Abstract
Lignin holds significant promise as a feedstock for biocrude production via hydrothermal liquefaction (HTL). Although lignin HTL has been widely studied, the specific depolymerization pathways associated with distinct lignin structures remain largely unexplored. This study investigates the HTL of four structurally diverse lignins: alkaline (AL), dealkaline (DAL), organosolv (OL), and lignosulfonate (LS) across 270–310 °C to elucidate structure-specific mechanisms governing biocrude yield and composition. AL and OL achieved the highest yields (16.8 ± 0.3% and 16.8 ± 2.5%), with AL-derived biocrude showing the highest carbon content (70.2 ± 0.0%) and HHV (31.0 ± 0.2 MJ/kg). In contrast, DAL and LS produced lower yields and inferior fuel quality due to higher sulfur content and lower carbon enrichment. The structures of AL and DAL, containing fewer methoxy groups, produced guaiacol-rich biocrudes (46.6% and 69.5%). Methylation in AL formed alkyl guaiacols and veratroles, while DAL favored side-chain oxidation. OL retained complex structures, forming syringols and desaspidinol, which contributed to heavier biocrude compounds. Sulfonate groups in LS were stabilized mostly as sulfides, leading to elevated sulfur content. These findings provide mechanistic insight into how lignin structure governs HTL behavior, enabling targeted control of biocrude yield and quality for renewable fuel production.
1. Introduction
Lignin, a major constituent of biomass, provides immense potential as a renewable liquid fuel source due to its wide availability and high carbon density. In fact, it is the second most abundant biopolymer on Earth after cellulose, which has an availability of 300 billion tons, with an annual increase of 20 billion tons [1,2]. Naturally, lignin is intensely bound with cellulose and hemicellulose, giving rigidity to the plant cell wall [3] and is separated during the various processes employed by biorefineries, such as the pulp and paper industry, and the bioethanol plant [4,5]. These processes produce lignin such as kraft, alkaline, dealkaline, organosolv, and lignosulfonate with distinct structural characteristics and varying reactivity, with an estimated global production of 500–3600 million tons annually [6].
Kraft lignin (KL), the most dominant type of lignin, has a market size of USD 1.7 billion [7] and is obtained from the kraft pulping process, which results in a highly modified condensed structure [8] with a significantly cleaved ether linkage (native linkage of lignin), making it challenging to depolymerize [9]. Lignosulfonate lignin (LS) with a market size of USD 1.62 billion [10] is obtained from the sulfite pulping process, which introduces sulfonate groups in the lignin structure, resulting in a highly reactive water-soluble lignin [8]. In contrast to KL and LS, the organosolv treatment process resulted in organosolv lignin (OL) with a sulfur-free structure close to native lignin and negligible ash content [11]. Although the current market for OL is lower compared to KL and LS, the production capacity is increasing significantly due to the rapid growth of the bioethanol industry [12]. Due to hydrophobicity, poor water solubility, and less reactivity, KL lignin goes through several treatment processes, producing alkaline (AL) and dealkaline (DAL) lignin with improved characteristics [13]. For instance, AL showed enhanced reactivity and water solubility due to the introduction of phenolate ions in its structure during the alkaline treatment of KL [14]. With the increase in KL production, the abundance of AL and DAL is also expected to grow significantly in the upcoming years. Apart from these, other types of lignin, such as deep eutectic solvent/ionic liquid extracted lignin, milled lignin, and enzymatic lignin, are available in the market, although in limited quantity. Despite the immense availability and utilization potential of lignin, almost 90% of the currently produced lignin is discarded as waste or burned as a low-grade fuel [15], contributing to greenhouse gas emissions [6,16]. The remaining tiny fraction produces low-value chemicals, including concrete additives, stabilizers, dispersants, and surfactants [17]. Thus, the valorization of waste lignin to liquid fuel offers a twofold advantage: providing a sustainable alternative for fuel production while reducing the burden of waste management.
In recent times, HTL is emerging as a promising technology to produce biocrude (a liquid biofuel precursor) from waste lignin feedstock. The HTL process employs the appealing properties of subcritical water, such as higher ionic product, enhanced diffusivity, and the ability to contribute both as a reactant and solvent [18,19]. Under HTL conditions, lignin depolymerizes into a nonpolar biocrude phase as well as solid char, water-soluble organics, and a gaseous phase [20,21]. However, one of the major challenges of producing biocrude from lignin is its wide structural variability, as well as the differences in reactivity resulting from the varying extraction process as discussed previously. For instance, Yang et al. [22] compared the HTL of KL and OL in a temperature range between 280 and 340 °C and found a maximum biocrude yield of 26.8% for KL, where the maximum biocrude yield was 35.8% for OL. OL-derived biocrude also showed significantly higher carbon content and lower oxygen content, especially at the lower temperature range. The molecular composition of biocrude also varied between lignin types, with OL biocrude showing a higher amount of syringols and methoxy furans, while KL biocrude showed a higher amount of guaiacol-containing compounds, indicating a compositional difference of biocrude. Lui et al. [23] found the biocrude yield in the order of AL (26.2%) > KL (25.9%) > OL (20%) during the HTL at 330 °C and 60 min retention time. Similar carbon (72.4%) and energy content (30.7 MJ/Kg) were observed in the biocrude from KL and AL, where OL showed lower carbon and energy content of 70.6% and 28.7 MJ/Kg, which is in contrast to the Yang et al. study [22]. During catalytic HTL, Halleraker et al. [24] found higher biocrude yield from OL (88.7–94.2%) than from KL (77.4–81.3%) in the identical experimental conditions. OL-derived biocrude showed significantly higher amounts of guaiacols and catechols types of compounds, especially at a higher temperature of 360 °C. In two separate studies, Hashmi et al. [25] found a maximum biocrude yield of 11.9% dominated by syringol and methoxy catechol from HTL of OL at 350 °C, while Kang et al. [26] found maximum yield of 29.5% with a dominant fraction of guaiacol and catechol from AL HTL at 300 °C.
The findings of these studies lead to the conjecture that biocrude yield and compound distribution vary significantly with lignin type which leads to varying fuel properties. Yet, the structure-specific formation pathways of these compounds remain largely unexplored. Furthermore, to the best of the authors’ knowledge, no prior study has systematically investigated the HTL of major types of lignin. This lack of comparative insight limits the mechanistic understanding necessary for advancing lignin valorization into biocrude. Additionally, most available studies on OL and AL focus narrowly on biocrude yield or property characterization at a single HTL temperature, despite evidence from Katongtung et al. [27] that HTL temperature significantly influences biocrude properties across different feedstocks.
To address these gaps, this study systematically investigates the HTL behavior of four structurally distinct lignins (AL, DAL, OL, and LS) across a consistent HTL temperature range of 270–310 °C, since the majority of the existing studies on lignin reported the maximum biocrude yield in this temperature range [22,28,29,30]. The primary objective is to conduct a comparative analysis of biocrude yield from different lignin types and to identify structure-specific depolymerization pathways that govern the fuel quality and molecular composition of the resulting biocrude. Overall, the insights gained from this study are expected to enhance the understanding of lignin behavior under HTL conditions and help assess the potential of various lignin types for sustainable liquid fuel production.
2. Materials and Methods
2.1. Materials
AL, DAL, and LS were purchased from TCI Chemicals (Portland, OR, USA), while OL was procured from BOC Sciences (Shirley, NY, USA). According to the manufacturers, LS is produced through sulfite pulping of needle-leaved and broad-leaved trees. DAL is derived from LS via partial desulfurization, oxidation, hydrolysis, and demethylation processes. Subsequently, AL is obtained through alkaline treatment of DAL. OL is extracted from wood using an organic solvent-based process. To determine the moisture content, a portion of each lignin sample was dried overnight in an oven at 105 °C. The measured moisture contents were 4.9 ± 0.3%, 7.5 ± 0.6%, 3.3 ± 0.6%, and 3.6 ± 0.1% for AL, DAL, OL, and LS, respectively. The dried lignins were further subjected to ultimate analysis and higher heating value (HHV) determination, the results of which are presented in Table 1.
High-performance liquid chromatography (HPLC) grade dichloromethane (DCM), used for biocrude extraction, was sourced from Fisher Scientific (Fair Lawn, NJ, USA). Ultra-high purity nitrogen and oxygen gases employed in thermogravimetric analysis (TGA) were supplied by NexAir (Melbourne, FL, USA). Standards for elemental analysis, including vanadium oxide and 5-tert-butyl-benzoxazol-2-yl thiophene (BBOT), were acquired from CE Elantech (Lakewood, NJ, USA).
2.2. Hydrothermal Liquefaction
Hydrothermal liquefaction (HTL) experiments were carried out in a 300 mL high-pressure batch reactor (Parr 4560, Moline, IL, USA) across a temperature range of 270–310 °C. In a typical run, a slurry was prepared containing 10 g of lignin and 80 g of water. The total water content included both the inherent moisture of the lignin and additional deionized (DI) water. After transferring the slurry into the reactor, it was sealed and heated via a ceramic fiber heater. Agitation at 120 rpm was maintained using a magnetic drive to ensure homogenous mixing and uniform temperature distribution. Once the desired temperature was reached, the reaction was held isothermally for 30 min. The system was then rapidly cooled using an ice-water bath. Gaseous products were released into a fume hood and were not subjected to further analysis. The reaction mixture was collected into a beaker, and the interior of the reactor, including the walls, thermowell, and impeller, was thoroughly rinsed with dichloromethane (DCM) followed by DI water. The rinses were combined with the reactor effluent and filtered through a 1 µm glass microfiber (GMF) filter under vacuum. The retained solids on the filter were dried at 105 °C overnight and designated as solid char. The filtrate was transferred to a separatory funnel and subjected to liquid-liquid extraction. The mixture was vigorously shaken and left to settle inside a fume hood for at least 4 h to ensure full phase separation. The aqueous bottom layer, containing water-soluble organics (WSO), was collected and dried in an oven at 105 °C. The DCM-rich upper phase containing biocrude was collected in a rotary evaporator flask, and DCM was removed under reduced pressure to isolate the biocrude product. All experiments were performed in duplicates to ensure reproducibility, and data are presented with standard deviation. The mass yields of the different product fractions were calculated using the following equations:
2.3. Biocrude Characterization
Elemental composition of the biocrude samples was determined using a FLASH EA 1112 Series elemental analyzer (Thermo Scientific, Greenland, NY, USA) equipped with a thermal conductivity detector (TCD). Approximately 2–3 mg of each biocrude sample was weighed for individual analysis. Vanadium oxide (V2O5) was employed as a combustion aid, while 5-tert-butyl-benzoxazol-2-yl thiophene (BBOT) was used as the calibration reference material. The higher heating value (HHV) of both the raw lignin and the resulting biocrude was estimated based on elemental composition using the Dulong formula [31], as expressed in the equation below, with each elemental term representing the corresponding mass fraction.
The boiling point distribution of the biocrude was assessed using a PerkinElmer TGA 4000 thermogravimetric analyzer (Waltham, MA, USA) operated under a nitrogen atmosphere at a flow rate of 10 mL/min [32]. The instrument offers a temperature accuracy of ±1 °C and a balance sensitivity of ±0.02%. To remove any residual dichloromethane (DCM), the sample was first heated from ambient temperature to 45 °C and held isothermally for 30 min. The temperature was then ramped at 10 °C/min up to 600 °C. After reaching the final temperature, air was introduced to oxidize the remaining biocrude for 10 min, followed by cooling to room temperature. Mass loss during the inert heating phase was used to estimate the distribution of various boiling point fractions within the biocrude. These were categorized into gasoline/naphtha (BP < 177 °C), kerosene (177–232 °C), diesel (232–343 °C), heavy gas oil (343–566 °C), and residues (BP > 566 °C) [20] fraction. The percentage of each fraction was calculated based on the corresponding mass loss within each temperature range.
Biocrude samples were analyzed using gas chromatography–mass spectrometry (GC-MS) on an Agilent 7890 GC system coupled with a 5975-mass selective detector (Agilent Technologies, Inc., Santa Clara, CA, USA). Separation was achieved using a Supelco Equity 1701 capillary column (Merck, Darmstadt, Germany). The injector temperature was set at 250 °C, operating with a 1:1 split ratio, and helium was used as the carrier gas at a constant flow rate of 5 mL/min. The oven temperature was initially held at 45 °C for 4 min, followed by a ramp up to 280 °C, where it was maintained for an additional 20 min. Compound identification was carried out by matching the acquired mass spectra against the NIST library, with care taken to confirm that each identified peak was distinct and free from co-elution.
3. Results and Discussion
3.1. Effect of Lignin Type and HTL Temperature on Biocrude Yield
The biocrude yield obtained from each lignin type at different HTL temperatures is presented in Figure 1, which shows different behavior across lignin types. AL showed a steady increase in biocrude yield from 14.9 ± 0.3% at 270 °C to 16.8 ± 0.3% at 310 °C. In contrast, DAL reached its maximum biocrude yield of 11.2 ± 1.8% at 290 °C, followed by a slight decline to 9.7 ± 1.8% at 310 °C. OL demonstrated its highest biocrude of 16.8 ± 2.5% at the lowest temperature of 270 °C, and remained relatively stable with increasing temperature (ANOVA, p-value = 0.574 > 0.05). LS showed a continuous decline in biocrude yield, decreasing from 12.6 ± 1.3% at 270 °C to 6.0 ± 1.8% at 310 °C. These trends indicate that the temperature response of biocrude yield is dependent on lignin type as well as HTL temperature.
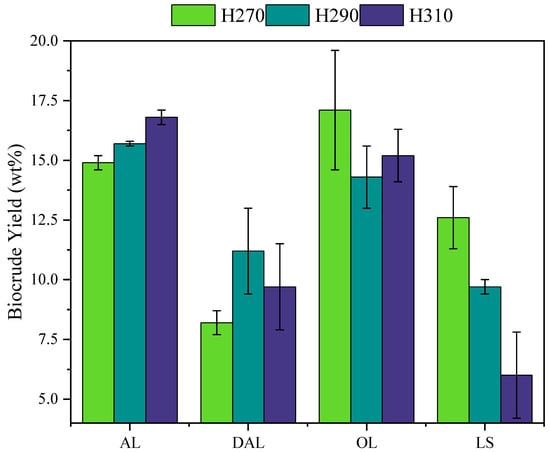
Figure 1.
Biocrude yield from different types of lignin and their variation with HTL temperatures.
The observed trends in biocrude yield can be explained based on WSO and char yields, as shown in Table S1, as well as the structural characteristics of each lignin type. The breakdown of the highly water-soluble structure of AL lignin leads to the maximum WSO yield at 270 °C (49.7 ± 0.5%), which decreased to 37.1 ± 0.9% with the increase of HTL temperature, indicating the repolymerization of WSO into the biocrude phase, increasing the yield [13,33]. A similar biocrude yield of 17.6% was also reported by Biswas et al. [30] during HTL of AL at 280 °C. Besides, Yang et al. [22] reported an increase of biocrude yield from 22.5% to 26.8% between 280 and 300 °C during HTL of KL, which shares structural similarities with AL. The maximum biocrude yield from DAL at 290 °C was also accompanied by the decrease of WSO yield from 24.1 ± 1.5 to 18.2 ± 2.4% indicating repolymerization of WSO compounds similar to AL. However, unlike AL, at a higher temperature of 310 °C, the WSO yield was stable, possibly indicating the secondary cracking of biocrude, which decreased the yield.
The maximum yield of OL at lower temperature is attributed to the higher abundance of β–O–4 linkages in OL structure, which can be readily cleaved and stabilized in the biocrude phase under mild conditions [34,35]. Although increasing temperature led to depolymerization of lignin structure, evidenced by the decreasing solid char yield from 68.9 ± 0.4% to 60.6 ± 1.7%, the depolymerized fragments were not stabilized into the biocrude phase, resulting in a stable biocrude yield. Hashmi et al. [25] similarly reported stable biocrude yields of around 10.5% within this temperature range during OL HTL. The lower yield reported in that study may result from the differences in the botanical origin of OL as well as from omitting a post-HTL char wash during extraction, potentially leaving biocrude adsorbed on the char surface. LS, due to its water-soluble structure, also exhibited a relatively high WSO yield comparable to that of AL. However, the stable solid char yield and the decrease in both biocrude and WSO yields with increasing temperature suggest secondary cracking of these products. This behavior may be attributed to the higher sulfur content of LS (as shown in Table 1), as sulfur-rich feedstocks are known to promote gaseous product formation during HTL [36]. Overall, AL and OL produced higher biocrude yields compared to DAL and LS. While OL achieved its maximum yield at the lowest temperature (270 °C), AL reached its maximum yield at 310 °C, reflecting differences in yield distribution among the lignin types. From an industrial perspective, achieving maximum yields at lower temperatures, as observed for OL, could reduce energy input and operational costs, whereas the higher temperature requirement for AL highlights the trade-off between yield optimization and process economics.
3.2. Effect of Lignin Type and HTL Temperature on Fuel Properties of Biocrude
After determining the biocrude yield from each type of lignin, the elemental composition and HHV were measured to assess biocrude quality, as presented in Table 1. AL-derived biocrude, along with a higher yield (as shown in Figure 1), exhibited the highest carbon percentage (68.2 ± 0.7 to 70.2 ± 0.0%) compared to other lignin-derived biocrude. It also resulted in biocrude with lower oxygen content (20.8 ± 0.1 to 24.1 ± 0.7%) compared to DAL and OL, which contributed to the enhanced HHV (28.1 ± 0.3 to 31.0 ± 0.2 MJ/kg). The higher percentage of carbon in AL biocrude was due to the enhanced demethoxylation of lignin structure during alkaline treatment, which removed the oxygen-containing methoxy groups [37], improving the carbon percentage. Similar elemental composition of AL biocrude was reported by Biswas et al. [30] during HTL with a metal-supported activated carbon catalyst. The carbon, oxygen, and HHV reported in that study were 70.3%, 20.8% and 31.1 MJ/Kg. Although the biocrude yield for OL did not change significantly with HTL temperature, the carbon content of OL biocrude increased with temperature from 64.6 ± 0.1 to 67.7 ± 0.0% due to enhanced deoxygenation at higher temperatures [20], as reflected by the reduced oxygen content from 27.9 ± 0.5 to 25.8 ± 0.0%. The enhanced carbon and reduced oxygen lead to the maximum HHV (27.0 ± 0.0 MJ/Kg) of OL biocrude at a higher HTL temperature of 310 °C. Yang et al. [22] also observed the enhancement of carbon content in biocrude with increasing temperature during HTL of OL. OL also leads to sulfur-free biocrude due to the absence of sulfur in the OL structure, which indicates significant potential since no desulfurization process will be required during the upgrading process of biocrude.
Along with lower biocrude yield, DAL and LS resulted in lower carbon content in the biocrude compared to AL and OL. This is due to the presence of a higher amount of sulfur (2.3 ± 1.0 to 5.2 ± 0.1% for DAL and 7.9 ± 0.2 to 11.6 ± 0.6% for LS) in biocrude from both lignin types, which was introduced during their extraction process with sodium sulfite [37,38]. It is noteworthy to mention that, unlike DAL and LS, AL-derived biocrude contains a significantly lower amount of sulfur, 0.4 ± 0.2 to 0.8 ± 0.1% despite having a higher content (3.66 ± 0.07%) in its raw form. This indicates efficient sulfur removal during HTL of AL, enhancing its suitability for fuel applications and potentially reducing the need for costly downstream desulfurization processes (similar to OL), thereby improving the economic feasibility of the industrial-scale conversion.

Table 1.
Elemental composition and HHV of raw lignins and their corresponding biocrude produced at different HTL temperatures.
Table 1.
Elemental composition and HHV of raw lignins and their corresponding biocrude produced at different HTL temperatures.
| Lignin Type | Temperature (°C) | Elemental Composition a | HHV c (MJ/kg) | ||||
|---|---|---|---|---|---|---|---|
| C (%) | H (%) | N (%) | S (%) | O b (%) | |||
| AL | Raw | 52.7 ± 0.2 | 4.9 ± 0.1 | 0.5 ± 0.1 | 3.7 ± 0.1 | 21.5 ± 0.7 | 21.5 ± 0.1 |
| 270 | 68.2 ± 0.7 | 6.4 ± 0.0 | 0.3 ± 0.2 | 0.4 ± 0.2 | 24.1 ± 0.7 | 28.1 ± 0.3 | |
| 290 | 70.2 ± 0.0 | 7.5 ± 0.1 | 0.3 ± 0.0 | 0.7 ± 0.0 | 20.8 ± 0.1 | 31.0 ± 0.2 | |
| 310 | 69.4 ± 0.3 | 6.7 ± 0.0 | 0.4 ± 0.2 | 0.8 ± 0.1 | 22.3 ± 0.2 | 29.3 ± 0.1 | |
| DAL | Raw | 48.4 ± 0.0 | 4.3 ± 0.0 | 0.6 ± 0.0 | 4.7 ± 0.1 | 29.8 ± 0.6 | 17.7 ± 0.0 |
| 270 | 63.1 ± 1.9 | 6.3 ± 0.4 | 0.2 ± 0.0 | 2.3 ± 1.0 | 28.0 ± 1.3 | 25.8 ± 1.4 | |
| 290 | 63.9 ± 0.5 | 7.1 ± 0.1 | 0.2 ± 0.0 | 5.2 ± 0.1 | 23.5 ± 0.6 | 28.3 ± 0.4 | |
| 310 | 62.0 ± 0.7 | 6.4 ± 0.2 | 0.2 ± 0.0 | 3.2 ± 0.2 | 28.4 ± 1.1 | 25.5 ± 0.7 | |
| OL | Raw | 62.2 ± 0.2 | 4.9 ± 0.0 | 1.1 ± 0.1 | 0.0 ± 0.0 | 31.9 ± 0.3 | 22.4 ± 0.2 |
| 270 | 64.6 ± 0.1 | 6.3 ± 0.0 | 0.5 ± 0.0 | 0.0 ± 0.0 | 27.9 ± 0.5 | 26.0 ± 0.1 | |
| 290 | 65.7 ± 0.3 | 6.4 ± 0.1 | 0.6 ± 0.2 | 0.0 ± 0.0 | 27.1 ± 0.6 | 26.7 ± 0.4 | |
| 310 | 67.7 ± 0.0 | 6.0 ± 0.0 | 0.4 ± 0.0 | 0.0 ± 0.0 | 25.8 ± 0.0 | 27.0 ± 0.0 | |
| LS | Raw | 37.8 ± 0.2 | 4.6 ± 0.3 | 0.6 ± 0.0 | 7.5 ± 0.1 | 22.9 ± 1.4 | 16.1 ± 0.1 |
| 270 | 64.5 ± 0.3 | 7.7 ± 0.1 | 0.3 ± 0.0 | 7.9 ± 0.2 | 19.7 ± 0.3 | 30.3 ± 0.2 | |
| 290 | 60.7 ± 0.5 | 6.8 ± 0.1 | 0.3 ± 0.0 | 11.6 ± 0.6 | 20.6 ± 0.7 | 27.9 ± 0.4 | |
| 310 | 63.0 ± 1.4 | 7.0 ± 0.2 | 0.3 ± 0.0 | 8.6 ± 2.7 | 21.0 ± 2.1 | 28.6 ± 0.9 | |
a Elemental composition is presented on a dry mass basis. b Oxygen percentage is calculated by the difference method. c HHV was calculated using the Dulong formula [31] and reported on a dry basis.
In addition to changes in elemental composition, the boiling point distribution of compounds in the biocrude varied notably across lignin types and HTL temperatures. To assess this, the compounds were classified into standard petroleum fractions based on weight loss across defined boiling point ranges. It is important to note that these fractions are not identical to their respective categories in composition, but they indicate biocrude components with volatility falling within the corresponding boiling point ranges. As shown in Figure 2, biocrude derived from all lignin types predominantly consisted of compounds that volatilize in the boiling point range of gasoline, kerosene, and diesel. The gasoline and kerosene fraction increased in both AL (from 28 to 36% and from 16 to 27%) and OL (from 17 to 25% and from 12 to 18%)
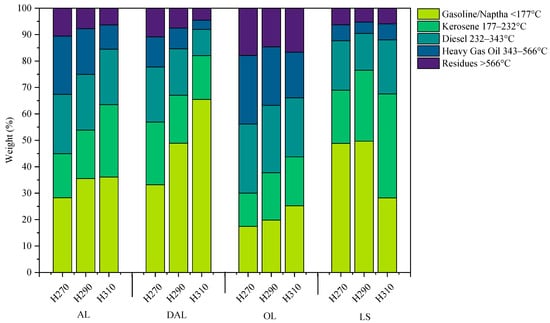
Figure 2.
Boiling point distribution of biocrude obtained from HTL of different types of lignin at varying HTL temperatures.
Although the gasoline fraction increased remarkably from 33 to 65% in the DAL biocrude, the kerosene fraction decreased from 23% to 16% with the increase in temperature. The increase of gasoline fraction in these lignin-derived biocrude was accompanied by the decrease of diesel, heavy gas oil, and residue fractions, indicating the conversion of heavy molecular weight compounds of biocrude into light compounds due to increased thermal cracking at high temperature [39,40]. In contrast, LS-derived biocrude showed the maximum amount of gasoline fraction (around 49%) at 270 and 290 °C, compared to other types of lignin, which decreased to 28% at 310 °C. Correspondingly, an increase in the kerosene and diesel fraction was observed at a higher HTL temperature of 310 °C. It is well known that lignosulfonate structure contains a notable amount of carbohydrate impurities [36,37,38], which leads to the formation of cyclic ketones (cyclopentanone, methyl cyclopentanone) during HTL [41]. These ketonic compounds typically have a lower boiling point (<177 °C). In addition, the sulfonate group of the LS structure could lead to the formation of highly volatile sulfur-containing compounds, explaining the higher fraction of gasoline at lower HTL temperature. However, with the increase of HTL temperature, these ketonic compounds might form heavier ketones with a side-chain, and sulfur-containing compounds could lead to gaseous product formation, lowering the gasoline fraction. It is worth mentioning that OL-derived biocrude exhibited significantly lower gasoline and kerosene fractions compared to other lignins at a particular HTL temperature. This behavior is attributed to the higher fraction of syringyl units in the OL structure [34], which degrade into syringol compounds containing a second methoxy group, resulting in higher boiling points. Moreover, OL retains a structure closest to native lignin and contains a higher abundance of side-chain-rich units [11]. Consequently, its depolymerization yields a greater fraction of compounds with complex side chains, explaining the lower amount of gasoline and kerosene fraction.
3.3. Molecular Compound Distribution of Biocrude and Its Formation Pathway
Biocrude derived from all types of lignin at 310 °C was analyzed using GC-MS to determine the molecular composition and obtain insights into their formation pathways. Due to the inherent limitations of GC-MS, only the volatile fraction of biocrude (Boiling point < 280 °C) was analyzed. The compounds with a match quality of more than 70% (with reference spectra of the NIST library) were categorized into multiple groups, and their corresponding peak areas are presented in Table 2. The total peak areas of these compounds correspond to 94%, 100%, 80.9% and 78% for AL, DAL, OL, and LS biocrude, respectively. The list of all the identified compounds of each category and their corresponding peak area was presented in Table S2.

Table 2.
Classification of compounds identified in biocrude derived from different lignin types at 310 °C and their corresponding peak area.
Table 2 shows that AL-derived biocrude is rich in guaiacol (46.6%) and alkyl guaiacols (26.2%) such as 4-methyl and 5-methyl guaiacol. The higher abundance of alkyl guaiacols might be correlated with the higher fraction of carbon in AL-derived biocrude, as shown in Table 1, due to the absence of oxygen in the alkyl group. It also uniquely contains veratroles (veratrole, 3-methyl veratrole) at 13%, which are absent in biocrudes from other lignin types. The formation of veratroles only under alkaline conditions was also reported in the HTL study of Lui et al. [23]. In DAL-derived biocrude, guaiacol content is even higher (69.5%), though it contains a lower proportion of alkyl guaiacols (10.9%) and a higher fraction of other complex guaiacol derivatives (11.6%) like apocynin and homovanillinic acid. In contrast, OL and LS-derived biocrudes contain significantly lower guaiacol levels (13.4% and 5.6%, respectively). OL biocrude is instead dominated by syringol-type compounds (26.8%) and unique side-chained aromatics such as desaspidinol (12.1%), which is in accordance with previous studies associating the depolymerization of OL [25,42,43]. LS-derived biocrude is characterized by high levels of sulfur-containing compounds (dimethyl trisulfide, 1,1-bis(methylthio)ethane), aliphatic carbonyls (2-cyclopenten-1-one derivatives), and catechols (4-methyl and 4-ethyl catechol), with peak areas of 18.3%, 15.3%, and 15.6%, respectively. The higher abundance of sulfur-containing compounds further justifies the higher percentage of sulfur in LS-derived biocrude, as shown in Table 1. Notably, LS and OL biocrudes exhibit higher proportions of phenol and alkyl phenol compounds, whereas alkyl phenols were not detected in AL and DAL biocrudes.
Next, to understand the distribution of compounds across different lignin-derived biocrudes, we explored their possible formation pathways (shown in Figure 3, Figure 4, Figure 5 and Figure 6) based on the structural characteristics of each lignin. The molecular structures of OL and LS were obtained from the PubChem database, while those of AL and DAL were reconstructed in ChemDraw 23.1.2 according to the reported structures in [44,45], respectively. During HTL, hydrolysis and thermal cracking depolymerize lignin into various monolignols, as shown in Figure 3, Figure 4, Figure 5 and Figure 6 for different types of lignin. Monolignol 1, a common intermediate across all lignins (only the OH group of the side-chain substituted with a sulfonate group for LS (1′)), undergoes side-chain cleavage at different positions to form guaiacol and 4-alkyl guaiacols, explaining their presence in all biocrudes. However, AL and DAL (Figure 3 and Figure 4) contain a higher abundance of inherent guaiacol in their structure, accounting for their elevated guaiacol content. In contrast, guaiacol and alkyl guaiacol intermediates in OL and LS are more susceptible to demethoxylation under HTL conditions, forming phenols and alkyl phenols [46]. This explains the higher abundance of these compounds in OL and LS biocrudes.
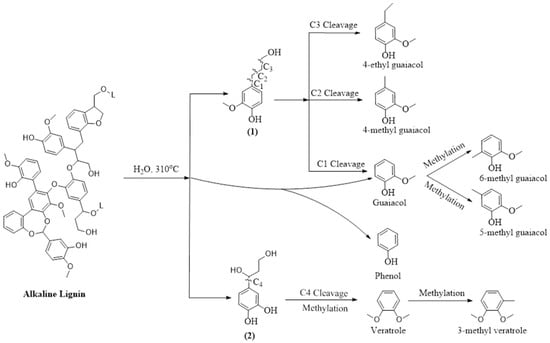
Figure 3.
Plausible formation pathways of compounds present in AL biocrude.
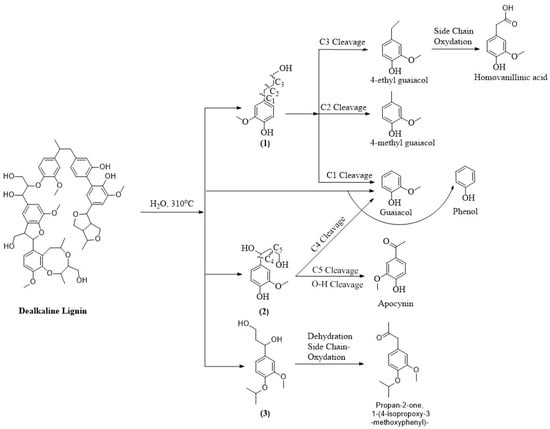
Figure 4.
Plausible formation pathways of compounds present in DAL biocrude.
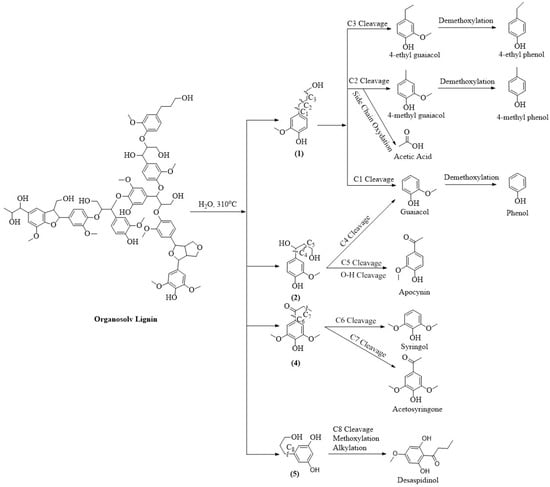
Figure 5.
Plausible formation pathways of compounds present in OL biocrude.
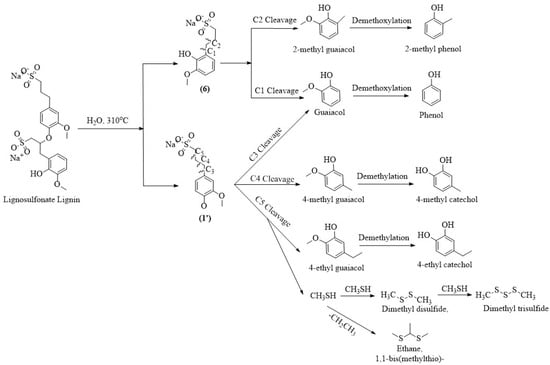
Figure 6.
Plausible formation pathways of compounds present in LS biocrude.
AL lignin also yields monolignol 2, where C4 cleavage followed by side-chain methylation leads to veratrole-type compounds. The exclusive presence of 5- and 6-methyl guaiacol further indicates enhanced methylation during AL liquefaction, promoted by alkaline conditions [23]. In DAL biocrude, complex guaiacol derivatives such as homovanillinic acid may form via side-chain oxidation of 4-ethyl guaiacol and apocynin via C5 cleavage and oxidation of monolignol 3, suggesting DAL’s higher tendency toward oxidative degradation. The elevated syringol content in OL biocrude can be attributed to the depolymerization of OL into monolignol 4 (exclusive to OL as shown in Figure 5), whose side-chain cleavage yields syringol-type compounds. Desaspidinol may form via side-chain cleavage of monolignol 5, followed by methoxylation and alkylation of the resulting butanal chain. In LS (Figure 6), catechols likely form via demethylation of guaiacols, while cleavage of sulfonate side chains may generate methanethiol (CH3SH), which contributes to the formation of sulfur-containing compounds [36]. Additionally, LS contains higher carbohydrate impurities (not shown in the reaction pathway since they are present as impurities) [47,48], whose depolymerization under HTL conditions can produce ketonic compounds [41], explaining their presence in LS-derived biocrude. However, it is important to note that the proposed reaction mechanism does not explain the non-volatile fraction, which may contain heavier, oxygen-rich compounds contributing to overall biocrude properties and yield. Therefore, the presence and potential reactivity of the non-volatile fraction should be considered in further studies.
4. Conclusions
This study systematically investigated the HTL of four structurally distinct lignin types over a temperature range of 270 to 310 °C and elucidated the structure-specific reaction mechanism that governs biocrude formation. AL lignin exhibited the highest biocrude yield of 16.8 % and produced biocrude with the highest carbon and energy content, as well as showed substantial desulfurization following HTL. OL lignin also yielded sulfur-free biocrude with comparable yields to AL and an increasing carbon content with temperature. In contrast, DAL and LS produced significantly lower biocrude yields, along with lower carbon and higher sulfur percentages. The distillation profiles of the biocrudes revealed dominant fractions within the gasoline, kerosene, and diesel boiling point ranges, with the gasoline fraction increasing with temperature in the AL, DAL, and OL biocrude. GC-MS analysis showed guaiacol-type compounds as predominant in AL and DAL biocrudes, while OL-derived biocrude was enriched with syringol derivatives, and LS biocrude contained a higher fraction of sulfur-containing, aliphatic ketones and catechol-type compounds. The reaction mechanisms indicate the dominance of methylation reactions in AL lignin, side-chain oxidation in DAL lignin, and demethoxylation in OL, while both demethoxylation and demethylation pathways appear prominent in LS lignin. Overall, AL and OL showed the most promise for biocrude production, although challenges related to both yield and fuel quality persist. Future research should prioritize strategies to enhance biocrude yield and upgrade its properties to enable a more sustainable contribution to renewable fuel systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en18174773/s1, Table S1. Yield of solid char and WSO from different types of lignin. Table S2. Detailed classification of compounds identified in HTL 310 °C biocrude from different types of lignin.
Author Contributions
M.M.R. Conceptualization, data curation, formal analysis, writing—original draft, writing—review, and editing. T.R. conceptualization, manuscript revision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the U.S. Department of Energy’s Office of Biological and Environmental Research under contracts DE-SC0024701.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to an internal agreement between the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chauhan, P.S.; Agrawal, R.; Satlewal, A.; Kumar, R.; Gupta, R.P.; Ramakumar, S.S.V. Next Generation Applications of Lignin Derived Commodity Products, Their Life Cycle, Techno-Economics and Societal Analysis. Int. J. Biol. Macromol. 2022, 197, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of Lignin from a Roadblock to Bridge for Lignocellulose Biorefineries: A Comprehensive Review. Sci. Total Environ. 2023, 861, 160560. [Google Scholar] [CrossRef] [PubMed]
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Bajaj, B.K. Application of Ionic Liquid and Alkali Pretreatment for Enhancing Saccharification of Sunflower Stalk Biomass for Potential Biofuel-Ethanol Production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Raj, T.; Mohanta, C.S.; Semwal, S.; Satlewal, A.; Gupta, R.P.; Puri, S.K.; Ramakumar, S.S.V.; Kumar, R. 2G Waste Lignin to Fuel and High Value-Added Chemicals: Approaches, Challenges and Future Outlook for Sustainable Development. Chemosphere 2021, 268, 129326. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; Vural-Gürsel, I.; Gosselink, R.; Junginger, M. Review of Life Cycle Assessments of Lignin and Derived Products: Lessons Learned. Sci. Total Environ. 2021, 770, 144656. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, J.; Innocent, M.T.; Wang, Q.; Xiang, H.; Tang, J.; Zhu, M. Lignin-Based Carbon Fibers: Formation, Modification and Potential Applications. Green Energy Environ. 2022, 7, 578–605. [Google Scholar] [CrossRef]
- Kraft Lignin Market. Market.us. Available online: https://market.us/report/global-kraft-lignin-market/ (accessed on 5 September 2025).
- Anukam, A.; Berghel, J.; Henrikson, G.; Frodeson, S.; Ståhl, M. A Review of the Mechanism of Bonding in Densified Biomass Pellets. Renew. Sustain. Energy Rev. 2021, 148, 111249. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zheng, F.; Zhang, Z.; Zhang, Y.; Long, D. Mechanism Insight into Photocatalytic Conversion of Lignin for Valuable Chemicals and Fuels Production: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2021, 147, 111217. [Google Scholar] [CrossRef]
- M.R.F. Lignosulfonate Market Size, Share & Industry Report 2034. Available online: https://www.marketresearchfuture.com/reports/lignosulfonate-market-26116 (accessed on 6 August 2025).
- Rabelo, S.C.; Nakasu, P.Y.S.; Scopel, E.; Araújo, M.F.; Cardoso, L.H.; Costa, A.C. da Organosolv Pretreatment for Biorefineries: Current Status, Perspectives, and Challenges. Bioresour. Technol. 2023, 369, 128331. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Valente, A.J.M.; Romano, A.; Antunes, F.E.; Medronho, B. Dissolution of Kraft Lignin in Alkaline Solutions. Int. J. Biol. Macromol. 2020, 148, 688–695. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Hu, S.; Zhou, X.; Zhang, H.; Yue, F. Reaction Pathways of Lignin Phenolation in Alkaline Medium and the Compatibility for Integrated Phenolation-Kraft Pulping Process. Ind. Crops Prod. 2025, 230, 121092. [Google Scholar] [CrossRef]
- Wang, H.; Male, J.; Wang, Y. Recent Advances in Hydrotreating of Pyrolysis Bio-Oil and Its Oxygen-Containing Model Compounds. ACS Catal. 2013, 3, 1047–1070. [Google Scholar] [CrossRef]
- Libretti, C.; Correa, L.S.; Meier, M.A.R. From Waste to Resource: Advancements in Sustainable Lignin Modification. Green Chem. 2024, 26, 4358–4386. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin Derived Carbon Materials: Current Status and Future Trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Abbas, S.C.; Alam, A.; Mian, M.M.; Walker, C.; Ni, Y. Hydrothermal Liquefaction of Sewage Sludge for Circular Bioeconomy: Focus on Lignocellulose Wastes, Microplastics, and Pharmaceuticals. J. Bioresour. Bioprod. 2025, 10, 170–186. [Google Scholar] [CrossRef]
- Motavaf, B.; Savage, P.E. Effect of Process Variables on Food Waste Valorization via Hydrothermal Liquefaction. ACS EST Eng. 2021, 1, 363–374. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. From Biomass to Biocrude: Innovations in Hydrothermal Liquefaction and Upgrading. Energy Convers. Manag. 2024, 302, 118093. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Z.; Li, B.; Zhang, H.; Wang, H. Experimental Study on Preparation of Bio-Oil by Hydrothermal Liquefaction of Three Kinds of Lignin. J. Fuel Chem. Technol. 2023, 51, 1084–1095. [Google Scholar] [CrossRef]
- Lui, Y.W.; Tao, Q.; Akien, G.R.; Yuen, A.K.L.; Montoya, A.; Chan, B.; Lui, M.Y. Hydrothermal Depolymerization of Different Lignins: Insights into Structures and Reactivities. Int. J. Biol. Macromol. 2025, 314, 144293. [Google Scholar] [CrossRef] [PubMed]
- Halleraker, H.V.; Kalogiannis, K.; Lappas, A.; Castro, R.C.A.; Roberto, I.C.; Mussatto, S.I.; Barth, T. The Consistency of Yields and Chemical Composition of HTL Bio-Oils from Lignins Produced by Different Preprocessing Technologies. Energies 2022, 15, 4707. [Google Scholar] [CrossRef]
- Hashmi, S.F.; Meriö-Talvio, H.; Hakonen, K.J.; Ruuttunen, K.; Sixta, H. Hydrothermolysis of Organosolv Lignin for the Production of Bio-Oil Rich in Monoaromatic Phenolic Compounds. Fuel Process. Technol. 2017, 168, 74–83. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Classified Separation of Lignin Hydrothermal Liquefied Products. Ind. Eng. Chem. Res. 2011, 50, 11288–11296. [Google Scholar] [CrossRef]
- Katongtung, T.; Onsree, T.; Tippayawong, N. Machine Learning Prediction of Biocrude Yields and Higher Heating Values from Hydrothermal Liquefaction of Wet Biomass and Wastes. Bioresour. Technol. 2022, 344, 126278. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.-E.; Vamling, L.; Olausson, L.; Andersson, S.-I.; Theliander, H. The Effect of Temperature on the Catalytic Conversion of Kraft Lignin Using Near-Critical Water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P.; Reddy, S.N. Catalytic (Copper) Hydrothermal Liquefaction for Lignin to Produce High Quality Bio-Oil and Nano Cu Carbon Hybrids Material. Chem. Eng. Sci. 2023, 270, 118548. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Catalytic Hydrothermal Liquefaction of Alkali Lignin over Activated Bio-Char Supported Bimetallic Catalyst. Bioresour. Technol. 2021, 337, 125439. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Saha, N.; Banivaheb, S.; Toufiq Reza, M. Towards Solvothermal Upcycling of Mixed Plastic Wastes: Depolymerization Pathways of Waste Plastics in Sub- and Supercritical Toluene. Energy Convers. Manag. X 2022, 13, 100158. [Google Scholar] [CrossRef]
- Helander, M.; Theliander, H.; Lawoko, M.; Henriksson, G.; Zhang, L.; Lindström, M.E. Fractionation of Technical Lignin: Molecular Mass and pH Effects. BioResources 2013, 8, 2270–2282. [Google Scholar] [CrossRef]
- Latham, K.G.; Matsakas, L.; Figueira, J.; Rova, U.; Christakopoulos, P.; Jansson, S. Examination of How Variations in Lignin Properties from Kraft and Organosolv Extraction Influence the Physicochemical Characteristics of Hydrothermal Carbon. J. Anal. Appl. Pyrolysis 2021, 155, 105095. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Cheng, H.; Lei, M.; Liang, J.; Tong, H. Theoretical Study of Bond Dissociation Energies for Lignin Model Compounds. J. Fuel Chem. Technol. 2015, 43, 429–436. [Google Scholar] [CrossRef]
- Wörner, M.; Werner, L.; Hornung, U.; Islongo Canabarro, N.; Baudouin, D.; Dahmen, N. The Impact of Sulfur-Containing Inorganic Compounds during the Depolymerization of Lignin by Hydrothermal Liquefaction of Black Liquor. Energy Fuels 2024, 38, 6036–6047. [Google Scholar] [CrossRef]
- TCI AMERICA. Lignin 8061-51-6. Available online: https://www.tcichemicals.com/US/en/p/L0045 (accessed on 13 May 2025).
- Deshpande, R.; Sundvall, L.; Grundberg, H.; Henriksson, G.; Lawoko, M. Structural Basis for Lignin Recalcitrance during Sulfite Pulping for Production of Dissolving Pulp from Pine Heartwood. Ind. Crops Prod. 2022, 177, 114391. [Google Scholar] [CrossRef]
- Xu, D.; Savage, P.E. Effect of Temperature, Water Loading, and Ru/C Catalyst on Water-Insoluble and Water-Soluble Biocrude Fractions from Hydrothermal Liquefaction of Algae. Bioresour. Technol. 2017, 239, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mohanty, K. Co-HTL of Domestic Sewage Sludge and Wastewater Treatment Derived Microalgal Biomass—An Integrated Biorefinery Approach for Sustainable Biocrude Production. Energy Convers. Manag. 2020, 204, 112312. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, M.; Jiang, H.; Yu, P.; Yang, W. Co-Liquefaction of Livestock Manure and Food Waste: Synergistic Effects and Product Combustion Performance. Appl. Energy 2023, 341, 121073. [Google Scholar] [CrossRef]
- Duan, D.; Zhao, Y.; Fan, L.; Dai, L.; Lv, J.; Ruan, R.; Wang, Y.; Liu, Y. Low-Power Microwave Radiation-Assisted Depolymerization of Ethanol Organosolv Lignin in Ethanol/Formic Acid Mixtures. BioResources 2017, 12, 5308–5320. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Pineda, A.; Romero, A.A.; Luque, R.; Labidi, J. Microwave-Assisted Depolymerisation of Organosolv Lignin via Mild Hydrogen-Free Hydrogenolysis: Catalyst Screening. Appl. Catal. B Environ. 2014, 145, 43–55. [Google Scholar] [CrossRef]
- Dutta, K.; Singh, A. Chemical Modification of Lignin and Thereafter Grafting with Lactic Acid for Flexible Polymer Film Preparation. J. Appl. Polym. Sci. 2022, 139, 52320. [Google Scholar] [CrossRef]
- Peng, M.; Muraishi, T.; Hou, X.; Zhao, M.; Kamiya, K.; Qian, E.W. Oxidative Depolymerization of Lignin to Vanillin and Lactic Acid in an Aqueous Solution. Fuel 2023, 348, 128486. [Google Scholar] [CrossRef]
- Wörner, M.; Barsuhn, A.; Zevaco, T.; Hornung, U.; Dahmen, N. From Pulp to Aromatic Products─Reaction Pathways of Lignin Depolymerization. Energy Fuels 2024, 38, 6020–6035. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Chemical and Thermal Characteristics of Ion-Exchanged Lignosulfonate. Molecules 2023, 28, 2755. [Google Scholar] [CrossRef] [PubMed]
- Khokarale, S.G.; Le-That, T.; Mikkola, J.-P. Carbohydrate Free Lignin: A Dissolution–Recovery Cycle of Sodium Lignosulfonate in a Switchable Ionic Liquid System. ACS Sustain. Chem. Eng. 2016, 4, 7032–7040. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).