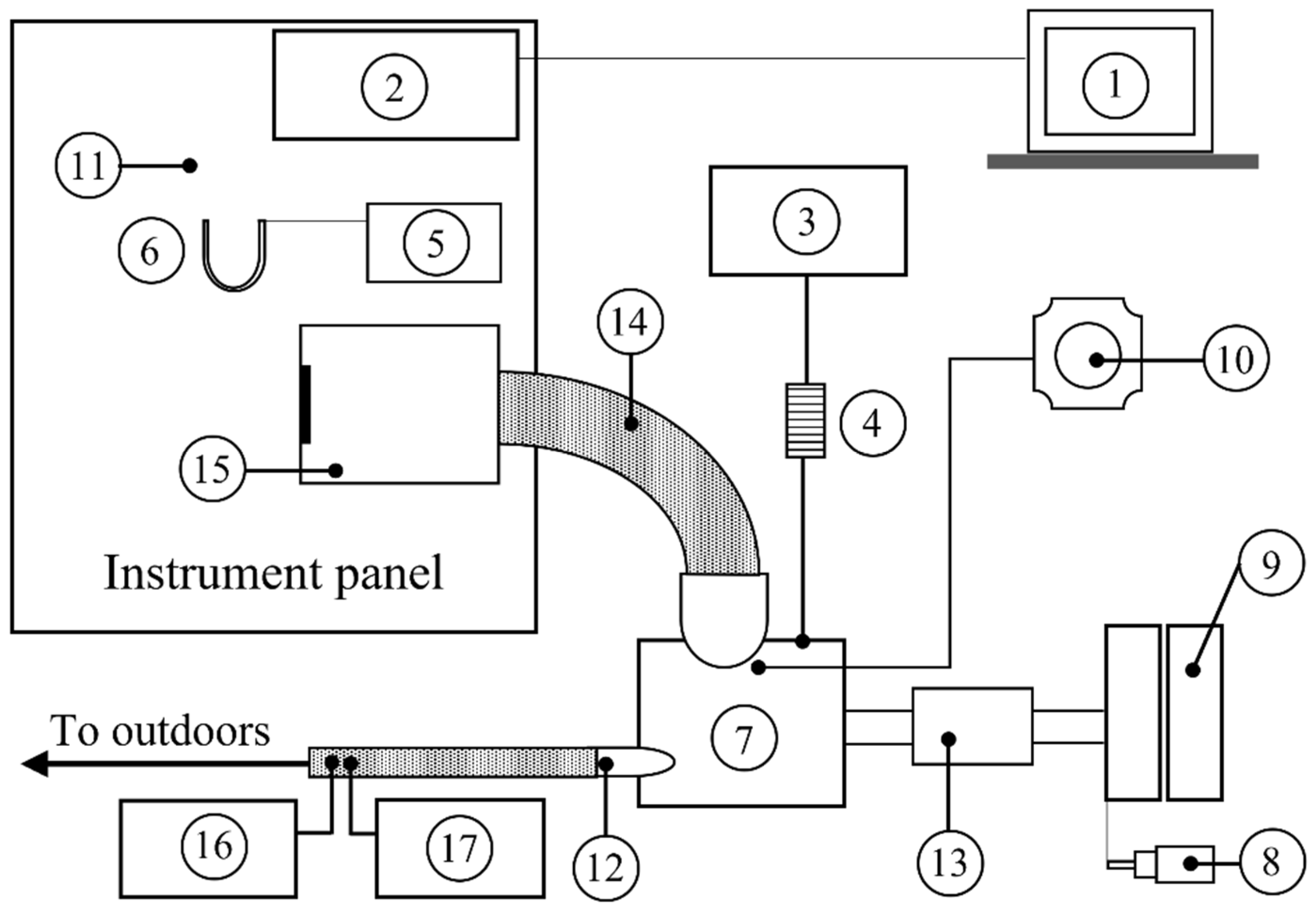
1. Introduction
Diesel engines remain pivotal power sources across transportation, agriculture, construction, and distributed power generation due to their high thermal efficiency, robust low-speed torque, and durability. However, the environmental and health burdens posed by nitrogen oxides (NO
x) and particulate matter (PM) emissions have prompted increasingly stringent regulations. International and regional policies, including the Paris Agreement, the European Union’s Euro 7 standards, and the greenhouse gas (GHG) reduction strategy of the International Maritime Organization (IMO), explicitly mandate further reductions in emissions [
1,
2,
3]. These pressures extend beyond mere emission control, driving a broader transition toward low-carbon fuel and powertrain solutions that shape the future sustainability of diesel technology. Consequently, advancements in aftertreatment systems, such as diesel oxidation catalysts (DOCs), diesel particulate filters (DPFs), and selective catalytic reduction (SCR), are being complemented by in-cylinder strategies that optimize fuel properties and combustion processes to reduce pollutants without compromising efficiency [
4,
5,
6]. Such integrated approaches offer cost-effective emission reduction potential while leveraging existing infrastructure, making them particularly viable for widespread adoption.
Among these, oxygenated alcohol–diesel blends have garnered sustained attention due to their ability to promote late-stage oxidation, suppress soot formation, and maintain compatibility with existing engine architectures [
7,
8]. A synthesis of review and experimental studies reveals consistent qualitative trends, albeit with condition-dependent variations. Generally, alcohols exhibit lower heating values (LHVs) and cetane numbers (CNs) compared with diesel, leading to an increase in brake-specific fuel consumption (BSFC) and brake-specific energy consumption (BSEC), whereas brake thermal efficiency (BTE) may remain comparable or slightly improve depending on operating conditions [
9,
10,
11]. Regarding emissions, reductions in carbon monoxide (CO), hydrocarbons (HCs), and smoke opacity are frequently observed. In contrast, NO
x responses hinge on the interplay of ignition delay, local equivalence ratio, temperature fields, and residence time [
12,
13,
14]. These behaviors are mediated by key physicochemical properties—such as CN, volatility, viscosity, latent heat of vaporization, and oxygen content—which govern spray atomization, evaporation, mixing, and heat release dynamics. Recent studies have expanded to include advanced optimization techniques, such as hydrogen addition and reactivity-controlled compression ignition (RCCI), to further enhance combustion stability [
15,
16]. However, heterogeneity in engine design, injection strategies, and aftertreatment systems contributes to discrepancies across studies, complicating integrative comparisons [
17,
18]. Emerging research explores surfactants, dual-fuel strategies, and hybrid blends with biodiesel or nano-additives, suggesting potential for simultaneous improvements in efficiency and emissions [
19,
20,
21]. These efforts underscore the capacity of alcohol fuels to mitigate the environmental impact of diesel engines while maintaining economic viability, aligning with global energy transition objectives.
Table 1 summarizes representative fuel properties of various alcohols. These values highlight the contrasting characteristics between lower and higher alcohols. For instance, lower alcohols, such as methanol and ethanol, demonstrate smoke reduction at low blend ratios; however, at higher ratios, they face phase-separation and cold-start/ignition-delay issues that often necessitate auxiliary strategies, such as the use of surfactants or dual-fuel approaches (e.g., alcohol port injection with diesel direct injection) [
22,
23,
24]. These limitations have shifted attention to higher alcohols, such as butanol, pentanol, and octanol, which generally provide higher LHVs and CNs, closer alignment with diesel’s viscosity and volatility, and superior miscibility, thereby enhancing blend stability and drop-in compatibility [
25,
26]. Moreover, advances in biomass conversion and CO
2-based synthesis pathways further position higher alcohols as integral components of renewable carbon cycles [
27]. Under transient/real-world-like operation, higher alcohol–diesel blends markedly suppress PM and often CO/HC without compromising BTE. Complementarily, well-to-wheel assessments indicate ~20–30% GHG reductions for conservative corn-based biobutanol pathways, with larger benefits for lignocellulosic acetone–butanol–ethanol (ABE)/butanol routes, underscoring their practicality [
28,
29].
Among these, n-pentanol (C
5H
12O) occupies a pragmatic middle ground. Compared with lower alcohols, it offers higher LHVs and CNs, alongside viscosity and density closely matched to diesel, enabling stable mid-to-high-ratio blending without engine modifications [
31,
32]. Its superior miscibility with diesel minimizes the risk of phase separation, and its elevated oxygen content promotes more complete combustion, thereby reducing smoke, CO, and HC emissions. However, its lower CN relative to diesel and higher oxygen content introduce condition-dependent trade-offs: at low-load/low-temperature, longer ignition delay can raise CO and HCs, whereas at high-load/high-speed, enhanced oxidation suppresses soot formation and stabilizes combustion, while NO
x tendencies remain regime-specific—governed by the balance of temperature field and residence time [
17,
33,
34,
35]. Recent studies on hybrid blends combining n-pentanol with biodiesel or nanoparticles have demonstrated synergistic benefits in specific conditions, further bolstering its environmental and operational advantages [
21]. These developments affirm n-pentanol’s potential to deliver eco-friendly solutions while preserving compatibility with existing diesel infrastructure, positioning it as a cost-effective pathway toward low-carbon engine operation.
Nevertheless, the practical deployment of higher alcohols, such as n-pentanol, also requires attention to long-term material compatibility with fuel system components. Prior studies on similar alcohol-based fuels have reported potential issues, such as swelling, degradation, or corrosion, in elastomers and metallic parts when exposed to alcohol-based fuels, suggesting that stabilizers or material upgrades may be necessary for sustained use [
25,
36,
37].
Despite these advances, limitations in comparability persist. First, while recent reviews and experiments have compiled results across a broad spectrum of engines, operating points, and alcohol–diesel blend types, cross-study synthesis is still hindered by heterogeneity in engine architectures, test protocols (steady-state vs. transient; full-load vs. part-load), and reporting practices. Most prior works have primarily investigated low-to-moderate n-pentanol ratios (typically ≤30%) under narrow operating ranges, such as steady-state conditions at a single speed or limited loads [
9,
11,
14,
17]. As a result, the condition-dependent nature of efficiency and emission responses, particularly potential reversals of efficiency penalties at higher loads or speeds, remains insufficiently understood.
Similar challenges in handling condition-dependent behaviors have also been addressed in other engineering fields through physics-informed, data-driven approaches. For example, recent reliability studies proposed physics-embedding multi-response regressors to capture time-variant responses under multiple conditions [
38], illustrating how cross-disciplinary methods can complement experimental datasets such as ours. Nevertheless, these insights underscore persistent gaps in alcohol–diesel combustion research, where the potential of n-pentanol at higher blending ratios remains underexplored.
Second, inconsistencies in reporting units, such as presenting emissions as concentrations (ppm, vol%) vs. mass-based metrics (g/kWh), hinder quantitative comparisons of fuel property effects across operating conditions, as demonstrated in PM correlations [
39]. These gaps undermine the empirical foundation needed to maximize n-pentanol’s potential, particularly in the context of sustainable energy transitions.
To address these shortcomings, this study systematically evaluates binary n-pentanol–diesel blends (10%, 30%, and 50% by volume) in a single-cylinder, air-cooled diesel engine across two engine speeds (1700 and 2700 rpm) and four brake mean effective pressure (BMEP) levels (0.25–0.49 MPa). By applying a consistent test protocol, we generate harmonized datasets that map performance (BTE, BSFC, and BSEC) and regulated emissions (NOx, CO, HC, CO2, and smoke opacity) across the speed–load matrix. This approach explicitly demonstrates that while efficiency penalties arise at light loads, these disadvantages diminish or even reverse at higher load/speed conditions. Such reversals, particularly in cases where high blend ratios (e.g., 50% n-pentanol blend) surpass diesel in BTE, highlight a novel contribution of this work, clarifying the practical feasibility of n-pentanol–diesel blends without requiring engine modification.
3. Results and Discussion
3.1. Brake Thermal Efficiency
Figure 2 shows that the BTE of D100 increases monotonically with load at both 1700 and 2700 rpm. At 1700 rpm, BTE climbs from 14.01% at 0.25 MPa to 17.73% at 0.49 MPa, and at 2700 rpm, it rises from 16.47% to 21.22% over the same range.
As load increases, all fuels follow a common upward trend for BTE. This reflects two intertwined effects: higher BMEP reduces the fraction of in-cylinder heat losses per unit work, and late-combustion oxidation becomes more complete under richer, higher-temperature conditions. Superimposed on this general behavior, n-pentanol blends exhibit efficiency penalties at light-to-medium loads (≤0.41 MPa). For example, at 1700 rpm and 0.25 MPa, BTE falls from 14.01% for D100 to 13.49% when using the D50P50 blend. This penalty arises from n-pentanol’s lower CN and higher latent heat, which extend ignition delay and increase charge cooling, together with its lower LHV that requires greater fuel mass flow. These combined effects promote incomplete combustion at light loads.
In addition,
Table 6 shows that the relative air/fuel ratio (λ) at 0.49 MPa decreases from about 0.99 for D100 to around 0.91 for D50P50. This indicates more pronounced local rich zones in naturally aspirated operation, the delayed oxidation of which is partly compensated by n-pentanol’s inherent oxygen content. Unlike boosted engines, where a higher λ is maintained, these results highlight that under rich conditions, the oxygenated nature of n-pentanol plays a more significant role, offering practical insights into optimizing operating strategies for pentanol blends.
At higher speeds, however, these drawbacks diminish or even reverse. Raising engine speed from 1700 to 2700 rpm increases in-cylinder temperatures and accelerates fuel vaporization, which shortens mixing delays and improves combustion completeness despite lower volumetric efficiency (
Table 7). Under these conditions, D50P50 actually surpasses D100: at 0.49 MPa and 2700 rpm, BTE reaches 21.85% compared with 21.22% for D100. This inversion reflects the combined influence of fuel-bound oxygen, which enhances the late-cycle oxidation of soot and CO, and higher volatility, which improves mixture uniformity, together with reductions in radiative and convective heat transfer losses (particularly through decreased soot radiation and lower blowby losses). These synergistic effects compensate for the lower energy density of n-pentanol under high-load/high-speed operation.
These trends are consistent with the prior literature. Jamrozik et al. [
9] reported that ethanol–diesel blends often yield BTE gains of 3–9% at 10–30% ratios, butanol causes only minor changes, and pentanol generally shows decreases of 1–17% depending on blend level and test conditions. In contrast, our results reveal penalties of ~5–10% at light-to-moderate loads, but efficiency gains of ~2–3% at high loads and speeds, highlighting a reversal relative to diesel and underscoring the novelty of demonstrating efficiency improvements for high-ratio pentanol blends.
In summary, n-pentanol blends impose modest BTE penalties at light loads due to lower CNs, high latent heat, and reduced LHVs. Still, these drawbacks diminish or invert at high loads and speeds, where enhanced mixing dynamics and oxygen-assisted oxidation close or even reverse the efficiency gap.
Section 3.2 and
Section 3.3 further corroborate these trends through BSEC and BSFC analyses.
3.2. Brake-Specific Energy Consumption
Figure 3 illustrates that the BSEC of D100 falls steadily with rising BMEP: at 1700 rpm, it drops from 25.68 MJ/kWh at 0.25 MPa to 20.29 MJ/kWh at 0.49 MPa, and at 2700 rpm, from 21.84 to 16.95 MJ/kWh over the same load range. This decline reflects the improved thermal efficiency and reduced incomplete combustion losses that accompany heavier loads.
BSEC is defined in Equation (5), where
is the fuel mass flow rate, and
is the brake power:
This metric represents the ratio of fuel energy input to brake work output. At light loads, BSEC is higher because a longer ignition delay shifts combustion to later in the expansion stroke, reducing the portion of fuel energy converted to work. Additional losses occur due to higher relative heat transfer and incomplete oxidation. At higher loads, elevated temperatures and pressures shorten the ignition delay, improve combustion completeness, and reduce relative heat losses, thereby increasing conversion efficiency.
When n-pentanol is blended with diesel, BSEC climbs at light and moderate loads (≤0.41 MPa). For example, at 1700 rpm and 0.25 MPa, D50P50 requires 26.67 MJ/kWh, about 4% more energy than D100, because n-pentanol’s lower LHV requires more fuel input; additionally, its lower CN and higher latent heat of vaporization extend ignition delay and intensify charge cooling through evaporative effects, thereby increasing heat losses at light loads. However, as the load increases, the BSEC penalty narrows; at 0.49 MPa, the D50P50 blend actually consumes slightly less energy than D100 under high-speed conditions, recording 16.46 MJ/kWh vs. 16.95 MJ/kWh at 2700 rpm. This reversal indicates that, at heavy loads, the fuel-bound oxygen in n-pentanol aids combustion chemistry so effectively (by facilitating complete oxidation in rich zones) that it offsets the lower energy density, particularly under enhanced turbulence at high speeds.
At 2700 rpm, all fuels show a further decline in BSEC because the higher in-cylinder temperatures accelerate fuel vaporization, and the intensified turbulence promotes faster air–fuel mixing, both of which cut down on heat losses and incomplete combustion fractions. While n-pentanol blends suffer a BSEC penalty at low speed, this disadvantage fades at moderate loads and disappears or even reverses at high load/high speed, where the cooling effect of latent heat is outweighed by the blend’s oxygen-driven oxidation support and improved combustion completeness. This inversion in BSEC accords with prior pentanol-blend studies reporting low-load energy penalties from reduced LHVs that attenuate, and may reverse, at higher load and speed as combustion efficiency improves [
33,
42].
In short, n-pentanol blends show higher energy demand at light loads due to lower LHVs and longer ignition delays. In contrast, this penalty diminishes at higher loads and speeds, where oxygen-assisted oxidation and volatility allow high blends like D50P50 to match or surpass diesel efficiency.
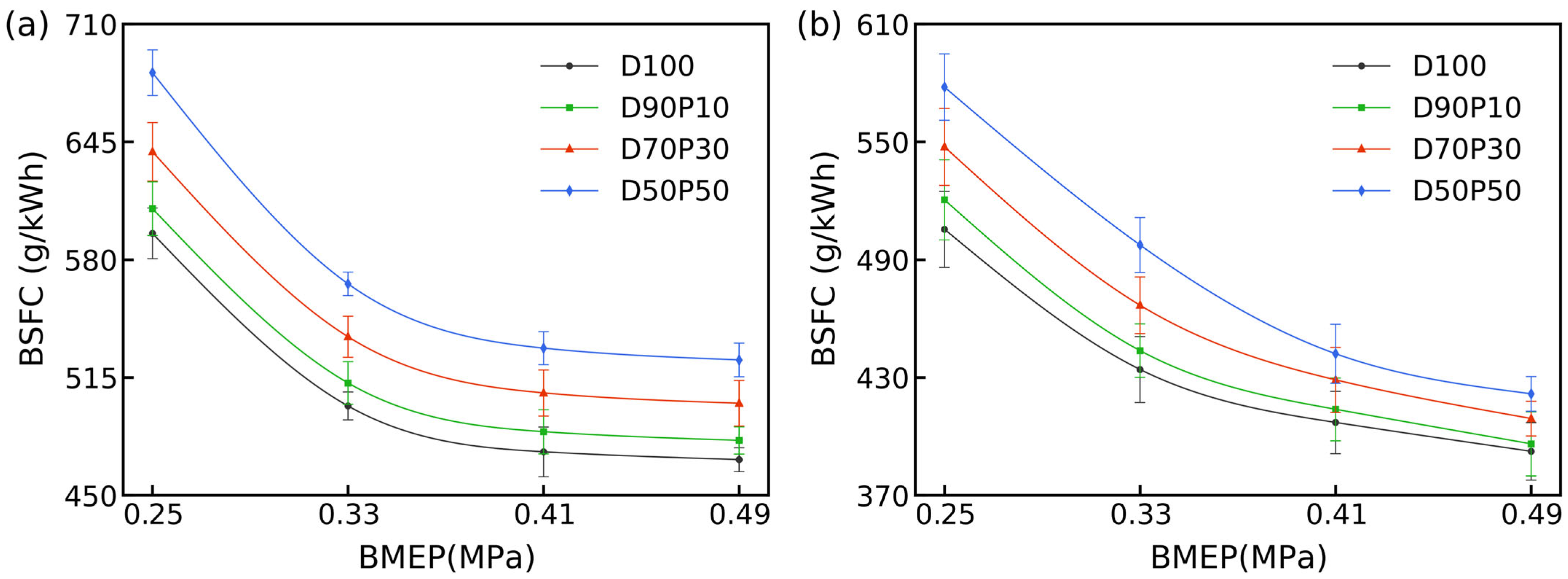
3.3. Brake-Specific Fuel Consumption
Figure 4 illustrates that the BSFC of D100 decreases monotonically with increasing BMEP: at 1700 rpm, it falls from 594.5 g/kWh at 0.25 MPa to 469.7 g/kWh at 0.49 MPa, and at 2700 rpm, from 505.5 to 392.4 g/kWh across the same load span. This pattern embodies the inherent trait of diesel engines, where fuel consumption per unit of work decreases as output increases.
Incorporating n-pentanol into diesel fuel elevates BSFC under low and intermediate loads (≤0.410 MPa). For instance, at 1700 rpm and 0.25 MPa, D50P50 requires 683.2 g/kWh, about 15% higher than D100. This penalty arises from n-pentanol’s lower LHV, which necessitates greater fuel mass for equivalent energy delivery. In addition, its lower CN and higher latent heat of vaporization prolong ignition delay and intensify charge cooling, thereby increasing incomplete combustion at low loads. With escalating load, though, the fuel-use drawback lessens: at 0.49 MPa and 1700 rpm, D50P50 uses 524.7 g/kWh (approximately 12% above D100), as the drop in λ is offset by the blend’s oxygen that supports oxidation and tempers consumption to some extent. The observed BSFC rise at light-to-mid loads and the narrowing gap at higher loads are consistent with prior reports on alcohol–diesel blends, which similarly attribute penalties at low loads to fuel properties but note mitigation at higher loads through enhanced combustion completeness [
17,
43].
Shifting to 2700 rpm brings a broader reduction in BSFC for all fuels, driven by improved air–fuel mixing and higher in-cylinder temperatures that accelerate vaporization and oxidation. Although n-pentanol blends incur a BSFC penalty at low speeds, this penalty contracts markedly at elevated speeds and mid-to-high loads (e.g., at 2700 rpm and 0.49 MPa, D50P50 requires 421.7 g/kWh, approximately 7% above D100). Such behavior reflects the mitigation of latent-heat cooling at high rpm, the oxidation assistance from fuel-bound oxygen compensating for the lower LHV, and the shift toward more premixed combustion, enhancing energy utilization.
In summary, high n-pentanol blends increase BSFC at low loads due to lower LHVs and longer ignition delays. Still, this penalty diminishes at high loads and speeds as fuel-bound oxygen and volatility enhance combustion completeness, underscoring their potential in mid- to high-load operation.
3.4. NOx Emissions
Figure 5 shows NO
x emissions vs. BMEP at (a) 1700 rpm and (b) 2700 rpm. For diesel (D100), NO
x climbs modestly from low to medium loads, rising from 13.77 g/kWh at 0.25 MPa to 14.19 g/kWh at 0.41 MPa at 1700 rpm, then drops to 10.14 g/kWh at 0.49 MPa. A similar pattern appears at 2700 rpm, with NO
x varying from 12.81 to 11.03 g/kWh up to 0.41 MPa before falling sharply to 7.28 g/kWh at 0.49 MPa.
All fuels follow the same basic pattern: NOx remains steady or rises slightly from low-to-medium loads (≤0.41 MPa) before falling at high loads (0.49 MPa). This behavior reflects two key effects at heavy load: the larger work term on a g/kWh basis, which normalizes emissions per unit output, and a decrease in λ, combined with improved in-cylinder mixing, which limits oxygen availability and shortens the high-temperature residence time for thermal-NOx formation.
Beyond residence time, the balance between premixed and diffusion combustion fractions also plays a role. At light loads, the longer ignition delay of n-pentanol increases the premixed portion; however, its high latent heat suppresses peak temperatures, thereby limiting NOx formation. At higher loads, enhanced volatility and fuel-bound oxygen reduce the diffusion fraction, thereby shrinking high-temperature regions and further curbing thermal NOx.
Against this shared trend, n-pentanol blends mirror D100 at lower loads but diverge at high loads; at 0.49 MPa, NO
x declines with blending ratio: D50P50 records 9.42 g/kWh at 1700 rpm (7% below D100) and 6.12 g/kWh at 2700 rpm (15% below D100). These high-load reductions are consistent with prior reports on pentanol–diesel blends, which show λ-driven moderation despite increased fueling [
44,
45]. In addition to the λ effect, pentanol’s fuel-bound oxygen and favorable atomization/volatility promote more complete late-cycle oxidation while tempering local peak temperatures in fuel-rich zones, further curbing NO
x at 0.49 MPa.
At higher speed (2700 rpm), these effects are amplified by stronger turbulence and shorter combustion duration, resulting in an even greater NOx reduction for D50P50 under high-load conditions.
To verify consistency between concentration-based and specific emissions,
Table 8 presents a comparison of NO
x values expressed in both ppm and g/kWh across all test conditions. The results show that both units yield consistent overall trends: NO
x decreases with increasing n-pentanol blending ratio, and D50P50 exhibits the lowest NO
x values relative to D100 in nearly all tested conditions. While absolute magnitudes differ between units because ppm values are sensitive to exhaust gas dilution (e.g., air–fuel ratio and volumetric flow), g/kWh values are normalized to engine work, and the relative trends in fuel behavior remain consistent. This confirms that the g/kWh-based reporting adopted in this study is robust and aligns with ppm-based values, reinforcing the reliability and generalizability of the findings.
In summary, high n-pentanol ratios magnify NOx reductions at high loads and speeds, highlighting their strong potential for concurrent NOx and PM control in compression-ignition engines.
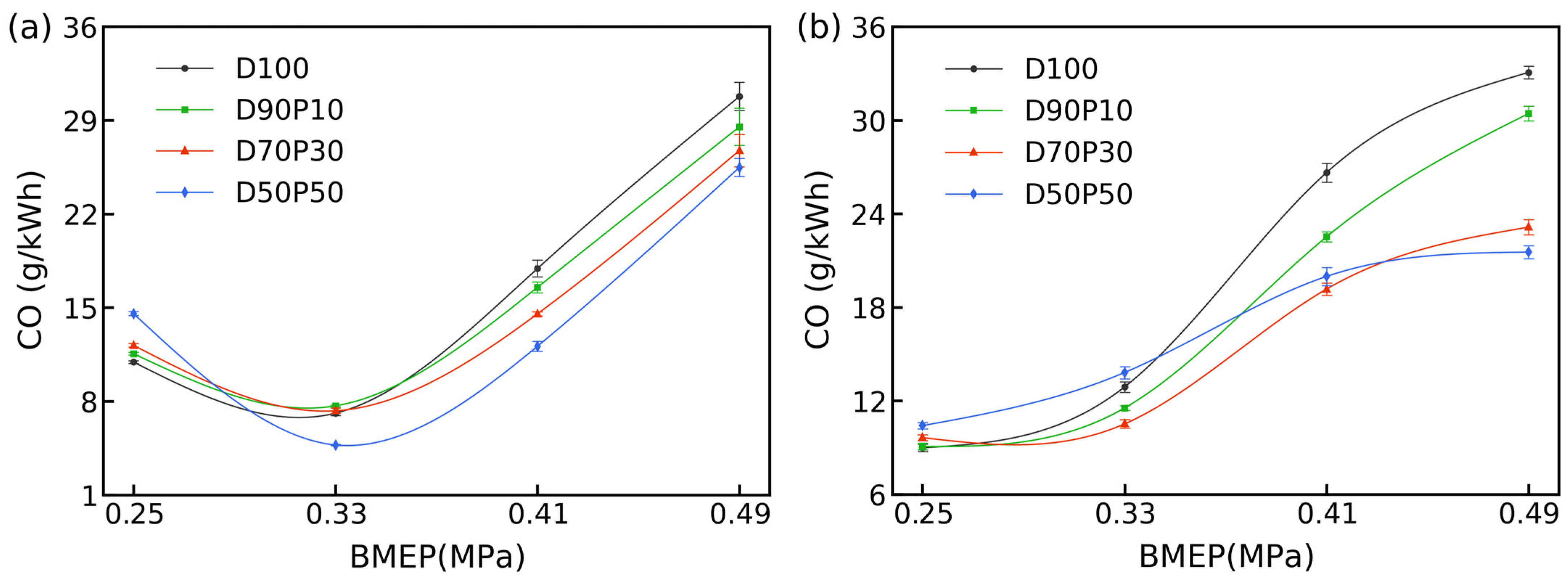
3.5. CO Emissions
Figure 6 presents CO emissions for D100, which trace a typical upward curve with load at both 1700 and 2700 rpm. At 1700 rpm, CO starts at 10.93 g/kWh at 0.25 MPa, dips slightly at 0.33 MPa, then surges to 17.92 g/kWh at 0.41 MPa and 30.79 g/kWh at 0.49 MPa. At 2700 rpm, D100 follows a similar trajectory, rising from 8.99 g/kWh at 0.25 MPa to 33.07 g/kWh at 0.49 MPa.
All fuels share a common trend of increasing CO with load, driven by a lower λ from fuel enrichment, expanded local fuel-rich zones, and spray evaporation delays. Against this backdrop, n-pentanol blends achieve substantial CO reduction at peak loads: D50P50 records 4.74 g/kWh at 1700 rpm/0.33 MPa (33% below D100) and 25.48 g/kWh at 0.49 MPa (17% lower). This improvement reflects n-pentanol’s fuel-bound oxygen, which promotes post-combustion CO oxidation to CO
2 through enriched local oxygen availability. The blend’s enhanced atomization and turbulence, driven by its lower viscosity and higher volatility, improve air–fuel mixing at higher loads. These high-load CO reductions are consistent with prior reports for pentanol–diesel blends, which attribute the decreases to fuel-bound oxygen and enhanced mixing [
11,
14].
When engine speed increases from 1700 to 2700 rpm, CO curves retain their shape but shift downward for all fuels as higher in-cylinder temperatures accelerate evaporation and stronger turbulence shorten mixing delays. Under these high-speed, high-load conditions, n-pentanol blends deliver even greater CO reductions, leveraging their oxygen content and mixing advantages more effectively.
To conclude, high n-pentanol blends increase CO emissions at low speeds and light loads due to ignition delay from lower CNs and mixing setbacks from high latent heat; however, at moderate-to-high loads and elevated speeds, the synergy of improved mixing from enhanced volatility and oxygen-assisted oxidation cuts CO by up to 35% for D50P50; this is consistent with the HC and smoke reductions reported in
Section 3.6 and
Section 3.8.
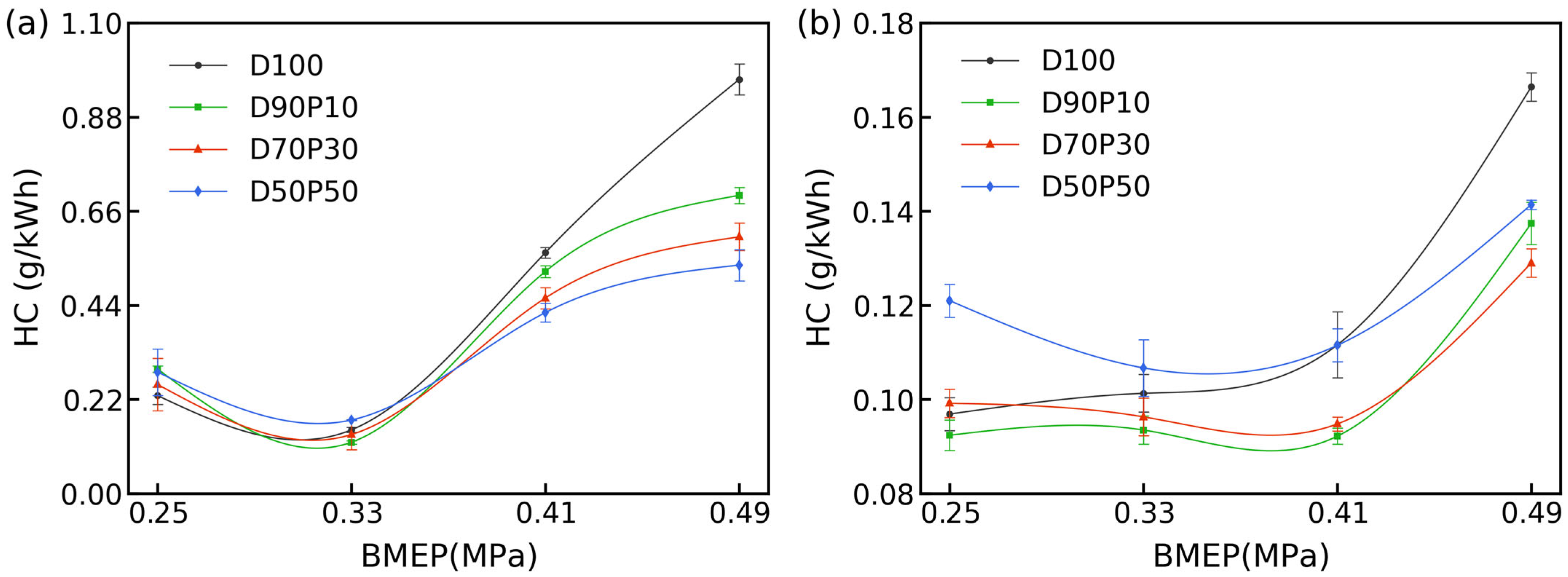
3.6. HC Emissions
Figure 7 shows that HC emissions for D100 increase with load at both 1700 and 2700 rpm, though the rate of rise differs by speed. At low-to-medium loads (≤0.41 MPa), HC climbs gradually or remains nearly constant before surging at peak load (0.49 MPa). For example, at 1700 rpm, HC increases from 0.229 g/kWh at 0.25 MPa to 0.563 g/kWh at 0.41 MPa and 0.968 g/kWh at 0.49 MPa; at 2700 rpm, it increases from 0.097 to 0.137 g/kWh and then to 0.166 g/kWh over the same range.
As load increases, more fuel is injected and volumetric efficiency falls, lowering λ and expanding local rich pockets. In these quench-layer regions near the cylinder walls, slower evaporation prolongs low-temperature exposure and boosts HC emissions. Under low-to-medium loads (≤0.41 MPa), n-pentanol blends (D90P10–D50P50) closely follow D100, but at peak load, their emissions drop sharply. For instance, at 1700 rpm/0.49 MPa, D50P50 records 0.534 g/kWh (~45% below D100), while D70P30 achieves a ~38% reduction. This improvement primarily arises from the blend’s fuel-bound oxygen, which facilitates post-combustion HC oxidation by supplying additional oxidants in fuel-rich zones. A further contribution comes from improved spray breakup and turbulence, enabled by n-pentanol’s higher volatility and lower viscosity, which reduce quench-layer transport at peak loads.
Increasing engine speed to 2700 rpm reduces HC emissions across the board by shortening combustion dwell times; simultaneously, higher in-cylinder temperatures improve evaporation and mixing. As a result, HC levels at 2700 rpm are lower than at 1700 rpm for every fuel, with n-pentanol blends showing a more pronounced drop in HC levels at medium-to-high loads, where spray-evaporation penalties are minimized and internal oxygen accelerates HC conversion. These speed- and load-dependent trends are consistent with prior reports on pentanol–diesel blends, which show amplified HC drops at higher speeds/loads [
11,
43].
In essence, HC emissions differ little between D100 and blends at light loads, but at heavy loads and high speeds, the combined effects of fuel-bound oxygen and improved mixing yield substantial HC mitigation for high-ratio blends. This load- and speed-dependent behavior highlights the environmental advantages of n-pentanol–diesel blends under demanding conditions.
3.7. CO2 Emissions
Figure 8 shows that CO
2 emissions for D100 decline steadily with increasing BMEP at both 1700 and 2700 rpm. At 1700 rpm, CO
2 drops from 1832.6 g/kWh at 0.25 MPa to 1427.1 g/kWh at 0.49 MPa; at 2700 rpm, it falls more sharply from 1549.7 to 1197.8 g/kWh over the same load span.
All fuels follow this downward trajectory because, although CO
2 production rises with fuel consumption, expressing it in g/kWh normalizes it by considering the larger output at higher loads. Blending with n-pentanol shifts the curves upward overall: D50P50 records 1890.6 g/kWh at light loads (0.25 MPa/1700 rpm), which is ~3% higher than D100. However, at heavy loads (0.49 MPa), it measures 1431.7 g/kWh, nearly matching the 1427.1 g/kWh for D100. The blend’s higher BSFC increases overall CO
2 emissions because more fuel mass is required due to its lower LHV. However, n-pentanol’s lower C/H ratio, stemming from its molecular structure (C
5H
12O), partly offsets this effect by producing less CO
2 per unit of fuel burned. At heavy loads, however, the blend’s improved thermal efficiency and fuel-bound oxygen-enhanced combustion compensate for this effect, bringing CO
2 emissions in line with commercial diesel. These load-dependent CO
2 outcomes are consistent with prior reports for pentanol–diesel blends, noting offsets from lower C/H ratios at heavy loads [
45,
46].
Raising speed to 2700 rpm reduces CO2 by 10–20% for all fuels, as gains in thermal efficiency outweigh the drop in volumetric efficiency. Blend-dependent differences become more pronounced: at 0.25 MPa, D50P50 emits 1592.0 g/kWh, compared with 1549.7 g/kWh for D100 (a 3% increase); however, at 0.49 MPa, it falls to 1156.9 g/kWh (~3% below D100). This reflects higher in-cylinder temperatures—which improve vaporization and mixing, enhancing the low C/H benefit—and shortened residence times limit CO2 formation.
Although this study reports CO
2 on a tailpipe basis, these results can also be linked to life-cycle GHG implications. Since n-pentanol can be produced from renewable biomass [
47], the lower CO
2 emissions observed at high blending ratios may translate into additional reductions on a well-to-wheel basis, thereby reinforcing the sustainability context noted in the Introduction.
In short, CO2 emissions decrease with both load and speed. Although high n-pentanol blends incur a slight CO2 penalty at light loads due to a higher BSFC from lower LHVs, they achieve comparable or lower CO2 under heavy-load, high-speed conditions owing to a lower C/H ratio and oxygen-enhanced efficiency, underscoring their promise for sustainable engine operation.
3.8. Smoke Opacity
Figure 9 shows that smoke opacity for D100 rises sharply with increasing BMEP at both 1700 and 2700 rpm. At 1700 rpm, opacity increases from 1.9% at 0.25 MPa to 61.8% at 0.49 MPa; at 2700 rpm, it climbs from 3.8% at 0.25 MPa to 61.1% at 0.49 MPa, with higher values in the medium-load range. This mirrors the typical diesel combustion trait where soot buildup intensifies with load.
As the load increases, all fuels exhibit rising smoke opacity due to a lower λ, which expands fuel-rich pockets and prolongs the residence of unburned carbon. While smoke levels are minimal at light loads, they surge at medium to high loads for every blend. High n-pentanol ratios, however, significantly reduce smoke: D50P50 records 40.0% opacity at 1700 rpm/0.49 MPa (~35% lower than D100’s 61.8%). This reduction arises from the blend’s inherent oxygen-enhancing soot oxidation during late combustion, by providing radicals for breaking down polycyclic aromatic hydrocarbons (PAHs) and other carbon precursors that would otherwise form soot. At light loads, the effect is muted, and occasionally, a slight increase is observed; however, under heavy loads, the smoke suppression is the overriding trend.
At 2700 rpm, smoke opacity is higher at low-to-medium loads than at 1700 rpm, but at high loads, it remains nearly constant (61.8% at 1700 rpm vs. 61.1% at 2700 rpm). Under these conditions, D50P50 achieves 15.6% opacity, 74% below D100, owing to enhanced turbulence from higher volatility, which reduces evaporation delays, and fuel-bound oxygen accelerates soot oxidation. These heavy-load, high-speed smoke reductions are consistent with prior reports for pentanol–diesel blends, which link decreases to oxygen-accelerated soot oxidation [
42,
48].
Comparatively, the magnitude of this reduction is within the ranges reported for advanced nano-additive approaches (typically 20–60% [
49]) and RCCI strategies (often 50–90% [
16]). Unlike these methods, however, n-pentanol blending offers drop-in compatibility without additional engine modifications or costly additives, underscoring its practical and cost-effective advantages for low-carbon diesel applications.
Smoke opacity, although not a direct measure of particle size, is a recognized surrogate for particulate emissions and correlates with the mass of soot. The substantial opacity decreases observed with high pentanol blends, therefore, imply a parallel reduction in PM. While size distributions were not measured here, similar trends have been reported for oxygenated fuels, where pentanol blends have been shown to reduce particle number (PN) concentrations [
28]. These outcomes are also relevant to emerging regulations such as Euro 7, which impose stricter limits on both PM mass and PN.
In summary, smoke opacity increases with load and speed due to impaired air–fuel mixing as the primary mechanism; however, n-pentanol blends leverage volatility, lower viscosity, and internal oxygen to enhance late-stage oxidation and achieve substantial reductions (up to 74%). This highlights their potential for sustainable PM mitigation, while also emphasizing the need for strategies to address minor increases in light load.
4. Conclusions
This study systematically evaluated the performance and emission characteristics of n-pentanol–diesel blends (D90P10, D70P30, and D50P50) across two speeds (1700 and 2700 rpm) and varying loads (0.25–0.49 MPa BMEP), providing a harmonized dataset to address gaps in prior research.
BTE increased with load and speed for all fuels, with n-pentanol blends showing penalties at light to medium loads due to a lower CN, higher latent heat, and a reduced LHV. These drawbacks were diminished or even reversed at high loads and speeds, where D50P50 outperformed D100 regarding efficiency. BSEC and BSFC followed similar patterns, underscoring the condition-dependent nature of fuel performance.
Emissions analysis revealed clear benefits under medium- to high-load conditions: smoke, CO, and HCs were substantially reduced through oxygen-assisted oxidation and improved mixing, while NOx showed regime-dependent behavior linked to residence time and combustion phasing. Slight CO2 increases at light loads, arising from higher BSFC, were offset at heavy loads by n-pentanol’s lower C/H ratio and improved thermal efficiency. Together, these results demonstrate that n-pentanol–diesel blends provide favorable efficiency–emission trade-offs in medium- to high-load regimes, with the high-load efficiency reversal of D50P50 underscoring both the novelty and practical significance of high-ratio blends.
Key practical considerations include the long-term compatibility of n-pentanol with fuel system materials, which may necessitate the use of additives or upgrades. Future work should examine durability at high blend ratios, transient/real-world cycles, and aftertreatment integration (e.g., SCR/DPF) to validate large-scale application. In practice, this suggests that n-pentanol blends are best deployed in medium- to high-loads, high-speed operations, such as those in heavy-duty vehicles and marine engines, where they can maximize efficiency while ensuring compliance with stringent emission standards. In contrast, adaptive or partial blending may be preferred at light loads to mitigate efficiency penalties.