Analysis of Thermal and Catalytic Pyrolysis Processes in Belém: A Socioeconomic Perspective
Abstract
1. Introduction
2. Methodology
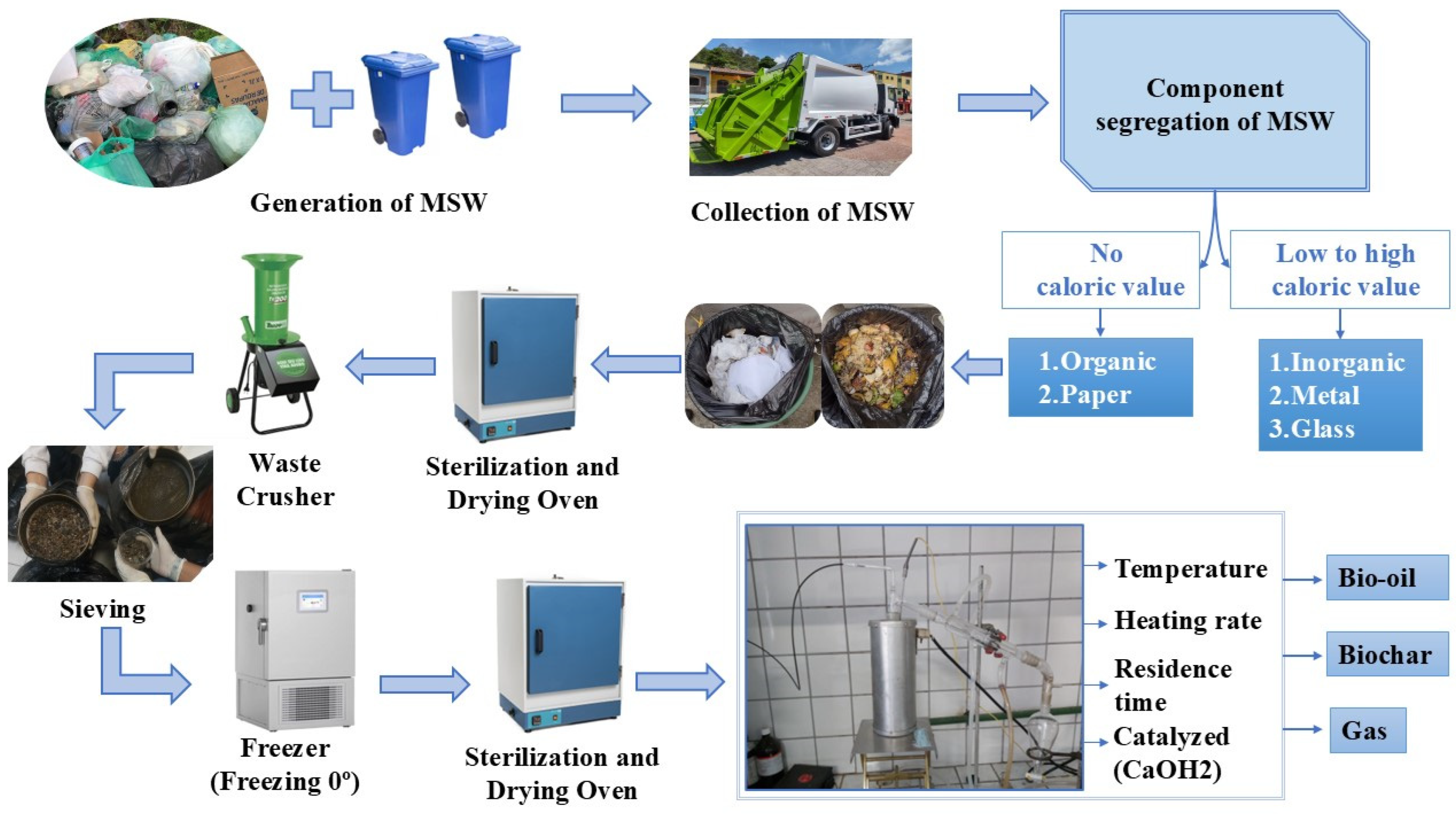
2.1. Gravimetric Composition of Urban Solid Waste
Pré-Tratamento das Amostras e Determinações Laboratoriais
2.2. Experimental Procedure
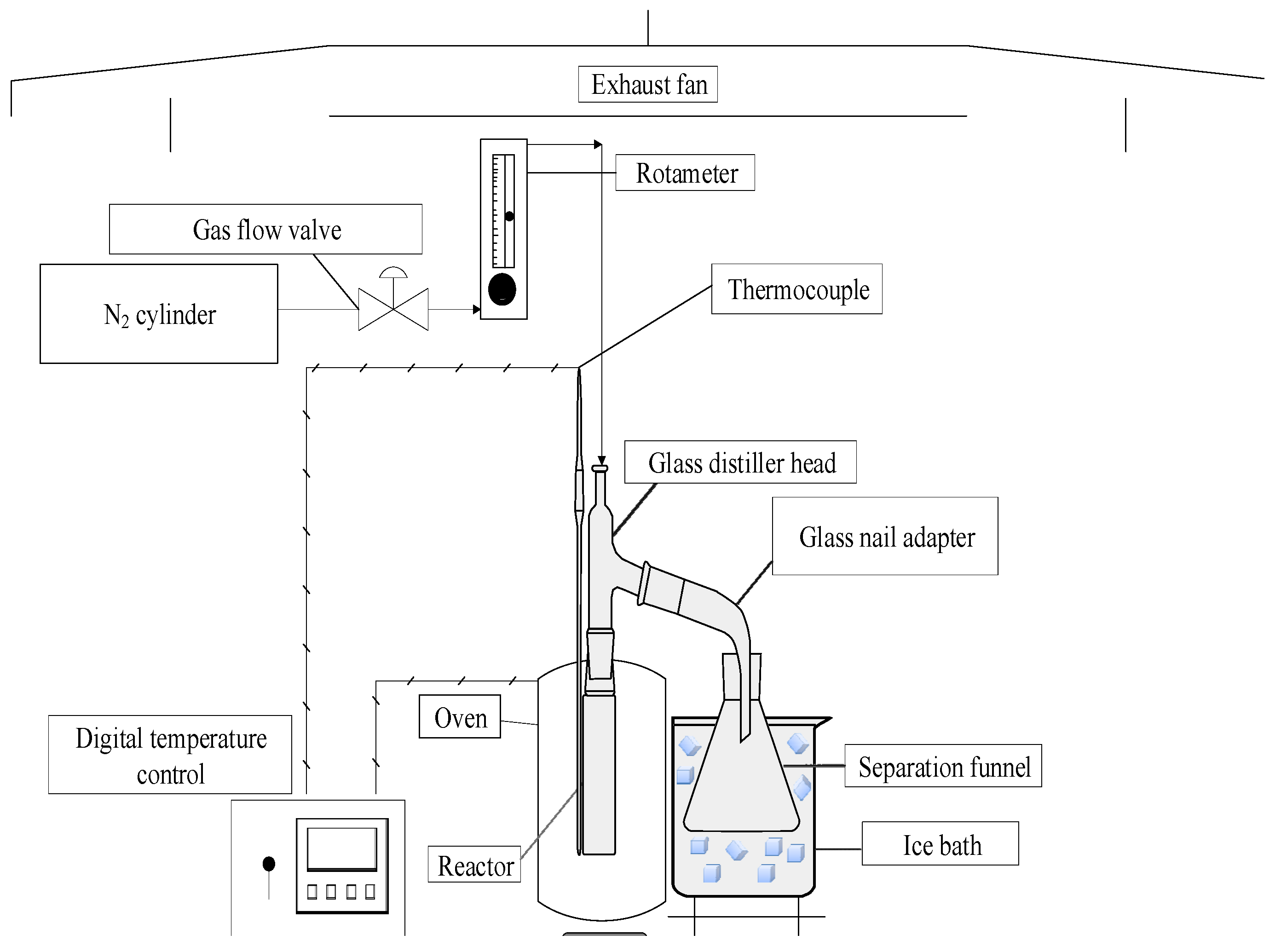
2.2.1. Pyrolysis Process Experimental Apparatus
2.2.2. Experimental Procedures
2.3. Physicochemical and Chemical Composition of Bio-Oil
2.3.1. Physicochemical Characterization of Bio-Oil and Aqueous Phase
2.3.2. Chemical Composition of Bio-Oil and Aqueous Phase
2.4. Characterization of Biochar
2.4.1. SEM and EDS Analysis
2.4.2. X-Ray Diffraction Analysis (XRD)
2.5. Yields from Bench-Scale Thermal and Catalytic Pyrolysis Experiments
3. Results
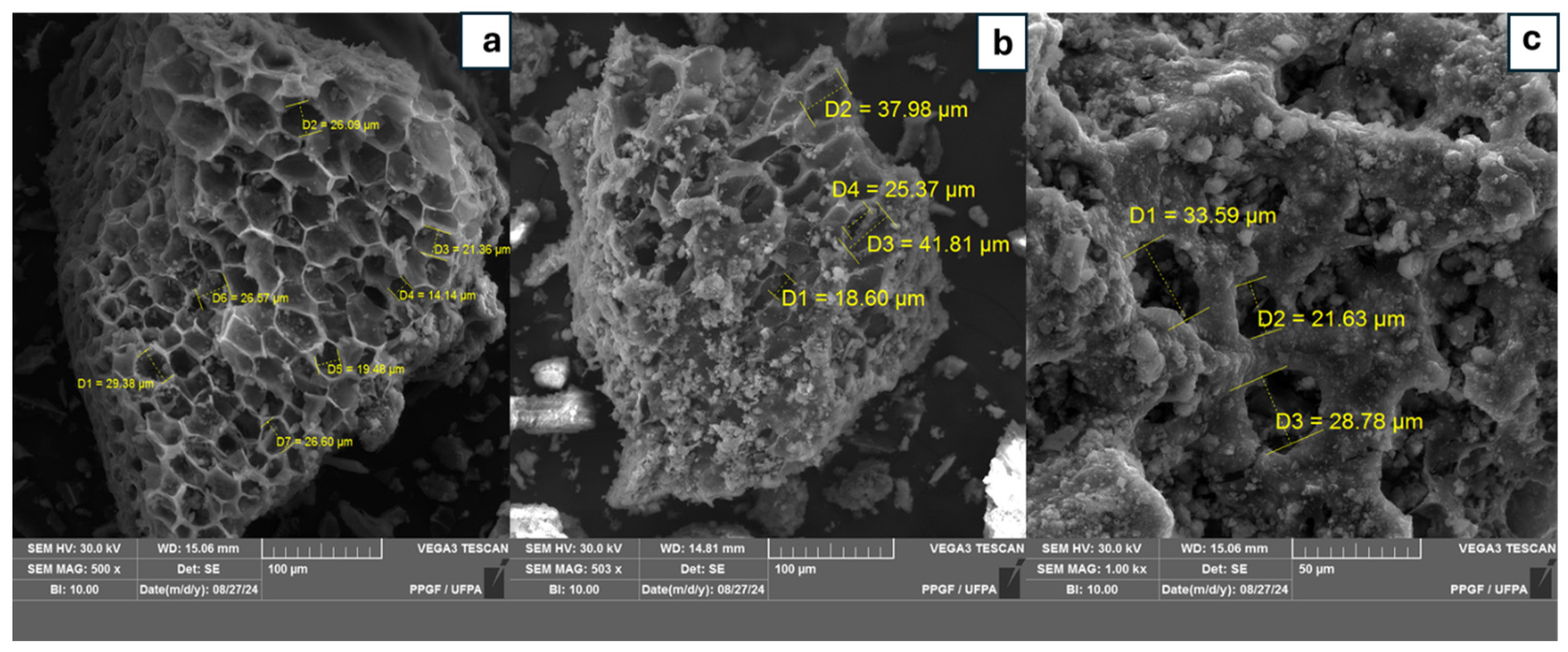
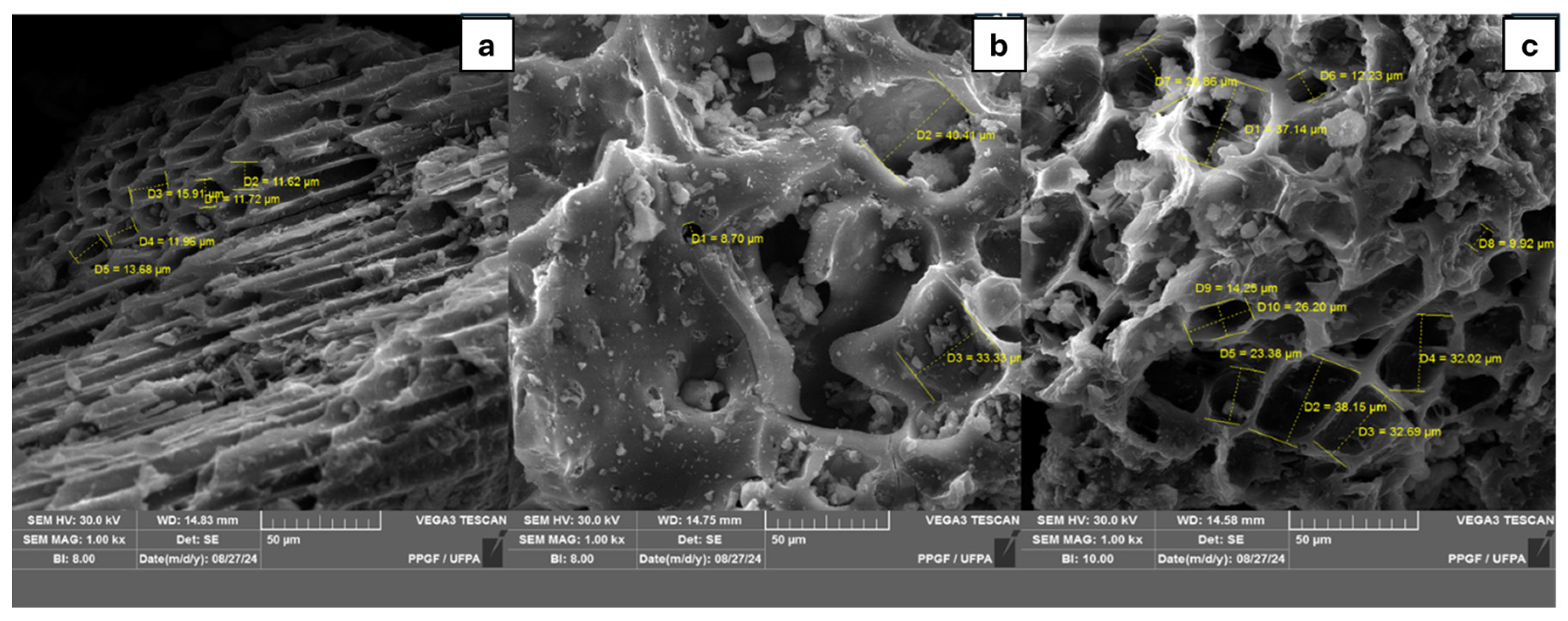
3.1. Scanning Electron Microscopy Analysis (SEM)
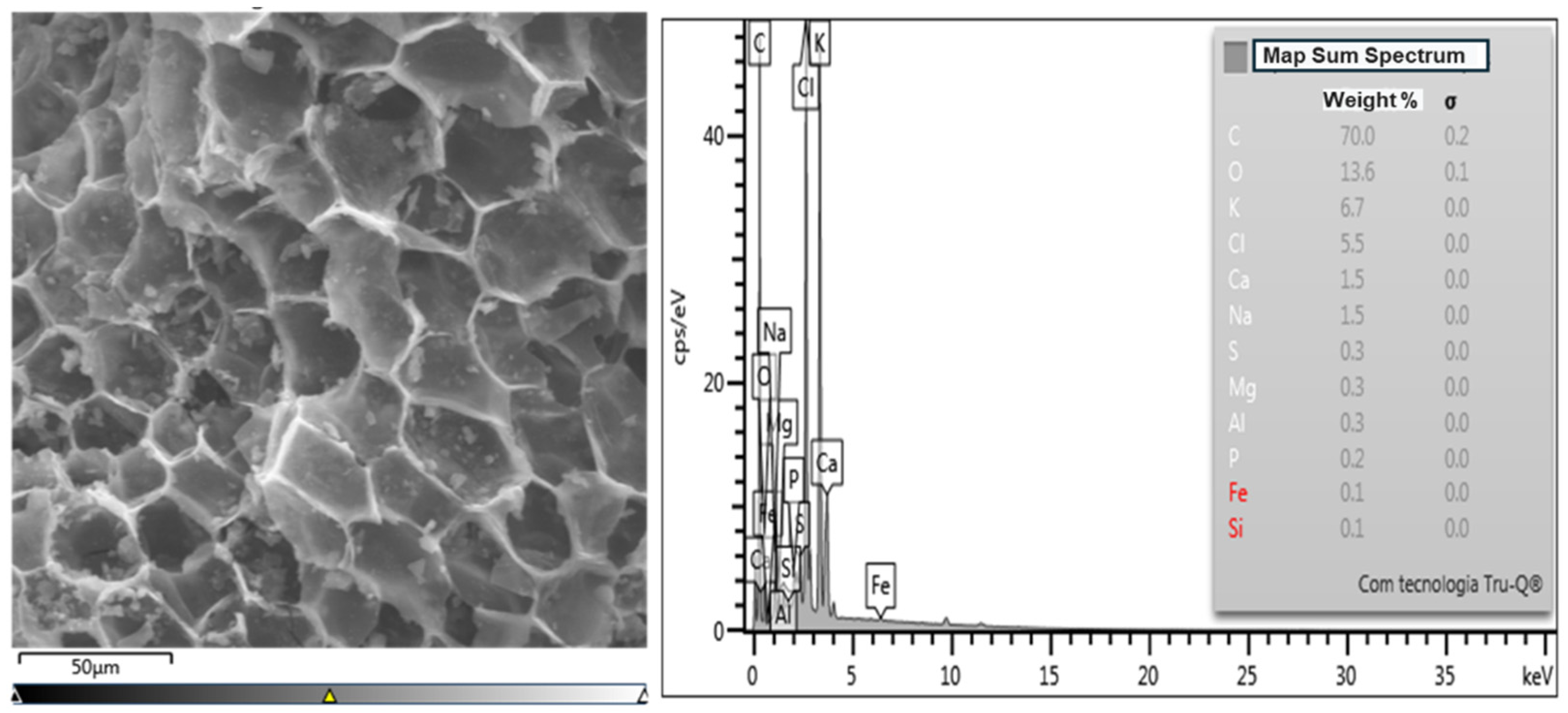
3.2. Energy-Dispersive Spectroscopy Analysis—EDS
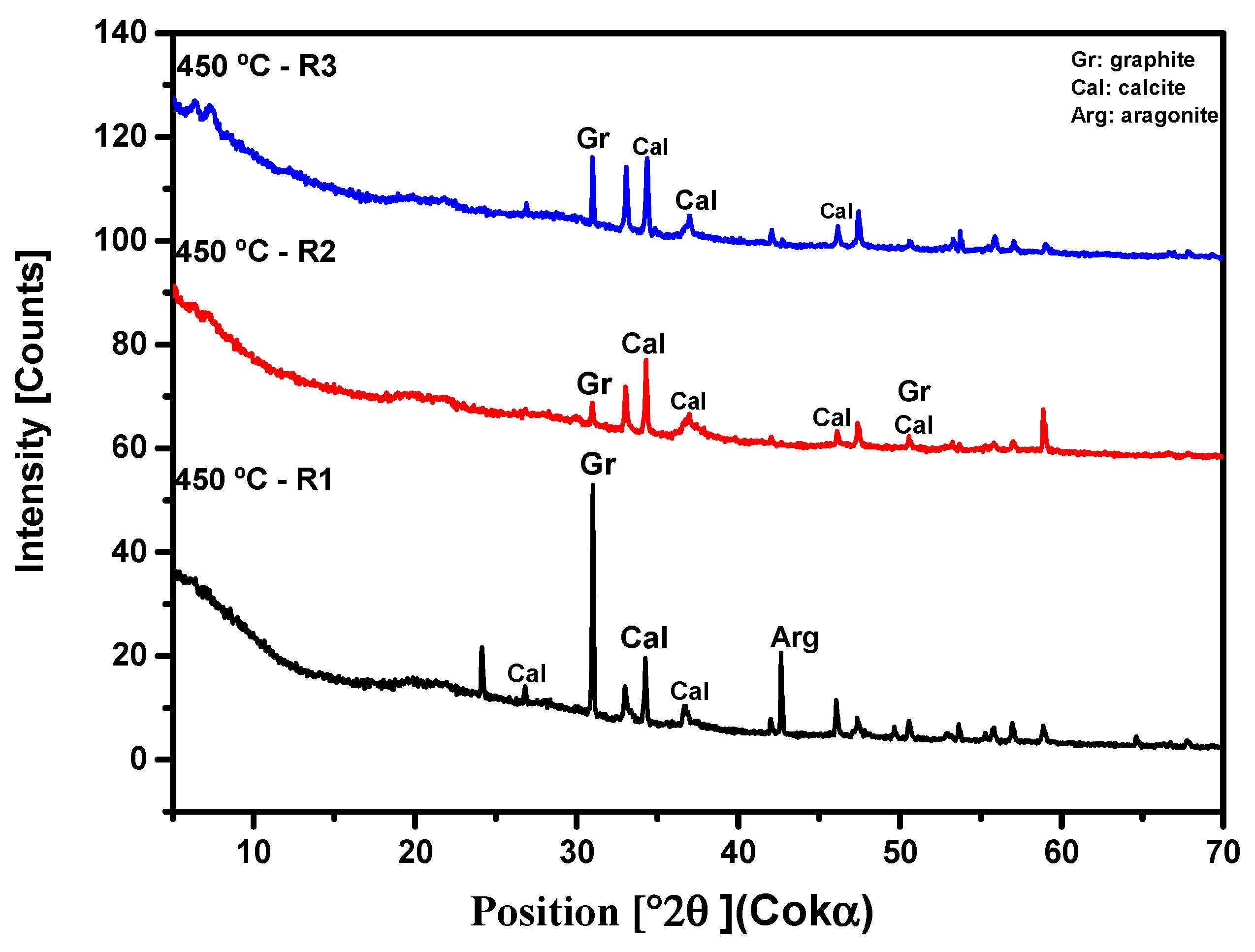
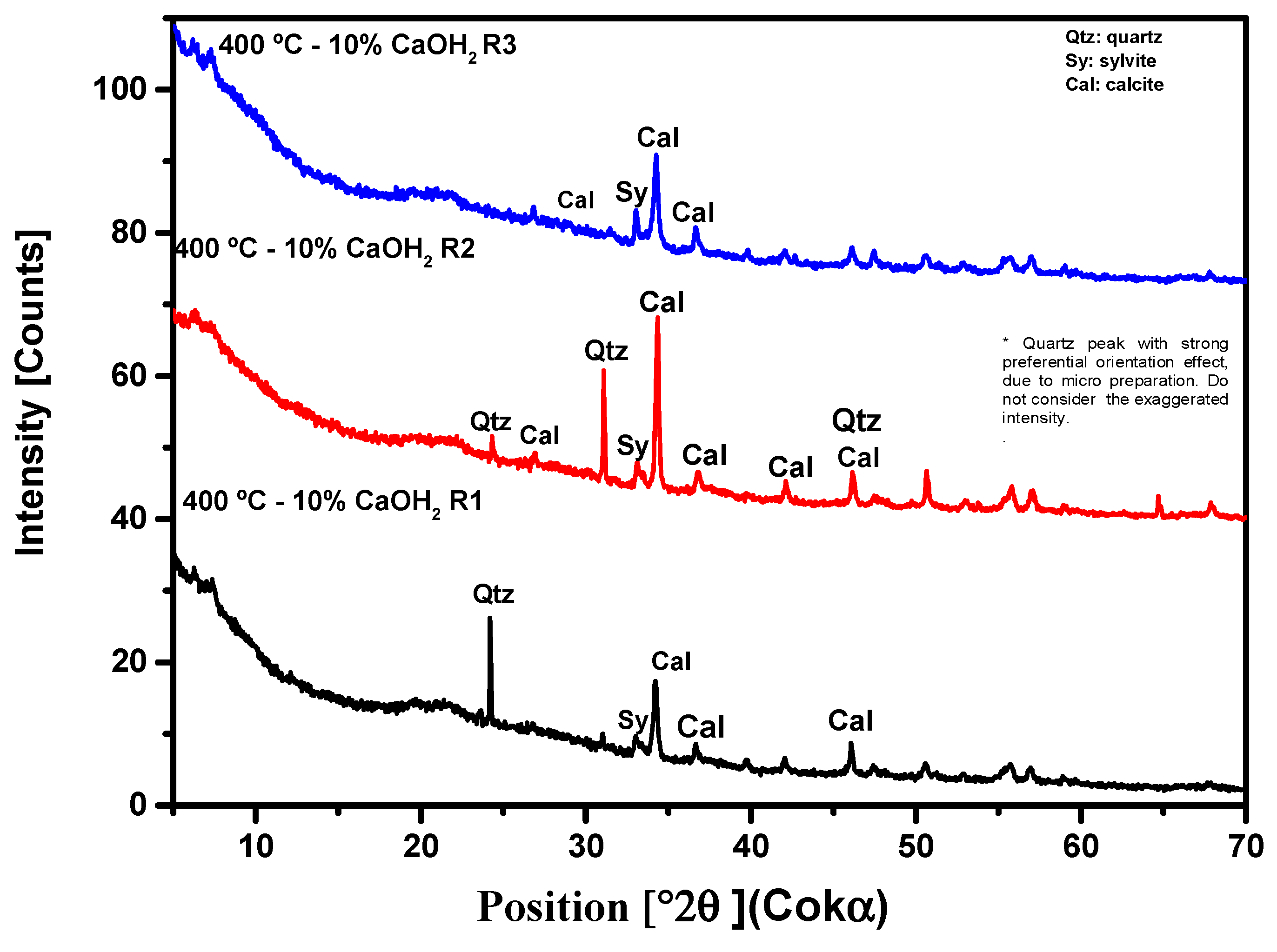
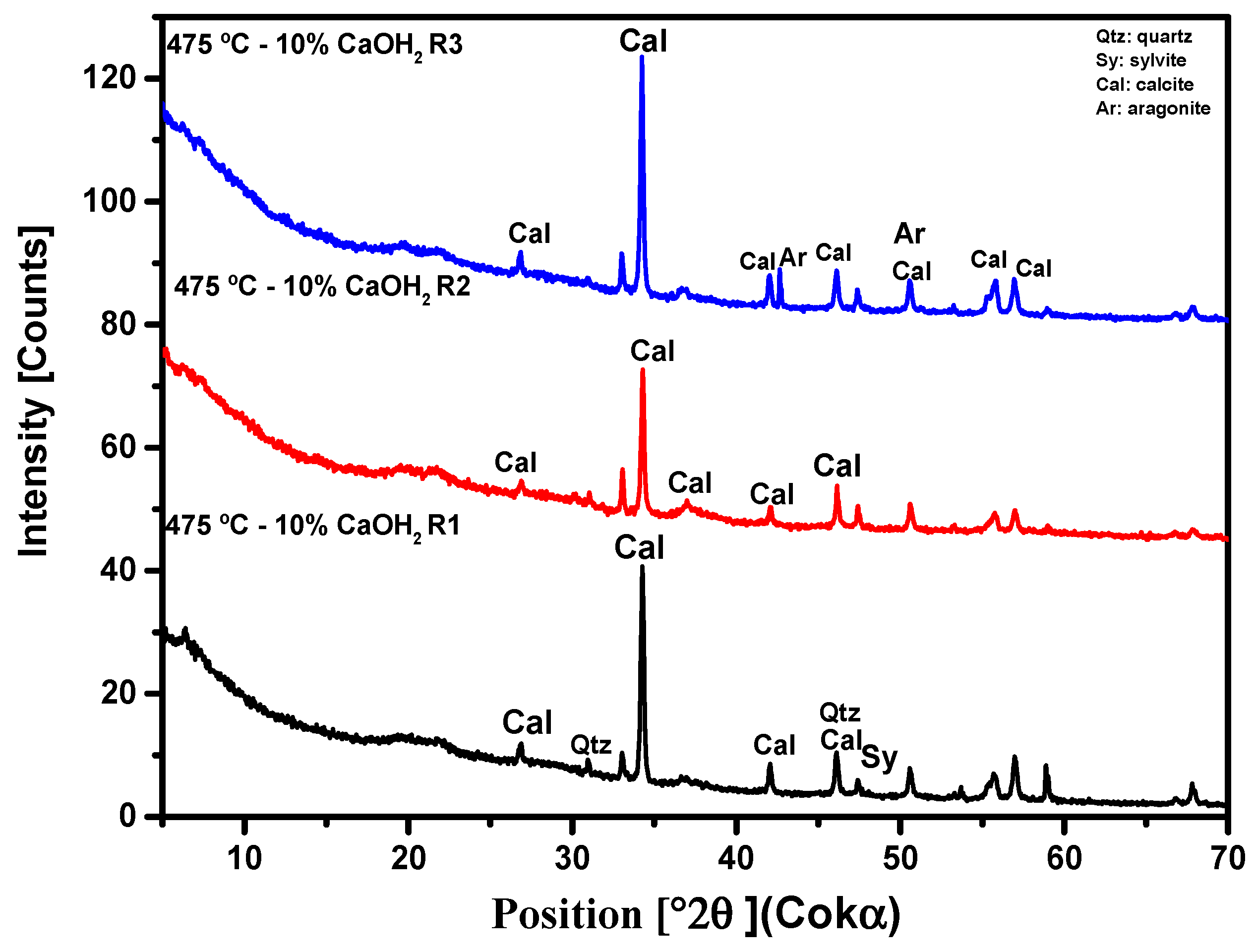
3.3. Crystallographic Analysis by X-Ray Diffractometry
3.4. Pyrolysis of MSW Fraction
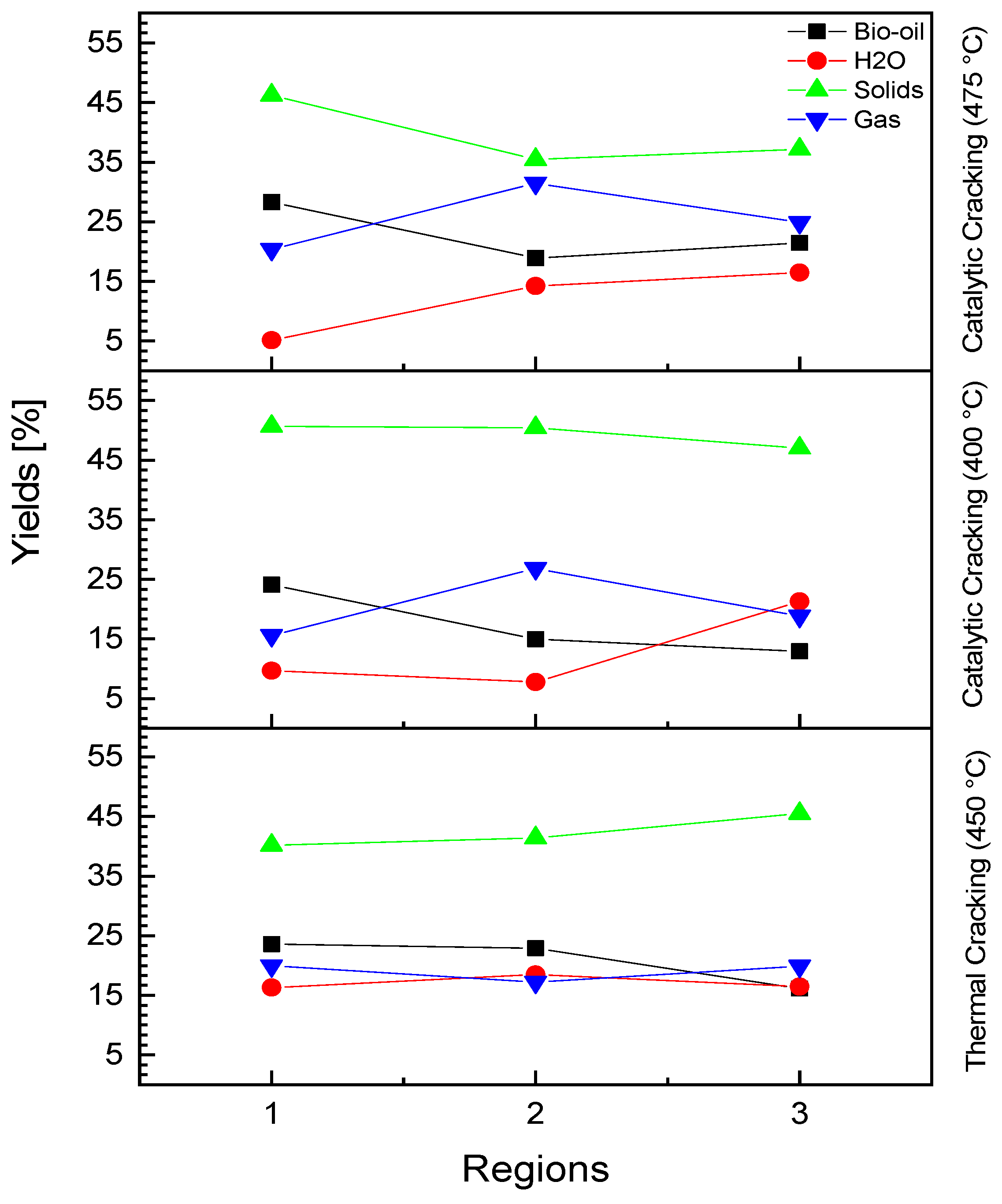
3.4.1. Process Conditions, Mass Balances, and Yields of Reaction Products
3.4.2. Physicochemical and Compositional Characterization of Bio-Oil
Acidity of Bio-Oil
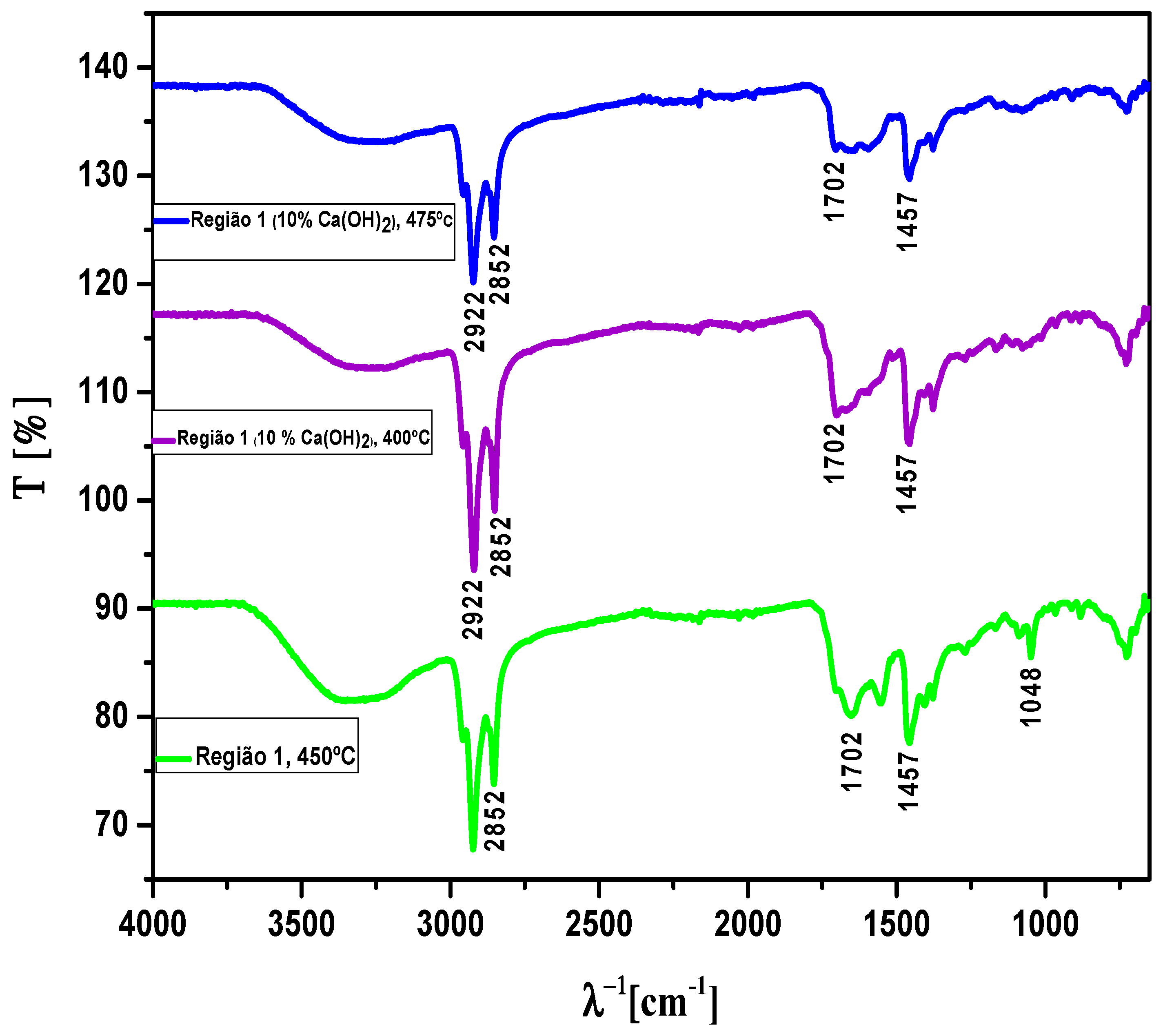
Fourier Transform in Infrared of Bio-Oil
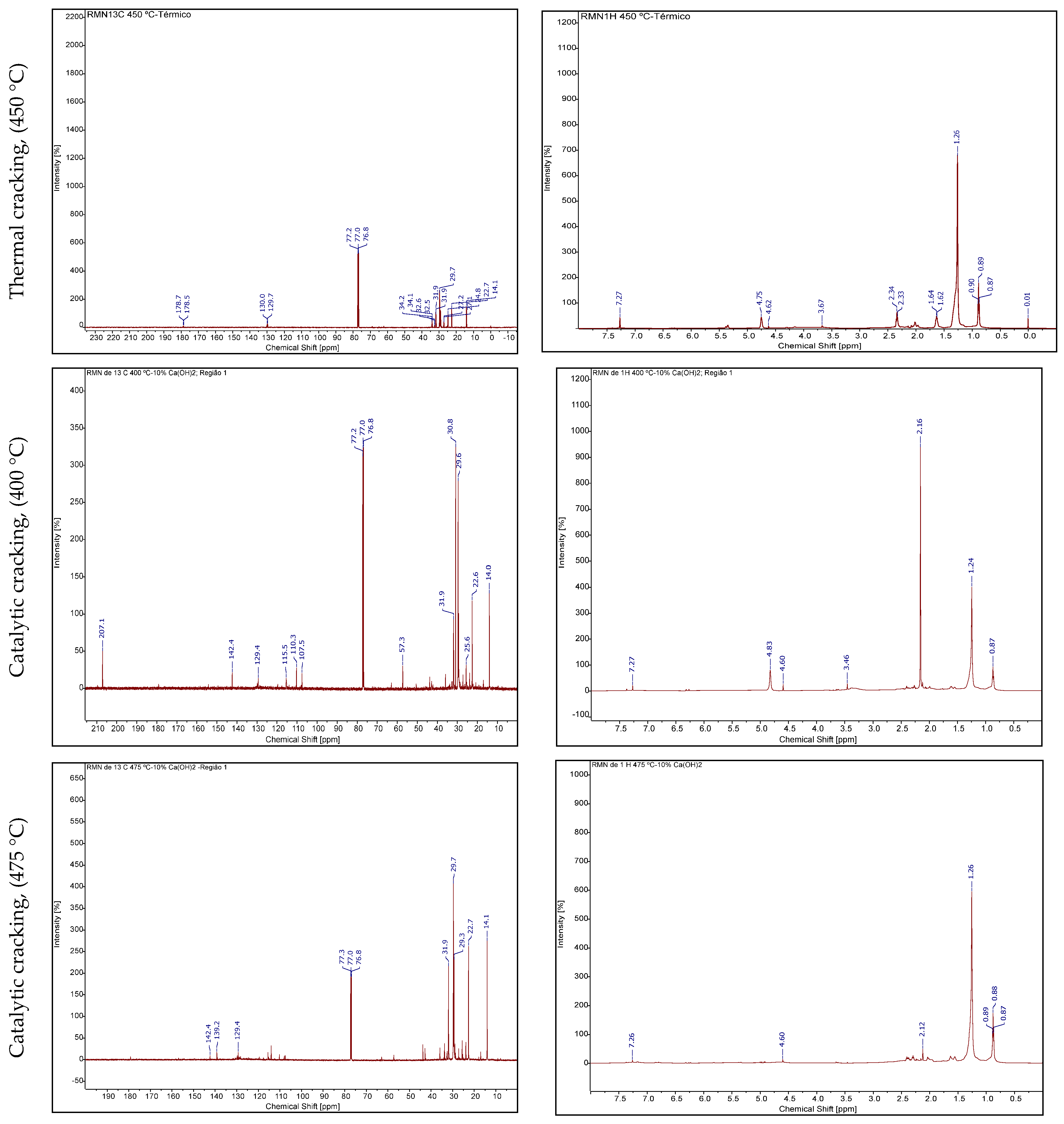
NMR of Bio-Oil
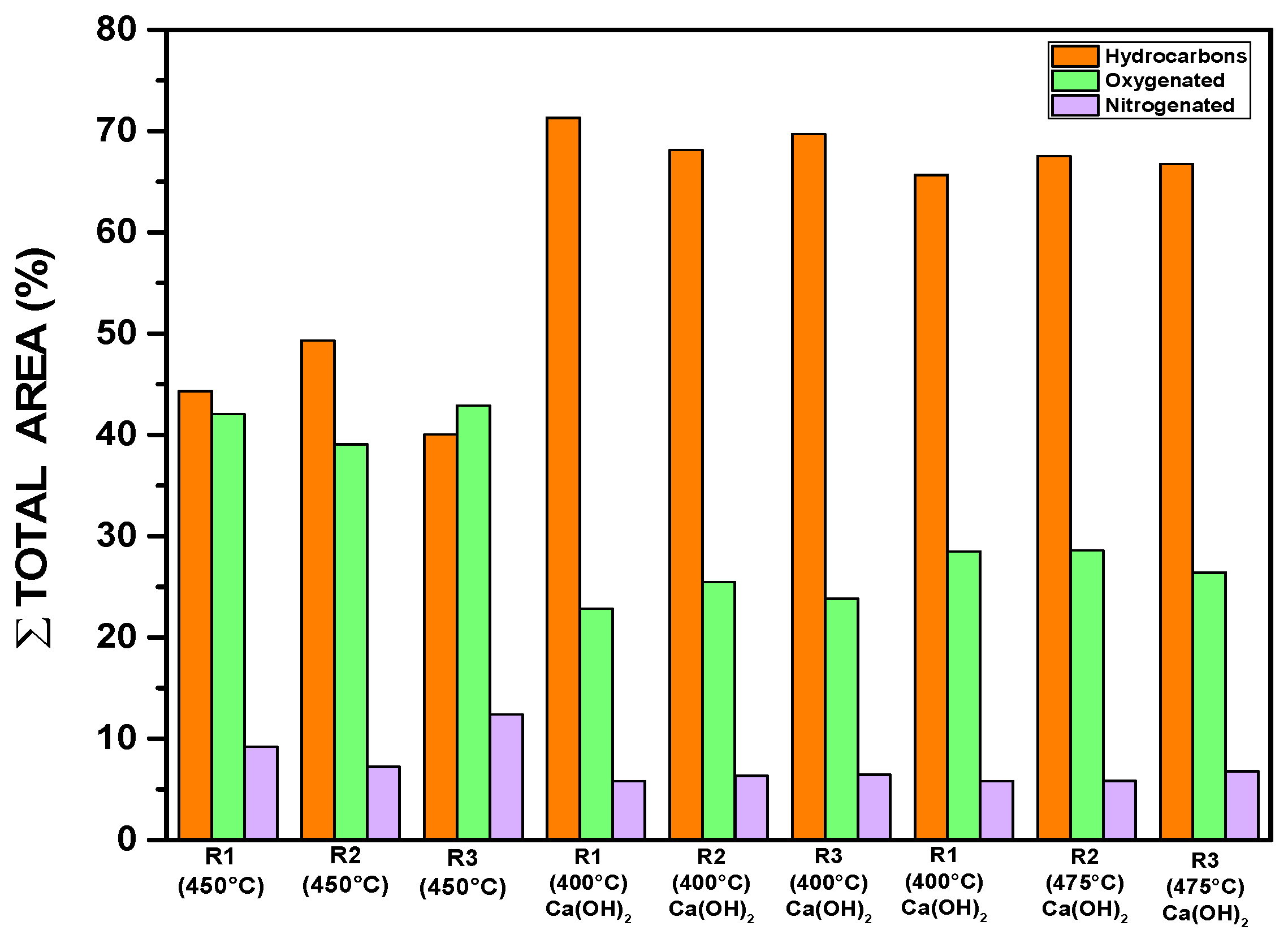
Gas Chromatography Analysis of Bio-Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: New York, NY, USA, 2012. [Google Scholar]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Association of Public Cleaning and Special Waste Companies—Abrelpe. Panora-Ma 2020; ABRELPE: São Paulo, Brazil, 2021. [Google Scholar]
- Silva, D.R.B.d.; Costa Filho, I.d.S.; Souza, W.C.S.d.; Santos, F.V.d.; Machado, P.C.d.F.; Brandão, I.W.d.S.; Pereira, F.C.; Assunação, M.A.d.C.; Silva, R.H.Y.; Russo, M.A.T.; et al. Quantitative and Qualitative Aspects of Urban Solid Waste in the Municipalities of Ananin-deua, Belém and Marituba. In Intersectoral Cooperation and Innovation: Tools for Sustainable Solid Waste Management; Pereira, C., Fricke, K., Eds.; Technische Universität Braunschweig: Braunschweig, Germany, 2022. [Google Scholar]
- United Nations Development Program (UNDP). Accompanying the 2030 Agenda for Sustainable Development: Initial Input from the United Nations System in Brazil on the Identification of National Indicators Relating to Sustainable Development Objectives/United Nations Development Program. Brasília: PNUD. 2015. Available online: https://brasil.un.org/pt-br/sdgs (accessed on 10 March 2018).
- Alam, S.; Rahman, K.S.; Rokonuzzaman, M.; Salam, P.A.; Miah, M.S.; Das, N.; Chowdhury, S.; Channumsin, S.; Sreesawet, S.; Channumsin, M. Selection of Waste to Energy Technologies for Municipal Solid Waste Management—Towards Achieving Sustainable Development Goals. Sustainability 2022, 14, 11913. [Google Scholar] [CrossRef]
- Menezes, R.O.; Castro, S.R.; Silva, J.B.G.; Teixeira, G.P.; Silva, M.A.M. Statistical analysis of the gravimetric characterization of household solid waste: Case study from the city of Juiz de Fora, Minas Gerais. Eng. Sanit. Ambient. 2019, 24, 271–282. [Google Scholar] [CrossRef]
- Brennan, R.; Healy, M.; Morrison, L.; Hynes, S.; Norton, D.; Clifford, E. Management of landfill leachate: The legacy of European Union Directives. Waste Manag. 2015, 55, 355–363. [Google Scholar] [CrossRef]
- Samadder, S.; Prabhakar, R.; Khan, D.; Kishan, D.; Chauhan, M. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Sci. Total Environ. 2016, 580, 593–601. [Google Scholar] [CrossRef]
- UNEP—Finance Initiative. Financing Circularity: Demystifying Finance for Circular Economies. 2020. Available online: https://www.unep.org/resources/report/financing-circularity-demystifying-finance-circular-economy (accessed on 12 October 2022).
- da Silva, R.C.P.; Costa, A.R.S.; El-Deir, S.G.; Jucá, J.F.T. Sectorization of household solid waste collection routes using multivariate techniques: Case study from the city of Recife, Brazil. Sanit. Environ. Eng. 2020, 25, 821–832. [Google Scholar] [CrossRef]
- Lenz, S.; Böhm, K.; Ottner, R.; Huber-Humer, M. Determination of leachate compounds relevant for landfill aftercare using FT-IR spectroscopy. Waste Manag. 2016, 55, 321–329. [Google Scholar] [CrossRef]
- Alsobky, A.; Ahmed, M.; Al Agroudy, S.; El Araby, K. A smart framework for municipal solid waste collection management: A case study in Greater Cairo Region. Ain Shams Eng. J. 2023, 14, 102183. [Google Scholar] [CrossRef]
- Banar, M.; Özcan, A. Characterization of the Municipal Solid Waste in Eskisehir City, Turkey. Environ. Eng. Sci. 2008, 25, 1213–1219. [Google Scholar] [CrossRef]
- Miezah, K.; Obiri-Danso, K.; Kádár, Z.; Fei-Baffoe, B.; Mensah, M. Municipal solid waste characterization and quantification as a measure towards effective waste management in Ghana. Waste Manag. 2015, 46, 15–27. [Google Scholar] [CrossRef]
- Ogwueleka, T.C. Survey of household waste composition and quantities in Abuja, Nigeria. Resour. Conserv. Recycl. 2013, 77, 52–60. [Google Scholar] [CrossRef]
- Costa, L.E.B.; Costa, S.K.; Rego, N.A.C.; Junior, M.F.d.S. Gravimetry of household urban solid waste and socioeconomic profile in the municipality of Salinas, Minas Gerais. Ibero-Am. J. Environ. Sci. 2012, 3, 73–90. [Google Scholar] [CrossRef]
- Ozcan, H.K.; Guvenc, S.Y.; Guvenc, L.; Demir, G. Municipal Solid Waste Characterization according to Different Income Levels: A Case Study. Sustainability 2016, 8, 1044. [Google Scholar] [CrossRef]
- Carneiro, P.F.T. Characterization and Evaluation of the Economic Potential of Selective Collection and Recycling of Household Solid Waste Generated in the Municipalities of Belém and Ananindeua. Master’s Thesis, Postgraduate Program in Civil Engineering (PPGEC), Institute of Technology (ITEC), Technological Center, Federal University of Pará, Belém, Brazil, 2006. Available online: http://www.repositorio.ufpa.br:8080/jspui/handle/2011/1899 (accessed on 4 October 2021).
- Frishammar, J.; Parida, V. Circular Business Model Transformation: A Roadmap for Incumbent Firms. Calif. Manag. Rev. 2019, 61, 5–29. [Google Scholar] [CrossRef]
- da Mota, S.; Mancio, A.; Lhamas, D.; de Abreu, D.; da Silva, M.; dos Santos, W.; de Castro, D.; de Oliveira, R.; Araújo, M.; Borges, L.E.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Abreu, D.H.S.d.; Machado, N.T.; Borges, L.E.P.; Gonzales, W.d.A. Study of the Thermocatalytic Cracking Process of Palm Oil (Elaeis guineensis) Neutralized on Semi-Pilot Scale and Laboratory Scale. In Proceedings of the 4th National Biofuels Symposium, Rio de Janeiro, Brazil, 13 September 2011. [Google Scholar]
- Santos, M.C. Study of the Thermocatalytic Cracking Process of Palm Oil Neutralization Sludge for Biofuel Production. Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, 2015; 241p. [Google Scholar]
- Almeida, H.D.S.; Corrêa, O.; Ferreira, C.; Ribeiro, H.; de Castro, D.; Pereira, M.; Mâncio, A.D.A.; Santos, M.; da Mota, S.; Souza, J.D.S.; et al. Diesel-like hydrocarbon fuels by catalytic cracking of fat, oils, and grease (FOG) from grease traps. J. Energy Inst. 2016, 90, 337–354. [Google Scholar] [CrossRef]
- Pereira, L.M. Study of the Sewage Sludge Cracking Process, at Different Scales, Aiming at Alternative Uses. Ph.D. Thesis, Federal University of Pará, Institute of technology, Postgraduate Program in Natural Resources Engineering of the Amazon, Belém, Brazil, 2019. [Google Scholar]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Grycová, B.; Koutník, I.; Pryszcz, A. Pyrolysis process for the treatment of food waste. Bioresour. Technol. 2016, 218, 1203–1207. [Google Scholar] [CrossRef]
- Yang, Y.; Heaven, S.; Venetsaneas, N.; Banks, C.J.; Bridgwater, A.V. Slow pyrolysis of organic fraction of municipal solid waste (OFMSW): Characterisation of products and screening of the aqueous liquid product for anaerobic digestion. Appl. Energy 2018, 213, 158–168. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Ben Hassen-Trabelsi, A.; Kraiem, T.; Naoui, S.; Belayouni, H. Pyrolysis of waste animal fats in a fixed-bed reactor: Production and characterization of bio-oil and bio- char. Waste Manag. 2014, 34, 210–218. [Google Scholar] [CrossRef] [PubMed]
- González, J.F.; Rom’an, S.; Encinar, J.M.; Martínez, G. Pyrolysis of various biomass residues and char utilization to produce activated carbons. J. Anal. Appl. Pyrol. 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Li, L.; Hu, Z.; Guo, P.; Jiang, Y. The catalytic pyrolysis of food waste by microwave heating. Bioresour. Technol. 2014, 166, 45–50. [Google Scholar] [CrossRef]
- Serio, M.; Kroo, E.; Florczak, E.; Wójtowicz, M.; Wignarajah, K.; Hogan, J.; Fisher, J. Pyrolysis of Mixed Solid Food, Paper, and Packaging Wastes; SAE International: Warrendale, PA, USA, 2008. [Google Scholar]
- Xu, F.; Ming, X.; Jia, R.; Zhao, M.; Wang, B.; Qiao, Y.; Tian, Y. Effects of operating parameters on products yield and volatiles composition during fast pyrolysis of food waste in the presence of hydrogen. Fuel Process Technol. 2020, 210, 106558. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J. Thermal processing of paper sludge and characterisation of its pyrolysis products. Waste Manag. 2009, 29, 1644–1648. [Google Scholar] [CrossRef]
- Islam, M.N.; Beg, M.R.A.; Islam, M.R. Pyrolytic oil from fixed bed pyrolysis of municipal solid waste and its characterization. Renew. Energy 2005, 30, 413–420. [Google Scholar] [CrossRef]
- Chen, C.; Jin, Y.; Chi, Y. Effects of moisture content and CaO on municipal solid waste pyrolysis in a fixed bed reactor. J. Anal. Appl. Pyrolysis 2014, 110, 108–112. [Google Scholar] [CrossRef]
- Biswal, B.; Kumar, S.; Singh, R.K. Production of hydrocarbon liquid by thermal pyrolysis of paper cup waste. J. Waste Manag. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Zhuang, X. Pyrolysis of waste paper: Characterization and composition of pyrolysis oil. Energy Sources 2005, 27, 867–873. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Tseng, C.-H. Pyrolysis products of uncoated printing and writing paper of MSW. Fuel 2002, 81, 719–725. [Google Scholar] [CrossRef]
- Jk, M.; Namjoshi, S.; Channiwala, S. Kinetics and pyrolysis of glossy paper waste. Int. J. Eng. Res. Gen. Sci. 2012, 2, 1067–1074. [Google Scholar]
- Sarkar, A.; Chowdhury, R. Co-pyrolysis of paper waste and mustard press cake in a semi-batch pyrolyzer—Optimization and bio-oil characterization. Int. J. Green Energy 2016, 13, 373–382. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Murakami, K.; Ota, M. On thermal stability and chemical kinetics of waste newspaper by thermogravimetric and pyrolysis analysis. J. Environ. Eng. 2008, 3, 1–12. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Tseng, C.-H.; Lin, J.-P. Pyrolysis product distribution of waste newspaper in MSW. J. Anal. Appl. Pyrol. 2003, 67, 41–53. [Google Scholar] [CrossRef]
- Veses, A.; Sanahuja-Parejo, O.; Callén, M.S.; Murillo, R.; García, T. A combined two-stage process of pyrolysis and catalytic cracking of municipal solid waste for the production of syngas and solid refuse-derived fuels. Waste Manag. 2020, 101, 171–179. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhong, D.; Zhang, B.; Qian, X. Pyrolysis of municipal solid waste in a fluidized bed for producing valuable pyrolytic oils. Clean Technol. Environ. Policy 2016, 18, 1111–1121. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guan, Y.; Cai, L. Influence of particle size on pyrolysis and gasification performance of municipal solid waste in a fixed bed reactor. Bioresour. Technol. 2010, 101, 6517–6520. [Google Scholar] [CrossRef]
- Mamad Gandidi, I.; Susila, M.D.; Agung Pambudi, N. Production of valuable pyrolytic oils from mixed Municipal Solid Waste (MSW) in Indonesia using non-isothermal and isothermal experimental. Case Stud. Therm. Eng. 2017, 10, 357–361. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, L.; Wu, D. Anaerobic pyrolysis characteristics of municipal solid waste under high temperature heat source. Energy Procedia 2015, 66, 197–200. [Google Scholar] [CrossRef]
- Wang, N.; Qian, K.; Chen, D.; Zhao, H.; Yin, L. Upgrading gas and oil products of the municipal solid waste pyrolysis process by exploiting in-situ interactions between the volatile compounds and the char. Waste Manag. 2020, 102, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Tursunov, O. A comparison of catalysts zeolite and calcined dolomite for gas production from pyrolysis of municipal solid waste (MSW). Ecol. Eng. 2014, 69, 237–243. [Google Scholar] [CrossRef]
- Baggio, P.; Baratieri, M.; Gasparella, A.; Longo, G.A. Energy and environmental analysis of an innovative system based on municipal solid waste (MSW) pyrolysis and combined cycle. Appl. Therm. Eng. 2008, 28, 136–144. [Google Scholar] [CrossRef]
- Fang, S.; Yu, Z.; Lin, Y.; Hu, S.; Liao, Y.; Ma, X. Thermogravimetric analysis of the co-pyrolysis of paper sludge and municipal solid waste. Energy Convers. Manag. 2015, 101, 626–631. [Google Scholar] [CrossRef]
- Paula, T.P.; Marques, M.F.V.; Marques, M.R.D.C. Influence of mesoporous structure ZSM-5 zeolite on the degradation of Urban plastics waste. J. Therm. Anal. 2019, 138, 3689–3699. [Google Scholar] [CrossRef]
- Vakalis, S.; Sotiropoulos, A.; Moustakas, K.; Malamis, D.; Vekkos, K.; Baratieri, M. Thermochemical valorization and characterization of household biowaste. J. Environ. Manag. 2017, 203, 648–654. [Google Scholar] [CrossRef]
- Lohri, C.R.; Faraji, A.; Ephata, E.; Rajabu, H.M.; Zurbrügg, C. Urban biowaste for solid fuel production: Waste suitability assessment and experimental carbonization in Dar es Salaam, Tanzania. Waste Manag. Res. J. A Sustain. Circ. Econ. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Phan, A.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. Characterisation of slow pyrolysis products from segregated wastes for energy production. J. Anal. Appl. Pyrolysis 2008, 81, 65–71. [Google Scholar] [CrossRef]
- Shi, H.; Mahinpey, N.; Aqsha, A.; Silbermann, R. Characterization, thermochemical conversion studies, and heating value modeling of municipal solid waste. Waste Manag. 2016, 48, 34–47. [Google Scholar] [CrossRef]
- Miskolczi, N.; Ateş, F.; Borsodi, N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: Contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013, 144, 370–379. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, H.-Y.; Xing, W.-L.; Song, L.-H.; Yang, L.; Yang, D.; Shu, X. Effects of various additives on the pyrolysis characteristics of municipal solid waste. Waste Manag. 2018, 78, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ates, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef] [PubMed]
- AlDayyat, E.; Saidan, M.; Al-Hamamre, Z.; Al-Addous, M.; Alkasrawi, M. Pyrolysis of Solid Waste for Bio-Oil and Char Production in Refugees’ Camp: A Case Study. Energies 2021, 14, 3861. [Google Scholar] [CrossRef]
- Chew, T.L.; Bhatia, S. Effect of catalyst additives on the production of biofuels from palm oil cracking in a transport riser reactor. Bioresour. Technol. 2009, 100, 2540–2545. [Google Scholar] [CrossRef]
- Sørum, L.; Grønli, M.G.; Hustad, J.E. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Guo, S.; Ma, X.; Zeng, C.; Wu, J. Thermal behavior and kinetics of municipal solid waste during pyrolysis and combustion process. Appl. Therm. Eng. 2016, 98, 400–408. [Google Scholar] [CrossRef]
- Konwer, D.; Taylor, S.E.; Gordon, B.E.; Otvos, J.W.; Calvin, M. Liquid fuels from Messua ferra L. seed oil. J. Am. Oil Chem. Soc. 1989, 66, 223–226. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 4th ed.; LTC: Rio de Janeiro, Brazil, 2009; 888p, ISBN 978-8521617167. [Google Scholar]
- Trazzi, P.A.; Higa, A.R.; Dieckow, J.; Mangrich, A.S.; Higa, R.C.V. Biocarvão:Realidade E Potencial De Uso No Meio Florestal. Ciência Florest. 2018, 28, 875–887. [Google Scholar] [CrossRef]
- Assunção, F.P.d.C.; Pereira, D.O.; da Silva, J.C.C.; Ferreira, J.F.H.; Bezerra, K.C.A.; Bernar, L.P.; Ferreira, C.C.; Costa, A.F.d.F.; Pereira, L.M.; da Paz, S.P.A.; et al. A Systematic Approach to Thermochemical Treatment of Municipal Household Solid Waste into Valuable Products: Analysis of Routes, Gravimetric Analysis, Pre-Treatment of Solid Mixtures, Thermochemical Processes, and Characterization of Bio-Oils and Bio-Adsorbents. Energies 2022, 15, 7971. [Google Scholar] [CrossRef]
- Pereira, D.O.; da Costa Assunção, F.P.; da Silva, J.C.C.; Ferreira, J.F.H.; Ferreira, R.B.P.; Lola, Á.L.; do Nascimento, Í.C.P.; Chaves, J.P.; do Nascimento, M.S.C.; da Silva Gouvêa, T.; et al. Prediction of Leachate Characteristics via an Analysis of the Solubilized Extract of the Organic Fraction of Domestic Solid Waste from the Municipality of Belém, PA. Sustainability 2023, 15, 15456. [Google Scholar] [CrossRef]
- Belém. Lei Municipal nº 9.656, de 30 de dezembro de 2020. Institui a Política Municipal de Saneamento Básico do Município de Belém, o Plano Municipal de Saneamento Básico (PMSB), e o Plano de Gestão Integrada de Resíduos Sólidos (PGIRS), em Atenção ao Disposto no Art. 9º da Lei Federal nº 11.445/2007, com as Atualizações Trazidas pela Lei nº 14.026/2020, o Novo Marco do Saneamento Básico, e dá Outras Providências. Belém, PA, 30 dez. 2020. Available online: https://arbel.belem.pa.gov.br/wp-content/uploads/2022/05/PMGIRS-INTEGRAL.pdf (accessed on 1 August 2022).
- IBGE. Instituto Brasileiro de Geografia e Estatística. Censo Brasileiro de 2010; IBGE: Rio de Janeiro, Brazil, 2012. Available online: https://censo2010.ibge.gov.br/ (accessed on 1 August 2022).
- NBR10.007; Amostragem de Resíduos Sólidos. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- Botti, L.; Battini, D.; Sgarbossa, F.; Mora, C. Door-to-Door Waste Collection: Analysis and Recommendations for Improving Ergonomics in an Italian Case Study. Waste Manag. 2020, 9, 149–160. [Google Scholar] [CrossRef] [PubMed]
- de Castro, D.R.; Ribeiro, H.D.S.; Guerreiro, L.H.; Bernar, L.P.; Bremer, S.J.; Santo, M.C.; Almeida, H.D.S.; Duvoisin, S.; Borges, L.P.; Machado, N.T. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- Cabrera-Penna, M.; Rodríguez-Páez, J. Calcium oxyhydroxide (CaO/Ca(OH)2) nanoparticles: Synthesis, characterization and evaluation of their capacity to degrade glyphosate-based herbicides (GBH). Adv. Powder Technol. 2020, 32, 237–253. [Google Scholar] [CrossRef]
- Hassani, E.; Feyzbar-Khalkhali-Nejad, F.; Rashti, A.; Oh, T.-S. Carbonation, Regeneration, and Cycle Stability of the Mechanically Activated Ca(OH)2 Sorbents for CO2 Capture: An In Situ X ray Diffraction Study. Ind. Eng. Chem. Res. 2020, 59, 11402–11411. [Google Scholar] [CrossRef]
- Gopu, C.; Gao, L.; Volpe, M.; Fiori, L.; Goldfarb, J.L. Valorizing municipal solid waste: Waste to energy and activated carbons for water treatment via pyrolysis. J. Anal. Appl. Pyrolysis 2018, 133, 48–58. [Google Scholar] [CrossRef]
- Lefebvre, D.; Williams, A.; Meersmans, J.; Kirk, G.J.D.; Sohi, S.; Goglio, P.; Smith, P. Modelling the potential for soil carbon sequestration using biochar from sugarcane residues in Brazil. Sci. Rep. 2020, 10, 19479. [Google Scholar] [CrossRef]
- Trang, P.T.T.; Dong, H.Q.; Toan, D.Q.; Hanh, N.T.X.; Thu, N.T. The Effects of Socio-economic Factors on Household Solid Waste Generation and Composition: A Case Study in Thu Dau Mot, Vietnam. Energy Procedia 2017, 107, 253–258. [Google Scholar] [CrossRef]
- Da Silva, G.P.C.; Assunção, F.P.d.C.; Pereira, D.O.; Ferreira, J.F.H.; Mathews, J.C.; Sandim, D.P.R.; Borges, H.R.; do Nascimento, M.S.C.; Mendonça, N.M.; de Sousa Brandão, I.W.; et al. Analysis of the Gravimetric Composition of Urban Solid Waste from the Municipality of Belém/PA. Sustainability 2024, 16, 5438. [Google Scholar] [CrossRef]
- Buah, W.K.; Cunliffe, A.M.; Williams, P.T. Characterization of products from the pyrolysis of municipal solid waste. Process Saf. Environ. Prot. 2007, 85, 450–457. [Google Scholar] [CrossRef]
- Lu, Q.; Xiong, W.; Li, W.; Guo, X.; Zhu, X. Catalytic pyrolysis of cellulose with sulfated metal oxides: A promising method for obtaining high yield of light furan compounds. Bioresour. Technol. 2009, 100, 4871–4876. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. Classification of municipal solid waste components for thermal conversion in waste-to-energy research. Fuel 2015, 145, 151–157. [Google Scholar] [CrossRef]
- Mati, A.; Buffi, M.; Dell’Orco, S.; Lombardi, G.; Ramiro, P.M.R.; Kersten, S.R.A.; Chiaramonti, D. Fractional Condensation of Fast Pyrolysis Bio-Oil to Improve Biocrude Quality towards Alternative Fuels Production. Appl. Sci. 2022, 12, 4822. [Google Scholar] [CrossRef]
- Ghanavati, H.; Nahvi, I.; Karimi, K. Organic fraction of municipal solid waste as a suitable feedstock for the production of lipid by oleaginous yeast Cryptococcus aerius. Waste Manag. 2015, 38, 141–148. [Google Scholar] [CrossRef] [PubMed]


| Socioeconomic Classification | |
|---|---|
| Classes | Family Income (Minimum/Basic Salary) |
| A | over 20 salaries |
| B | from 10 to 20 salaries |
| C | from 04 to 10 salaries |
| D | from 02 to 04 salaries |
| E | up to 02 salaries |
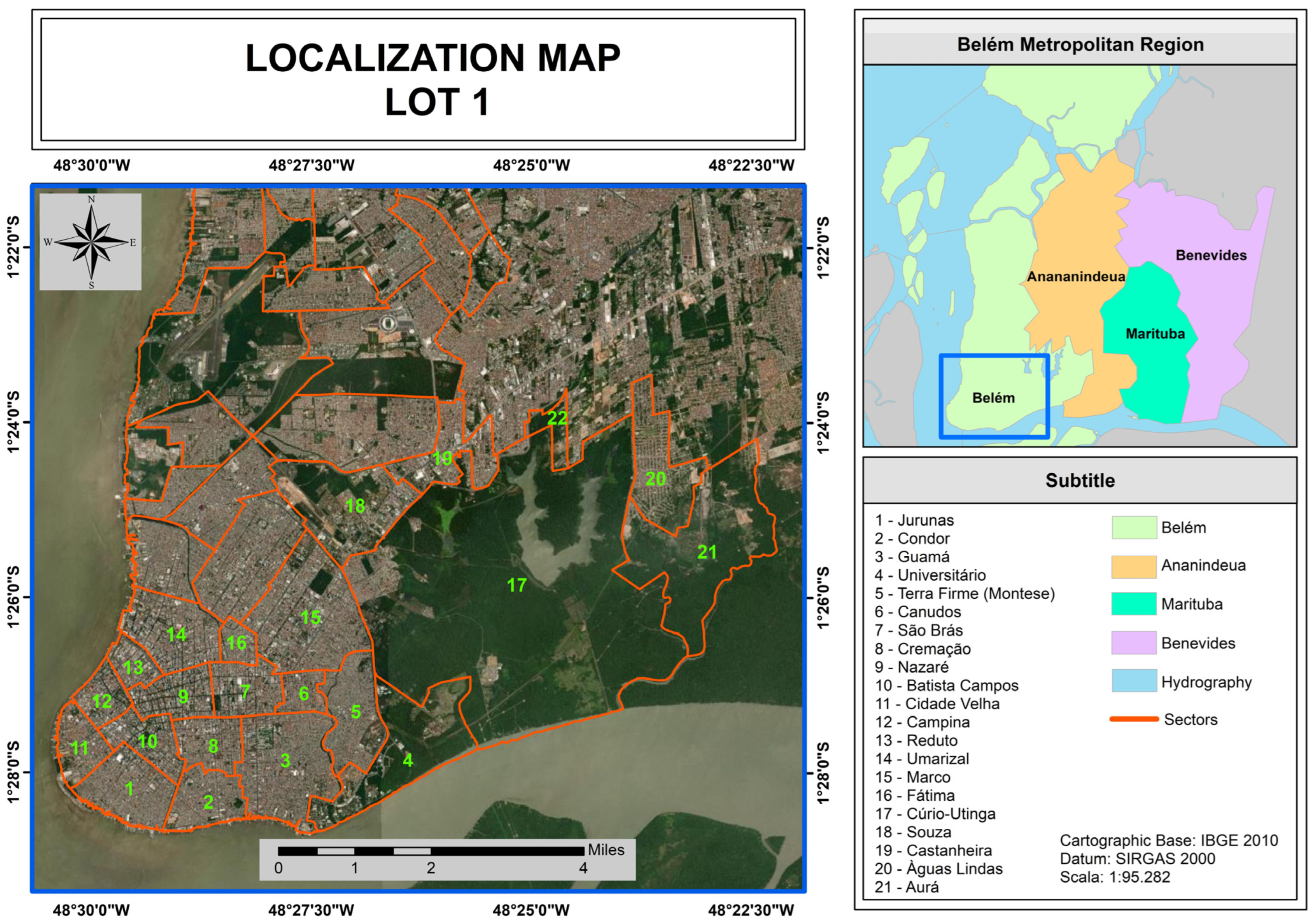
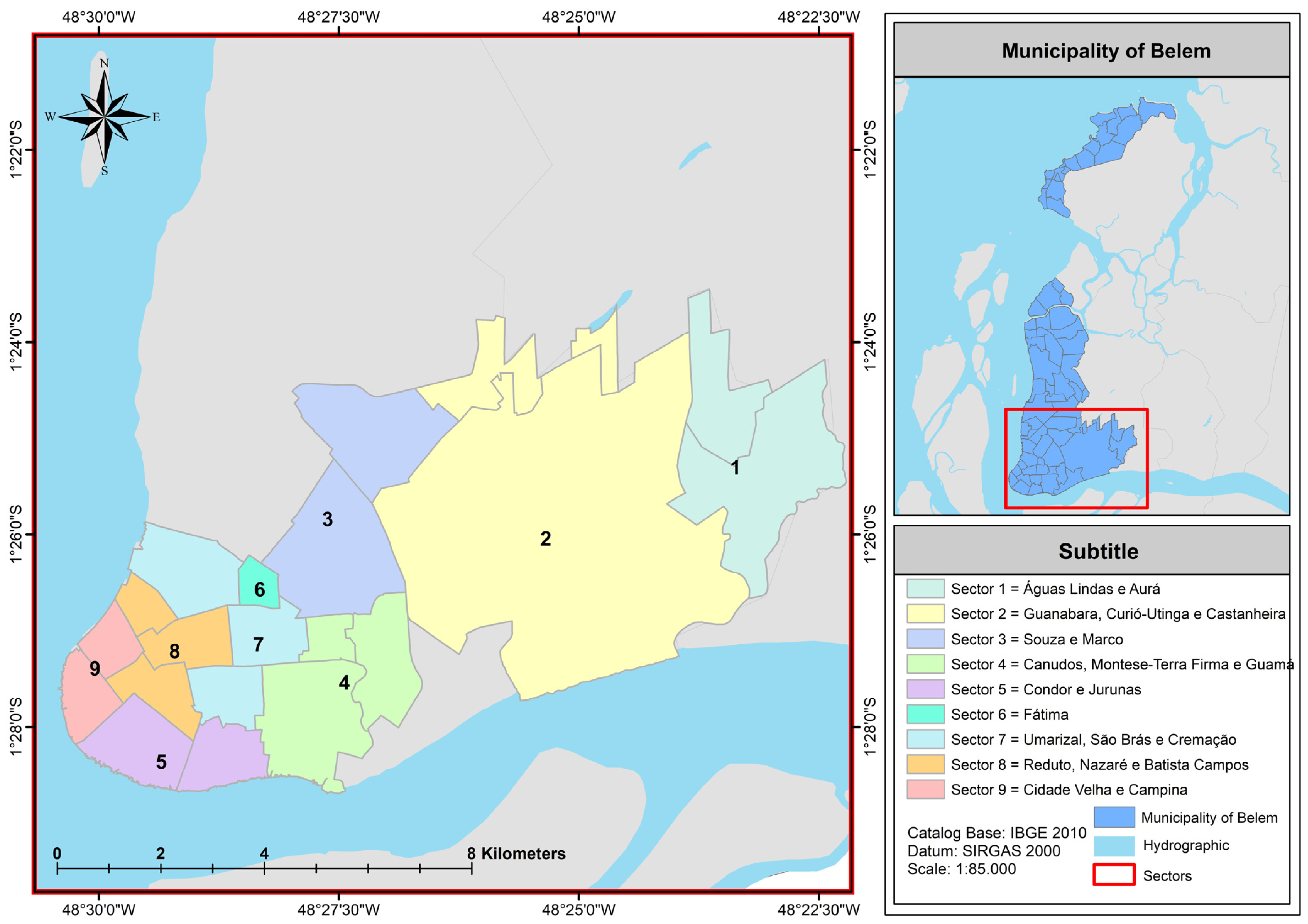
| Region | Sectors | Neighborhoods |
|---|---|---|
| 1 | 1, 2, and 3 | Aurá, Águas Lindas, Curió-Utinga, Guanabara, Castanheira, Souza, and Marco |
| 2 | 4, 5, and 6 | Canudos, Terra Firme, Guamá, Condor, Jurunas, and Fátima |
| 3 | 7, 8, and 9 | Umarizal, São Brás, Cremação, Batista Campos, Nazaré, Reduto, Campina, and Cidade Velha |
| Experiments | Material | Catalyst Mass Ca(OH)2 (%) | Temperature (°C) | Retention Time (min.) |
|---|---|---|---|---|
| Region (1) | F.O. + Paper | 0 | 450 | 1 h 20 |
| Region (2) | F.O. + Paper | 0 | 450 | 1 h 20 |
| Region (3) | F.O. + Paper | 0 | 450 | 1 h 20 |
| Region (1) | F.O. + Paper | 10 | 400 | 1 h 20 |
| Region (2) | F.O. + Paper | 10 | 400 | 1 h 20 |
| Region (3) | F.O. + Paper | 10 | 400 | 1 h 20 |
| Region (1) | F.O. + Paper | 10 | 475 | 1 h 20 |
| Region (2) | F.O. + Paper | 10 | 475 | 1 h 20 |
| Region (3) | F.O. + Paper | 10 | 475 | 1 h 20 |
| Mass Percentages and Atomic Masses of Biochars Obtained by Thermal Pyrolysis at 450 °C | ||||||
|---|---|---|---|---|---|---|
| Chemical Elements | (R1) 450 °C | (R2) 450 °C | (R3) 450 °C | |||
| Mass [wt.%] | SD | Mass [wt.%] | SD | Mass [wt.%] | SD | |
| C | 70.0 | 0.2 | 60.4 | 0.1 | 62.0 | 0.2 |
| O | 13.6 | 0.1 | 26.3 | 0.1 | 22.2 | 0.2 |
| K | 6.7 | 0.0 | 1.6 | 0.0 | 2.5 | 0.0 |
| Cl | 5.5 | 0.0 | 1.6 | 0.0 | 3.0 | 0.0 |
| Ca | 1.5 | 0.0 | 8.4 | 0.0 | 7.2 | 0.0 |
| Na | 1.5 | 0.0 | 1.5 | 0.0 | 1.6 | 0.0 |
| S | 0.3 | 0.0 | - | - | - | 0.0 |
| Mg | 0.3 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 |
| Al | 0.3 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 |
| P | 0.2 | 0.0 | 0.2 | 0.0 | 0.4 | 0.0 |
| Fe | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 |
| Si | 0.1 | 0.0 | - | - | 0.5 | 0.0 |
| Cu | - | - | - | - | 0.1 | 0.0 |
| Mass Percentages and Atomic Masses of Biochars Obtained by Catalytic Pyrolysis at 400 °C | ||||||
|---|---|---|---|---|---|---|
| Chemical Elements | (R1) 400 °C | (R2) 400 °C | (R3) 400 °C | |||
| Mass [wt.%] | SD | Mass [wt.%] | SD | Mass [wt.%] | SD | |
| C | 62.5 | 0.1 | 60.5 | 0.1 | 56.7 | 0.1 |
| O | 18.9 | 0.1 | 24.5 | 0.1 | 28.8 | 0.1 |
| K | 2.9 | 0.0 | 2.4 | 0.0 | 1.1 | 0.0 |
| Cl | 4.1 | 0.0 | 3.4 | 0.0 | 1.1 | 0.0 |
| Ca | 6.8 | 0.0 | 6.2 | 0.0 | 9.5 | 0.0 |
| Na | 2.3 | 0.0 | 2.3 | 0.0 | 1.4 | 0.0 |
| S | 0.2 | 0.0 | - | 0.0 | - | 0.0 |
| Mg | 0.4 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 |
| Al | 0.2 | 0.0 | - | 0.0 | 0.6 | 0.0 |
| P | 0.6 | 0.0 | - | 0.0 | 0.3 | 0.0 |
| Fe | 0.7 | 0.0 | 0.4 | 0.0 | - | 0.0 |
| Si | 0.4 | 0.0 | - | 0.0 | 0.2 | 0.0 |
| Mass Percentages and Atomic Masses of Biochars Obtained by Catalytic Pyrolysis at 475 °C | ||||||
|---|---|---|---|---|---|---|
| Chemical Elements | (R1) 475 °C | (R2) 475 °C | (R3) 475 °C | |||
| Mass [wt.%] | SD | Mass [wt.%] | SD | Mass [wt.%] | SD | |
| C | 77.6 | 0.1 | 70.4 | 0.1 | 45.0 | 0.1 |
| O | 18.2 | 0.1 | 19.6 | 0.1 | 32.4 | 0.1 |
| K | 1.5 | 0.0 | 1.7 | 0.0 | 2.6 | 0.0 |
| Cl | 0.5 | 0.0 | 1.7 | 0.0 | 2.6 | 0.0 |
| Ca | 0.8 | 0.0 | 1.3 | 0.0 | 14.2 | 0.0 |
| Na | 0.9 | 0.0 | 1.2 | 0.0 | 1.5 | 0.0 |
| S | - | - | - | - | 0.1 | 0.0 |
| Mg | 0.4 | 0.0 | 0.1 | 0.0 | 0.4 | 0.0 |
| Al | - | - | - | - | 0.1 | 0.0 |
| P | - | - | 0.2 | 0.0 | 0.5 | 0.0 |
| Fe | 0.1 | 0.0 | - | - | 0.4 | 0.0 |
| Si | - | - | 0.1 | 0.0 | 0.2 | 0.0 |
| Cu | - | - | 0.1 | 0.0 | 0.1 | 0.0 |
| Process Parameters | Region 1 | Region 2 | Region 3 |
|---|---|---|---|
| 450 [°C] | 450 [°C] | 450 [°C] | |
| 0.0 (wt.) | 0.0 (wt.) | 0.0 (wt.) | |
| Mass of urban solid wastes [g] | 40.1 | 40.11 | 40.57 |
| Cracking time [min] | 20 | 20 | 20 |
| Initial cracking temperature [°C] | 334 | 363 | 364 |
| Mass of solids (coke) [g] | 16.11 | 16.61 | 19.28 |
| Mass of bio-oil [g] | 9.45 | 9.19 | 6.54 |
| Mass of H2O [g] | 6.54 | 7.42 | 6.68 |
| Mass of gas [g] | 8.00 | 6.89 | 8.07 |
| Yield of bio-oil [%] | 23.57 | 22.91 | 16.12 |
| Yield of H2O [%] | 16.31 | 18.49 | 16.46 |
| Yield of solids [%] | 40.17 | 41.41 | 45.52 |
| Yield of gas [%] | 19.95 | 17.21 | 19.89 |
| Process Parameters | Region 1 | Region 2 | Region 3 |
|---|---|---|---|
| 400 [°C] | 400 [°C] | 400 [°C] | |
| 10.0 (wt.) | 10.0 (wt.) | 10.0 (wt.) | |
| Mass of urban solid wastes [g] | 30.0 | 30.0 | 30.03 |
| Mass of Ca(OH)2 [g] | 3.0 | 3.0 | 3.0 |
| Cracking time [min] | 20 | 20 | 20 |
| Initial cracking temperature [°C] | 351 | 327 | 328 |
| Mass of solids (coke) [g] | 15.2 | 15.13 | 14.12 |
| Mass of bio-oil [g] | 7.23 | 4.49 | 3.88 |
| Mass of H2O [g] | 2.90 | 2.33 | 6.39 |
| Mass of gas [g] | 4.67 | 8.05 | 5.65 |
| Yield of bio-oil [%] | 24.10 | 14.97 | 12.92 |
| Yield of H2O [%] | 9.66 | 7.77 | 21.27 |
| Yield of solids [%] | 50.67 | 50.43 | 47.01 |
| Yield of gas [%] | 15.57 | 26.83 | 18.81 |
| Process Parameters | Region 1 | Region 2 | Region 3 |
|---|---|---|---|
| 475 [°C] | 475 [°C] | 475 [°C] | |
| 10.0 (wt.) | 10.0 (wt.) | 10.0 (wt.) | |
| Mass of urban solid wastes [g] | 30.0 | 30.08 | 30.06 |
| Mass of Ca(OH)2 [g] | 3.0 | 3.02 | 3.00 |
| Cracking time [min] | 20 | 20 | 20 |
| Initial cracking temperature [°C] | 364 | 381 | 340 |
| Mass of solids (coke) [g] | 13.87 | 10.66 | 11.18 |
| Mass of bio-oil [g] | 8.48 | 5.68 | 6.45 |
| Mass of H2O [g] | 1.53 | 4.28 | 4.95 |
| Mass of gas [g] | 6.10 | 9.47 | 7.48 |
| Yield of bio-oil [%] | 28.28 | 18.88 | 21.45 |
| Yield of H2O [%] | 5.11 | 14.22 | 16.46 |
| Yield of solids [%] | 46.24 | 35.43 | 37.19 |
| Yield of gas [%] | 20.35 | 31.48 | 24.88 |
| Physicochemical Property | Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R1 | R2 | R3 | R1 | R2 | R3 | |
| Acid Index | 450 °C | 450 °C | 450 °C | 400 °C Ca(OH)2 | 400 °C Ca(OH)2 | 400 °C Ca(OH)2 | 475 °C Ca(OH)2 | 475 °C Ca(OH)2 | 475 °C Ca(OH)2 |
| I.ABio-Oil [mg KOH/g] | 116.8 | 115.1 | 115.3 | 34.45 | 34.41 | 40.0 | 36.36 | 36.31 | 37.20 |
| I.AAqueous Phase [mg KOH/g] | 69.01 | 65.55 | 76.23 | 42.3 | 42.1 | 42.0 | 43.4 | 4.2 | 40.0 |
| Temperature [°C] | Concentration [%area.] | |||
|---|---|---|---|---|
| Hydrocarbons | Oxygenated | Nitrogenated | Chlorinated | |
| 450 (R1) | 44.34 | 42.09 | 9.23 | 4.34 |
| 450 (R2) | 49.34 | 39.09 | 7.23 | 4.34 |
| 450 (R3) | 40.07 | 42.91 | 12.40 | 4.34 |
| 400 (R1) | 71.32 | 22.85 | 5.82 | - |
| 400 (R2) | 68.16 | 25.48 | 6.36 | - |
| 400 (R3) | 69.70 | 23.83 | 6.47 | - |
| 475 (R1) | 65.69 | 28.48 | 5.82 | - |
| 475 (R2) | 67.53 | 28.63 | 5.84 | - |
| 475 (R3) | 66.77 | 26.40 | 6.82 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, F.P.d.C.; da Silva, J.C.C.; Soares Almeida, F.F.; Santos, M.C.; da Paz, S.P.A.; de Castro, D.A.R.; Ferreira, J.F.H.; Mendonça, N.M.; do Nascimento, M.S.C.; Pereira, J.A.R.; et al. Analysis of Thermal and Catalytic Pyrolysis Processes in Belém: A Socioeconomic Perspective. Energies 2025, 18, 4532. https://doi.org/10.3390/en18174532
Assunção FPdC, da Silva JCC, Soares Almeida FF, Santos MC, da Paz SPA, de Castro DAR, Ferreira JFH, Mendonça NM, do Nascimento MSC, Pereira JAR, et al. Analysis of Thermal and Catalytic Pyrolysis Processes in Belém: A Socioeconomic Perspective. Energies. 2025; 18(17):4532. https://doi.org/10.3390/en18174532
Chicago/Turabian StyleAssunção, Fernanda Paula da Costa, Jéssica Cristina Conte da Silva, Fernando Felipe Soares Almeida, Marcelo Costa Santos, Simone Patrícia Aranha da Paz, Douglas Alberto Rocha de Castro, Jorge Fernando Hungria Ferreira, Neyson Martins Mendonça, Mel Safira Cruz do Nascimento, José Almir Rodrigues Pereira, and et al. 2025. "Analysis of Thermal and Catalytic Pyrolysis Processes in Belém: A Socioeconomic Perspective" Energies 18, no. 17: 4532. https://doi.org/10.3390/en18174532
APA StyleAssunção, F. P. d. C., da Silva, J. C. C., Soares Almeida, F. F., Santos, M. C., da Paz, S. P. A., de Castro, D. A. R., Ferreira, J. F. H., Mendonça, N. M., do Nascimento, M. S. C., Pereira, J. A. R., Almeida, A. C. P., Junior, S. D., Borges, L. E. P., & Machado, N. T. (2025). Analysis of Thermal and Catalytic Pyrolysis Processes in Belém: A Socioeconomic Perspective. Energies, 18(17), 4532. https://doi.org/10.3390/en18174532






