Thermal and Kinetic Study of Waste Polypropylene, Cardboard, Wood Biomass, and Their Blends: A Thermogravimetry Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods
2.2.1. Thermogravimetric Analysis
2.2.2. Activation Energy Determination
2.2.3. Statistical Analysis
3. Results
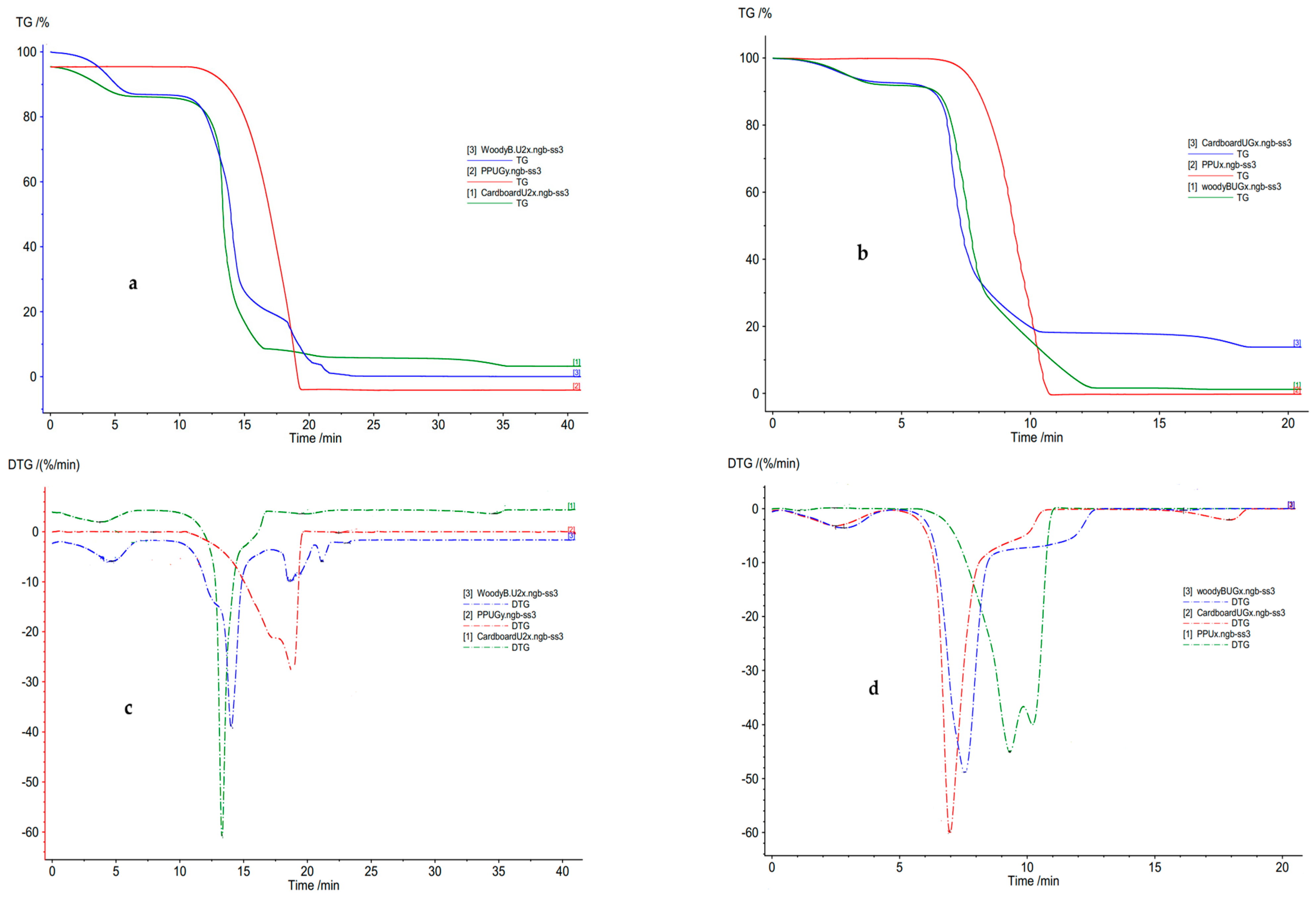
3.1. Thermal Parameters for Individual Feedstocks
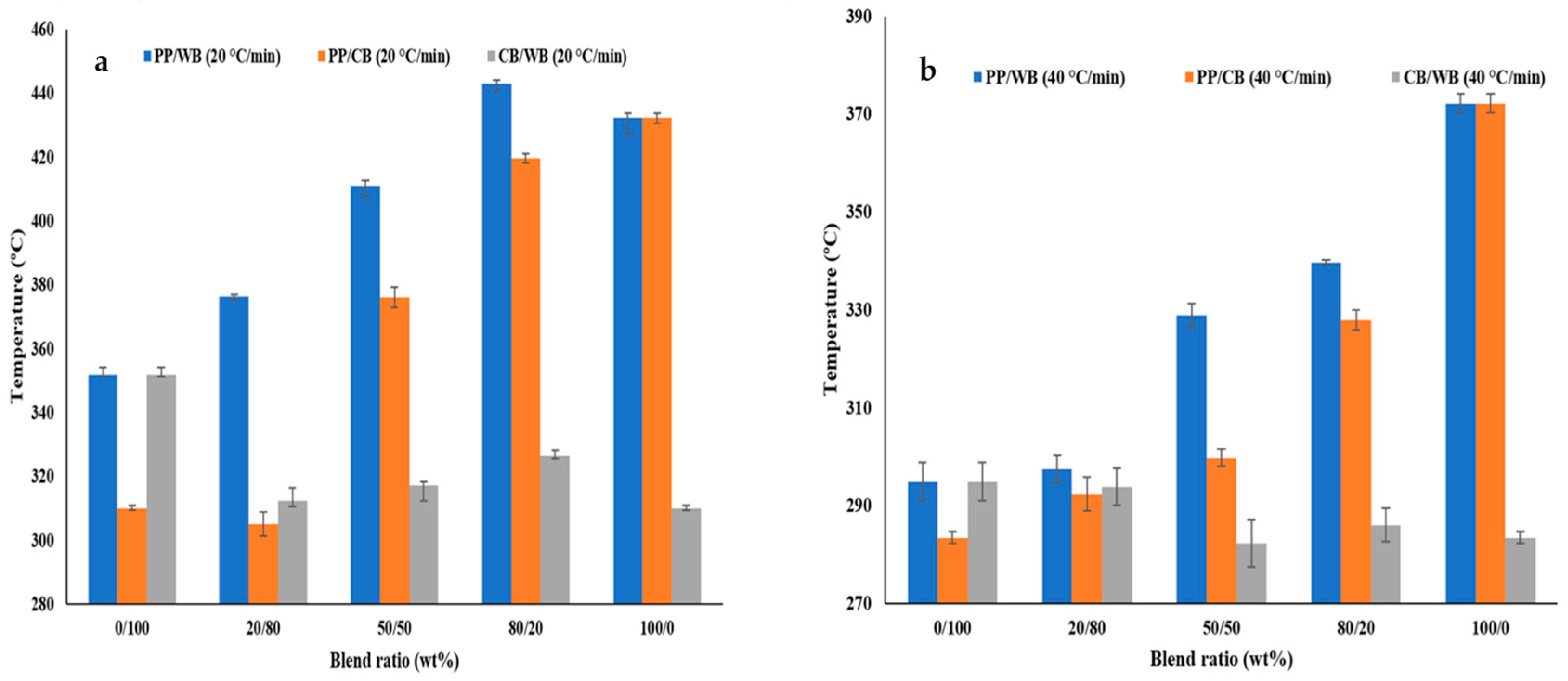
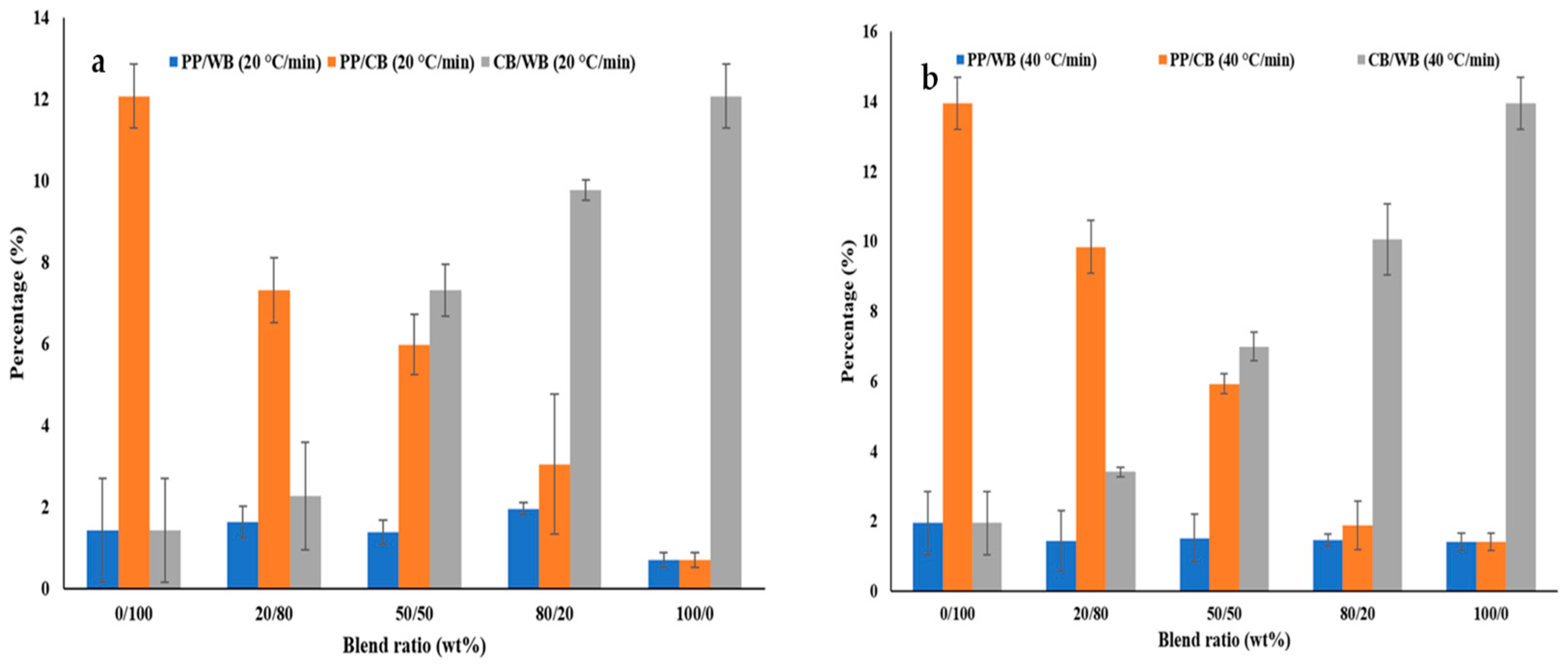
3.2. Thermal Parameters of Blended Feedstocks
3.3. Kinetic Analysis for Individual Feedstocks and Blends
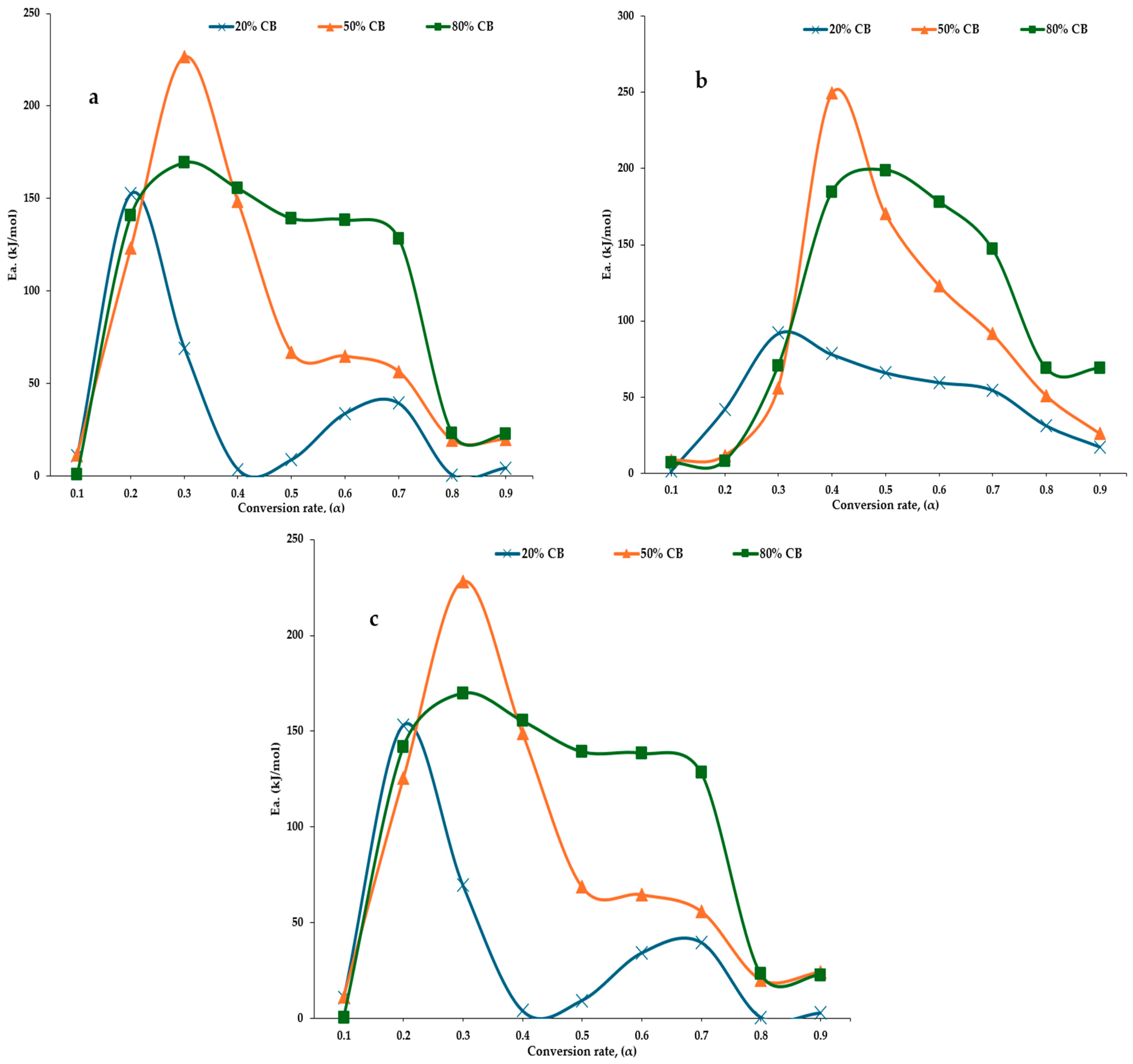
3.4. Activation Energies of the Blended Feedstocks
4. Discussion
4.1. Thermal Parameters
4.2. Activation Energy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biancini, G.; Moradi, R.; Cioccolanti, L.; Marchetti, B.; Moglie, M.; Del Zotto, L. A general model for air gasification of heterogenous municipal solid waste. Energy Convers. Manag. 2023, 278, 116749. [Google Scholar] [CrossRef]
- Veksha, A.; Giannis, A.; Yuan, G.; Tng, J.; Chan, W.P.; Chang, V.W.-C.; Lisak, G.; Lim, T.-T. Distribution and modeling of tar compounds produced during downdraft gasification of municipal solid waste. Renew. Energy 2019, 136, 1294–1303. [Google Scholar] [CrossRef]
- Gao, P.; Hu, Z.; Sheng, Y.; Pan, W.; Tang, L.; Chen, Y.; Chen, X.; Wang, F. Migration characteristics of chlorine during pyrolysis of municipal solid waste pellets. Waste Manag. 2023, 172, 208–215. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics: A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals: A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Alaedini, A.H.; Tourani, H.K.; Saidi, M. A review of waste-to-hydrogen conversion technologies for solid oxide fuel cell (SOFC) applications: Aspect of gasification process and catalyst development. J. Environ. Manag. 2023, 329, 117077. [Google Scholar] [CrossRef]
- Lora, E.E.S. Gasification of municipal solid waste for power generation in Brazil, a review of available technologies and their environmental benefits. J. Chem. Chem. Eng. 2016, 10, 249–255. [Google Scholar] [CrossRef]
- Weiland, F.; Lundin, L.; Celebi, M.; van der Vlist, K.; Moradian, F. Aspects of chemical recycling of complex plastic waste via the gasification route. Waste Manag. 2021, 126, 65–77. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Ahmed, U.; Abdul Jameel, A.G.; Zahid, U.; Usman, M.; Ahmad, N. Simulation and modelling of hydrogen production from waste plastics: Technoeconomic analysis. Polymers 2022, 14, 2056. [Google Scholar] [CrossRef]
- Bashir, M.A.; Tuo, J.; Weidman, J.; Soong, Y.; Gray, M.L.; Shi, F.; Wang, P. Plastic waste gasification for low-carbon hydrogen production: A comprehensive review. Energy Adv. 2025, 4, 330–363. [Google Scholar] [CrossRef]
- Mojaver, P.; Chitsaz, A.; Hasannejad, S.J.; Khalilian, M. Plastic Waste Gasification. In Plastic Waste Treatment and Management: Gasification Processes; Springer: Berlin/Heidelberg, Germany, 2023; pp. 47–60. [Google Scholar]
- Chai, Y.; Gao, N.; Wang, M.; Wu, C. H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem. Eng. J. 2020, 382, 122947. [Google Scholar] [CrossRef]
- Du, S.; Yuan, S.; Zhou, Q. Numerical investigation of co-gasification of coal and PET in a fluidized bed reactor. Renew. Energy 2021, 172, 424–439. [Google Scholar] [CrossRef]
- Xu, H.; Shi, B. Design and system evaluation of mixed waste plastic gasification process based on integrated gasification combined cycle system. Processes 2022, 10, 499. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Gesese, T.N.; Admase, A.T.; Asrade, E.D.; Getahun, E. Biomass Gasification for Sustainable Energy Production: Effect of Operational Parameters on Product Gas; InTech: London, UK, 2025. [Google Scholar]
- Lackner, M.; Fei, Q.; Guo, S.; Yang, N.; Guan, X.; Hu, P. Biomass gasification as a scalable, green route to combined heat and power (CHP) and synthesis gas for materials: A review. Fuels 2024, 5, 625–649. [Google Scholar] [CrossRef]
- Allesina, G.; Pedrazzi, S. Barriers to success: A technical review on the limits and possible future roles of small scale gasifiers. Energies 2021, 14, 6711. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Zhang, J.-l.; Guo, J.; Wang, G.-w.; Xu, T.; Chai, Y.-f.; Zheng, C.-l.; Xu, R.-s. Kinetics of petroleum coke/biomass blends during co-gasification. Int. J. Miner. Metall. Mater. 2016, 23, 1001–1010. [Google Scholar] [CrossRef]
- Pachamaanickam, R.I.; Navaneethakrishnan, S.V.M. Thermogravimetric assessment and multi-model kinetic study of Indian fish tail nut biomass. React. Kinet. Mech. Catal. 2025. [Google Scholar] [CrossRef]
- Kirsanovs, V.; Žandeckis, A.; Rochas, C. Biomass gasification thermodynamic model including tar and char. Agron. Res. 2016, 14, 1321–1331. [Google Scholar]
- Ibrahim, S.; Samy, M. Syngas Compositions, Cold gas and Carbon conversion efficiencies for different Coal gasification processes and all Coal ranks. J. Min. Mech. Eng. 2020, 1, 59–72. [Google Scholar] [CrossRef]
- Vamvuka, D.; Deli, M.; Stratakis, A. Effect of Mineral Additives on Fusion Behavior of Agricultural Residue Ashes during Combustion or Co-combustion with Lignite. J. Energy Power Technol. 2021, 3, 1–27. [Google Scholar] [CrossRef]
- Meng, Z.; Xu, R.; Feng, Q.; Lin, X.; Li, M. Effect of residual carbon on crystallization and solidification behavior of coal gasification coarse slag. Int. J. Coal Sci. Technol. 2025, 12, 1. [Google Scholar] [CrossRef]
- Ten Have, I.C.; van den Brink, R.Y.; Marie-Rose, S.C.; Meirer, F.; Weckhuysen, B.M. Using biomass gasification mineral residue as catalyst to produce light olefins from CO, CO2, and H2 mixtures. ChemSusChem 2022, 15, e202200436. [Google Scholar] [CrossRef]
- Wehrung, Q.; Bernasconi, D.; Michel, F.; Destefanis, E.; Caviglia, C.; Curetti, N.; Mezni, M.; Pavese, A.; Pastero, L. Accelerated Carbonation of Waste Incineration Residues: Reactor Design and Process Layout from Laboratory to Field Scales—A Review. Clean Technol. 2025, 7, 58. [Google Scholar] [CrossRef]
- Kwon, G.; Cho, D.-W.; Park, J.; Bhatnagar, A.; Song, H. A review of plastic pollution and their treatment technology: A circular economy platform by thermochemical pathway. Chem. Eng. J. 2023, 464, 142771. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Janajreh, I.; Adeyemi, I.; Elagroudy, S. Gasification feasibility of polyethylene, polypropylene, polystyrene waste and their mixture: Experimental studies and modeling. Sustain. Energy Technol. Assess. 2020, 39, 100684. [Google Scholar] [CrossRef]
- Felix, C.B.; Chen, W.-H.; Ubando, A.T.; Park, Y.-K.; Lin, K.-Y.A.; Pugazhendhi, A.; Nguyen, T.-B.; Dong, C.-D. A comprehensive review of thermogravimetric analysis in lignocellulosic and algal biomass gasification. Chem. Eng. J. 2022, 445, 136730. [Google Scholar] [CrossRef]
- Adeyemi, I.; Janajreh, I.; Arink, T.; Ghenai, C. Gasification behavior of coal and woody biomass: Validation and parametrical study. Appl. Energy 2017, 185, 1007–1018. [Google Scholar] [CrossRef]
- Chanda, M.; Roy, S.K. Industrial Polymers, Specialty Polymers, and Their Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A TG-FTIR approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar]
- Jafri, Y.; Waldheim, L.; Lundgren, J. Emerging gasification technologies for waste & biomass. IEA Bioenergy 2020, 10, 14–75. [Google Scholar]
- Bai, Y.; Fu, L.; Zhang, X. Co-gasification of biomass and plastic wastes using a kinetic model in ASPEN plus. J. Mater. Cycles Waste Manag. 2025, 27, 2198–2206. [Google Scholar] [CrossRef]
- Manić, N.; Janković, B.; Dodevski, V. Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J. Therm. Anal. Calorim. 2021, 143, 3419–3438. [Google Scholar] [CrossRef]
- Das, P. Pyrolysis study of a waste plastic mixture through different kinetic models using isothermal and nonisothermal mechanism. RSC Adv. 2024, 14, 25599–25618. [Google Scholar] [CrossRef]
- Saghir, M.; Rehan, M.; Nizami, A.-S. Recent trends in gasification based waste-to-energy. In Gasification for Low-Grade Feedstock; IntechOpen: London, UK, 2018. [Google Scholar]
- Abd-Elghany, M.; Klapötke, T.M. A review on differential scanning calorimetry technique and its importance in the field of energetic materials. Phys. Sci. Rev. 2018, 3, 20170103. [Google Scholar] [CrossRef]
- Vyazovkin, S. Conversion dependence of activation energy for model DSC curves of consecutive reactions. Thermochim. Acta 1994, 236, 1–13. [Google Scholar] [CrossRef]
- Mandal, S.; Mohalik, N.K.; Ray, S.K.; Khan, A.M.; Mishra, D.; Pandey, J.K. A comparative kinetic study between TGA & DSC techniques using model-free and model-based analyses to assess spontaneous combustion propensity of Indian coals. Process Saf. Environ. Prot. 2022, 159, 1113–1126. [Google Scholar]
- Snegirev, A.Y.; Talalov, V.; Stepanov, V.; Harris, J. Formal kinetics of polystyrene pyrolysis in non-oxidizing atmosphere. Thermochim. Acta 2012, 548, 17–26. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry: Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Akahira, T. Trans. Joint convention of four electrical institutes. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Campbell, S.D.; Sell, D.; Jenkins, R.P.; Whiting, E.B.; Fan, J.A.; Werner, D.H. Review of numerical optimization techniques for meta-device design. Opt. Mater. Express 2019, 9, 1842–1863. [Google Scholar] [CrossRef]
- Strasser, C. Terminology in Pharmacy—Thermal Analysis Provides the Overall Picture; ASTM: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Garenne, A.; Beck, P.; Montes-Hernandez, G.; Chiriac, R.; Toche, F.; Quirico, E.; Bonal, L.; Schmitt, B. The abundance and stability of “water” in type 1 and 2 carbonaceous chondrites (CI, CM and CR). Geochim. Cosmochim. Acta 2014, 137, 93–112. [Google Scholar]
- Yorulmaz, S.Y.; Atimtay, A.T. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process. Technol. 2009, 90, 939–946. [Google Scholar] [CrossRef]
- Zholdassov, Y.S.; Kwok, R.W.; Shlain, M.A.; Patel, M.; Marianski, M.; Braunschweig, A.B. Kinetics of primary mechanochemical covalent-bond-forming reactions. RSC Mechanochem. 2024, 1, 11–32. [Google Scholar] [CrossRef]
- Selim, K.; Özkar, S.; Yilmaz, L. Thermal characterization of glycidyl azide polymer (GAP) and GAP-based binders for composite propellants. J. Appl. Polym. Sci. 2000, 77, 538–546. [Google Scholar] [CrossRef]
- Wang, K.; Addiego, F.; Bahlouli, N.; Ahzi, S.; Rémond, Y.; Toniazzo, V.; Muller, R. Analysis of thermomechanical reprocessing effects on polypropylene/ethylene octene copolymer blends. Polym. Degrad. Stab. 2012, 97, 1475–1484. [Google Scholar] [CrossRef]
- Delgado, B.; González, D.L.; Godbout, S.; Lagacé, R.; Giroir-Fendler, A.; Ramirez, A.A. A study of torrefied cardboard characterization and applications: Composition, oxidation kinetics and methane adsorption. Sci. Total Environ. 2017, 593, 406–417. [Google Scholar] [CrossRef]
- Acharya, B.; Sule, I.; Dutta, A. A review on advances of torrefaction technologies for biomass processing. Biomass Convers. Biorefinery 2012, 2, 349–369. [Google Scholar] [CrossRef]
- Shankar Tumuluru, J.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W.; Blasiak, W. Characteristics of waste printing paper and cardboard in a reactor pyrolyzed by preheated agents. Fuel Process. Technol. 2013, 116, 63–71. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J.; Teixeira, S.; Soares, D.; Ferreira, M.; Teixeira, J. Influence of operating conditions on the thermal behavior and kinetics of pine wood particles using thermogravimetric analysis. Energies 2020, 13, 2756. [Google Scholar] [CrossRef]
- Chen, G.-B.; Huang, K.-C. A study of copyrolysis characteristics of sewage sludge and waste polypropylene. Int. J. Energy Res. 2023, 2023, 1406397. [Google Scholar] [CrossRef]
- Campo, E.A. Selection of Polymeric Materials: How to Select Design Properties from Different Standards; William Andrew: Norwich, NY, USA, 2008. [Google Scholar]
- Mentes, D.; Nagy, G.; Szabó, T.J.; Hornyák-Mester, E.; Fiser, B.; Viskolcz, B.; Póliska, C. Combustion behaviour of plastic waste–A case study of PP, HDPE, PET, and mixed PES-EL. J. Clean. Prod. 2023, 402, 136850. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, X.; Li, S.; Mikulčić, H.; Bešenić, T.; Deng, S.; Vujanović, M.; Tan, H.; Kumfer, B.M. Synergistic effects during co-pyrolysis of biomass and plastic: Gas, tar, soot, char products and thermogravimetric study. J. Energy Inst. 2019, 92, 108–117. [Google Scholar] [CrossRef]
- Rahman, A.; Adeboye, O.; Adebayo, A.; Salleh, M. Behaviour and Some Properties of Wood Plastic Composite Made from Recycled Polypropylene and Rubberwood. Jordan J. Mech. Ind. Eng. 2023, 17, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M. Polypropylene-based wood-plastic composites: Effect of using a coupling agent derived from a renewable resource. Maderas. Cienc. Tecnol. 2017, 19, 265–272. [Google Scholar]
- Stančin, H.; Mikulcic, H.; Manic, N.; Stojiljkovic, D.; Vujanović, M. Thermogravimetric and kinetic analysis of waste biomass and plastic mixtures. J. Sustain. Dev. Energy Water Environ. Syst. 2023, 11, 1–17. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Mikulčić, H.; Jin, Q.; Stančin, H.; Wang, X.; Li, S.; Tan, H.; Duić, N. Thermogravimetric analysis investigation of polyurethane plastic thermal properties under different atmospheric conditions. J. Sustain. Dev. Energy Water Environ. Syst. 2019, 7, 355–367. [Google Scholar] [CrossRef]
- Leyssens, G.; Trouvé, G.; Caplain, I.; Schönnenbeck, C.; Cazier, F. Energetic performances and environmental impact of the combustion of cardboard/sawdust in a domestic boiler. Fuel 2014, 122, 21–27. [Google Scholar] [CrossRef]
- Tappi, T. Ash in wood, pulp, paper and paperboard: Combustion at 525 C. TAPPI Test Methods T 1993, 211, 3–4. [Google Scholar]
- Ali, M.F.; Ahmed, S.; Qureshi, M.S. Catalytic coprocessing of coal and petroleum residues with waste plastics to produce transportation fuels. Fuel Process. Technol. 2011, 92, 1109–1120. [Google Scholar] [CrossRef]
- Paik, P.; Kar, K.K. Kinetics of thermal degradation and estimation of lifetime for polypropylene particles: Effects of particle size. Polym. Degrad. Stab. 2008, 93, 24–35. [Google Scholar] [CrossRef]
- Pang, Y.; Zhu, X.; Li, N.; Wang, Z. Study on CO2 co-gasification of cellulose and high-density polyethylene via TG-FTIR and ReaxFF MD. Process Saf. Environ. Prot. 2024, 186, 1471–1480. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. Effect of the melt flow index and melt flow rate on the thermal degradation kinetics of commercial polyolefins. J. Appl. Polym. Sci. 2012, 126, 1739–1745. [Google Scholar] [CrossRef]
- Shen, D.; Gu, S.; Jin, B.; Fang, M. Thermal degradation mechanisms of wood under inert and oxidative environments using DAEM methods. Bioresour. Technol. 2011, 102, 2047–2052. [Google Scholar] [CrossRef]
- Olabisi, A.S.; Balogun, A.O.; Oni, T.O.; Fakinle, B.S.; Sotoudehnia, F.; McDonald, A.G.; Ikubanni, P.P. Physicochemical characterization of woody lignocellulosic biomass and charcoal for bio-energy heat generation. Sci. Rep. 2023, 13, 19242. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Evaluating the role of feedstock composition and component interactions on biomass gasification. Fuel 2025, 381, 133528. [Google Scholar] [CrossRef]
- Klein, H.C.; Cheng, X.; Smith, J.C.; Shen, T. Transfer matrix approach to the hydrogen-bonding in cellulose Iα fibrils describes the recalcitrance to thermal deconstruction. J. Chem. Phys. 2011, 135, 085106. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Mamleev, V.; Bourbigot, S.; Bras, M.L.; Lefebvre, J. Three model-free methods for calculation of activation energy in TG. J. Therm. Anal. Calorim. 2004, 78, 1009–1027. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340, 53–68. [Google Scholar]
- Kabir, A.S.; Yuan, Z.; Kuboki, T.; Xu, C.C. De-polymerization of industrial lignins to improve the thermo-oxidative stability of polyolefins. Ind. Crops Prod. 2018, 120, 238–249. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T.H. Degradation behavior of polypropylene during reprocessing and its biocomposites: Thermal and oxidative degradation kinetics. Polymers 2020, 12, 1627. [Google Scholar] [CrossRef]



| CB (wt%) | WB (wt%) | Waste PP (wt%) |
|---|---|---|
| 50 | 50 | - |
| 80 | 20 | - |
| 20 | 80 | - |
| 100 | - | - |
| - | - | 100 |
| - | 80 | 20 |
| - | 50 | 50 |
| - | 20 | 80 |
| 80 | - | 20 |
| 50 | - | 50 |
| 20 | - | 80 |
| - | 100 | - |
| Apparatus/Controls | Description |
|---|---|
| Furnace | Silicon Carbide (0 °C to 1600 °C). Operational heating rates range from 0 °C/min to 50 °C/min. |
| Gas Controls | Purge Gas MFC—Air (N2/O2 (80/20) at a flowrate of 50 L/min |
| Protective Gas MFC—Air (N2/O2 (80/20) at a flowrate of 20 L/min | |
| Crucibles | Al2O3 (temperature range of 0 °C to 1564 °C) |
| Temperature Resolution | 0.001 °C |
| Method | Equation | Plot | Slope | Reference |
|---|---|---|---|---|
| Friedmann | [43,44] | |||
| Kissinger–Akahira–Sunose (KAS) | [45,46,47] | |||
| Numerical Optimization | [48] |
| Conversion Rate (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | Mean (kJ/mol) | |
| Friedmann | PP (r2 = 0.9966) | 120.10 | 68.90 | 54.25 | 57.80 | 77.22 | 81.81 | 54.61 | 32.82 | 17.99 | 63.70 |
| CB (r2 = 0.9947) | 59.33 | 73.21 | 130.89 | 121.62 | 110.79 | 157.78 | 181.69 | 102.98 | 107.80 | 116.20 | |
| WB (r2 = 0.9998) | 231.51 | 262.71 | 304.84 | 200.09 | 95.54 | 59.61 | 54.13 | 36.25 | 9.12 | 139.31 | |
| KAS | PP (r2 = 0.8753) | 84.22 | 60.49 | 59.86 | 48.32 | 64.31 | 66.62 | 50.21 | 40.26 | 34.74 | 56.36 |
| CB (r2 = 1.0000) | 73.21 | 99.13 | 117.98 | 146.49 | 160.81 | 165.59 | 162.51 | 141.13 | 45.50 | 113.48 | |
| WB (r2 = 0.9980) | 125.74 | 190.6 | 235.678 | 227.37 | 169.38 | 120.31 | 85.36 | 54.83 | 36.56 | 138.43 | |
| Num. Op. | PP (r2 = 0.9998) | 119.66 | 68.49 | 53.76 | 57.93 | 77.72 | 81.47 | 54.92 | 32.88 | 17.99 | 63.89 |
| CB (r2 = 0.9964) | 58.71 | 140.10 | 120.50 | 122.30 | 111.54 | 158.61 | 120.48 | 108.66 | 107.48 | 116.27 | |
| WB (r2 = 0.9999) | 231.31 | 262.76 | 304.94 | 200.09 | 95.54 | 59.62 | 54.02 | 36.02 | 9.06 | 139.26 | |
| Blend | Blend Ratio (w/w%) | Model | R2 Value | Mean Ea (kJ/mol) |
|---|---|---|---|---|
| PP/CB | 20/80 | Friedmann | 0.9932 | 22.54 |
| KAS | 1.0000 | 35.41 | ||
| Num. Op. | 0.9952 | 26.21 | ||
| 50/50 | Friedmann | 0.9932 | 27.83 | |
| KAS | 1.0000 | 37.14 | ||
| Num. Op. | 0.9952 | 27.22 | ||
| 80/20 | Friedmann | 0.9735 | 46.17 | |
| KAS | 1.0000 | 43.32 | ||
| Num. Op. | 0.9983 | 46.96 | ||
| PP/WB | 20/80 | Friedmann | 0.9932 | 25.39 |
| KAS | 1.0000 | 37.14 | ||
| Num. Op. | 0.9952 | 27.21 | ||
| 50/50 | Friedmann | 0.9988 | 82.89 | |
| KAS | 1.0000 | 66.00 | ||
| Num. Op. | 0.9992 | 83.72 | ||
| 80/20 | Friedmann | 0.9610 | 55.10 | |
| KAS | 1.0000 | 63.28 | ||
| Num. Op. | 0.9860 | 50.83 | ||
| CB/WB | 20/80 | Friedmann | 0.9979 | 32.40 |
| KAS | 0.9999 | 48.76 | ||
| Num. Op. | 0.9987 | 32.97 | ||
| 50/50 | Friedmann | 0.9982 | 79.42 | |
| KAS | 0.9999 | 82.90 | ||
| Num. Op. | 0.9986 | 80.56 | ||
| 80/20 | Friedmann | 0.9981 | 101.89 | |
| KAS | 0.9995 | 102.02 | ||
| Num. Op. | 0.9983 | 102.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonsu, M.J.D.; Palmer, G.; Yee, L.; Du Toit, E.; Rahman, M.S.; McIntosh, S. Thermal and Kinetic Study of Waste Polypropylene, Cardboard, Wood Biomass, and Their Blends: A Thermogravimetry Approach. Energies 2025, 18, 5193. https://doi.org/10.3390/en18195193
Bonsu MJD, Palmer G, Yee L, Du Toit E, Rahman MS, McIntosh S. Thermal and Kinetic Study of Waste Polypropylene, Cardboard, Wood Biomass, and Their Blends: A Thermogravimetry Approach. Energies. 2025; 18(19):5193. https://doi.org/10.3390/en18195193
Chicago/Turabian StyleBonsu, Martinson Joy Dadson, Graeme Palmer, Lachlan Yee, Ernest Du Toit, Md Sydur Rahman, and Shane McIntosh. 2025. "Thermal and Kinetic Study of Waste Polypropylene, Cardboard, Wood Biomass, and Their Blends: A Thermogravimetry Approach" Energies 18, no. 19: 5193. https://doi.org/10.3390/en18195193
APA StyleBonsu, M. J. D., Palmer, G., Yee, L., Du Toit, E., Rahman, M. S., & McIntosh, S. (2025). Thermal and Kinetic Study of Waste Polypropylene, Cardboard, Wood Biomass, and Their Blends: A Thermogravimetry Approach. Energies, 18(19), 5193. https://doi.org/10.3390/en18195193






