Can Hydrogen Be Produced Cost-Effectively from Heavy Oil Reservoirs?
Abstract
1. Introduction
2. Methodology
2.1. Reservoir Modeling
2.1.1. Reservoir Model
2.1.2. Fluid Model
2.1.3. Reaction Kinetics Model
2.1.4. Injection Strategy
2.1.5. Grid Configuration Selection Justification
2.1.6. Reservoir Model Validation
2.2. Economic Modeling
2.2.1. Injection Cost Components
Lease Equipment Costs for Injection Well
Annual Operating and Maintenance Costs
CO2 Supply and Conveying Costs
Air Source and Cost
2.2.2. Carbon Dioxide (CO2) Cost Components
CO2 Source and Cost
Calculation of Injection Pressure and CO2 Pressurizing Expenses
2.2.3. Water Cost Components
Water Procurement Cost
Steam/Air Injection Cost
2.2.4. Production Cost Components
Cost of Production Equipment
Cost of Fluid Lifting
Cost of Syngas and Liquid Separation
Cost of Separating Oil and Water Mixture
Production Revenue, Tax and Royalty Separation
2.2.5. Carbon Dioxide Recycling Cost Components
Gas Treatment: Gas Separation and Compression
2.2.6. Parameters Utilized in the Economic Modeling
2.2.7. Reservoir Classification Process
3. Results and Discussion
3.1. Technical Aspects of Hydrogen Generation
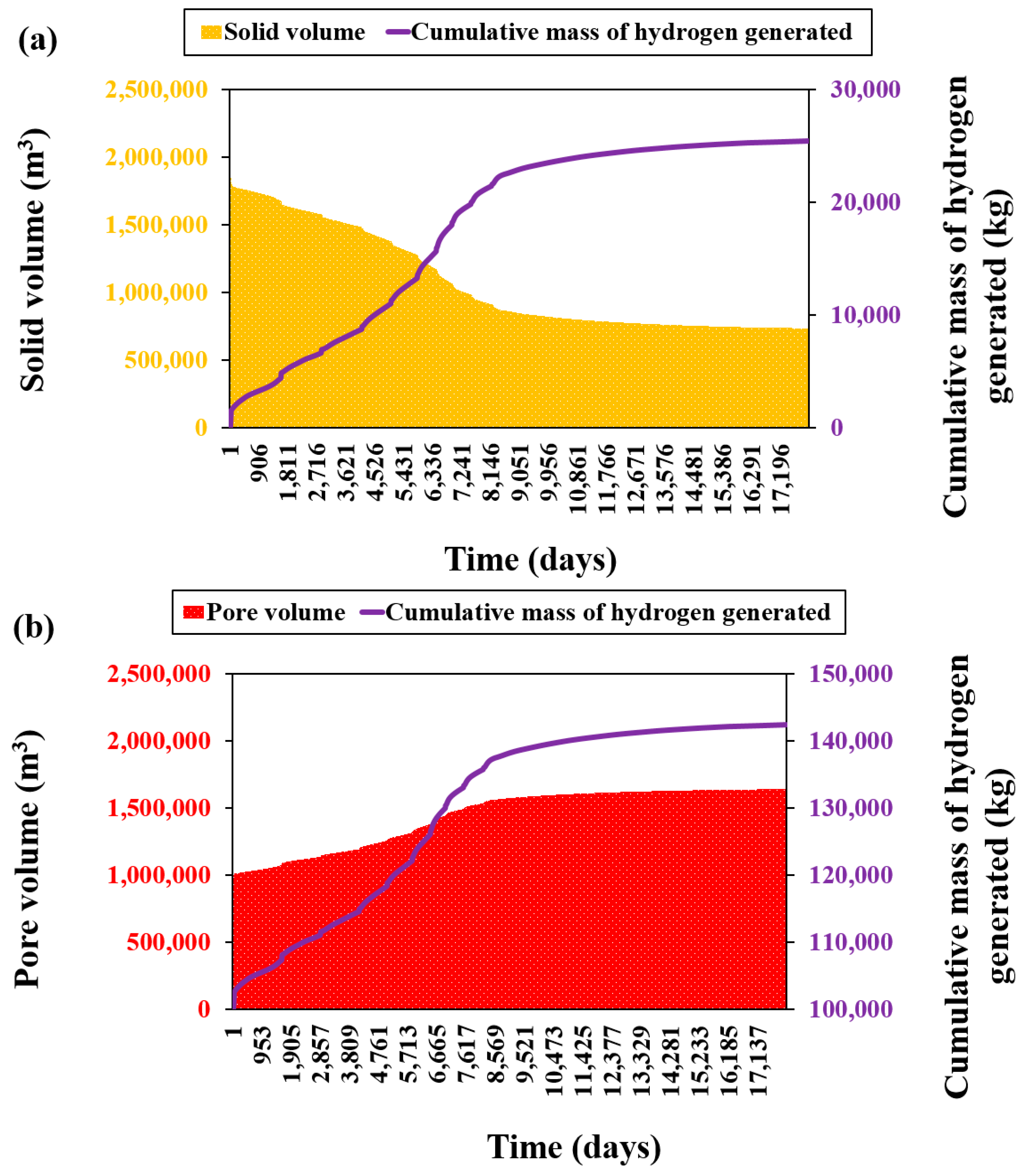
3.1.1. Analysis of Hydrogen Yield and Gas Composition
Impact of Injection Strategy on Hydrogen Generation
Temperature Dependence of Hydrogen Generation
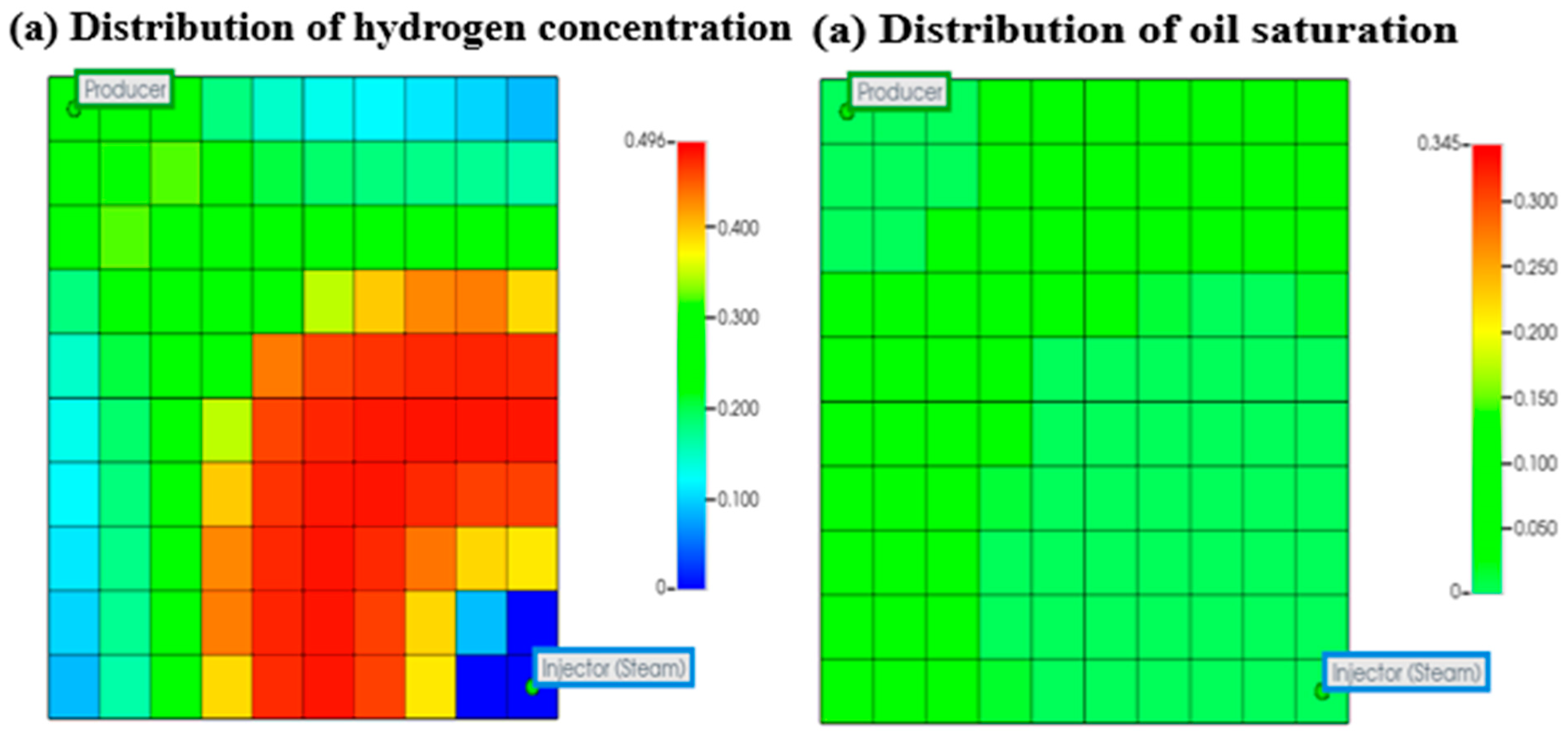
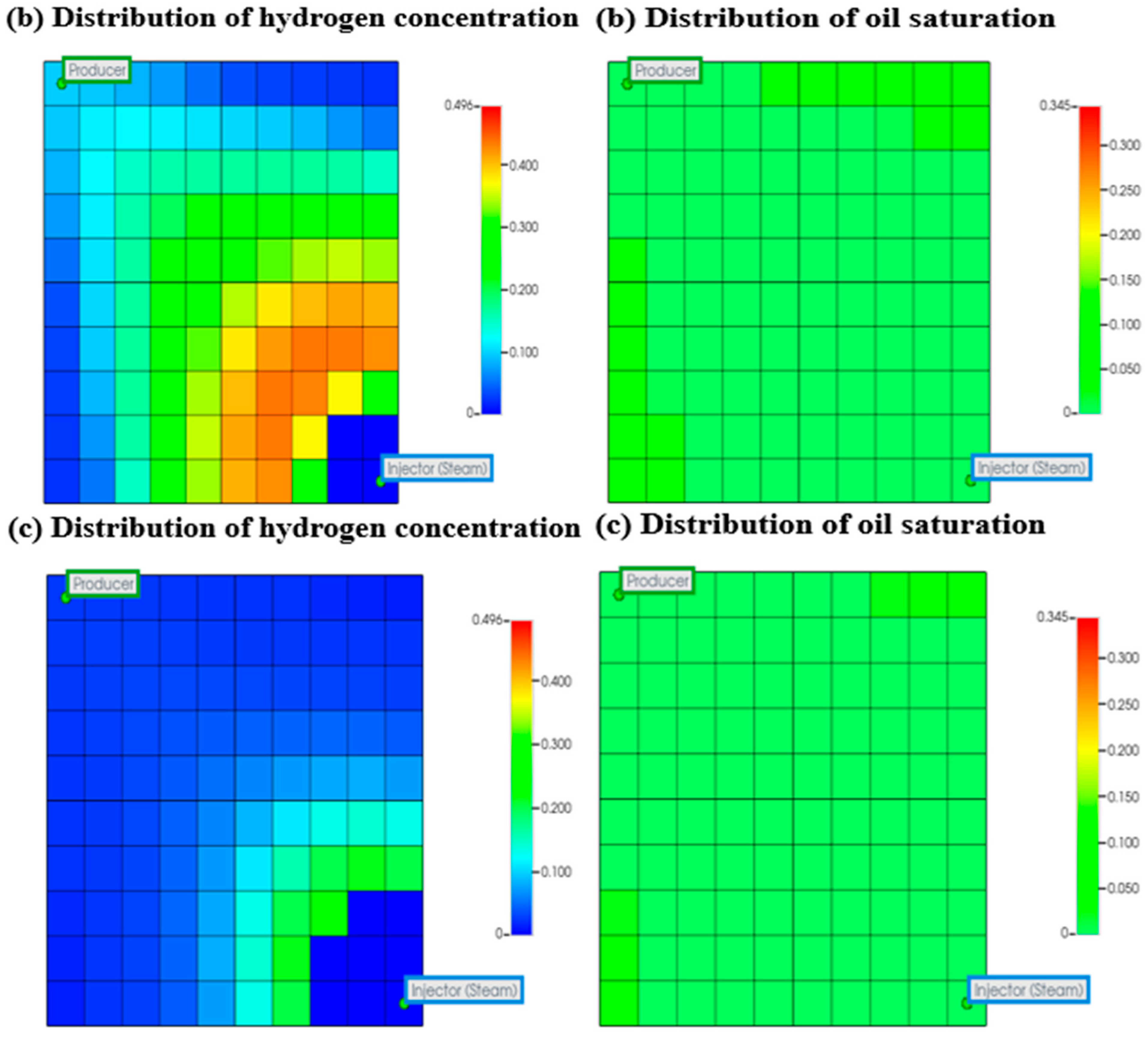
3.1.2. Analysis of Combustion Front Propagation and Hydrogen Diffusion
Impact of Combustion Front Propagation on Hydrogen Generation
Impact of Underground Hydrogen Migration and Diffusion on Hydrogen Generation
3.1.3. Impact of Formation Damage on Hydrogen Generation
3.2. Economic Aspects of Hydrogen Production
3.2.1. Cost and Profitability Analysis of Hydrogen Production
Capital and Operating Costs
- ▪
- Capital expenditures (CAPEX): Total initial capital expenditures for the infrastructure required for hydrogen production are estimated at US$ 340 million. This covers:
- US$ 245 million for the construction of air separation units (ASUs);
- US$ 65 million for hydrogen purification systems;
- US$ 20 million for hydrogen pipeline infrastructure;
- US$ 10 million for production well development.
- ▪
- Operating expenditures (OPEX): Annual operating costs, which include labor, maintenance, and administrative expenses, are estimated at US$ 1.8 million per year, broken down as follows:
- US$ 1.37 million for labor and staffing;
- US$ 0.4 million for administrative and operational expenses;
- US$ 0.03 million for facility insurance.
Revenue from Hydrogen Production
Cost per Kilogram of Hydrogen Produced
- ▪
- Cyclic steam/air injection: With a total hydrogen production of 1,818,836 kg, the cost per kilogram of hydrogen is:
- ▪
- CO2 + O2 injection: With a total hydrogen production of 701,928 kg, the cost per kilogram of hydrogen is:
Economic Comparison with Competing Methods
Opportunities for Cost Reduction
3.2.2. Breakeven Analysis
3.3. Comparative Techno-Economic Analysis of Hydrogen and Oil Recovery Strategies
3.4. Discussion
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Wang, L.; Chen, W.; Zhang, C. Assessing the impact of hydrogen trade towards low-carbon energy transition. Appl. Energy 2024, 376, 124233. [Google Scholar] [CrossRef]
- Hille, E. Europe’s energy crisis: Are geopolitical risks in source countries of fossil fuels accelerating the transition to renewable energy? Energy Econ. 2023, 127, 107061. [Google Scholar] [CrossRef]
- Zaiter, I.; Ramadan, M.; Bouabid, A.; El-Fadel, M.; Mezher, T. Potential utilization of hydrogen in the UAE’s industrial sector. Energy 2023, 280, 128108. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J.; Ejike, C. Evaluating reservoir suitability for large-scale hydrogen storage: A preliminary assessment considering reservoir properties. Energy Geosci. 2024, 5, 100318. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Ajori, S.; Taghikhani, V.; Moghanloo, R.G.; Yoo, C.K. Sustainable hydrogen production from flare gas and produced water: A United States case study. Energy 2024, 306, 132435. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J. Review on clean hydrogen generation from petroleum reservoirs: Fundamentals, mechanisms, and field applications. Int. J. Hydrogen Energy 2023, 48, 38188–38222. [Google Scholar] [CrossRef]
- Smith, E.K.; Barakat, S.M.; Akande, O.; Ogbaga, C.C.; Okoye, P.U.; Okolie, J.A. Subsurface combustion and gasification for hydrogen production: Reaction mechanism, techno-economic and lifecycle assessment. Chem. Eng. J. 2023, 480, 148095. [Google Scholar] [CrossRef]
- Ikpeka, P.; Alozieuwa, E.; Duru, U.I.; Ugwu, J. A parametric study on in-situ hydrogen production from hydrocarbon reservoirs—Effect of reservoir and well properties. Int. J. Hydrogen Energy 2024, 80, 733–742. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J. Probing the Mechanism and Impact of Light Oil Oxidation on In Situ Hydrogen Production from Petroleum Reservoirs: A Combined SARA-Based Experimental and Numerical Investigation. Energy Fuels 2024, 38, 17649–17661. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J.; Ikpeka, P.M. Comparative Numerical Study on the Optimization of Double-Simultaneous Gas Injection Techniques for Enhanced Hydrogen Production from Depleted Light Oil Reservoirs. Energy Fuels 2024, 38, 16358–16370. [Google Scholar] [CrossRef]
- Hanfi, M.A.; Alade, O.S.; Tanimu, A.; Mahmoud, M.; Alarifi, S.A. Catalytic and Noncatalytic in Situ Hydrogen Production from Heavy Oil: A Review of Experimental Studies. ACS Omega 2024, 9, 50118–50133. [Google Scholar] [CrossRef]
- Alkan, H.; Bauer, J.F.; Burachok, O.; Kowollik, P.; Olbricht, M.; Amro, M. Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective. Appl. Sci. 2024, 14, 6217. [Google Scholar] [CrossRef]
- Johnson, L.A.; Fahy, L.J.; Romanowski, L.J.; Barbour, R.V.; Thomas, K.P. An Echoing In-Situ Combustion Oil Recovery Project in a Utah Tar Sand. J. Pet. Technol. 1980, 32, 295–305. [Google Scholar] [CrossRef]
- Hajdo, L.E.; Hallam, R.J.; Vorndran, L.D.L. Hydrogen Generation During In-Situ Combustion. In Proceedings of the Society of Petroleum Engineers—SPE California Regional Meeting, CRM, Bakersfield, CA, USA, 27–29 March 1985; pp. 675–689. [Google Scholar] [CrossRef]
- Hallam, R.J.; Hajdo, L.E.; Donnelly, J.K.; Baron, R.P. Thermal Recovery of Bitumen at Wolf Lake. SPE Reserv. Eng. 1989, 4, 178–186. [Google Scholar] [CrossRef]
- Innovative Energy Technologies Program Project 01-019: Whitesands Experimental Project—Open Government. n.d. Available online: https://open.alberta.ca/dataset/ietp-project-01-019 (accessed on 21 June 2024).
- Kerrobert THAI Public Data. n.d. Available online: https://petrobank.ca/sf/thai/thai.htm (accessed on 21 June 2024).
- Turta, A.; Singhal, A.; Sierra, R.; Greaves, M.; Islam, M. Evaluation of the First Field Piloting of the THAI Process: Athabasca Pilot. In Proceedings of the Society of Petroleum Engineers—SPE Canadian Energy Technology Conference and Exhibition, CET, Calgary, AB, Canada, 15–16 March 2023; pp. 15–16. [Google Scholar] [CrossRef]
- Sheng, J.J. Techno-Economic Analysis of Hydrogen Generation in Hydrocarbon Reservoirs. SPE J. 2024, 29, 5752–5760. [Google Scholar] [CrossRef]
- Alkan, H.; Fabian Bauer, J.; Burachok, O.; Kowollik, P.; Olbricht, M.; Amro, M. Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: Is it feasible? In Proceedings of the Fourth EAGE Global Energy Transition Conference and Exhibition, Paris, France, 14–17 November 2023; Volume 2023, pp. 1–5. [Google Scholar] [CrossRef]
- Belgrave, J.D.M.; Moore, M.G.; Ursenbach, M.G.; Bennion, D.W. A Comprehensive Approach to In-Situ Combustion Modeling. SPE Adv. Technol. Ser. 1993, 1, 98–107. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J. A new modelling approach for in-situ hydrogen production from heavy oil reservoirs: Sensitivity analysis and process mechanisms. Energy 2024, 302, 131817. [Google Scholar] [CrossRef]
- Yang, S.; Huang, S.; Jiang, Q.; Yu, C.; Zhou, X. Experimental study of hydrogen generation from in-situ heavy oil gasification. Fuel 2022, 313, 122640. [Google Scholar] [CrossRef]
- Afanasev, P.; Smirnov, A.; Ulyanova, A.; Popov, E.; Cheremisin, A. Experimental Study of Catalytically Enhanced Cyclic Steam-Air Stimulation for In Situ Hydrogen Generation and Heavy Oil Upgrading. Catalysts 2023, 13, 1172. [Google Scholar] [CrossRef]
- He, H.; Li, Q.; Tang, J.; Liu, P.; Zheng, H.; Zhao, F.; Guan, W.; Guo, E.; Xi, C. Study of hydrogen generation from heavy oil gasification based on ramped temperature oxidation experiments. Int. J. Hydrogen Energy 2023, 48, 2161–2170. [Google Scholar] [CrossRef]
- Song, P.; Li, Y.; Yin, Z.; Ifticene, M.A.; Yuan, Q. Simulation of hydrogen generation via in-situ combustion gasification of heavy oil. Int. J. Hydrogen Energy 2024, 49, 925–936. [Google Scholar] [CrossRef]
- Newman, L.L. Oxygen in Production of Hydrogen or Synthesis Gas. Ind. Eng. Chem. 2002, 40, 559–582. [Google Scholar] [CrossRef]
- Parry, V.F.; Wagner, E.; Koth, A.; Goodman, J. Gasification of Subbituminous Coal and Lignite in Externally Heated Retorts. Ind. Eng. Chem. 2002, 40, 627–641. [Google Scholar] [CrossRef]
- Wright, C.C.; Barclay, K.M.; Mitchell, R.F. Production of Hydrogen and Synthesis Gas by Oxygen Gasification of Solid Fuel. Ind. Eng. Chem. 2002, 40, 592–600. [Google Scholar] [CrossRef]
- Eastman, D. Synthesis Gas by Partial Oxidation. Ind. Eng. Chem. 2002, 48, 1118–1122. [Google Scholar] [CrossRef]
- Eastman, D. 13. The Production of Synthesis Gas by Partial Oxidation 1959. In Proceedings of the 5th World Petroleum Congress, New York, NY, USA, 30 May–5 June 1959. [Google Scholar]
- Pichler, H. The Oil Gasification by Internal Heating in Continuous Process 1967. In Proceedings of the 7th World Petroleum Congress, Mexico City, Mexico, 2–9 April 1967. [Google Scholar]
- Jones, J.M. Hydrogen Production by Partial Oxidation 1971. In Proceedings of the 8th World Petroleum Congress, Moscow, Russia, 13–18 June 1971. [Google Scholar]
- Strelzoff, S. Partial Oxidation for Syngas and Fuel. Hydrocarb. Process 1974, 53, 79–87. [Google Scholar]
- Reed, C.L.; Kuhre, C.J. Hydrogen Production from Partial Oxidation of Residual Fuel Oil. ACS Symp. Ser. 1980, 116, 95–121. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Leskiw, C.; Gates, I.D. Potential for Hydrogen Generation during In Situ Combustion of Bitumen. In Proceedings of the 71st European Association of Geoscientists and Engineers Conference and Exhibition 2009: Balancing Global Resources Incorporating SPE EUROPEC, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar] [CrossRef]
- Sadeghbeigi, R. Fluid Catalytic Cracking Handbook: An Expert Guide to the Practical Operation, Design, and Optimization of FCC Units, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2012; 361p. [Google Scholar] [CrossRef]
- Tayari, F.; Blumsack, S.; Dilmore, R.; Mohaghegh, S.D. Techno-economic assessment of industrial CO2 storage in depleted shale gas reservoirs. J. Unconv. Oil Gas. Resour. 2015, 11, 82–94. [Google Scholar] [CrossRef]
- Tayari, F.; Blumsack, S.; Johns, R.T.; Tham, S.; Ghosh, S. Techno-economic assessment of reservoir heterogeneity and permeability variation on economic value of enhanced oil recovery by gas and foam flooding. J. Pet. Sci. Eng. 2018, 166, 913–923. [Google Scholar] [CrossRef]
- ProtonH2’s Patented ISHGTM Process—ProtonH2. n.d. Available online: https://protonh2.com/our-process/ (accessed on 10 June 2024).
- Thermophysical Properties of Fluid Systems. n.d. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 15 June 2024).
- Sigurdardottir, S.B.; DuChanois, R.M.; Epsztein, R.; Pinelo, M.; Elimelech, M. Energy barriers to anion transport in polyelectrolyte multilayer nanofiltration membranes: Role of intra-pore diffusion. J. Memb. Sci. 2020, 603, 117921. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, M.K.; Lee, J.Y.; Lee, Y.M.; Zoh, K.D. Degradation kinetics and pathway of 1H-benzotriazole during UV/chlorination process. Chem. Eng. J. 2019, 359, 1502–1508. [Google Scholar] [CrossRef]
- Ling, H.; Liu, S.; Gao, H.; Liang, Z. Effect of heat-stable salts on absorption/desorption performance of aqueous monoethanolamine (MEA) solution during carbon dioxide capture process. Sep. Purif. Technol. 2019, 212, 822–833. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Fang, J.; Ding, L.X.; Wang, H. A nano-silica modified polyimide nanofiber separator with enhanced thermal and wetting properties for high safety lithium-ion batteries. J. Memb. Sci. 2017, 537, 248–254. [Google Scholar] [CrossRef]
- Oil & Gas Market Analysis and Trends|S&P Global. n.d. Available online: https://www.spglobal.com/commodityinsights/en/ci/industry/oil-gas.html (accessed on 16 June 2024).
- U.S. Department of Energy. DOE National Clean Hydrogen Strategy and Roadmap. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/clean-hydrogen-strategy-roadmap.pdf?Status=Master (accessed on 20 December 2024).
- Vickers, J.; Peterson, D.; Randolph, K.; Irwin, L.; Desantis, D.; Hamdan, M. DOE Hydrogen and Fuel Cells Program Record Title: Cost of Electrolytic Hydrogen Production with Existing Technology 2000. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/20004-cost-electrolytic-hydrogen-production.pdf?Status=Master (accessed on 20 December 2024).
- Mulder, M. Economic Analysis of Energy Markets: An Introduction. Lect. Notes Energy 2021, 80, 1–11. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Bölük, G.; Kaplan, R. Effectiveness of renewable energy incentives on sustainability: Evidence from dynamic panel data analysis for the EU countries and Turkey. Environ. Sci. Pollut. Res. 2022, 29, 26613–26630. [Google Scholar] [CrossRef] [PubMed]
- Qadir, S.A.; Al-Motairi, H.; Tahir, F.; Al-Fagih, L. Incentives and strategies for financing the renewable energy transition: A review. Energy Rep. 2021, 7, 3590–3606. [Google Scholar] [CrossRef]
- Dilmore, R.M. An Assessment of Gate-To-Gate Environmental Life Cycle Performance of Water-Alternating-Gas CO2-Enhanced Oil Recovery in the Permian Basin; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2010. [CrossRef]
- Ferguson, R.C.; Nichols, C.; Van Leeuwen, T.; Kuuskraa, V.A. Storing CO2 with enhanced oil recovery. Energy Procedia 2009, 1, 1989–1996. [Google Scholar] [CrossRef]
- Maja, A.; Domagoj, V.; Gabriela, G.L.; Lucija, J. Simulation Analysis of CO2-EOR Process and Feasibility of CO2 Storage during EOR. Energies 2021, 14, 1154. [Google Scholar] [CrossRef]
- McCollum, D.L.; Ogden, J.M. UC Davis Research Reports Title Techno-Economic Models for Carbon Dioxide Compression, Transport, and Storage and Correlations for Estimating Carbon Dioxide Density and Viscosity Permalink. Available online: https://escholarship.org/uc/item/1zg00532 (accessed on 20 December 2024).
- Chen, F.; Morosuk, T. Exergetic and Economic Evaluation of CO2 Liquefaction Processes. Energies 2021, 14, 7174. [Google Scholar] [CrossRef]
- Electricity Cost in Texas: 2024 Electric Rates|EnergySage. n.d. Available online: https://www.energysage.com/local-data/electricity-cost/tx/ (accessed on 15 June 2024).
- Dake, L.P. Fundamentals of Reservoir Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 8, 464p. [Google Scholar]
- Ahmed, T. Reservoir Engineering Handbook, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Green, D.W.; Willhite, G.P. Introduction to Eor Processes. In Enhanced Oil Recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Gluyas, J.G. Swarbrick REdward. In Petroleum Geoscience; Wiley: Hoboken, NJ, USA, 2004; p. 359. [Google Scholar]
- Yuan, Q.; Jie, X.; Ren, B. High-Purity, CO2-Free Hydrogen Generation from Crude Oils in Crushed Rocks Using Microwave Heating. In Proceedings of the SPE Annual Technical Conference and Exhibition 2021, Dubai, United Arab Emirates, 21–23 September 2021. [Google Scholar] [CrossRef]
- Song, P.; Li, Y.; Yin, Z.; Yuan, Q. Hydrogen Generation from Heavy Oils via In-Situ Combustion Gasification. In Proceedings of the SPE Western Regional Meeting Proceedings 2023, Anchorage, AK, USA, 22–25 May 2023. [Google Scholar] [CrossRef]
- Williams, J.M.; Bourtsalas, A.C. Assessment of Co-Gasification Methods for Hydrogen Production from Biomass and Plastic Wastes. Energies 2023, 16, 7548. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Jia, H.; Pu, W.F.; Wang, L.L.; Peng, H. Sensitivity studies on the oxidation behavior of crude oil in porous media. Energy Fuels 2012, 26, 6815–6823. [Google Scholar] [CrossRef]
- Sheng, J. An analytical model to estimate the oxidation time required to reach spontaneous ignition with heat loss being considered. Pet. Sci. Technol. 2024, 43, 662–675. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, E. An Analytical model to estimate the time delay to reach spontaneous ignition considering heat loss in oil reservoirs. Pet. Sci. 2024, 21, 2469–2474. [Google Scholar] [CrossRef]
- Abu-Khamsin, S.A.; Iddris, A.; Aggour, M.A. The spontaneous ignition potential of a super-light crude oil. Fuel 2001, 80, 1415–1420. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, C.-Y.; Pu, W.-F.; Chen, Q.-Y.; Yuan, C.-D.; Varfolomeev, M.A.; Sudakov, V. Heat front propagation in shale oil reservoirs during air injection: Experimental and numerical studies. Pet. Sci. 2024, 21, 3379–3389. [Google Scholar] [CrossRef]
- Burger, J.G. Spontaneous Ignition in Oil Reservoirs. Soc. Pet. Eng. J. 1976, 16, 73–81. [Google Scholar] [CrossRef]
- Wu, C.; Fulton, P. Experimental Simulation of the Zones Preceding the Combustion Front of an In-Situ Combustion Process. Soc. Pet. Eng. J. 1971, 11, 38–46. [Google Scholar] [CrossRef]
- Storey, B.M.; Worden, R.H.; McNamara, D.D. The Geoscience of In-Situ Combustion and High-Pressure Air Injection. Geosciences 2022, 12, 340. [Google Scholar] [CrossRef]
- Lysyy, M.; Føyen, T.; Johannesen, E.B.; Fernø, M.; Ersland, G. Hydrogen Relative Permeability Hysteresis in Underground Storage. Geophys. Res. Lett. 2022, 49, e2022GL100364. [Google Scholar] [CrossRef]
- Zhang, Y.; Bijeljic, B.; Gao, Y.; Goodarzi, S.; Foroughi, S.; Blunt, M.J. Pore-Scale Observations of Hydrogen Trapping and Migration in Porous Rock: Demonstrating the Effect of Ostwald Ripening. Geophys. Res. Lett. 2023, 50, e2022GL102383. [Google Scholar] [CrossRef]
- Thaysen, E.M.; Jangda, Z.; Hassanpouryouzband, A.; Menke, H.; Singh, K.; Butler, I.B.; Heinemann, N.; Edlmann, K. Hydrogen wettability and capillary pressure in Clashach sandstone for underground hydrogen storage. J. Energy Storage 2024, 97, 112916. [Google Scholar] [CrossRef]
- Zeng, L.; Hosseini, M.; Keshavarz, A.; Iglauer, S.; Lu, Y.; Xie, Q. Hydrogen wettability in carbonate reservoirs: Implication for underground hydrogen storage from geochemical perspective. Int. J. Hydrogen Energy 2022, 47, 25357–25366. [Google Scholar] [CrossRef]
- Saajanlehto, M.; Uusi-Kyyny, P.; Alopaeus, V. Hydrogen solubility in heavy oil systems: Experiments and modeling. Fuel 2014, 137, 393–404. [Google Scholar] [CrossRef]
- Al-Abduwani, F.A.H.; Shirzadi, A.; van den Broek, W.M.G.T.; Currie, P.K. Formation Damage vs. Solid Particles Deposition Profile During Laboratory-Simulated Produced-Water Reinjection. SPE J. 2005, 10, 138–151. [Google Scholar] [CrossRef]
- Chang, F.; Civan, F. Modeling of Formation Damage Due to Physical and Chemical Interactions Between Fluids and Reservoir Rocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991. [Google Scholar] [CrossRef]
- Tan, Q.; You, L.; Kang, Y.; Xu, C. Formation damage mechanisms in tight carbonate reservoirs: The typical illustrations in Qaidam Basin and Sichuan Basin, China. J. Nat. Gas. Sci. Eng. 2021, 95, 104193. [Google Scholar] [CrossRef]
- He, J.; Okere, C.J.; Su, G.; Hu, P.; Zhang, L.; Xiong, W.; Li, Z. Formation damage mitigation mechanism for coalbed methane wells via refracturing with fuzzy-ball fluid as temporary blocking agents. J. Nat. Gas. Sci. Eng. 2021, 90, 103956. [Google Scholar] [CrossRef]
- Liu, H.; Su, G.; Okere, C.J.; Li, G.; Wang, X.; Cai, Y.; Wu, T.; Zheng, L. Working fluid-induced formation damage evaluation for commingled production of multi-layer natural gas reservoirs with flow rate method. Energy 2022, 239, 122107. [Google Scholar] [CrossRef]
- Okere, C.J.; Su, G.; Zheng, L.; Cai, Y.; Li, Z.; Liu, H. Experimental, algorithmic, and theoretical analyses for selecting an optimal laboratory method to evaluate working fluid damage in coal bed methane reservoirs. Fuel 2020, 282, 118513. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J.; Fan, L.-K.; Huang, X.-W.; Zheng, L.-H.; Wei, P.-F. Experimental study on the degree and damage-control mechanisms of fuzzy-ball-induced damage in single and multi-layer commingled tight reservoirs. Pet. Sci. 2023, 20, 3598–3609. [Google Scholar] [CrossRef]
- Okere, C.J.; Zheng, L. Experimental Study on the Mechanisms and Degree of Fuzzy-Ball Induced Damage in Single and Double-Layer Tight Reservoirs: Implications for Optimal Laboratory Design and Strategic Field Application of Low-Damaging Fluids. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Shojaee, A.; Kord, S.; Miri, R.; Mohammadzadeh, O. Reactive transport modeling of scale precipitation and deposition during incompatible water injection in carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2024, 14, 515–534. [Google Scholar] [CrossRef]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Economics—SGH2 Energy. n.d. Available online: https://www.sgh2energy.com/economics (accessed on 8 December 2024).
- Hydrogen Electrolysis: The Future of Cost-Effective Green Hydrogen Production|Energy Central. n.d. Available online: https://energycentral.com/c/pip/hydrogen-electrolysis-future-cost-effective-green-hydrogen-production (accessed on 8 December 2024).
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of hydrogen production costs in Steam-Methane Reforming considering integration with electrolysis and CO2 capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J.; Ikpeka, P.M. Which Offers Greater Techno-Economic Potential: Oil or Hydrogen Production from Light Oil Reservoirs? Geoscience 2025, 15, 214. [Google Scholar] [CrossRef]
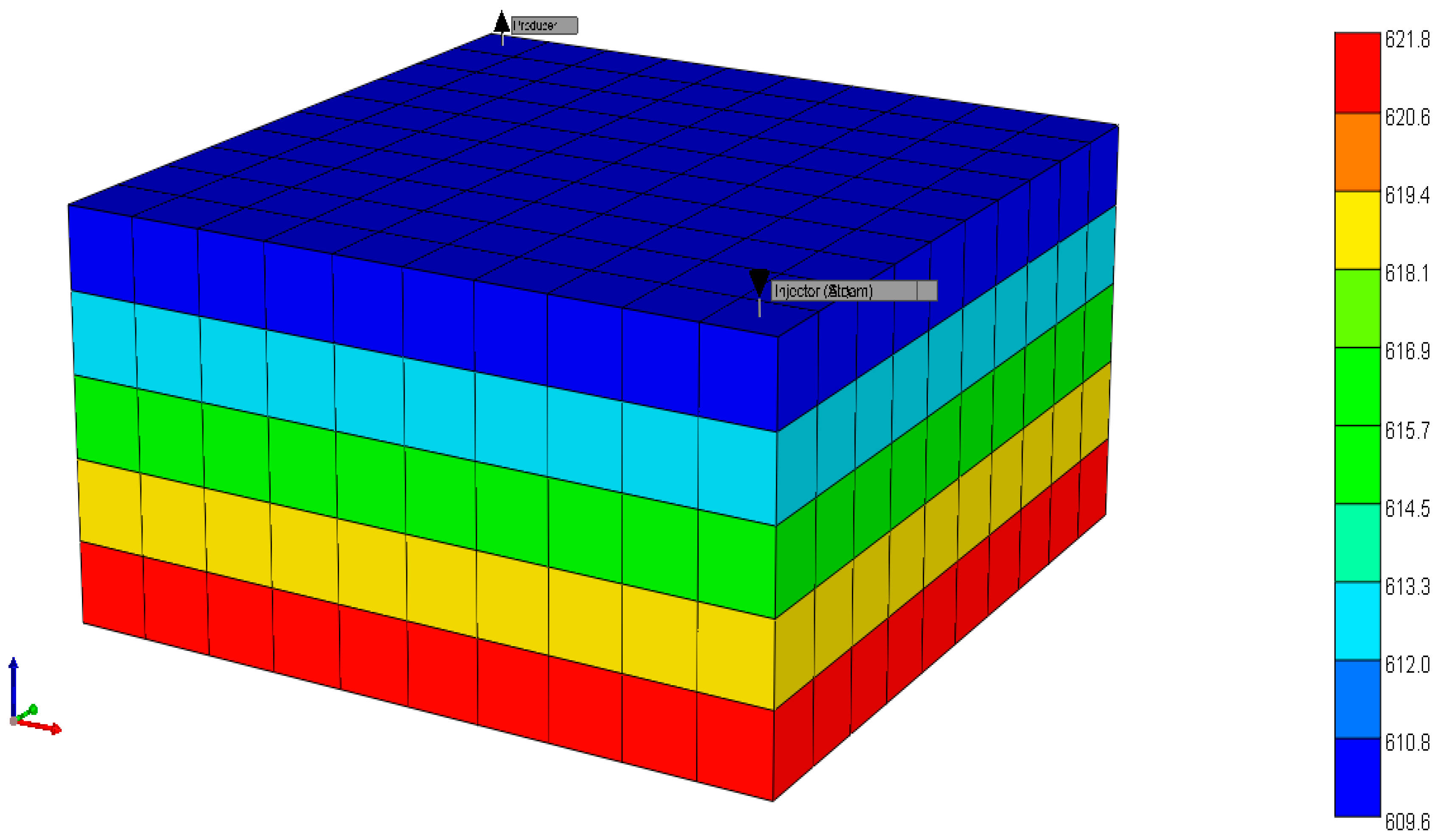
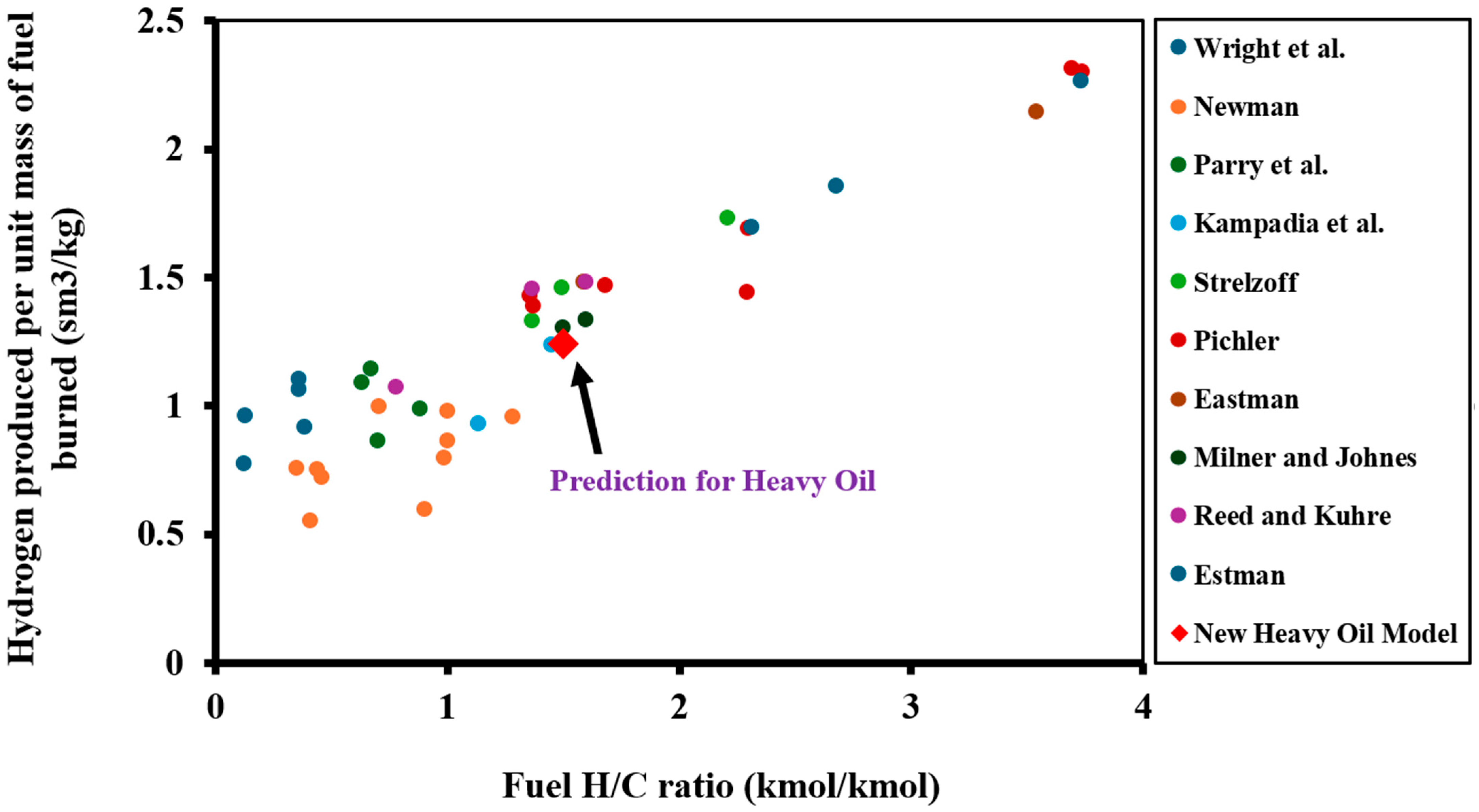


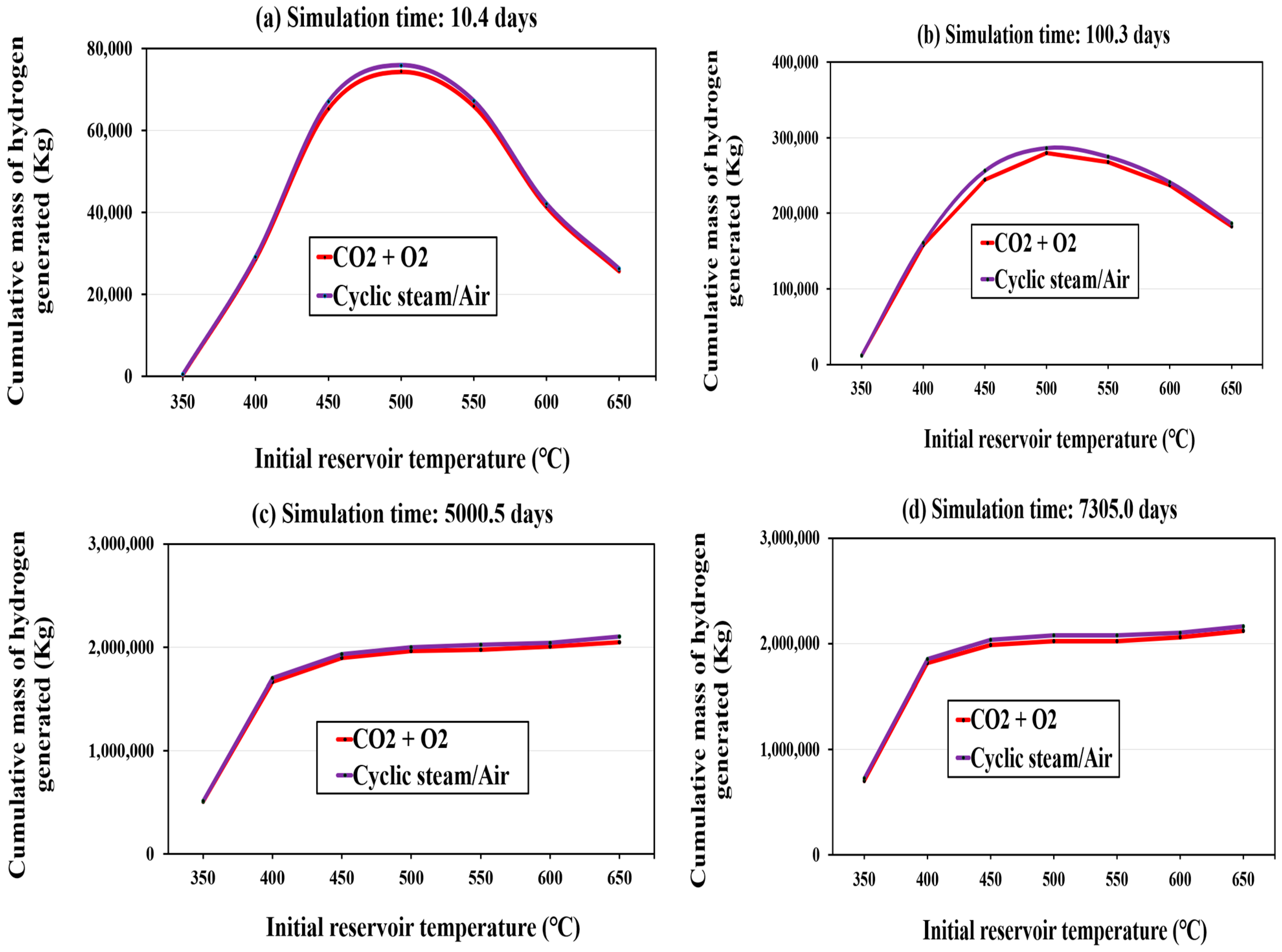
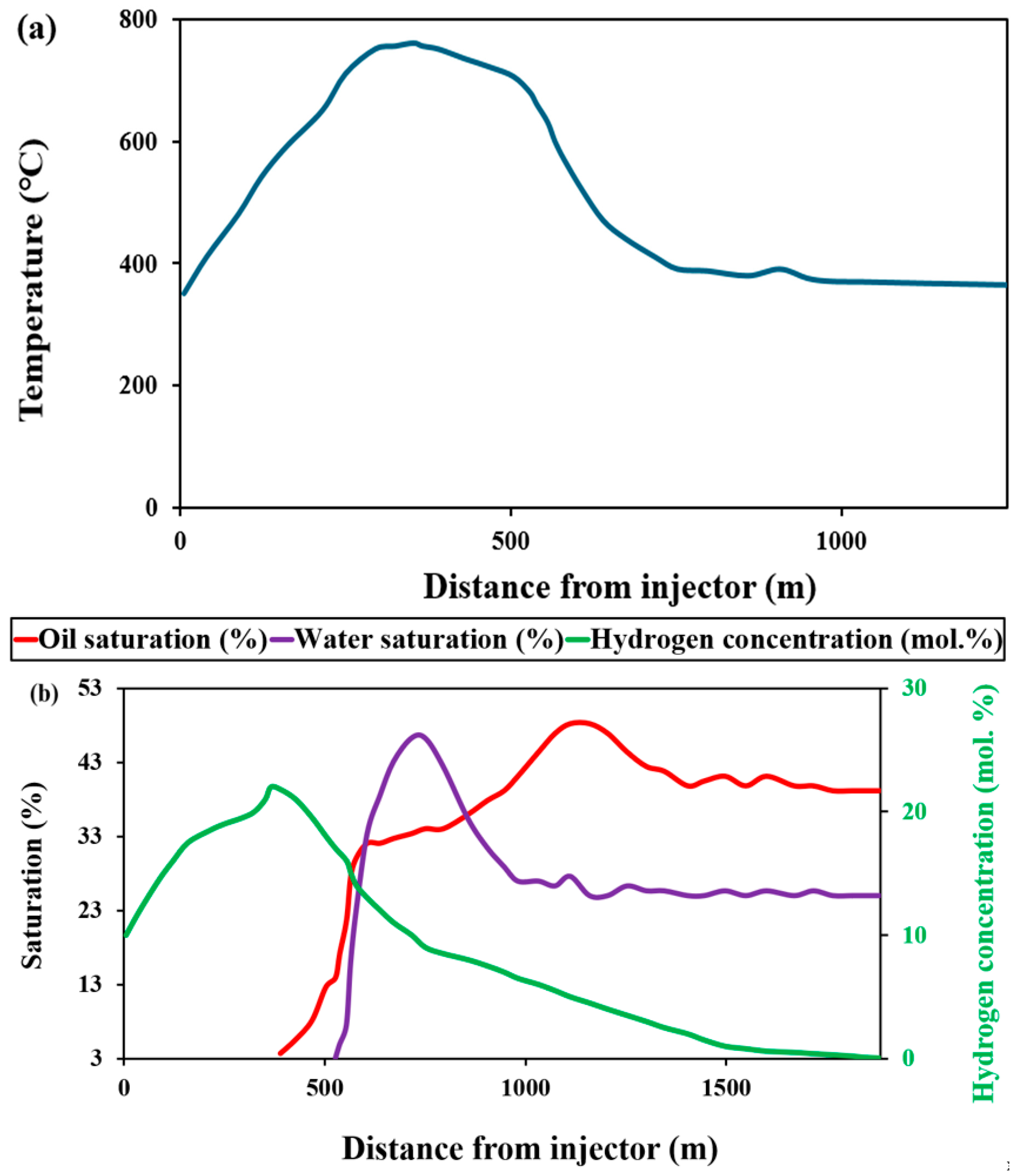
| Year | Project Title | Location | Porosity (%) | Permeability (mD) | Max. Yield (mol. %) | H2 Source |
|---|---|---|---|---|---|---|
| 1977 | Utah Tar Sand | United States | 31 | 600–700 | 14 | [16] |
| 1979 | Marguerite Lake | Canada | 30 | 1000–3000 | 33 | [17] |
| 1985 | Wolf Lake | Canada | 32 | 1000–3000 | 25 | [18] |
| 2006 | Whitesands | Canada | 36 | 3000–12,000 | 10 | [19] |
| 2009 | Kerrobert | Canada | 32 | 2000–6000 | 7 | [20] |
| 2023 | Whitesands | Canada | 34 | 200–7000 | 15 | [21] |
| Grid Configuration | Number of Active Blocks | Cumulative H2 Produced (kg) | Cumulative CO2 Produced (m3) | Average Temperature at Combustion Front (°C) |
|---|---|---|---|---|
| 5 × 5 × 3 | 75 | 300,246 | 2421.85 | 705.4 |
| 7 × 7 × 3 | 147 | 305,142 | 2451.24 | 707.9 |
| 10 × 10 × 5 | 500 | 310,245 | 2482.56 | 710.2 |
| 12 × 12 × 7 | 1008 | 310,812 | 2485.76 | 711.1 |
| 15 × 15 × 10 | 2250 | 311,025 | 2486.98 | 712.3 |
| Injection | Production | CO2 Recycling |
|---|---|---|
| Cost of equipment leased | Cost of production (equipment and Separation costs) | Cost of gas compression and processing (pumping, compression, and separation costs). |
| Annual operation-and-maintenance costs | Tax, royalty, social responsibilities, and revenue | |
| Cost of CO2 distribution within the system | ||
| Steam cost | ||
| Air source and cost | ||
| CO2 source and cost |
| Components | Computation Technique |
|---|---|
| Injection | |
| Equipment cost ($) | 60.682/ft + 85,997 |
| Annual operating and maintenance costs | |
| 0.0027/ft2–30.548/ft + 88,879 |
| 0.0031/ft2–34.465/ft + 106,019 |
| 0.0053/ft2–57.067/ft + 162,380 |
| CO2 supply and distribution system costs | $200,000 |
| Air source and cost | $50,000 with $5000 per year operating cost |
| CO2 purchase cost ($/Mscf) | 2.5 percent of oil price |
| CO2 pressurizing cost | |
| Pump capital cost ($) | (1.944 × 103 × Wp) + 0.1224 × 106 |
| 13 Cents per kWh |
| $0.14 per barrel |
| Steam/air pumping and injection cost | 13 Cents per kWh |
| Production | |
| Cost of producing equipment | 32.516/ft-21,146 |
| Cost of fluid lifting | $0.25 per barrel of produced fluids |
| Cost of syngas and liquid separation | $1000 per square meter with 10% annual maintenance cost |
| Cost of water/oil separation | $1.917 per barrel |
| Production revenue, tax and royalty | |
| 2% of the produced hydrogen value |
| 10% of the produced hydrogen value |
| CO2 recycling | |
| Gas treatment: Gas separation and compression ($) | $500 of gas production rate |
| Compression costs | |
| $2500 per horsepower |
| 13 Cents per kWh |
| Reservoir Property | Ultra-Low-Quality Reservoirs (ULQR) | Low-Quality Reservoirs (LQR) | Moderate-Quality Reservoirs (MQR) | High-Quality Reservoirs (HQR) |
|---|---|---|---|---|
| Permeability | <0.1 | 0.1–1 md | 1–10 md | >10 md |
| Porosity | <5% | 5–10% | 10–20% | >20% |
| Injection Strategy | Reservoir | Cum. Mass of H2 Prod. (kg) | Cum. Mass of Oil Prod. (kg) | Cum. Mass of CO2 Prod. (kg) | Cum. Mass of CO2 Inj. (kg) | Total Revenue from H2 ($) | Total Revenue from Oil ($) | Total Cost for New CO2 Procurement ($) |
|---|---|---|---|---|---|---|---|---|
| Steam/air | ULQR | 157.74 | 5146.17 | 55.60 | 0 | 473.22 | 2589.48 | 0 |
| LQR | 195.48 | 7106.34 | 73.15 | 0 | 586.44 | 3575.52 | 0 | |
| MQR | 14,579.03 | 1,227,232.25 | 4541.80 | 0 | 43,737.09 | 617,390.93 | 0 | |
| HQR | 3,714,918 | 53,639,076 | 46,950,988 | 0 | 11,144,754 | 27,000,000 | 0 | |
| CO2 + O2 | ULQR | 19.58 | 1106.34 | 93.12 | 782.74 | 58.74 | 556.69 | 18.79 |
| LQR | 46.48 | 3385.06 | 138.68 | 1472.04 | 139.44 | 1703.52 | 35.33 | |
| MQR | 13,575.51 | 1,127,300.13 | 19,542.98 | 24,643.49 | 40,726.53 | 567,764.45 | 591.44 | |
| HQR | 2,697,763.75 | 42,623,424 | 116,356,432 | 360,118,356 | 8,093,291.25 | 21,427,202.52 | 8,642,840.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okere, C.J.; Sheng, J.J. Can Hydrogen Be Produced Cost-Effectively from Heavy Oil Reservoirs? Energies 2025, 18, 5539. https://doi.org/10.3390/en18205539
Okere CJ, Sheng JJ. Can Hydrogen Be Produced Cost-Effectively from Heavy Oil Reservoirs? Energies. 2025; 18(20):5539. https://doi.org/10.3390/en18205539
Chicago/Turabian StyleOkere, Chinedu J., and James J. Sheng. 2025. "Can Hydrogen Be Produced Cost-Effectively from Heavy Oil Reservoirs?" Energies 18, no. 20: 5539. https://doi.org/10.3390/en18205539
APA StyleOkere, C. J., & Sheng, J. J. (2025). Can Hydrogen Be Produced Cost-Effectively from Heavy Oil Reservoirs? Energies, 18(20), 5539. https://doi.org/10.3390/en18205539






