1. Introduction
The term ‘decarbonization’ has been appearing quite frequently in the press lately. It should be noted that this is a broad concept encompassing many directions for the development of production, transport, science, and technology.
Therefore, one proposed option is to store energy in the form of fuel cells or hydrogen [
1,
2,
3]. Ammonia is also proposed for use as a fuel, as it is easy to store and transport as a hydrogen-containing fuel, and its combustion is not accompanied by CO
2 emissions. At the present stage, technologies for the production, storage, and transportation of ammonia are already quite well developed. In the first half of the 20th century, the Haber-Bosch process was developed, in which atmospheric nitrogen is bound into industrially produced ammonia [
4]. The prospects and problems of this type of fuel are well covered in the review [
5]. A new technologies and materials are being developed to improve the process’s efficiency, selectivity, and environmental friendliness [
6]. Proposals for using a mixture of hydrogen and ammonia in gas turbines for electricity generation are also found in the literature. For instance, study [
7] examined issues related to the applicability of ammonia as a fuel. The following problems were noted: low ignitability and burning velocity, and the continued probability of NOx emissions. These problems can be solved through knowledge of the dynamics and chemical composition during the combustion process. Numerical research on the use of ammonia and hydrogen in the combustion of a dual-fuel blend under high pressure is presented in [
8]. At elevated pressure and temperature, more stable combustion regimes for ammonia are realized. In the case of a significant hydrogen concentration in the fuel mixture, a transition to stabilization on the injector is possible, which could lead to a reduced service life for combustion chamber elements. Good agreement of the obtained results with experimental data is noted.
The work [
9] studied a laminar flame of a premixed ammonia–hydrogen-air mixture depending on the composition and fuel equivalence ratio. The detailed chemical reaction mechanism used was tested by comparing the simulated flame speed with experimental results. Dependencies of the burning velocity on the ratio in the ammonia–hydrogen mixture, the equivalence ratio, and the initial parameters (pressure and temperature) were obtained. The maximum burning velocity is achieved at an equivalence ratio of φ = 1.1. Given that a complete abandonment of hydrocarbons for electricity generation is not seriously considered, there is also growing interest in the development of gas turbine systems capable of operating efficiently on mixtures of hydrocarbon fuels and hydrogen [
10], across a range of hydrogen concentrations in the fuel mixture from 0 to 100%. Research results presented in [
11,
12] indicate the potential for increased efficiency in engines using hydrogen/methane mixtures compared to pure hydrogen.
Thus, the study of the properties of fuel mixtures where one component is hydrogen and the second can be either gaseous hydrocarbons (methane, propane) or ammonia is highly relevant. It should be noted that the issues of combustion stabilization remain relevant and cannot be resolved without studying the properties of fuel mixtures in which one component is hydrogen, and the second component can be either gaseous hydrocarbons (methane, propane) or ammonia. It is important to consider that in the case of hydrocarbon fuels, the reaction zone contains a significant number of charge carriers (~1011–1012 cm−3). This gives high sensitivity of the flame to the external electric field. Particular attention is paid to weak electric fields with reduced electric field strength (E[V/cm]/P[Torr] < 2), where ohmic heating and the excitation of neutral molecules through inelastic collisions with electrons can be neglected. In this regime, electrical forces with a power of just a few watts can significantly alter the combustion process, which has a heat release rate three orders of magnitude higher.
The application of non-contact methods such as PIV (Particle Image Velocimetry), PLIF (Planar Laser-Induced Fluorescence), and spectral band imaging at the emission wavelengths of OH, CH, and C2* radicals has allowed for the formulation of a hypothesis regarding the localized effect of the electric field on combustion zones [
13]. Results from experimental and computational studies presented in works [
14,
15,
16,
17] provide confirmation for this hypothesis. This suggests that positive ions are present exclusively in the flame front (the reaction zone), and their concentration is determined by chemi-ionization reactions.
The first model of the chemi-ionization reaction chain was presented by Calcote H.F.: CH + O → CHO
+ + e
−, where the CHO
+ ion rapidly proceeds in subsequent chain reactions CHO
+ + X → HX
+ + CO (for example, CHO
+ + H
2O → H
3O
+ + CO) [
18]. Although numerous kinetic schemes for various hydrocarbons have been developed to date [
19,
20,
21], the starting point remains the reaction of atomic oxygen with the free CH radical, as identified by Calcote H.F.
Optical methods like PLIF and the detection of chemiluminescence and fluorescent emission are used to study reaction kinetics [
22,
23]. During the combustion of lean hydrocarbon mixtures, the local fuel equivalence ratio and the dimensions of heat release zones can be determined from the ratio of the flame’s intrinsic chemiluminescence intensities from CH* and OH* radicals. In study [
24], using this approach, it was shown that for a methane mole fraction in the CH
4/H
2 fuel mixture exceeding 40%, the CH chemiluminescence intensity depends linearly on the amount of methane. This holds true regardless of the combustion type (diffusion or kinetic). The same dependence is demonstrated by the flame conductivity when an external electric field is applied, determining the electrochemical property of the flame, namely the relationship between conductivity and the composition of the reaction mixture.
The aim of this study was to determine the influence of an electric field on diffusion flame front deformation and combustion stabilization modes for promising NH3/CH4/H2 fuel mixtures. The addition of hydrogen, on the one hand, reduces the ion concentration, on the other, increases the chemical activity of the fuel. This paper presents the results of a study of the electrochemical properties and flame stabilization. The effects of transverse and longitudinal electric fields on the diffusion flame were experimentally studied. The volt-ampere characteristics of the flame are presented as combustion modes are changed under the influence of an external electric field.
2. Materials and Methods
The diffusion flame of a mixture of combustible gases (hydrogen, methane, ammonia) was chosen as the object of observation. The diffusion combustion regime is relatively simple to implement, requires a minimal number of control parameters, and provides good flame stabilization over a wide range of flow velocities.
Figure 1 shows the experimental setup. The flow rate and percentage composition of the methane–hydrogen mixture were set using a UFPGS-2 fuel mixture generator; the outlet diameter of the diffusion burner was d = 2 mm. A transverse field at a given field strength ensures maximum flame front deformation, potentially reducing flame length. Orienting the field along the flame maximizes the stabilizing effect of the ion wind. For an electric field directed transverse to the jet flow, the electrodes consisted of two thick needles positioned
L = 10–12 mm above the nozzle exit.
The diffusion flame of a mixture of combustible gases (hydrogen, methane, ammonia) was chosen as the object of observation. The diffusion combustion regime is relatively simple to implement, requires a minimal number of control parameters, and provides good flame stabilization over a wide range of flow velocities.
Figure 1 shows the experimental setup. The flow rate and percentage composition of the methane–hydrogen mixture were set using a UFPGS-2 fuel mixture generator; the outlet diameter of the diffusion burner was d = 2 mm. For an electric field directed transverse to the jet flow, the electrodes consisted of two thick needles positioned 10–12 mm above the nozzle exit. For the electric field aligned with the flow direction, the nozzle served as one electrode, while the second electrode was a metal ring spanned with parallel conductors, positioned above the burner at a height of
L = 12.5 cm. A voltage of up to 4.5 kV was supplied from a power source (DC or pulsed-periodic). The stabilization zone in raised flame mode (ignition points) was located between the electrode and the burner. Electrical characteristics were recorded during the experiment: flowing current and applied voltage. A V7-78/1 voltmeter (basic error of 0.0035%) and a resistor with an accuracy class of 0.01 for precision measurements were used in the current measurement circuit. The intensity of chemiluminescence at the wavelengths of the OH* and CH* radicals was determined from images captured by a Nanogate-24/3 optoelectronic digital camera using appropriate interference filters. For different fuel mixture compositions, the regimes of flame blow-off and reattachment to the burner were determined.
To study the combustion process, the optical method of Hilbert imaging was chosen. The Hilbert transform is a special case of linear filtering. Since the intensity transmittance of a Hilbert filter is unity, the filtered image contains all the energy of the original optical signal without loss. Compared to the classical schlieren method, Hilbert imaging also offers increased image contrast. For Hilbert visualization, a diagnostic complex based on the IAB-451 optical shading device was used, the schematic of which is presented in
Figure 2: light source 1 (an LED with a wavelength of λ = 537 nm), a collimator lens 2, and a slit aperture 3 located in the front Fourier plane of objective lens 4. A quadrant Hilbert filter 6 is positioned in the focal plane of objective lens 5, its orientation aligned with the aperture 3. Lens 7 is selected to provide the necessary resolution when the signal is registered by the digital camera 8.
Figure 2 also shows the general principle of forming the observation object 9 (a diffusion flame): 13–15—gas cylinders, 10–12—flow meters. Processing the images obtained through Hilbert visualization yields the phase structure of the object under study (the flame):
where
z is the direction along the scanning beam, x and y are the horizontal and vertical coordinates in the image plane,
n(
x,
y,
z) is the refractive index of the medium in the disturbance region, and
n0 is the refractive index of the unperturbed medium. By applying the Abel transform for axially symmetric functions, the refractive index distribution is reconstructed [
25]. To determine the temperature distribution and estimate the composition (concentrations of the fuel, oxidizer, and combustion products) across the flame cross-section, a procedure detailed in work [
26] was employed.
The physicochemical properties of the fuel mixture (laminar flame speed, characteristic combustion time, critical gradient for flashback) were studied separately based on literature data and a series of experiments using a Bunsen burner.
3. Results
When voltage is applied to the electrodes with an electric field directed across the fuel jet, a displacement of the combustion zone occurs on the side of the negative electrode while the position of the flame front on the positive electrode side remains unchanged (see
Figure 3). During the displacement, the flame front is deformed. The definition of the degree of deformation was proposed by Williams [
27] and can be written as:
where
γ—is the degree of deformation and
A—is the area of an infinitesimal element of the flame surface.
In our case, the expression for deformation splits into the change in surface area over time (due to motion under the applied electric field) and the curvature of the surface due to electric forces:
where
Su—is the laminar flame speed and
xn—is the coordinate normal to the surface. The dynamic change in deformation was estimated in [
24] to be approximately Δγ ≈ 50 s
−1. To determine the curvature of the flame front due to electric forces, we apply the approach proposed in [
28], which yields an additional approximately 35 s
−1. Thus, the total value of the degree of deformation will be γ ≈ 85 s
−1.
A.M. Klimov considered the conditions for changing the combustion regime in relation to kinetic flame [
29]. He introduced the parameter
Ka = γ × τ, which characterizes the heat transfer from the reaction zone with combustion temperature Tb to the fresh mixture with temperature T
0, taking into account the curvature and stretching of the combustion front. At
Ka > 1, the flame front ceases to be continuous, and ruptures and reconnections occur. Following analogy, from a comparison of the characteristic combustion and deformation times in our case, we obtain
Ka* ≈ 0.18 < 1, which corresponds to the conditions of a laminar flame according to A.M. Klimov’s limit. In other words, despite fairly strong flame disturbances under the influence of electrical forces, the laminar combustion regime is maintained.
Unfortunately, due to the loss of flame symmetry, it was not possible to perform a reconstruction of the spatial distribution of the refractive index and, consequently, the temperature using Hilbert visualization.
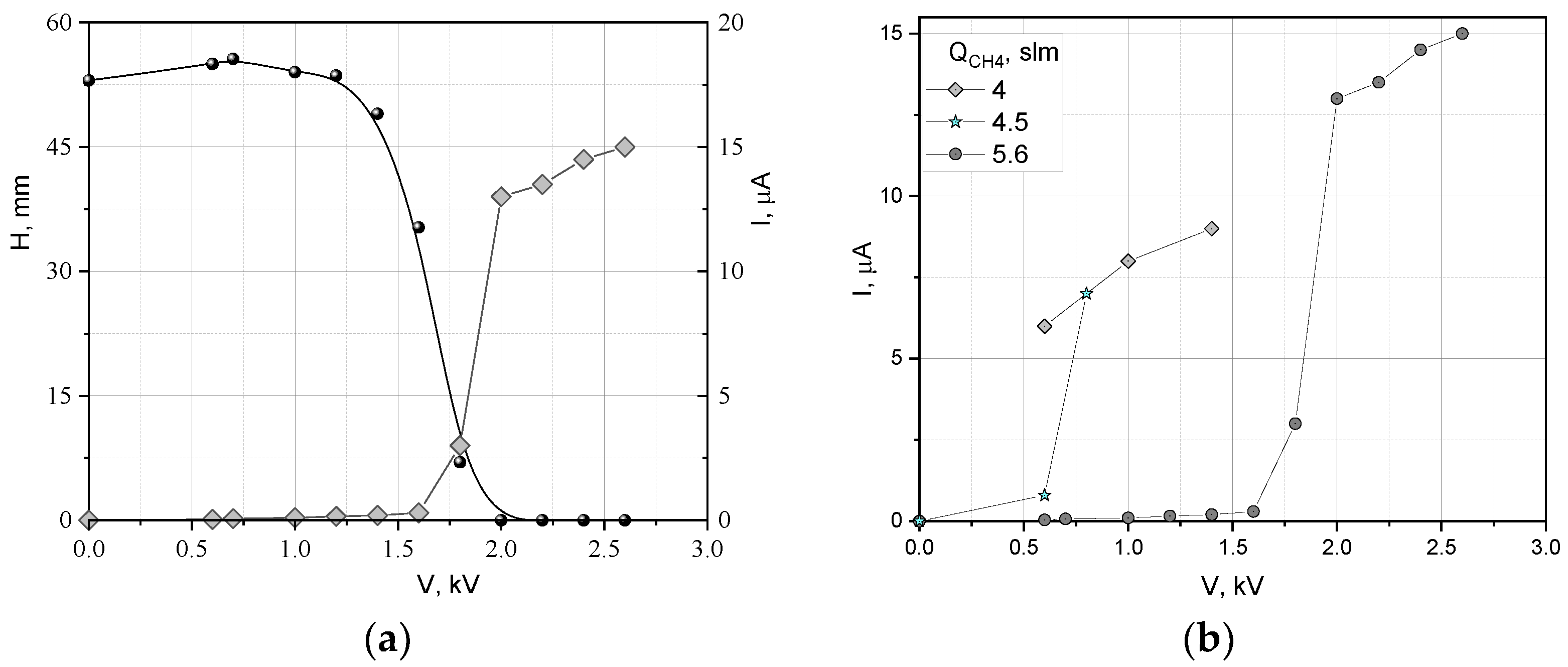
When an electric field directed along the flow axis was applied, a change in the stabilization regime occurred at voltages U > 1.6 kV. The lifted flame reattached to the burner rim. This change in the stabilization regime was accompanied by an abrupt change in the volt-ampere characteristic (VAC) of the flame (see
Figure 4a). After the flame reattached to the burner, the magnitude of the current flowing through it increased by two orders of magnitude.
It should be noted that changing the co-flow velocity affects the conditions for flame reattachment to the burner, but the VAC of the reattached flame remains linear with the same slope (see
Figure 4b), i.e., the resistance of the flame section between the electrodes is preserved. A similar dependence was noted in [
30] for propane diluted with nitrogen; however, the authors observed a nonlinear dependence of current on voltage.
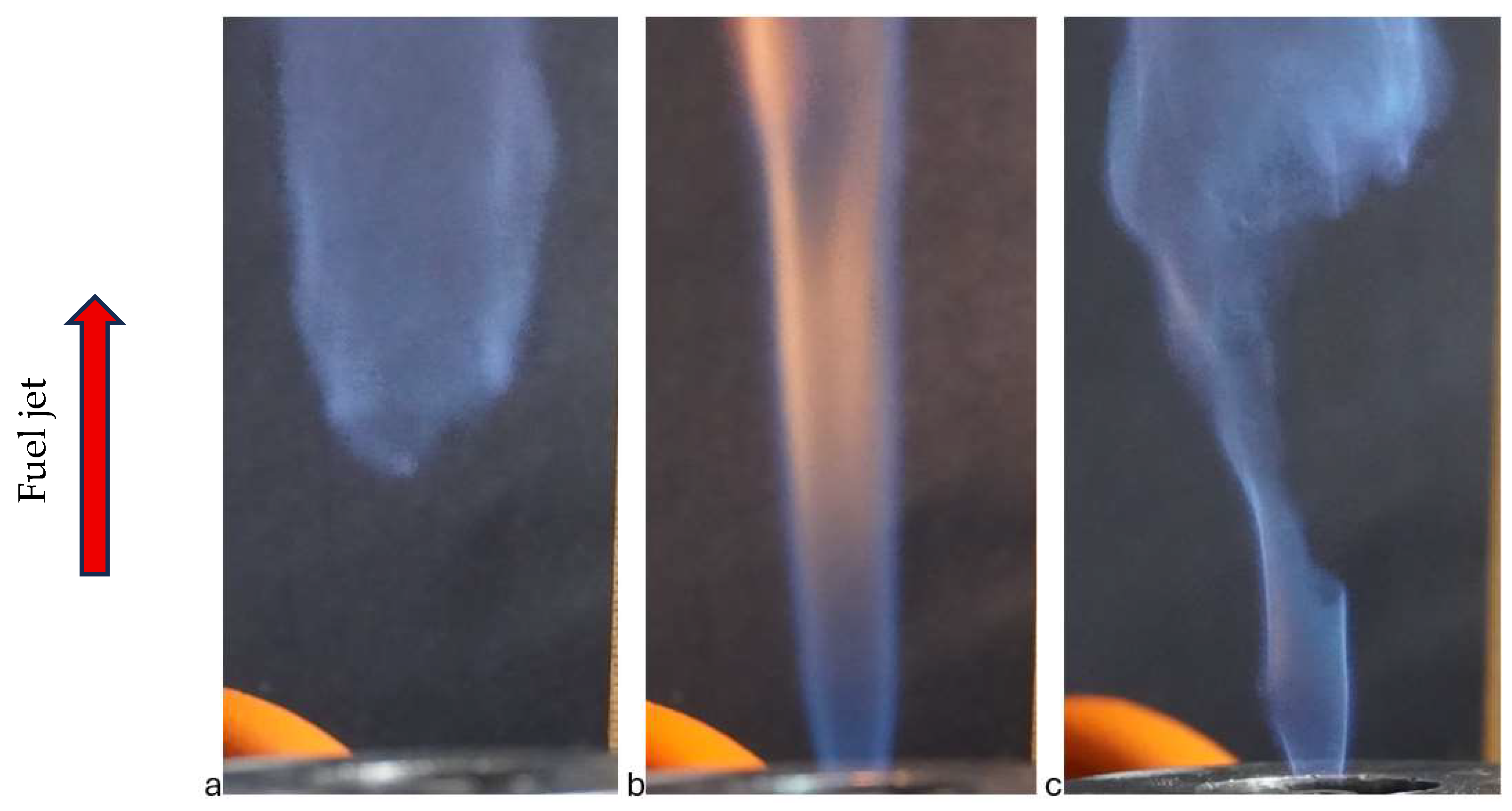
Furthermore, the reattachment of the flame to the burner rim was not instantaneous. Increasing the voltage caused a portion of the flame to touch the burner rim, followed by a stabilization pattern where part of the flame remained lifted and part was reattached (see
Figure 5). The images clearly show that the combustion regime is laminar in the reattached part of the flame and turbulent in the lifted part.
Flame attachment to the nozzle edge under the influence of an electric field was observed in [
31] while maintaining the laminar combustion regime.
Figure 6 shows Hilbert visualization of the stabilization regimes. The flow spreading near the burner rim, caused by ion motion along the flame, is clearly visible. Frames exhibiting symmetry were selected for temperature distribution reconstruction. The profile was built at a height of h/d ≈ 30, where x is the coordinate across the axis of the fuel jet. Temperature was reconstructed based on the Hilbertogram using air as a reference and under the equilibrium approximation (for details, see [
26]).
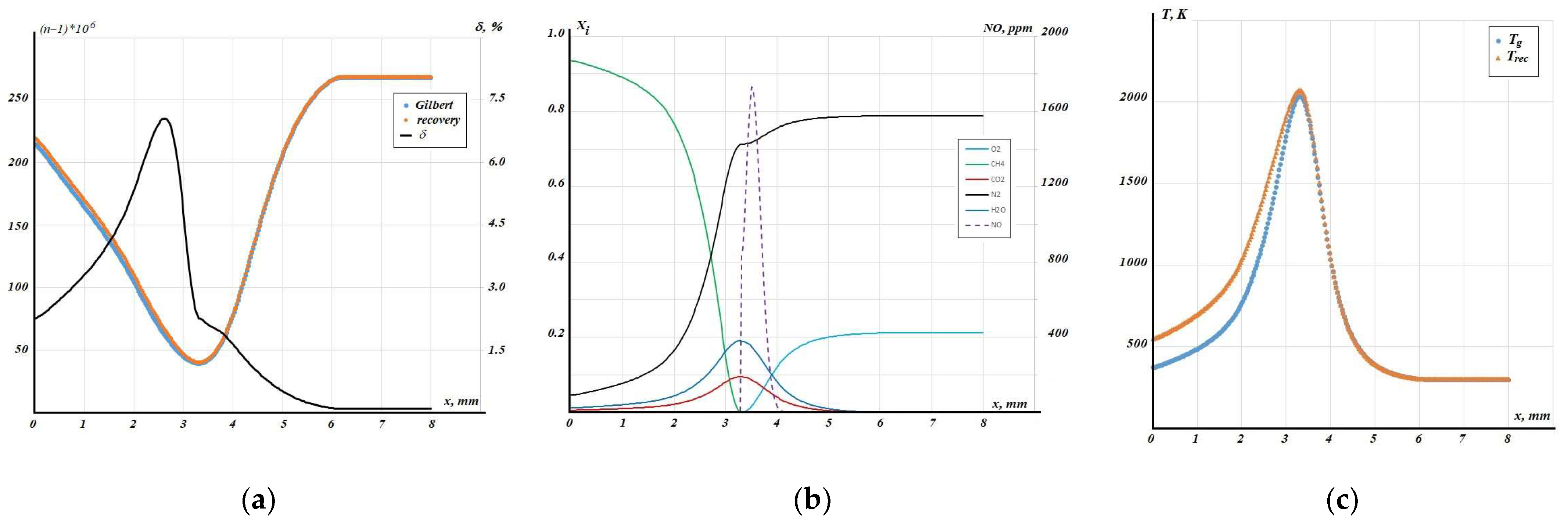
Based on the equilibrium composition, the distribution of the refractive index was determined (
Figure 7a). Surprisingly, the maximum relative deviation of the obtained refractive index from the value determined from the Hilbertogram is not located at the hottest point but in the fuel-rich flame region (δ ≈ 7%). Meanwhile, the maximum absolute deviation in the refractive index is approximately 5.5 × 10
−6, and at the temperature maximum, it is less than 1 × 10
−6.
From the results of composition reconstruction under the equilibrium approximation (
Figure 7b,
Xi volume fractions), it can be noted that the region of nitrogen oxide (NO
x) formation is located in the lean part of the flame (in the post-combustion zone).
The application of the equilibrium approach overestimates the amount of NO formed in the flame, while good agreement is observed with calculated data on nitrogen oxide production during methane combustion in air for the equilibrium approximation [
32]. A significant discrepancy in temperature (see
Figure 7c) is observed only on the jet axis, as the optical properties of methane and air differ considerably. Unfortunately, direct temperature measurement in the presence of electric fields is impossible; nevertheless, it can be stated with confidence that during reconstruction based on air, the temperature value on the axis is underestimated, while for the equilibrium approximation, it is most likely overestimated. It should also be noted that for the obtained distributions, it was not possible to apply the regularization procedure as was performed in study [
26]. When comparing with data obtained for the case of electric field impact on the flame, the reduced temperature function was used
where T
0 is the air temperature. The plots in
Figure 8 indicate a shift in the flame towards the axis under the influence of the electric field. Solid lines—without electric field, dashed lines—when a field is applied. A similar effect was registered by colleagues from the South China University of Technology in experiments with ethanol vapor combustion in an electric field [
33].
In the case of methane, under the influence of the electric field, a broadening of the flame also occurs, i.e., the zone of oxidation reactions shifts towards the axis and increases in size.
The use of ammonia–hydrogen fuel mixtures for power plants appears promising, considering that even at a 30% molar fraction of H2, the chemical activity (or the maximum critical velocity gradient for flashback) of such fuel is close to that of pure methane. Therefore, experimental studies were conducted on the effect of the hydrogen additive amount on the electrochemical properties of the mixtures.
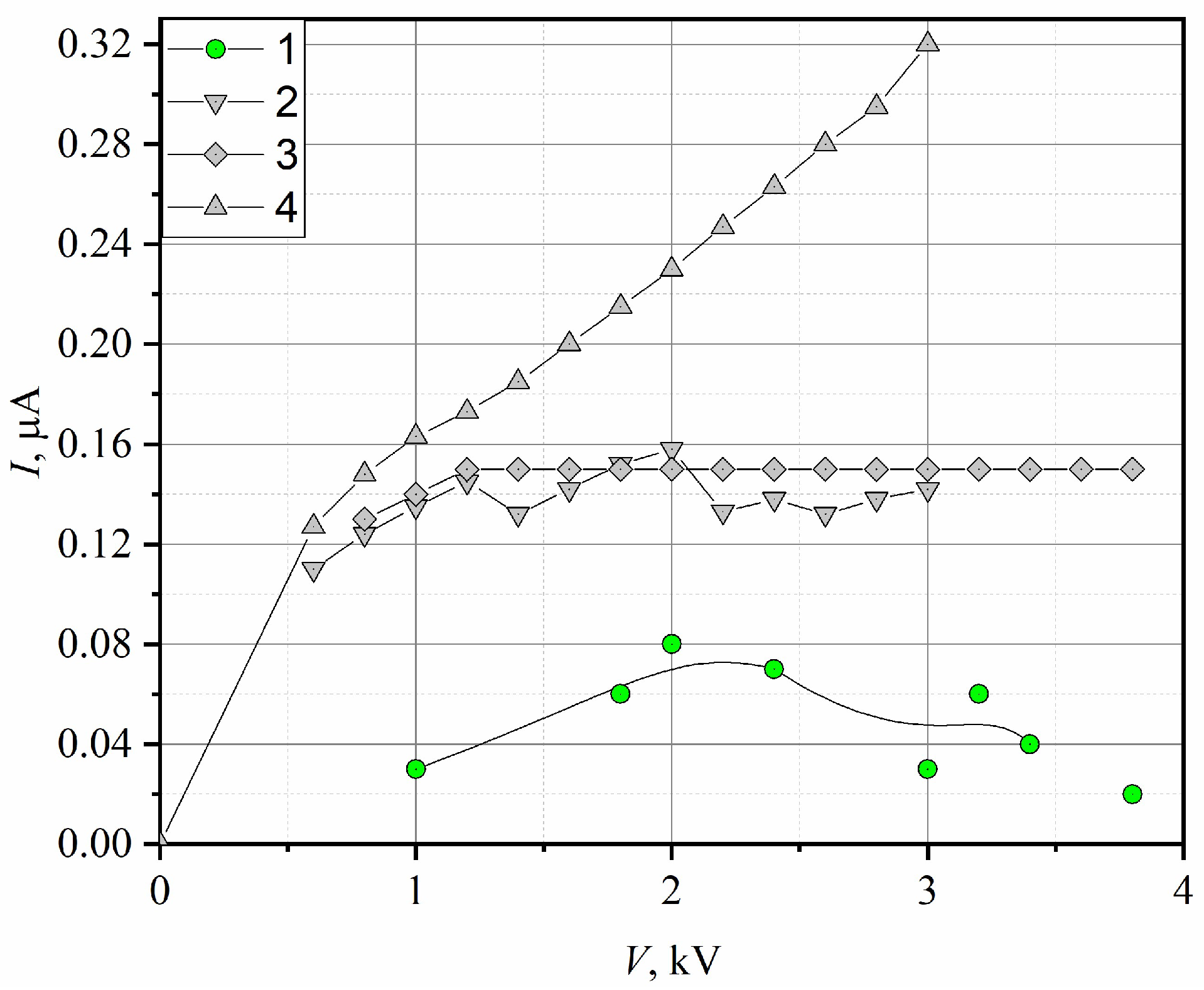
A diffusion flame of pure hydrogen has low conductivity and quickly reaches current saturation (
Figure 9, curve 1), with
Isat = 0.06 µA. Additions of 12% and 20% H
2 by volume lead to an increase in the saturation current to
Isat = 0.15 µA (
Figure 9, curves 2 and 3). For a hydrogen fraction of 30%, a qualitative change in the VAC (volt-ampere characteristic) occurs: the current exits the saturation regime and begins to grow with an increase in the voltage on the electrodes (
Figure 9, curve 4).
Thus, the flame of the blended fuel begins to possess electrical properties, which enables the creation of combustion control systems based on the action of a weak electric field. However, the current level remains almost two orders of magnitude lower than during hydrocarbon fuel combustion.
The use of ammonia–hydrogen fuel mixtures for power plants appears promising, considering that even at a 30% molar fraction of H2, the chemical activity (or the maximum critical velocity gradient for flashback) of such fuel is close to that of pure methane.
Therefore, experimental studies were conducted on the effect of the hydrogen additive amount on the electrochemical properties of the mixtures. Thus, the flame of the blended fuel begins to possess electrical properties, which enables the creation of combustion control systems based on the action of a weak electric field. However, the current level remains almost two orders of magnitude lower than during hydrocarbon fuel combustion.
4. Conclusions
Based on Hilbert visualization and recorded current-voltage characteristics (CVC), the electrochemical properties of flames from blended fuels based on methane and ammonia were determined.
The combustion of methane–hydrogen mixtures was studied under two spatial configurations of the electric field. In the case of a transverse electric field, a change in the degree of flame front stretching was determined. It was shown that despite relatively strong flame perturbations caused by electric forces, the laminar combustion regime is maintained. A transversely oriented field creates the maximum flame deformation, which expands the combustion region and reduces the flame length without compromising combustion stability (Ka* < 1).
In a longitudinal electric field, a scenario involving a change in the stabilization regime from a lifted flame to one attached to the rim was observed. A regime was recorded where part of the flame was lifted and part was attached. Furthermore, the combustion regime in the attached part of the flame was laminar, while it was turbulent in the lifted part. The change in stabilization regime was accompanied by an abrupt change in the flame’s CVC. After the flame attached to the burner, the magnitude of the current flowing through it increased by two orders of magnitude.
Results from reconstructing temperature and composition based on Hilbert visualization data under the equilibrium approximation indicated that the region of nitrogen oxide (NOx) formation is located in the lean part of the flame (in the post-combustion zone). The obtained data indicate a shift in the flame towards the axis under the influence of an electric field.
For ammonia–hydrogen fuel mixtures, it was shown that at a volumetric hydrogen fraction of 30%, a qualitative change in the CVC occurs: the current exits the saturation regime and begins to increase with rising voltage on the electrodes, although its value remains an order of magnitude lower than in the case of hydrocarbon fuel.
Thus, it can be stated that flames of blended fuels based on methane and ammonia possess electrochemical properties that enable control over stabilization using an external electric field. Furthermore, for methane–hydrogen mixtures, active control of the combustion regime can also be implemented.