Oil Extraction from the Spent Coffee Grounds and Its Conversion into Biodiesel
Abstract
1. Introduction
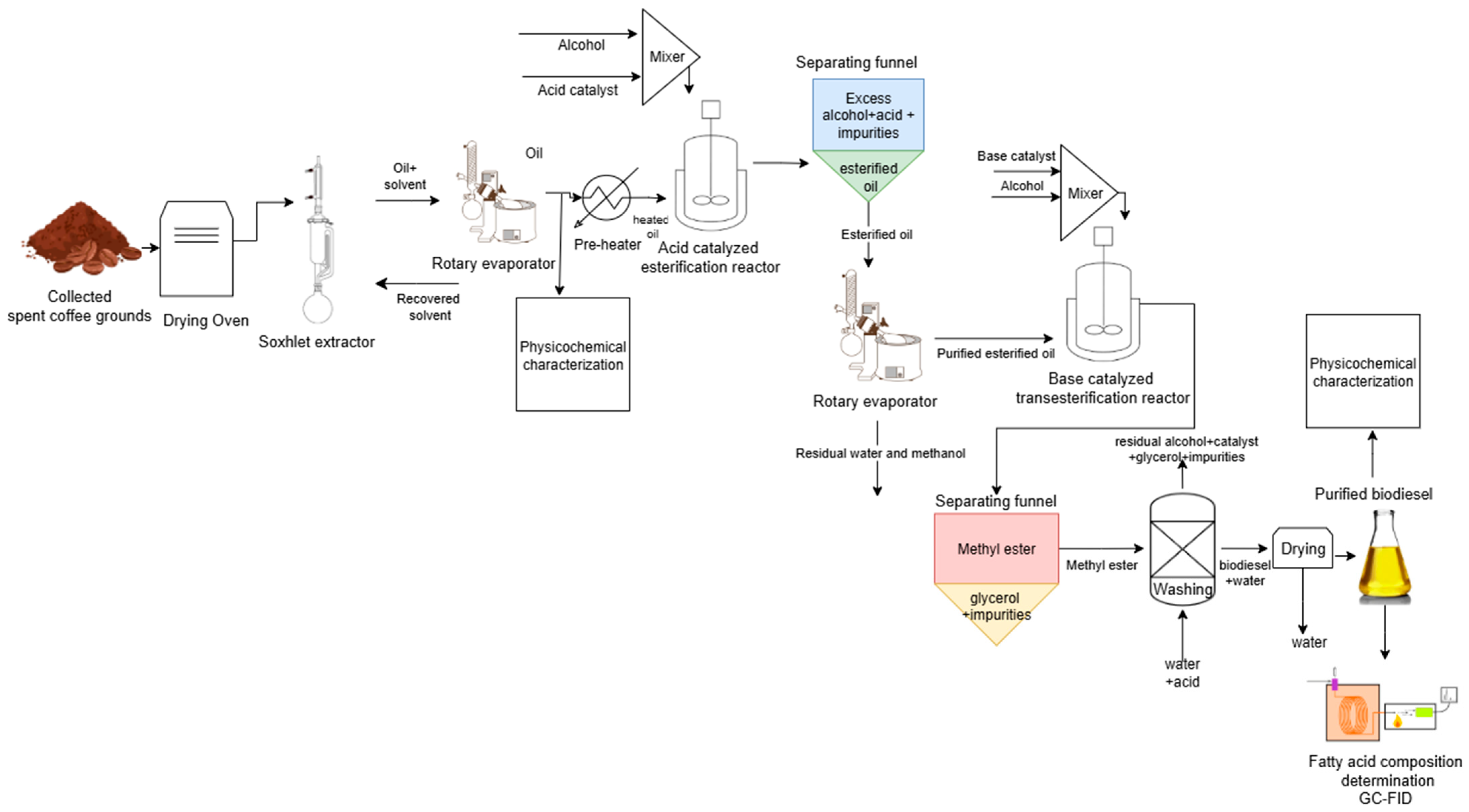
2. Experimental Spent Coffee Ground Oil Extraction
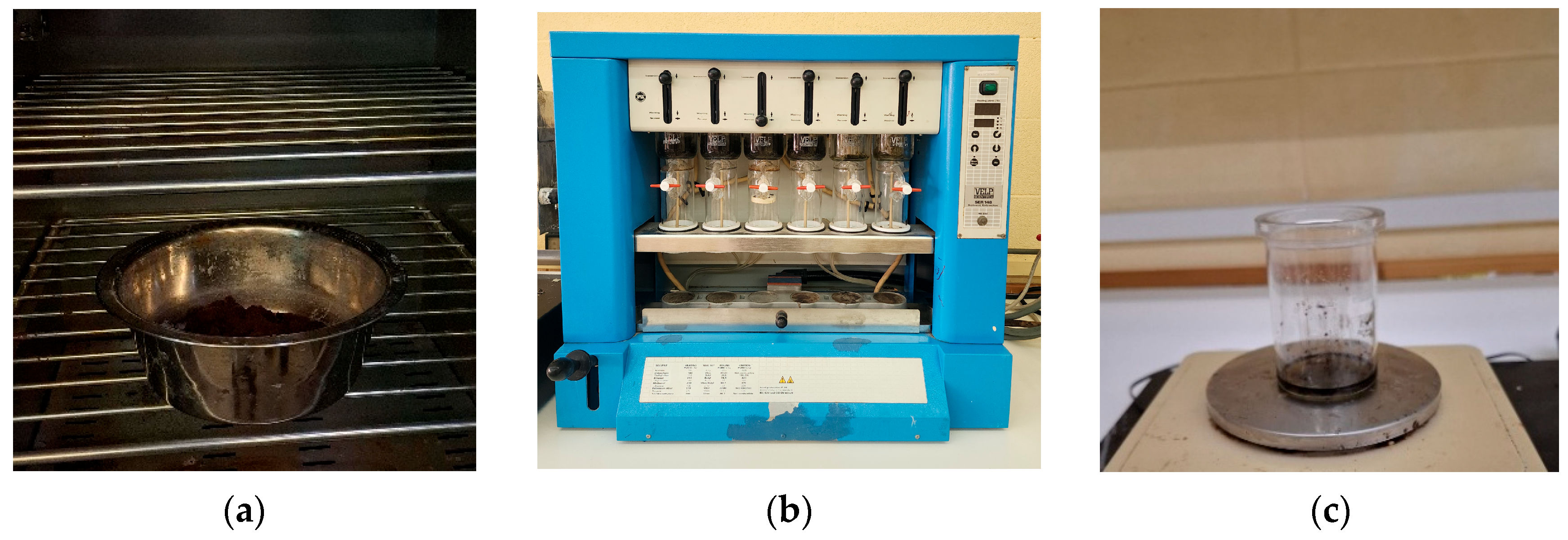
2.1. Collection and Drying
2.2. Oil Extraction
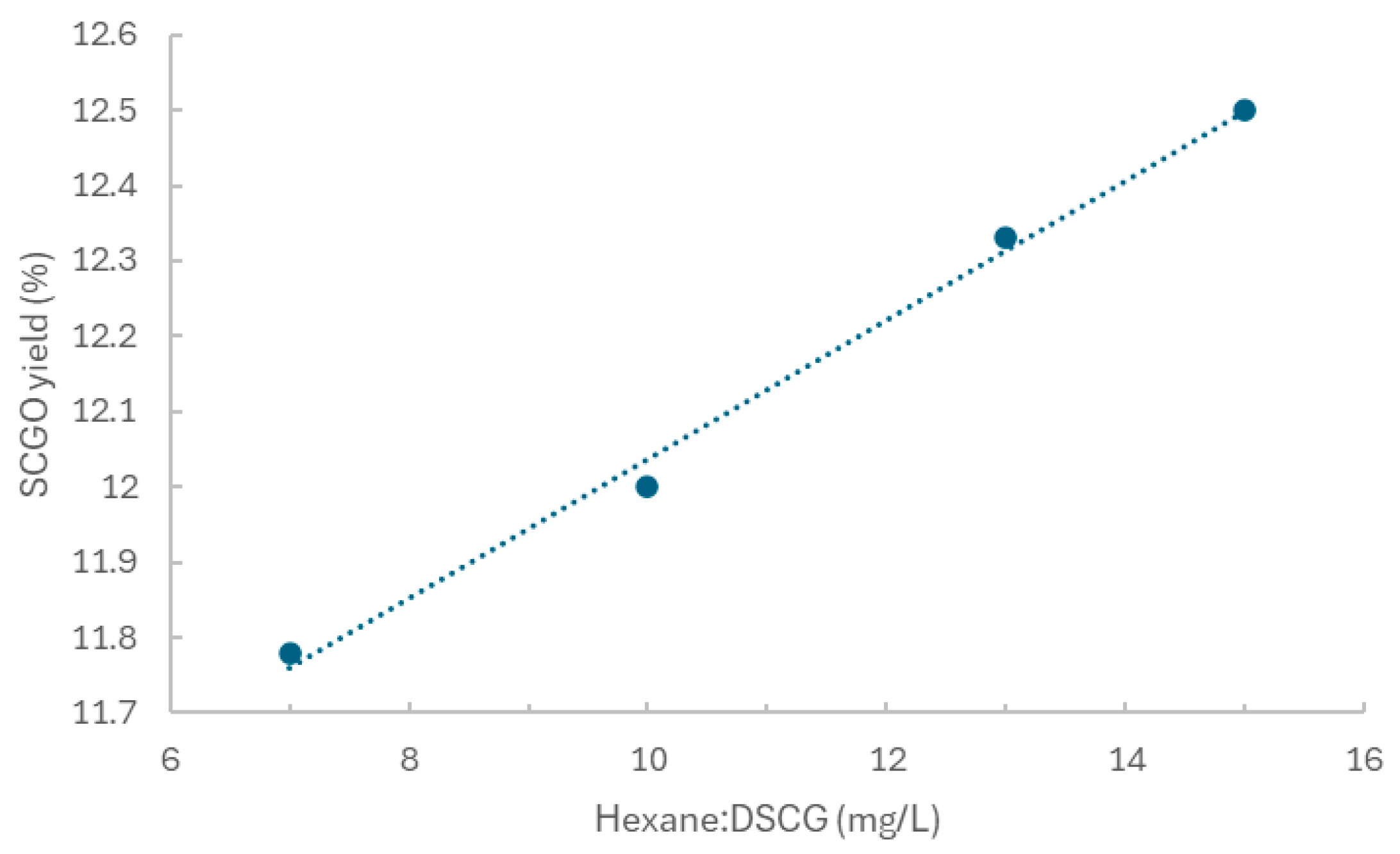
2.3. Influence of Hexane to Dried Spent Coffee Ground Ratio
2.4. Acid Value and Free Fatty Acid Determination
2.5. Peroxide Value
3. Reactors Design and Kinetic Modeling
3.1. Chemical Reactions
3.1.1. Acid Catalyzed Esterification
3.1.2. Base Catalyzed Transesterification
3.2. Process Flowrates and Catalyst Requirements
3.3. Kinetic Modeling
3.4. Reactor Type Selection and Design
3.5. Reactor Design Results
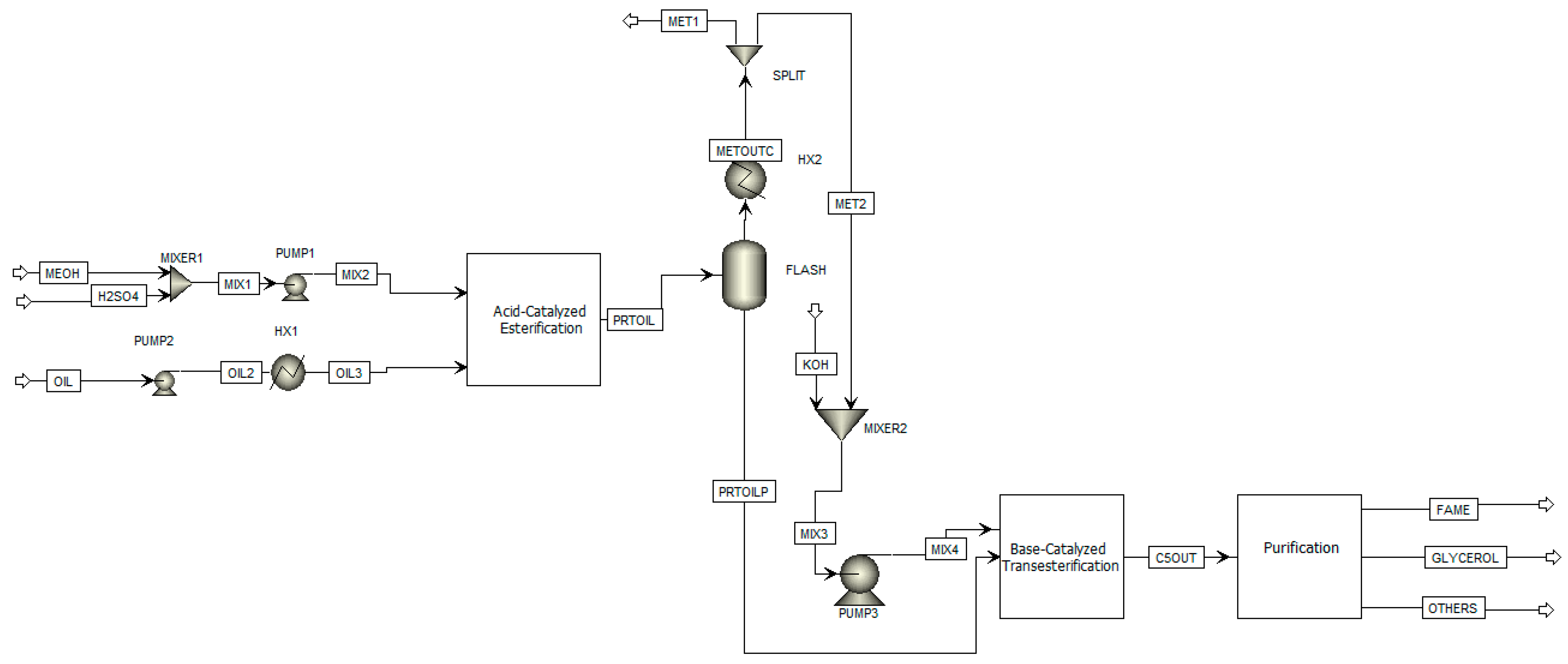
4. Aspen Plus Process Simulation: Oil to Biodiesel
4.1. Components
4.2. Process Flowsheet
4.2.1. Streams Inputs
4.2.2. Blocks Inputs
5. Results
5.1. Model Validation and Results
5.2. Sensitivity Analysis
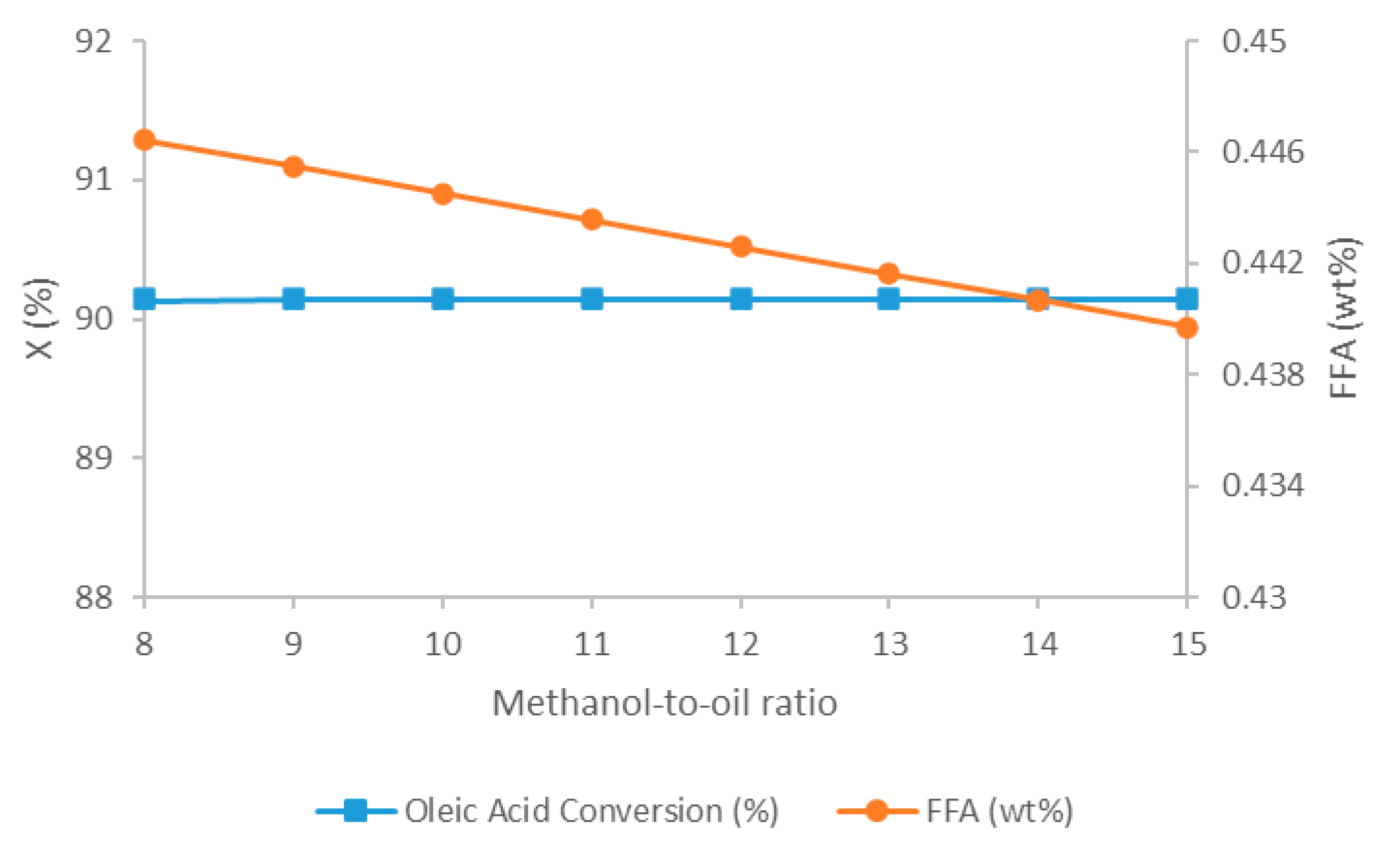
5.2.1. Impact of Methanol-to-Oil Ratio on Oleic Acid Conversion and FFA Content
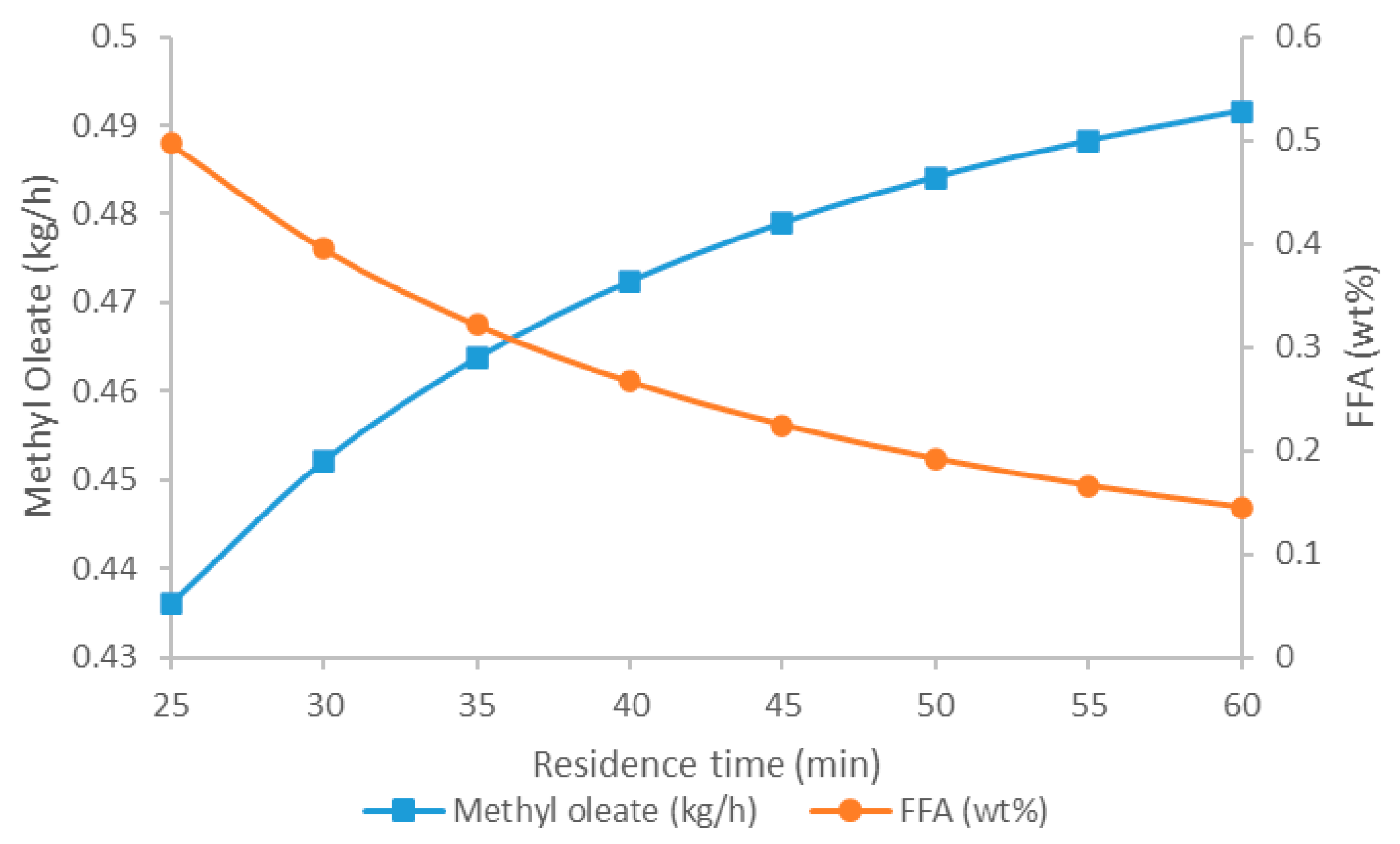
5.2.2. Impact of Residence Time on Methyl Oleate Produced and FFA Content
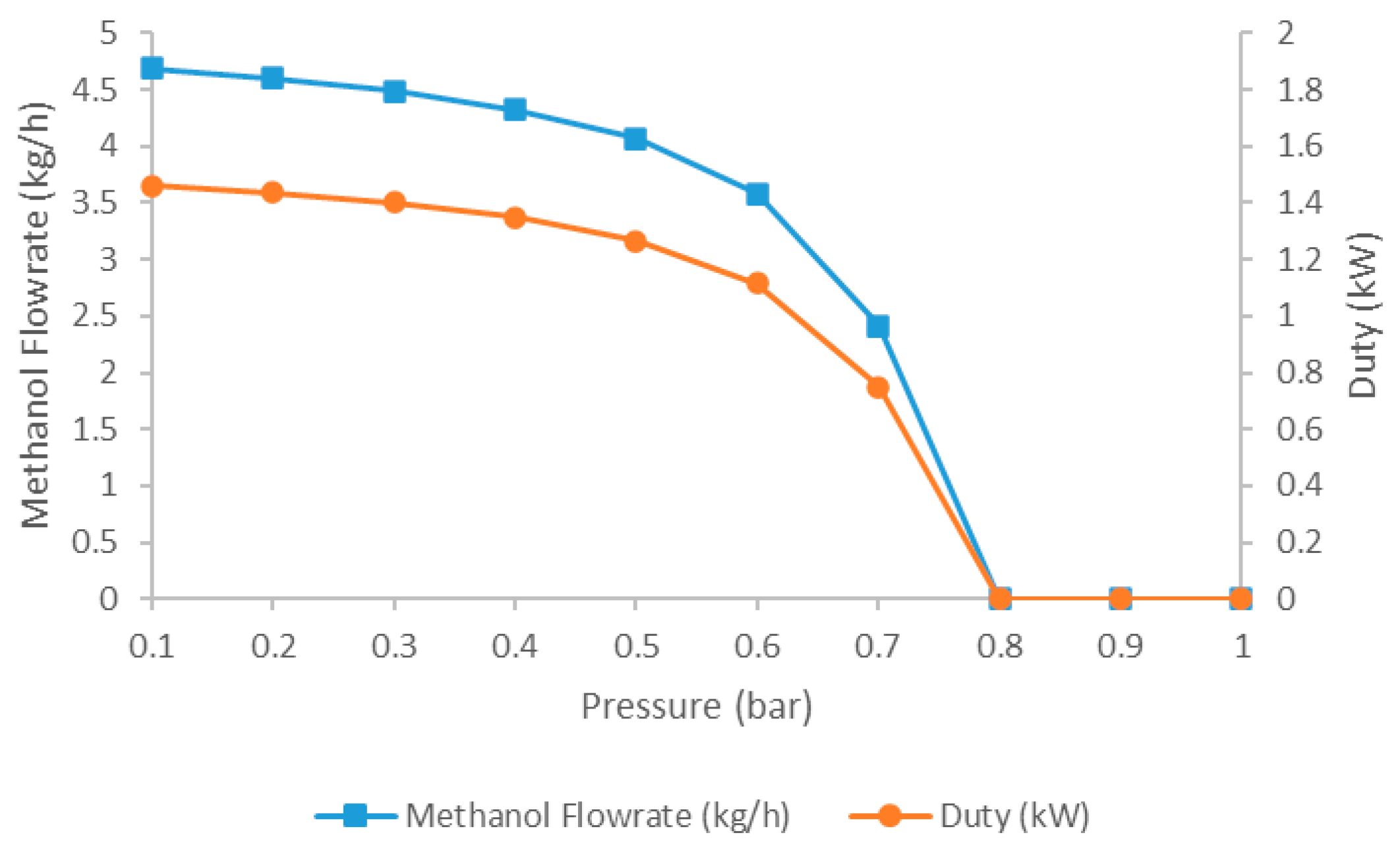
5.2.3. Impact of the Flash Drum Pressure on Methanol Recovery
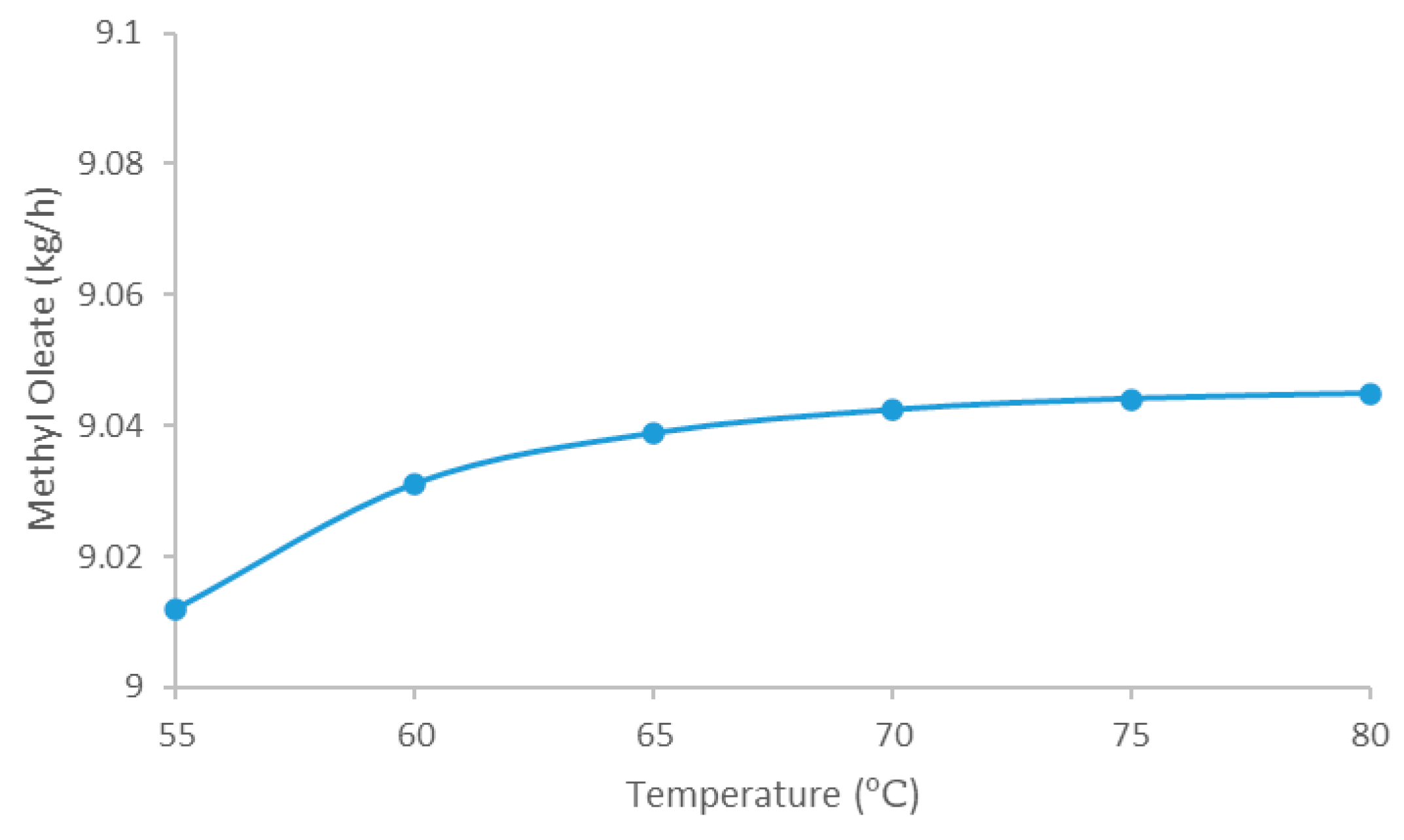
5.2.4. Impact of Base Catalyzed Transesterification Reactor Temperature on the Biodiesel Production
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCG | Spent Coffee Grounds |
| SE | Soxhlet Extraction |
| UAE | Ultrasonic-Assisted Extraction |
| FFA | Free Faty Acid |
| ASTM | American Society for Testing and Materials |
| FAME | Fatty Acid Methyl Ester |
| GC | Gas Chromatography |
| FID | Flame Ionization Detector |
| CSTR | Continuous Stirred Tank Reactor |
| SGCO | Spent Coffee Ground Oil |
| VCF | Volume Correction Factor |
| AV | Acid Value |
| PV | Peroxide value |
| AOAC | Association of Official Agricultural Chemists |
| WCO | Waste Cooling Oil |
| PFR | Plug Flow Reactor |
| DSCG | Dried Spent Coffee Ground |
| PPM | Parts Per Million (10−6) |
| PPB | Parts Per Billion (10−9) |
Appendix A

References
- As’ad, S. Why Renewable Energy Gained Attention and Demand Globally? Nat. Environ. Pollut. Technol. 2024, 23, 467–473. [Google Scholar] [CrossRef]
- Shahzad, U. The Need For Renewable Energy Sources. Int. J. Inf. Technol. Electr. Eng. 2015, 15, 16–18. [Google Scholar]
- Atabani, A.E.; Al-Muhtaseb, A.H.; Kumar, G.; Saratale, G.D.; Aslam, M.; Khan, H.A.; Said, Z.; Mahmoud, E. Valorization of Spent Coffee Grounds into Biofuels and Value-Added Products: Pathway towards Integrated Bio-Refinery. Fuel 2019, 254, 115640. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, T.; Huang, Z.; Wang, J.; Cheng, H.; Yang, Y. Integration of Spent Coffee Grounds Valorization for Co-Production of Biodiesel and Activated Carbon: An Energy And Techno-Economic Case Assessment in China. J. Clean. Prod. 2021, 324, 129187. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Engel, D. Coffee Waste and the Environment: Grounds for Concern. Available online: https://stir-tea-coffee.com/api/content/796516ae-f7bc-11ec-9252-12274efc5439/ (accessed on 12 February 2025).
- Acosta Suasnabar Eusterio, H.; Camarena Taxa Lizzette, P.; Ordonez Galvez Juan, J.; Elmer, A.B. Oil Extracted from Coffee Grounds to Obtain Biodiesel as Renewable Energy. Chem. Eng. Trans. 2023, 105, 517–522. [Google Scholar] [CrossRef]
- Jeníček, L.; Tunklová, B.; Malaťák, J.; Neškudla, M.; Velebil, J. Use of Spent Coffee Ground as an Alternative Fuel and Possible Soil Amendment. Materials. 2022, 15, 6722. [Google Scholar] [CrossRef]
- Supang, W.; Ngamprasertsith, S.; Sakdasri, W.; Sawangkeaw, R. Biodiesel Production from Spent Coffee Grounds by Using Ethanolic Extraction and Supercritical Transesterification. BioEnergy Res. 2024, 17, 2429–2439. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Rasul, M.G.; Hassan, N.M.S.; Mofijur, M. Waste Coffee Oil: A Promising Source for Biodiesel Production. Energy Procedia 2019, 160, 677–682. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil Extracted from Spent Coffee Grounds as a Renewable Source for Fatty Acid Methyl Ester Manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.M.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent Coffee Grounds for Biodiesel Production and Other Applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef]
- Chbeir, R. The Lebanese Coffee Market: A Brewing Success; Blominvest Bank s.a.l: Beirut, Lebanon, 2017. [Google Scholar]
- Mofijur, M.; Kusumo, F.; Fattah, I.M.R.; Mahmudul, H.M.; Rasul, M.G.; Shamsuddin, A.H.; Mahlia, T.M.I. Resource Recovery from Waste Coffee Grounds Using Ultrasonic-Assisted Technology for Bioenergy Production. Energies 2020, 13, 1770. [Google Scholar] [CrossRef]
- Haile, M. Integrated Volarization of Spent Coffee Grounds to Biofuels. Biofuel Res. J. 2014, 1, 65–69. [Google Scholar] [CrossRef]
- Somnuk, K.; Eawlex, P.; Prateepchaikul, G. Optimization of Coffee Oil Extraction From Spent Coffee Grounds Using Four Solvents and Prototype-Scale Extraction Using Circulation Process. Agric. Nat. Resour. 2017, 51, 181–189. [Google Scholar] [CrossRef]
- Westling, E. Valorising Spent Coffee Grounds: An Assessment of Possibilities in the Municipality of Stockholm. Master’s Thesis, KTH, School of Architecture and the Built Environment (ABE), Sustainable Development, Environmental Science and Engineering, Stockholm, Sweden, 2023. [Google Scholar]
- Tinoco-Caicedo Diana, L.; Mero-Benavides, M.; Cordova-Molina, K.; Estrada-Ordonez, D.; Blanco-Marigorta Ana, M. Oil Extraction from Spent Coffee Grounds: Experimental Studies and Exergoeconomic Analysis. Chem. Eng. Trans. 2023, 102, 295–300. [Google Scholar] [CrossRef]
- Kusuma, J.; Indartono, Y.S.; Mujahidin, D. Biodiesel and Activated Carbon from Arabica Spent Coffee Grounds. MethodsX 2023, 10, 102185. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Harahap, F.A.U.; Heru, H.; Darni, Y.; Ginting, S.B. Extraction and Characterization of Coffee Oil From Instant-Coffee Waste. JBAT 2019, 8, 59–64. [Google Scholar] [CrossRef]
- EN 14214; Liquid Petroleum Products-Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications-Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2012.
- ASTM D6751-24; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2024.
- SER 148 Series—Solvent Extractors. Available online: https://www.velp.com/en-ww/ser-148-series-solvent-extractors.aspx (accessed on 17 June 2025).
- Panpraneecharoen, S.; Chumanee, S. Optimization of the Oil Extraction, Study the Chemical and Physical Properties of Arabica Spent Coffee Grounds. Sci. Technol. Asia 2020, 25, 12–19. [Google Scholar] [CrossRef]
- Najdanovic-Visak, V.; Lee, F.Y.-L.; Tavares, M.T.; Armstrong, A. Kinetics of Extraction and in Situ Transesterification of Oils from Spent Coffee Grounds. J. Environ. Chem. Eng. 2017, 5, 2611–2616. [Google Scholar] [CrossRef]
- Krinski, I.M.; Leite, V.R.; Moura, L.M.; Mariani, V.C. Critical Extraction Parameters for Maximizing Oil Yield from Spent Coffee Grounds. Energies 2025, 18, 1346. [Google Scholar] [CrossRef]
- Tham, M.W.; Liew, K.C. Influence of Different Extraction Temperatures and Methanol Solvent Percentages on the Total Phenols and Total Flavonoids from the Heartwood and Bark of Acacia auriculiformis. Eur. J. Wood Prod. 2014, 72, 67–72. [Google Scholar] [CrossRef]
- Volume Correction Factors—Diesel Fuel. Available online: https://www.ic.gc.ca/eic/site/mc-mc.nsf/vwapj/VCF_Diesel.pdf/$file/VCF_Diesel.pdf (accessed on 25 January 2025).
- Abdullah, M.; Bulent Koc, A. Oil Removal from Waste Coffee Grounds Using Two-Phase Solvent Extraction Enhanced with Ultrasonication. Renew. Energy 2013, 50, 965–970. [Google Scholar] [CrossRef]
- Bharati, D. Estimation of Free Fatty Acid (FFA). Biology Discussion. 2016. Available online: https://www.biologydiscussion.com/plants/estimation-of-free-fatty-acid/57212 (accessed on 28 March 2025).
- Jalkh, R. Oil Extracts from Spent Coffee Grounds and Waste Cooking Oil to Modify the Physical Properties of Recycled Asphalt Binder; American University of Beirut: Beirut, Lebanon, 2016. [Google Scholar]
- Dordevic, D.; Gablo, N.; Zelenkova, L.; Dordevic, S.; Tremlova, B. Utilization of Spent Coffee Grounds as a Food By-Product to Produce Edible Films Based on κ-Carrageenan with Biodegradable and Active Properties. Foods 2024, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- Firuz Alam, P. Simulation Study of Distillation, Stripping, and Flash Technology for an Energy Efficient Methanol Recovery Unit in Biodiesel Production Processes. Master’s Thesis, University of Regina, Regina, SK, Canada, 2013. [Google Scholar]
- DeVincentis, J. Aspen Plus Biodiesel Model; Aspen Technology: Bedford, MA, USA, 2012. [Google Scholar]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Silitonga, A.S.; Fattah, I.M.R.; Mahlia, T.M.I. Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource. Catalysts 2022, 12, 1472. [Google Scholar] [CrossRef]
- Gu, J.; Lee, A.; Choe, C.; Lim, H. Comparative Study of Biofuel Production Based on Spent Coffee Grounds Transesterification and Pyrolysis: Process Simulation, Techno-Economic, and Life Cycle Assessment. J. Clean. Prod. 2023, 428, 139308. [Google Scholar] [CrossRef]
- Kottititum, B.; Chakton, K.; Srinophakun, T.R. Simulation Approach to Biodiesel Production from Palm Oil by Conventional and Reactive Distillation Processes. Agric. Nat. Resour. 2014, 48, 139–149. [Google Scholar]
- Raja, K.V. Optimization of Biodiesel Production Process Using Aspen Plus; National Institute of Technology Rourkela: Rourkela, India, 2015. [Google Scholar]
- Morad, N.A.; Kamal, A.A.M.; Panau, F.; Yew, T.W. Liquid Specific Heat Capacity Estimation for Fatty Acids, Triacylglycerols, and Vegetable Oils Based on Their Fatty Acid Composition. J. Am. Oil Chem. Soc. 2000, 77, 1001–1005. [Google Scholar] [CrossRef]
- Prankl, H.; Korbitz, W.; Mittelbach, M.; Worgetter, M. Review on Biodiesel Standardization World-Wide; IEA Bioenergy: Wieselburg, Österreich, 2004. [Google Scholar]

| Run | 1 | 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| SCGO yield (wt%) | 13.0 | 12.4 | 12.0 | 12.0 | 13.4 | 13.4 | 13.4 | 13.4 | 13.4 | 13.4 |
| Titration Run | Vb (mL) |
|---|---|
| 1 | 1.9 |
| 2 | 1.7 |
| 3 | 1.7 |
| Parameter | Acid Catalyzed Esterification | Base Catalyzed Transesterification |
|---|---|---|
| Pseudo-rate constant, k′ (min−1) | 0.062 | 0.051 |
| Desired conversion, X | 90% | 95% |
| Residence time, τ (min) | 34.94 | 33.56 |
| Volumetric flowrate, (m3/h) | 0.0172 | 0.0156 |
| Reactor volume, V (m3) | 0.01 | 0.0087 |
| Number of reactors in series | 2 | 3 |
| Reactor type | CSTR | CSTR |
| Reaction temperature | 60 °C | 60 °C |
| Methanol-to-oil molar ratio | 12:1 | 9:1 |
| Catalyst | H2SO4 (1% v/v) | KOH (1% w/w) |
| ID | Type | Component Name | Formula |
|---|---|---|---|
| TRIOL-01 | Conventional | Triolein | C57H104O6 |
| METHANOL | Conventional | Methanol | CH4O |
| METHY-01 | Conventional | Methyl-oleate | C19H36O2 |
| GLYCEROL | Conventional | Glycerol | C3H8O3 |
| H2SO4 | Conventional | Sulfuric-acid | H2SO4 |
| KOH | Conventional | Potassium-hydroxide | KOH |
| WATER | Conventional | Water | H2O |
| OLEIC-01 | Conventional | Oleic-acid | C18H34O2 |
| Stream | Stream Name | Mass Flowrate (kg/h) | Mass Composition |
|---|---|---|---|
| Oil feed | OIL | 10 | wtriolein = 0.951 woleic acid = 0.049 |
| Methanol | MEOH | 4.7964 | wmethanol = 1 |
| Sulfuric acid | H2SO4 | 0.2013 | wsulfuric acid = 1 |
| Potassium hydroxide | KOH | 0.1 | wKOH = 1 |
| Block | Block Name | Operating Conditions |
|---|---|---|
| Acid catalyzed esterification reactors | C1 and C2 | T = 60 °C P = 4 bar Residence time = 34.94 min |
| Cooler | HX2 | T = 25 °C |
| Flash drum | FLASH | T = 60 °C P = 0.5 bar |
| Base catalyzed transesterification reactors | C3, C4, and C5 | T = 60 °C P = 4 bar Residence time = 33.56 min |
| Methanol recovery distillation column | METRECOV | Number of stages = 7 Condenser type = Total Reboiler type = Kettle Feed stage (above stage) = 4 Distillate rate = 2.25 kg/h Mass reflux ratio = 2 Pressure at stage 1 = 0.5 bar |
| Separator | SEP | Split fractions for stream EST2:
|
| Glycerol recovery distillation column | GLYCECOL | Number of stages = 6 Condenser type = Total Reboiler type = Kettle Feed stage (above stage) = 4 Distillate rate = 1 kg/h Mass reflux ratio = 2 Condenser pressure = 0.4 bar Column pressure drop = 0.1 bar |
| FAME recovery distillation column | FAMECOL | Number of stages = 6 Condenser type = Partial Reboiler type = Kettle Feed stage (above stage) = 4 Bottom rate = 1 kg/h Mass reflux ratio = 1 Condenser pressure = 0.1 bar Condenser temperature = 148.3 °C |
| Stream ID | OIL | MIX1 | PRTOIL | METOUT | PRTOILP | C5OUT | FAME | GLYCEROL |
|---|---|---|---|---|---|---|---|---|
| Component | Mass Flowrate (kg/h) | |||||||
| Triolein | 9.51 | 9.51 | Trace | 9.51 | 0.47 | Trace | 0.01 | |
| Methanol | 4.80 | 4.75 | 4.07 | 0.68 | 3.29 | 0.01 | 0.03 | |
| Methyl Oleate | 0.46 | 3 PPM | 0.46 | 9.55 | 9.03 | |||
| Glycerol | 0.94 | 0.94 | ||||||
| H2SO4 | 0.20 | 0.20 | 5 PPM | 0.20 | 0.20 | 0.20 | ||
| KOH | 0.10 | 0.10 | ||||||
| Water | 0.03 | 0.02 | 0.01 | 0.03 | 400 PPM | 0.02 | ||
| Oleic Acid | 0.49 | 0.05 | 30 PPB | 0.05 | 0.05 | 0.03 | 0.01 | |
| Total | 10.00 | 5.00 | 15.00 | 4.08 | 10.91 | 14.62 | 9.07 | 1.30 |
| Study | Focus | Main Findings and Biodiesel Yield |
|---|---|---|
| Mofijur et al. [14] | Experimental | The biodiesel yield was tested against the reaction time. Results showed that the biodiesel yield increased from 74% for a residence time of 30 min, until reaching a peak of 98.25% at 60 min. |
| Haile [15] | Experimental | A biodiesel yield of 82% w/w of SCGO was obtained after purification |
| Al-Hamamre et al. [11] | Experimental | A biodiesel yield varying from 68.75% to 85.5% was obtained through a one-step esterification. The yield increased to reach 99% by adaption a two steps transesterification process. |
| Gu et al. [36] | Simulation | The biodiesel yield was not clearly stated. The final biodiesel stream, having a purity ranging between 99.6 and 99.78 wt%, has a mass flowrate varying from 45.6 to 376.64 kg/h for an extracted oil flowrate between 54 and 433 kg/h. This leads to a biodiesel yield ranging between 84.44 and 86.93 wt%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harb, R.; Salloum Abou Jaoudeh, L. Oil Extraction from the Spent Coffee Grounds and Its Conversion into Biodiesel. Energies 2025, 18, 4603. https://doi.org/10.3390/en18174603
Harb R, Salloum Abou Jaoudeh L. Oil Extraction from the Spent Coffee Grounds and Its Conversion into Biodiesel. Energies. 2025; 18(17):4603. https://doi.org/10.3390/en18174603
Chicago/Turabian StyleHarb, Rita, and Lara Salloum Abou Jaoudeh. 2025. "Oil Extraction from the Spent Coffee Grounds and Its Conversion into Biodiesel" Energies 18, no. 17: 4603. https://doi.org/10.3390/en18174603
APA StyleHarb, R., & Salloum Abou Jaoudeh, L. (2025). Oil Extraction from the Spent Coffee Grounds and Its Conversion into Biodiesel. Energies, 18(17), 4603. https://doi.org/10.3390/en18174603





