Sand-Based Thermal Storage System for Human-Powered Energy Generation: A Review
Abstract
1. Introduction
2. Development of Phase Change Thermal Storage Materials in Energy Storage
2.1. Fundamental Principles and Technological Evolution of Phase Change Materials
2.1.1. Solid–Liquid and Solid–Solid Phase Change Mechanisms
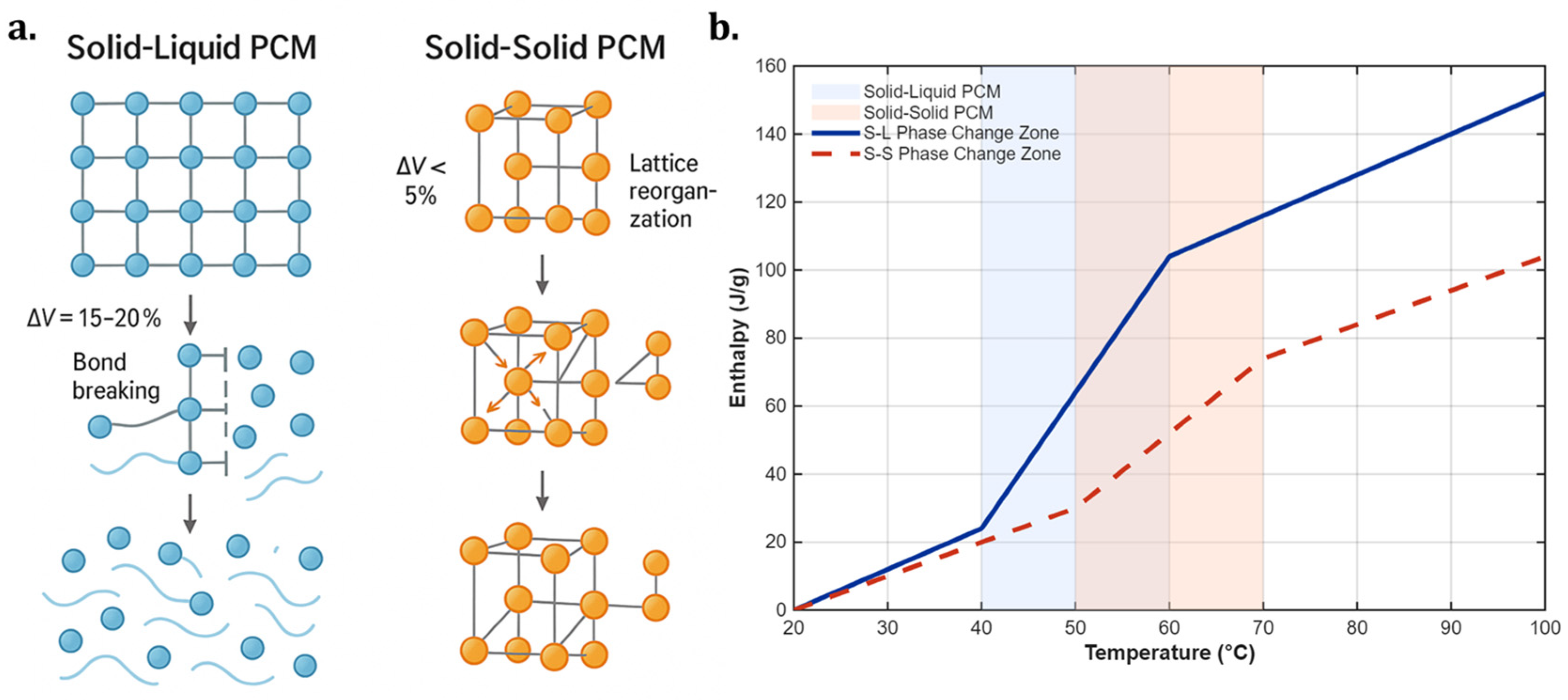
2.1.2. Thermal Conductivity Enhancement Strategies for Nanocomposite Phase Change Materials
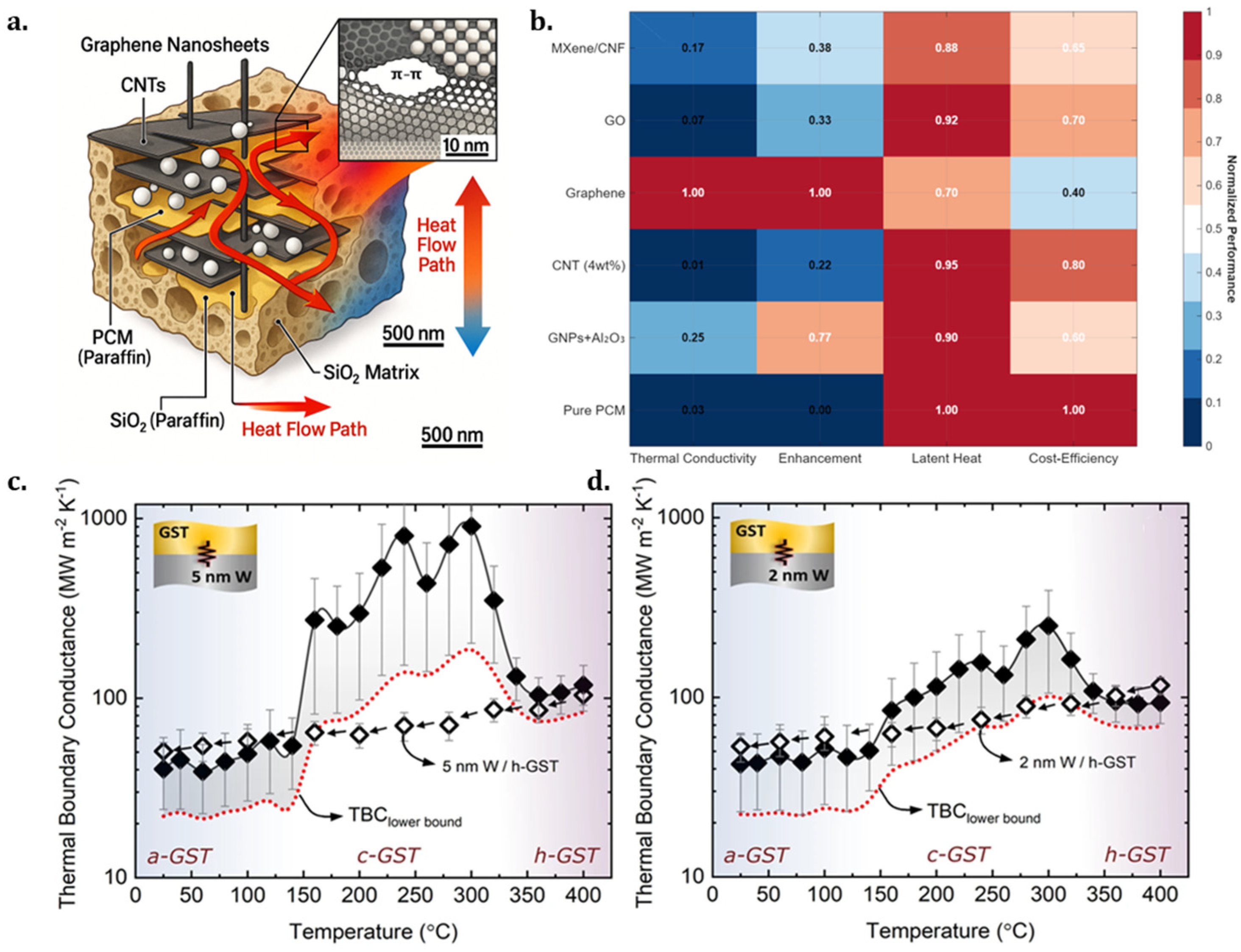
2.2. Historical Application Scenarios of Phase Change Thermal Storage Technology
2.2.1. Solar Thermal Storage and Building Energy Conservation
2.2.2. Electronic Device Thermal Management and Battery Thermal Protection
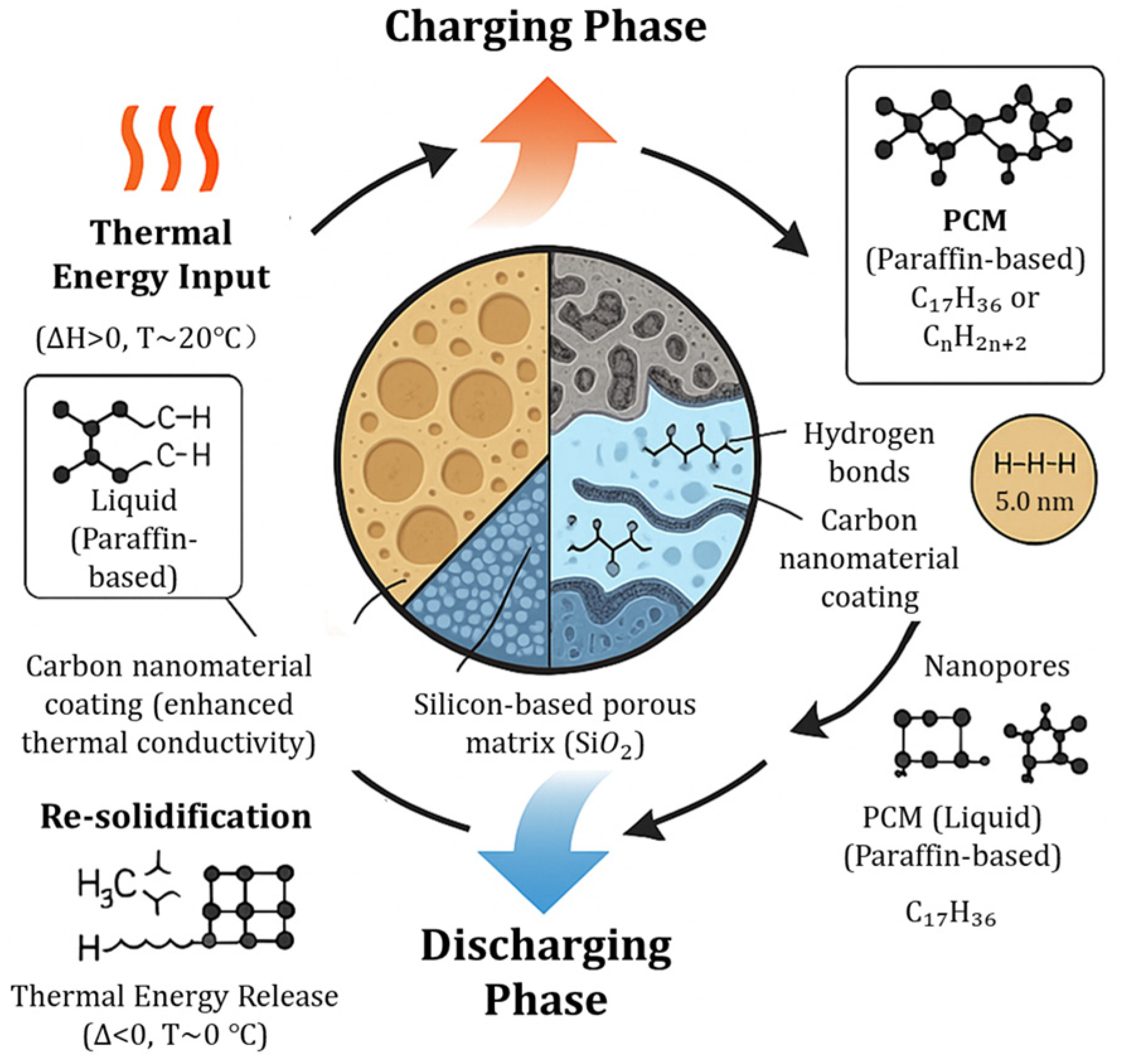
3. Basic Principles and Technical Classification of Sand Batteries
3.1. Core Mechanisms of Sand as Thermal Storage Medium
3.1.1. Thermal Storage Characteristics of Silicon-Based Porous Structures

3.1.2. Physicochemical Processes of Thermal Energy Storage and Conversion
3.2. Technical Classification of Sand Batteries
3.2.1. Sand-Based Composite Materials Based on Solid–Solid Phase Changes
3.2.2. Nano-Doped Sand-Based Phase Change Systems
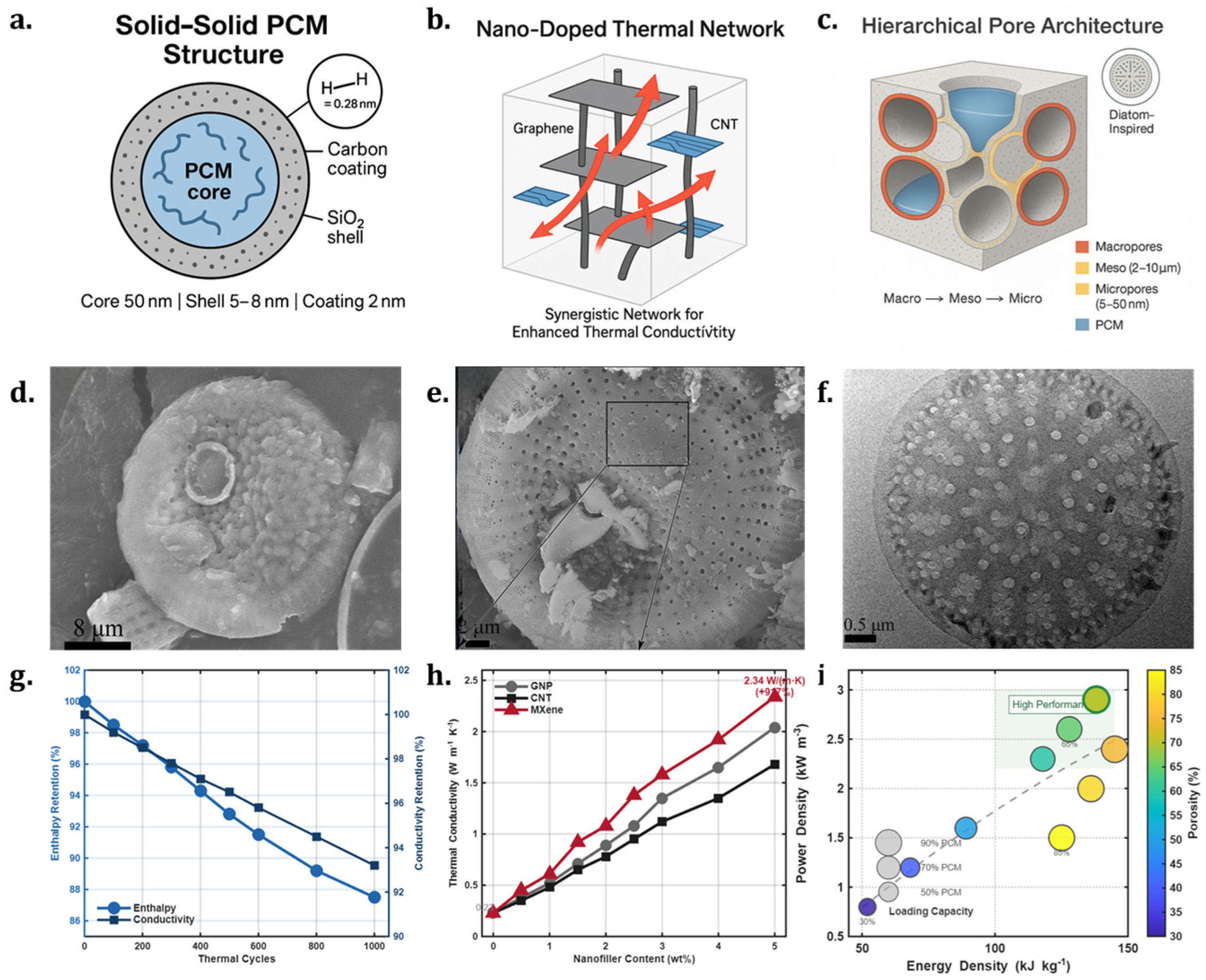
3.2.3. Multi-Level Pore Structure Sand-Based Thermal Storage Devices
| Classification | Structure | Thermal Conductivity (W/m·K) | PCM Loading (%) | Cycle Life (Cycles) | Main Advantage | Optimal Use | Refs. |
|---|---|---|---|---|---|---|---|
| Solid–Solid | Core–shell SiO2; 2–50 nm pores | 0.89–1.8 | 92–95 | 500–3000 | Zero leakage; <5% volume change | Stationary storage; building integration | [35,37,41,69] |
| Nano-Doped | GNPs/CNTs/MXene; 1–5 wt% | 1.9–2.34 | 75–92 | 1000–2000 | 269% conductivity boost; 2.7 s response | Rapid charge–discharge; HPEG | [35,73,76,78,79] |
| Hierarchical Pore | Macro–meso–micro; 50 nm–5 μm | 0.82–3.6 | 90–98 | 1000–3000 | Highest loading; anisotropic (3.6:1) | High energy density; solar thermal | [34,35,41,82,83] |
3.3. PCM Selection Criteria for Sand-Based Thermal Storage Systems
3.3.1. Operating Temperature Range and PCM Classification
3.3.2. Chemical Compatibility and Interfacial Stability Considerations
4. Current Research Progress and Representative Achievements
4.1. Performance Optimization of Sand-Based Composite Phase Change Materials
4.1.1. Thermal Conductivity Enhancement Technologies
4.1.2. Cycling Stability and Leakage Prevention Solutions
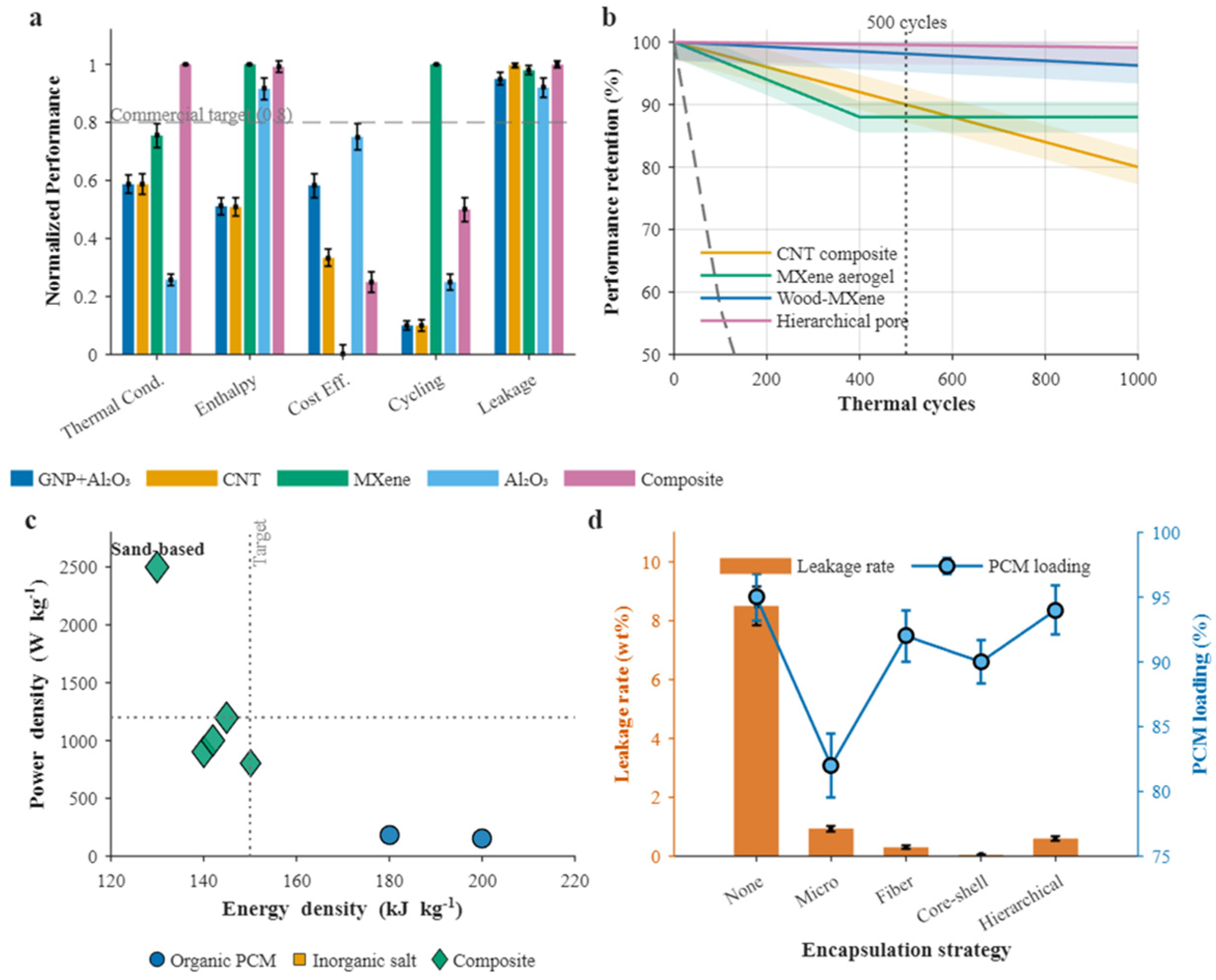
4.2. Energy Conversion Integration in HPEG
4.2.1. Mechanical–Thermal–Electrical Energy Synergistic Conversion Mechanisms
4.2.2. Miniaturized Thermal Storage Module Design Cases
5. Key Challenges and Technical Bottlenecks
5.1. Material-Level Limiting Factors
5.1.1. Thermal Cycling Degradation and Interface Stability Issues
5.1.2. Balance Between Energy Density and Power Density
5.2. System Integration Challenges
5.2.1. Dynamic Thermal Management Control Strategies
5.2.2. Environmental Adaptability
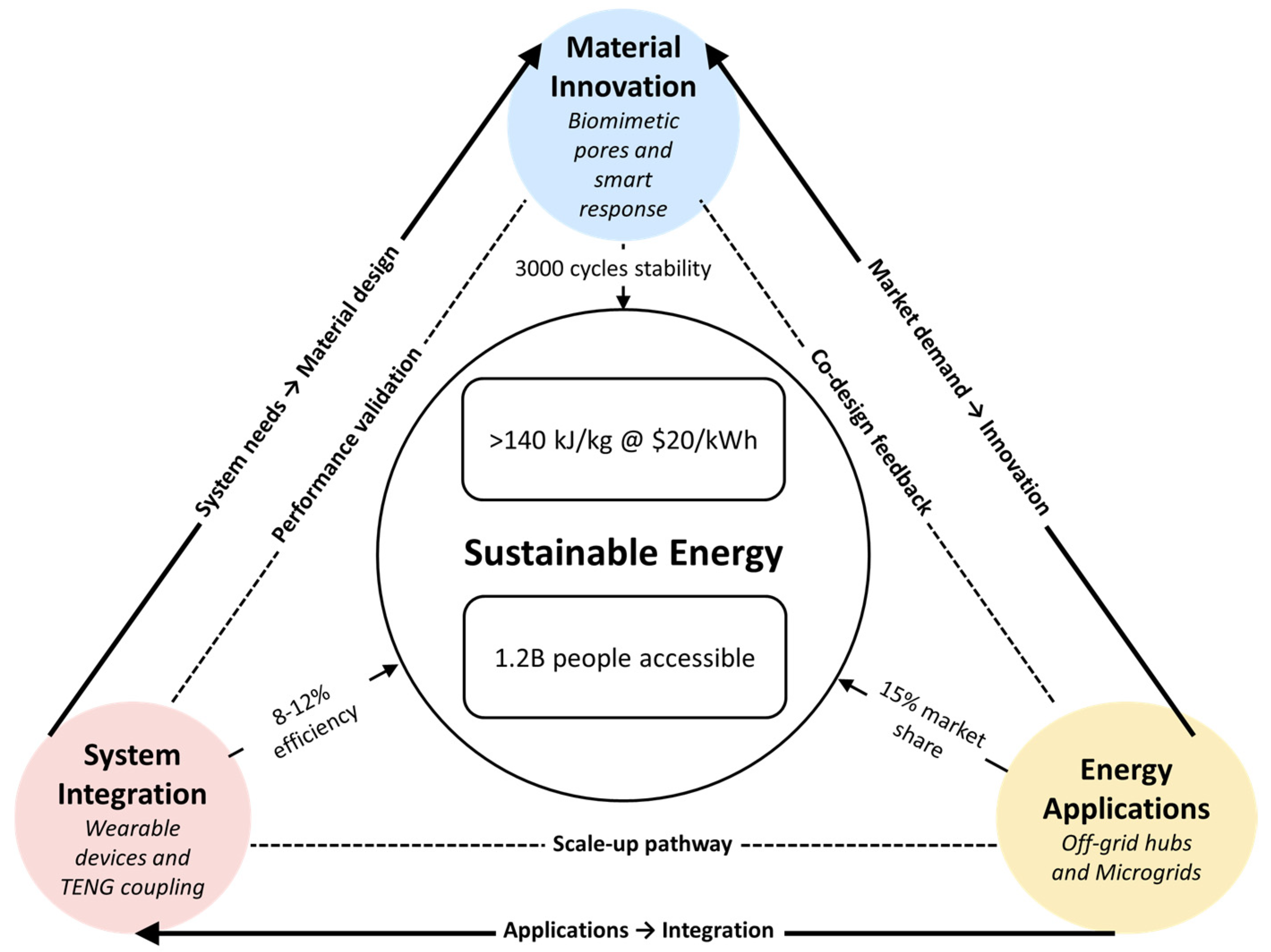
6. Future Development Directions and Innovation Pathways
6.1. Development of Novel Sand-Based Composite Materials
6.1.1. Biomimetic Multi-Level Pore Structure Design
6.1.2. Intelligent Responsive Phase Change Systems
6.2. System-Level Innovation Applications
6.2.1. Off-Grid Community Energy Hubs: Human-Powered Storage Integration
6.2.2. Distributed Microgrid Energy Storage Nodes
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPEG | Human-powered electricity generation |
| PCM | Phase Change Material |
| TENG | Triboelectric Nanogenerator |
| CNT | Carbon Nanotube |
| GNP | Graphene Nanoplatelet/Nanosheet |
| PEG | Polyethylene Glycol |
| ALD | Atomic Layer Deposition |
| UV | Ultraviolet |
| RH | Relative Humidity |
| ZIF-8 | Zeolitic Imidazolate Framework-8 |
| SEM | Scanning Electron Microscope/Microscopy |
References
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.-Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- He, Y.; Xie, Y.; Qiao, Y.; Qin, J.; Tang, Y. Estimation of underground hydrogen storage capacity in depleted gas reservoirs using CO2 as cushion gas. Appl. Energy 2024, 375, 124093. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B. Towards underground hydrogen storage: A review of barriers. Renew. Sustain. Energy Rev. 2022, 162, 112451. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Miocic, J.M.; Hangx, S.J.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.J.; Schmidt-Hattenberger, C. Enabling large-scale hydrogen storage in porous media–the scientific challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Li, Q. Reservoir Science: A Multi-Coupling Communication Platform to Promote Energy Transformation, Climate Change and Environmental Protection. Reserv. Sci. 2025, 1, 1–2. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Ouda, M.; Sanad, A.A.A.; Krishna, A.; Kandah, M.; Kurdi, J. Advancing environmental sustainability: A comprehensive review of waste-based composite materials for efficient electromagnetic shielding and absorption. IEEE Access 2025, 13, 15028–15061. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Szewerda, K.; Michalak, D.; Matusiak, P.; Kowol, D. Concept of Adapting the Liquidated Underground Mine Workings into High-Temperature Sand Thermal Energy Storage. Appl. Sci. 2025, 15, 3868. [Google Scholar] [CrossRef]
- Malik, F.H.; Hussain, G.A.; Alsmadi, Y.M.; Haider, Z.M.; Mansoor, W.; Lehtonen, M. Integrating Energy Storage Technologies with Renewable Energy Sources: A Pathway Toward Sustainable Power Grids. Sustainability 2025, 17, 4097. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.-L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Qiu, L.; Yan, K.; Feng, Y.; Liu, X.; Zhang, X. Bionic hierarchical porous aluminum nitride ceramic composite phase change material with excellent heat transfer and storage performance. Compos. Commun. 2021, 27, 100892. [Google Scholar] [CrossRef]
- An, S.; Shi, B.; Jiang, M.; Fu, B.; Song, C.; Tao, P.; Shang, W.; Deng, T. Biological and bioinspired thermal energy regulation and utilization. Chem. Rev. 2023, 123, 7081–7118. [Google Scholar] [CrossRef]
- Zhao, F.; Yuan, W.; Chen, H.; Fu, H.; Li, Z.; Xiao, J.; Feng, Y. Advances in Organic Porous Polymeric-Supported Photothermal Phase Change Materials. Carbon Energy 2025, 7, e719. [Google Scholar] [CrossRef]
- Yu, M.; Li, S.; Zhang, X.; Zhao, Y. Techno-economic analysis of air source heat pump combined with latent thermal energy storage applied for space heating in China. Appl. Therm. Eng. 2021, 185, 116434. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, F.; Yang, L.; Shi, X.; Zhang, G.; Shuai, Y. Thermal performance analysis of PCM capsules packed-bed system with biomimetic leaf hierarchical porous structure. J. Therm. Sci. 2021, 30, 1559–1571. [Google Scholar] [CrossRef]
- Homa, M.; Sornek, K.; Goryl, W.; Papis-Frączek, K.; Żak, P.; Dańko, R. Numerical analysis of the prototype of the high-temperature thermal energy storage based on sand bed. Energy 2025, 333, 137472. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, R.; Jin, X.; Zhang, X.; Bao, G.; Qin, D. Bamboo-derived phase change material with hierarchical structure for thermal energy storage of building. J. Energy Storage 2023, 62, 106911. [Google Scholar] [CrossRef]
- Ding, Q.; Li, R.; Liu, Q.; Cui, W. Human-powered electricity generation: Current technologies, challenges, and potential application in sustainable society construction. Energy Convers. Manag. X 2025, 28, 101239. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Yi, F.; Zi, Y.; Lin, J.; Wang, X.; Xu, Y.; Wang, Z.L. Sustainably powering wearable electronics solely by biomechanical energy. Nat. Commun. 2016, 7, 12744. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Z.L. Toward a new era of sustainable energy: Advanced triboelectric nanogenerator for harvesting high entropy energy. Small 2022, 18, 2107034. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric nanogenerator (TENG)—Sparking an energy and sensor revolution. Adv. Energy Mater. 2020, 10, 2000137. [Google Scholar] [CrossRef]
- Dharmasena, R.D.I.G.; Jayawardena, K.; Mills, C.; Deane, J.; Anguita, J.; Dorey, R.; Silva, S. Triboelectric nanogenerators: Providing a fundamental framework. Energy Environ. Sci. 2017, 10, 1801–1811. [Google Scholar] [CrossRef]
- So, M.Y.; Xu, B. Adaptive Ultra-Low Resilience Woven Triboelectric Nanogenerators for High-Performance Wearable Energy Harvesting and Motion Sensing. Small 2025, 21, 2501116. [Google Scholar] [CrossRef]
- Liu, L.; Guo, X.; Lee, C. Promoting smart cities into the 5G era with multi-field Internet of Things (IoT) applications powered with advanced mechanical energy harvesters. Nano Energy 2021, 88, 106304. [Google Scholar] [CrossRef]
- Rashid, F.L.; Al-Obaidi, M.A.; Dulaimi, A.; Bahlol, H.Y.; Hasan, A. Recent advances, development, and impact of using phase change materials as thermal energy storage in different solar energy systems: A review. Designs 2023, 7, 66. [Google Scholar] [CrossRef]
- Abdullah, M.; Obayedullah, M.; Musfika, S.A. Recent Advances in Phase Change Energy Storage Materials: Developments and Applications. Int. J. Energy Res. 2025, 2025, 6668430. [Google Scholar] [CrossRef]
- Ding, Q.; Cui, W. Stochastic Biomechanical Modeling of Human-Powered Electricity Generation: A Comprehensive Framework with Advanced Monte Carlo Uncertainty Quantification. Energies 2025, 18, 4821. [Google Scholar] [CrossRef]
- Falchetta, G.; Pachauri, S.; Parkinson, S.; Byers, E. A high-resolution gridded dataset to assess electrification in sub-Saharan Africa. Sci. Data 2019, 6, 110. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, Y.; Feng, X.; Naayagi, R.; Soong, B.H. Transactive energy systems in active distribution networks: A comprehensive review. CSEE J. Power Energy Syst. 2022, 8, 1302–1317. [Google Scholar] [CrossRef]
- Ali, S.A.; Habib, K.; Younas, M.; Rahman, S.; Das, L.; Rubbi, F.; Mulk, W.U.; Rezakazemi, M. Advancements in thermal energy storage: A review of material innovations and strategic approaches for phase change materials. Energy Fuels 2024, 38, 19336–19392. [Google Scholar] [CrossRef]
- Wang, G.; Tang, Z.; Gao, Y.; Liu, P.; Li, Y.; Li, A.; Chen, X. Phase change thermal storage materials for interdisciplinary applications. Chem. Rev. 2023, 123, 6953–7024. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Xiong, F.; Aftab, W.; Qin, M.; Zou, R. Emerging solid-to-solid phase-change materials for thermal-energy harvesting, storage, and utilization. Adv. Mater. 2022, 34, 2202457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Tang, Z.; Gao, Y.; Liu, P.; Liu, C.; Chen, X. Flexible engineering of advanced phase change materials. Iscience 2022, 25, 104226. [Google Scholar] [CrossRef]
- Ge, Z.; Ye, F.; Ding, Y. Composite materials for thermal energy storage: Enhancing performance through microstructures. ChemSusChem 2014, 7, 1318–1325. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Wang, K.; Xue, R.; Liu, X.; Yang, Q. Flexible polyolefin elastomer/paraffin wax/alumina/graphene nanoplatelets phase change materials with enhanced thermal conductivity and mechanical performance for solar conversion and thermal energy storage applications. Polymers 2024, 16, 362. [Google Scholar] [CrossRef]
- Qiao, J.; He, C.; Guo, Z.; Lin, F.; Liu, M.; Liu, X.; Liu, Y.; Huang, Z.; Mi, R.; Min, X. Flexible highly thermally conductive PCM film prepared by centrifugal electrospinning for wearable thermal management. Materials 2024, 17, 4963. [Google Scholar] [CrossRef]
- Diao, X.; Li, Y.; Zhao, Z.; Wang, P.; Feng, Y.; Zhao, Z.; Guan, C.; Gao, H.; Zhang, X.; Wang, G. Nickel-Induced Dual Carbon Networks Encapsulating Phase Change Materials for Photothermal Conversion and Storage. ACS Appl. Mater. Interfaces 2024, 16, 66192–66200. [Google Scholar] [CrossRef]
- Yang, D.; Tu, S.; Chen, J.; Zhang, H.; Chen, W.; Hu, D.; Lin, J. Phase change composite microcapsules with low-dimensional thermally conductive nanofillers: Preparation, performance, and applications. Polymers 2023, 15, 1562. [Google Scholar] [CrossRef]
- ASTM D7896-19; Standard Test Method for Thermal Conductivity, Thermal Diffusivity, and Volumetric Heat Capacity of Engine Coolants and Related Fluids by Transient Hot Wire Liquid Thermal Conductivity Method. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 22007-2:2022; Plastics—Determination of Thermal Conductivity and Thermal Diffusivity—Part 2: Transient Plane Heat Source (Hot Disc) Method. ISO: Geneva, Switzerland, 2022.
- Naveenkumar, R.; Ravichandran, M.; Mohanavel, V.; Karthick, A.; Aswin, L.S.R.L.; Priyanka, S.S.H.; Kumar, S.K.; Kumar, S.P. Review on phase change materials for solar energy storage applications. Environ. Sci. Pollut. Res. 2022, 29, 9491–9532. [Google Scholar] [CrossRef]
- Cui, W.; Si, T.; Li, X.; Li, X.; Lu, L.; Ma, T.; Wang, Q. Heat transfer enhancement of phase change materials embedded with metal foam for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2022, 169, 112912. [Google Scholar] [CrossRef]
- Aramesh, M.; Shabani, B. Metal foam-phase change material composites for thermal energy storage: A review of performance parameters. Renew. Sustain. Energy Rev. 2022, 155, 111919. [Google Scholar] [CrossRef]
- Hu, X.; Gong, X.; Zhu, F.; Xing, X.; Li, Z.; Zhang, X. Thermal analysis and optimization of metal foam PCM-based heat sink for thermal management of electronic devices. Renew. Energy 2023, 212, 227–237. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Du, Z.; Yu, J.; Yang, X.; Yan, J. Effect of fin-metal foam structure on thermal energy storage: An experimental study. Renew. Energy 2021, 172, 57–70. [Google Scholar] [CrossRef]
- Baccega, E.; Vallese, L.; Bottarelli, M. Enhancement of thermal conductivity of paraffin pcm with metal foams. Int. J. Thermophys. 2025, 46, 35. [Google Scholar] [CrossRef]
- Wang, C.; Lin, T.; Li, N.; Zheng, H. Heat transfer enhancement of phase change composite material: Copper foam/paraffin. Renew. Energy 2016, 96, 960–965. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.; Yang, B.; Cheng, H.; Wei, P.; He, Y.-L. Design of non-uniformly distributed annular fins for a shell-and-tube thermal energy storage unit. Appl. Energy 2020, 279, 115772. [Google Scholar] [CrossRef]
- Khan, L.A.; Khan, M.M. Role of orientation of fins in performance enhancement of a latent thermal energy storage unit. Appl. Therm. Eng. 2020, 175, 115408. [Google Scholar] [CrossRef]
- Tiari, S.; Hockins, A.; Mahdavi, M. Numerical study of a latent heat thermal energy storage system enhanced by varying fin configurations. Case Stud. Therm. Eng. 2021, 25, 100999. [Google Scholar] [CrossRef]
- Abdulateef, A.M.; Mat, S.; Abdulateef, J.; Sopian, K.; Al-Abidi, A.A. Geometric and design parameters of fins employed for enhancing thermal energy storage systems: A review. Renew. Sustain. Energy Rev. 2018, 82, 1620–1635. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Li, Z.; Wang, G.; Zhao, L.; Yan, Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Xiao, T.; Hu, Z.; He, Y.-L. Design and operating evaluation of a finned shell-and-tube thermal energy storage unit filled with metal foam. Appl. Energy 2020, 261, 114385. [Google Scholar] [CrossRef]
- Voronin, D.V.; Ivanov, E.; Gushchin, P.; Fakhrullin, R.; Vinokurov, V. Clay composites for thermal energy storage: A review. Molecules 2020, 25, 1504. [Google Scholar] [CrossRef]
- Jing, J.-H.; Wu, H.-Y.; Shao, Y.-W.; Qi, X.-D.; Yang, J.-H.; Wang, Y. Melamine foam-supported form-stable phase change materials with simultaneous thermal energy storage and shape memory properties for thermal management of electronic devices. ACS Appl. Mater. Interfaces 2019, 11, 19252–19259. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jia, S.; Niu, Y.; Lv, X.; Fu, H.; Zhang, Y.; Liu, D.; Wang, B.; Li, Q. Bean-pod-inspired 3D-printed phase change microlattices for solar-thermal energy harvesting and storage. Small 2021, 17, 2101093. [Google Scholar] [CrossRef]
- Mu, Y.; Han, M.; Wu, B.; Wang, Y.; Li, Z.; Li, J.; Li, Z.; Wang, S.; Wan, J.; Zeng, L. Nitrogen, oxygen-codoped vertical graphene arrays coated 3D flexible carbon nanofibers with high silicon content as an ultrastable anode for superior lithium storage. Adv. Sci. 2022, 9, 2104685. [Google Scholar] [CrossRef]
- Han, G.G.; Li, H.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Leow, W.R.; Chen, X. Thermal-responsive polymers for enhancing safety of electrochemical storage devices. Adv. Mater. 2018, 30, 1704347. [Google Scholar] [CrossRef] [PubMed]
- Teja, P.N.S.; Gugulothu, S.K.; Sastry, G.R.; Burra, B.; Bhurat, S.S. Numerical analysis of nanomaterial-based sustainable latent heat thermal energy storage system by improving thermal characteristics of phase change material. Environ. Sci. Pollut. Res. 2022, 29, 50937–50950. [Google Scholar] [CrossRef]
- Ge, M.; Lu, Y.; Ercius, P.; Rong, J.; Fang, X.; Mecklenburg, M.; Zhou, C. Large-scale fabrication, 3D tomography, and lithium-ion battery application of porous silicon. Nano Lett. 2014, 14, 261–268. [Google Scholar] [CrossRef]
- Wang, F.; Sun, L.; Zi, W.; Zhao, B.; Du, H. Solution synthesis of porous silicon particles as an anode material for lithium ion batteries. Chem.–A Eur. J. 2019, 25, 9071–9077. [Google Scholar] [CrossRef]
- Wang, F.; Kobina, F. The influence of geological factors and transmission fluids on the exploitation of reservoir geothermal resources: Factor discussion and mechanism analysis. Reserv. Sci. 2025, 1, 3–18. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, G.; Zhang, W.; Zhao, H.; Wang, C.; Qiu, S.; Wei, Y. Diatom-Templated Synthesis of Ordered Meso/Macroporous Hierarchical Materials; Wiley Online Library: Hoboken, NJ, USA, 2006. [Google Scholar]
- Qian, T.; Li, J.; Deng, Y. Pore structure modified diatomite-supported PEG composites for thermal energy storage. Sci. Rep. 2016, 6, 32392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Mao, Y.; Miljkovic, N. Nano-Enhanced Graphite/Phase Change Material/Graphene Composite for Sustainable and Efficient Passive Thermal Management. Adv. Sci. 2024, 11, 2402190. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.K.; Gupta, L.R.; Gehlot, A.; Sikarwar, V.S. Advances in phase change materials and nanomaterials for applications in thermal energy storage. Environ. Sci. Pollut. Res. 2024, 31, 6649–6677. [Google Scholar] [CrossRef]
- Yang, S.; Shi, H.-Y.; Liu, J.; Lai, Y.-Y.; Bayer, Ö.; Fan, L.-W. Supercooled erythritol for high-performance seasonal thermal energy storage. Nat. Commun. 2024, 15, 4948. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xia, K.; Yu, T.; Gao, F.; Zhang, Q.; Zhu, L.; Ye, Z.; Yang, S.; Ma, Y.; Lu, J. Multifunctional smart fabrics with integration of self-cleaning, energy harvesting, and thermal management properties. ACS Nano 2024, 18, 31085–31097. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, T.; Wu, J.; Xu, T.; Wang, X.; Han, X.; Cui, H.; Xu, X.; Pan, C.; Li, X. Energy conversion analysis of multilayered triboelectric nanogenerators for synergistic rain and solar energy harvesting. Adv. Mater. 2022, 34, 2202238. [Google Scholar] [CrossRef]
- Shi, T.; Liu, H.; Wang, X. Unidirectionally structured magnetic phase-change composite based on carbonized polyimide/kevlar nanofiber complex aerogel for boosting solar-thermo-electric energy conversion. ACS Appl. Mater. Interfaces 2024, 16, 10180–10195. [Google Scholar] [CrossRef]
- Tao, Z.; Zou, H.; Li, M.; Ren, S.; Xu, J.; Lin, J.; Yang, M.; Feng, Y.; Wang, G. Polypyrrole coated carbon nanotube aerogel composite phase change materials with enhanced thermal conductivity, high solar-/electro-thermal energy conversion and storage. J. Colloid Interface Sci. 2023, 629, 632–643. [Google Scholar] [CrossRef]
- Lin, L.; Xie, Y.; Niu, S.; Wang, S.; Yang, P.-K.; Wang, Z.L. Robust triboelectric nanogenerator based on rolling electrification and electrostatic induction at an instantaneous energy conversion efficiency of ∼55%. ACS Nano 2015, 9, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Zhu, X.; Tian, Y.; Cheng, J.; Zhang, J. Phase change energy storage material with photocuring, photothermal conversion, and self-cleaning performance via a two-layer structure. ACS Appl. Mater. Interfaces 2022, 14, 57299–57310. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, D.; DasMahapatra, S.; Singh, K. Integration of silicon nanostructures for health and energy applications using MACE: A cost-effective process. Nanotechnology 2024, 35, 423001. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Li, J.; Feng, W.; Nian, H.e. Enhanced thermal conductivity of form-stable phase change composite with single-walled carbon nanotubes for thermal energy storage. Sci. Rep. 2017, 7, 44710. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for energy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Y.; Zhang, J.; Xie, Y.; Guo, H.; He, M.; Shi, X.; Mei, Y.; Sheng, X.; Xie, D. Leakage proof, flame-retardant, and electromagnetic shield wood morphology genetic composite phase change materials for solar thermal energy harvesting. Nano-Micro Lett. 2024, 16, 196. [Google Scholar] [CrossRef]
- Liu, K.; He, Z.; Luo, Y.; Lin, P.; Chen, Y. Massive fabrication of flexible, form-stable, and self-repairing brine phase change material gels toward smart cold chain logistics. ACS Appl. Mater. Interfaces 2023, 15, 17091–17102. [Google Scholar] [CrossRef]
- Leong, K.Y.; Hasbi, S.; Ahmad, K.K.; Jali, N.M.; Ong, H.C.; Din, M.M. Thermal properties evaluation of paraffin wax enhanced with carbon nanotubes as latent heat thermal energy storage. J. Energy Storage 2022, 52, 105027. [Google Scholar] [CrossRef]
- Bharathi, A.L.K.; Manikandan, C.; Bhuvanesh, M.; Kalaiselvam, S. Experimental investigation on the thermal storage performance of nanocomposite-enhanced fatty acid eutectic PCM and the effect of ultrasonic vibration for application in cold storage. J. Energy Storage 2024, 101, 113797. [Google Scholar] [CrossRef]
- Van Ravensteijn, B.G.; Donkers, P.A.; Ruliaman, R.C.; Eversdijk, J.; Fischer, H.R.; Huanan, H.P.; Adan, O.C. Encapsulation of salt hydrates by polymer coatings for low-temperature heat storage applications. ACS Appl. Polym. Mater. 2021, 3, 1712–1726. [Google Scholar] [CrossRef]
- Behera, U.S.; Poddar, S.; Deshmukh, M.P.; Sangwai, J.S.; Byun, H.-S. Comprehensive review on the role of nanoparticles and nanofluids in chemical enhanced oil recovery: Interfacial phenomenon, compatibility, scalability, and economic viability. Energy Fuels 2024, 38, 13760–13795. [Google Scholar] [CrossRef]
- Wadee, A.; Walker, P.; McCullen, N.; Ferrandiz-Mas, V. The effect of thermal cycling on the thermal and chemical stability of paraffin phase change materials (PCMs) composites. Mater. Struct. 2025, 58, 25. [Google Scholar] [CrossRef]
- Weingrill, H.; Resch-Fauster, K.; Lucyshyn, T.; Zauner, C. High-density polyethylene as phase-change material: Long-term stability and aging. Polym. Test. 2019, 76, 433–442. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Yu, Z.; Lam, R.H.; Hui, D.; Lau, D. Molecular dynamics simulations on adhesion of epoxy-silica interface in salt environment. Compos. Part B Eng. 2017, 131, 165–172. [Google Scholar] [CrossRef]
- Awan, M.B.; Ma, Z.; Lin, W.; Pandey, A.; Tyagi, V. A characteristic-oriented strategy for ranking and near-optimal selection of phase change materials for thermal energy storage in building applications. J. Energy Storage 2023, 57, 106301. [Google Scholar] [CrossRef]
- Kemaloglu, S.; Ozkoc, G.; Aytac, A. Properties of thermally conductive micro and nano size boron nitride reinforced silicon rubber composites. Thermochim. Acta 2010, 499, 40–47. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Sehrawat, R.; Sahdev, R.K.; Tiwari, S. Heat storage material: A hope in solar thermal. Environ. Sci. Pollut. Res. 2023, 30, 11175–11198. [Google Scholar] [CrossRef]
- Zhu, C.; Hao, Y.; Wu, H.; Chen, M.; Quan, B.; Liu, S.; Hu, X.; Liu, S.; Ji, Q.; Lu, X. Self-assembly of binderless MXene aerogel for multiple-scenario and responsive phase change composites with ultrahigh thermal energy storage density and exceptional electromagnetic interference shielding. Nano-Micro Lett. 2024, 16, 57. [Google Scholar] [CrossRef]
- Wang, Z.L. From contact electrification to triboelectric nanogenerators. Rep. Prog. Phys. 2021, 84, 096502. [Google Scholar] [CrossRef]
- Wu, H.; Shan, C.; Fu, S.; Li, K.; Wang, J.; Xu, S.; Li, G.; Zhao, Q.; Guo, H.; Hu, C. Efficient energy conversion mechanism and energy storage strategy for triboelectric nanogenerators. Nat. Commun. 2024, 15, 6558. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Zhang, B.; Huang, J. Silver attached graphene-based aerogel composite phase change material and the enhancement of thermal conductivity. Materials 2020, 13, 3271. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, Z.; Cao, X.; Sun, Q.; Yuan, Y.; Li, T. Ultraflexible, cost-effective and scalable polymer-based phase change composites via chemical cross-linking for wearable thermal management. Nat. Commun. 2023, 14, 8060. [Google Scholar] [CrossRef]
- Cui, P.; Li, G.; Zhang, Q. Enhancing energy conversion efficiency of electromagnetic repulsion mechanisms through resistance coefficient optimization model. Sci. Rep. 2024, 14, 26144. [Google Scholar] [CrossRef] [PubMed]
- Saher, S.; Johnston, S.; Esther-Kelvin, R.; Pringle, J.M.; MacFarlane, D.R.; Matuszek, K. Trimodal thermal energy storage material for renewable energy applications. Nature 2024, 636, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tian, H.; Zhang, W.; Zhou, J.-J.; Pang, X. Development of NaCl–MgCl2–CaCl2 ternary salt for high-temperature thermal energy storage using machine learning. ACS Appl. Mater. Interfaces 2023, 16, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Manj, R.Z.A.; Chen, X.; Rehman, W.U.; Zhu, G.; Luo, W.; Yang, J. Big potential from silicon-based porous nanomaterials: In field of energy storage and sensors. Front. Chem. 2018, 6, 539. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Hua, S.; Wang, H. Experimental investigation of grid storage modes effect on aging of LiFePO4 battery modules. Front. Energy Res. 2025, 13, 1528691. [Google Scholar] [CrossRef]
- Sun, S.; Brown, W.; Tan, X.; Rui, B.; Xiong, G.; Wang, Y.; Zeng, D.; Xu, J. Aging matters: How degradation pathways govern thermal runaway in lithium-ion batteries. J. Energy Chem. 2025, in press. [Google Scholar] [CrossRef]
- Kirkaldy, N.; Samieian, M.A.; Offer, G.J.; Marinescu, M.; Patel, Y. Lithium-ion battery degradation: Comprehensive cycle ageing data and analysis for commercial 21700 cells. J. Power Sources 2024, 603, 234185. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of heterostructure materials in energy storage: A review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, Y.; Du, M.; Hou, X.; Liu, J.; Ke, H.; Wei, Q. Ultralight and flexible carbon foam-based phase change composites with high latent-heat capacity and photothermal conversion capability. ACS Appl. Mater. Interfaces 2019, 11, 31997–32007. [Google Scholar] [CrossRef]
- Su, H.; Lin, P.; Li, D.; Chen, Y. Reduced graphene oxide/cellulose sodium aerogel-supported eutectic phase change material gel demonstrating superior energy conversion and storage capacity toward high-performance personal thermal management. ACS Appl. Mater. Interfaces 2024, 16, 3334–3347. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Li, J.; Li, X. Preparation and performance of solid thermal energy storage materials based on low-grade pyrophyllite minerals. Heliyon 2024, 10, e26871. [Google Scholar] [CrossRef]
- Rahman, U.U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.M.; Khan, A. MXenes as emerging materials: Synthesis, properties, and applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Swoboda, T.; Klinar, K.; Abbasi, S.; Brem, G.; Kitanovski, A.; Rojo, M.M. Thermal rectification in multilayer phase change material structures for energy storage applications. Iscience 2021, 24, 102843. [Google Scholar] [CrossRef]
- Zhou, J.; Fei, H.; He, Q.; Li, P.; Pan, Y.; Liang, X. Structural characteristics and thermal performances of lauric-myristic-palmitic acid introduced into modified water hyacinth porous biochar for thermal energy storage. Sci. Total Environ. 2023, 882, 163670. [Google Scholar] [CrossRef]
- Wu, J.; Ansari, U. From CO2 Sequestration to Hydrogen Storage: Further Utilization of Depleted Gas Reservoirs. Reserv. Sci. 2025, 1, 19–35. [Google Scholar] [CrossRef]
- Kelsall, C.C.; Buznitsky, K.; Henry, A. Technoeconomic analysis of thermal energy grid storage using graphite and tin. arXiv 2021, arXiv:2106.07624. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Ji, R.; Zhang, H.; Xu, F.; Huang, P.; Zou, Y.; Chu, H.; Lin, X.; Sun, L. Multielement synergetic effect of boron nitride and multiwalled carbon nanotubes for the fabrication of novel shape-stabilized phase-change composites with enhanced thermal conductivity. ACS Appl. Mater. Interfaces 2020, 12, 41398–41409. [Google Scholar] [CrossRef]
- Liang, R.; Yuan, B.; Zhang, F.; Feng, W. Azopyridine polymers in organic phase change materials for high energy density photothermal storage and controlled release. Angew. Chem. 2025, 137, e202419165. [Google Scholar] [CrossRef]
- Aftab, W.; Shi, J.; Jin, Y.; Usman, A.; Qin, M.; Ashraf, Z.; Shen, Z.; Zhong, R.; Zou, R. Phase engineered composite phase change materials for thermal energy manipulation. Small 2024, 20, 2312134. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Lee, D.-S.; Chen, W.-H.; Lin, Y.-L.; Luo, D.; Park, Y.-K.; Bandala, A. An overview of commercialization and marketization of thermoelectric generators for low-temperature waste heat recovery. Iscience 2023, 26, 107874. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Fayaz, M.; Ayoub, N.; Islam, I.; Ghosh, S.; Rubab, S.; Khandy, S.A. Oxide thermoelectric materials: A review of emerging strategies for efficient waste heat recovery. J. Power Sources 2025, 654, 237806. [Google Scholar] [CrossRef]
- Bu, Z.; Zhang, X.; Hu, Y.; Chen, Z.; Lin, S.; Li, W.; Xiao, C.; Pei, Y. A record thermoelectric efficiency in tellurium-free modules for low-grade waste heat recovery. Nat. Commun. 2022, 13, 237. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, P.; Liu, R.; Yuan, L.; Meng, X.; Tao, G.; Chen, J.; Ran, Q.; Hong, J.; Liu, J. Integration of zinc anode and cement: Unlocking scalable energy storage. Natl. Sci. Rev. 2024, 11, nwae309. [Google Scholar] [CrossRef]
- Kalantar-Neyestanaki, H.; Soltani, M. Uncertainty-cognizant model predictive control for energy management of residential buildings with pvt and thermal energy storage. arXiv 2022, arXiv:2201.08909. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Lee, J.-H.; Kim, C.-H.; Gautam, J.; Heo, K.; Hussain, S.; Ikram, M.; AlObaid, A.A.; Lee, S.-Y. A review of rechargeable zinc–air batteries: Recent progress and future perspectives. Nano-Micro Lett. 2024, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Farakhor, A.; Wu, D.; Wang, Y.; Fang, H. A novel modular, reconfigurable battery energy storage system: Design, control, and experimentation. IEEE Trans. Transp. Electrif. 2022, 9, 2878–2890. [Google Scholar] [CrossRef]
- Shuai, H.; Li, F.; Pulgar-Painemal, H.; Xue, Y. Branching dueling Q-network-based online scheduling of a microgrid with distributed energy storage systems. IEEE Trans. Smart Grid 2021, 12, 5479–5482. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.; Zhang, X.; Zhu, Y.; Chen, H. Finite-time thermodynamics modeling and analysis on compressed air energy storage systems with thermal storage. Renew. Sustain. Energy Rev. 2021, 138, 110656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Zeng, L.; Zeng, Y.; Song, C.; Lei, L.; Cui, W. Sand-Based Thermal Storage System for Human-Powered Energy Generation: A Review. Energies 2025, 18, 5869. https://doi.org/10.3390/en18225869
Ding Q, Zeng L, Zeng Y, Song C, Lei L, Cui W. Sand-Based Thermal Storage System for Human-Powered Energy Generation: A Review. Energies. 2025; 18(22):5869. https://doi.org/10.3390/en18225869
Chicago/Turabian StyleDing, Qirui, Lili Zeng, Ying Zeng, Changhui Song, Liang Lei, and Weicheng Cui. 2025. "Sand-Based Thermal Storage System for Human-Powered Energy Generation: A Review" Energies 18, no. 22: 5869. https://doi.org/10.3390/en18225869
APA StyleDing, Q., Zeng, L., Zeng, Y., Song, C., Lei, L., & Cui, W. (2025). Sand-Based Thermal Storage System for Human-Powered Energy Generation: A Review. Energies, 18(22), 5869. https://doi.org/10.3390/en18225869








