Impact of Ion Crossover on Mass Transfer Polarization Regulation in High-Power Vanadium Flow Batteries
Abstract
1. Introduction
2. VFB Modeling
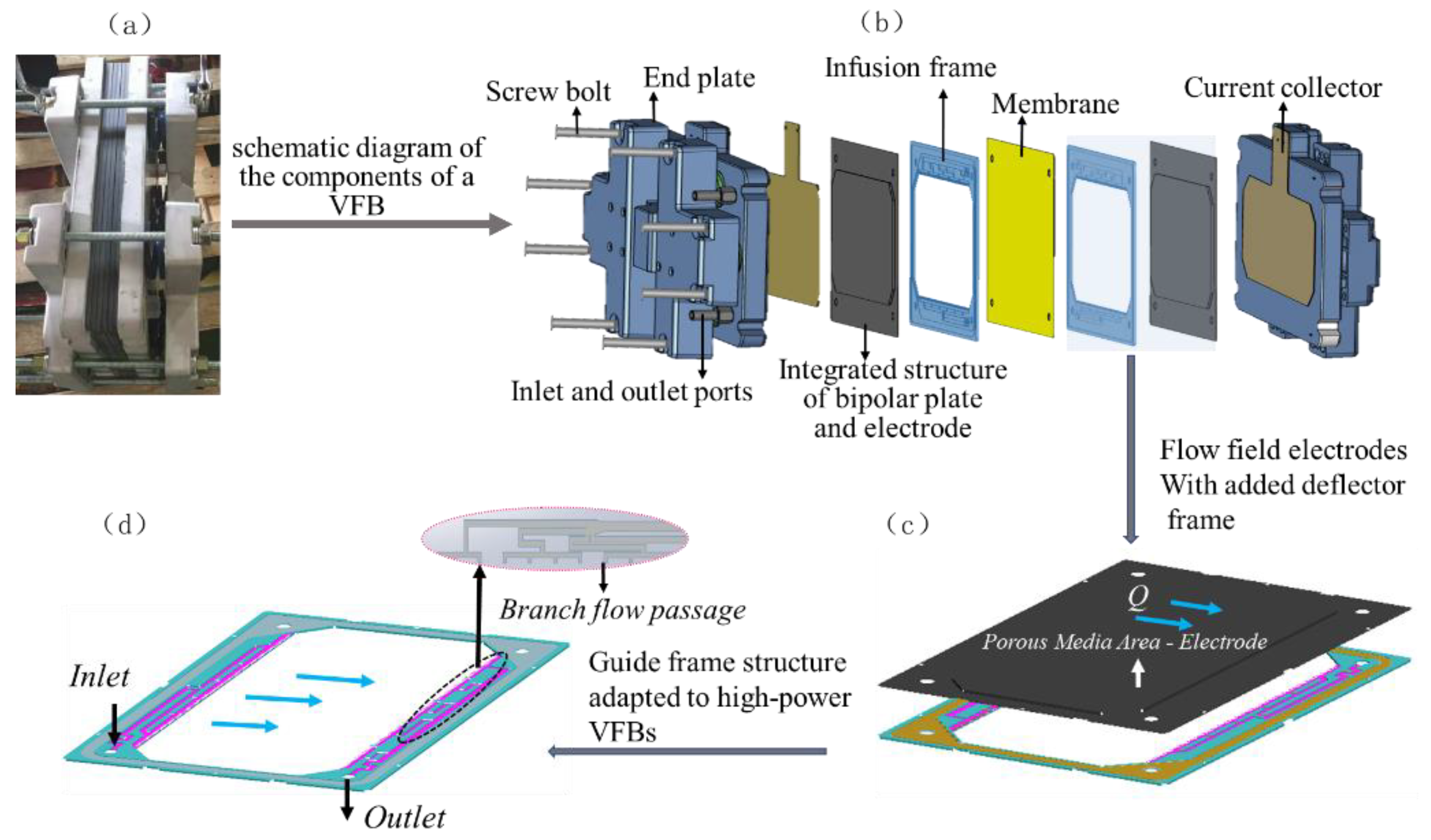
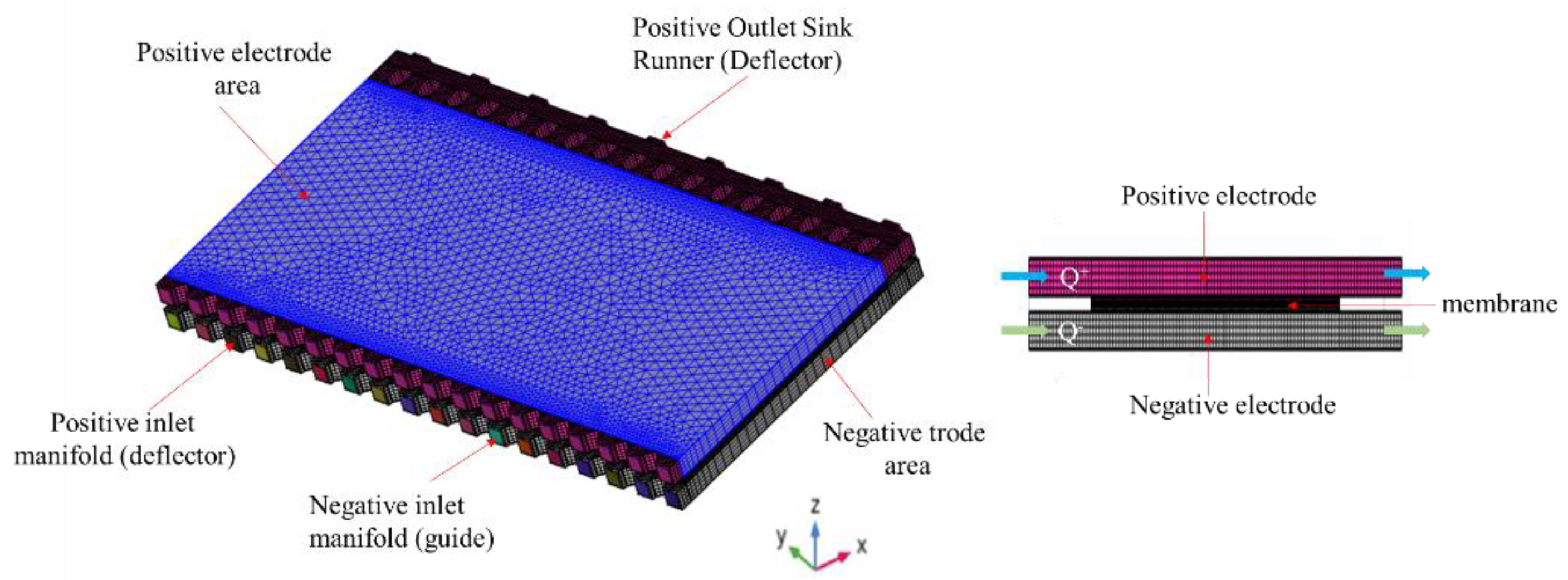
2.1. Battery Model and Computational Domain
2.2. Mass Transfer Polarization Theory
2.3. Ion Crossover Reactions
3. Relationship Between Ion Crossover and Mass Transfer Polarization Regulation
3.1. Quantification of Ion Crossover Impact on Mass Transfer Characteristics
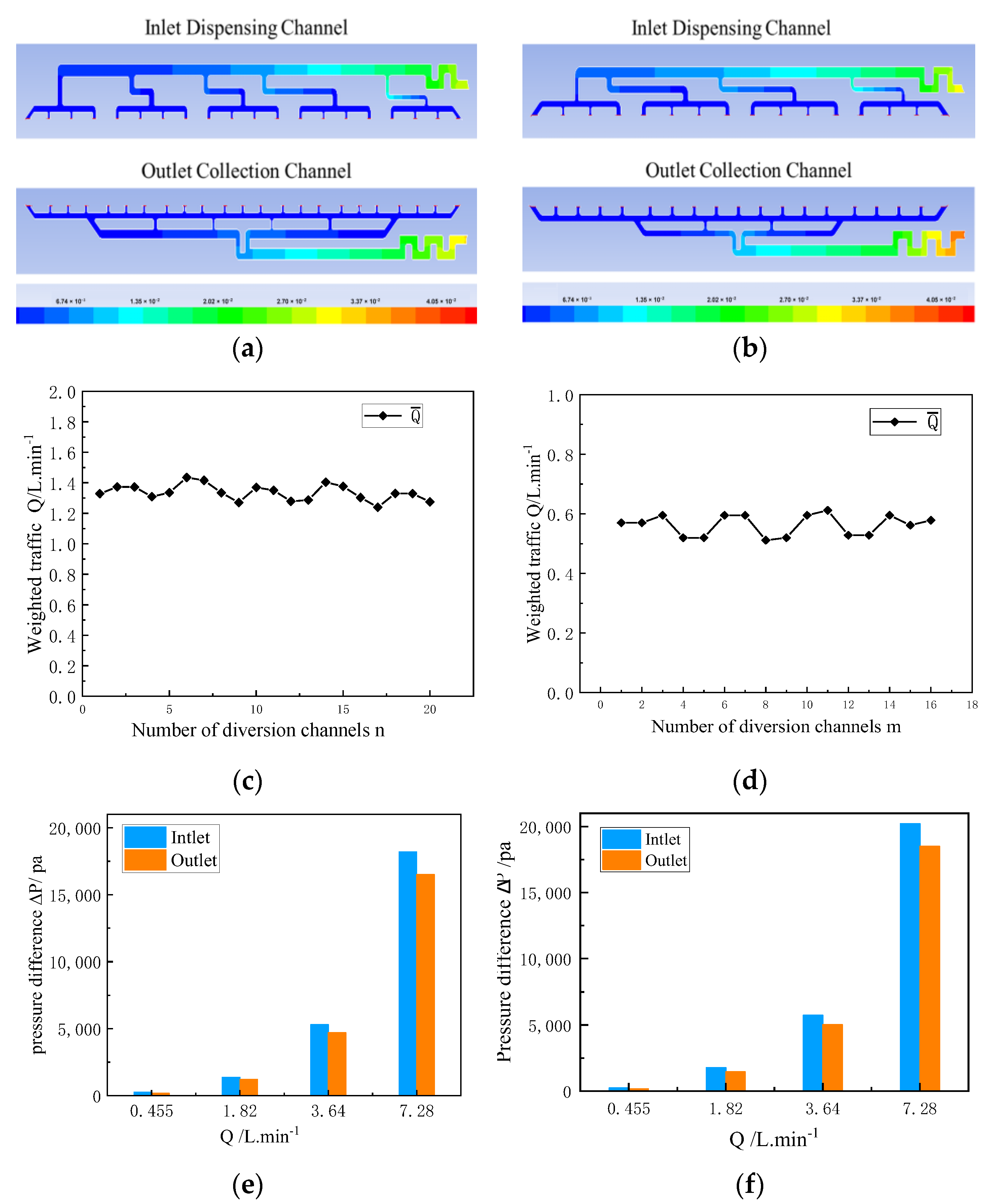
3.2. Mass Transfer Regulation Design Considering Ion Crossover
4. Results and Discussion
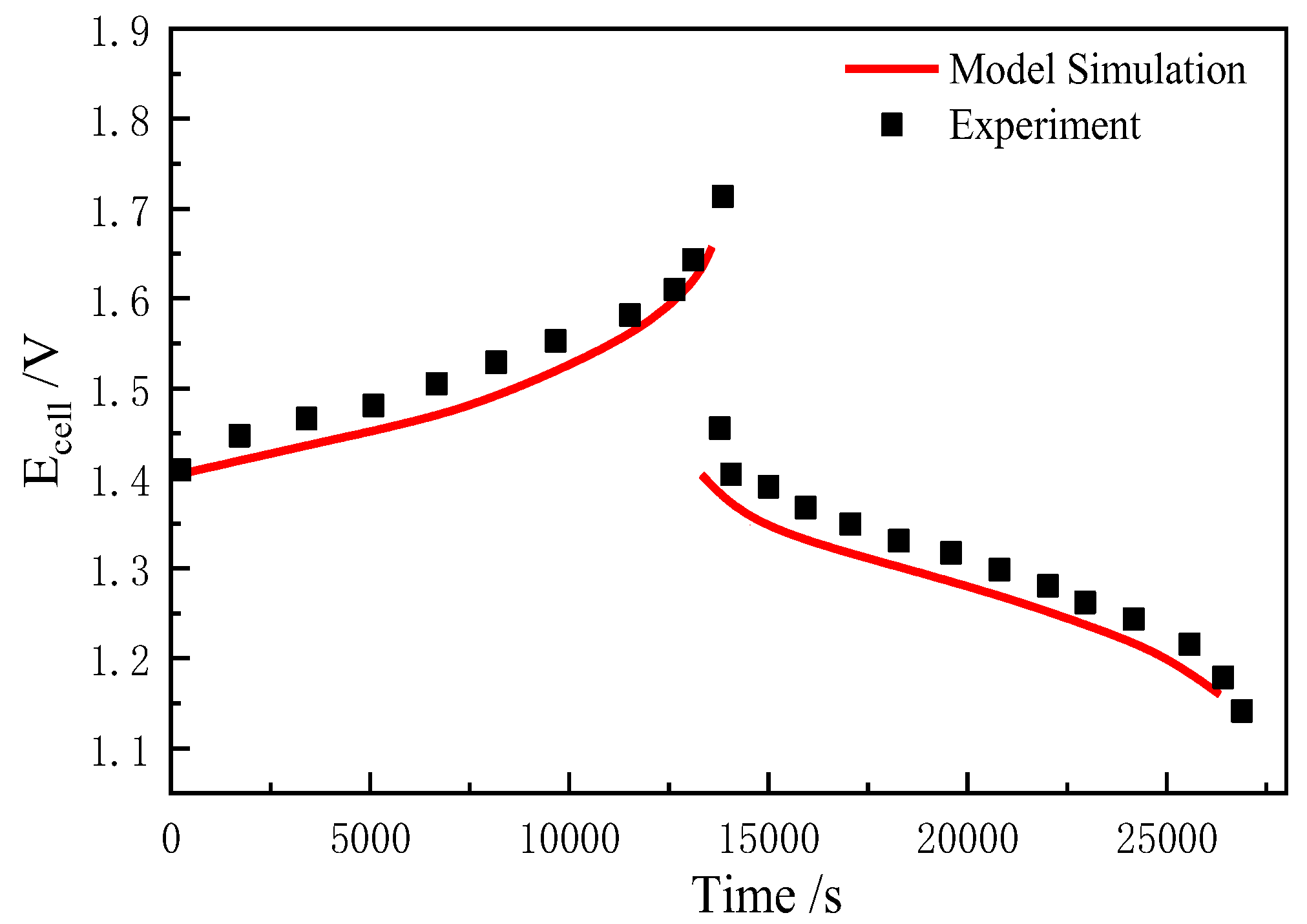
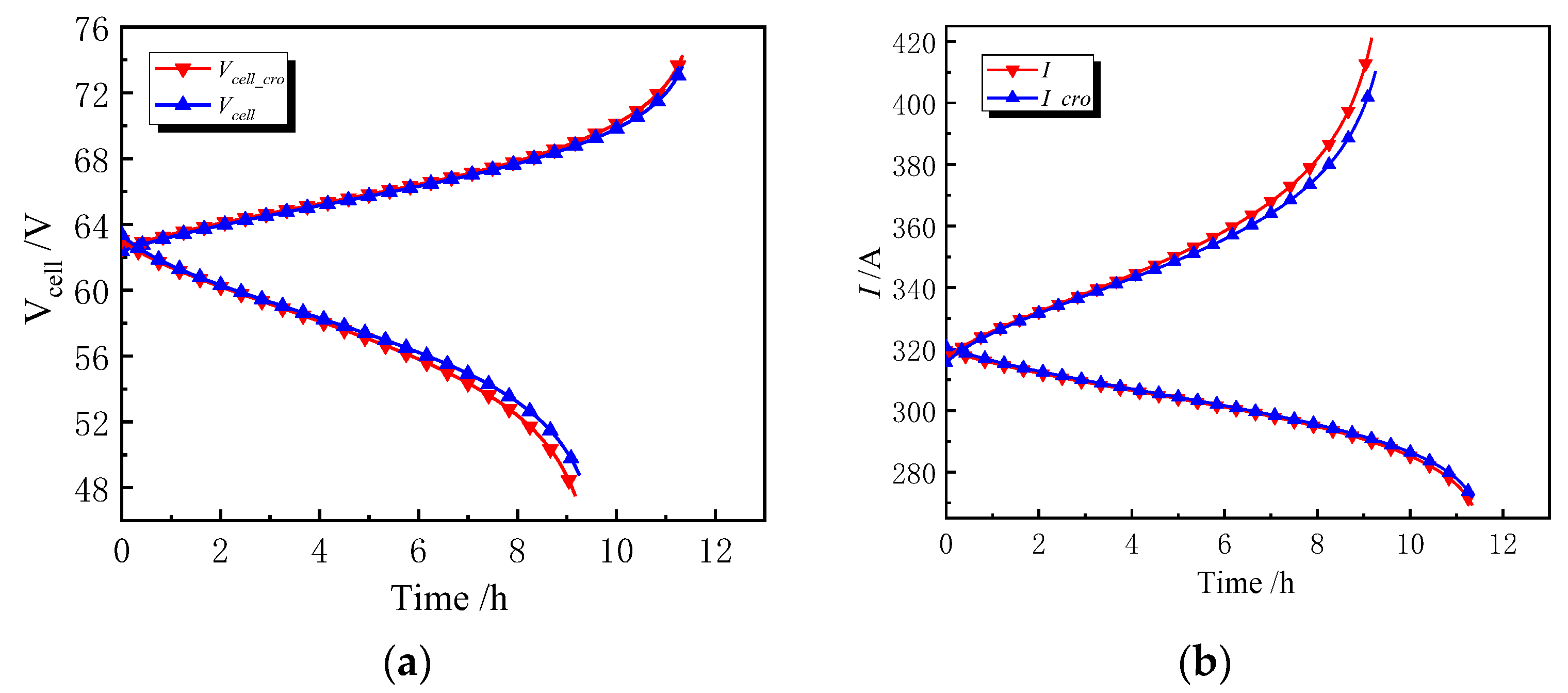
4.1. Model Validation
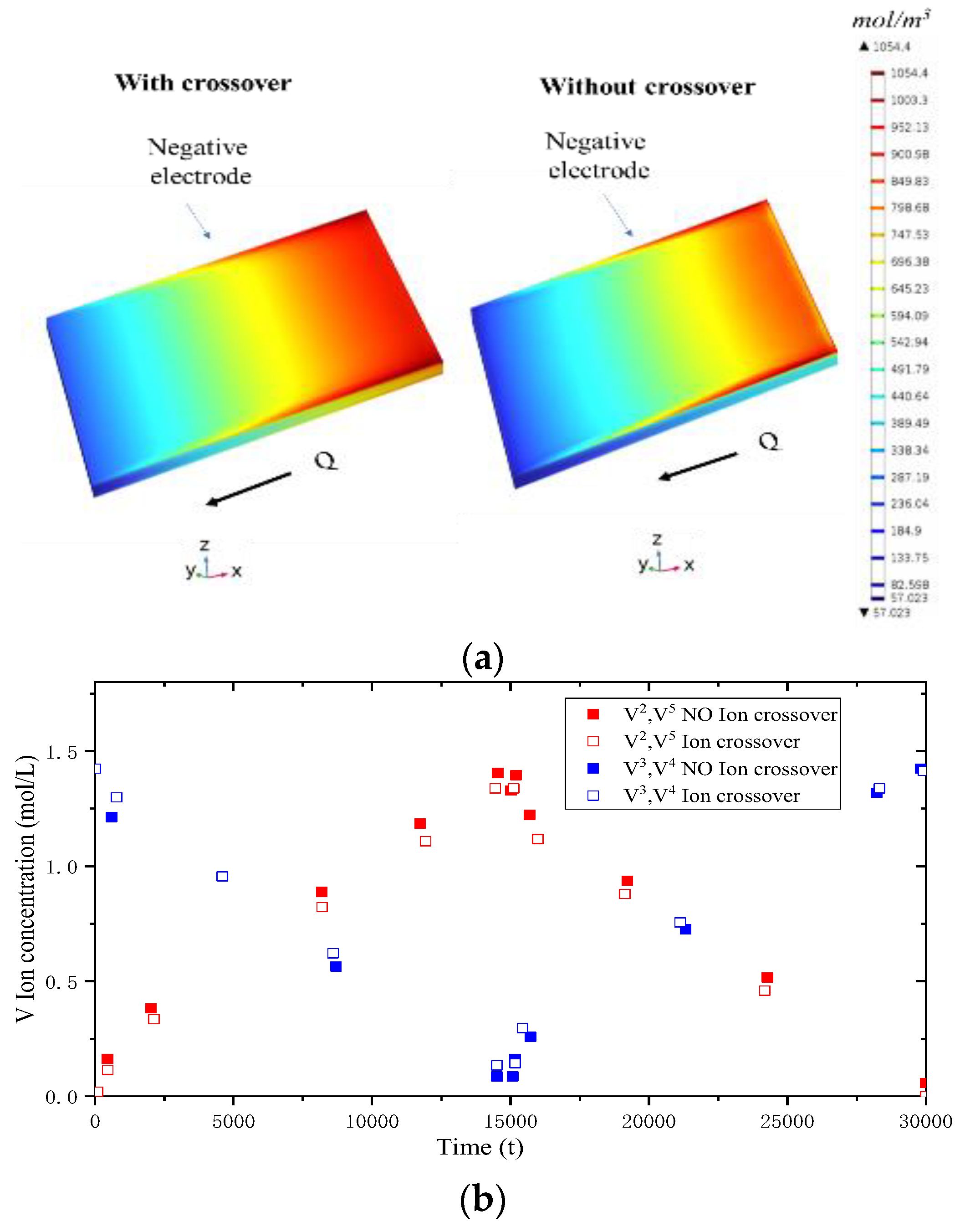
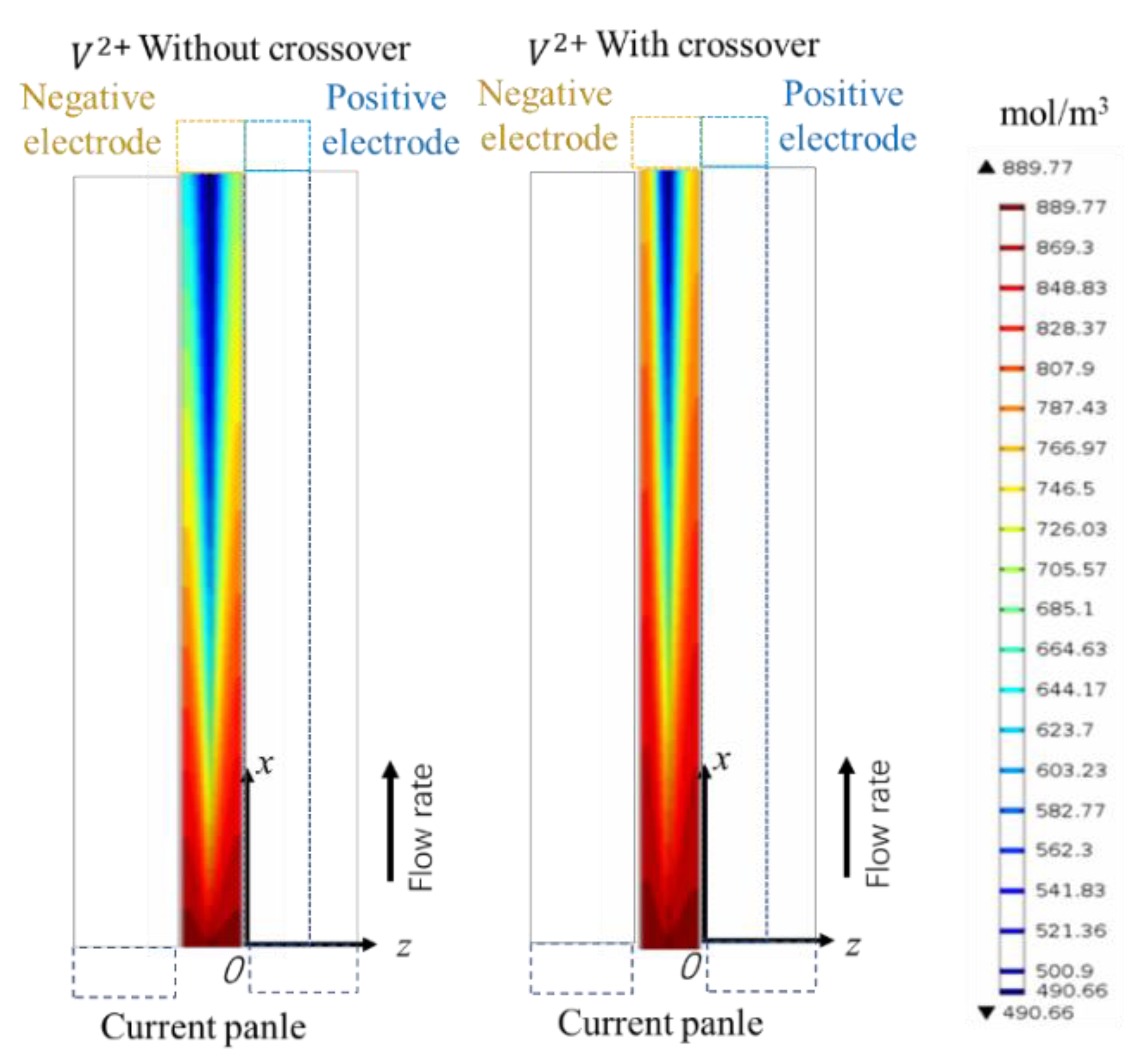
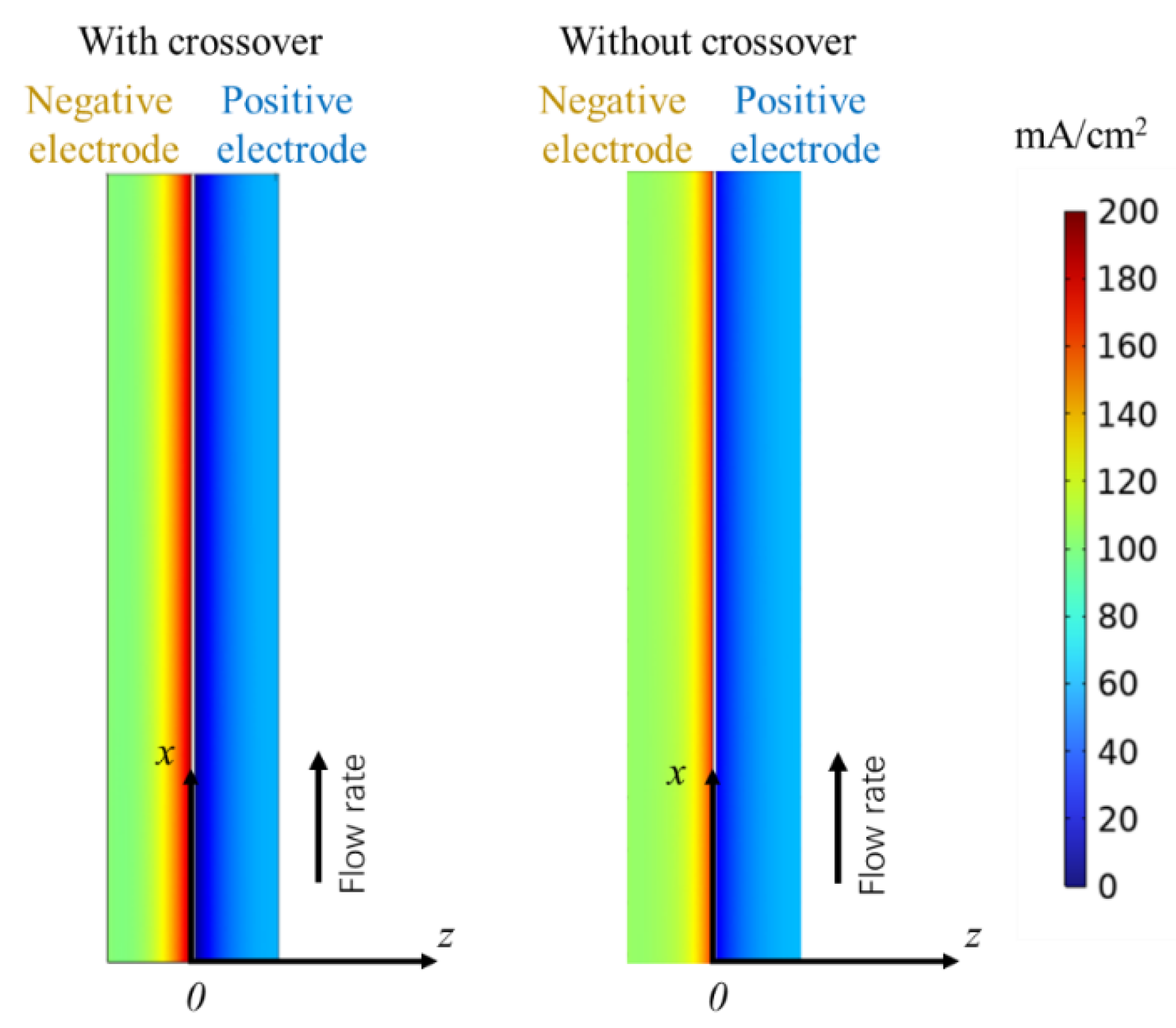
4.2. Ion Crossover Characteristics
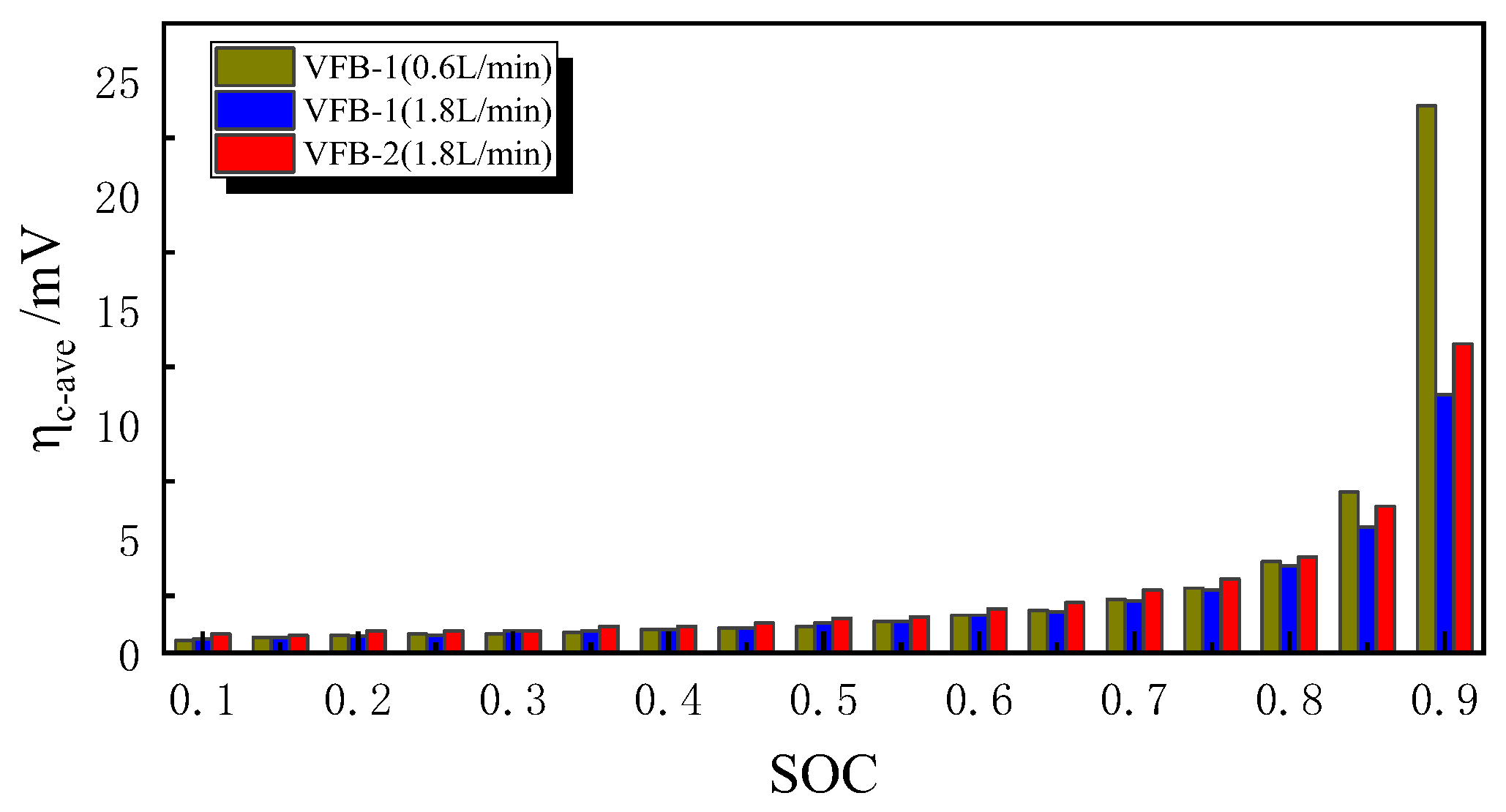
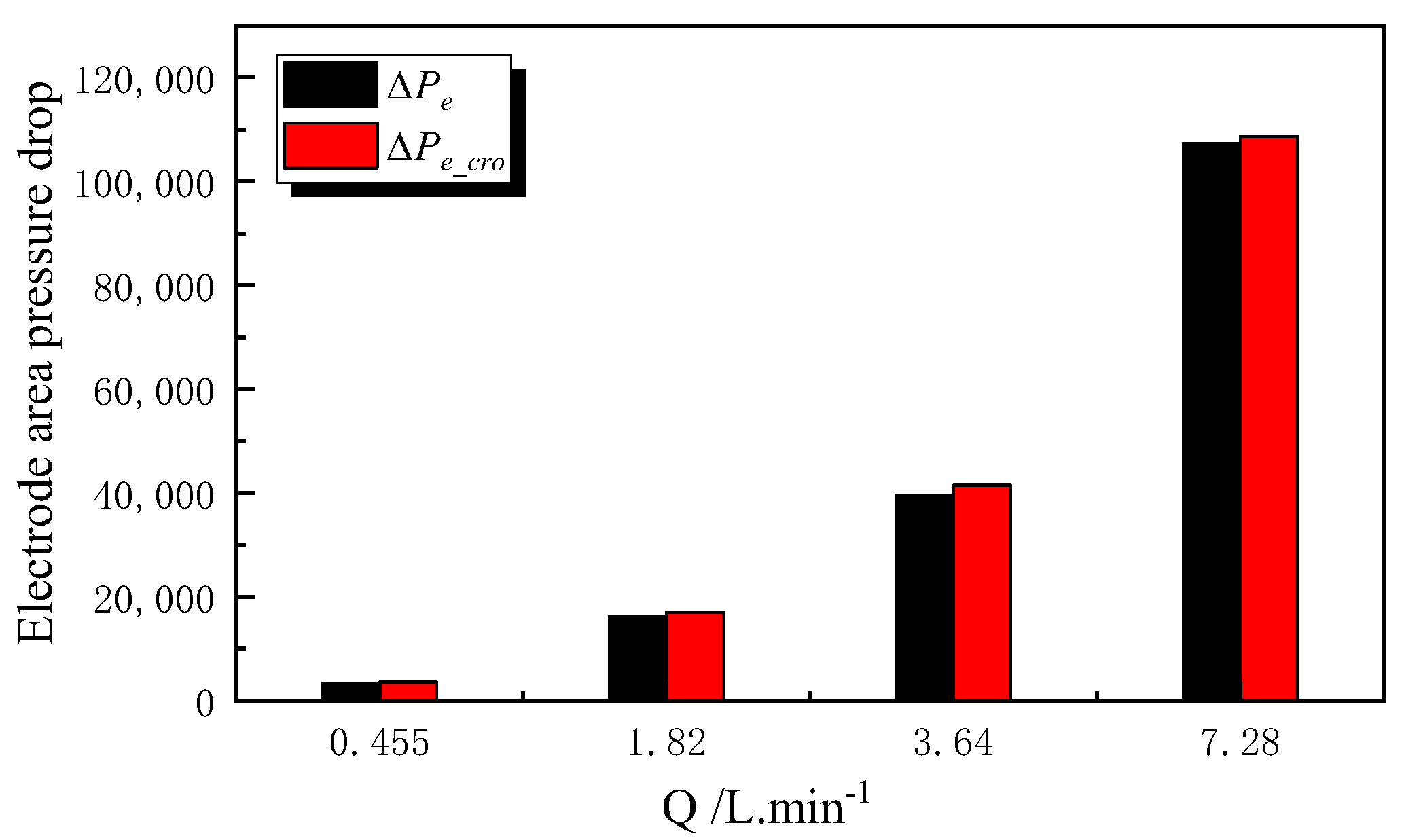
4.3. Quantification of Ion Crossover Impact on Mass Transfer Polarization Regulation
5. Conclusions
- (1)
- Model Validation and Error Analysis: The simulated charge/discharge voltage curves agree well with experimental data, with errors within ±2% and a maximum error of 1.5% (charging phase). This indicates the established 3D numerical model can accurately describe the mass transfer behavior of high-power VFB modules during actual operation. Limitations of the model include neglecting the Donnan potential and ionic interactions, which produces modeling errors, which may affect long-term accuracy. Future models could use the Poisson–Boltzmann equation to address this coupling.
- (2)
- Ion Crossover Characteristics and Distribution: The degree of ion crossover is positively correlated with the concentration gradient across the membrane between the positive and negative electrodes. Simulations show ion crossover has a more significant impact on the concentration distribution and current density distribution in the negative electrolyte. During charging, ion crossover causes an average reactant concentration distribution deviation of 3–5%, and reduces the average reaction current density in the negative and positive electrodes by about 11% and 5%, respectively.
- (3)
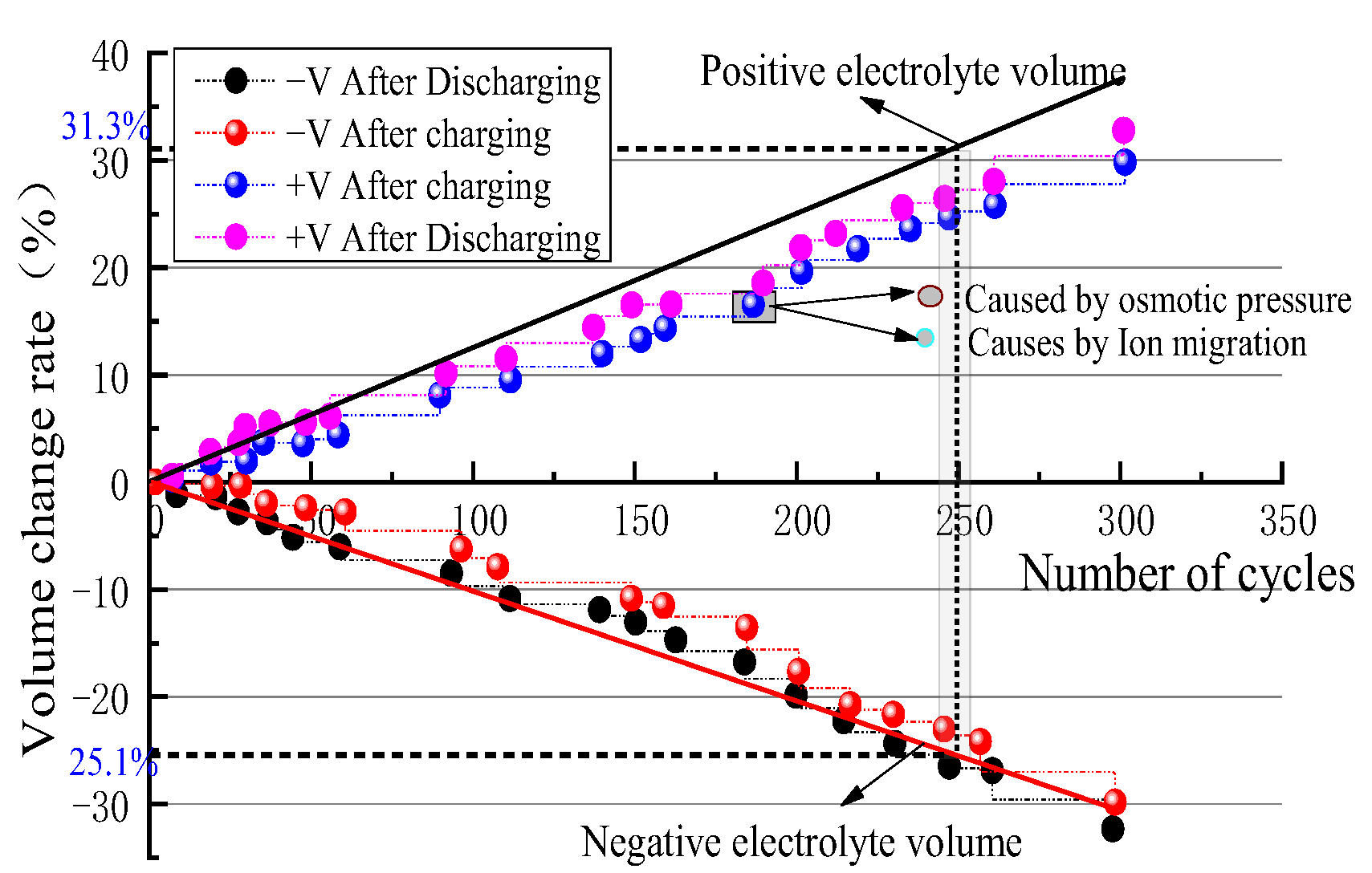
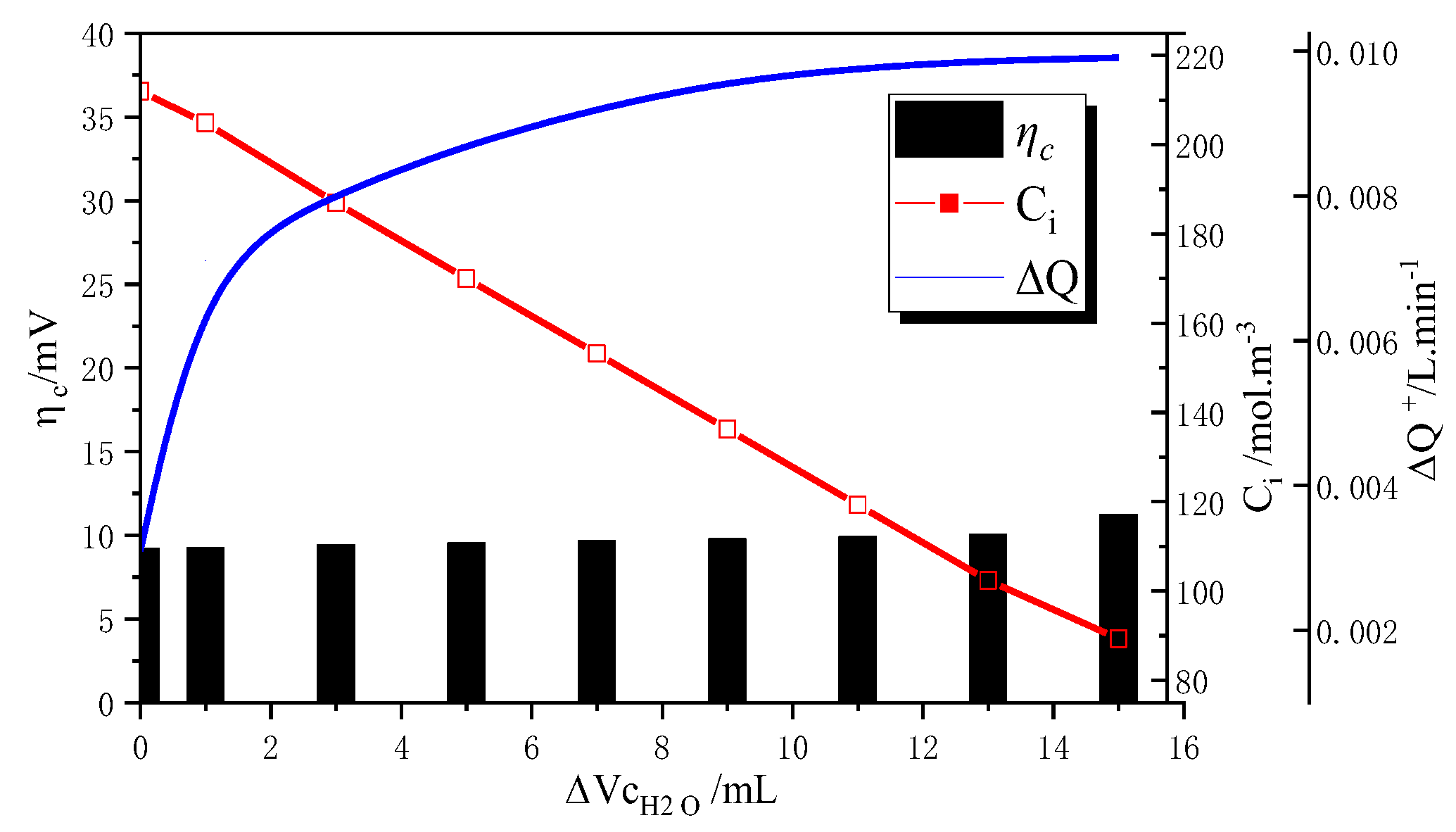
- Impact of Ion Crossover on Mass Transfer Polarization Regulation: In high-power VFBs, ion crossover significantly affects the electrode reaction zone pressure drop, charge/discharge performance curves, and system efficiency by inducing a flow rate difference ΔQ between positive and negative electrolytes. Specifically, it increases the electrode region pressure drop, causing the charging voltage to increase by ~0.85% and the discharging voltage to decrease by ~1.1%; it significantly reduces system efficiency, causing a Coulombic efficiency (CE) decrease of ~1.1% at the optimized flow point and an Energy efficiency (EE) decrease of ~1.5% during high-flow-rate operation. The 1.5% EE loss is critical for grid-scale VFBs. Future work will optimize channel width/layout using genetic algorithms to minimize ΔQ impact, with experimental validation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- M2 PRESSWIRE. Vanadium Redox Flow Battery (VFB) Market 2018 Global Analysis, Opportunities and Forecast to 2025; Normans Media Ltd.: Coventry, UK, 2018. [Google Scholar]
- Zeng, C.; Kim, S.; Chen, Y.; Fu, Y.; Bao, J.; Xu, Z.; Wang, W. In-situ characterization of kinetics and mass transfer for vanadium redox flow batteries. Electrochem. Soc. 2023, 170, 030507. [Google Scholar] [CrossRef]
- Yan, T.; Lin, H.; Xu, Y.; Ma, K.; Liu, L.; Xi, J. In situ mapping of oxygen evolution reaction in aqueous flow batteries. Power Sources 2024, 592, 233919. [Google Scholar] [CrossRef]
- Huang, Z.; Mu, A.; Wu, L. Critical issues in all-vanadium redox flow batteries. ACS Sustain. Chem. Eng. 2022, 10, 7993–8003. [Google Scholar] [CrossRef]
- Yeonho, A.; Dukjoon, K. High efficiency of vanadium flow battery using pore-filled anion exchange membranes. Ind. Eng. Chem. 2022, 110, 395–404. [Google Scholar]
- Jiang, S.; Li, Y.; Wang, H.; Li, L.; Wei, Q.; Shi, H. SPEEK composite membrane with ultrahigh selectivity for vanadium flow battery. Membr. Sci. 2024, 699, 122663. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Zhang, K.; Zhang, D.; Hou, S.; Zhao, J.; He, H.; Wu, X.; Liu, J.; Yan, C. Modulating sulfur-vacancy defect in MoS2−x for vanadium flow batteries. Energy Storage Mater. 2024, 69, 103442. [Google Scholar] [CrossRef]
- Xavier, M.M.; Sivasankaran, S.; George, A. Stable ion exchange membranes for vanadium batteries. Power Sources 2024, 591, 233857. [Google Scholar]
- Wang, X.; Ye, Q. Aggravation of side reactions by insufficient liquid supply in vanadium flow battery. Energy Storage Sci. Technol. 2022, 11, 1455–1467. [Google Scholar]
- Chou, Y.-S.; Yen, S.-C.; Arpornwichanop, A.; Singh, B.; Chen, Y.-S. Mathematical model to Study vanadium ion crossover in an All-Vanadium Redox Flow Battery. ACS Sustain. Chem. Eng. 2021, 9, 5377–5387. [Google Scholar] [CrossRef]
- Lawton, J.S.; Jones, A.M.; Tang, Z.; Aaron, D.S.; Mench, M.M. Polarization losses in vanadium flow batteries. Electrochem. Soc. 2017, 164, A2987–A2994. [Google Scholar] [CrossRef]
- Oh, K.; Moazzam, M.; Gwak, G.; Ju, H. Water crossover phenomena in all-vanadium redox flow batteries. Electrochim. Acta 2019, 297, 101–111. [Google Scholar] [CrossRef]
- Yang, B.Y. Study on Hydration Structure and Permeation Mechanism of Vanadium Ions. Master’s Thesis, Dalian University of Technology, Dalian, China, 2019; pp. 115–177. [Google Scholar]
- Sharma, H.; Kumar, M. Enhancing power density using modified serpentine channels. Power Sources 2021, 494, 229753. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Z.; Fan, X.; Li, X. Comparison of snake shaped and finger shaped flow channels in all vanadium flow batteries. Energy Storage Sci. Technol. 2022, 11, 10. [Google Scholar]
- Huang, Z.; Mu, A.; Wu, L.; Wang, H. Flow field design and flow rate optimization. Energy 2021, 234, 121266. [Google Scholar]
- Wang, Z.Y.; Guo, Z.X.; Fan, X.Z.; Zhao, T.S. Comparative study between serpentine and interdigitated flow fields for vanadium redox flow batteries. Energy Storage Sci. Technol. 2022, 11, 1121–1130. [Google Scholar]
- Ali, E.; Kwon, H.; Kim, J.; Park, H. Numerical study on serpentine flow channels. Energy Storage 2020, 32, 101802. [Google Scholar] [CrossRef]
- Al-Yasiri, M.; Park, J. Channel geometry of vanadium flow batteries. Electrochem. Soc. 2017, 164, A1970–A1982. [Google Scholar] [CrossRef]
- Zheng, Q.; Xing, F.; Li, X.; Ning, G.; Zhang, H. Mass transport polarization regulation in flow-through vanadium battery. Power Sources 2016, 324, 402–411. [Google Scholar] [CrossRef]
- Pan, J. Model Study of Vanadium Flow Battery. Ph.D. Thesis, Central South University, Changsha, China, 2025. [Google Scholar]
- Li, Z.; Wang, F.; Huang, Z. Numerical Analysis and Research on Mass Transfer Performance of Vanadium Redox Flow Battery Based on Novel Spiral Flow Field. Electrochem. Soc. 2024, 171, 060512. [Google Scholar] [CrossRef]
- Kim, J.; Park, H. Mass transfer in flow batteries characterized by comparison of electrical potentials according to regions of porous electrodes. Energy Storage 2022, 55, 715–723. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Kim, Y.-B.; Jung, S. Capacity fade prediction for vanadium redox flow batteries during long-term operations. Appl. Energy 2024, 356, 122329. [Google Scholar]
- Xiong, R.; Xiong, B.; Zhang, Q.; Shi, S.; Su, Y.; Zhang, D. Capacity fading model of vanadium redox flow battery considering water molecules migration. Int. Green Energy 2022, 19, 1613–1622. [Google Scholar] [CrossRef]
- Weber, R.; Schubert, C.; Poisl, B.; Pettinger, K.-H. Analyzing Experimental Design and Input Data Variation of a Vanadium Redox Flow Battery Model. Batteries 2023, 9, 122. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Xiong, J.; Yang, L.; Pan, G.; Yan, C.; Tang, A. Electrolyte transfer mechanism and optimization strategy for vanadium flow batteries adopting a Nafion membrane. Power Sources 2020, 447, 227503. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.G.; Jiang, Z.Y.; Yin, Y.; Chen, L. The numerical study of vanadium redox flow battery performance with different electrode morphologies and electrolyte inflow patterns. Energy Storage 2020, 28, 101941. [Google Scholar] [CrossRef]
- Firouzabadi, S.M.Z.; Naji, L.; Ghadiri, L. Comparative study on graphene oxide and sulfonated graphene oxide reinforced polyethersulfone-based cation-exchange membrane. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134392. [Google Scholar] [CrossRef]
- Li, T.; Xing, F.; Liu, T.; Sun, J.; Shi, D.; Zhang, H.; Li, X. Cost, performance prediction and optimization of a vanadium flow battery by machine-learning. Energy Environ. Sci. 2020, 13, 4353–4361. [Google Scholar] [CrossRef]




| Study | Ion Coupling Scheme | Error Compensation | Spatiotemporal Analysis | Key Limitations |
|---|---|---|---|---|
| Zheng et al. [20] | Static crossover factor | Not considered | Steady-state only | Neglects ΔQ-induced polarization |
| Huang et al. [16] | Empirical water migration | Flow field optimization only | 2D simulation | Omits dynamic SOC effects |
| Sharma et al. [14] | CFD flow optimization | N/A | Local current density | No ion crossover coupling |
| This work | Real-time ΔQ compensation | Objective function | 3D transient SOC mapping | Requires membrane parameter calibration |
| Parameter | Channel Area (m2) | Electrode Area (m2) | Electrode Thickness (m) | Inlet Velocity (L·min−1) | Outlet Pressure (Pa) | Mesh Count |
|---|---|---|---|---|---|---|
| value | 0.006 | 0.24 | 0.004 | 1.2 | 12,154,640 | hexahedral elements |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| 1500 mol/m3 | 0.929 | ||
| 4.095 × 10−6 m2 s−1 | 103 S m−1 | ||
| 3.538 × 10−6 m2 s−1 | 0.15 | ||
| 5.261 × 10−6 m2 s−1 | T | 25 °C | |
| 1.933 × 10−6 m2 s−1 | 5.1 × 10−2 kg/mol | ||
| 5.1 × 10−2 kg/mol | |||
| 6.5 × 10−2 kg/mol | |||
| 8.3 × 10−2 kg/mol | |||
| 4.4 cm | |||
| 1350 kg/m3 | 4.45 Pa·s | ||
| 1500 mol/m3 |
| Structure | Inlet Channels (n) | Outlet Channels (m) | Avg. Channel Width (mm) | Secondary Channel Width (mm) |
|---|---|---|---|---|
| VFB-1 | 20 | 23 | 2.7 | 2.8 |
| VFB-2 | 16 | 19 | 2.5 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Song, Z.; Li, Z. Impact of Ion Crossover on Mass Transfer Polarization Regulation in High-Power Vanadium Flow Batteries. Energies 2025, 18, 5192. https://doi.org/10.3390/en18195192
Li J, Song Z, Li Z. Impact of Ion Crossover on Mass Transfer Polarization Regulation in High-Power Vanadium Flow Batteries. Energies. 2025; 18(19):5192. https://doi.org/10.3390/en18195192
Chicago/Turabian StyleLi, Jianbin, Zhengxiang Song, and Zihan Li. 2025. "Impact of Ion Crossover on Mass Transfer Polarization Regulation in High-Power Vanadium Flow Batteries" Energies 18, no. 19: 5192. https://doi.org/10.3390/en18195192
APA StyleLi, J., Song, Z., & Li, Z. (2025). Impact of Ion Crossover on Mass Transfer Polarization Regulation in High-Power Vanadium Flow Batteries. Energies, 18(19), 5192. https://doi.org/10.3390/en18195192






