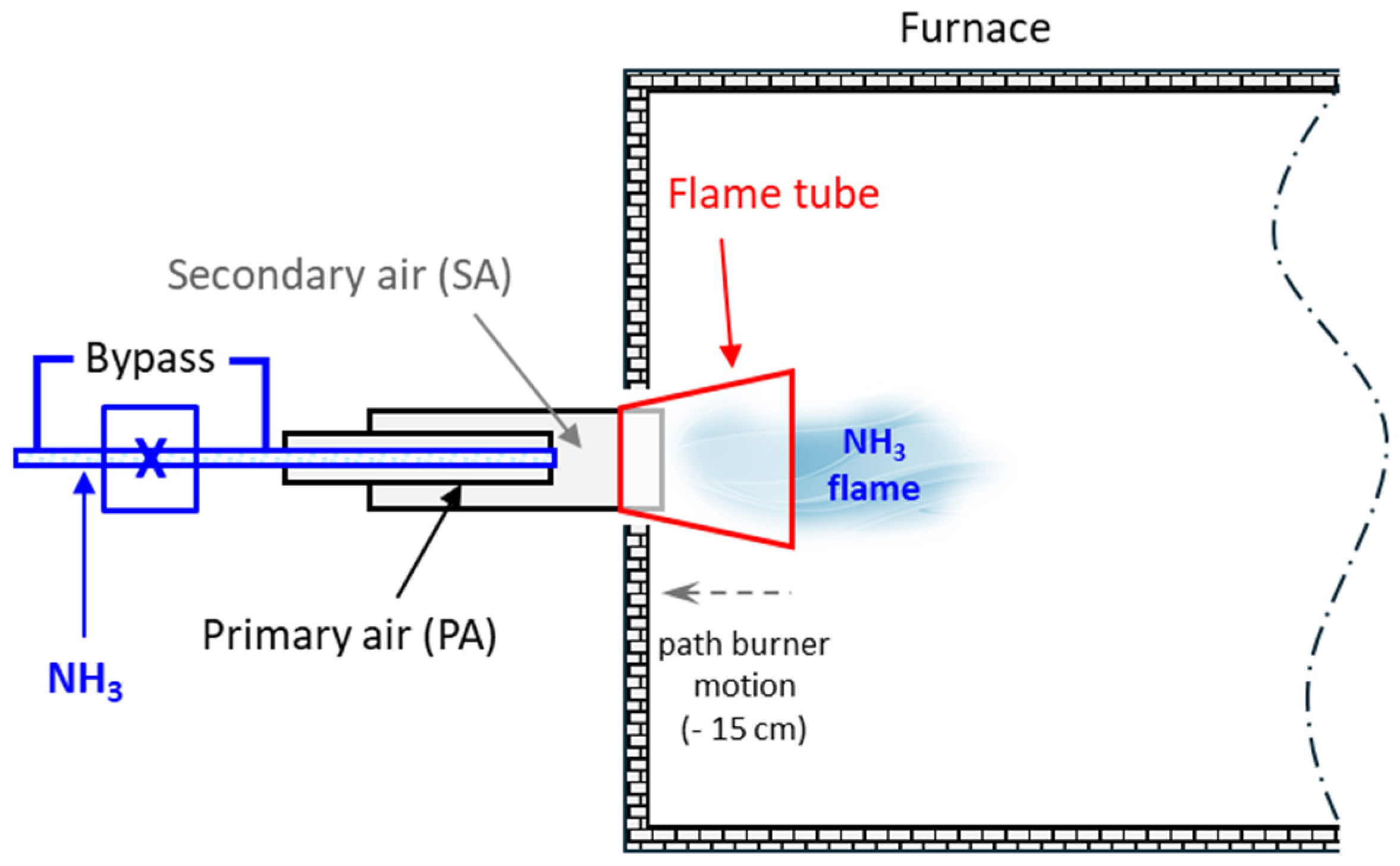
2.2. Burner
2.3. Oscillator
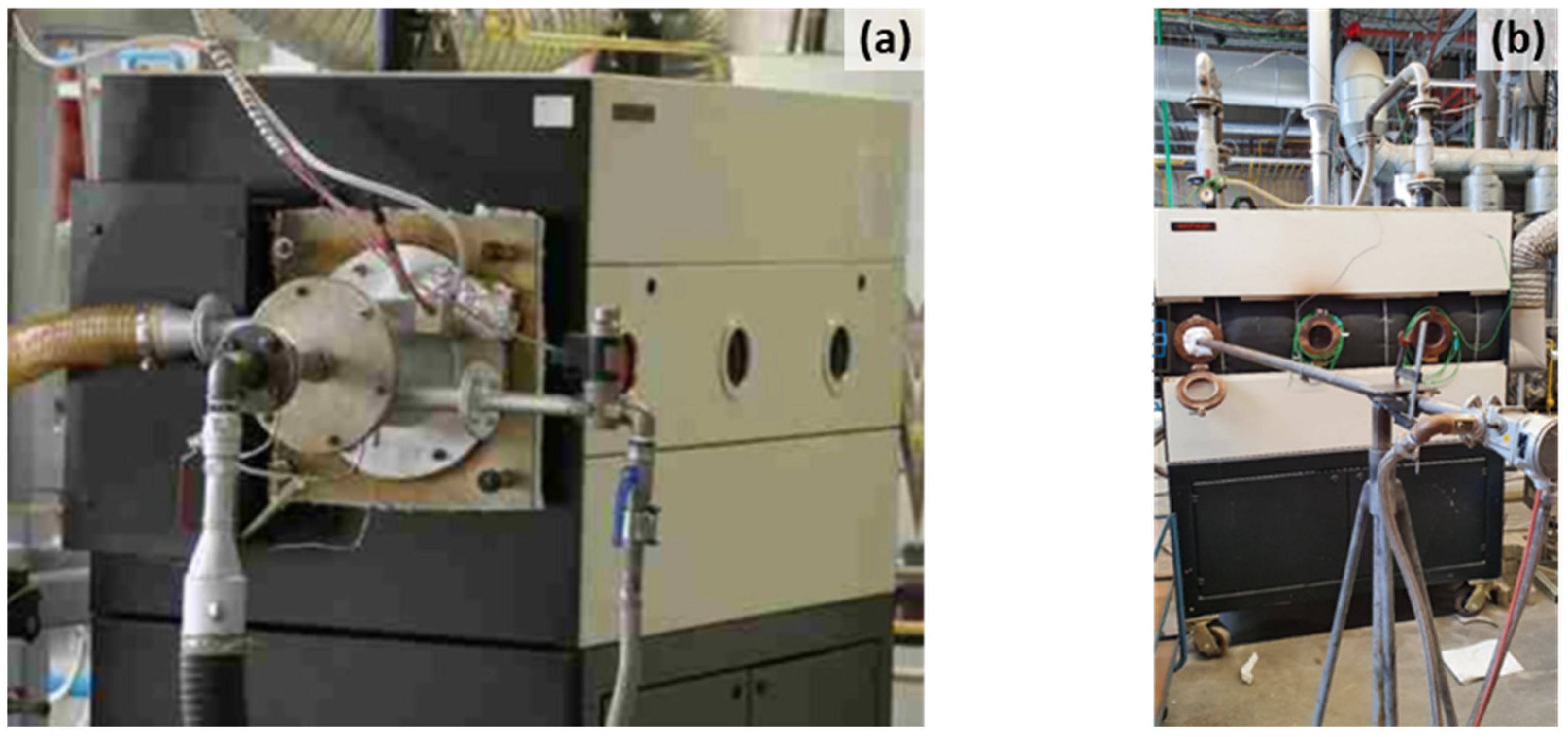
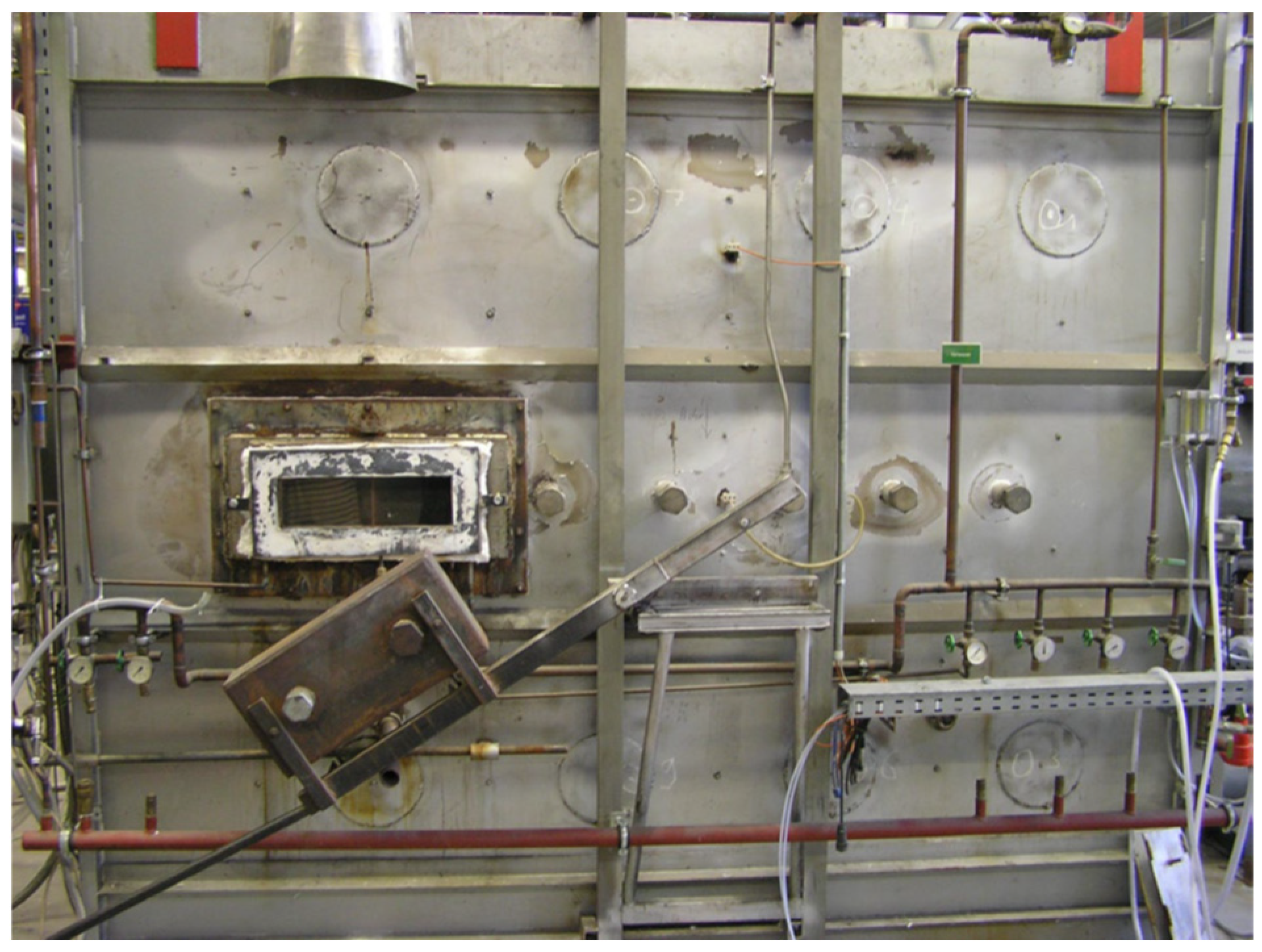
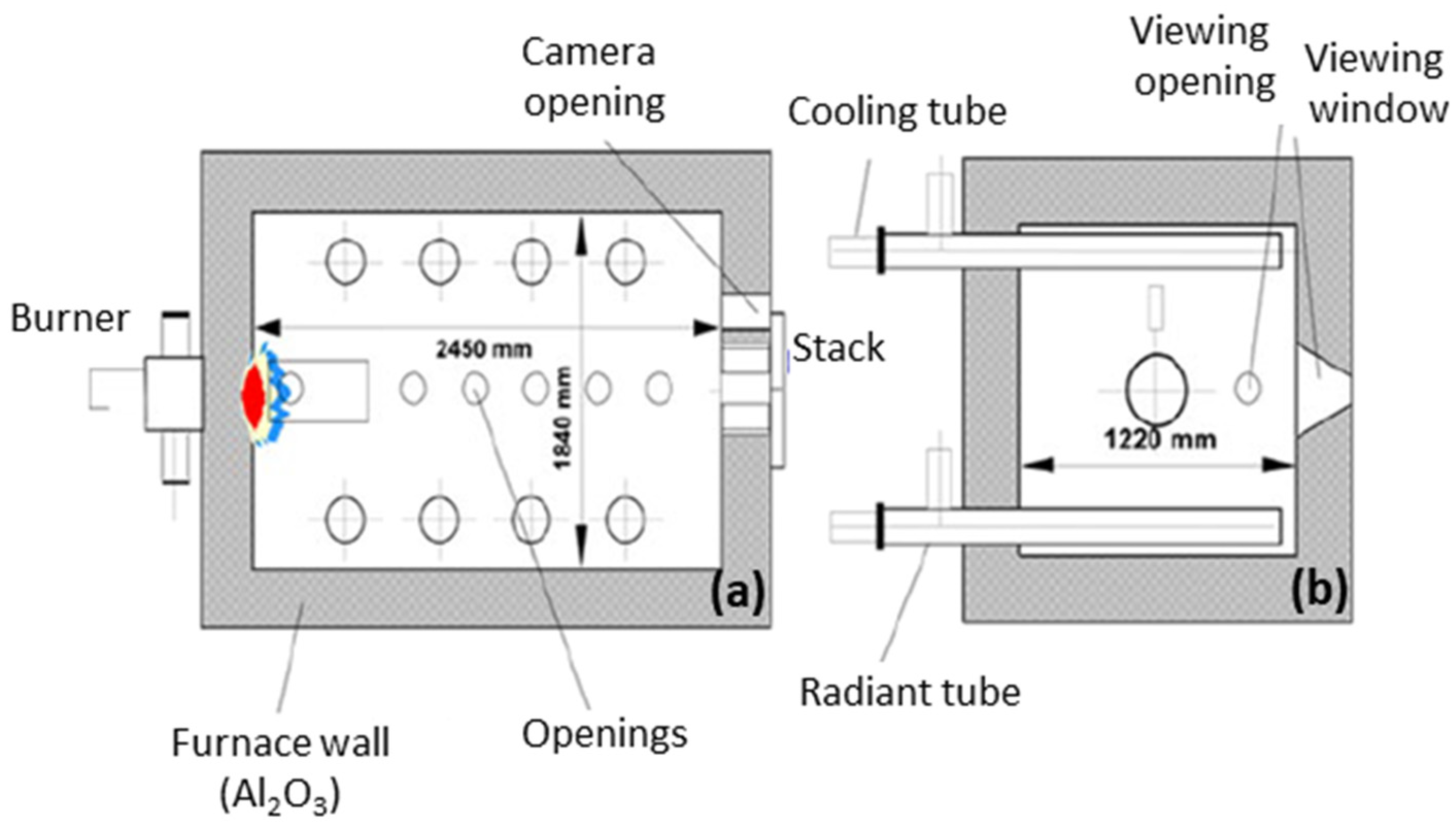
2.4. Test Furnace Systems
2.5. Flue Gas Analytics
2.6. Economic Analysis
3. Results
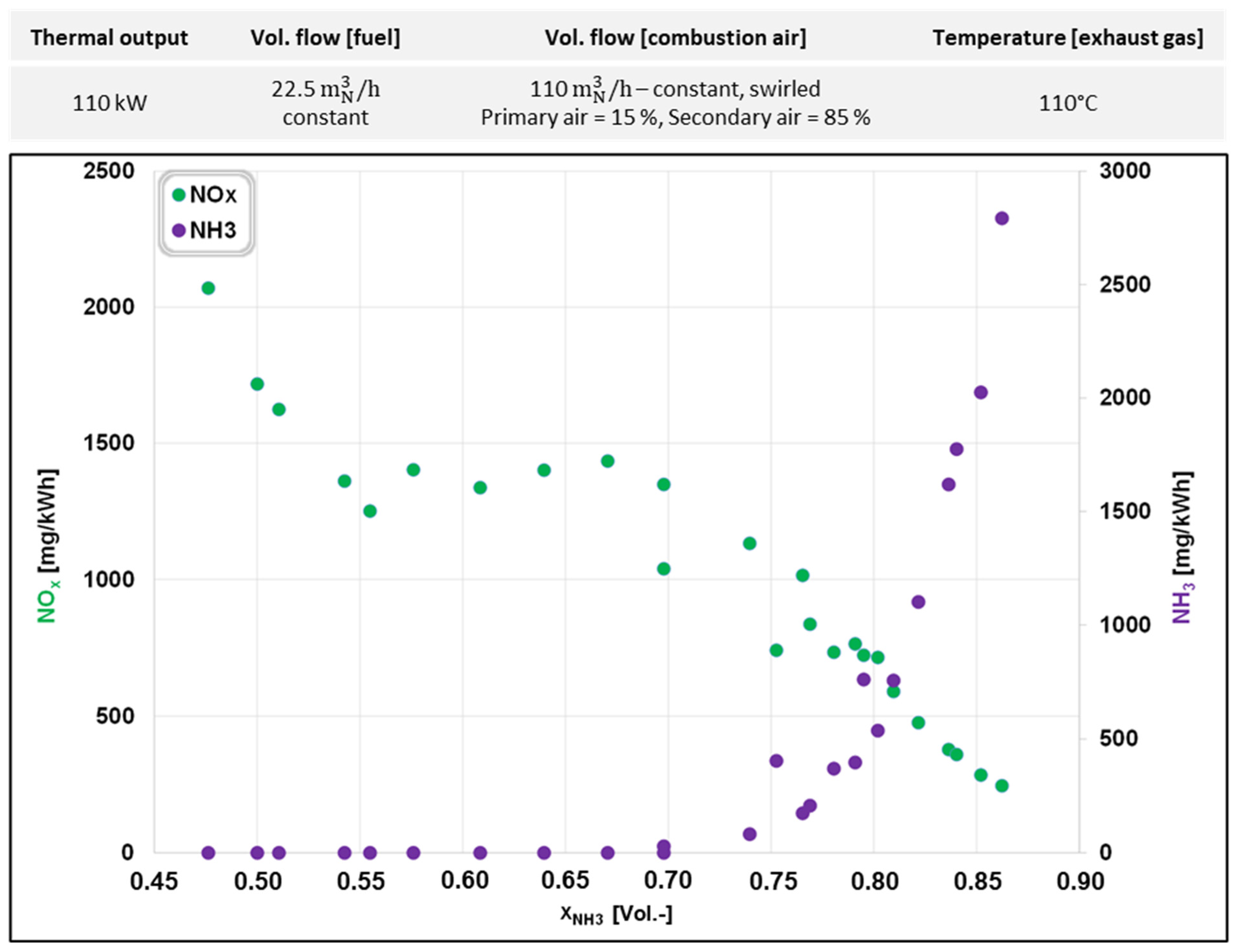
3.1. NH3 Combustion in a Water-Cooled Combustion Chamber
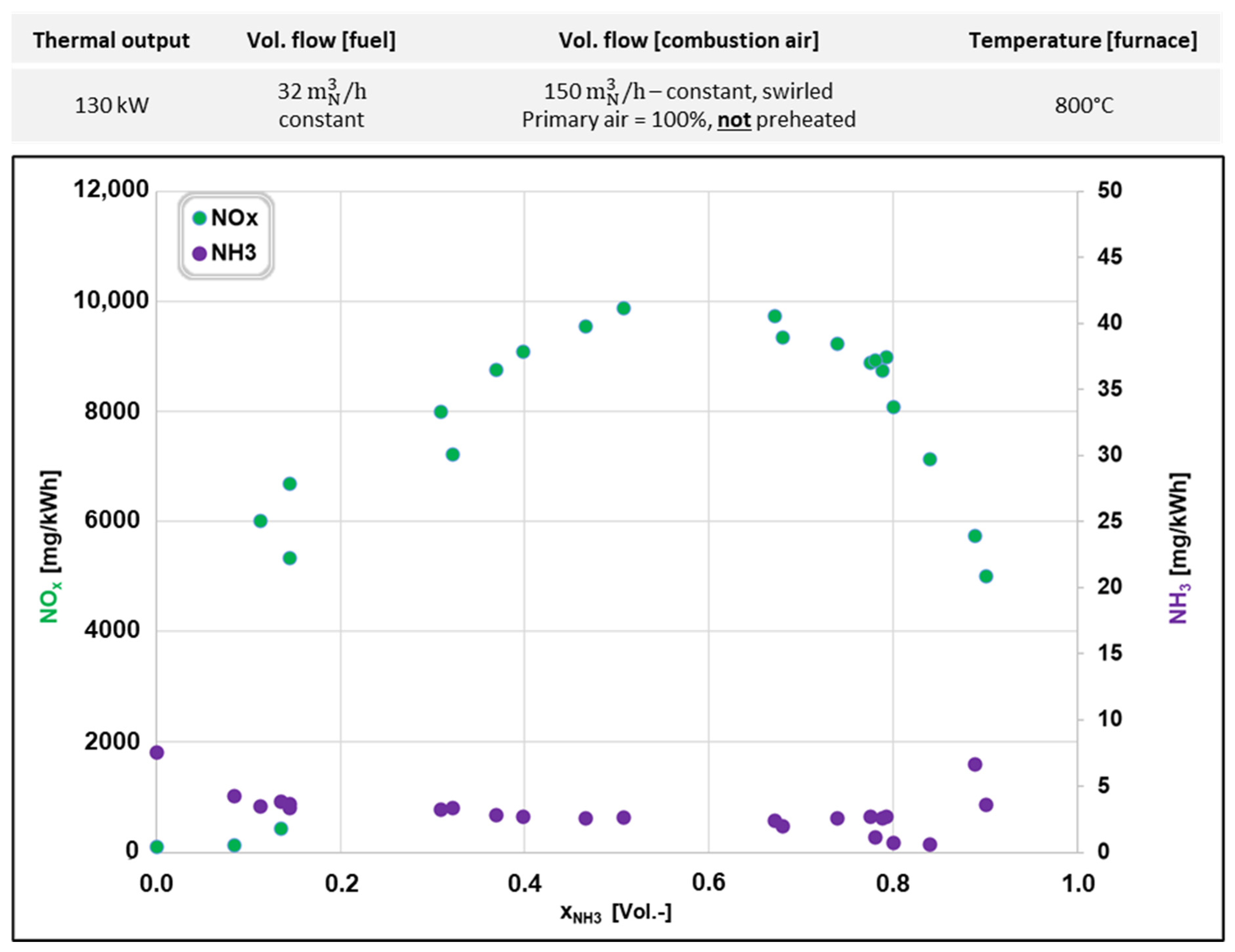
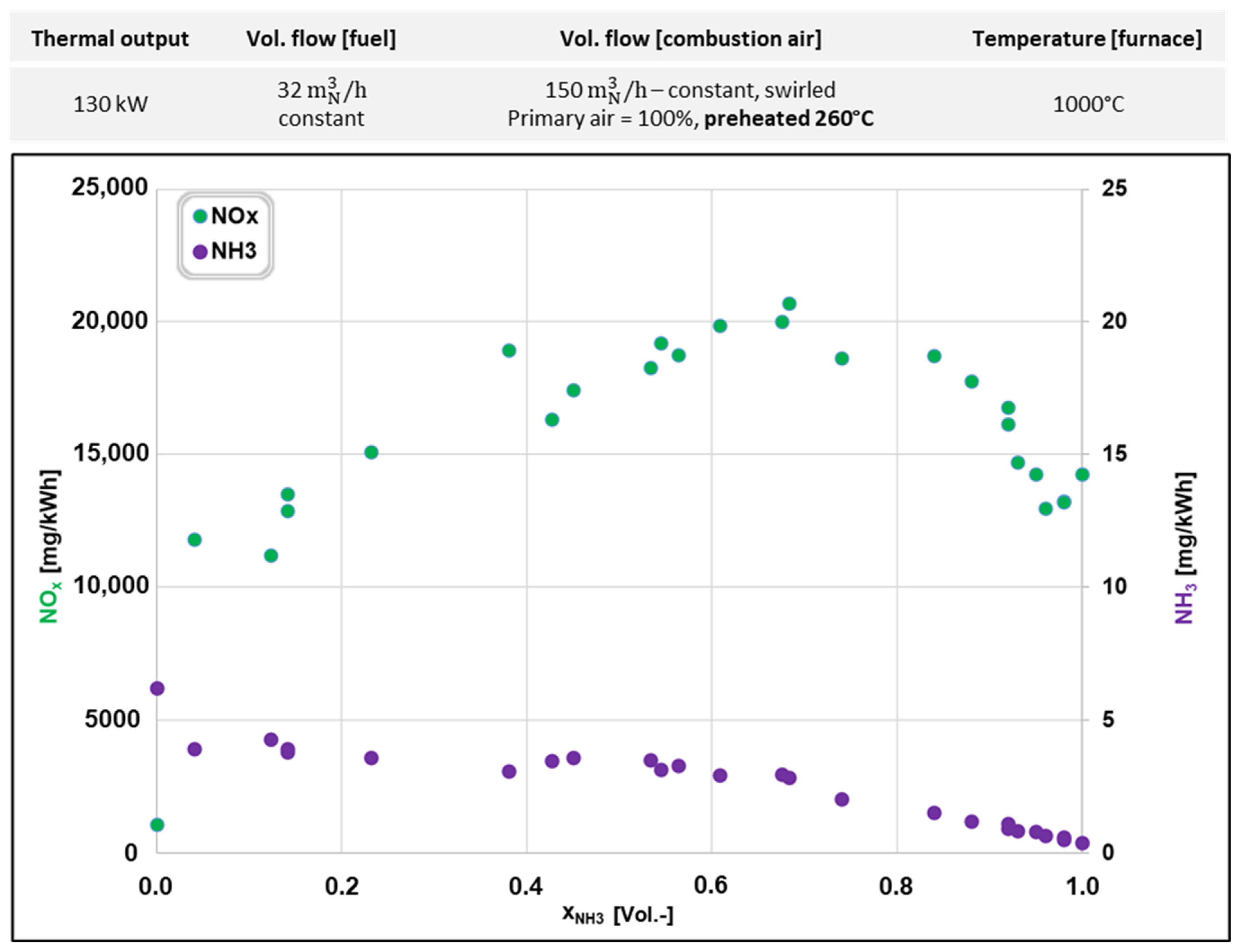
3.2. NH3 Combustion in an Isothermal Combustion Chamber
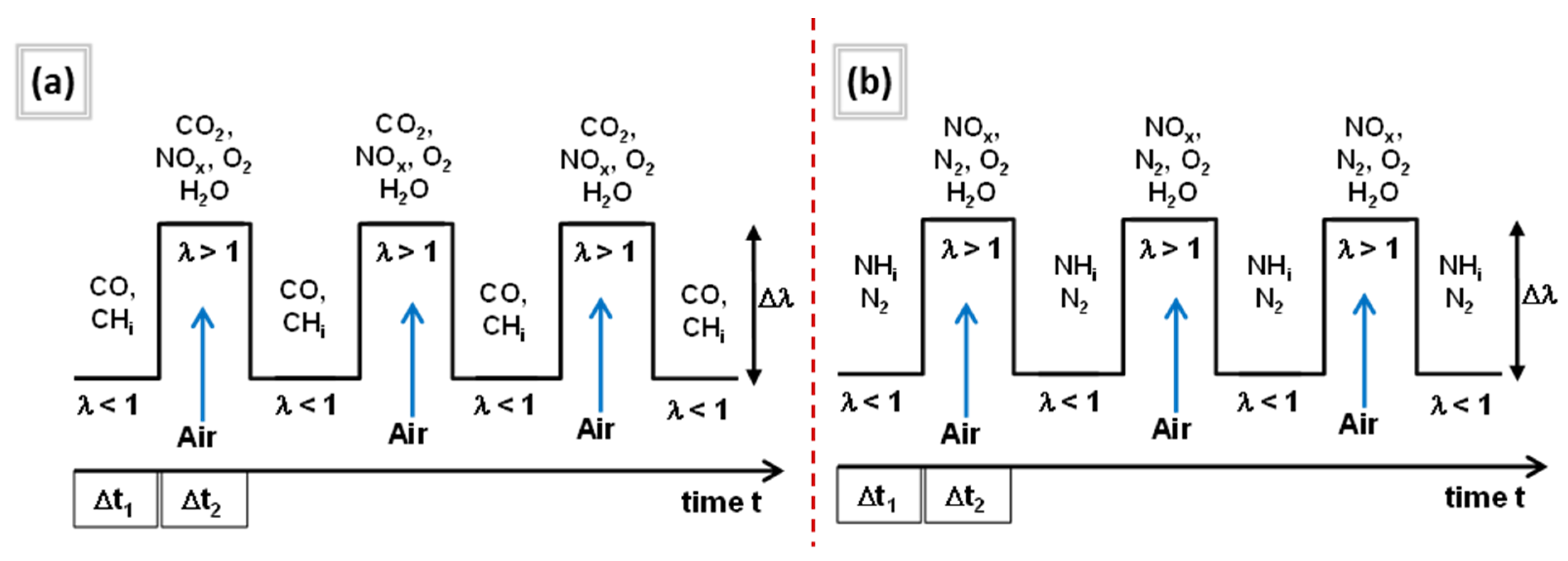
3.3. NOx Reduction Through Oscillating Combustion of Ammonia in an Isothermal Combustion Chamber
3.4. Economic Analysis
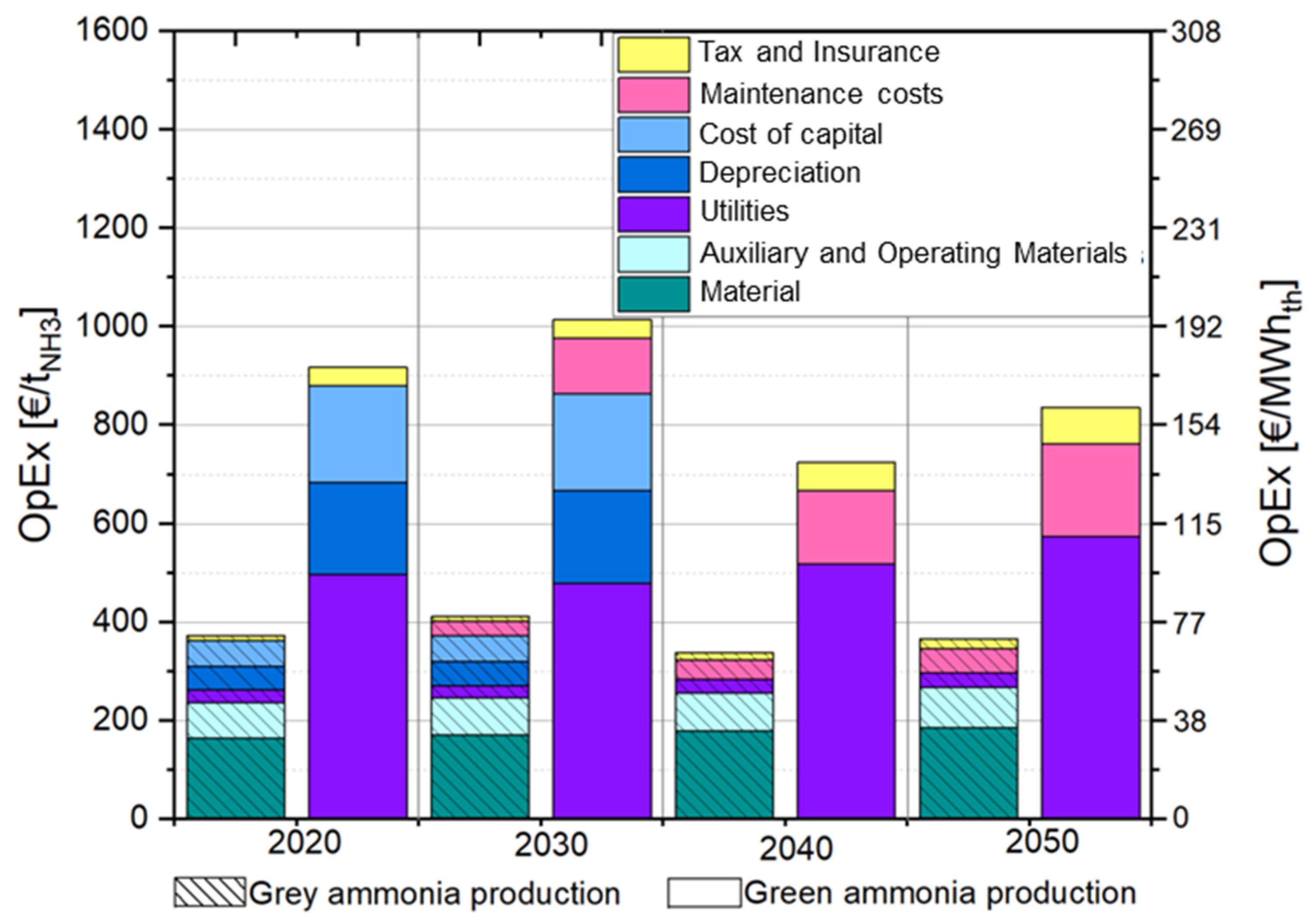
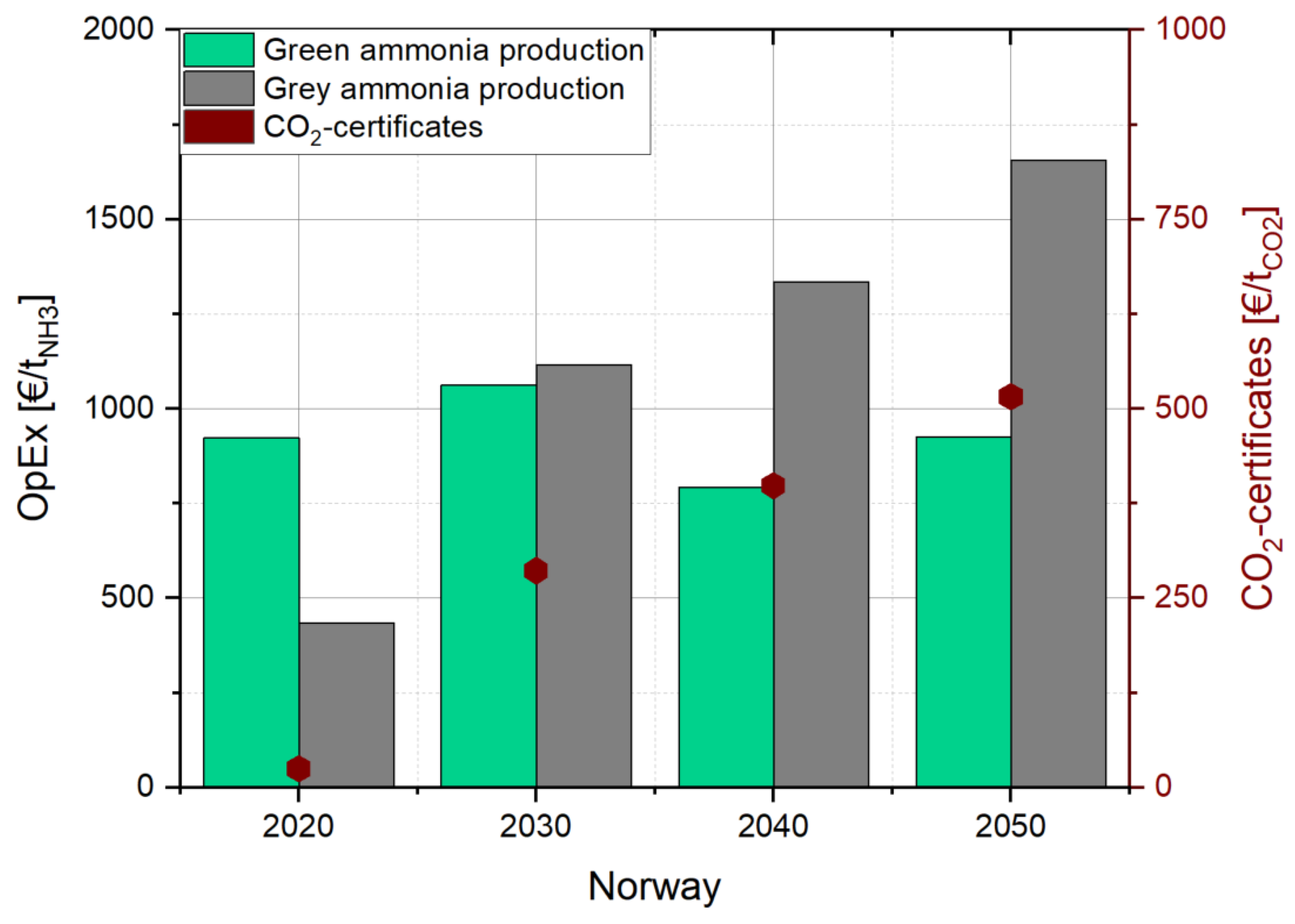
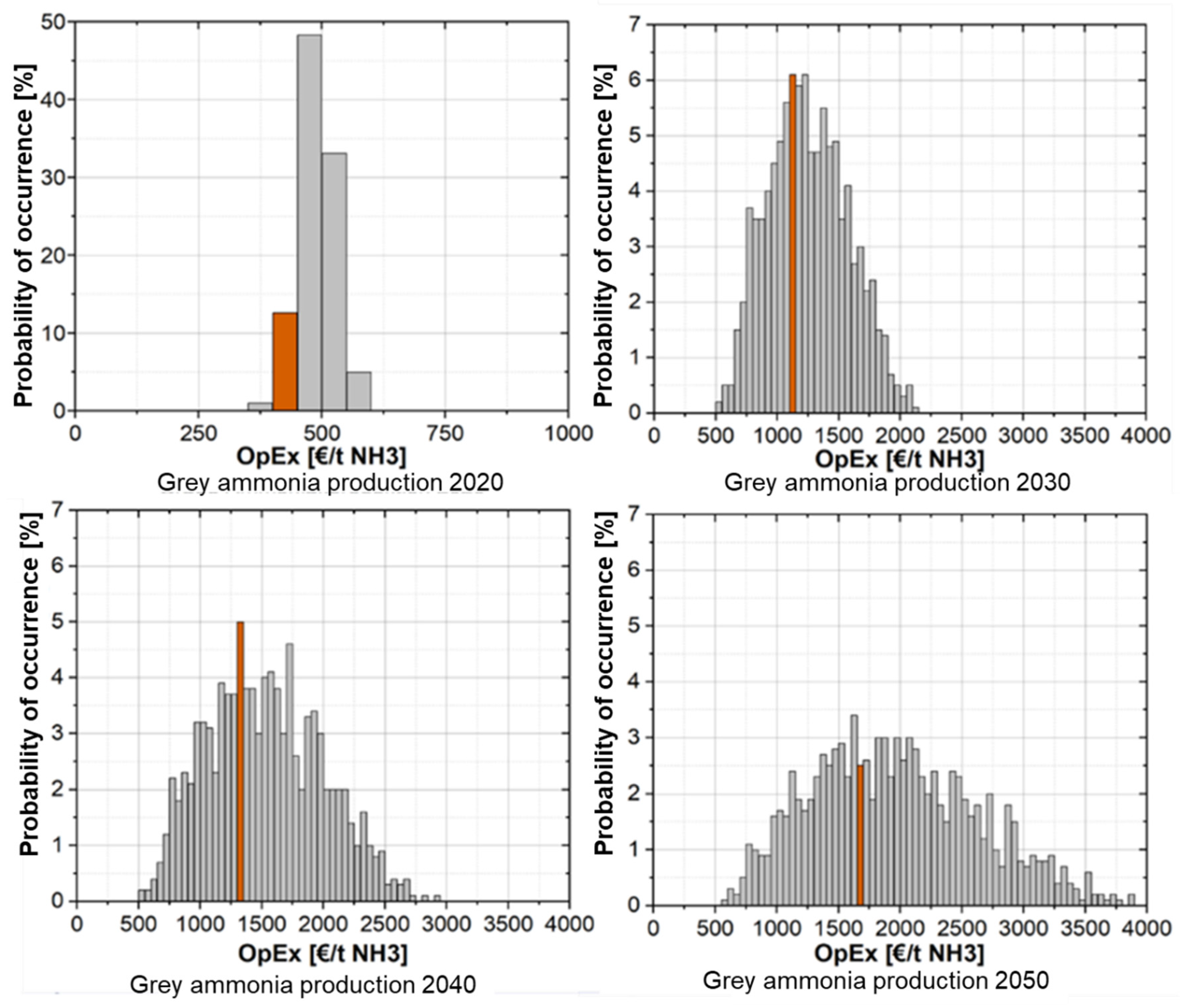
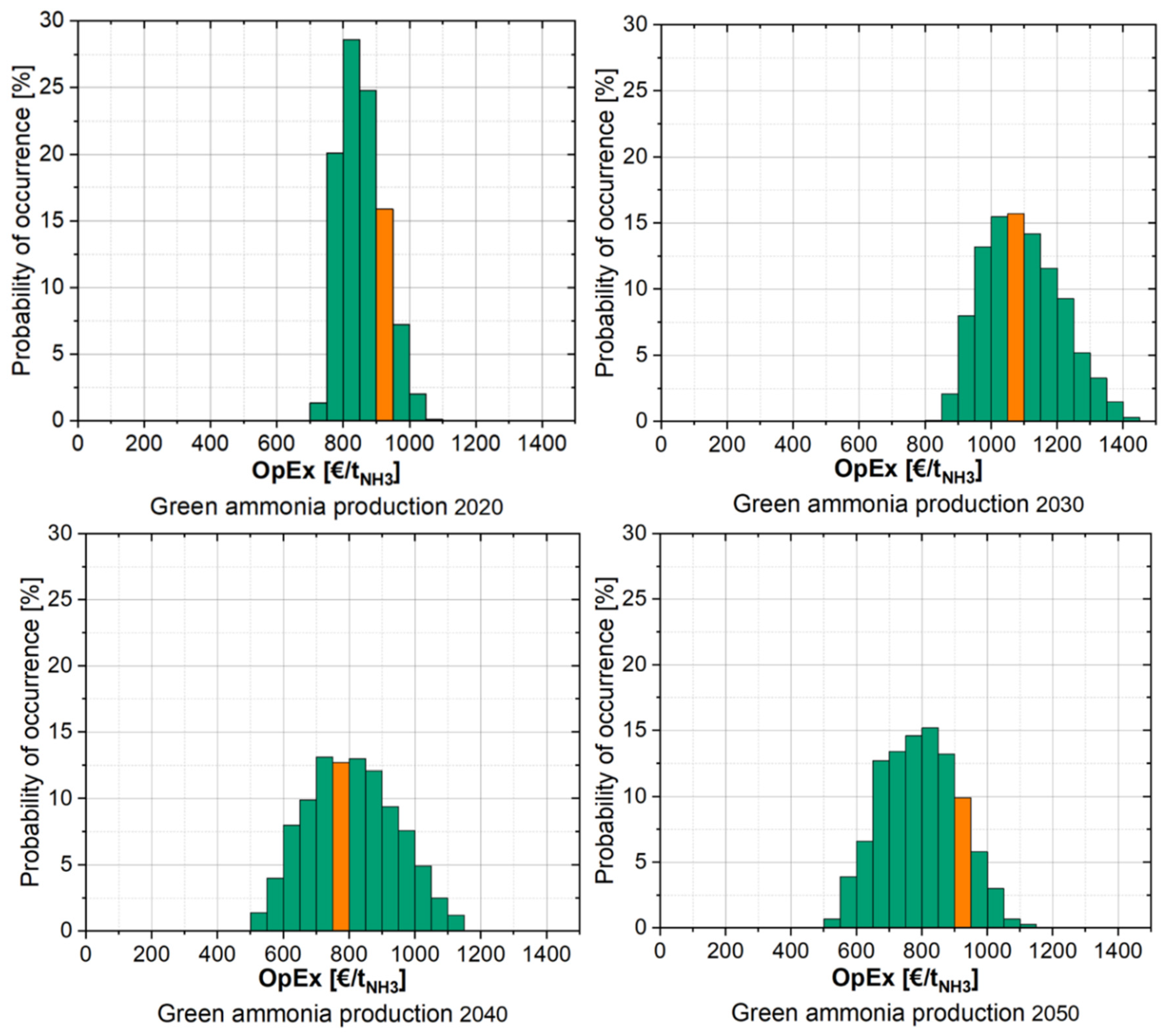
3.4.1. Cost Analysis of Ammonia Production in Norway Without CO2 Pricing
3.4.2. Cost Analysis of Ammonia Production in Norway with CO2 Pricing
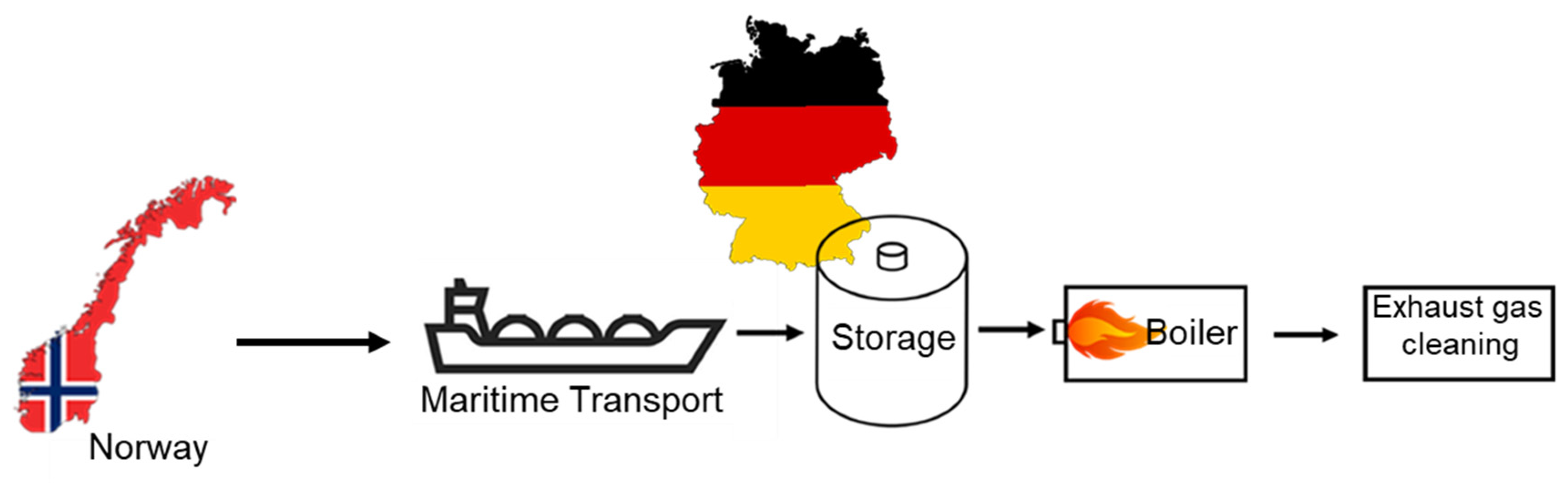
3.4.3. Cost Analysis of the Entire Ammonia Process Chain
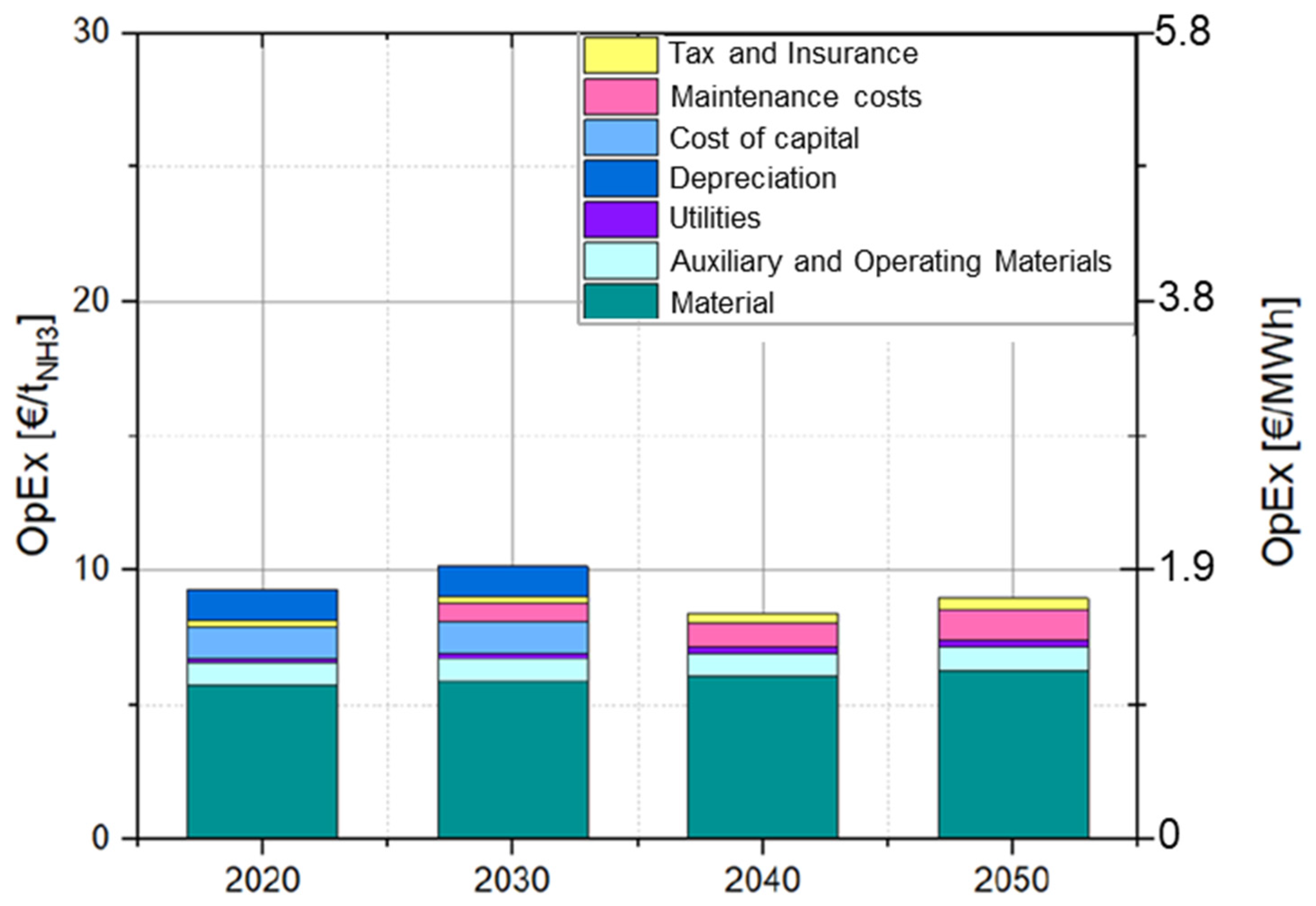
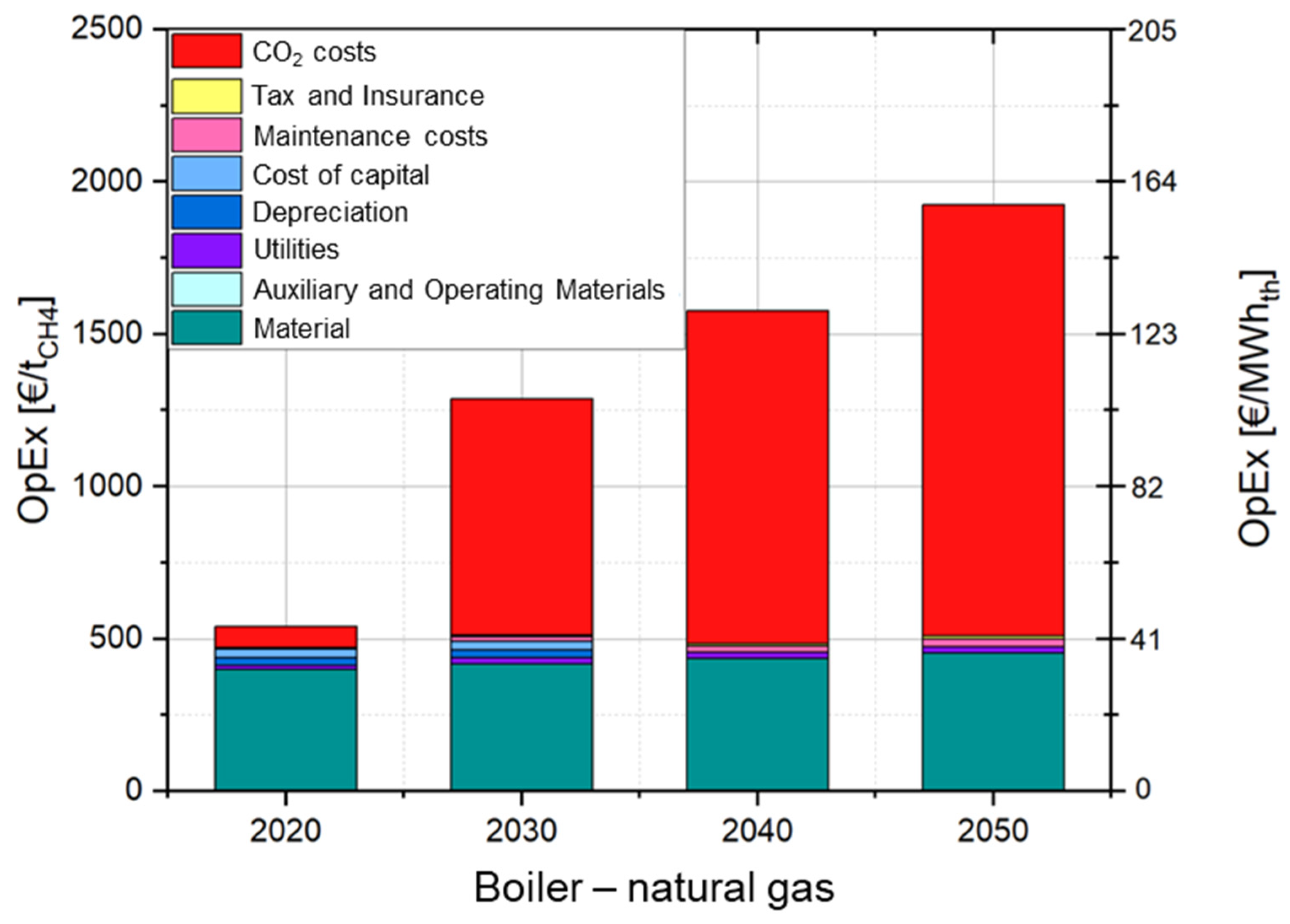
3.4.4. Cost Analysis of Heat Generation by Boiler Firing with Natural Gas and Ammonia
4. Discussion
4.1. NH3 Flame Stabilization and Combustion Under Oscillating Conditions
4.2. Production Cost of Ammonia and Heat
5. Conclusions
Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. Overview of Greenhouse Gases|Greenhouse Gas (GHG) Emissions|US EPA. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 29 April 2025).
- UNFCCC. What is the Kyoto Protocol? Available online: https://unfccc.int/kyoto_protocol (accessed on 29 April 2025).
- UNFCCC. The Paris Agreement. Available online: http://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 30 July 2025).
- Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.; Hodgetts, R.Y.; Bakker, J.M.; Vallana, F.M.F.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Patonia, A.; Poudineh, R. Ammonia as a Storage Solution for Future Decarbonized Energy Systems; OIES Paper EL No. 42; The Oxford Institute for Energy Studies: Oxford, UK, 2020. [Google Scholar]
- Cheng, Q.; Muhammad, A.; Kaario, O.; Ahmad, Z.; Martti, L. Ammonia as a sustainable fuel: Review and novel strategies. Renew. Sustain. Energy Rev. 2024, 207, 114995. [Google Scholar] [CrossRef]
- Kumar, L.; Sleiti, A.K. Systematic review on ammonia as a sustainable fuel for combustion. Renew. Sustain. Energy Rev. 2024, 202, 114699. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Herbinet, O.; Bartocci, P.; Dana, A.G. On the use of ammonia as a fuel—A perspective. Fuel Commun. 2022, 11, 100064. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- NIST Thermo-Physical Properties of Fluid Systems. 2017. Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 29 April 2025).
- Heldebrant, D.J.; Karkamkar, A.; Linehan, J.C.; Autrey, T. Synthesis of ammonia borane for hydrogen storage applications. Energy Environ. Sci. 2008, 1, 156. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Chen, X.; Shore, S.G. Ammonia borane, past as prolog. J. Organomet. Chem. 2014, 751, 60–66. [Google Scholar] [CrossRef]
- Petit, J.-F.; Miele, P.; Demirci, U.B. Ammonia borane H 3 N BH 3 for solid-state chemical hydrogen storage: Different samples with different thermal behaviors. Int. J. Hydrogen Energy 2016, 41, 15462–15470. [Google Scholar] [CrossRef]
- Rassat, S.D.; Aardahl, C.L.; Autrey, T.; Smith, R.S. Thermal Stability of Ammonia Borane: A Case Study for Exothermic Hydrogen Storage Materials. Energy Fuels 2010, 24, 2596–2606. [Google Scholar] [CrossRef]
- Gao, L.; Fang, H.; Li, Z.; Yu, X.; Fan, K. Liquefaction of Solid-State BH3NH3 by Gaseous NH3. Inorg. Chem. 2011, 50, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a Renewable Energy Transportation Media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- NASA. X-15 Hypersonic Research Program; Dryden Flight Research Center: Edwards, CA, USA, 2002. Available online: https://www.nasa.gov/wp-content/uploads/2021/09/120306main_FS-052-DFRC.pdf?emrc=9ae601 (accessed on 14 July 2025).
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia combustion properties and performance in gas-turbine burners. Symp. Combust. 1967, 11, 985–992. [Google Scholar] [CrossRef]
- Bull, M.G. Development of an Ammonia-Burning Gas Turbine Engine; DA-44-009-AMC-824(T); Solar Turbines International: San Diego, CA, USA, 1968. [Google Scholar]
- Pratt, D.T. Performance of Ammonia-Fired Gas-Turbine Combustors; US Deptartment of the Army: Fort Belvoir, VA, USA, 1967. [Google Scholar] [CrossRef]
- SIP Energy Carriers. Available online: https://www.jst.go.jp/sip/pdf/SIP_energycarriers2016_en.pdf (accessed on 25 April 2025).
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Ito, S.; Uchida, M.; Suda, T.; Fujimori, T. Development of Ammonia Gas Turbine Co-Generation Technology. IHI Eng. Rev. 2020, 53, 6. [Google Scholar]
- Ammonia Energy Association. REFUEL Is Back on Track. Available online: https://ammoniaenergy.org/articles/refuel-is-back-on-track/ (accessed on 29 April 2025).
- ARPA-E Homepage. Available online: https://arpa-e.energy.gov/?q=arpa-e-programs/refuel (accessed on 29 April 2025).
- West Central Research and Outreach Center. Renewable Energy Ammonia Production Research Continues at the WCROC. Available online: https://wcroc.cfans.umn.edu/news/arpa-e-ammonia-project (accessed on 29 April 2025).
- Ammonia Energy Association. NH3 Fuel Association Chapter Launching in Australia. Available online: https://ammoniaenergy.org/articles/nh3-fuel-association-chapter-launching-in-australia/ (accessed on 29 April 2025).
- Ammonia Industry. Yara: Solar Ammonia Pilot Plant, for Start-Up in 2019. Available online: https://ammoniaenergy.org/articles/yara-solar-ammonia-pilot-plant-for-start-up-in-2019/ (accessed on 29 April 2025).
- Siemens Energy. Power-to-X: A Closer Look at e-Ammonia, 2021. Available online: https://p3.aprimocdn.net/siemensenergy/4e089afc-eac1-45eb-ab45-b08400b001ba/20211012-WP-eAmmonia-pdf_Original%20file.pdf (accessed on 14 July 2025).
- Bañares-Alcántara, R.; Dericks, G., III; Fiaschetti, M.; Grünewald, P.; Lopez, J.M.; Tsang, E.; Yang, A.; Ye, L.; Zhao, S. Analysis of Islanded Ammonia-Based Energy Storage Systems; University of Oxford: Oxford, UK, 2015. [Google Scholar]
- Howell, D. Revolutionary Disruption Coming to the Energy Sector. The Japan Times. Available online: https://www.japantimes.co.jp/opinion/2017/03/10/commentary/world-commentary/revolutionary-disruption-coming-energy-sector/ (accessed on 14 July 2025).
- Afman, M.; Hers, S.; Scholten, T.; ISPT. Power to Ammonia; ISPT: Amersfoort, The Nethertlands, 2017; Available online: https://ispt.eu/media/DR-20-09-Power-to-Ammonia-2017-publication.pdf (accessed on 30 April 2025).
- DOE. The Future of Ammonia-Based Fuel and Ammonia for Energy Storage & Delivery; Abu Dhabi Department of Energy (DOE): Abu Dhabi, United Arab Emirates, 2024. Available online: https://www.doe.gov.ae/-/media/Project/DOE/Department-Of-Energy/Media-Center-Publications/English-Files/DOE-Future-Foresight-Reports---AmoniaENG.pdf (accessed on 30 April 2025).
- National Research Council Canada. Ammonia Shows Promise as Fuel for Hard-to-Decarbonize Sectors. Available online: https://nrc.canada.ca/en/stories/ammonia-shows-promise-fuel-hard-decarbonize-sectors (accessed on 30 April 2025).
- Natural Resources Canada. Hydrogen Strategy for Canada: Seizing the Opportunities for Hydrogen: A Call to Action; Natural Resources Canada: Ottawa, ON, Canada, 2020. [Google Scholar]
- Frigo, S.; Gentili, R. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. Int. J. Hydrogen Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.-C. Demonstration of Compression-Ignition Engine Combustion Using Ammonia in Reducing Greenhouse Gas Emissions. Energy Fuels 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Gillespie, D.E.; Bohac, S.V. Exhaust Emissions from a Stoichiometric, Ammonia and Gasoline Dual Fueled Spark Ignition Engine. In Proceedings of the ASME 2009 Internal Combustion Engine Division Spring Technical Conference, Milwaukee, WI, USA, 3–6 May 2009; pp. 135–141. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.-C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Mørch, C.; Bjerre, A.; Gøttrup, M.; Sorenson, S.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Gill, S.S.; Chatha, G.S.; Tsolakis, A.; Golunski, S.E.; York, A.P.E. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrogen Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Jackson, D.; Verreault, J. Flammability limits of NH3–H2–N2–air mixtures at elevated initial temperatures. Combust. Flame 2006, 144, 53–63. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-H.; Kim, H.-S.; Kim, H.-J.; Kang, S.-H.; Ryu, J.-H.; Shim, S.-S. Reduction of NOx Emission from the Cement Industry in South Korea: A Review. Atmosphere 2022, 13, 121. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Ammonia combustion at elevated pressure and temperature conditions. Fuel 2010, 89, 3540–3545. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Kwon, O. Effects of ammonia substitution on hydrogen/air flame propagation and emissions. Int. J. Hydrogen Energy 2010, 35, 11332–11341. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Evans, B. Using Local Green Energy and Ammonia to Power Gas Turbine Generators. In Proceedings of the 10th NH3 Fuel Conference, Sacramento, CA, USA, 23–24 September 2013; Available online: https://ammoniaenergy.org/wp-content/uploads/2019/12/nh3fcx-brian-evans.pdf (accessed on 30 April 2025).
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Pugh, D.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary study on lean premixed combustion of ammonia-hydrogen for swirling gas turbine combustors. Int. J. Hydrogen Energy 2017, 42, 24495–24503. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Cairns, E.J.; Simons, E.L.; Tevebaugh, A.D. Ammonia–Oxygen Fuel Cell. Nature 1968, 217, 780–781. [Google Scholar] [CrossRef]
- Ganley, J.C. An intermediate-temperature direct ammonia fuel cell with a molten alkaline hydroxide electrolyte. J. Power Sources 2008, 178, 44–47. [Google Scholar] [CrossRef]
- Hejze, T.; Besenhard, J.; Kordesch, K.; Cifrain, M.; Aronsson, R. Current status of combined systems using alkaline fuel cells and ammonia as a hydrogen carrier. J. Power Sources 2008, 176, 490–493. [Google Scholar] [CrossRef]
- Yang, J.; Muroyama, H.; Matsui, T.; Eguchi, K. Development of a direct ammonia-fueled molten hydroxide fuel cell. J. Power Sources 2014, 245, 277–282. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Direct Ammonia Alkaline Anion-Exchange Membrane Fuel Cells. Electrochem. Solid-State Lett. 2010, 13, B83. [Google Scholar] [CrossRef]
- Maffei, N.; Pelletier, L.; Charland, J.P.; McFarlan, A. A Direct Ammonia Fuel Cell Using Barium Cerate Proton Conducting Electrolyte Doped with Gadolinium and Praseodymium. Fuel Cells 2007, 7, 323–328. [Google Scholar] [CrossRef]
- Ni, M. Thermo-electrochemical modeling of ammonia-fueled solid oxide fuel cells considering ammonia thermal decomposition in the anode. Int. J. Hydrogen Energy 2011, 36, 3153–3166. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic Fuel-Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M.K. Electrochemical modeling of ammonia-fed solid oxide fuel cells based on proton conducting electrolyte. J. Power Sources 2008, 183, 687–692. [Google Scholar] [CrossRef]
- Kojima, Y.; Miyaoka, H.; Ichikawa, T. Ammonia-Based Hydrogen Storage Materials. In Advanced Materials for Clean Energy; CRC Press: Boca Raton, FL, USA, 2015; pp. 497–526. [Google Scholar] [CrossRef]
- Zakaznov, V.F.; Kursheva, L.A.; Fedina, Z.I. Determination of normal flame velocity and critical diameter of flame extinction in ammonia-air mixture. Combust. Explos. Shock Waves 1978, 14, 710–713. [Google Scholar] [CrossRef]
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning velocity measurements of nitrogen-containing compounds. J. Hazard. Mater. 2008, 155, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Huang, Z.; He, J.; Miao, H. Experimental and numerical study on laminar burning velocities and flame instabilities of hydrogen–air mixtures at elevated pressures and temperatures. Int. J. Hydrogen Energy 2009, 34, 8741–8755. [Google Scholar] [CrossRef]
- Varea, E.; Modica, V.; Vandel, A.; Renou, B. Measurement of laminar burning velocity and Markstein length relative to fresh gases using a new postprocessing procedure: Application to laminar spherical flames for methane, ethanol and isooctane/air mixtures. Combust. Flame 2012, 159, 577–590. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Somarathne, K.K.A.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/hydrogen/air premixed flames at elevated pressures. Int. J. Hydrogen Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Osaka, Y.; Zeng, T. Numerical study on effect of oxygen content in combustion air on ammonia combustion. Energy 2015, 93, 2053–2068. [Google Scholar] [CrossRef]
- Zietz, U.; Baumgärtel, G. The laminar flame speeds of propane-ammonia-air mixtures. Combust. Flame 1969, 13, 329–330. [Google Scholar] [CrossRef]
- Bockhorn, H.; Fetting, F.; Mende, J. The laminar flame velocities of propane/ammonia mixtures. Combust. Flame 1972, 18, 471–473. [Google Scholar] [CrossRef]
- Pfahl, U.; Ross, M.; Shepherd, J.; Pasamehmetoglu, K.; Unal, C. Flammability limits, ignition energy, and flame speeds in H2–CH4–NH3–N2O–O2–N2 mixtures. Combust. Flame 2000, 123, 140–158. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Park, J.; Kwon, O. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, X.; An, Z.; Okafor, E.C.; Guiberti, T.F.; Wang, J.; Huang, Z. Flame stabilization and emission characteristics of ammonia combustion in lab-scale gas turbine combustors: Recent progress and prospects. Prog. Energy Combust. Sci. 2024, 106, 101193. [Google Scholar] [CrossRef]
- Zeldovich, Y.B. The oxidation of nitrogen in combustion and explosions. Acta Physicochim. URSS 1946, 21, 577–628. [Google Scholar]
- Fisher, C.J. A study of rich ammonia/oxygen/nitrogen flames. Combust. Flame 1977, 30, 143–149. [Google Scholar] [CrossRef]
- Dasch, C.J.; Blint, R. A Mechanistic and Experimental Study of Ammonia Flames. Combust. Sci. Technol. 1984, 41, 223–244. [Google Scholar] [CrossRef]
- Vandooren, J.; Bian, J.; Van Tiggelen, P. Comparison of experimental and calculated structures of an ammonia-nitric oxide flame. Importance of the NH2 + NO reaction. Combust. Flame 1994, 98, 402–410. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 1994, 99, 253–276. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Kinetics in Ammonia-Containing Premixed Flames and a Preliminary Investigation of Their Use as Fuel in Spark Ignition Engines. Combust. Sci. Technol. 2009, 181, 1092–1106. [Google Scholar] [CrossRef]
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
- Duynslaegher; Jeanmart, H.; Vandooren, J. Use of ammonia as a fuel for SI engine. In Proceedings of the European Combustion Meeting, Vienna, Austria, 14–17 April 2009; pp. 1–6. [Google Scholar]
- Shmakov, A.; Korobeinichev, O.; Rybitskaya, I.; Chernov, A.; Knyazkov, D.; Bolshova, T.; Konnov, A. Formation and consumption of NO in H2 + O2 + N2 flames doped with NO or NH3 at atmospheric pressure. Combust. Flame 2010, 157, 556–565. [Google Scholar] [CrossRef]
- Mansha, M.; Hassan, J.S.; Saleemi, A.R.; Ghauri, B.M. Detailed Kinetic Mechanism of CNG Combustion in an IC Engine. Adv. Chem. Eng. Sci. 2011, 1, 102–117. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A. Chemical Kinetic Mechanism Study on Premixed Combustion of Ammonia/Hydrogen Fuels for Gas Turbine Use. J. Eng. Gas Turbines Power 2017, 139, 081504. [Google Scholar] [CrossRef]
- Wu, M.; Cova-Bonillo, A.; Gabana, P.; Brinklow, G.; Khedkar, N.; Herreros, J.; Rezaei, S.Z.; Tsolakis, A.; Millington, P.; Clave, S.A.; et al. Addressing the challenge of ammonia slip and nitrous oxide emissions from zero-carbon fuelled engines through catalytic aftertreatment solutions. Int. J. Hydrogen Energy 2024, 94, 848–861. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Gutmark, E. Overview of fundamental kinetic mechanisms and emission mitigation in ammonia combustion. Chem. Eng. J. 2023, 458, 141391. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, Y. Numerical investigation on the combustion and emission characteristics of ammonia in a low-speed two-stroke marine engine. Fuel 2022, 314, 122727. [Google Scholar] [CrossRef]
- Fenimore, C.P.; Jones, G.W. Oxidation of Ammonia in Flames. J. Phys. Chem. 1961, 65, 298–303. [Google Scholar] [CrossRef]
- Setchell, R.E.; Miller, J.A. Raman scattering measurements of nitric oxide in ammonia/oxygen flames. Combust. Flame 1978, 33, 23–32. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Jójka, J.; Ślefarski, R. Dimensionally reduced modeling of nitric oxide formation for premixed methane-air flames with ammonia content. Fuel 2018, 217, 98–105. [Google Scholar] [CrossRef]
- US EPA. Nitrous Oxide Emissions. Available online: https://www.epa.gov/ghgemissions/nitrous-oxide-emissions (accessed on 16 May 2025).
- Stocker, T.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- US EPA. Basic Information About NO2. Available online: https://www.epa.gov/no2-pollution/basic-information-about-no2 (accessed on 16 May 2025).
- Evans, M. Control Techniques for Nitrogen Oxides Emissions from Stationary Sources; Environmental Protection Agency: Research Triangle Park, NC, USA, 1978. [Google Scholar]
- Liu, B.; Bao, B.; Wang, Y.; Xu, H. Numerical simulation of flow, combustion and NO emission of a fuel-staged industrial gas burner. J. Energy Inst. 2017, 90, 441–451. [Google Scholar] [CrossRef]
- Hanraths, N.; Tolkmitt, F.; Berndt, P.; Djordjevic, N. Numerical Study on NOx Reduction in Pulse Detonation Combustion by Using Steam Injection Decoupled from Detonation Development. J. Eng. Gas Turbines Power 2018, 140, 121008. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total. Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Miller, J.; Branch, M.; Kee, R. A chemical kinetic model for the selective reduction of nitric oxide by ammonia. Combust. Flame 1981, 43, 81–98. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Selim, M.A. Reduced Reaction Mechanisms for Ammonia Oxidation in Premixed Laminar Flames. Combust. Sci. Technol. 1994, 99, 277–298. [Google Scholar] [CrossRef]
- Radojevic, M. Reduction of nitrogen oxides in flue gases. Environ. Pollut. 1998, 102, 685–689. [Google Scholar] [CrossRef]
- Chung, S.J.; Pillai, K.C.; Moon, I.S. A sustainable environmentally friendly NOx removal process using Ag(II)/Ag(I)-mediated electrochemical oxidation. Sep. Purif. Technol. 2009, 65, 156–163. [Google Scholar] [CrossRef]
- Shao, J.; Hansen, K.K. NOx reduction on Ag electrochemical cells with a K-Pt-Al2O3 adsorption layer. J. Electrochem. Soc. 2013, 160, H294. [Google Scholar] [CrossRef]
- Shao, J.; Hansen, K.K. Characterization of LSM/CGO Symmetric Cells Modified by NOx Adsorbents for Electrochemical NOx Removal with Impedance Spectroscopy. J. Electrochem. Soc. 2013, 160, H494–H501. [Google Scholar] [CrossRef]
- Hamamoto, K.; Suzuki, T.; Fujishiro, Y.; Awano, M. Tubular Solid Oxide Electrolysis Cell for NOx Decomposition. J. Electrochem. Soc. 2011, 158, B1050–B1053. [Google Scholar] [CrossRef]
- Hansen, K.K. Activation/Deactivation Phenomena in the Electrochemical Reduction of Nitric oxide and Oxygen on LSM perovskites. Int. J. Electrochem. Sci. 2018, 13, 4782–4791. [Google Scholar] [CrossRef]
- Friedberg, A.Z.; Hansen, K.K. NOx and propene conversion on La0.85Sr0.15MnO3+d/Ce0.9Gd0.1O1.95 symmetrical cells. J. Electrochem. Sci. Eng. 2017, 7, 153–166. [Google Scholar] [CrossRef][Green Version]
- Zabetta, E.C.; Hupa, M.; Saviharju, K. Reducing NOx Emissions Using Fuel Staging, Air Staging, and Selective Noncatalytic Reduction in Synergy. Ind. Eng. Chem. Res. 2005, 44, 4552–4561. [Google Scholar] [CrossRef]
- Chen, S.; Cole, J.; Heap, M.; Kramlich, J.; Mccarthy, J.; Pershing, D. Advanced NOx reduction processes using-NH and -CN compounds in conjunction with staged air addition. Symp. Int. Combust. 1989, 22, 1135–1145. [Google Scholar] [CrossRef]
- Wendt, J.; Sternling, C.; Matovich, M. Reduction of sulfur trioxide and nitrogen oxides by secondary fuel injection. Symp. Int. Combust. 1973, 14, 897–904. [Google Scholar] [CrossRef]
- Smoot, L.; Hill, S.; Xu, H. NOx control through reburning. Prog. Energy Combust. Sci. 1998, 24, 385–408. [Google Scholar] [CrossRef]
- Su, S.; Xiang, J.; Sun, L.; Hu, S.; Zhang, Z.; Zhu, J. Application of gaseous fuel reburning for controlling nitric oxide emissions in boilers. Fuel Process. Technol. 2009, 90, 396–402. [Google Scholar] [CrossRef]
- What’s the Best Way to Remove NOx? Available online: https://www.linde-gas.com/industries/refining/emission-abatement-and-control/nox-removal (accessed on 11 June 2025).
- MARTIN GmbH. NOx_Reduktion18; MARTIN GmbH: Munchen, Germany, 2013; Available online: https://www.martingmbh.de/media/files/Technologie/NOx_Reduktion18.pdf (accessed on 11 June 2025).
- Gehrmann, H.-J.; Jaeger, B.; Wirtz, S.; Scherer, V.; Aleksandrov, K.; Hauser, M.; Stapf, D.; Pollmeier, G.; Danz, P. Oscillating Combustion—Primary Measure to Reduce Nitrogen Oxide in a Grate Furnace–Experiments and Simulations. Processes 2021, 9, 2210. [Google Scholar] [CrossRef]
- Cho, H.-C.; Cho, K.-W.; Kim, H.-J. NOX Emission Characteristics in Radiant Tube Burner with Oscillating Combustion Technology. Trans. Korean Soc. Mech. Eng. B 2008, 32, 100–106. [Google Scholar] [CrossRef]
- Wagner, J.C. Nox Emission Reduction by Oscillating Combustion; Gas Technology Institute: Des Plaines, IL, USA, 2004. [Google Scholar]
- Pattabathula, V.; Richardson, J. Introduction to ammonia production. Back Basics. 2016. Available online: https://www.aiche.org/sites/default/files/cep/20160969.pdf (accessed on 2 October 2025).
- European Commission. Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals-Ammonia, Acids and Fertilisers; European Comission: Brussels, Belgium, 2007. [Google Scholar]
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Lasocki, J. Ammonia and conventional engine fuels: Comparative environmental impact assessment. In Proceedings of the 10th Conference on Interdisciplinary Problems in Environmental Protection and Engineering EKO-DOK, Polanica-Zdroj, Poland, 16–18 April 2018. [Google Scholar] [CrossRef]
- Frattini, D.; Cinti, G.; Bidini, G.; Desideri, U.; Cioffi, R.; Jannelli, E. A system approach in energy evaluation of different renewable energies sources integration in ammonia production plants. Renew. Energy 2016, 99, 472–482. [Google Scholar] [CrossRef]
- Bora, N.; Singh, A.K.; Pal, P.; Sahoo, U.K.; Seth, D.; Rathore, D.; Bhadra, S.; Sevda, S.; Venkatramanan, V.; Prasad, S.; et al. Green ammonia production: Process technologies and challenges. Fuel 2024, 369, 131808. [Google Scholar] [CrossRef]
- Spillias, S.; Kareiva, P.; Ruckelshaus, M.; McDonald-Madden, E. Renewable energy targets may undermine their sustainability. Nat. Clim. Change 2020, 10, 974–976. [Google Scholar] [CrossRef]
- Enerdata. Renewables in Electricity Production|Statistics Map by Region. Available online: https://yearbook.enerdata.net/renewables/renewable-in-electricity-production-share.html (accessed on 3 July 2025).
- IEA. Norway 2022 Energy Policy Review; IEA: Paris, France, 2022; Available online: https://iea.blob.core.windows.net/assets/de28c6a6-8240-41d9-9082-a5dd65d9f3eb/NORWAY2022.pdf (accessed on 3 July 2025).
- Stromerzeugung nach Energieträgern in Norwegen 2024. Statista. Available online: https://de.statista.com/statistik/daten/studie/1292636/umfrage/struktur-der-stromerzeugung-in-norwegen/ (accessed on 15 July 2025).
- Glossary Beginning with L. Clean Energy Wire. Available online: https://www.cleanenergywire.org/glossary/letter_l (accessed on 15 July 2025).
- DX4000 FTIR Gas Analyzer Helps Study Flue Gases at Savonia University. Gasmet.de. Available online: https://www.gasmet.com/de/cases/dx4000-ftir-gas-analyzer-helps-study-flue-gases-at-savonia-university/ (accessed on 15 July 2025).
- Calcmet Software: Analyzing Multiple Gases with the New FTIR Analyzer. Gasmet.de. Available online: https://www.gasmet.com/de/blog/calcmet-software-analyzing-multiple-gases-with-the-new-ftir-analyzer/ (accessed on 15 July 2025).
- Saarinen, P.; Kauppinen, J. Multicomponent Analysis of FT-IR Spectra. Appl. Spectrosc. 1991, 45, 953–963. [Google Scholar] [CrossRef]
- About @RISK. Available online: https://help.palisade.com/v8_8/en/@RISK/About/About/About-RISK.htm (accessed on 3 July 2025).
- Operating Expense Definition and How It Compares to Capital Expenses. Investopedia. Available online: https://www.investopedia.com/terms/o/operating_expense.asp (accessed on 3 July 2025).
- Fernanado, J.; James, M. Capital Expenditure (CapEx) Definition, Formula, and Examples. Investopedia. Available online: https://www.investopedia.com/terms/c/capitalexpenditure.asp (accessed on 3 July 2025).
- Kunysz, D.O. Kostenschätzung im Chemischen Anlagenbau; Springer Nature: Dordrecht, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Zero Instrument. Understanding Ammonia Slip: Causes, Impacts, and Mitigation Strategies. Just Measure It. Available online: https://zeroinstrument.com/understanding-ammonia-slip-causes-impacts-and-mitigation-strategies/ (accessed on 2 September 2025).
- Bundesministerium der Justiz. 44. BimSchV—Vierundvierzigste Verordnung zur Durchführung des Bundes-Immissionsschutzgesetzes; Bundesministerium der Justiz: Berlin, Germany, 2019; Available online: https://www.gesetze-im-internet.de/bimschv_44/BJNR080410019.html (accessed on 3 September 2025).
- Carvalho, M.d.G.; Fiveland, W.A.; Lockwood, F.C.; Papadopoulos, C. Combustion Technologies for a Clean Environment; Taylor & Francis: London, UK, 2022. [Google Scholar]
- 17_10_26_MA_Power_to_Ammoniak_Michael_Heberl.pdf. Available online: https://opus4.kobv.de/opus4-oth-regensburg/frontdoor/deliver/index/docId/2216/file/17_10_26_MA_Power_to_Ammoniak_Michael_Heberl.pdf (accessed on 3 July 2025).
- Hydrogen Center Bavaria|Zentrum Wasserstoff.Bayern (H2.B). Available online: https://h2.bayern/en/ (accessed on 3 July 2025).
- Heat Roadmap Europe. EU28 Fuel Prices for 2015, 2030 and 2025; Heat Roadmap Europe: Aalborg, Denmark, 2017; Available online: https://heatroadmap.eu/wp-content/uploads/2020/01/HRE4_D6.1-Future-fuel-price-review.pdf (accessed on 15 July 2025).
- Moritz, M.; Schönfisch, M.; Schulte, S. Estimating global production and supply costs for green hydrogen and hydrogen-based green energy commodities. Int. J. Hydrogen Energy 2022, 48, 9139–9154. [Google Scholar] [CrossRef]
- Demineralized Water Cost. Intratec.us. Available online: https://www.intratec.us/solutions/industry-economics-worldwide/utility/demineralized-water-cost (accessed on 15 July 2025).
- Commodity Price Index. HWWI. Available online: https://www.hwwi.org/en/data-offers/commodity-price-index/ (accessed on 3 July 2025).
- European Union. Consolidated Text: Directive 2003/87/EC of the European Parliament and of the Council of 13 October 2003 Establishing a System for Greenhouse Gas Emission Allowance Trading Within the Union and Amending Council Directive 96/61/EC (Text with EEA Relevance); European Commission: Brussels, Belgium, 2024; Available online: https://eur-lex.europa.eu/eli/dir/2003/87/2024-03-01/eng (accessed on 3 July 2025).
- Örtl, E. Klimaschutzbeitrag Verschiedener CO2-Preispfade in den BEHG-Sektoren Verkehr, Gebäude und Industrie; Umweltbundesamt: Dessau-Roßlau, Germany, 2022; Available online: https://www.umweltbundesamt.de/publikationen/klimaschutzbeitrag-verschiedener-co2-preispfade-in (accessed on 3 July 2025).
- Kreidelmeyer, S. Strompreisprognose. 2023. Available online: https://www.prognos.com/de/projekt/strompreisprognose-2023 (accessed on 2 October 2025).
- Feenstra, M.; Monteiro, J.; Akker, J.T.v.D.; Abu-Zahra, M.R.; Gilling, E.; Goetheer, E. Ship-based carbon capture onboard of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Control. 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Nagai, Y. Study on using hydrogen and ammonia as fuels: Combustion characteristics and NOx formation. Int. J. Energy Res. 2014, 38, 1214–1223. [Google Scholar] [CrossRef]
- Mashruk, S.; Okafor, E.; Kovaleva, M.; Alnasif, A.; Pugh, D.; Hayakawa, A.; Valera-Medina, A. Evolution of N2O production at lean combustion condition in NH3/H2/air premixed swirling flames. Combust. Flame 2022, 244, 112299. [Google Scholar] [CrossRef]




| Component Flue Gas | Concentration Range | Concentration Range | Concentration Range |
|---|---|---|---|
| [mg/m3] | [Vol. %] | [ppm] | |
| NH3 | 0 to 5000 | ||
| H2O | 0 to 30 | ||
| CO | 0 to 2 | ||
| CO2 | 0 to 20 | ||
| N2O | 0 to 180 | ||
| NO | 0 to 1100 | ||
| NO2 dry | 0 to 250 | ||
| NOx dry | 0 to 1300 |
| Parameter | First Experimental Series | Second Experimental Series | |
|---|---|---|---|
| Volume flow (NH3) | [m3N/h] | 10.7–19.4 | 0–32.5 |
| Volume flow (natural gas) | [m3N/h] | 11.8–3.1 | 12–0 |
| Total volume flow fuel (NH3 + natural gas) | [m3N/h] | 22.5 | 32.5 |
| NH3 fraction | [%] | 48–86 | 0–100 |
| Natural gas fraction | [%] | 52–14 | 100–0 |
| Volume flow (combustion air) | [m3N/h] | 110 | 150 |
| Primary air/secondary air | [%] | 15/85 | 100/0 |
| Air ratio, λ | [-] | 1.24–1.06 | 1.06–1.97 |
| Swirl | [-] | Yes | Yes |
| Temperature (combustion air) | [°C] | 25 | 25/260 |
| Oscillation frequency | [Hz] | 0 | 0–2 |
| Furnace type | Water-cooled | Isothermal | |
| Furnace interior temperature | [°C] | 800 | 800/1000 |
| Thermal output furnace | [kW] | 110 | 130 |
| Projection Costs | 2020 | 2030 | 2040 | 2050 | Location | Reference |
|---|---|---|---|---|---|---|
| Natural gas [€/MWh] | 33 | 34 | 36 | 37 | EU | [154] |
| Electricity [€/MWh] | 42 | 41 | 44 | 49 | NOR | [155] |
| Demineralized water [] | 0.44 | 0.45 | 0.45 | 0.45 | NOR | [156,157] |
| Cost Estimate | 2020 | 2030 | 2040 | 2050 | Location | Reference |
|---|---|---|---|---|---|---|
| CO2 certificate [] | 25 | 282 | 398 | 515 | EU | [159,160] |
| CapEx [Millions of EUR] | OpEx ] | ||||
|---|---|---|---|---|---|
| 2020 | 2030 | 2040 | 2050 | ||
| Maritime transport | - | 2.6 | 2.6 | 2.6 | 2.6 |
| Storage | 16.4 | 16.6 | 23 | 13.3 | 14.6 |
| Boiler firing | 30 | 26.5 | 35.6 | 18.2 | 20.8 |
| Flue gas treatment | 3.5 | 9.5 | 10.4 | 8.5 | 9.1 |
| Operation Mode | Investment SNCR and SCR | Reduction Rate [%] | 2020 | 2030 | |
|---|---|---|---|---|---|
| SNCR | SCR | ] | |||
| Without oscillation (14,000 mg/kWh) | EUR 3.5 million | 86 | 95 | 21.8 | 23.2 |
| With oscillation (3000 mg/kWh) | 70 | 90 | 9.5 | 10.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrov, K.; Gehrmann, H.-J.; Wiebe, J.; Stapf, D. Ammonia—A Fuel of the Future? Economies of Production and Control of NOx Emissions via Oscillating NH3 Combustion for Process Heat Generation. Energies 2025, 18, 5948. https://doi.org/10.3390/en18225948
Aleksandrov K, Gehrmann H-J, Wiebe J, Stapf D. Ammonia—A Fuel of the Future? Economies of Production and Control of NOx Emissions via Oscillating NH3 Combustion for Process Heat Generation. Energies. 2025; 18(22):5948. https://doi.org/10.3390/en18225948
Chicago/Turabian StyleAleksandrov, Krasimir, Hans-Joachim Gehrmann, Janine Wiebe, and Dieter Stapf. 2025. "Ammonia—A Fuel of the Future? Economies of Production and Control of NOx Emissions via Oscillating NH3 Combustion for Process Heat Generation" Energies 18, no. 22: 5948. https://doi.org/10.3390/en18225948
APA StyleAleksandrov, K., Gehrmann, H.-J., Wiebe, J., & Stapf, D. (2025). Ammonia—A Fuel of the Future? Economies of Production and Control of NOx Emissions via Oscillating NH3 Combustion for Process Heat Generation. Energies, 18(22), 5948. https://doi.org/10.3390/en18225948







