Opportunities and Challenges in Reducing the Complexity of the Fischer–Tropsch Gas Loop of Smaller-Scale Facilities for the Production of Renewable Hydrocarbons
Abstract
1. Introduction
- How do the gas loop designs compare in terms of carbon monoxide conversion and efficiency?
- How much tail gas recycling is required to achieve an adequate efficiency?
- What H2/CO ratio is required for the syngas to maintain a feed gas H2/CO ratio of 1.9?
- What influence does the syngas carbon dioxide content have on the efficiency and the required syngas H2/CO ratio?
2. Materials and Methods
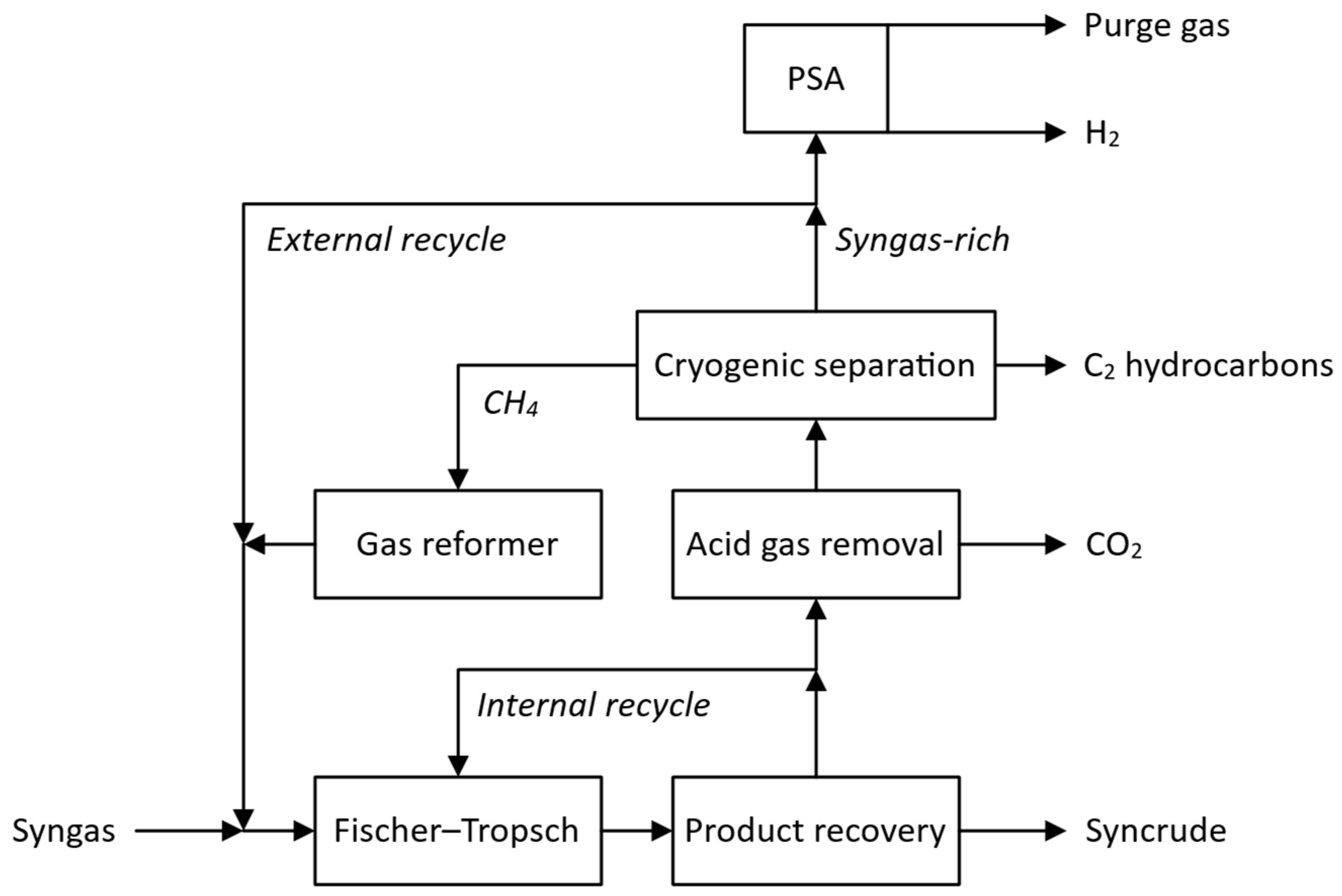
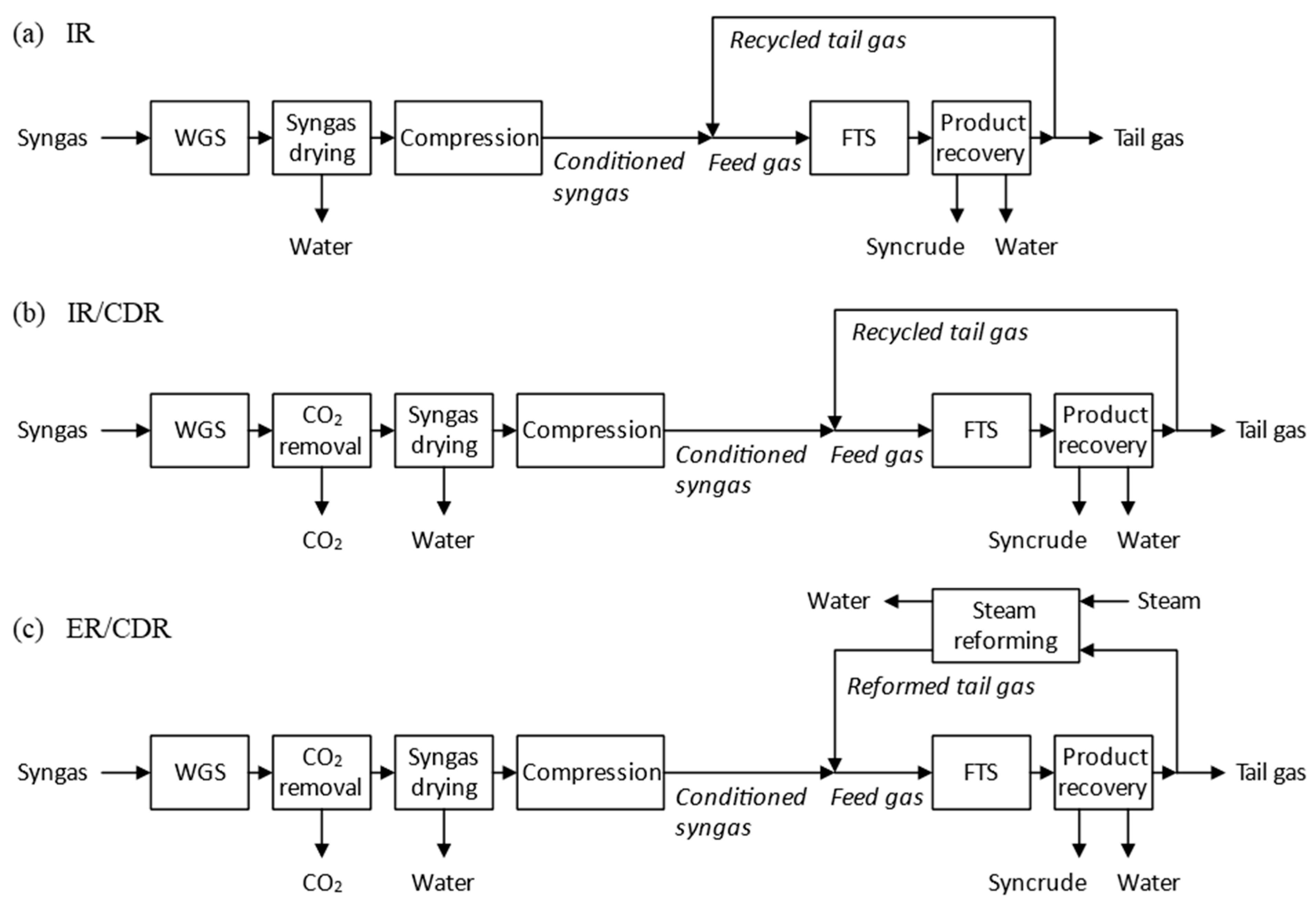
2.1. Gas Loop Designs
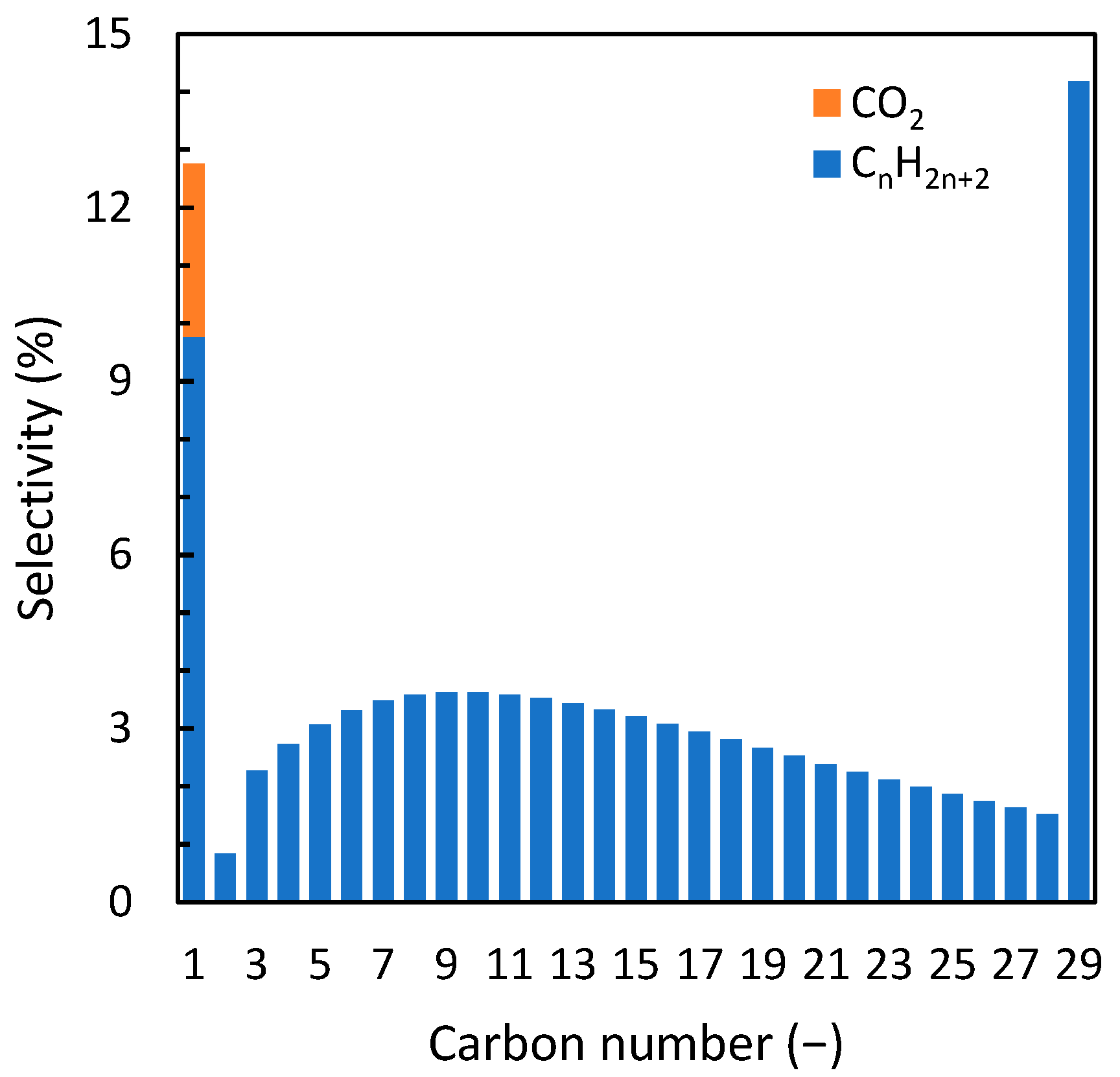
2.2. Model Description
2.3. Evaluation
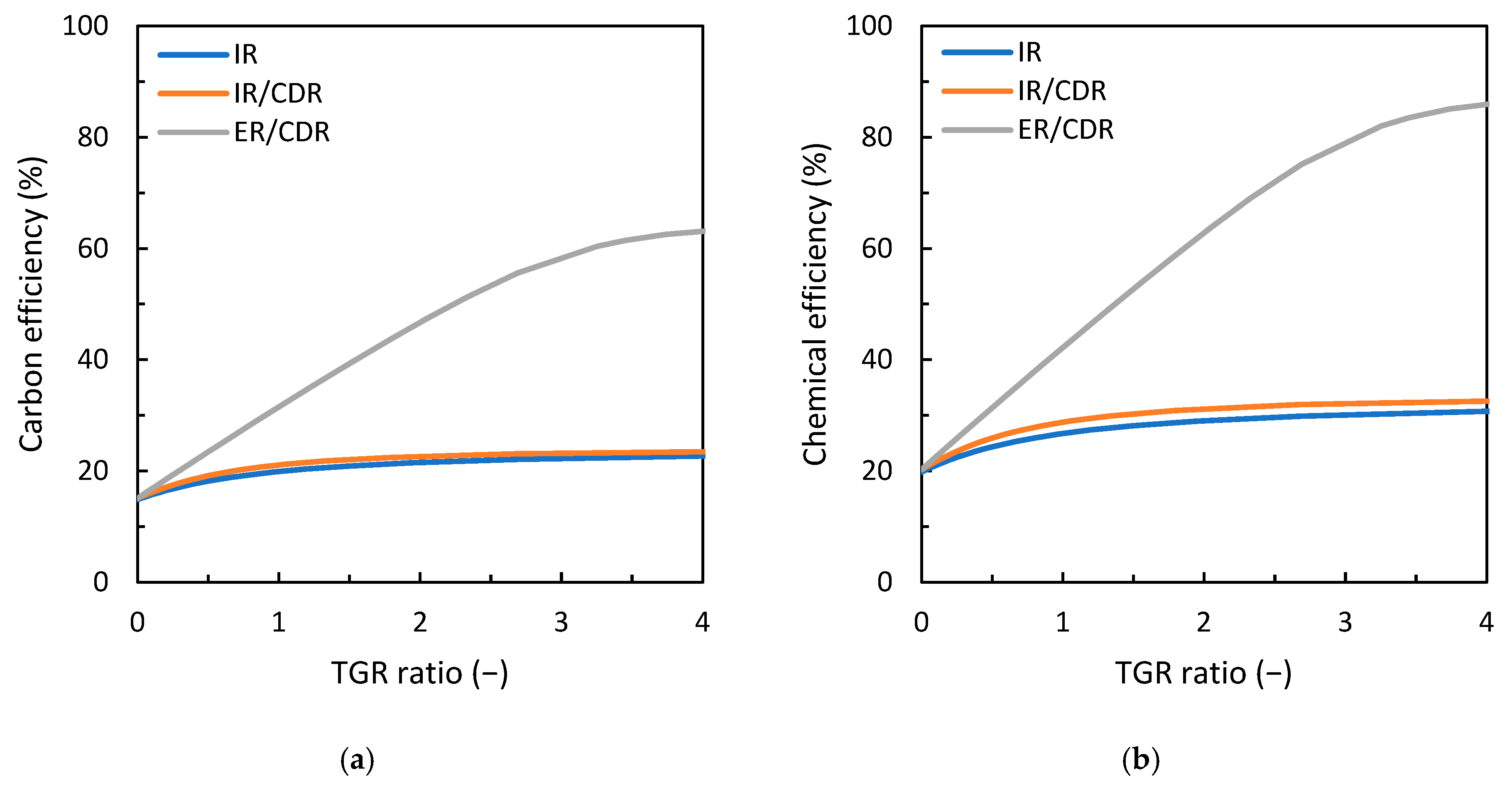
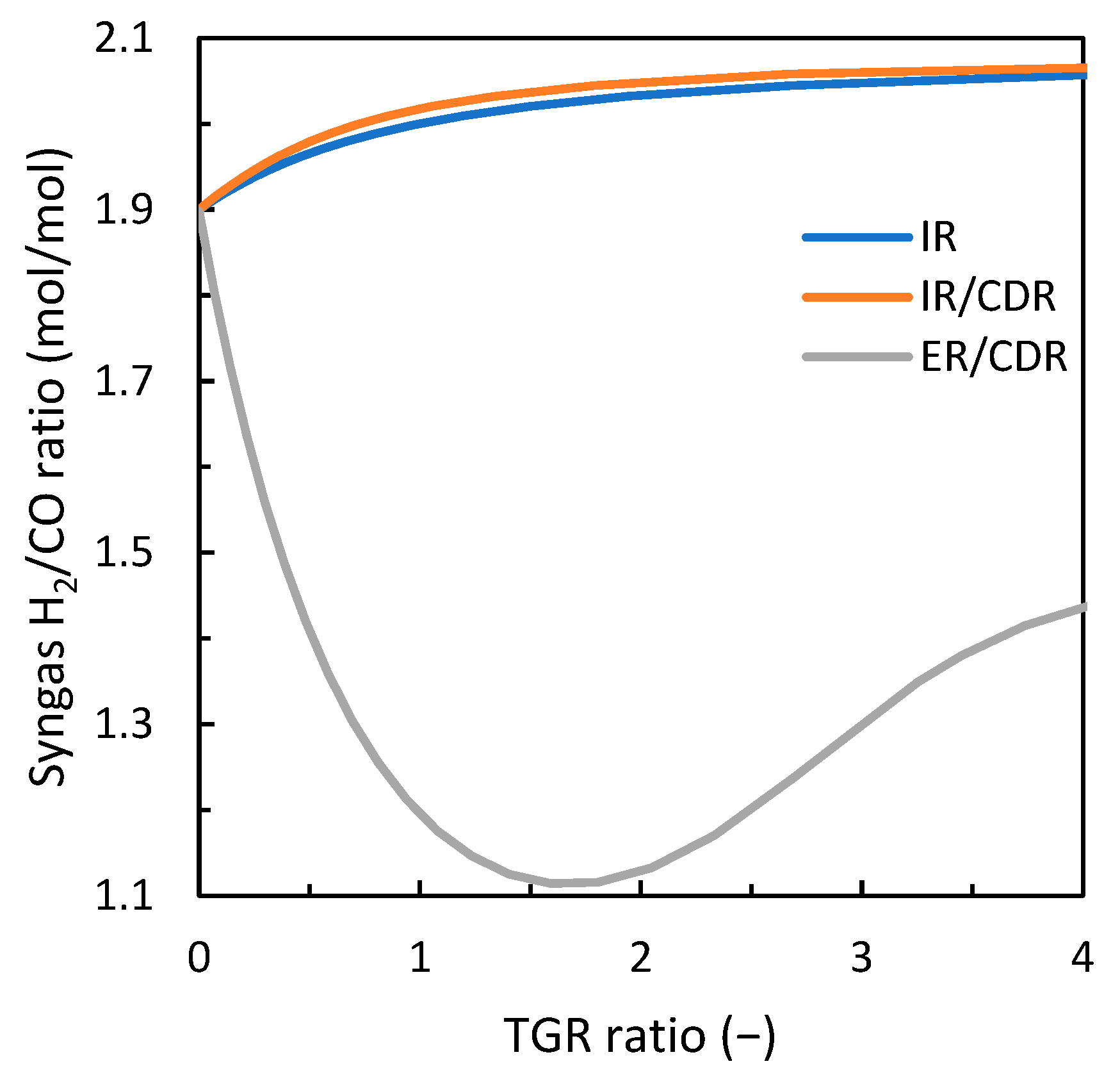
3. Results
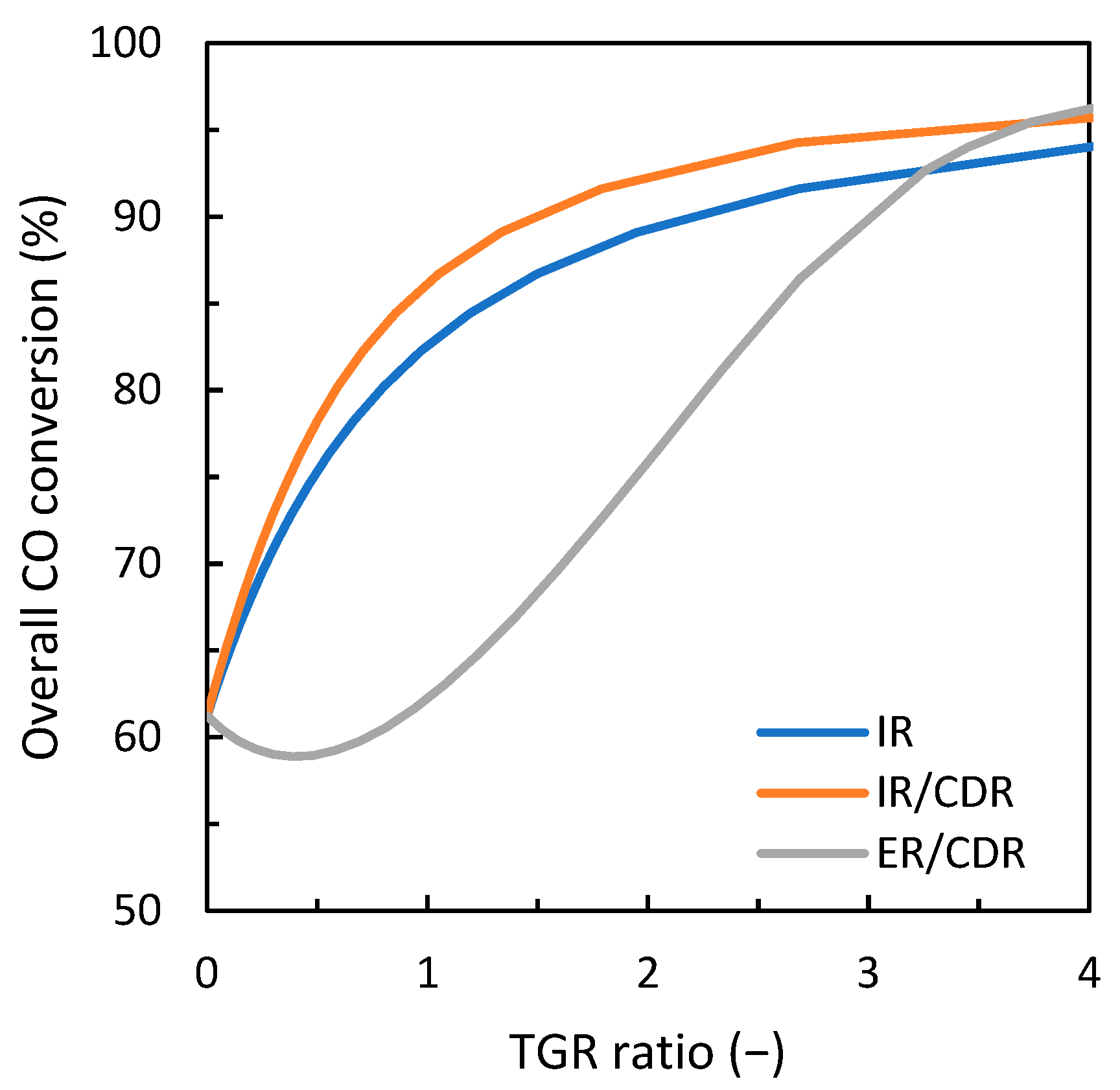
3.1. Comparison of Gas Loop Designs
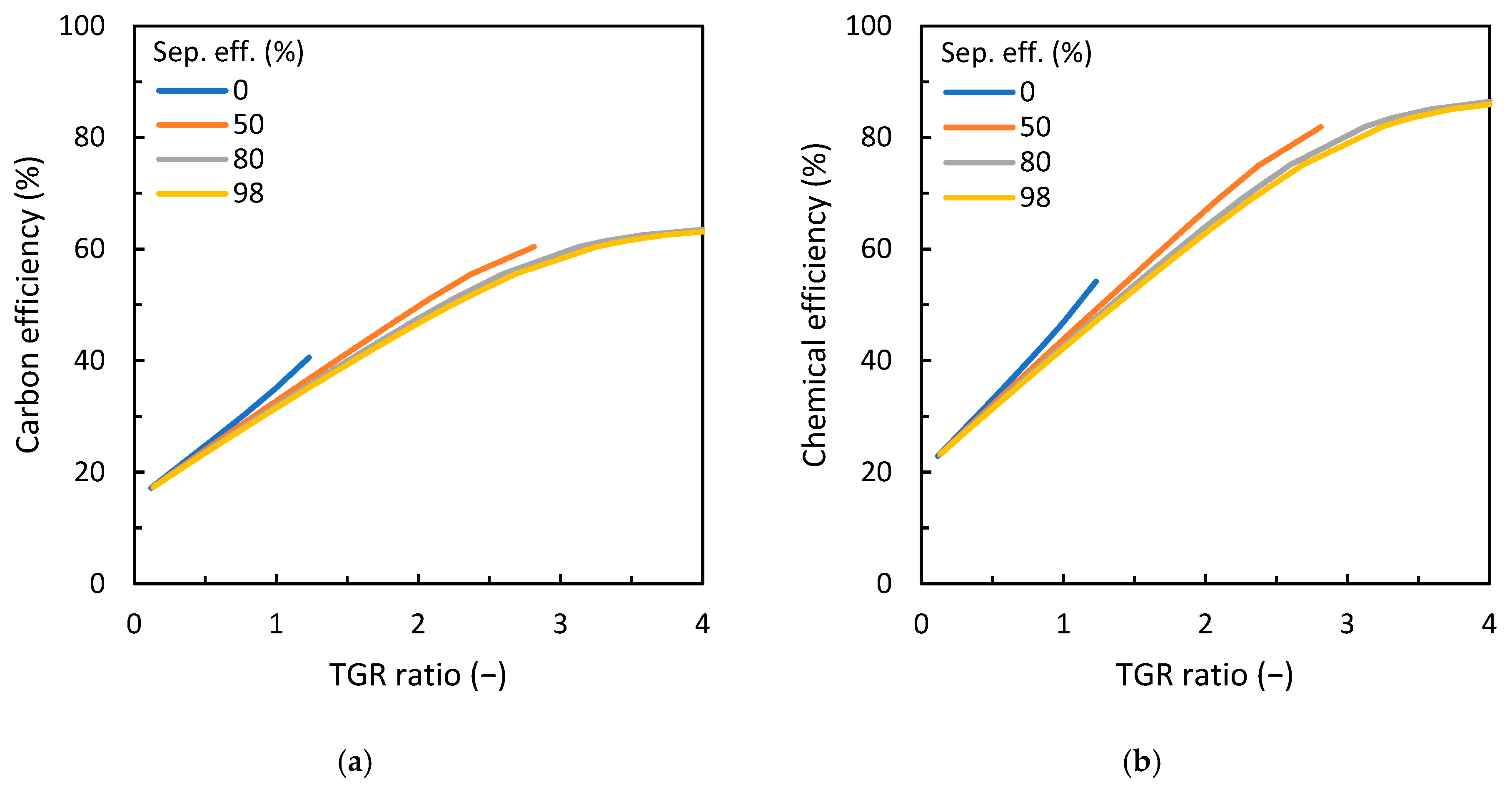
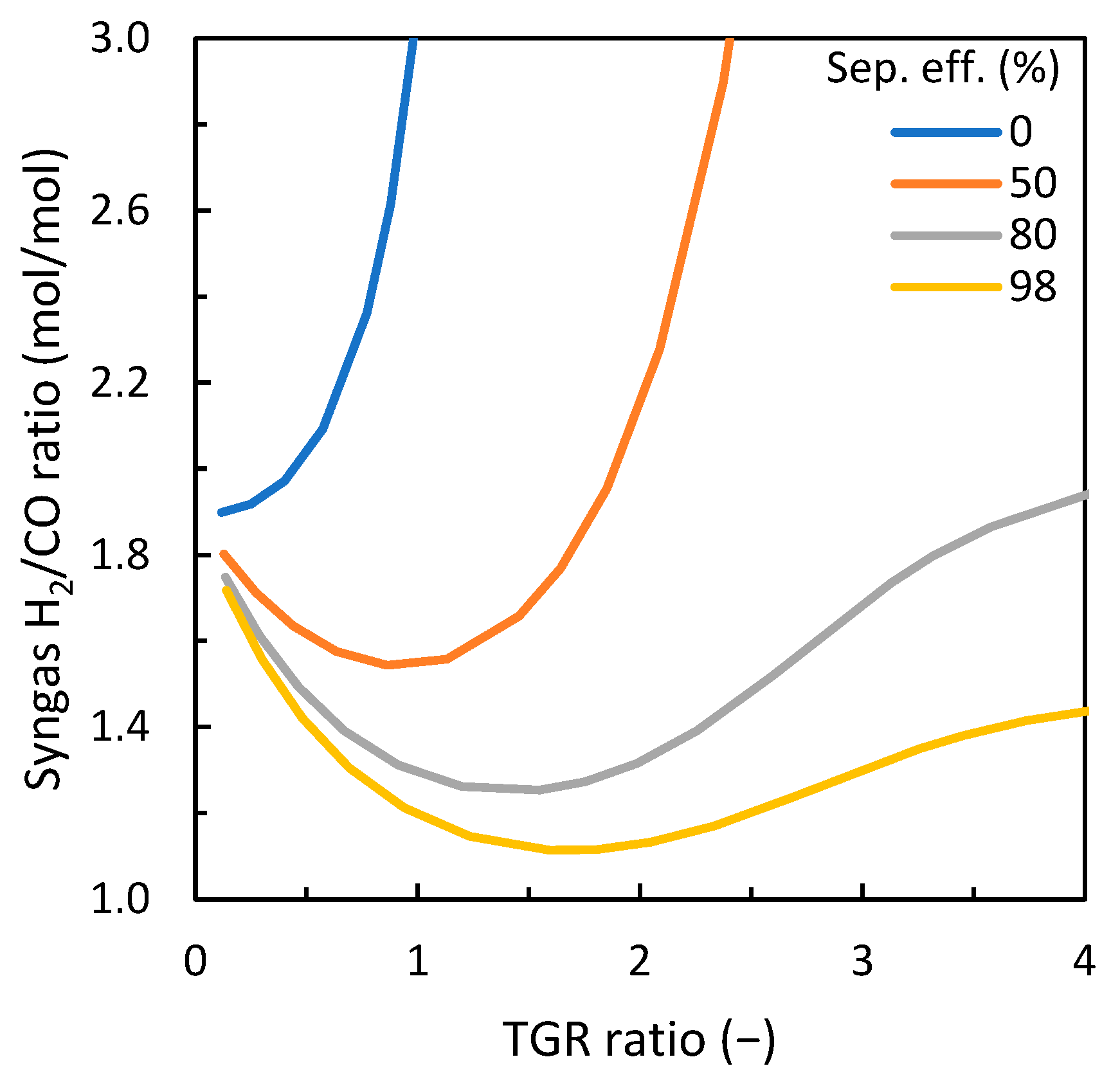
3.2. Influence of Carbon Dioxide Content in ER/CDR Configuration
3.3. Discussion
3.3.1. Gas Loop Design Intent
3.3.2. Investigation of Gas Loop Simulation Results
3.3.3. Comparability of Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTL | Biomass-to-Liquids |
| CTL | Coal-to-Liquids |
| GTL | Gas-to-Liquids |
| CDR | Carbon dioxide removal |
| Cn | Hydrocarbon with n carbon atoms |
| DFB | Dual fluidized bed |
| eASF | Extended Anderson–Schulz–Flory |
| ER | External recycle |
| FT | Fischer–Tropsch |
| FTS | Fischer–Tropsch synthesis |
| IR | Internal recycle |
| LHV | Lower heating value |
| PSA | Pressure swing adsorption |
| PTL | Power-to-Liquids |
| PBTL | Power- and Biomass-to-Liquids |
| SG | Syngas |
| TG | Tail gas |
| TGR | Tail gas recycling |
| WGS | Water–gas shift |
| WTL | Waste-to-Liquids |
| XTL | Feed-to-Liquids |
Appendix A
References
- Grubler, A.; Johansson, T.B.; Mundaca, L.; Nakicenovic, N.; Pachauri, S.; Riahi, K.; Rogner, H.-H.; Strupeit, L.; Kolp, P.; Krey, V.; et al. Energy Primer. In Global Energy Assessment (GEA); Johansson, T.B., Nakicenovic, N., Patwardhan, A., Gomez-Echeverri, L., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 99–150. ISBN 978-0-511-79367-7. [Google Scholar]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2023. [Google Scholar]
- IEA. World Energy Outlook 2024; International Energy Agency: Paris, France, 2024. [Google Scholar]
- IRENA. World Energy Transitions Outlook 2024: 1.5 °C Pathway; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2024. [Google Scholar]
- IEA. Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach; International Energy Agency: Paris, France, 2023. [Google Scholar]
- Maitlis, P.M. What Is Fischer–Tropsch? In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 1–15. ISBN 978-3-527-32945-8. [Google Scholar]
- King, D.L.; De Klerk, A. Overview of Feed-to-Liquid (XTL) Conversion. In ACS Symposium Series; De Klerk, A., King, D.L., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1084, pp. 1–24. ISBN 978-0-8412-2681-4. [Google Scholar]
- Stranges, A. Germany’s Synthetic Fuel Industry, 1927–1945. In The German Chemical Industry in the Twentieth Century; Lesch, J.E., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 147–216. ISBN 978-90-481-5529-3. [Google Scholar]
- Stranges, A.N. A History of the Fischer-Tropsch Synthesis in Germany 1926–45. In Fischer-Tropsch Synthesis, Catalyst and Catalysis; Davis, B.H., Occelli, M.L., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 163, pp. 1–27. ISBN 978-0-444-52221-4. [Google Scholar]
- Schulz, H. Short History and Present Trends of Fischer–Tropsch Synthesis. Appl. Catal. Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch Process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Martinelli, M.; Gnanamani, M.K.; LeViness, S.; Jacobs, G.; Shafer, W.D. An Overview of Fischer-Tropsch Synthesis: XtL Processes, Catalysts and Reactors. Appl. Catal. Gen. 2020, 608, 117740. [Google Scholar] [CrossRef]
- De Klerk, A.; Maitlis, P.M. What Can We Do with Fischer–Tropsch Products? In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 81–105. ISBN 978-3-527-32945-8. [Google Scholar]
- Ali, S.A.; Bangash, I.A.; Sajjad, H.; Karim, M.A.; Ahmad, F.; Ahmad, M.; Habib, K.; Nasir Shah, S.; Sami, A.; Laghari, Z.A.; et al. Review on the Role of Electrofuels in Decarbonizing Hard-to-Abate Transportation Sectors: Advances, Challenges, and Future Directions. Energy Fuels 2025, 39, 5051–5098. [Google Scholar] [CrossRef]
- De Klerk, A. Fischer–Tropsch Refining, 1st ed.; Wiley: Weinheim, Germany, 2011; ISBN 978-3-527-32605-1. [Google Scholar]
- Vogt, E.T.C.; Weckhuysen, B.M. The Refinery of the Future. Nature 2024, 629, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Dry, M.E.; Steynberg, A.P. Commercial FT Process Applications. In Fischer-Tropsch Technology; Steynberg, A.P., Dry, M.E., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 406–481. ISBN 978-0-444-51354-0. [Google Scholar]
- Cornelisse, R.; Gort, R.; Westerink, P. Starting up Mega-Projects, Experiences with the Start-Up of Pearl GTL. In Proceedings of the SPE Production and Operations Symposium, Doha, Qatar, 14–16 May 2012; Society of Petroleum Engineers: Doha, Qatar, 2012; pp. 1386–1390. [Google Scholar]
- Zennaro, R. Fischer–Tropsch Process Economics. In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 149–169. ISBN 978-3-527-32945-8. [Google Scholar]
- Maitlis, P.M.; De Klerk, A. New Directions, Challenges, and Opportunities. In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 337–358. ISBN 978-3-527-32945-8. [Google Scholar]
- Li, Y.; De Klerk, A. Industrial Case Studies. In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 107–129. ISBN 978-3-527-32945-8. [Google Scholar]
- De Klerk, A. Fischer–Tropsch Fuels Refinery Design. Energy Environ. Sci. 2011, 4, 1177. [Google Scholar] [CrossRef]
- Zennaro, R.; Ricci, M.; Bua, L.; Querci, C.; Carnelli, L.; d’Arminio Monforte, A. Syngas: The Basis of Fischer–Tropsch. In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 17–51. ISBN 978-3-527-32945-8. [Google Scholar]
- Habermeyer, F.; Weyand, J.; Maier, S.; Kurkela, E.; Dietrich, R.-U. Power Biomass to Liquid—An Option for Europe’s Sustainable and Independent Aviation Fuel Production. Biomass Convers. Biorefinery 2024, 14, 16199–16217. [Google Scholar] [CrossRef]
- Klüh, D.; Gaderer, M. Integrating a Fischer Tropsch Process into a Pulp Mill—A Techno-Economic Assessment. Energy 2023, 285, 129015. [Google Scholar] [CrossRef]
- Pratschner, S.; Hammerschmid, M.; Müller, F.J.; Müller, S.; Winter, F. Simulation of a Pilot Scale Power-to-Liquid Plant Producing Synthetic Fuel and Wax by Combining Fischer–Tropsch Synthesis and SOEC. Energies 2022, 15, 4134. [Google Scholar] [CrossRef]
- Arlt, S. Renewable Carbon Refinery Based on Fischer–Tropsch Synthesis for Industrial Applications. Ph.D. Dissertation, Technische Universität Wien: Vienna, Austria, 2025. [Google Scholar] [CrossRef]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Steam Gasification of Various Feedstocks at a Dual Fluidised Bed Gasifier: Impacts of Operation Conditions and Bed Materials. Biomass Convers. Biorefinery 2011, 1, 39–53. [Google Scholar] [CrossRef]
- Förtsch, D.; Pabst, K.; Groß-Hardt, E. The Product Distribution in Fischer–Tropsch Synthesis: An Extension of the ASF Model to Describe Common Deviations. Chem. Eng. Sci. 2015, 138, 333–346. [Google Scholar] [CrossRef]
- Reimert, R.; Marschner, F.; Renner, H.-J.; Boll, W.; Supp, E.; Brejc, M.; Liebner, W.; Schaub, G. Gas Production, 2. Processes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 423–482. ISBN 978-3-527-30673-2. [Google Scholar]
- Carbo, M.C.; Jansen, D.; Boon, J.; Dijkstra, J.W.; Van Den Brink, R.W.; Verkooijen, A.H.M. Staged Water-Gas Shift Configuration: Key to Efficiency Penalty Reduction during Pre-Combustion Decarbonisation in IGCC. Energy Procedia 2009, 1, 661–668. [Google Scholar] [CrossRef]
- Bartholomé, E.; Biekert, E.; Hellmann, H.; Ley, H.; Weigert, W.M.; Weise, E. Wachse bis Zündhölzer. In Ullmanns Encyklopädie der technischen Chemie; Verl. Chemie: Weinheim, Germany, 1983; ISBN 978-3-527-20024-5. [Google Scholar]
- De Klerk, A.; Li, Y.; Zennaro, R. Fischer–Tropsch Technology. In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., De Klerk, A., Eds.; Wiley: Weinheim, Germany, 2013; pp. 53–79. ISBN 978-3-527-32945-8. [Google Scholar]
- De Klerk, A. Fischer–Tropsch Process. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Weinheim, Germany, 2013; pp. 1–20. ISBN 978-0-471-48494-3. [Google Scholar]
- Dry, M.E. Chemical Concepts Used for Engineering Purposes. In Fischer-Tropsch Technology; Steynberg, A.P., Dry, M.E., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 196–257. ISBN 978-0-444-51354-0. [Google Scholar]
- LeViness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer-Tropsch Synthesis Technology—New Advances on State-of-the-Art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- Dry, M.E. FT Catalysts. In Fischer-Tropsch Technology; Steynberg, A.P., Dry, M.E., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 533–600. ISBN 978-0-444-51354-0. [Google Scholar]
- LeViness, S. Velocys Fischer-Tropsch Synthesis Technology—New Advances on the State of the Art. In Proceedings of the 245th ACS National Meeting, New Orleans, LA, USA, 7–11 April 2013. [Google Scholar]
- Steynberg, A.P.; Dry, M.E.; Davis, B.H.; Breman, B.B. Fischer-Tropsch Reactors. In Fischer-Tropsch Technology; Steynberg, A.P., Dry, M.E., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 64–195. ISBN 978-0-444-51354-0. [Google Scholar]
- Hofbauer, H.; Rauch, R. Stoichiometric Water Consumption of Steam Gasification by the FICFB-Gasification Process. In Progress in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Wiley: Weinheim, Germany, 2001; pp. 199–208. ISBN 978-0-632-05533-3. [Google Scholar]
- Mauerhofer, A.M.; Fuchs, J.; Müller, S.; Benedikt, F.; Schmid, J.C.; Hofbauer, H. CO2 Gasification in a Dual Fluidized Bed Reactor System: Impact on the Product Gas Composition. Fuel 2019, 253, 1605–1616. [Google Scholar] [CrossRef]
- Jager, B. Developments in Fischer-Tropsch Technology. In Natural Gas Conversion V; Parmaliana, A., Sanfilippo, D., Frusteri, F., Vaccari, A., Arena, F., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; Volume 119, pp. 25–34. ISBN 978-0-444-82967-2. [Google Scholar]
- Aasberg-Petersen, K.; Christensen, T.S.; Dybkjaer, I.; Sehested, J.; Østberg, M.; Coertzen, R.M.; Keyser, M.J.; Steynberg, A.P. Synthesis Gas Production for FT Synthesis. In Fischer-Tropsch Technology; Steynberg, A.P., Dry, M.E., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 258–405. ISBN 978-0-444-51354-0. [Google Scholar]
- Fuchs, J.; Schmid, J.C.; Müller, S.; Hofbauer, H. Dual Fluidized Bed Gasification of Biomass with Selective Carbon Dioxide Removal and Limestone as Bed Material: A Review. Renew. Sustain. Energy Rev. 2019, 107, 212–231. [Google Scholar] [CrossRef]
- Müller, S.; Groß, P.; Rauch, R.; Zweiler, R.; Aichernig, C.; Fuchs, M.; Hofbauer, H. Production of Diesel from Biomass and Wind Power—Energy Storage by the Use of the Fischer-Tropsch Process. Biomass Convers. Biorefinery 2018, 8, 275–282. [Google Scholar] [CrossRef]
- Hillestad, M.; Ostadi, M.; Alamo Serrano, G.d.; Rytter, E.; Austbø, B.; Pharoah, J.G.; Burheim, O.S. Improving Carbon Efficiency and Profitability of the Biomass to Liquid Process with Hydrogen from Renewable Power. Fuel 2018, 234, 1431–1451. [Google Scholar] [CrossRef]
- Ostadi, M.; Rytter, E.; Hillestad, M. Boosting Carbon Efficiency of the Biomass to Liquid Process with Hydrogen from Power: The Effect of H2/CO Ratio to the Fischer-Tropsch Reactors on the Production and Power Consumption. Biomass Bioenergy 2019, 127, 105282. [Google Scholar] [CrossRef]
- Gruber, H.; Groß, P.; Rauch, R.; Reichhold, A.; Zweiler, R.; Aichernig, C.; Müller, S.; Ataimisch, N.; Hofbauer, H. Fischer-Tropsch Products from Biomass-Derived Syngas and Renewable Hydrogen. Biomass Convers. Biorefinery 2021, 11, 2281–2292. [Google Scholar] [CrossRef]
- Benedikt, F.; Müller, S.; Hofbauer, H. 1 MW Scale-up of the Advanced Fuel Flexible Dual Fluidized Bed Steam Gasification Process by Process Simulation. In Proceedings of the ICPS 19, Vienna, Austria, 18–20 November 2019; Technische Universität Wien: Vienna, Austria, 2019; pp. 131–139. [Google Scholar]
- De Klerk, A. Indirect Liquefaction Carbon Efficiency. In ACS Symposium Series; De Klerk, A., King, D.L., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1084, pp. 215–235. ISBN 978-0-8412-2681-4. [Google Scholar]
- Gibson, E.J.; Hall, C.C. Fischer-tropsch Synthesis with Cobalt Catalysts. II. The Effect of Nitrogen, Carbon Dioxide and Methane in the Synthesis Gas. J. Appl. Chem. 1954, 4, 464–468. [Google Scholar] [CrossRef]
| Compound | Volume fraction (%) | ||
|---|---|---|---|
| Reference [28] | Assumed (Dry) | Assumed | |
| Hydrogen | 36–42 | 39.00 | 32.01 |
| Carbon monoxide | 19–24 | 21.50 | 17.65 |
| Carbon dioxide | 20–25 | 22.50 | 18.47 |
| Methane | 9–12 | 10.50 | 8.62 |
| Ethene | 2.0–2.6 | – a | – a |
| Ethane | 1.3–1.8 | 3.85 | 3.16 |
| Propane | 0.3–0.6 | 0.45 | 0.37 |
| Nitrogen | – | 2.20 b | 1.80 |
| Water | – | – | 17.92 c |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Per pass CO conversion | 60.00 | % | |
| Chain growth probability of first distribution | 0.90 | – | |
| Chain growth probability of second distribution | 0.90 | – | |
| Re-adsorption probability of C2 | 0.50 | – | |
| Enhancement factor of C1 | 0.50 | – | |
| Molar fraction of second distribution | 0.00 | – | |
| CO2 selectivity | 3.00 | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arlt, S.; Köffler, T.; Wustinger, I.; Aichernig, C.; Rauch, R.; Hofbauer, H.; Weber, G. Opportunities and Challenges in Reducing the Complexity of the Fischer–Tropsch Gas Loop of Smaller-Scale Facilities for the Production of Renewable Hydrocarbons. Energies 2025, 18, 5479. https://doi.org/10.3390/en18205479
Arlt S, Köffler T, Wustinger I, Aichernig C, Rauch R, Hofbauer H, Weber G. Opportunities and Challenges in Reducing the Complexity of the Fischer–Tropsch Gas Loop of Smaller-Scale Facilities for the Production of Renewable Hydrocarbons. Energies. 2025; 18(20):5479. https://doi.org/10.3390/en18205479
Chicago/Turabian StyleArlt, Stefan, Theresa Köffler, Imanuel Wustinger, Christian Aichernig, Reinhard Rauch, Hermann Hofbauer, and Gerald Weber. 2025. "Opportunities and Challenges in Reducing the Complexity of the Fischer–Tropsch Gas Loop of Smaller-Scale Facilities for the Production of Renewable Hydrocarbons" Energies 18, no. 20: 5479. https://doi.org/10.3390/en18205479
APA StyleArlt, S., Köffler, T., Wustinger, I., Aichernig, C., Rauch, R., Hofbauer, H., & Weber, G. (2025). Opportunities and Challenges in Reducing the Complexity of the Fischer–Tropsch Gas Loop of Smaller-Scale Facilities for the Production of Renewable Hydrocarbons. Energies, 18(20), 5479. https://doi.org/10.3390/en18205479







