Innovative Calcium L-Lactate/PDMS-Based Composite Foams as Core for Sandwich Materials for the Thermopassive Regulation of Buildings
Abstract
1. Introduction
2. Materials and Methods
2.1. CaL-PDMS Foam Synthesis and Characterization
2.2. Physicochemical and Morphological Characterization of the Composite Foams
2.3. Hydration–Dehydration Cycle Through Thermogravimetric Dynamic Vapor Sorption System
2.4. Dehydration Enthalpy Measurements
3. Results
3.1. Chemical Analysis
3.2. Microstructural Analysis
3.3. Hydration–Dehydration Thermochemical Behavior
3.4. Dehydration Enthalpy Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament and the Council of the European Union. Directive (EU) 2024/1275 of the European Parliament and of the Council on the Energy Performance of Buildings; 2024. Available online: https://eur-lex.europa.eu/eli/dir/2024/1275/oj (accessed on 20 October 2025).
- Huang, Q.; Aftab, W.; Cheng, Y.; Li, Y. Form-Stable Metal Ion Coordination Organic Sorbent Aerogel and Its Application in Low Temperature Microwave-Powered Sorption Thermochemical Energy Storage. J. Energy Storage 2025, 114, 115810. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside Porous Matrix” for Adsorption Heat Transformation: A Current State-of-the-Art and New Trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, Z.; Wang, D.; Ju, Z.; Zhang, Z.; Zhang, H.; Zhu, Y.; Jiang, Z. Synthesis and Properties of Biomass Derived Carbon/PEG Composite as Photothermal Conversion Effective Phase Change Material for Functional Concrete. Cem. Concr. Compos. 2024, 149, 105495. [Google Scholar] [CrossRef]
- Duan, S.; Wang, L.; Zhao, Z.; Zhang, C. Experimental Study on Thermal Performance of an Integrated PCM Trombe Wall. Renew Energy 2021, 163, 1932–1941. [Google Scholar] [CrossRef]
- He, J.W.; Huang, X.Y.; Shu, Z.Y.; Huang, R.N.; Cai, Y.; Lv, Y.; Zhao, F.Y. Preliminary Study of a Dual Channel Solar Photocatalytic Ventilation Wall System for Year-Round Air Purification and Building Energy Savings. J. Build. Eng. 2024, 82, 108261. [Google Scholar] [CrossRef]
- Gao, F.; Xiao, X.; Shu, Z.; Zhong, K.; Wang, Y.; Li, M. Investigation of Thermoregulation Effect of Stabilized Phase Change Gypsum Board with Different Structures in Buildings. Sustainability 2024, 16, 6929. [Google Scholar] [CrossRef]
- Jeong, S.G.; Wi, S.; Chang, S.J.; Lee, J.; Kim, S. An Experimental Study on Applying Organic PCMs to Gypsum-Cement Board for Improving Thermal Performance of Buildings in Different Climates. Energy Build. 2019, 190, 183–194. [Google Scholar] [CrossRef]
- Abbasi Hattan, H.; Madhkhan, M.; Marani, A. Thermal and Mechanical Properties of Building External Walls Plastered with Cement Mortar Incorporating Shape-Stabilized Phase Change Materials (SSPCMs). Constr. Build. Mater. 2021, 270, 121385. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Du, Y.; Xu, T.; Zou, T.; Wei, S.; Zhang, D. Development of CaCl2·6H2O-Mannitol/SiO2 Shape-Stabilized Composite Phase Change Material for the Radiant Cooling Panel System. Energy Build. 2024, 313. [Google Scholar] [CrossRef]
- Jiao, K.; Lu, L.; Zhao, L.; Wang, G. Towards Passive Building Thermal Regulation: A State-of-the-Art Review on Recent Progress of PCM-Integrated Building Envelopes. Sustainability 2024, 16, 6482. [Google Scholar] [CrossRef]
- Mastronardo, E.; La Mazza, E.; Palamara, D.; Piperopoulos, E.; Iannazzo, D.; Proverbio, E.; Milone, C. Organic Salt Hydrate as a Novel Paradigm for Thermal Energy Storage. Energies 2022, 15, 4339. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A Systematic Multi-Step Screening of Numerous Salt Hydrates for Low Temperature Thermochemical Energy Storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Michel, B.; Mazet, N.; Mauran, S.; Stitou, D.; Xu, J. Thermochemical Process for Seasonal Storage of Solar Energy: Characterization and Modeling of a High Density Reactive Bed. Energy 2012, 47, 553–563. [Google Scholar] [CrossRef]
- Grevel, K.D.; Majzlan, J. Internally Consistent Thermodynamic Data for Magnesium Sulfate Hydrates. Geochim. Cosmochim. Acta 2009, 73, 6805–6815. [Google Scholar] [CrossRef]
- Ferchaud, C.J.; Zondag, H.A.; Veldhuis, J.B.J.; De Boer, R. Study of the Reversible Water Vapour Sorption Process of MgSO 4.7H2O and MgCl2.6H2O under the Conditions of Seasonal Solar Heat Storage. J. Phys. Conf. Ser. 2012, 395, 012069. [Google Scholar] [CrossRef]
- Michel, B.; Neveu, P.; Mazet, N. Comparison of Closed and Open Thermochemical Processes, for Long-Term Thermal Energy Storage Applications. Energy 2014, 72, 702–716. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Caprì, A.; Cannilla, C.; Gordeeva, L.G.; Aristov, Y.I. Development of “Salt in Porous Matrix” Composites Based on LiCl for Sorption Thermal Energy Storage. Energy 2020, 208, 118338. [Google Scholar] [CrossRef]
- Shervani, S.; Strong, C.; Tezel, F.H. Simultaneous Impregnation and Microencapsulation of CaCl2 Using Silica Gel and Methyl Cellulose for Thermal Energy Storage Applications. Sci. Rep. 2024, 14, 7183. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Liu, X.; Yang, F.; Guan, L.; Sani, S.; Sun, C.; Wu, Y. Development of MgSO4/Mesoporous Silica Composites for Thermochemical Energy Storage: The Role of Porous Structure on Water Adsorption. Energy Rep. 2022, 8, 4913–4921. [Google Scholar] [CrossRef]
- Fenwick, O.; Coutiño-Gonzalez, E.; Grandjean, D.; Baekelant, W.; Richard, F.; Bonacchi, S.; De Vos, D.; Lievens, P.; Roeffaers, M.; Hofkens, J.; et al. Tuning the Energetics and Tailoring the Optical Properties of Silver Clusters Confined in Zeolites. Nat. Mater. 2016, 15, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Hongois, S.; Kuznik, F.; Stevens, P.; Roux, J.J. Development and Characterisation of a New MgSO4-Zeolite Composite for Long-Term Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1831–1837. [Google Scholar] [CrossRef]
- Whiting, G.; Grondin, D.; Bennici, S.; Auroux, A. Heats of Water Sorption Studies on Zeolite-MgSO4 Composites as Potential Thermochemical Heat Storage Materials. Sol. Energy Mater. Sol. Cells 2013, 112, 112–119. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, J.; Wang, X.; Huang, H.; Wang, Y.; Deng, L. Development of Lithium Hydroxide-Metal Organic Framework-Derived Porous Carbon Composite Materials for Efficient Low Temperature Thermal Energy Storage. Microporous Mesoporous Mater. 2021, 328, 111455. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Padamurthy, A.; Nandanavanam, J.; Rajagopalan, P. Preparation and Characterization of Metal Organic Framework Based Composite Materials for Thermochemical Energy Storage Applications. Appl. Surf. Sci. Adv. 2022, 11, 100309. [Google Scholar] [CrossRef]
- Zhou, S.W.; Xia, C.C.; Hu, Y.S.; Zhou, Y.; Zhang, P.Y. Damage Modeling of Basaltic Rock Subjected to Cyclic Temperature and Uniaxial Stress. Int. J. Rock Mech. Min. Sci. 2015, 77, 163–173. [Google Scholar] [CrossRef]
- Li, K.-S.; Chen, L.-X.; Yang, S.-Q.; Song, G.-L. Multiscale Deterioration of Physical and Triaxial Mechanical Behaviors of Fine-Grained Sandstone Under Cyclic Freezing–Thawing: Experimental Observation and Microstructural Interpretation. Rock Mech. Rock Eng. 2025, 58, 10827–10851. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L.; Park, J.H.; Kim, K.S. Synthesis of a Novel Siloxane-Containing Diamine for Increasing Flexibility of Epoxy Resins. Mater. Sci. Eng. A 2005, 399, 377–381. [Google Scholar] [CrossRef]
- Taherizadeh, A.; Simon, A.; Richter, H.; Stelter, M.; Voigt, I. Development and Investigation of a Multilayer PDMS/Zeolite Composite Membrane for CO2 Separation Applications. Sep. Purif. Technol. 2024, 346, 127344. [Google Scholar] [CrossRef]
- Fauvel, M.; Trybala, A.; Tseluiko, D.; Starov, V.M.; Bandulasena, H.C.H. Stability of Two-Dimensional Liquid Foams under Externally Applied Electric Fields. Langmuir 2022, 38, 6305–6321. [Google Scholar] [CrossRef]
- Zambotti, A.; Valentini, F.; Lodi, E.; Pegoretti, A.; Tyrpekl, V.; Kohúteková, S.; Sorarù, G.D.; Kloda, M.; Biesuz, M. Thermochemical Heat Storage Performances of Magnesium Sulphate Confined in Polymer-Derived SiOC Aerogels. J. Alloys Compd. 2022, 895, 162592. [Google Scholar] [CrossRef]
- Mastronardo, E.; Piperopoulos, E.; Palamara, D.; Frazzica, A.; Calabrese, L. Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams. Appl. Sci. 2022, 12, 1510. [Google Scholar] [CrossRef]
- Calabrese, L.; Palamara, D.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; Proverbio, E. Deviceful LiCl Salt Hydrate Confinement into a Macroporous Silicone Foam for Low-Temperature Heat Storage Application. J. Sci. Adv. Mater. Devices 2022, 7, 100463. [Google Scholar] [CrossRef]
- He, X.; Yang, M.; Hu, F.; Jiang, G.; Shen, Y. Comparative Study on the Foaming and Fireproof Properties of PDMS Foam Composites with Different Inorganic Fillers. Buildings 2025, 15, 1172. [Google Scholar] [CrossRef]
- You, L.; Liu, B.; Hua, H.; Jiang, H.; Yin, C.; Wen, F. Energy Storage Performance of Polymer-Based Dielectric Composites with Two-Dimensional Fillers. Nanomaterials 2023, 13, 2842. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Bae, E.J.; Lee, M.H.; Han, M.; Kim, B.J.; Cho, S.Y. Highly Flexible and Durable Thermoelectric Power Generator Using CNT/PDMS Foam by Rapid Solvent Evaporation. Small 2022, 18, 2106108. [Google Scholar] [CrossRef]
- Chhetri, S.; Nguyen, A.T.; Song, S.; Gaillard, N.; Severa, G.; Ma, T.; Yoon, S.H.; Lee, W. Flexible Graphite Nanoflake/Polydimethylsiloxane Nanocomposites with Promising Solar-Thermal Conversion Performance. ACS Appl. Energy Mater. 2023, 6, 2582–2593. [Google Scholar] [CrossRef]
- De Maere D’aertrycke, J.B.; Morlot, J.; Robeyns, K.; Filinchuk, Y.; Leyssens, T. Exploring the Solid-State Phases and Thermodynamics of Calcium L-Lactate. Food Chem. 2020, 325, 126884. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Shiraishi, S.; Otsuka, M. Characterization of Dehydration and Hydration Behavior of Calcium Lactate Pentahydrate and Its Anhydrate. Colloids Surf. B Biointerfaces 2005, 46, 135–141. [Google Scholar] [CrossRef]
- Mastronardo, E.; Previti, E.; Calabrese, L.; Milone, C. Experimental Assessment of Calcium L-Lactate as Thermochemical Heat Storage Material. J. Energy Storage 2025, 108, 115065. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Assessment of the Hydration/Dehydration Behaviour of MgSO4∙7H2O Filled Cellular Foams for Sorption Storage Applications through Morphological and Thermo-Gravimetric Analyses. Sustain. Mater. Technol. 2018, 17, e00073. [Google Scholar] [CrossRef]
- Miao, K.; Lu, Z.; Cao, J.; Zhang, H.; Li, D. Effect of Polydimethylsiloxane on the Mid-Temperature Strength of Gelcast Al2O3 Ceramic Parts. Mater. Des. 2016, 89, 810–814. [Google Scholar] [CrossRef]
- De Souza Neto, F.N.; Araújo, O.A.; Guilherme, L.R.; Garg, V.K.; Oliveira, A.C.; De Souza, P.E.N.; Franco Júnior, A. Particles That Slide over the Water Surface: Synthesis and Characterization of Iron Oxides Particles Coated with PDMS, with Hydrophobic and Magnetic Properties. Mater. Chem. Phys. 2015, 162, 100–105. [Google Scholar] [CrossRef]
- Brancato, V.; Calabrese, L.; Palomba, V.; Frazzica, A.; Fullana-Puig, M.; Solé, A.; Cabeza, L.F. MgSO4·7H2O Filled Macro Cellular Foams: An Innovative Composite Sorbent for Thermo-Chemical Energy Storage Applications for Solar Buildings. Sol. Energy 2018, 173, 1278–1286. [Google Scholar] [CrossRef]
- Baglioni, M.; Bartoletti, A.; Bozec, L.; Chelazzi, D.; Giorgi, R.; Odlyha, M.; Pianorsi, D.; Poggi, G.; Baglioni, P. Nanomaterials for the Cleaning and PH Adjustment of Vegetable-Tanned Leather. Appl. Phys. A Mater. Sci. Process 2016, 122, 1–11. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Frazzica, A. Experimental Evaluation of the Hydrothermal Stability of a Silicone/Zeolite Composite Foam for Solar Adsorption Heating and Cooling Application. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Chen, C.; Yu, H.; Lai, T.; Guo, J.; Qin, M.; Qu, Z.; Feng, Y.; Feng, W. Flexible and Elastic Thermal Regulator for Multimode Intelligent Temperature Control. SusMat 2023, 3, 843–858. [Google Scholar] [CrossRef]
- Krom, J.; Meister, K.; Vilgis, T.A. Simple Method to Assess Foam Structure and Stability Using Hydrophobin and BSA as Model Systems. ChemPhysChem 2024, 25, e202400050. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Cai, J.; Cheng, S. Bubble Characterization and Bubble Population on SiC Material Surface in Subcooled Boiling Flow. Int. J. Therm. Sci. 2025, 208, 109447. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Gullì, G.; Freni, A.; Proverbio, E. Zeolite Filled Siloxane Composite Foams: Compression Property. J. Appl. Polym. Sci. 2018, 135, 46145. [Google Scholar] [CrossRef]
- Liao, W.; Wang, P.; Xu, Z.; Huang, X. Microfluidic Foaming of Polydimethylsiloxane (PDMS). Mater. Lett. 2025, 379, 137653. [Google Scholar] [CrossRef]
- Haily, E.; Ait Ousaleh, H.; Zari, N.; Faik, A.; Bouhfid, R.; Qaiss, A. Use of a Form-Stable Phase Change Material to Improve Thermal Properties of Phosphate Sludge-Based Geopolymer Mortar. Constr. Build. Mater. 2023, 386, 131570. [Google Scholar] [CrossRef]
- Tieger, E.; Kiss, V.; Pokol, G.; Finta, Z.; Dušek, M.; Rohlíček, J.; Skořepová, E.; Brázda, P. Studies on the Crystal Structure and Arrangement of Water in Sitagliptin L-Tartrate Hydrates. CrystEngComm 2016, 18, 3819–3831. [Google Scholar] [CrossRef]
- Al-Jalali, M.A.; Aljghami, I.F.; Mahzia, Y.M. Voigt Deconvolution Method and Its Applications to Pure Oxygen Absorption Spectrum at 1270 Nm Band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 157, 34–40. [Google Scholar] [CrossRef]
- Mahlin, D.; Berggren, J.; Alderborn, R. Moisture-Induced Surface Crystallization of Spray-Dried Amorphous Lactose Particles Studied by Atomic Force Microscopy. J. Pharm. Sci. 2004, 93, 29–37. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Arunachalam, S.; Arslanoğlu, H.; Gencel, O. Porous Biochar/Heptadecane Composite Phase Change Material with Leak-Proof, High Thermal Energy Storage Capacity and Enhanced Thermal Conductivity. Powder Technol. 2021, 394, 1017–1025. [Google Scholar] [CrossRef]
- Moulakhnif, K.; Ait Ousaleh, H.; Sair, S.; Bouhaj, Y.; El Majd, A.; Ghazoui, M.; Faik, A.; El Bouari, A. Renewable Approaches to Building Heat: Exploring Cutting-Edge Innovations in Thermochemical Energy Storage for Building Heating. Energy Build. 2024, 318, 114421. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, W.; Lv, Z.; Huang, Z.; Gao, W. A Novel Composite Phase Change Material of Stearic Acid/Carbonized Sunflower Straw for Thermal Energy Storage. Mater. Lett. 2018, 215, 42–45. [Google Scholar] [CrossRef]
- Fang, Y.; Ahmad, M.R.; Lao, J.C.; Qian, L.P.; Dai, J.G. Development of Artificial Geopolymer Aggregates with Thermal Energy Storage Capacity. Cem. Concr. Compos. 2023, 135, 104834. [Google Scholar] [CrossRef]
- Gencel, O.; Hekimoglu, G.; Sarı, A.; Ustaoglu, A.; Subasi, S.; Marasli, M.; Erdogmus, E.; Memon, S.A. Glass Fiber Reinforced Gypsum Composites with Microencapsulated PCM as Novel Building Thermal Energy Storage Material. Constr. Build. Mater. 2022, 340, 127788. [Google Scholar] [CrossRef]
- Erdogmus, E.; Yaras, A.; Ustaoglu, A.; Hekimoğlu, G.; Sarı, A.; Gencel, O. Thermal Performance Analysis of Novel Foam Concrete Composites with PCM for Energy Storage and Environmental Benefits in Buildings. Energy Build. 2023, 296, 113413. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Wang, X.; Cao, X.; Yang, X.; Hu, S. Preparation and Characterization of Lauric-Myristic-Palmitic Acid Ternary Eutectic Mixtures/Expanded Graphite Composite Phase Change Material for Thermal Energy Storage. Chem. Eng. J. 2013, 231, 214–219. [Google Scholar] [CrossRef]
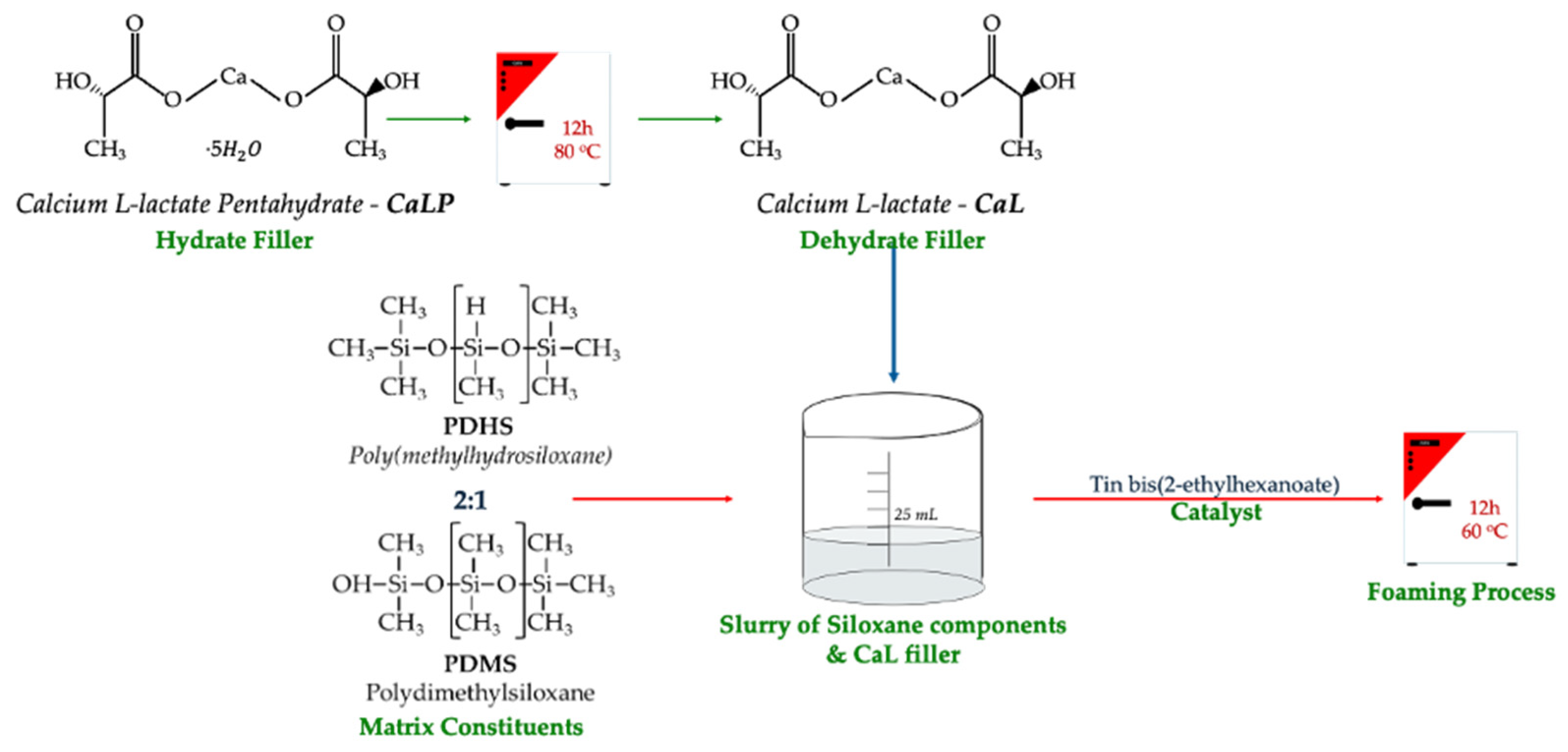



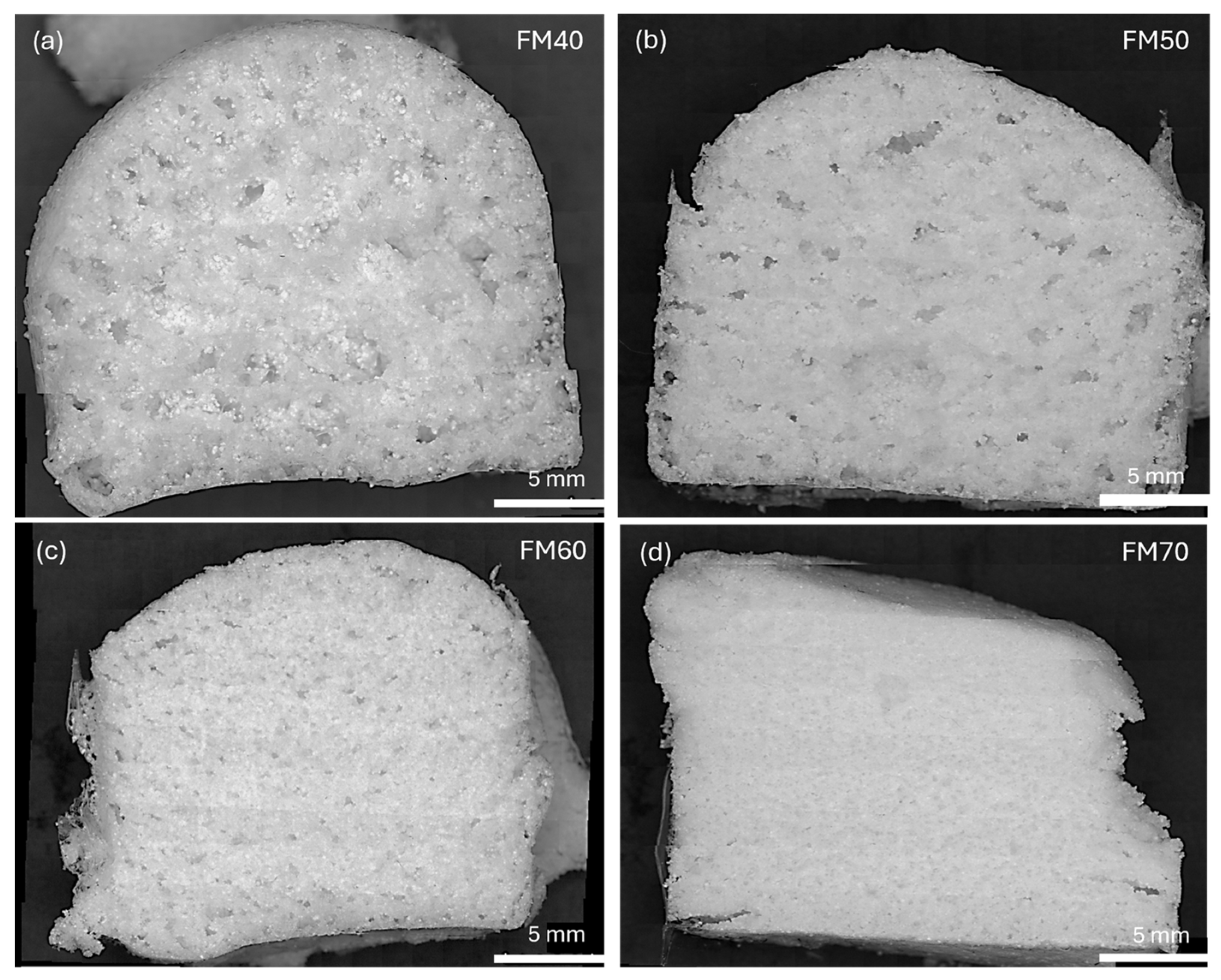
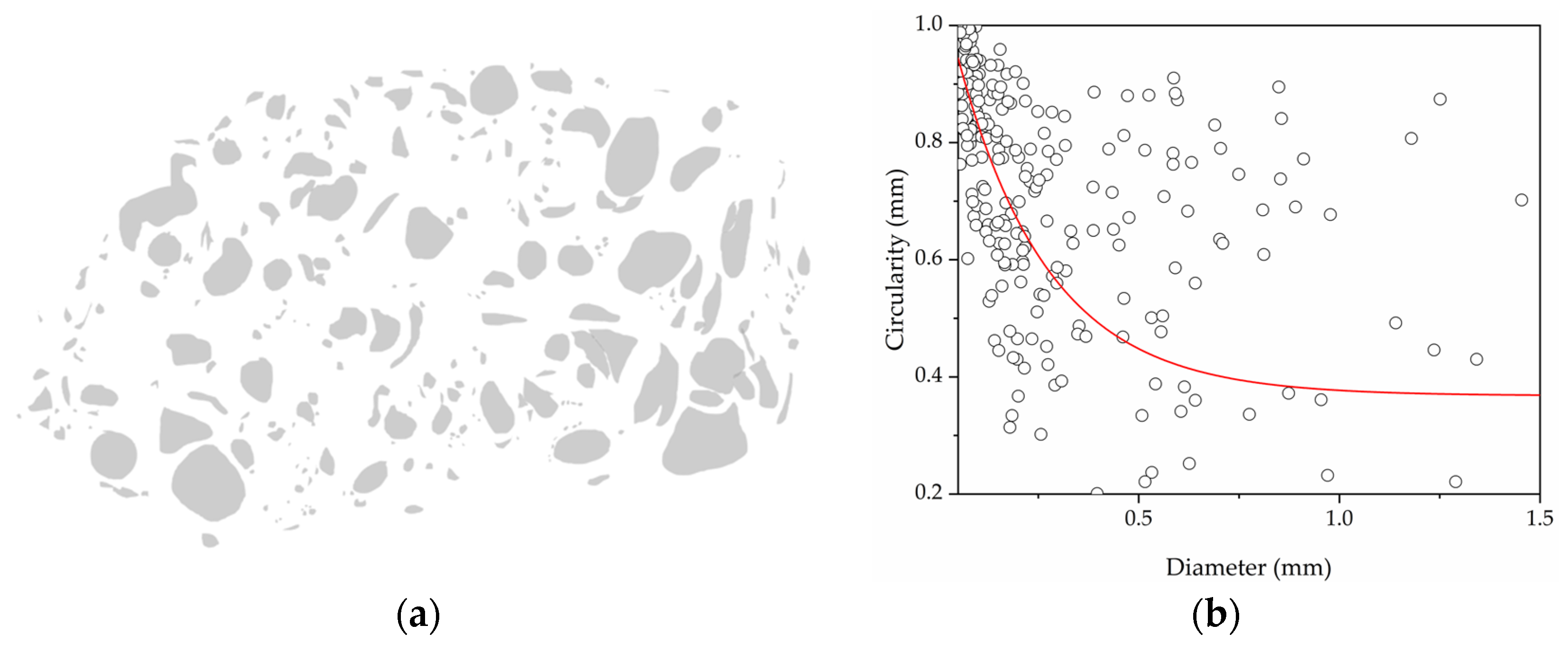
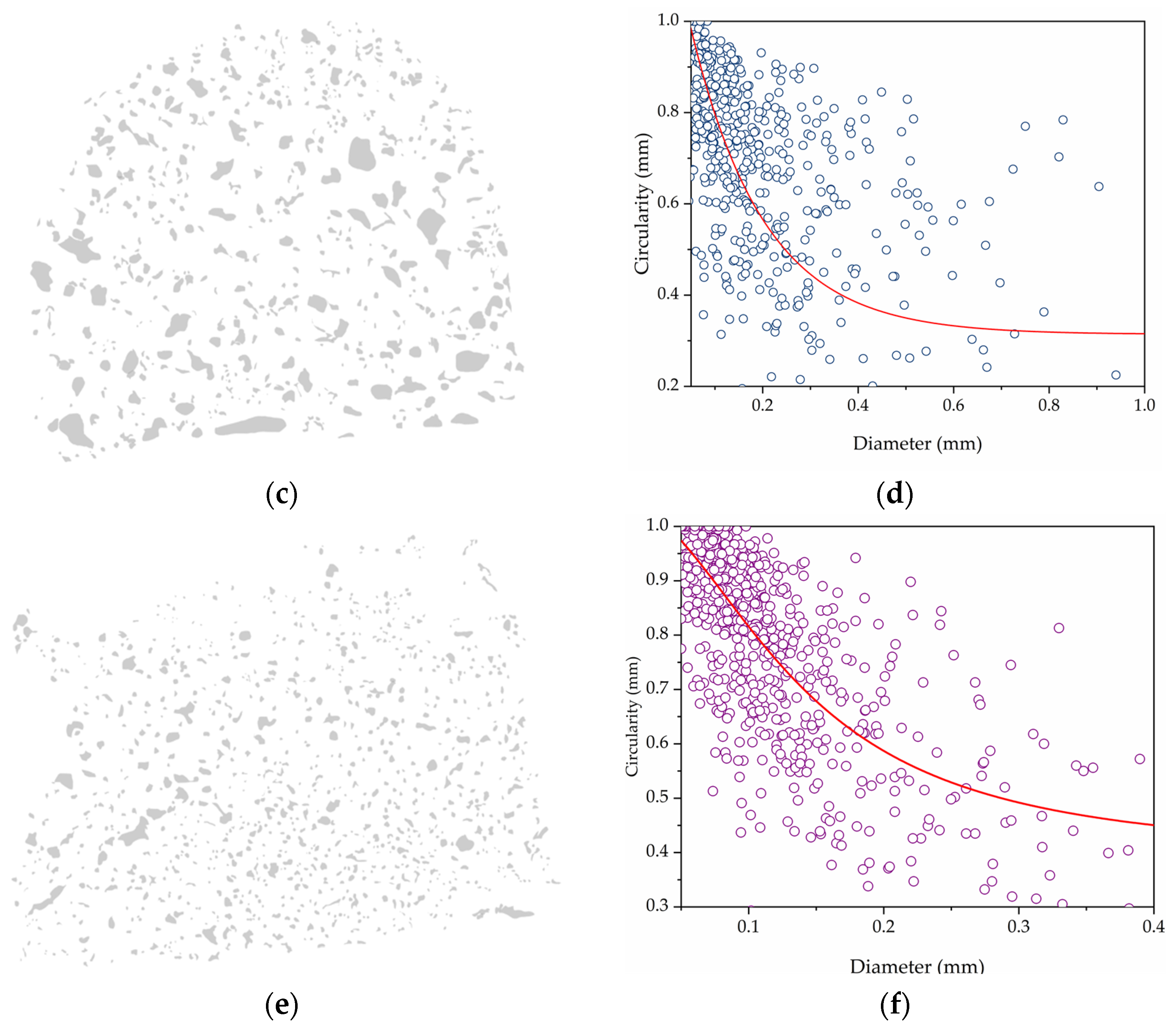

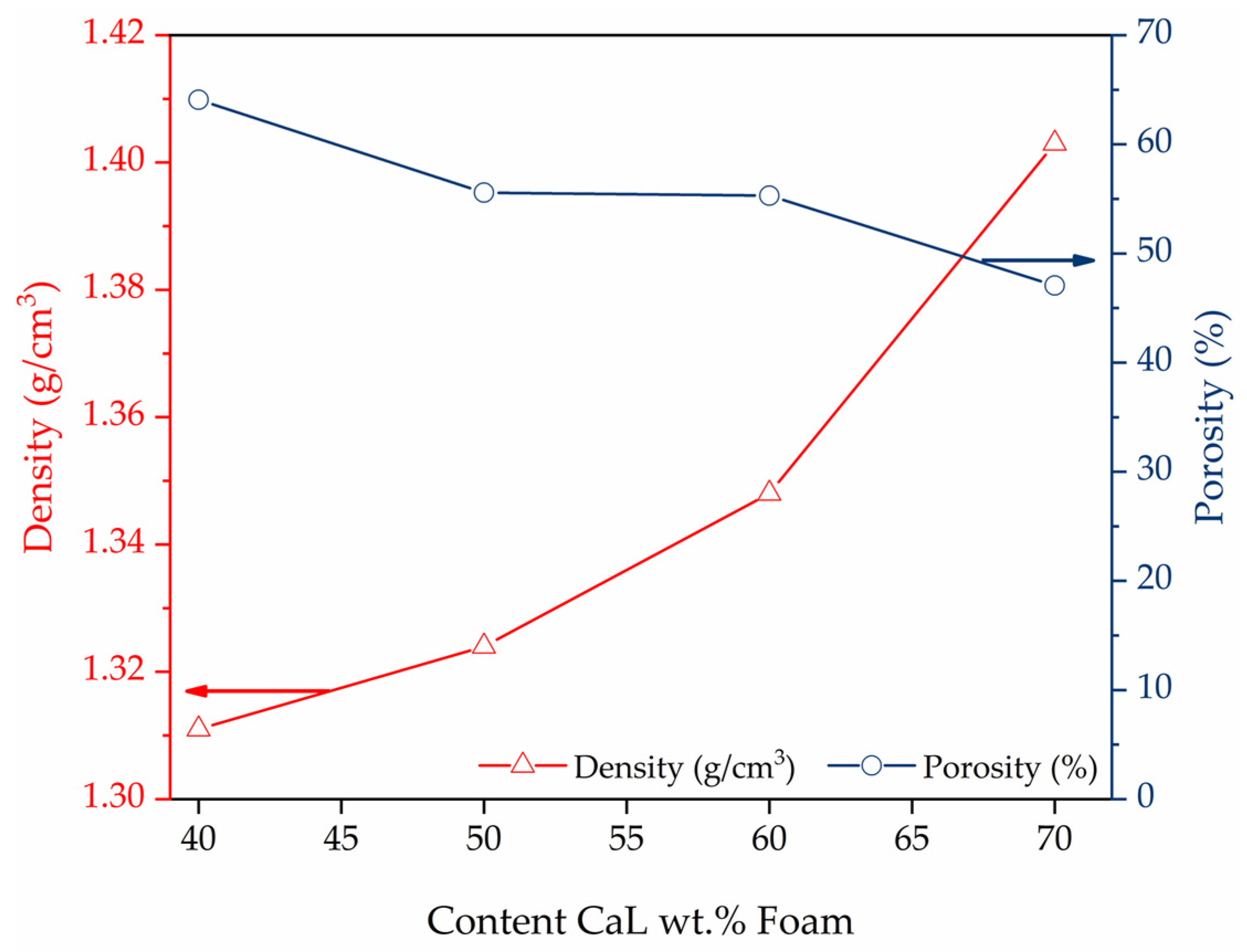
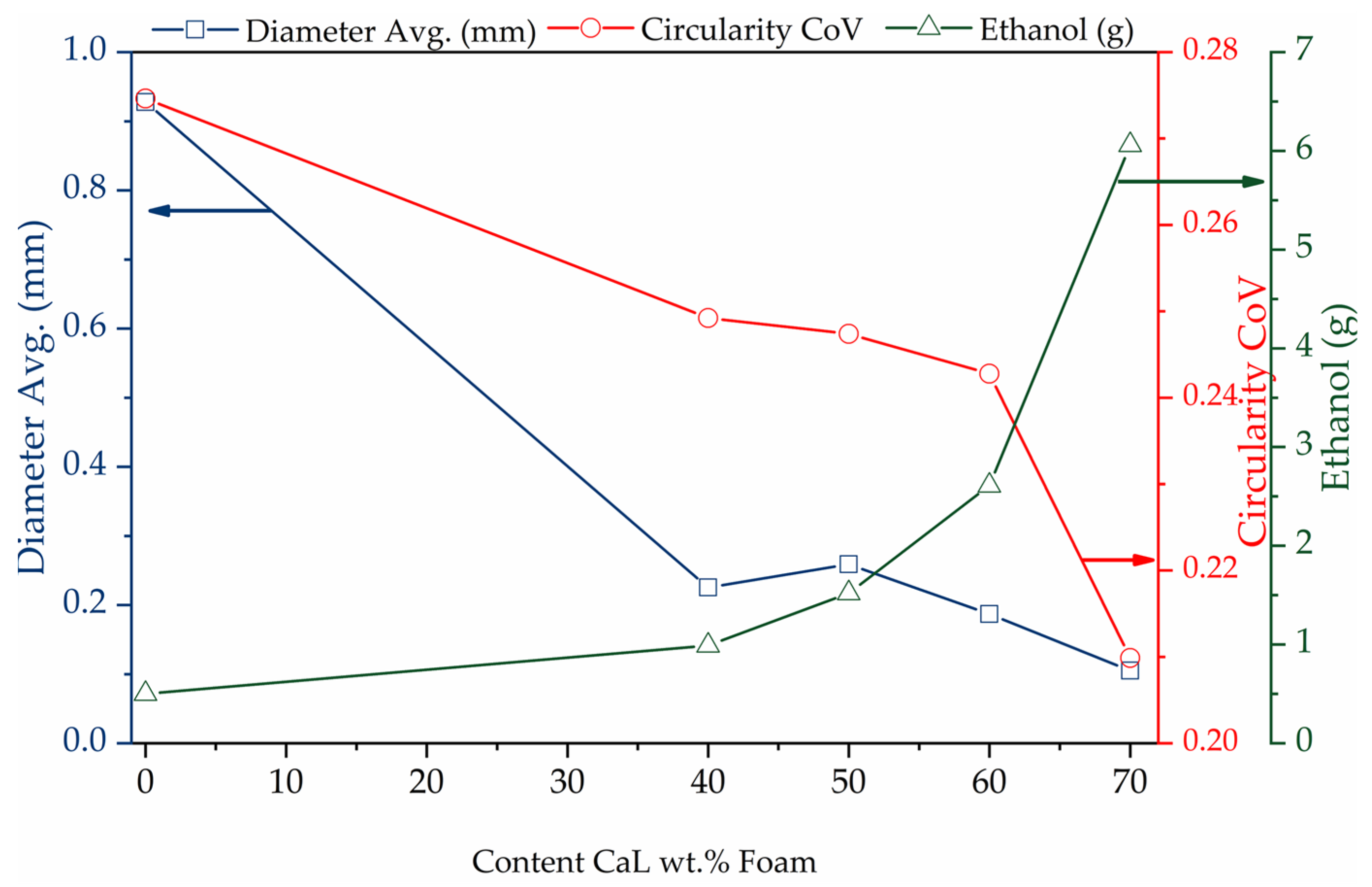
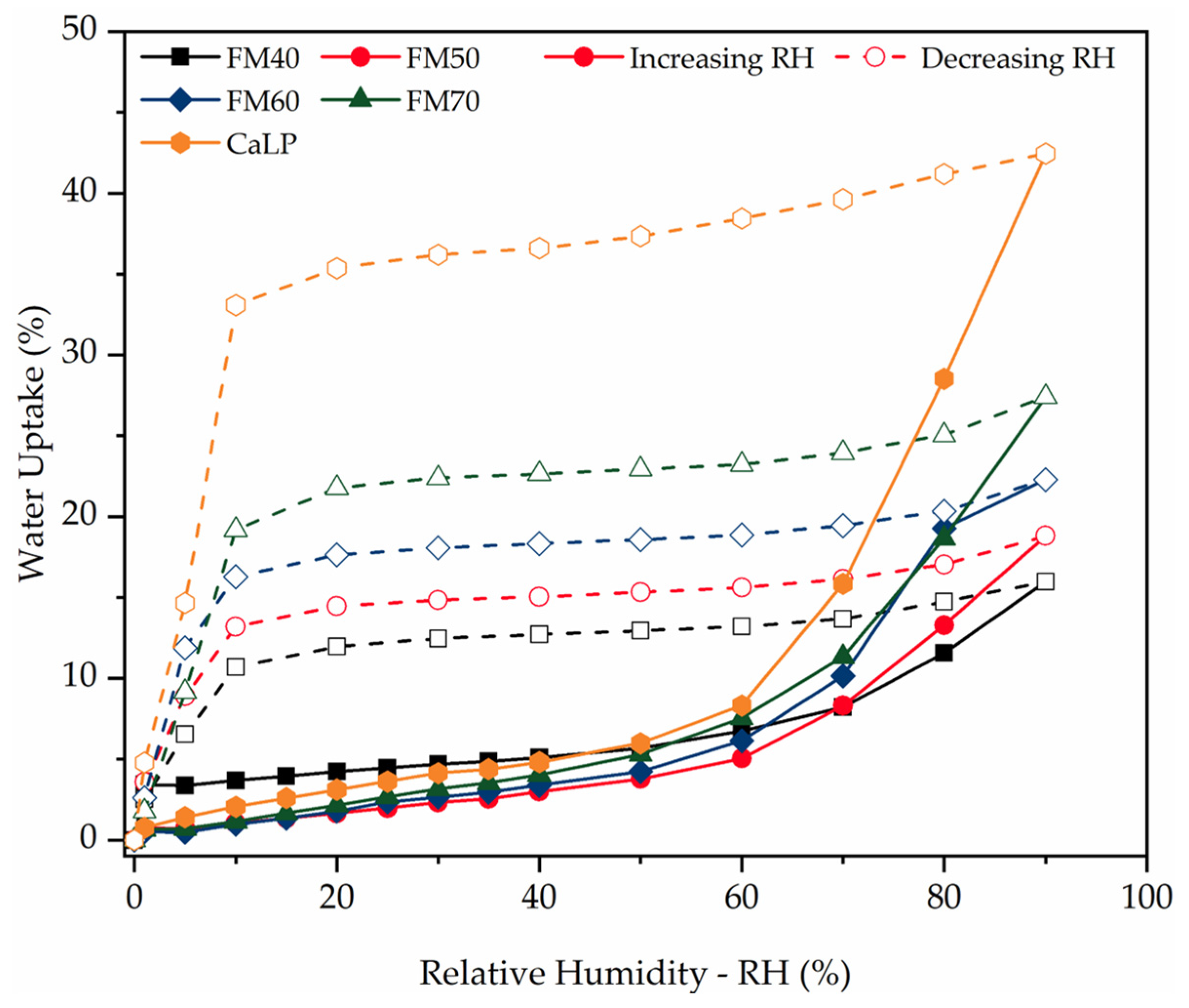

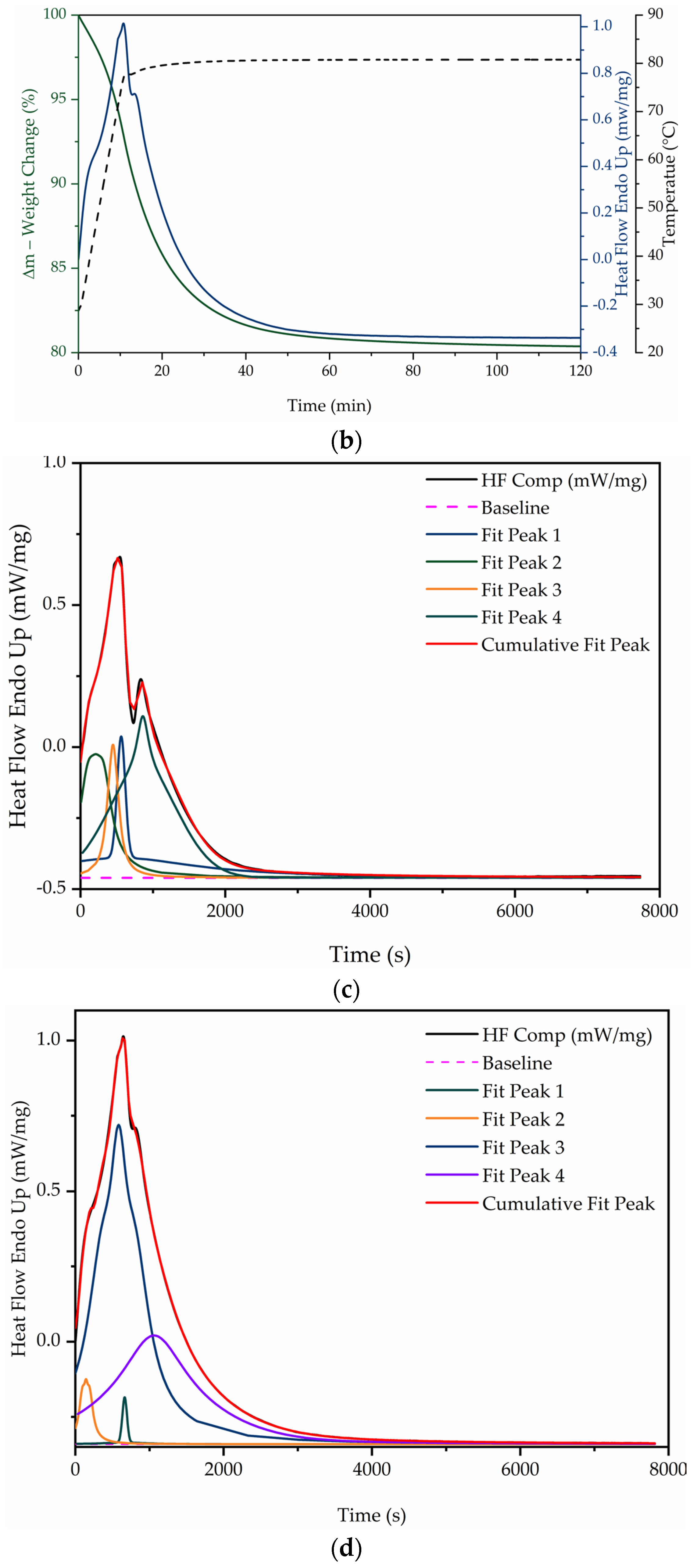
| Sample | PDMS (g) | PHMS (g) | EtOH (g) | CaL (g) | wt.% | Weight (g) |
|---|---|---|---|---|---|---|
| FM0 | 2.04 | 1.09 | 0.72 | 0 | 0 | 1.0 |
| FM40 | 2.02 | 1.12 | 0.99 | 2.19 | 41.09 | 3.64 |
| FM50 | 2.17 | 1.20 | 1.52 | 3.41 | 50.29 | 6.75 |
| FM60 | 2.16 | 1.19 | 2.61 | 5.13 | 60.50 | 9.02 |
| FM70 | 2.00 | 1.01 | 6.06 | 7.15 | 70.37 | 8.56 |
| Sample | Avg. Diam. (mm) | Circularity CoV |
|---|---|---|
| FM0 | 0.927 | 0.274 |
| FM40 | 0.225 | 0.249 |
| FM50 | 0.258 | 0.247 |
| FM60 | 0.186 | 0.242 |
| FM70 | 0.104 | 0.209 |
| Theoretical Values | |||||
|---|---|---|---|---|---|
| Sample | (%) | ||||
| FM40 | 17.40 | 2.11 | |||
| FM50 | 21.75 | 2.64 | |||
| FM60 | 26.10 | 3.16 | |||
| FM70 | 30.45 | 3.69 | |||
| Experimental Values | |||||
| Sample | |||||
| FM40 | 36.85 | 4.47 | 15.76 | 1.91 | |
| FM50 | 37.63 | 4.56 | 20.40 | 2.47 | |
| FM60 | 37.15 | 4.51 | 24.35 | 2.95 | |
| FM70 | 39.17 | 4.75 | 30.22 | 3.66 | |
| CaLP | 43.5 | 5.27 | -- | -- | |
| Theoretical Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samp. | |||||||||
| FM40 | 11.68 | 451 | 1.68 | ||||||
| FM50 | 14.60 | 563 | 2.10 | ||||||
| FM60 | 17.52 | 676 | 2.52 | ||||||
| FM70 | 20.44 | 789 | 2.94 | ||||||
| CaLP [42] | 29.2 | -- | 5.0 | ||||||
| Experimental Values | |||||||||
| Samp. | |||||||||
| FM40 | 713 ± 3.3 | 293 ± 3.7 | 924 ± 5.3 | 380 ± 4.9 | 11.30 | 1.54 | |||
| FM50 | 1020 ± 7.8 | 538 ± 6.9 | 1374 ± 10.5 | 726 ± 9.3 | 14.80 | 2.10 | |||
| FM60 | 1045 ± 3.7 | 621 ± 9.0 | 1452 ± 5.2 | 864 ± 12.5 | 18.88 | 2.82 | |||
| FM70 | 998 ± 5.4 | 687 ± 9.1 | 1412 ± 7.7 | 955 ± 12.6 | 19.66 | 2.96 | |||
| CaLP [42] | 1127 | -- | 1696 | -- | 24.52 | 4.2 | |||
| Composite Host Matrix | Active Phase | Content (wt.%) | Storage Mechanism | ΔHcomp. (kJ/kg) | Reference |
|---|---|---|---|---|---|
| PDMS | CaL | 70 | TCES | 687 | This Work |
| Trombe Wall | Decanoic Acid–Lauric Acid | 55–45 | PCM | 133 | [6] |
| Modified Activated Carbon | Sodium Acetate–Trihydrate–Urea | 48 | PCM | 103 | [49] |
| Carbonized Lemon Peel | n-Heptadecane | 65 | PCM | 141 | [58] |
| Porous Carbon | MgSO4-MgCl2 | 60 | PCM | 1840 | [59] |
| Expanded Perlite | CaCl2 | 30 | PCM | 2166 | [59] |
| Carbonized Sunflower Straw | Stearic Acid | 83.7 | PCM | 186 | [60] |
| Expanded Perlite | Paraffin | 30 | PCM | 11 | [61] |
| Polyurethane | Lauric–Capric Acid | 20 | PCM | 164 | [54] |
| Attapulgite | Lauric–Capric Acid | 30 | PCM | 74 | [62] |
| Water treatment sludge | Methyl Palmitate | 35 | PCM | 86 | [63] |
| Expanded Graphite | Paraffin | 10 | PCM | 178 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Gutiérrez, M.; Previti, E.; Orfila, M.; Acquaro, I.; Calabrese, L.; Milone, C.; Mastronardo, E. Innovative Calcium L-Lactate/PDMS-Based Composite Foams as Core for Sandwich Materials for the Thermopassive Regulation of Buildings. Energies 2025, 18, 5940. https://doi.org/10.3390/en18225940
Ávila-Gutiérrez M, Previti E, Orfila M, Acquaro I, Calabrese L, Milone C, Mastronardo E. Innovative Calcium L-Lactate/PDMS-Based Composite Foams as Core for Sandwich Materials for the Thermopassive Regulation of Buildings. Energies. 2025; 18(22):5940. https://doi.org/10.3390/en18225940
Chicago/Turabian StyleÁvila-Gutiérrez, Mario, Emanuele Previti, María Orfila, Ilenia Acquaro, Luigi Calabrese, Candida Milone, and Emanuela Mastronardo. 2025. "Innovative Calcium L-Lactate/PDMS-Based Composite Foams as Core for Sandwich Materials for the Thermopassive Regulation of Buildings" Energies 18, no. 22: 5940. https://doi.org/10.3390/en18225940
APA StyleÁvila-Gutiérrez, M., Previti, E., Orfila, M., Acquaro, I., Calabrese, L., Milone, C., & Mastronardo, E. (2025). Innovative Calcium L-Lactate/PDMS-Based Composite Foams as Core for Sandwich Materials for the Thermopassive Regulation of Buildings. Energies, 18(22), 5940. https://doi.org/10.3390/en18225940











