The Torrefaction of Agricultural and Industrial Residues: Thermogravimetric Analysis, Characterization of the Products and TG-FTIR Analysis of the Gas Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Torrefaction Process
2.3. Characterization of Raw Materials and Solid Products
3. Results
3.1. Mass and Energy Yield, and the Energetic Value of the Products
3.2. Analylsis of Ash Content, Volatile Matter and Fixed Carbon
3.3. Functional and Structural Characteristics of the Samples
3.4. Thermogravimetric Analysis
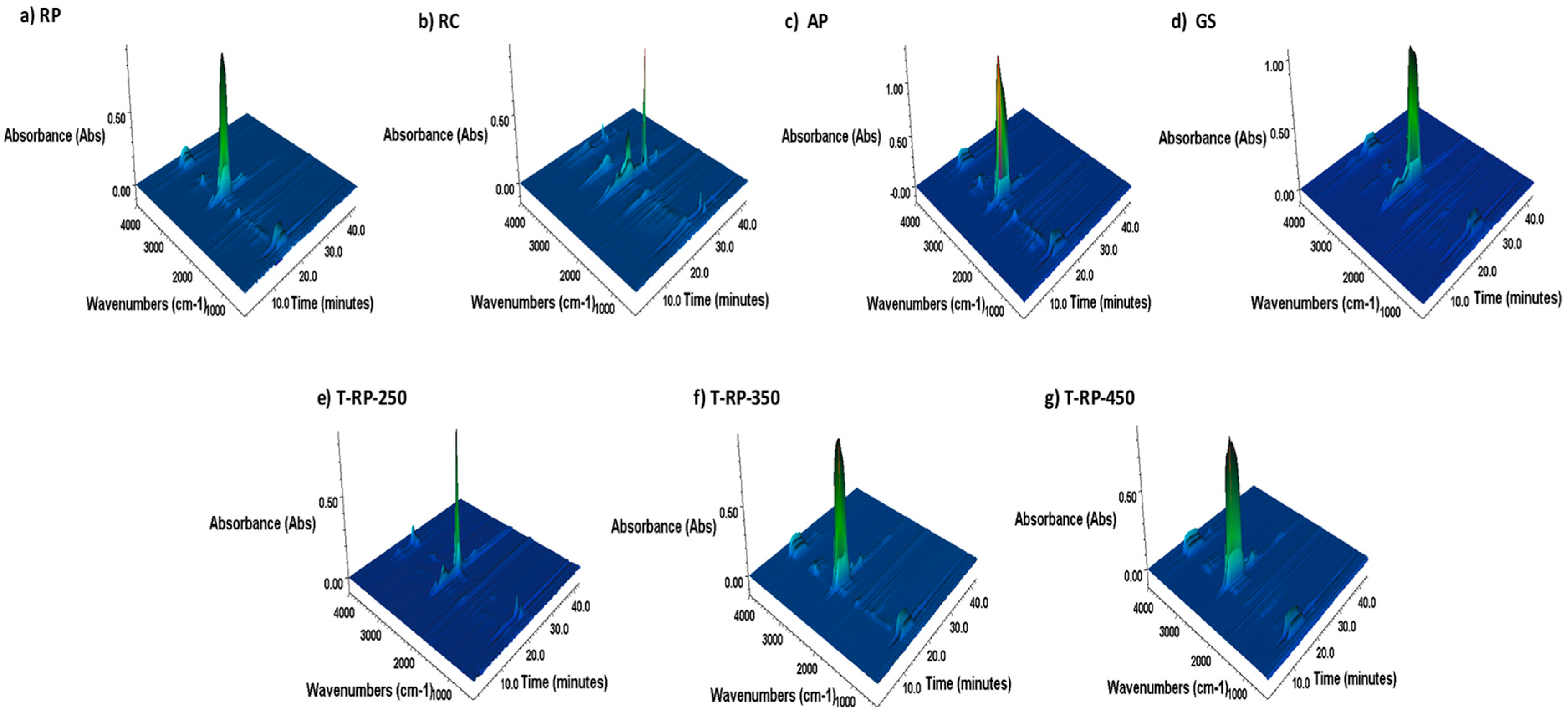
3.5. TGA-FTIR Analysis of Gaseous Products Released During Pyrolysis and Combustion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Apple pomace |
| DTG | Derivative thermogravimetric curve |
| EY | Energy yield |
| FTIR | Fourier transform infrared spectroscopy |
| GS | Grape seeds |
| HHV | Higher heating value |
| MY | Mass yield |
| RP | Rosemary pomace |
| RC | Rosemary cake |
| TGA | Thermogravimetric analysis |
References
- Zhang, J.; Gu, J.; Shan, R.; Yuan, H.; Chen, Y. Advances in thermochemical valorization of biomass towards carbon neutrality. Resour. Conserv. Recycl. 2025, 212, 107905. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste biomass valorization for the production of biofuels and value-added products: A comprehensive review of thermochemical, biological and integrated processes. Process Saf. Environ. Prot. 2022, 159, 323–344. [Google Scholar] [CrossRef]
- Ashfaq, M.M.; Bilgic Tüzemen, G.; Noor, A. Exploiting agricultural biomass via thermochemical processes for sustainable hydrogen and bioenergy: A critical review. Int. J. Hydrogen Energy 2024, 84, 1068–1084. [Google Scholar] [CrossRef]
- Seraj, S.; Azargohar, R.; Dalai, A.K. Dry torrefaction and hydrothermal carbonization of biomass to fuel pellets. Renew. Sust. Energ. Rev. 2025, 210, 115186. [Google Scholar] [CrossRef]
- Ivanovski, M.; Urbancl, D.; Petrovič, A.; Stergar, J.; Goričanec, D.; Simonič, M. Improving Lignocellulosic and Non-Lignocellulosic Biomass Characteristics through Torrefaction Process. Appl. Sci. 2022, 12, 12210. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, M.; Bai, Y.; Su, W.; Song, X.; Lv, P.; Wang, J.; Yu, G. Torrefaction of sludge under CO2 atmosphere to improve the fuel properties for high temperature gasification with coal. Thermochim. Acta 2022, 713, 179249. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sust. Energ. Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, C.; Liu, T.; Xu, M.; Ling, P.; Wen, W.; Li, R. Combustion and co-combustion of biochar: Combustion performance and pollutant emissions. Appl. Energy 2024, 376, 124292. [Google Scholar] [CrossRef]
- Dira, A.; Harrou, A.; Elmouwahidi, A.; Bendahhou, A.; Carrasco-Marin, F.; Gharibi, E. Assessment of the electrical and energetic properties of rosemary residue biochars and their wettability. Biomass Bioenergy 2025, 201, 108065. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B. Rosemary Essential Oil Extraction and Residue Valorization by Means of Polyphenol Recovery. Biol. Life Sci. Forum 2023, 26, 8. [Google Scholar] [CrossRef]
- Rebolledo-Leiva, R.; Estévez, S.; Hernández, D.; Feijoo, G.; Moreira, M.T.; González-García, S. Apple Pomace Integrated Biorefinery for Biofuels Production: A Techno-Economic and Environmental Sustainability Analysis. Resources 2024, 13, 156. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple Pomace: A Versatile Substrate for Biotechnological Applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, A.; Barbanera, M. Hydrochar from winemaking industry wastes as solid biofuel: A thermal and kinetic analysis of pyrolysis and combustion. Fuel 2024, 372, 132256. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Lezza, A.; Anderlini, B.; Malferrari, D.; Romagnoli, M.; Roncaglia, F. Technological Prospects of Biochar Derived from Viticulture Waste: Characterization and Application Perspectives. Energies 2024, 17, 3421. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef]
- Pardo, R.; Taboada-Ruiz, L.; Fuente, E.; Ruiz, B.; Díaz-Somoano, M.; Calvo, L.F.; Paniagua, S. Exploring the potential of conventional and flash pyrolysis methods for the valorisation of grape seed and chestnut shell biomass from agri-food industry waste. Biomass Bioenergy 2023, 177, 106942. [Google Scholar] [CrossRef]
- Madadian, E.; Rahimi, J.; Mohebbi, M.; Simakov, D.S.A. Grape pomace as an energy source for the food industry: A thermochemical and kinetic analysis. Food Bioprod. Process. 2022, 132, 177–187. [Google Scholar] [CrossRef]
- Karod, M.; Orton, K.A.; Elkasabi, Y.; Mullen, C.A.; Harman-Ware, A.E.; Iisa, K.; Goldfarb, J.L. Recovery of value-added compounds through fast pyrolysis of apple pomace hydrochar. J. Anal. Appl. Pyrolysis 2025, 185, 106868. [Google Scholar] [CrossRef]
- ASTM_D7582-15(2015); Standard Test Method for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTMInternational: WestConshohocken, PA, USA, 2015.
- UNI EN 14918:2019; Solid Recovered Fuels—Method for the Determination of Calorific Value. UNI: Milano, Italy, 2019.
- Petrovič, A.; Cenčič Predikaka, T.; Škodič, L.; Vohl, S.; Čuček, L. Hydrothermal co-carbonization of sewage sludge and whey: Enhancement of product properties and potential application in agriculture. Fuel 2023, 350, 128807. [Google Scholar] [CrossRef]
- Bimal, A.; Ranjan, R.P.; Animesh, D. Qualitative and kinetic analysis of torrefaction of lignocellulosic biomass using DSC-TGA-FTIR. AIMS Energy 2015, 3, 760–773. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Valenzuela-Medina, D.; Bilbao-Sainz, C.; Klamczynski, A.K.; Avena-Bustillos, R.J.; Milczarek, R.R.; Du, W.-X.; Glenn, G.M.; Orts, W.J. Torrefaction of pomaces and nut shells. Bioresour. Technol. 2015, 177, 58–65. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.J.; Oh, K.C.; Cho, L.H.; Jeon, Y.K.; Kim, D.H. Evaluation of the Optimal Conditions for Oxygen-Rich and Oxygen-Lean Torrefaction of Forestry Byproduct as a Fuel. Energies 2023, 16, 4763. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Ivashchuk, O.; Atamanyuk, V.; Chyzhovych, R.; Manastyrska, V.; Sobechko, I. Evaluation of the Apple Pomace Use as a Raw Material for Alternative Solid Fuel. J. Sustain. Dev. Energy Water Environ. Syst. 2024, 12, 1120529. [Google Scholar] [CrossRef]
- Ferreira, C.I.A.; Calisto, V.; Cuerda-Correa, E.M.; Otero, M.; Nadais, H.; Esteves, V.I. Comparative valorisation of agricultural and industrial biowastes by combustion and pyrolysis. Bioresour. Technol. 2016, 218, 918–925. [Google Scholar] [CrossRef]
- Pulidori, E.; Gonzalez-Rivera, J.; Pelosi, C.; Ferrari, C.; Bernazzani, L.; Bramanti, E.; Tiné, M.R.; Duce, C. Thermochemical Evaluation of Different Waste Biomasses (Citrus Peels, Aromatic Herbs, and Poultry Feathers) towards Their Use for Energy Production. Thermo 2023, 3, 66–75. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sust. Energ. Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Zhao, Y.; Xie, X.; Yang, S.; Hua, D.; Wang, C.; Li, T. Effect of Torrefaction on the Physiochemical Characteristics and Pyrolysis of the Corn Stalk. Polymers 2023, 15, 4069. [Google Scholar] [CrossRef] [PubMed]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sust. Energ. Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effects of torrefaction on lignin-rich biomass (hazelnut shell): Structural variations. J. Renew. Sustain. Energy 2017, 9, 063102. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, X.; Huang, G.; Ren, S.; Dong, L.; Sun, T.; Liu, P.; Li, Y.; Lei, T.; Cai, J. Insight into lignocellulosic biomass torrefaction kinetics with case study of pinewood sawdust torrefaction. Renew. Energy 2023, 215, 118941. [Google Scholar] [CrossRef]
- Gajera, B.; Tyagi, U.; Sarma, A.K.; Jha, M.K. Impact of torrefaction on thermal behavior of wheat straw and groundnut stalk biomass: Kinetic and thermodynamic study. Fuel Commun. 2022, 12, 100073. [Google Scholar] [CrossRef]
- Zhang, D.; Han, P.; Zheng, H.; Yan, Z. Torrefaction of walnut oil processing wastes by superheated steam: Effects on products characteristics. Sci. Total Environ. 2022, 830, 154649. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Nepal, R.; Kim, H.J.; Poudel, J.; Oh, S.C. A study on torrefaction of spent coffee ground to improve its fuel properties. Fuel 2022, 318, 123643. [Google Scholar] [CrossRef]
- Parodi, E.; La Nasa, J.; Ribechini, E.; Petri, A.; Piccolo, O. Extraction of proteins and residual oil from flax (Linum usitatissimum), camelina (Camelina sativa), and sunflower (Helianthus annuus) oilseed press cakes. Biomass Convers. Biorefin. 2023, 13, 1915–1926. [Google Scholar] [CrossRef]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Kumar, P.; Subbarao, P.M.V.; Kala, L.D.; Vijay, V.K. Thermogravimetry and associated characteristics of pearl millet cob and eucalyptus biomass using differential thermal gravimetric analysis for thermochemical gasification. Therm. Sci. Eng. Prog. 2021, 26, 101104. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Zeng, J.; Singh, D.; Chen, S. Thermal decomposition kinetics of wheat straw treated by Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2011, 65, 410–414. [Google Scholar] [CrossRef]
- Tian, X.; Dai, L.; Wang, Y.; Zeng, Z.; Zhang, S.; Jiang, L.; Yang, X.; Yue, L.; Liu, Y.; Ruan, R. Influence of torrefaction pretreatment on corncobs: A study on fundamental characteristics, thermal behavior, and kinetic. Bioresour. Technol. 2020, 297, 122490. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of Torrefaction on Biomass Physicochemical Characteristics and the Resulting Pyrolysis Behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Tapasvi, D.; Khalil, R.; Várhegyi, G.; Skreiberg, Ø.; Tran, K.-Q.; Grønli, M. Kinetic Behavior of Torrefied Biomass in an Oxidative Environment. Energy Fuels 2013, 27, 1050–1060. [Google Scholar] [CrossRef]
- Barzegar, R.; Yozgatligil, A.; Olgun, H.; Atimtay, A.T. TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J. Energy Inst. 2020, 93, 889–898. [Google Scholar] [CrossRef]
- Singara Veloo, K.; Lau, A.K.; Sokhansanj, S. Structural and molecular modifications of woody biomass under minimal torrefaction conditions toward making durable pellets. GSC Adv. Res. Rev. 2024, 20, 129–138. [Google Scholar] [CrossRef]
- Kopczyński, M.; Plis, A.; Zuwała, J. Thermogravimetric and Kinetic Analysis of Raw and Torrefied Biomass Combustion. Chem. Process Eng. 2015, 36, 209–223. [Google Scholar] [CrossRef]
- Ucar, S.; Ozkan, A.R. Characterization of products from the pyrolysis of rapeseed oil cake. Bioresour. Technol. 2008, 99, 8771–8776. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, W.; Rokni, E.; Yang, X.; Levendis, Y.A. Evolution of Gases From the Pyrolysis of Raw and Torrefied Biomass and From the Oxy-Combustion of Their Bio-Chars. J. Energy Resour. Technol. 2021, 144, 021901. [Google Scholar] [CrossRef]
- Merdun, H.; Yıldırım, M. Pyrolysis and combustion of industrial hemp, coal and their blends for thermal analysis by thermogravimetric analysis/Fourier transform infrared spectrometer. Waste Manag. Res. 2025, 43, 192–206. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of Torrefaction on the Pyrolysis Behavior and Bio-Oil Properties of Rice Husk by Using TG-FTIR and Py-GC/MS. Energy Fuels 2014, 28, 5857–5863. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Quek, A.; Betha, R.; Balasubramanian, R. TGA–FTIR investigation of co-combustion characteristics of blends of hydrothermally carbonized oil palm biomass (EFB) and coal. Fuel Process. Technol. 2014, 118, 228–234. [Google Scholar] [CrossRef]
- Singh, K.; Zondlo, J. Characterization of fuel properties for coal and torrefied biomass mixtures. J. Energy Inst. 2017, 90, 505–512. [Google Scholar] [CrossRef]
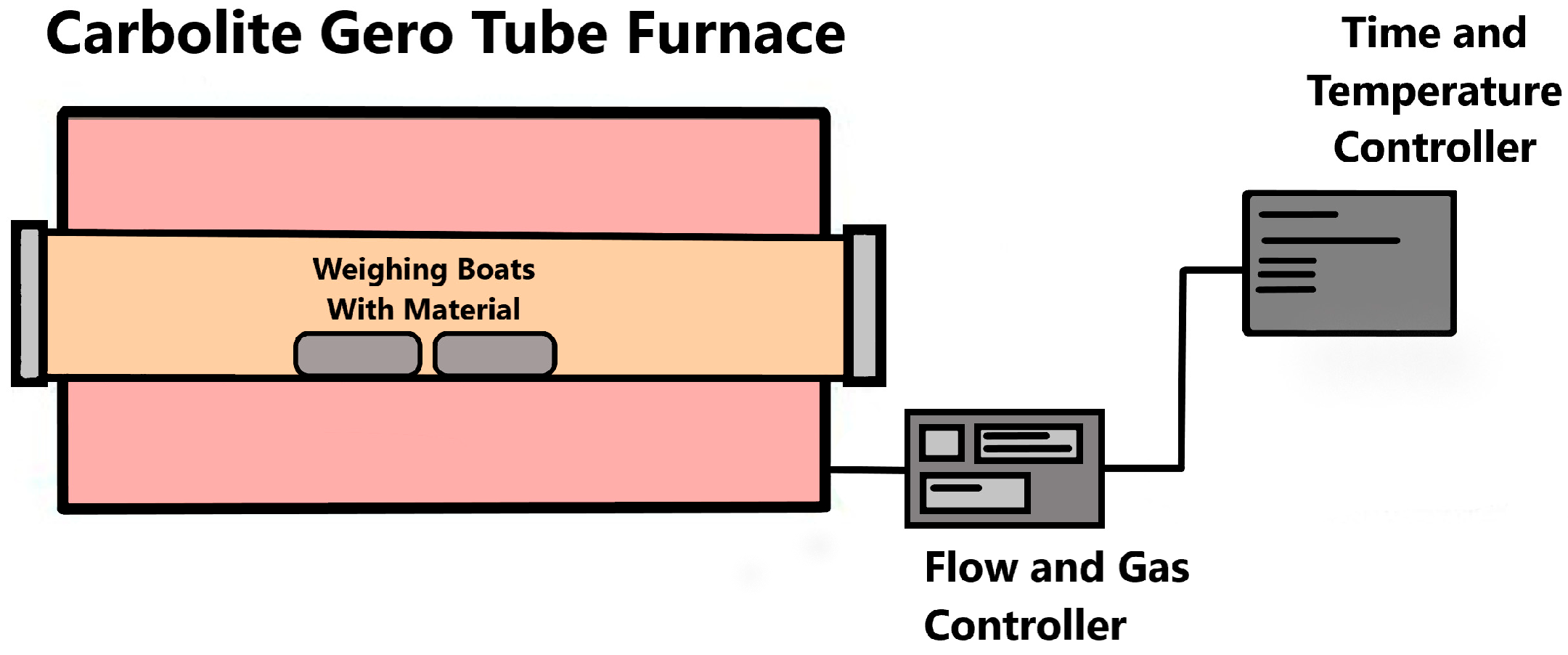


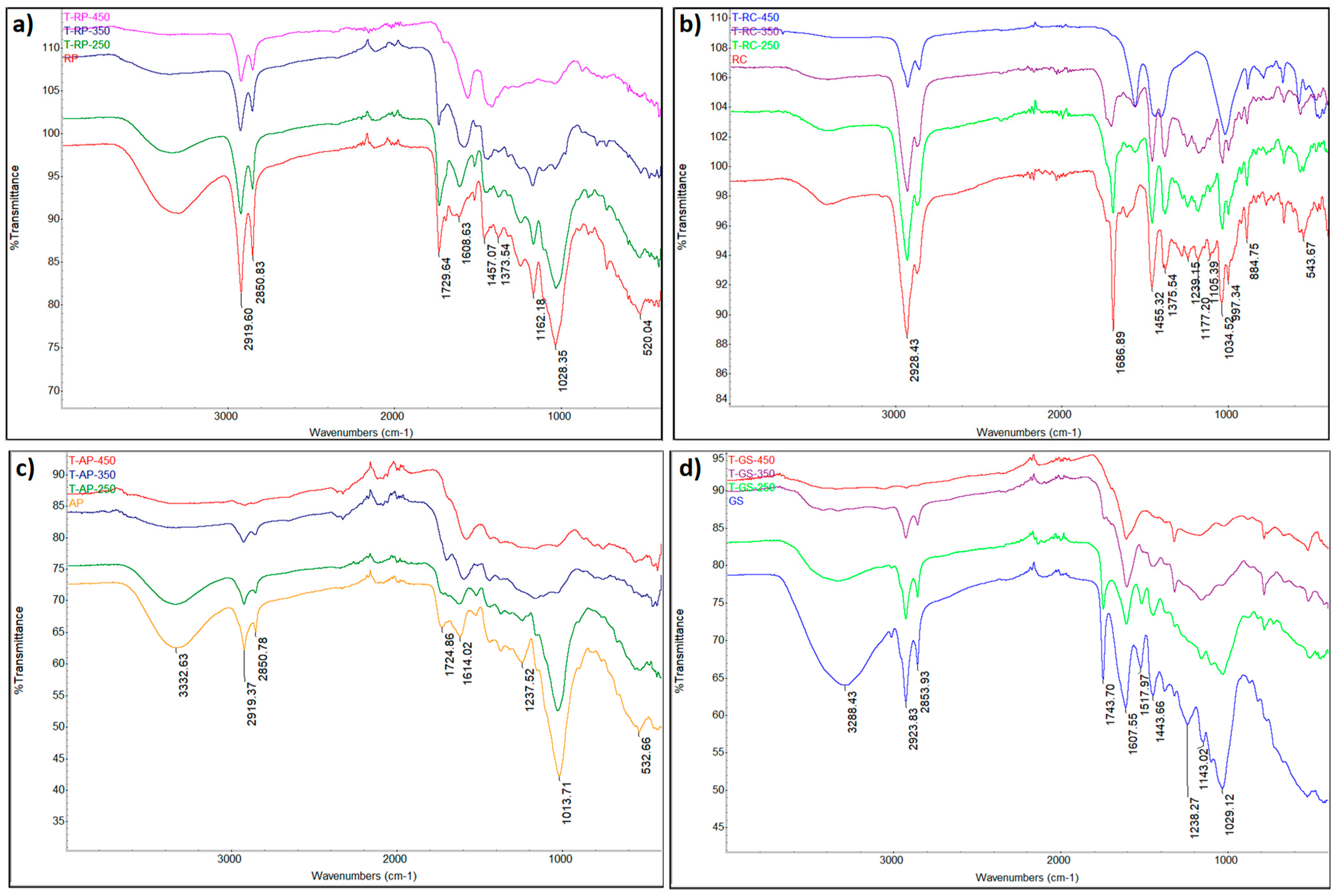
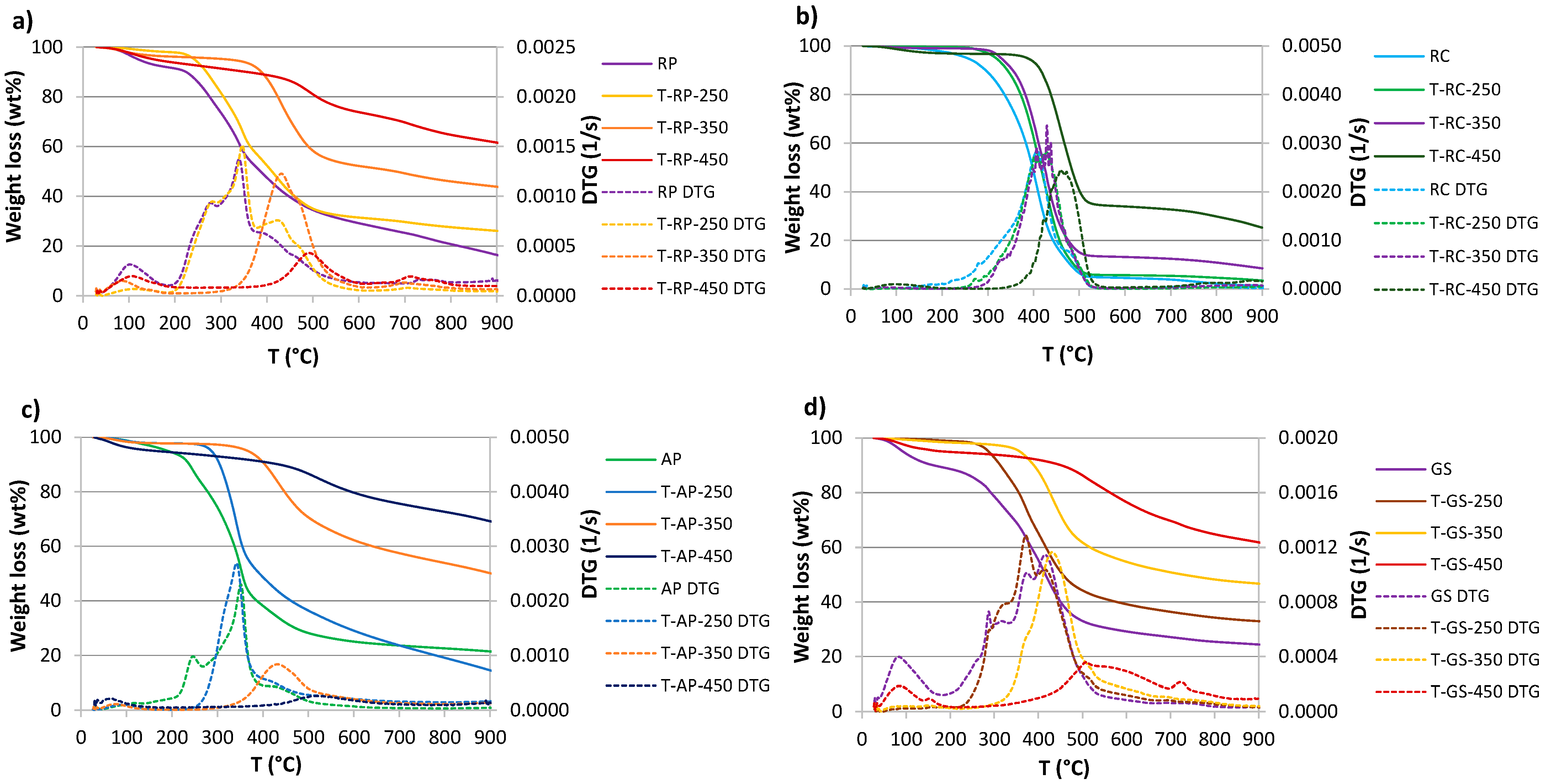

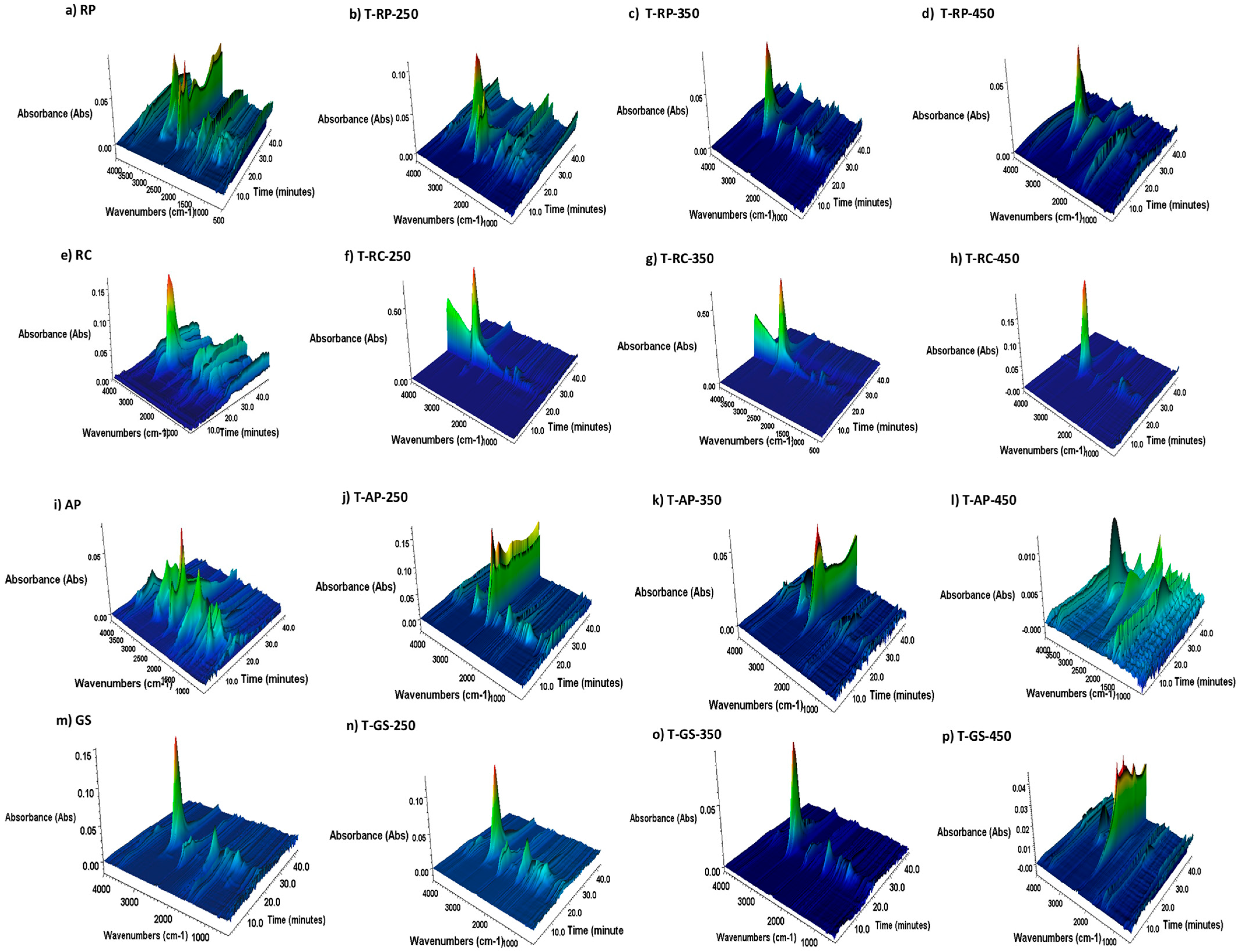
| Material | Process | Operating Temperature [°C] | Operation Time [min] | Sample Name |
|---|---|---|---|---|
| Rosemary pomace | Raw | / | / | RP |
| Torrefied | 250 | 45 | T-RP-250 | |
| 350 | T-RP-350 | |||
| 450 | T-RP-450 | |||
| Rosemary cake | Raw | / | / | RC |
| Torrefied | 250 | 45 | T-RC-250 | |
| 350 | T-RC-350 | |||
| 450 | T-RC-450 | |||
| Grape seeds | Raw | / | / | GS |
| Torrefied | 250 | 45 | T-GS-250 | |
| 350 | T-GS-350 | |||
| 450 | T-GS-450 | |||
| Apple pomace | Raw | / | / | AP |
| Torrefied | 250 | 45 | T-AP-250 | |
| 350 | T-AP-350 | |||
| 450 | T-AP-450 |
| Sample | Ti (°C) | Tmax (°C) | Tb (°C) | DTGmax (1/s) | Weight Loss (wt.%) | Residue (wt.%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1st Step | 2nd Step | 3rd Step | Total | ||||||
| RP | 266.6 | 339.6 | 547.4 | 0.00137 | 8.17 | 61.68 | 13.73 | 83.59 | 16.41 |
| T-RP-250 | 275.4 | 346.5 | 521.6 | 0.00151 | 2.02 | 66.65 | 5.19 | 73.86 | 26.14 |
| T-RP-350 | 357.3 | 431.5 | 508.7 | 0.00123 | 4.05 | 45.49 | 6.64 | 56.19 | 43.81 |
| T-RP-450 | 442.6 | 491.1 | 541.2 | 0.00043 | 6.31 | 19.85 | 12.31 | 38.47 | 61.53 |
| RC | 346.0 | 408.1 | 514.3 | 0.00283 | 2.21 | 93.31 | 3.21 | 98.72 | 1.28 |
| T-RC-250 | 357.3 | 428.6 | 518.2 | 0.00282 | 0.42 | 93.86 | 2.27 | 96.55 | 3.45 |
| T-RC-350 | 379.2 | 429.2 | 512.9 | 0.00336 | 1.40 | 85.62 | 4.43 | 91.46 | 8.54 |
| T-RC-450 | 415.6 | 459.0 | 528.8 | 0.00245 | 3.19 | 62.72 | 8.72 | 74.63 | 25.37 |
| AP | 290.8 | 351.2 | 499.2 | 0.00230 | 3.21 | 71.93 | 4.43 | 79.58 | 20.42 |
| T-AP-250 | 303.1 | 341.4 | 669.9 | 0.00269 | 2.19 | 72.52 | 11.03 | 85.74 | 14.26 |
| T-AP-350 | 375.8 | 432.5 | 635.0 | 0.00083 | 2.28 | 40.92 | 6.76 | 49.96 | 50.04 |
| T-AP-450 | 448.6 | 518.5 | 610.7 | 0.00026 | 5.03 | 19.53 | 6.23 | 30.79 | 69.21 |
| GS | 312.4 | 415.4 | 521.2 | 0.00115 | 10.66 | 61.52 | 3.34 | 75.53 | 24.47 |
| T-GS-250 | 308.9 | 371.6 | 539.5 | 0.00129 | 3.61 | 60.36 | 3.20 | 67.16 | 32.84 |
| T-GS-350 | 375.8 | 431.1 | 600.6 | 0.00116 | 2.74 | 45.08 | 5.51 | 53.33 | 46.67 |
| T-GS-450 | 430.1 | 508.4 | 633.7 | 0.00036 | 5.66 | 28.33 | 4.20 | 38.19 | 61.81 |
| Sample | Ti (°C) | Tmax (°C) | Tb (°C) | DTGmax (1/s) | Weight Loss (wt.%) | Residue (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1st Step | 2nd Step | 3rd Step | Total | ||||||
| RP | 256.5 | 312.1 | 386.9 | 0.00770 | 8.13 | 84.01 | 1.60 | 93.74 | 6.26 |
| T-RP-250 | 264.0 | 436.0 | 487.1 | 0.00627 | 6.16 | 85.31 | 2.99 | 94.46 | 5.54 |
| T-RP-350 | 268.5 | 277.7 | 380.3 | 0.00726 | 5.58 | 77.10 | 7.33 | 90.01 | 9.99 |
| T-RP-450 | 260.8 | 265.8 | 385.8 | 0.00417 | 7.81 | 67.31 | 10.09 | 85.21 | 14.79 |
| RC | 300.7 | 588.4 | 664.4 | 0.00498 | 10.53 | 83.29 | 4.69 | 98.51 | 1.49 |
| AP | 282.1 | 300.9 | 402.0 | 0.00860 | 9.90 | 88.36 | 0.27 | 98.53 | 1.47 |
| GS | 265.6 | 472.8 | 529.7 | 0.00257 | 11.24 | 83.63 | 2.74 | 97.61 | 2.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbancl, D.; Agačević, D.; Gradišnik, E.; Šket, A.; Štajnfelzer, N.; Goričanec, D.; Petrovič, A. The Torrefaction of Agricultural and Industrial Residues: Thermogravimetric Analysis, Characterization of the Products and TG-FTIR Analysis of the Gas Phase. Energies 2025, 18, 4648. https://doi.org/10.3390/en18174648
Urbancl D, Agačević D, Gradišnik E, Šket A, Štajnfelzer N, Goričanec D, Petrovič A. The Torrefaction of Agricultural and Industrial Residues: Thermogravimetric Analysis, Characterization of the Products and TG-FTIR Analysis of the Gas Phase. Energies. 2025; 18(17):4648. https://doi.org/10.3390/en18174648
Chicago/Turabian StyleUrbancl, Danijela, Deniz Agačević, Eva Gradišnik, Anja Šket, Nina Štajnfelzer, Darko Goričanec, and Aleksandra Petrovič. 2025. "The Torrefaction of Agricultural and Industrial Residues: Thermogravimetric Analysis, Characterization of the Products and TG-FTIR Analysis of the Gas Phase" Energies 18, no. 17: 4648. https://doi.org/10.3390/en18174648
APA StyleUrbancl, D., Agačević, D., Gradišnik, E., Šket, A., Štajnfelzer, N., Goričanec, D., & Petrovič, A. (2025). The Torrefaction of Agricultural and Industrial Residues: Thermogravimetric Analysis, Characterization of the Products and TG-FTIR Analysis of the Gas Phase. Energies, 18(17), 4648. https://doi.org/10.3390/en18174648






