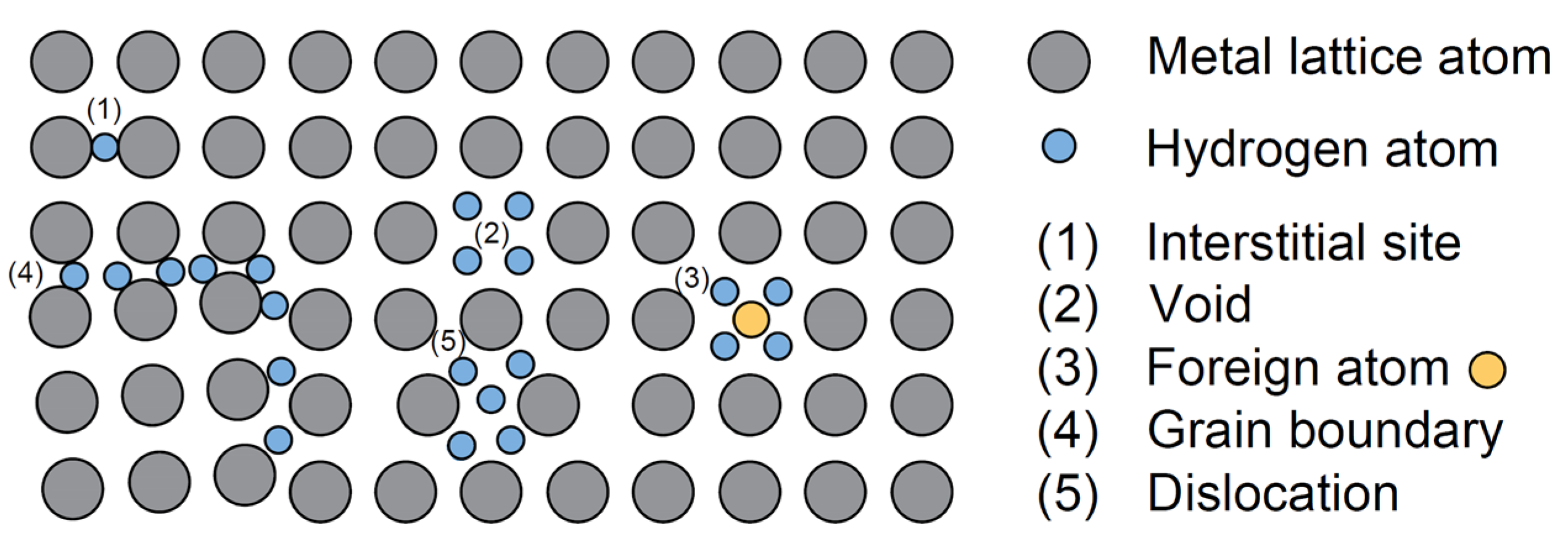
Hydrogen Safety in Energy Infrastructure: A Review
Abstract
Highlights
- Clarifies hydrogen transport mechanisms and material compatibility in metals and polymers.
- Identifies material-specific safety challenges and mitigation measures across hydrogen infrastructure.
- Emphasizes the need for harmonization of safety standards for hydrogen systems.
- Highlights the lack of experimental data for PEX-AL-PEX multilayer pipes used in indoor hydrogen piping.
- Stresses the importance of testing under real-world conditions to validate material performance.
Abstract
1. Introduction
2. Theoretical Foundation
2.1. Physical and Chemical Properties of Hydrogen
2.2. Hydrogen Production
2.2.1. Production Methods
2.2.2. Electrolyzers
2.3. Hydrogen Storage and Distribution
3. Hydrogen Transport
3.1. Hydrogen Transport Mechanism in Metal Materials
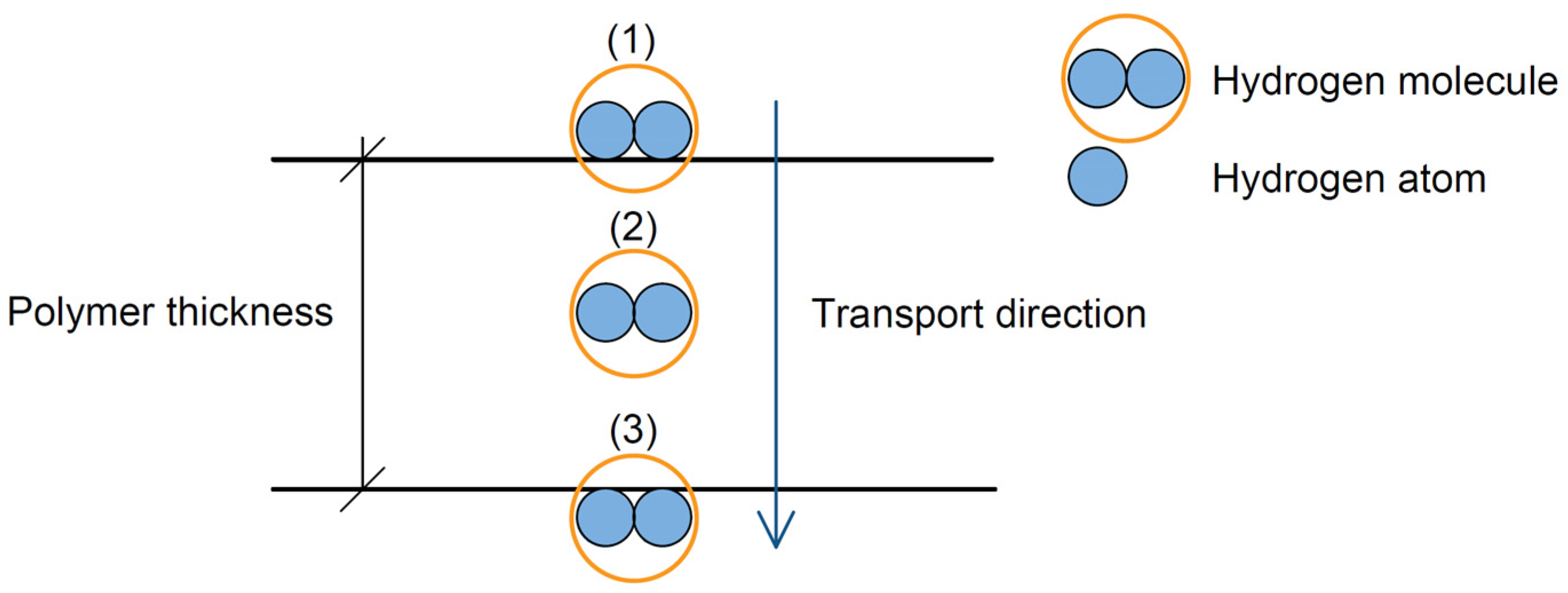
3.2. Hydrogen Transport Mechanism in Polymer Materials (PE)
3.3. Hydrogen Sources in Metal and Polymer
3.4. Hydrogen Traps and Types of Hydrogen
4. Compatibility of Gas Pipeline Materials with Hydrogen
4.1. Hydrogen Embrittlement
4.2. Metal Pipeline—Recommendation
5. Factors Affecting Permeation (Diffusion) of Hydrogen and HE
6. Hydrogen Current Topics
6.1. Hydrogen Blending in Natural Gas
6.2. Hydrogen Refueling Stations
6.3. Hydrogen Fuel Cells
6.4. Hydrogen Permeation in Nuclear Fusion Reactors
6.5. Hydrogen-Fueled Gas Turbines
7. Hydrogen Leakage
Methods of Verifying Tightness and Detection of Hydrogen Leakage
8. Hydrogen Applications in the Czech Republic
9. Future Challenges
10. Conclusions
- Conduct experimental research on internal piping made from PEX-AL-PEX multilayer pipe.
- Evaluate next-generation materials, such as high-entropy alloys and ceramic matrix composites, under real operational conditions.
- Develop and validate risk-mitigation methods, such as cost-effective pipe-in-pipe design solutions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEL | Alkaline Electrolysis |
| AEM | Anion Exchange Membrane |
| AFC | Alkaline Fuel Cell |
| AIDE | Adsorption Induced Dislocation Emission |
| AISI | American Iron and Steel Institute |
| BCC | Body Centered Cubic |
| BEV | Battery Electric Vehicle |
| CCS | Carbon Capture and Storage |
| CCU | Carbon Capture and Utilization |
| CLAM | China Low Activation Martensitic |
| CLF | China Low-activation Ferrite |
| CMC | Ceramic Matrix Composite |
| D-T | Deuterium-Tritium |
| FCC | Face Centered Cubic |
| FCEV | Fuel Cell Electric Vehicle |
| FCV | Fuel Cell Vehicle |
| GHRS | Gaseous Hydrogen Refueling Station |
| H2DI | Hydrogen Direct Injection |
| HAC | Hydrogen-Assisted Cracking |
| HBNG | Hydrogen-Blended Natural Gas |
| HCNG | Hydrogen Enriched-Compressed Natural Gas |
| HCP | Hexagonal Close-Packed |
| HE | Hydrogen Embrittlement |
| HEA | High-Entropy Alloy |
| HEDE | Hydrogen Enhanced Decohesion |
| HELP | Hydrogen Enhanced Localized Plasticity |
| HESIV | Hydrogen-Enhanced Strain-Induced Vacancy |
| HGE | Hydrogen Gas Embrittlement |
| HIC | Hydrogen-Induced Cracking |
| HRS | Hydrogen Refueling Station |
| CHP | Combined Heat and Power |
| LFL | Lower Flammability Limit |
| LH2 | Liquid Hydrogen |
| LHRS | Liquid Hydrogen Refueling Station |
| LOHC | Liquid Organic Hydrogen Carrier |
| MCFC | Molten Carbonate Fuel Cell |
| MHHS | Metal Hydride Hydrogen Storage |
| MIE | The Minimum Ignition Energy |
| MLG | Multi-Layer Graphene |
| MOF | Metal–Organic Framework |
| P2G | Power to Gas |
| PAFC | Phosphoric Acid Fuel Cell |
| PE | Polyethylene |
| PEM | Polymer-Electrolyte Membrane |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PRD | Pressure Relief Devices |
| PSV | Pressure Safety Valve |
| RAFM | Reduced-Activation Ferritic-Martensitic |
| RFNBO | Renewable Fuels of Non-Biological Origin |
| SCC | Stress Corrosion Cracking |
| SCR | Selective Catalytic Reduction |
| SMR | Steam Methane Reforming |
| SOEC | Solid Oxide Electrolyzer Cell |
| SOFC | Solid Oxide Fuel Cell |
| SRB | Sulfate-Reducing Bacteria |
| SS | Stainless Steel |
| TPB | Tritium Permeation Barrier |
| TPRD | Thermal Pressure Relief Device |
| TSC | Thermal Spray Coating |
| VARS | Variable Absorption Refrigeration System |
References
- Peng, S.; He, Q.; Peng, D.; Ouyang, X.; Zhang, X.; Chai, C.; Zhang, L.; Sun, X.; Deng, H.; Hu, W.; et al. Equilibrium Distribution and Diffusion of Mixed Hydrogen-Methane Gas in Gravity Field. Fuel 2024, 358, 130193. [Google Scholar] [CrossRef]
- EU Hydrogen Strategy Under the EU Green Deal|European Hydrogen Observatory. Available online: https://observatory.clean-hydrogen.europa.eu/eu-policy/eu-hydrogen-strategy-under-eu-green-deal (accessed on 10 December 2024).
- Wu, X.; Zhang, H.; Yang, M.; Jia, W.; Qiu, Y.; Lan, L. From the Perspective of New Technology of Blending Hydrogen into Natural Gas Pipelines Transmission: Mechanism, Experimental Study, and Suggestions for Further Work of Hydrogen Embrittlement in High-Strength Pipeline Steels. Int. J. Hydrogen Energy 2022, 47, 8071–8090. [Google Scholar] [CrossRef]
- Lecture 1 Introduction of Hydrogen Safety for Responders LEVEL I Firefighter. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L1_HyResponder_Level1_2002231.pdf (accessed on 14 October 2025).
- Habib, M.A.; Abdulrahman, G.A.Q.; Alquaity, A.B.S.; Qasem, N.A.A. Hydrogen Combustion, Production, and Applications: A Review. Alexandria Eng. J. 2024, 100, 182–207. [Google Scholar] [CrossRef]
- Li, Q.; Ghadiani, H.; Jalilvand, V.; Alam, T.; Farhat, Z.; Islam, M. Hydrogen Impact: A Review on Diffusibility, Embrittlement Mechanisms, and Characterization. Materials 2024, 17, 965. [Google Scholar] [CrossRef]
- Calabrese, M.; Portarapillo, M.; Di Nardo, A.; Venezia, V.; Turco, M.; Luciani, G.; Di Benedetto, A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies 2024, 17, 1350. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, H.; Dong, X.; Teng, C.; Zhang, A.; Kong, S.; Zhou, J.; Zhang, X.-C.; Tu, S.-T. Current Challenges in the Utilization of Hydrogen Energy-a Focused Review on the Issue of Hydrogen-Induced Damage and Embrittlement. Adv. Appl. Energy 2024, 14, 100168. [Google Scholar] [CrossRef]
- Guo, L.; Su, J.; Wang, Z.; Shi, J.; Guan, X.; Cao, W.; Ou, Z. Hydrogen Safety: An Obstacle That Must Be Overcome on the Road towards Future Hydrogen Economy. Int. J. Hydrogen Energy 2024, 51, 1055–1078. [Google Scholar] [CrossRef]
- ČSN ISO 19880-1; Gaseous Hydrogen—Fuelling Stations—Part 1: General Requirements. Czech Agency for Standardization: Prague, Czech Republic, 2020.
- Hydrogen Usage and Consumption Solutions|TÜV SÜD. Available online: https://www.tuvsud.com/en-us/industries/energy/conventional-power/hydrogen-services/hydrogen-consumption (accessed on 16 January 2025).
- Rosenow, J. A Meta-Review of 54 Studies on Hydrogen Heating. Cell Rep. Sustain. 2024, 1, 100010. [Google Scholar] [CrossRef]
- Hydrogen Applications—World Hydrogen Energy Organization. Available online: https://worldhydrogenenergy.org/hydrogen-energy/hydrogen-applications/?utm_source (accessed on 10 December 2024).
- Hydrogen Energy Solutions|TÜV SÜD|TÜV SÜD Czech. Available online: https://www.tuvsud.com/cs-cz/odvetvi/energetika/konvencni-energie/vodikova-energie (accessed on 16 January 2025).
- Hydrogen in Industry—Areas of Application and Role in the Transformation of the Sector—Ses Hydrogen. Available online: https://seshydrogen.com/en/hydrogen-in-industry-areas-of-application-and-role-in-the-transformation-of-the-sector/?utm_source (accessed on 10 December 2024).
- Why We Need Green Hydrogen—State of the Planet. Available online: https://news.climate.columbia.edu/2021/01/07/need-green-hydrogen/ (accessed on 2 January 2025).
- Vodík: Nejlehčí Prvek Je Největší Výzva. Available online: https://oenergetice.cz/vodik/vodik-nejlehci-prvek-nejvetsi-vyzva (accessed on 19 November 2024).
- Lecture 2 Properties of Hydrogen Relevant to Safety LEVEL IV Specialist Officer. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L2_HyResponder_Level4_2302201.pdf (accessed on 14 October 2025).
- ČSN EN IEC 60079-10-1; Ed. 3 Explosive Atmospheres—Part 10-1: Classification of Areas—Explosive Gas Atmospheres. Czech Agency for Standardization: Prague, Czech Republic, 2021.
- Lecture 8 Ignition Sources and Prevention of Ignition LEVEL I Firefighter. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L8_HyResponder_Level1_230220.pdf (accessed on 14 October 2025).
- European Industrial Gases Association Hydrogen Pipeline Systems. Available online: https://www.eiga.eu/uploads/documents/DOC121.pdf (accessed on 14 October 2025).
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen Energy Systems: Technologies, Trends, and Future Prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef]
- Lecture 10 Dealing with Hydrogen Explosions LEVEL I Firefighter. Available online: https://ctif.org/sites/default/files/2023-05/L10_HyResponder_Level1_210618.pdf (accessed on 14 October 2025).
- Lecture 6 Harm Criteria for People and Property LEVEL II Crew Commander. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L6_HyResponder_Level2_230220.pdf (accessed on 14 October 2025).
- Lecture 5 Safety of Liquid Hydrogen LEVEL I Firefighter. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L5_HyResponder_Level1_230220.pdf (accessed on 14 October 2025).
- Mohammed Abbas, A.H.; Cheralathan, K.K.; Porpatham, E.; Arumugam, S.K. Hydrogen Generation Using Methanol Steam Reforming—Catalysts, Reactors, and Thermo-Chemical Recuperation. Renew. Sustain. Energy Rev. 2024, 191, 114147. [Google Scholar] [CrossRef]
- ČSN ISO 14687; Hydrogen Fuel Quality—Product Specification. Czech Agency for Standardization: Prague, Czech Republic, 2020.
- Erdener, B.C.; Sergi, B.; Guerra, O.J.; Lazaro Chueca, A.; Pambour, K.; Brancucci, C.; Hodge, B.-M. A Review of Technical and Regulatory Limits for Hydrogen Blending in Natural Gas Pipelines. Int. J. Hydrogen Energy 2023, 48, 5595–5617. [Google Scholar] [CrossRef]
- FAQ—Česká Vodíková Technologická Platforma. Available online: https://www.hytep.cz/en/about-hydrogen/faq (accessed on 16 January 2025).
- Riemer, M.; Duscha, V. Carbon Capture in Blue Hydrogen Production Is Not Where It Is Supposed to Be—Evaluating the Gap between Practical Experience and Literature Estimates. Appl. Energy 2023, 349, 121622. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue Hydrogen Production from Natural Gas Reservoirs: A Review of Application and Feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A Comprehensive Review on Hydrogen Production from Coal Gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Vodíková Strategie České Republiky Aktualizace 2024. Available online: https://mpo.gov.cz/assets/cz/prumysl/strategicke-projekty/2024/7/Vodikova-strategie-CR-aktualizace-2024.pdf (accessed on 14 October 2025).
- Ozturk, M.; Dincer, I. A Comprehensive Review on Power-to-Gas with Hydrogen Options for Cleaner Applications. Int. J. Hydrogen Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Khawaja, M.K.; Al-Mohamad, R.; Salameh, T.; Alkhalidi, A. Strategies to Promote Nuclear Energy Utilization in Hydrogen Production. Int. J. Hydrogen Energy 2024, 82, 36–46. [Google Scholar] [CrossRef]
- Safety and Efficiency in Your Hydrogen Production Projects|TÜV SÜD. Available online: https://www.tuvsud.com/en-us/themes/hydrogen/explore-the-hydrogen-value-chain/hydrogen-production (accessed on 16 January 2025).
- Jiao, H.; Tsigkou, K.; Elsamahy, T.; Pispas, K.; Sun, J.; Manthos, G.; Schagerl, M.; Sventzouri, E.; Al-Tohamy, R.; Kornaros, M.; et al. Recent Advances in Sustainable Hydrogen Production from Microalgae: Mechanisms, Challenges, and Future Perspectives. Ecotoxicol. Environ. Saf. 2024, 270, 115908. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiao, Y.; He, C.; Ruan, R.; Hu, J.; Ren, J.; Toniolo, S.; Jiang, D.; Lu, C.; Li, Y.; et al. Biological Fermentation Pilot-Scale Systems and Evaluation for Commercial Viability towards Sustainable Biohydrogen Production. Nat. Commun. 2024, 15, 4539. [Google Scholar] [CrossRef]
- Hydrogen Production: Electrolysis|Department of Energy. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis (accessed on 3 January 2025).
- Akyüz, E.S.; Telli, E.; Farsak, M. Hydrogen Generation Electrolyzers: Paving the Way for Sustainable Energy. Int. J. Hydrogen Energy 2024, 81, 1338–1362. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen Production by Water Electrolysis Technologies: A Review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Wei, X.; Sharma, S.; Waeber, A.; Wen, D.; Sampathkumar, S.N.; Margni, M.; Maréchal, F.; Van Herle, J. Comparative Life Cycle Analysis of Electrolyzer Technologies for Hydrogen Production: Manufacturing and Operations. Joule 2024, 8, 3347–3372. [Google Scholar] [CrossRef]
- Chand, K.; Paladino, O. Recent Developments of Membranes and Electrocatalysts for the Hydrogen Production by Anion Exchange Membrane Water Electrolysers: A Review. Arab. J. Chem. 2023, 16, 104451. [Google Scholar] [CrossRef]
- Arunachalam, M.; Han, D.S. Efficient Solar-Powered PEM Electrolysis for Sustainable Hydrogen Production: An Integrated Approach. Emergent Mater. 2024, 7, 1401–1415. [Google Scholar] [CrossRef]
- Institute of Chemical Process Fundamentals of the Czech Academy of Sciences (CAS). Rychlý Vodíkový Elektrolyzér Může Přinést Revoluci v Energetické Stabilitě. Available online: https://www.icpf.cas.cz/wp-content/uploads/2024/09/UCHP_Elektrolyzer.pdf (accessed on 14 October 2025).
- Cozzolino, R.; Bella, G. A Review of Electrolyzer-Based Systems Providing Grid Ancillary Services: Current Status, Market, Challenges and Future Directions. Front. Energy Res. 2024, 12, 1358333. [Google Scholar] [CrossRef]
- Flis, G.; Wakim, G. Solid Oxide Electrolysis: A Technology Status Assessment. Available online: https://cdn.catf.us/wp-content/uploads/2023/11/15092028/solid-oxide-electrolysis-report.pdf (accessed on 14 October 2025).
- Fallah Vostakola, M.; Ozcan, H.; El-Emam, R.S.; Amini Horri, B. Recent Advances in High-Temperature Steam Electrolysis with Solid Oxide Electrolysers for Green Hydrogen Production. Energies 2023, 16, 3327. [Google Scholar] [CrossRef]
- Lecture 3 Hydrogen Storage LEVEL I Firefighter. Available online: https://ctif.org/sites/default/files/2023-05/L3_HyResponder_Level1_210616.pdf (accessed on 14 October 2025).
- Wang, C.; Zhang, J.; Liu, C.; Hu, Q.; Zhang, R.; Xu, X.; Yang, H.; Ning, Y.; Li, Y. Study on Hydrogen Embrittlement Susceptibility of X80 Steel through In-Situ Gaseous Hydrogen Permeation and Slow Strain Rate Tensile Tests. Int. J. Hydrogen Energy 2023, 48, 243–256. [Google Scholar] [CrossRef]
- Tackie-Otoo, B.N.; Haq, M.B. A Comprehensive Review on Geo-Storage of H2 in Salt Caverns: Prospect and Research Advances. Fuel 2024, 356, 129609. [Google Scholar] [CrossRef]
- Pinzón, M.; García-Carpintero, R.; de la Osa, A.R.; Romero, A.; Abad-Correa, D.; Sánchez, P. Ammonia as a Hydrogen Carrier: An Energy Approach. Energy Convers. Manag. 2024, 321, 118998. [Google Scholar] [CrossRef]
- Lin, A.; Bagnato, G. Revolutionising Energy Storage: The Latest Breakthrough in Liquid Organic Hydrogen Carriers. Int. J. Hydrogen Energy 2024, 63, 315–329. [Google Scholar] [CrossRef]
- Hydrogen Storage, Transportation & Distribution|TÜV SÜD. Available online: https://www.tuvsud.com/en-us/industries/energy/conventional-power/hydrogen-services/hydrogen-storage-transportation-and-distribution (accessed on 16 January 2025).
- Lecture 4 Compatibility of Hydrogen with Different Materials LEVEL I Firefighter. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L4_HyResponder_Level1_230220.pdf (accessed on 14 October 2025).
- Zheng, D.; Li, J.; Liu, B.; Yu, B.; Yang, Y.; Han, D.; Li, J.; Huang, Z. Molecular Dynamics Investigations into the Hydrogen Permeation Mechanism of Polyethylene Pipeline Material. J. Mol. Liq. 2022, 368, 120773. [Google Scholar] [CrossRef]
- Röthig, M.; Hoschke, J.; Tapia, C.; Venezuela, J.; Atrens, A. A Review of Gas Phase Inhibition of Gaseous Hydrogen Embrittlement in Pipeline Steels. Int. J. Hydrogen Energy 2024, 60, 1239–1265. [Google Scholar] [CrossRef]
- Sobola, D.; Dallaev, R. Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science. Energies 2024, 17, 2972. [Google Scholar] [CrossRef]
- Kirchheim, R.; Pundt, A. Hydrogen in Metals. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 2597–2705. [Google Scholar]
- ČSN EN ISO 11114-2; Gas Cylinders—Compatibility of Cylinder and Valve Materials with Gas Contents—Part 2: Non-Metallic Materials. Czech Agency for Standardization: Prague, Czech Republic, 2001.
- 9: Diffusion—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/09%3A_Diffusion (accessed on 13 January 2025).
- Sun, Y.; Cheng, Y.F. Thermodynamics of Spontaneous Dissociation and Dissociative Adsorption of Hydrogen Molecules and Hydrogen Atom Adsorption and Absorption on Steel under Pipelining Conditions. Int. J. Hydrogen Energy 2021, 46, 34469–34486. [Google Scholar] [CrossRef]
- Bandlamudi, G.C.; Wetegrove, M.; Warr, L.N.; Wartmann, J.; Kruth, A. Fine Gas Purification Approaches for High Purity Hydrogen Production from Ammonia. Energy Technol. 2024, 13, 2301023. [Google Scholar] [CrossRef]
- Kürten, D.; Khader, I.; Kailer, A. Determining the Effective Hydrogen Diffusion Coefficient in 100Cr6. Mater. Corros. 2020, 71, 918–923. [Google Scholar] [CrossRef]
- Cupertino-Malheiros, L.; Oudriss, A.; Thébault, F.; Piette, M.; Feaugas, X. Hydrogen Diffusion and Trapping in Low-Alloy Tempered Martensitic Steels. Metall. Mater. Trans. A 2023, 54, 1159–1173. [Google Scholar] [CrossRef]
- Rhode, M.; Nietzke, J.; Mente, T.; Richter, T.; Kannengiesser, T. Characterization of Hydrogen Diffusion in Offshore Steel S420G2+M Multi-Layer Submerged Arc Welded Joint. J. Mater. Eng. Perform. 2022, 31, 7018–7030. [Google Scholar] [CrossRef]
- Drexler, A.; Helic, B.; Silvayeh, Z.; Mraczek, K.; Sommitsch, C.; Domitner, J. The Role of Hydrogen Diffusion, Trapping and Desorption in Dual Phase Steels. J. Mater. Sci. 2022, 57, 4789–4805. [Google Scholar] [CrossRef]
- Wang, H.; Ming, H.; Wang, J.; Ke, W.; Han, E.-H. Hydrogen Permeation Behavior at Different Positions in the Normal Direction of X42 and X52 Pipeline Steels. Int. J. Hydrogen Energy 2024, 72, 1105–1115. [Google Scholar] [CrossRef]
- Lee, J.; Park, C.; Park, H.; Kang, N. Effective Hydrogen Diffusion Coefficient for CoCrFeMnNi High-Entropy Alloy and Microstructural Behaviors after Hydrogen Permeation. Int. J. Hydrogen Energy 2020, 45, 10227–10232. [Google Scholar] [CrossRef]
- Padhy, G.K.; Ramasubbu, V.; Parvathavarthini, N.; Wu, C.S.; Albert, S.K. Influence of Temperature and Alloying on the Apparent Diffusivity of Hydrogen in High Strength Steel. Int. J. Hydrogen Energy 2015, 40, 6714–6725. [Google Scholar] [CrossRef]
- Lee, S.K.; Yun, S.-H.; Joo, H.G.; Noh, S.J. Deuterium Transport and Isotope Effects in Type 316L Stainless Steel at High Temperatures for Nuclear Fusion and Nuclear Hydrogen Technology Applications. Curr. Appl. Phys. 2014, 14, 1385–1388. [Google Scholar] [CrossRef]
- Esteban, G.A.; Peña, A.; Urra, I.; Legarda, F.; Riccardi, B. Hydrogen Transport and Trapping in EUROFER’97. J. Nucl. Mater. 2007, 367–370, 473–477. [Google Scholar] [CrossRef]
- Wang, B.; Liu, L.; Xiang, X.; Rao, Y.; Ye, X.; Chen, C.A. Diffusive Transport Parameters of Deuterium through China Reduced Activation Ferritic-Martensitic Steels. J. Nucl. Mater. 2016, 470, 30–33. [Google Scholar] [CrossRef]
- Zheng, D.; Li, J.; Yu, B.; Huang, Z.; Zhang, Y.; Yang, Y.; Han, D.; Li, J. Molecular Dynamics Study of Hydrogen Dissolution and Diffusion in Different Nonmetallic Pipe Materials. In Proceedings of the Computational Science—ICCS 2023, Prague, Czech Republic, 3–5 July 2023; Springer Nature: Cham, Switzerland, 2023; pp. 361–368. [Google Scholar]
- Yao, C.; Ming, H.; Chen, J.; Wang, J.; Han, E.H. Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel. Coatings 2023, 13, 280. [Google Scholar] [CrossRef]
- Tiwari, G.P.; Bose, A.; Chakravartty, J.K.; Wadekar, S.L.; Totlani, M.K.; Arya, R.N.; Fotedar, R.K. A Study of Internal Hydrogen Embrittlement of Steels. Mater. Sci. Eng. A 2000, 286, 269–281. [Google Scholar] [CrossRef]
- Zheng, S.; Qin, Y.; Li, W.; Huang, F.; Qiang, Y.; Yang, S.; Wen, L.; Jin, Y. Effect of Hydrogen Traps on Hydrogen Permeation in X80 Pipeline Steel—A Joint Experimental and Modelling Study. Int. J. Hydrogen Energy 2023, 48, 4773–4788. [Google Scholar] [CrossRef]
- Sun, D.; Wu, M.; Xie, F.; Gong, K. Hydrogen Permeation Behavior of X70 Pipeline Steel Simultaneously Affected by Tensile Stress and Sulfate-Reducing Bacteria. Int. J. Hydrogen Energy 2019, 44, 24065–24074. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Masoumi, M. Different Aspects of Hydrogen Diffusion Behavior in Pipeline Steel. J. Mater. Res. Technol. 2023, 24, 4762–4783. [Google Scholar] [CrossRef]
- Yang, W.; Peng, K.; Zhang, L.; Ren, Q. Deformation and Fracture of Non-Metallic Inclusions in Steel at Different Temperatures. J. Mater. Res. Technol. 2020, 9, 15016–15022. [Google Scholar] [CrossRef]
- Thomas, A.; Szpunar, J.A. Hydrogen Diffusion and Trapping in X70 Pipeline Steel. Int. J. Hydrogen Energy 2020, 45, 2390–2404. [Google Scholar] [CrossRef]
- Schaffner, T. Characterisation and Modelling of Hydrogen Transport and Hydrogen-Dependent Stress Limits of Ultrahigh-Strength Multiphase Steels. 2018. Available online: https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/docId/6233 (accessed on 14 October 2025).
- ČSN EN ISO 11114-1; Gas Cylinders—Compatibility of Cylinder and Valve Materials with Gas Contents—Part 1: Metallic Materials. Czech Agency for Standardization: Prague, Czech Republic, 2021.
- Li, X.; Huang, Q.; Liu, Y.; Zhao, B.; Li, J. Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials 2023, 16, 5366. [Google Scholar] [CrossRef] [PubMed]
- ČSN EN ISO 11114-4; Transportable Gas Cylinders—Compatibility of Cylinder and Valve Materials with Gas Contents—Part 4: Test Methods for Selecting Steels Resistant to Hydrogen Embrittlement. Czech Agency for Standardization: Prague, Czech Republic, 2019.
- Wu, M.; Zhu, H.; Wang, J.; Wang, J.; Zhu, J. Hydrogen Diffusion in Ni-Doped Iron Structure: A First-Principles Study. Chem. Phys. Lett. 2023, 831, 140844. [Google Scholar] [CrossRef]
- AIGA Standard for Hydrogen Piping Systems At User Locations. Available online: https://www.asiaiga.org/uploaded_docs/en_AIGA_087_20_Hydrogen_Piping_at_User_Locations.pdf (accessed on 14 October 2025).
- Sun, B.; Wang, D.; Lu, X.; Wan, D.; Ponge, D.; Zhang, X. Current Challenges and Opportunities Toward Understanding Hydrogen Embrittlement Mechanisms in Advanced High-Strength Steels: A Review. Acta Metall. Sin. English Lett. 2021, 34, 741–754. [Google Scholar] [CrossRef]
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of Existing Steel Pipeline Infrastructure for Gaseous Hydrogen Storage and Transport: A Review of Factors Affecting Hydrogen Induced Degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534. [Google Scholar] [CrossRef]
- Ghadiani, H.; Farhat, Z.; Alam, T.; Islam, M.A. Assessing Hydrogen Embrittlement in Pipeline Steels for Natural Gas-Hydrogen Blends: Implications for Existing Infrastructure. Solids 2024, 5, 375–393. [Google Scholar] [CrossRef]
- Javeria, U.; Kim, S.J. Investigation of Hydrogen Embrittlement in Steel Alloys: Mechanism, Factors, Advanced Methods and Materials, Applications, Challenges, and Future Directions: A Review. J. Mater. Res. Technol. 2025, 38, 1276–1301. [Google Scholar] [CrossRef]
- Jia, L.; Hu, C.; Zhang, M.; Dong, H.; Zhu, X.; Cheng, L.; Xue, H.; Zhao, J.; Wu, K. Interplay between H Atoms and Characteristic Microstructure Features in 2100 MPa Grade Full Pearlite Steel Wire for Bridge Cable. Int. J. Hydrogen Energy 2025, 172, 151226. [Google Scholar] [CrossRef]
- Hasan, M.S.; Kapci, M.F.; Bal, B.; Koyama, M.; Bayat, H.; Xu, W. An Atomistic Study on the HELP Mechanism of Hydrogen Embrittlement in Pure Metal Fe. Int. J. Hydrogen Energy 2024, 57, 60–68. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, H.; Yao, N.; Lu, T.; Lu, X.; Lu, F.; Li, W.; Sun, B.; Zhang, X.-C.; Tu, S.-T. Temperature Dependence of Hydrogen Embrittlement Behavior in a Medium-Entropy Alloy. Acta Mater. 2025, 298, 121396. [Google Scholar] [CrossRef]
- Guzmán, A.A.; Jeon, J.; Hartmaier, A.; Janisch, R. Hydrogen Embrittlement at Cleavage Planes and Grain Boundaries in Bcc Iron—Revisiting the First-Principles Cohesive Zone Model. Materials 2020, 13, 5785. [Google Scholar] [CrossRef]
- Shen, S.; Song, X.; Li, Q.; Li, X.; Zhu, R.; Yang, G. Effect of CrxCy–NiCr Coating on the Hydrogen Embrittlement of 17-4 PH Stainless Steel Using the Smooth Bar Tensile Test. J. Mater. Sci. 2019, 54, 7356–7368. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen Trapping and Embrittlement in Metals—A Review. Int. J. Hydrogen Energy 2025, 136, 789–821. [Google Scholar] [CrossRef]
- Chiari, L.; Mizukami, R.; Nishiwaki, T. Hydrogen-Induced Vacancy Formation Process in Austenitic Stainless Steel 304. ISIJ Int. 2025, 65, ISIJINT-2024-391. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. English Lett. 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Nagumo, M.; Takai, K. The Predominant Role of Strain-Induced Vacancies in Hydrogen Embrittlement of Steels: Overview. Acta Mater. 2019, 165, 722–733. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, R.; Wang, C.; Liu, C.; Zhang, J.; Li, Y. Experimental Study on the Temperature Dependence of Gaseous Hydrogen Permeation and Hydrogen Embrittlement Susceptibility of X52 Pipeline Steel. Eng. Fail. Anal. 2024, 155, 107746. [Google Scholar] [CrossRef]
- Li, Y.; Gong, B.; Li, X.; Deng, C.; Wang, D. Specimen Thickness Effect on the Property of Hydrogen Embrittlement in Single Edge Notch Tension Testing of High Strength Pipeline Steel. Int. J. Hydrogen Energy 2018, 43, 15575–15585. [Google Scholar] [CrossRef]
- Capelle, J.; Dmytrakh, I.; Pluvinage, G. Comparative Assessment of Electrochemical Hydrogen Absorption by Pipeline Steels with Different Strength. Corros. Sci. 2010, 52, 1554–1559. [Google Scholar] [CrossRef]
- Peng, X.Y.; Cheng, Y.F. A Comparison of Hydrogen Permeation and the Resulting Corrosion Enhancement of X65 and X80 Pipeline Steels. Can. Metall. Q. 2014, 53, 107–111. [Google Scholar] [CrossRef]
- ČSN EN ISO 17081; Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique. Czech Agency for Standardization: Prague, Czech Republic, 2014.
- Cheng, L.; Li, L.; Zhang, X.; Liu, J.; Wu, K. Numerical Simulation of Hydrogen Permeation in Steels. Electrochim. Acta 2018, 270, 77–86. [Google Scholar] [CrossRef]
- Cheng, W.; Song, B.; Lu, K.; Mao, J. The Effect of V8C7 Size on Hydrogen Diffusion Behavior and Hydrogen Induced Cracking in Pipeline Steel. Int. J. Hydrogen Energy 2024, 50, 94–107. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, W.; Li, S.; Li, X.; Sun, C.; Sun, J.; Jiang, W. Insights into the Role of CO in Inhibiting Hydrogen Embrittlement of X80 Steel Weld at Different Hydrogen Blending Ratios. Int. J. Hydrogen Energy 2024, 50, 292–302. [Google Scholar] [CrossRef]
- Wang, H.; Tong, Z.; Zhou, G.; Zhang, C.; Zhou, H.; Wang, Y.; Zheng, W. Research and Demonstration on Hydrogen Compatibility of Pipelines: A Review of Current Status and Challenges. Int. J. Hydrogen Energy 2022, 47, 28585–28604. [Google Scholar] [CrossRef]
- Nyrkova, L.I.; Klymenko, A.V.; Osadchuk, S.O.; Kovalenko, S.Y. Comparative Investigation of Electrolytic Hydrogenation of Pipe Assortment Steel under Cathodic Polarization. Int. J. Hydrogen Energy 2024, 49, 1075–1087. [Google Scholar] [CrossRef]
- Cheng, W.; Song, B.; Mao, J.; Li, L. Role of Vanadium Content in Hydrogen Diffusion Behavior in X80 Pipeline Steel. Ironmak. Steelmak. 2023, 50, 257–265. [Google Scholar] [CrossRef]
- Omura, T.; Sawada, H.; Kobayashi, K.; Arai, Y. Effects of Alloying Elements on Hydrogen Diffusion in Iron. ISIJ Int. 2021, 61, 1287–1293. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Song, W.; Wang, H.-B.; Qi, J.-T.; Zeng, R.-C.; Ren, H.; Jiang, W.-C.; Meng, H.-B.; Li, Y.-X. Advances in Reducing Hydrogen Effect of Pipeline Steels on Hydrogen-Blended Natural Gas Transportation: A Systematic Review of Mitigation Strategies. Renew. Sustain. Energy Rev. 2024, 189, 113950. [Google Scholar] [CrossRef]
- Dong, C.F.; Li, X.G.; Liu, Z.Y.; Zhang, Y.R. Hydrogen-Induced Cracking and Healing Behaviour of X70 Steel. J. Alloys Compd. 2009, 484, 966–972. [Google Scholar] [CrossRef]
- Moshref-Javadi, M.; Edris, H.; Shafyei, A.; Salimi-Jazi, H.; Abdolvand, E. Evaluation of Hydrogen Permeation through Standalone Thermally Sprayed Coatings of AISI 316L Stainless Steel. Int. J. Hydrogen Energy 2018, 43, 4657–4670. [Google Scholar] [CrossRef]
- Vargas, F.; Latorre, G.; Uribe, I. Behavior of Thermal Spray Coatings against Hydrogen Attack. CTyF—Cienc. Tecnol. Futur. 2003, 2, 65–74. [Google Scholar] [CrossRef]
- Shi, K.; Xiao, S.; Ruan, Q.; Wu, H.; Chen, G.; Zhou, C.; Jiang, S.; Xi, K.; He, M.; Chu, P.K. Hydrogen Permeation Behavior and Mechanism of Multi-Layered Graphene Coatings and Mitigation of Hydrogen Embrittlement of Pipe Steel. Appl. Surf. Sci. 2022, 573, 151529. [Google Scholar] [CrossRef]
- Wetegrove, M.; Duarte, M.J.; Taube, K.; Rohloff, M.; Gopalan, H.; Scheu, C.; Dehm, G.; Kruth, A. Preventing Hydrogen Embrittlement: The Role of Barrier Coatings for the Hydrogen Economy. Hydrogen 2023, 4, 307–322. [Google Scholar] [CrossRef]
- Cai, K.; Jiang, B. Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures. Coatings 2025, 15, 518. [Google Scholar] [CrossRef]
- Yin, P.; Li, X.; Lu, W.; Chen, Y.; Yang, Z.; Zhang, B.; Guo, Y.; Ding, J.; Cao, R. Effect of Cathodic Potentials on the Hydrogen Embrittlement Susceptibility of 10Ni5CrMo Steel. Int. J. Electrochem. Sci. 2019, 14, 8479–8493. [Google Scholar] [CrossRef]
- Li, H.; Venezuela, J.; Zhou, Q.; Shi, Z.; Dong, F.; Yan, M.; Knibbe, R.; Zhang, M.; Atrens, A. Effect of Cold Deformation on the Hydrogen Permeation in a Dual-Phase Advanced High-Strength Steel. Electrochim. Acta 2022, 424, 140619. [Google Scholar] [CrossRef]
- Shang, J.; Guo, J.; Hua, Z.; Xing, B.; Cui, T.; Wei, H. Effects of Plastic Deformation on Hydrogen Trapping and Hydrogen Distribution in X80 Pipeline Steel. Int. J. Hydrogen Energy 2024, 136, 1306–1316. [Google Scholar] [CrossRef]
- Yin, C.; Wu, Y.; Xu, Z.; Ye, D.; Yao, J.; Chen, J.; Liu, Q.; Ge, X.; Ding, M. A Novel Strategy for Estimating the Diffusion Behavior of Hydrogen in Metallic Materials with the Combined Effect of Stress Corrosion and Hydrogenation: Case Study of 2.25Cr-1Mo-0.25V High-Strength Steel. Vacuum 2024, 219, 112751. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, P.; Zhang, B.; Lei, B.; Feng, Z.; Wang, J.; Shao, Y.; Meng, G.; Wang, Y.; Wang, F. Effects of La3+ on the Hydrogen Permeation and Evolution Kinetics in X70 Pipeline Steel. J. Pipeline Sci. Eng. 2023, 3, 100107. [Google Scholar] [CrossRef]
- Plennevaux, C.; Kittel, J.; Frégonèse, M.; Normand, B.; Ropital, F.; Grosjean, F.; Cassagne, T. Contribution of CO2 on Hydrogen Evolution and Hydrogen Permeation in Low Alloy Steels Exposed to H2S Environment. Electrochem. Commun. 2013, 26, 17–20. [Google Scholar] [CrossRef]
- Du, Q.; Liu, Z.; Yang, J.; Luo, Z.; Wang, H.; Bai, X.; Jiang, B.; Zeng, D. Hydrogen Permeation and Hydrogen Damage Behavior of High Strength Casing Steel in Acidic Environment. Int. J. Electrochem. Sci. 2023, 18, 100136. [Google Scholar] [CrossRef]
- CHENG, S.; GUPTA, V.; LIN, J. Synthesis and Hydrogen Permeation Properties of Asymmetric Proton-Conducting Ceramic Membranes. Solid State Ionics 2005, 176, 2653–2662. [Google Scholar] [CrossRef]
- Heidari, M.; Safekordi, A.; Zamaniyan, A.; Ganji Babakhani, E.; Amanipour, M. Comparison of Microstructure and Hydrogen Permeability of Perovskite Type ACe0.9Y0.1O3-δ (A Is Sr, Ba, La, and BaSr) Membranes. Int. J. Hydrogen Energy 2015, 40, 6559–6565. [Google Scholar] [CrossRef]
- Islam, A.; Alam, T.; Sheibley, N.; Edmonson, K.; Burns, D.; Hernandez, M. Hydrogen Blending in Natural Gas Pipelines: A Comprehensive Review of Material Compatibility and Safety Considerations. Int. J. Hydrogen Energy 2024, 93, 1429–1461. [Google Scholar] [CrossRef]
- Molina, S.; Gomez-Soriano, J.; Lopez-Juarez, M.; Olcina-Girona, M. Evaluation of the Environmental Impact of HCNG Light-Duty Vehicles in the 2020–2050 Transition towards the Hydrogen Economy. Energy Convers. Manag. 2024, 301, 117968. [Google Scholar] [CrossRef]
- Sun, R.; Pu, L.; He, Y.; Yan, T.; Tan, H.; Lei, G.; Li, Y. Investigation of the Leakage and Diffusion Characteristics of Hydrogen-Addition Natural Gas from Indoor Pipelines. Int. J. Hydrogen Energy 2023, 48, 38922–38934. [Google Scholar] [CrossRef]
- Emami, S.D.; Rajabi, M.; Che Hassan, C.R.; Hamid, M.D.A.; Kasmani, R.M.; Mazangi, M. Experimental Study on Premixed Hydrogen/Air and Hydrogen–Methane/Air Mixtures Explosion in 90 Degree Bend Pipeline. Int. J. Hydrogen Energy 2013, 38, 14115–14120. [Google Scholar] [CrossRef]
- Shang, J.; Wang, J.Z.; Chen, W.F.; Wei, H.T.; Zheng, J.Y.; Hua, Z.L.; Zhang, L.; Gu, C.H. Different Effects of Pure Hydrogen vs. Hydrogen/Natural Gas Mixture on Fracture Toughness Degradation of Two Carbon Steels. Mater. Lett. 2021, 296, 129924. [Google Scholar] [CrossRef]
- American Gas Association Impacts of Hydrogen Blending on Gas Piping Materials. Available online: https://www.aga.org/wp-content/uploads/2023/08/Impacts-of-Hydrogen-Blending-on-Gas-Piping-Ma_.pdf (accessed on 14 October 2025).
- Zhu, J.; Pan, J.; Zhang, Y.; Li, Y.; Li, H.; Feng, H.; Chen, D.; Kou, Y.; Yang, R. Leakage and Diffusion Behavior of a Buried Pipeline of Hydrogen-Blended Natural Gas. Int. J. Hydrogen Energy 2023, 48, 11592–11610. [Google Scholar] [CrossRef]
- Klopffer, M.-H.; Berne, P.; Espuche, É. Development of Innovating Materials for Distributing Mixtures of Hydrogen and Natural Gas. Study of the Barrier Properties and Durability of Polymer Pipes. Oil Gas Sci. Technol.—Rev. IFP Energies Nouv. 2015, 70, 305–315. [Google Scholar] [CrossRef]
- Télessy, K.; Barner, L.; Holz, F. Repurposing Natural Gas Pipelines for Hydrogen: Limits and Options from a Case Study in Germany. Int. J. Hydrogen Energy 2024, 80, 821–831. [Google Scholar] [CrossRef]
- Vianello, P. Pipe-in-Pipe Solutions for Hydrogen Transport Employing Fibre-Reinforced Polymers: Material Assessment and Application Evaluation 2 State of the Art 3 Roughness Tests on FRP. Available online: https://www.politesi.polimi.it/retrieve/aeab6aab-7370-4a2f-8ff6-ee5fe0e774df/2023_10_Vianello_Executive Summary_02.pdf (accessed on 14 October 2025).
- Ohřev Vodíkem Se Sníženým Obsahem CO2|Viessmann CZ. Available online: https://www.viessmann.cz/cs/rady-a-tipy/technologie/vytapeni-vodikem.html?utm (accessed on 22 January 2025).
- Fang, Z.; Zhang, S.; Huang, X.; Hu, Y.; Xu, Q. Performance of Three Typical Domestic Gas Stoves Operated with Methane-Hydrogen Mixture. Case Stud. Therm. Eng. 2023, 41, 102631. [Google Scholar] [CrossRef]
- Genovese, M.; Fragiacomo, P. Hydrogen Refueling Station: Overview of the Technological Status and Research Enhancement. J. Energy Storage 2023, 61, 106758. [Google Scholar] [CrossRef]
- Guideline for Small Scale Hydrogen Production AIGA 125/23. Available online: https://www.asiaiga.org/uploaded_docs/en_en_AIGA_125_23_Small_Scale_H2_Production_.pdf (accessed on 14 October 2025).
- Apostolou, D.; Xydis, G. A Literature Review on Hydrogen Refuelling Stations and Infrastructure. Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2019, 113, 109292. [Google Scholar] [CrossRef]
- Lecture 12 Hydrogen Refuelling Stations & Infrastructure LEVEL I Firefighter. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L12_HyResponder_L1_230220.pdf (accessed on 14 October 2025).
- SAE International J2601_202005; Fueling Protocols for Light Duty Gaseous Hydrogen Surface Vehicles—SAE International. SAE International: Pittsburgh, PA, USA, 2020. Available online: https://www.sae.org/standards/j2601_202005-fueling-protocols-light-duty-gaseous-hydrogen-surface-vehicles (accessed on 20 January 2025).
- ČSN ISO 19880-8; Gaseous Hydrogen—Fuelling Stations Part 8: Fuel Quality Control. Czech Agency for Standardization: Prague, Czech Republic, 2020. Available online: https://www.technicke-normy-csn.cz/csn-iso-19880-8-656525-214585.html (accessed on 20 January 2025).
- Schneider, J.; Dang-Nhu, G.; Hart, N.; Groth, K. ISO 19880-1, Hydrogen Fueling Station and Vehicle Interface Safety Technical Report (ICHS # 116). Available online: https://h2tools.org/sites/default/files/2019-08/paper_255.pdf (accessed on 14 October 2025).
- SAE International J2799_202406; Hydrogen Surface Vehicle to Station, Communications Hardware and Software—SAE International. SAE International: Pittsburgh, PA, USA, 2024. Available online: https://www.sae.org/standards/content/j2799_202406/ (accessed on 22 January 2025).
- Genovese, M.; Blekhman, D.; Fragiacomo, P. An Exploration of Safety Measures in Hydrogen Refueling Stations: Delving into Hydrogen Equipment and Technical Performance. Hydrogen 2024, 5, 102–122. [Google Scholar] [CrossRef]
- Hy Responder Lecture 12 Hydrogen Refuelling Stations & Infrastructure Level IV Specialist Officer All Levels to Be Determined. Available online: https://hyresponder.eu/wp-content/uploads/2021/06/L12_HyResponder_L4_210622.pdf (accessed on 14 October 2025).
- ČSN 73 6060; Filling Station for Motor Fuels Czech Agency for Standardization: Prague, Czech Republic, 2018.
- TPG 304 03; Plnicí Stanice Stlačeného Vodíku pro Mobilní Zařízení. Czech Gas Association: Prague, Czech Republic, 2020. Available online: https://www.technicke-normy-csn.cz/csn-73-6060-736060-223686.html (accessed on 14 October 2025).
- Karol, V.; Sharma, P.; Singh, A.; Goel, D.; Kaur, S. Review of Energy Storage Devices: Fuel Cells, Hydrogen Storage Fuel Cells, Rechargeable Batteries, PV Solar Cells. In Materials for Boosting Energy Storage. Volume 3: Advances in Sustainable Energy Technologies; American Chemical Society: Washington, DC, USA, 2024; Volume 3, pp. 1–15. [Google Scholar] [CrossRef]
- Fuel Cell Material—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/fuel-cell-material (accessed on 27 February 2025).
- Mancino, A.; Menale, C.; Vellucci, F.; Pasquali, M.; Bubbico, R. PEM Fuel Cell Applications in Road Transport. Energies 2023, 16, 6129. [Google Scholar] [CrossRef]
- Arsalis, A.; Georghiou, G.E.; Papanastasiou, P. Recent Research Progress in Hybrid Photovoltaic–Regenerative Hydrogen Fuel Cell Microgrid Systems. Energies 2022, 15, 3512. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Energy-Engineering; Elsevier Science: Amsterdam, The Netherlands, 2012; ISBN 9780123877109. [Google Scholar]
- BMW IX5 Puts Hydrogen Power To Use In Acceleration Test: Video. Available online: https://fuelcellsworks.com/news/bmw-ix5-puts-hydrogen-power-to-use-in-acceleration-test-video (accessed on 26 February 2025).
- Tuncer, D.; Yilmaz Ulu, E. Contribution of Regenerative Suspension Module to Charge Efficiency and Range in Hydrogen Fuel Cell Electric Vehicles. Int. J. Hydrogen Energy 2024, 75, 547–556. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, X.; Sun, C. The Alternative Path for Fossil Oil: Electric Vehicles or Hydrogen Fuel Cell Vehicles? J. Environ. Manage. 2023, 341, 118019. [Google Scholar] [CrossRef]
- Goyal, H.; Jones, P.; Bajwa, A.; Parsons, D.; Akehurst, S.; Davy, M.H.; Leach, F.C.; Esposito, S. Design Trends and Challenges in Hydrogen Direct Injection (H2DI) Internal Combustion Engines—A Review. Int. J. Hydrogen Energy 2024, 86, 1179–1194. [Google Scholar] [CrossRef]
- Gandiglio, M.; Ferrero, D.; Lanzini, A.; Santarelli, M. Fuel Cell Cogeneneration for Building Sector: European Status. REHVA J. 2020, 57, 21–25. [Google Scholar]
- Elkhatib, R.; Kaoutari, T.; Louahlia, H. Green Hydrogen Energy Source for a Residential Fuel Cell Micro-Combined Heat and Power. Appl. Therm. Eng. 2024, 248, 123194. [Google Scholar] [CrossRef]
- Shboul, B.; Zayed, M.E.; Tariq, R.; Ashraf, W.M.; Odat, A.-S.; Rehman, S.; Abdelrazik, A.S.; Krzywanski, J. New Hybrid Photovoltaic-Fuel Cell System for Green Hydrogen and Power Production: Performance Optimization Assisted with Gaussian Process Regression Method. Int. J. Hydrogen Energy 2024, 59, 1214–1229. [Google Scholar] [CrossRef]
- SFC Energy AG. EFOY Hybrid Power. Available online: https://www.marinea.fi/files/products/EFOY-Hybrid-Power-Flyer-EN.pdf?srsltid=AfmBOooi-3hxAXFzCHuJJO_3_JAjR3P3y001a7HLtmjVbaV0RAd-84rS (accessed on 14 October 2025).
- Bhogilla, S.; Pandoh, A.; Singh, U.R. Cogeneration System Combining Reversible PEM Fuel Cell, and Metal Hydride Hydrogen Storage Enabling Renewable Energy Storage: Thermodynamic Performance Assessment. Int. J. Hydrogen Energy 2024, 52, 1147–1155. [Google Scholar] [CrossRef]
- Katayama, K.; Izumino, J.; Matsuura, H.; Fukada, S. Evaluation of Hydrogen Permeation Rate through Zirconium Pipe. Nucl. Mater. Energy 2018, 16, 12–18. [Google Scholar] [CrossRef]
- Kunisada, Y.; Sano, R.; Sakaguchi, N. Unveiling the Origin of Diffusion Suppression of Hydrogen Isotopes at the α-Al2O3(0001)/α-Cr2O3(0001) Interfaces. Int. J. Hydrogen Energy 2025, 97, 1327–1334. [Google Scholar] [CrossRef]
- Houben, A.; Scheuer, J.; Rasiński, M.; Kreter, A.; Unterberg, B.; Linsmeier, C. Hydrogen Permeation and Retention in Deuterium Plasma Exposed 316L ITER Steel. Nucl. Mater. Energy 2020, 25, 100878. [Google Scholar] [CrossRef]
- Zilnyk, K.D.; Oliveira, V.B.; Sandim, H.R.Z.; Möslang, A.; Raabe, D. Martensitic Transformation in Eurofer-97 and ODS-Eurofer Steels: A Comparative Study. J. Nucl. Mater. 2015, 462, 360–367. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Wang, Z.; Ma, B.; Ye, X.; Yang, F.; Rao, Y.; Jiang, C.; Chen, C. Diffusion of Hydrogen in China Reduced Activation Ferritic-Martensitic Steels at Low Temperatures. Fusion Eng. Des. 2019, 143, 59–65. [Google Scholar] [CrossRef]
- Houben, A.; Engels, J.; Rasiński, M.; Linsmeier, C. Comparison of the Hydrogen Permeation through Fusion Relevant Steels and the Influence of Oxidized and Rough Surfaces. Nucl. Mater. Energy 2019, 19, 55–58. [Google Scholar] [CrossRef]
- Zhou, H.; Hirooka, Y.; Ashikawa, N.; Muroga, T.; Sagara, A. Gas- and Plasma-Driven Hydrogen Permeation through a Reduced Activation Ferritic Steel Alloy F82H. J. Nucl. Mater. 2014, 455, 470–474. [Google Scholar] [CrossRef]
- Serra, E.; Perujo, A.; Benamati, G. Influence of Traps on the Deuterium Behaviour in the Low Activation Martensitic Steels F82H and Batman. J. Nucl. Mater. 1997, 245, 108–114. [Google Scholar] [CrossRef]
- Esteban, G.A.; Perujo, A.; Douglas, K.; Sedano, L.A. Tritium Diffusive Transport Parameters and Trapping Effects in the Reduced Activating Martensitic Steel OPTIFER-IVb. J. Nucl. Mater. 2000, 281, 34–41. [Google Scholar] [CrossRef]
- Esteban, G.A.; Perujo, A.; Sedano, L.A.; Legarda, F.; Mancinelli, B.; Douglas, K. Diffusive Transport Parameters and Surface Rate Constants of Deuterium in Incoloy 800. J. Nucl. Mater. 2002, 300, 1–6. [Google Scholar] [CrossRef]
- Aiello, A.; Ricapito, I.; Benamati, G.; Valentini, R. Hydrogen Isotopes Permeability in Eurofer 97 Martensitic Steel. Fusion Sci. Technol. 2002, 41, 872–876. [Google Scholar] [CrossRef]
- Ouyang, Y.J.; Yu, G.; Hu, L.; Wang, X.J.; Xu, W.J.; Zhan, Y.G.; Zhang, X.Y. Influence of Ni or Pd Coatings on Oxidation of Permeated Hydrogen. Surf. Eng. 2013, 29, 312–317. [Google Scholar] [CrossRef]
- Anderton, M.D.; Baus, C.; Davis, T.P.; Pearson, R.; Mukai, K.; Pollard, J.; Taylor, K.; Kirk, S.; Hagues, J. Novel High Temperature Tritium Blanket Designs for Confined Spaces in Spherical Tokamak Fusion Reactors. Fusion Eng. Des. 2025, 210, 114732. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Murakami, Y.; Yamaguchi, H.; Yamamoto, T.; Yonetsu, D.; Noborio, K.; Konishi, S. Re-Evaluation of SiC Permeation Coefficients at High Temperatures. Fusion Eng. Des. 2016, 109–111, 1286–1290. [Google Scholar] [CrossRef]
- HYFLEXPOWER Consortium Successfully Operates a Gas Turbine with 100 Percent Renewable Hydrogen, a World First. Available online: https://www.siemens-energy.com/global/en/home/press-releases/hyflexpower-consortium-successfully-operates-a-gas-turbine-with-.html (accessed on 27 February 2025).
- Amrouche, F.; Bari, O.K.; Boudjemaa, L. Investigating the Effect of Using Hydrogen as Fuel on Gas Turbine Performance Operating under Lean Conditions at the Hassi R’mel Gas Site. Int. J. Hydrogen Energy 2024, 140, 1095–1110. [Google Scholar] [CrossRef]
- Zhou, H.; Xue, J.; Gao, H.; Ma, N. Hydrogen-Fueled Gas Turbines in Future Energy System. Int. J. Hydrogen Energy 2024, 64, 569–582. [Google Scholar] [CrossRef]
- Stefan, E.; Talic, B.; Larring, Y.; Gruber, A.; Peters, T.A. Materials Challenges in Hydrogen-Fuelled Gas Turbines. Int. Mater. Rev. 2022, 67, 461–486. [Google Scholar] [CrossRef]
- Alnaeli, M.; Alnajideen, M.; Navaratne, R.; Shi, H.; Czyzewski, P.; Wang, P.; Eckart, S.; Alsaegh, A.; Alnasif, A.; Mashruk, S.; et al. High-Temperature Materials for Complex Components in Ammonia/Hydrogen Gas Turbines: A Critical Review. Energies 2023, 16, 6973. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G.; Mazzocchi, L. Using Hydrogen as Gas Turbine Fuel. J. Eng. Gas Turbines Power 2005, 127, 73–80. [Google Scholar] [CrossRef]
- Varela, C.; Wang, Y.; Tonarely, M.; Ahmed, K.; Gou, J. Ceramic Matrix Composites for H2 Combustion. Available online: https://netl.doe.gov/sites/default/files/netl-file/24UTSR/24UTSR_Gou.pdf (accessed on 14 October 2025).
- ČSN EN ISO 15848-1; Industrial Valves—Measurement, Test and Qualification Procedures for Fugitive Emissions—Part 1: Classification System and Qualification Procedures for Type Testing of Valves. Czech Agency for Standardization: Prague, Czech Republic, 2015.
- Yang, N.; Deng, J.; Wang, C.; Bai, Z.; Qu, J. High Pressure Hydrogen Leakage Diffusion: Research Progress. Int. J. Hydrogen Energy 2024, 50, 1029–1046. [Google Scholar] [CrossRef]
- De Stefano, M.; Rocourt, X.; Sochet, I.; Daudey, N. Hydrogen Dispersion in a Closed Environment. Int. J. Hydrogen Energy 2019, 44, 9031–9040. [Google Scholar] [CrossRef]
- Wang, T.; Huang, T.; Hu, S.; Li, Y.; Yang, F.; Ouyang, M. Simulation and Risk Assessment of Hydrogen Leakage in Hydrogen Production Container. Int. J. Hydrogen Energy 2023, 48, 20096–20111. [Google Scholar] [CrossRef]
- Tato, R.I. V Lecture 11 Confined Spaces LEVEL IV Specialist Officer. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L11_HyResponder_Level4_230220.pdf (accessed on 14 October 2025).
- Chen, H.; Mao, Z. The Study on the Results of Hydrogen Pipeline Leakage Accident of Different Factors. IOP Conf. Ser. Earth Environ. Sci. 2017, 64, 012002. [Google Scholar] [CrossRef]
- Liang, Y.; Pan, X.; Zhang, C.; Xie, B.; Liu, S. The Simulation and Analysis of Leakage and Explosion at a Renewable Hydrogen Refuelling Station. Int. J. Hydrogen Energy 2019, 44, 22608–22619. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Jia, W.; Chen, Y.; Wang, J. Study on Multicomponent Leakage and Diffusion Characteristics of Hydrogen-Blended Natural Gas in Utility Tunnels. Int. J. Hydrogen Energy 2023, 50, 740–760. [Google Scholar] [CrossRef]
- ČSN EN 12245; Transportable Gas Cylinders—Fully Wrapped Composite Cylinders. Czech Agency for Standardization: Prague, Czech Republic, 2022.
- ASME Hydrogen Piping and Pipelines B31.12-2023. Available online: https://haisms.ir/images/iso/638477715664883795ASME%20B31.12-2023.pdf (accessed on 14 October 2025).
- ČSN EN ISO 15848-2; Industrial Valves—Measurement, Test and Qualification Procedures for Fugitive Emissions—Part 2: Production Acceptance Test of Valves. Czech Agency for Standardization: Prague, Czech Republic, 2015.
- ČSN EN ISO 17268; Gaseous Hydrogen Land Vehicle Refuelling Connection Devices. Czech Agency for Standardization: Prague, Czech Republic, 2022.
- ČSN EN ISO 11114-5; Gas Cylinders—Compatibility of Cylinder and Valve Materials with Gas Contents—Part 5: Test Methods for Evaluating Plastic Liners. Czech Agency for Standardization: Prague, Czech Republic, 2022.
- Hydrogen Production Services|TÜV SÜD. Available online: https://www.tuvsud.com/en-us/industries/energy/conventional-power/hydrogen-services/hydrogen-production (accessed on 22 January 2025).
- 2JCP Získává Největší Zakázku Na Montáž Vodíkových Elektrolyzérů ve Své Historii—TZB-Info. Available online: https://vytapeni.tzb-info.cz/vytapime-plynem/28485-2jcp-ziskava-nejvetsi-zakazku-na-montaz-vodikovych-elektrolyzeru-ve-sve-historii (accessed on 9 June 2025).
- Press. Available online: https://www.solarisbus.com/en/press/significant-hydrogen-project-in-the-czech-republic-involving-solaris-2187 (accessed on 18 June 2025).
- ORLEN Group Launches Hydrogen Refuelling Station in Czech Republic. Available online: https://www.orlen.pl/en/about-the-company/media/press-releases/archive/2023/march-2023/ORLEN-Group-launches-hydrogen-refuelling-station-in-Czech-Republic (accessed on 18 June 2025).
- Poul, D.; Jia, X.; Pavlas, M.; Stehlík, P. Model Development and Implementation of Techno-Economic Assessment of Hydrogen Logistics Value Chain: A Case Study of Selected Regions in the Czech Republic. Energies 2025, 18, 1741. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Lin, L.; Bao, W.; Zhang, B.; Ai, B. Development of Standards for Hydrogen Safety. E3S Web Conf. 2020, 194, 02013. [Google Scholar] [CrossRef]
- Xiaolu, C.; Yanmei, Y.; Wei, B.; Bing, L.; Xiao, T.; Junfu, L.; Ling, L. Research on Standards and Standard System of Hydrogen Pipeline in China. E3S Web Conf. 2024, 520, 04016. [Google Scholar] [CrossRef]
- An, Y.; Loh, T.Y.; Sujith Bhaskara, P.; Sin, S.M.I. Review of Safety Regulations, Codes, and Standards (RCS) for Hydrogen Distribution and Application. Int. J. Green Energy 2024, 21, 1757–1765. [Google Scholar] [CrossRef]
- Hua, Z.; Gao, R.; Xing, B.; Shang, J.; Zheng, J.; Peng, W.; Zhao, Y. State-of-the-Art and Knowledge Gaps in Gaseous Hydrogen Pipelines: From the Perspective of Materials, Design, and Integrity Management. J. Zhejiang Univ. A 2025, 26, 87–108. [Google Scholar] [CrossRef]

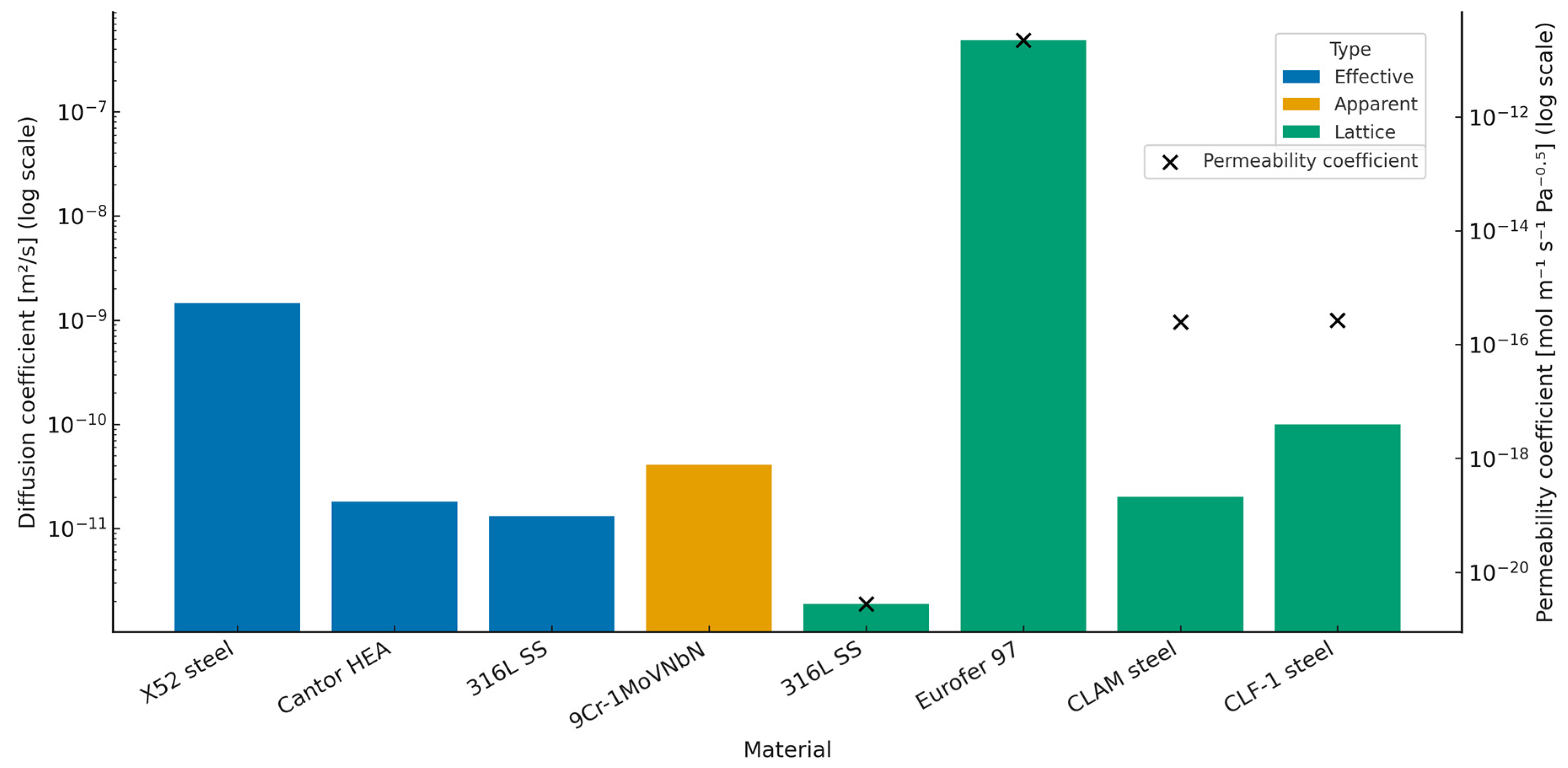
| Material | Gas | Diffusion Coefficient [m2/s] | Permeability Coefficient [mol m−1 s−1 Pa−0.5] | Type |
|---|---|---|---|---|
| X52 high-strength, low-alloy steel [68] | Hydrogen | ~1.20–1.70 × 10−9 (for layers of different hardness) | Effective | |
| Cantor HEA–CoCrFeMnNi [69] | Hydrogen | 1.81 × 10−11 | Effective | |
| 316L stainless steel [69] | Hydrogen | 1.31 × 10−11 | Effective | |
| 9Cr–1MoVNbN high-strength [70] | Hydrogen | f(T) = 4.08 × 10−11 | Apparent | |
| 316L stainless steel [71] | Hydrogen | f(R,T) = 1.89 × 10−12 | f(R,T) = 2.78 × 10−21 | Lattice |
| Eurofer 97–9CrWVTa [72] | Hydrogen | f(R,T) = 4.86 × 10−7 | f(R,T) = 2.23 × 10−11 | Lattice |
| Martensitic steel–CLAM [73] | Deuterium | f(R,T) = 2.02 × 10−11 | f(R,T) = 2.47 × 10−16 | Lattice |
| Martensitic steel–CLF-1 [73] | Deuterium | f(R,T) = 9.94 × 10−11 | f(R,T) = 2.65 × 10−16 | Lattice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorovičová, E.; Pospíšil, J. Hydrogen Safety in Energy Infrastructure: A Review. Energies 2025, 18, 5470. https://doi.org/10.3390/en18205470
Gregorovičová E, Pospíšil J. Hydrogen Safety in Energy Infrastructure: A Review. Energies. 2025; 18(20):5470. https://doi.org/10.3390/en18205470
Chicago/Turabian StyleGregorovičová, Eva, and Jiří Pospíšil. 2025. "Hydrogen Safety in Energy Infrastructure: A Review" Energies 18, no. 20: 5470. https://doi.org/10.3390/en18205470
APA StyleGregorovičová, E., & Pospíšil, J. (2025). Hydrogen Safety in Energy Infrastructure: A Review. Energies, 18(20), 5470. https://doi.org/10.3390/en18205470