Catalytic Role of Nickel in Hydrogen Storage and Release Using Dibenzyltoluene as a Liquid Organic Hydrogen Carrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Catalyst Preparation
2.4. Characterization
3. Results and Discussion
3.1. Influence of the Impregnation Order on Catalytic Activity
- Simultaneous impregnation—(denoted Pt-Nisimul); the corresponding amounts of Pt and Ni precursors were dissolved together in a single aqueous solution, into which the support was added. The resulting mixture was then dried, and the material was calcined (450 °C).
- Sequential impregnation—Pt followed by Ni (denoted Pt-Niseq); initially, only the Pt precursor was dissolved and impregnated onto the support. After calcination (450 °C), the material was then introduced into another aqueous solution of the Ni precursor, followed by a second drying and calcination step (500 °C).
- Sequential impregnation—Ni followed by Pt (denoted Ni-Ptseq); this procedure is analogous to the previous one, with the order of metal impregnation reversed.
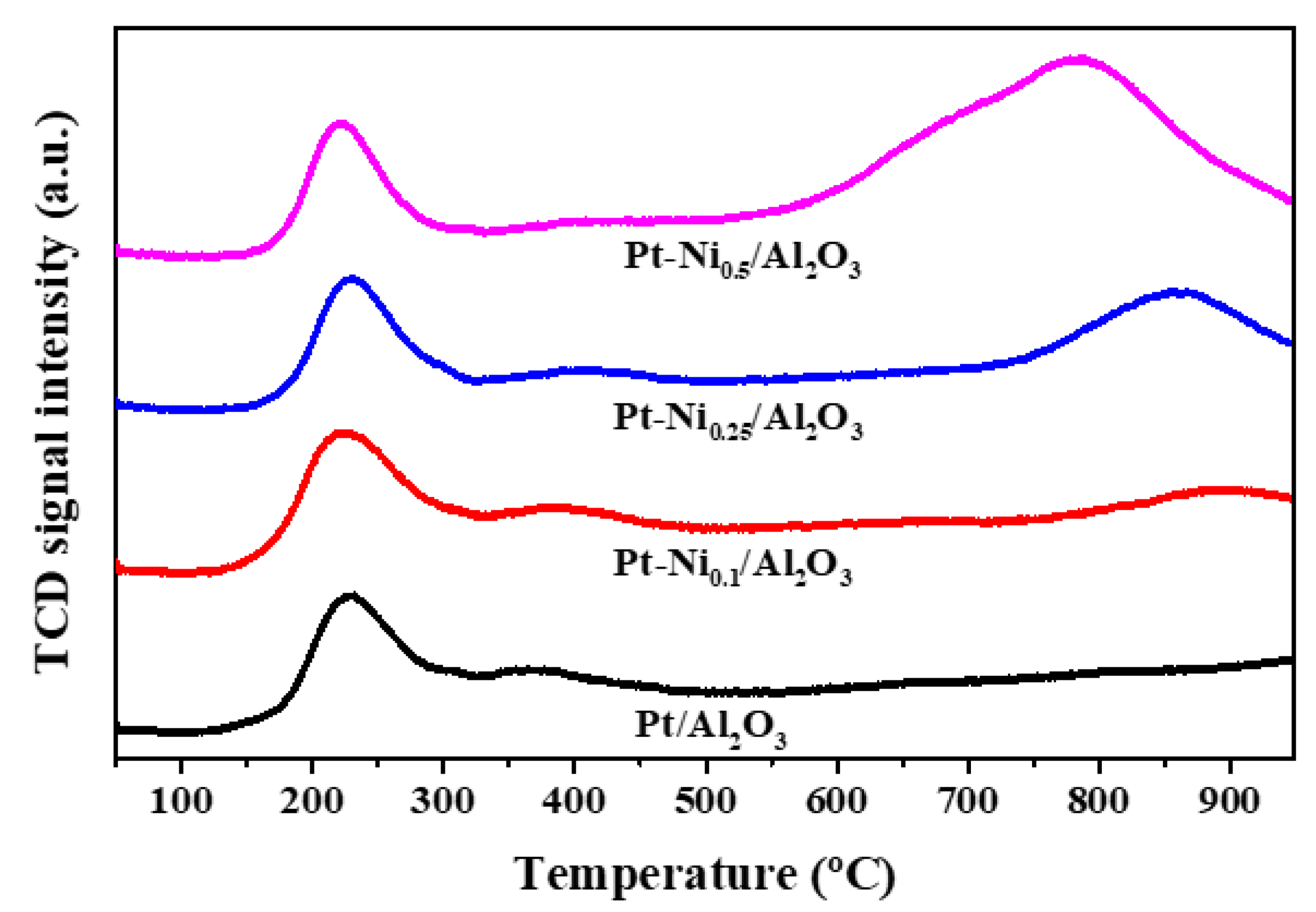
3.2. Catalyst Characterization
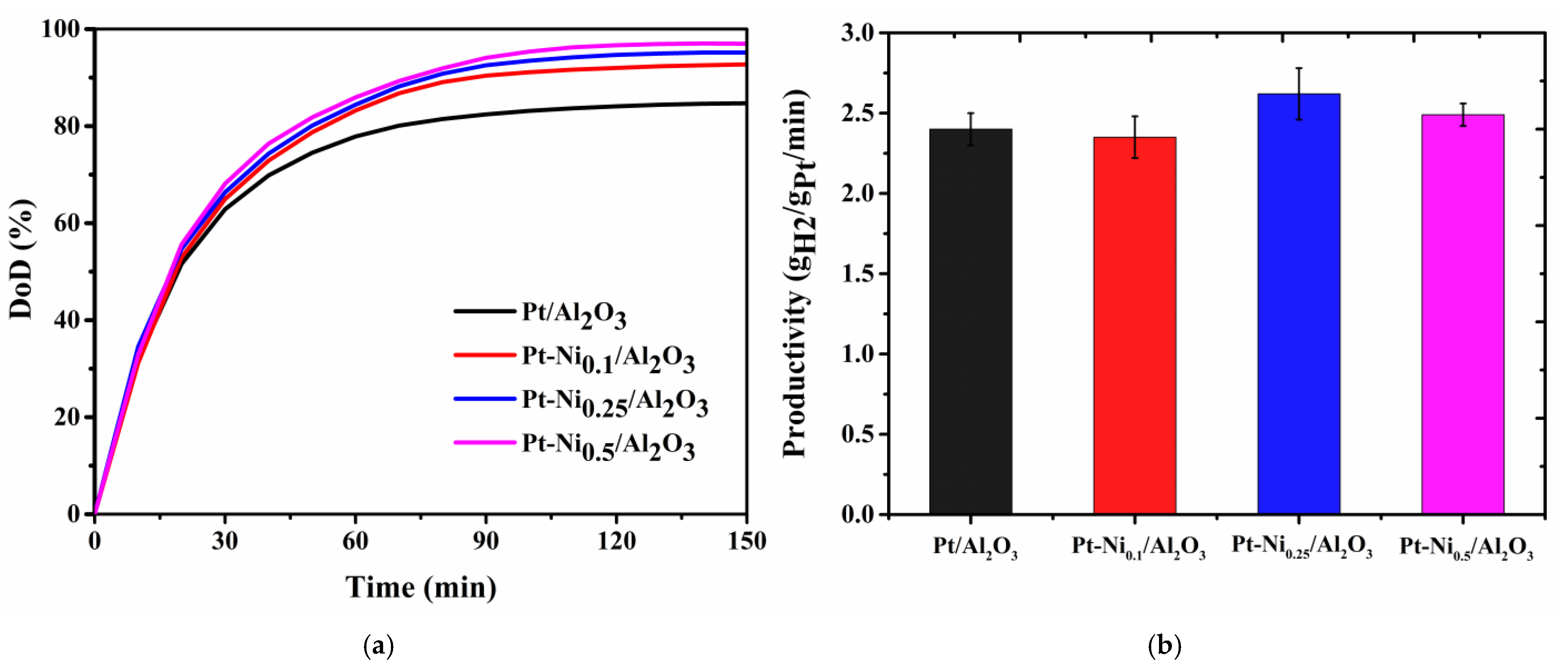
3.3. Catalytic Activity in the Dehydrogenation of H18-DBT
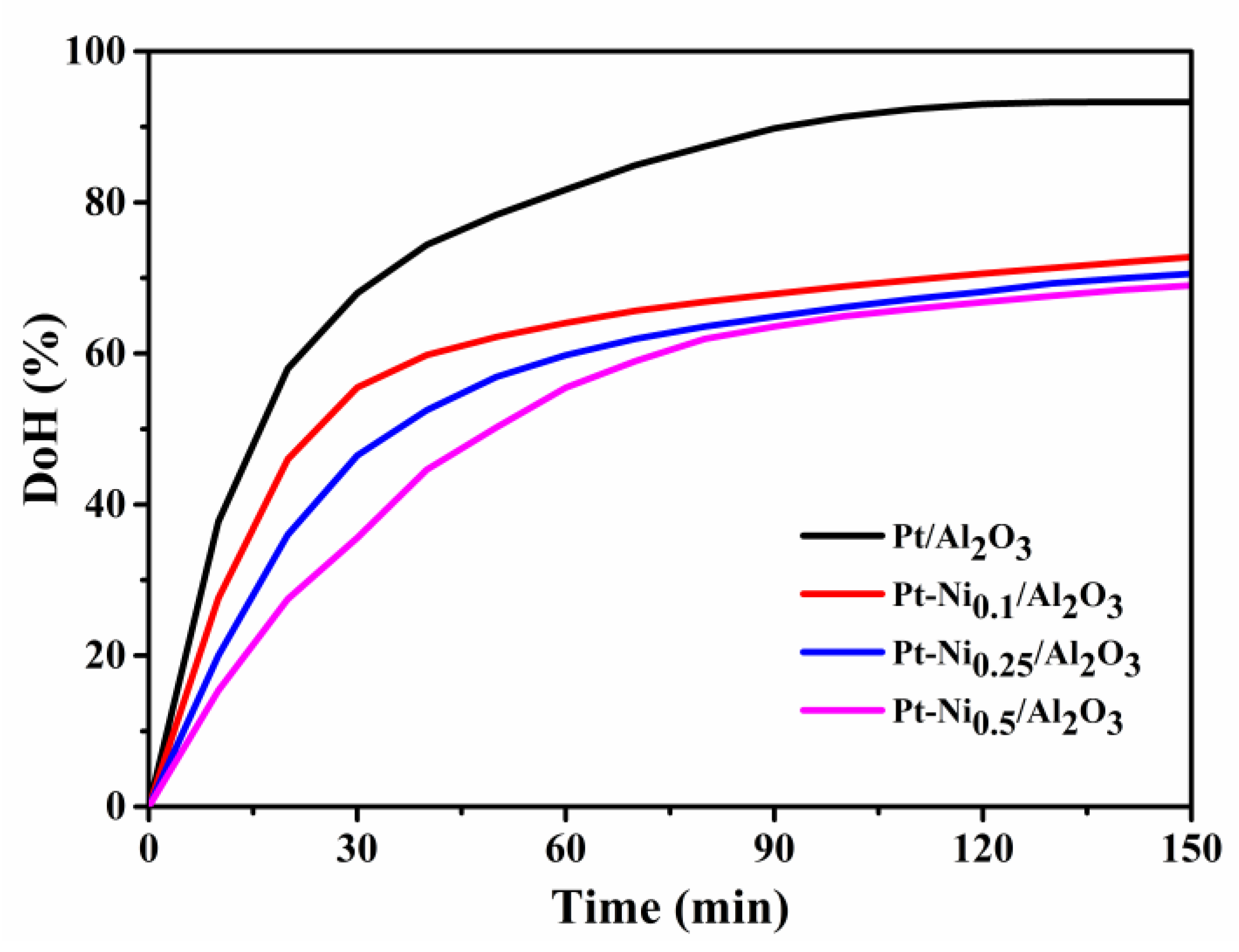
3.4. Catalytic Activity in the Hydrogenation of H0-DBT
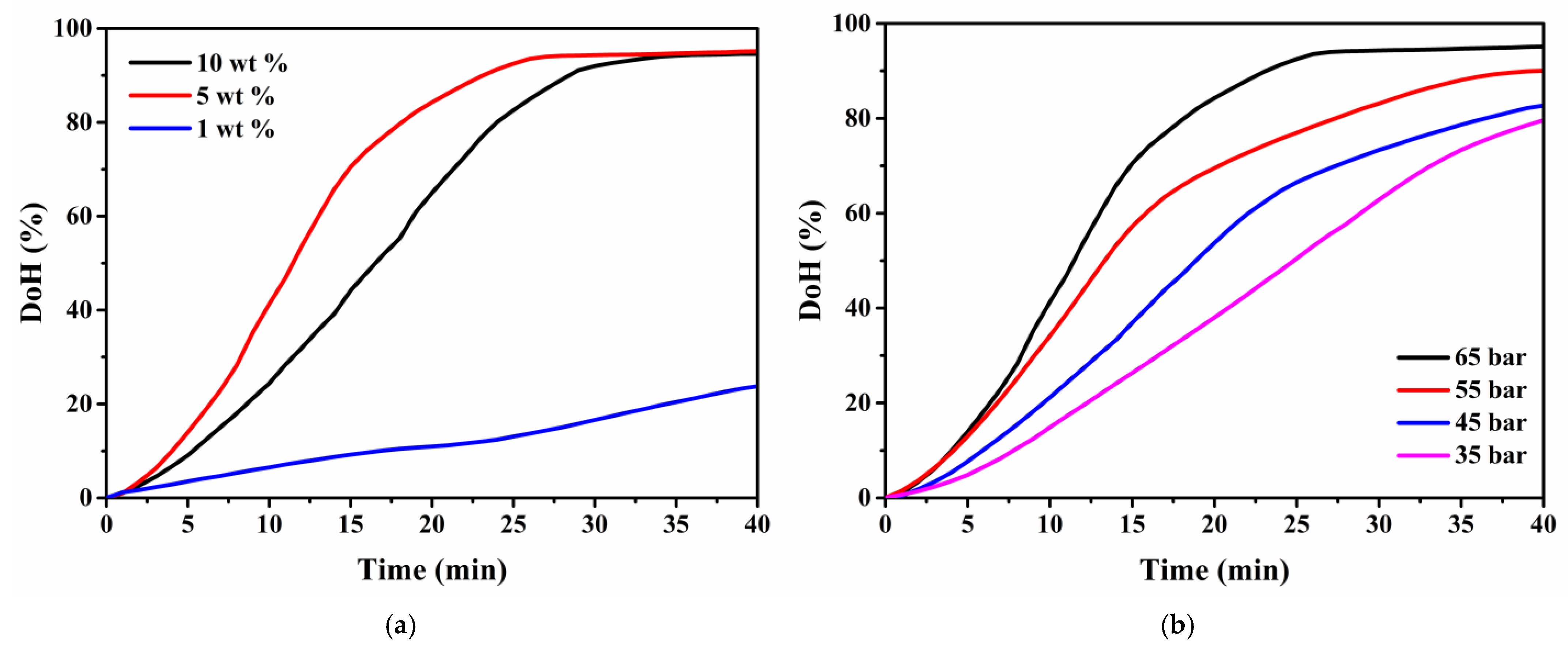
3.5. Noble Metal-Free Catalyst Alternative for Hydrogenation of H0-DBT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, W.; Zhang, Y.; Chen, X.; Sha, A.; Xiong, Z.; Luo, Y.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. The Easily Overlooked Effect of Global Warming: Diffusion of Heavy Metals. Toxics 2024, 12, 400. [Google Scholar] [PubMed]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Gayen, D.; Chatterjee, R.; Roy, S. A Review on Environmental Impacts of Renewable Energy for Sustainable Development. Int. J. Environ. Sci. Technol. 2024, 21, 5285–5310. [Google Scholar]
- Wang, W.; Yuan, B.; Sun, Q.; Wennersten, R. Application of Energy Storage in Integrated Energy Systems—A Solution to Fluctuation and Uncertainty of Renewable Energy. J. Energy Storage 2022, 52, 104812. [Google Scholar]
- Cabrera, G.; Mora, M.; Gil-Burgos, J.P.; Visbal, R.; Machuca-Martínez, F.; Mosquera-Vargas, E. Liquid Organic Hydrogen Carrier Concepts and Catalysts for Hydrogenation and Dehydrogenation Reactions. Molecules 2024, 29, 4938. [Google Scholar]
- Chilunda, M.D.; Talipov, S.A.; Farooq, H.M.U.; Biddinger, E.J. Electrochemical Cycling of Liquid Organic Hydrogen Carriers as a Sustainable Approach for Hydrogen Storage and Transportation. ACS Sustain. Chem. Eng. 2025, 13, 1174–1195. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Venkateswaran, P.S.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A Sustainable Fuel for Future of the Transport Sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an Energy Vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, H.; Baimbetov, D.; Syrlybekkyzy, S.; Akhmetov, B.B.; Abbas, Q. Development Status and Future Prospects of Hydrogen Energy Technology: Production, Storage, and Cost Analysis. Adv. Energy Sustain. Res. 2025, 2400451. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, M.; Zhou, Y.; Dong, X.; Shen, J. Thermodynamics Analysis of Hydrogen Storage Based on Compressed Gaseous Hydrogen, Liquid Hydrogen and Cryo-Compressed Hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)–Assessment Based on Chemical and Economic Properties. Int. J. Hydrog. Energy 2019, 44, 6631–6654. [Google Scholar]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid Organic Hydrogen Carriers for Transportation and Storing of Renewable Energy—Review and Discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar]
- Munyentwali, A.; Tan, K.C.; He, T. Advancements in the Development of Liquid Organic Hydrogen Carrier Systems and Their Applications in the Hydrogen Economy. Prog. Nat. Sci. Mater. Int. 2024, 34, 825–839. [Google Scholar]
- Zhou, L.; Sun, L.; Xu, L.; Wan, C.; An, Y.; Ye, M. Recent Developments of Effective Catalysts for Hydrogen Storage Technology Using N-Ethylcarbazole. Catalysts 2020, 10, 648. [Google Scholar] [CrossRef]
- Chen, C.-X.; Cao, J.-P.; Jiang, W.; Tang, W.; Zhang, C.; Yao, N.-Y.; Wang, H.-Y.; Zhao, X.-Y. Effect of Pt Particle Size on Methylcyclohexane Dehydrogenation over Pt/Al2O3 Catalysts. Fuel 2024, 360, 130607. [Google Scholar]
- Rüde, T.; Dürr, S.; Preuster, P.; Wolf, M.; Wasserscheid, P. Benzyltoluene/Perhydro Benzyltoluene–Pushing the Performance Limits of Pure Hydrocarbon Liquid Organic Hydrogen Carrier (LOHC) Systems. Sustain. Energy Fuels 2022, 6, 1541–1553. [Google Scholar]
- Modisha, P.; Bessarabov, D. Stress Tolerance Assessment of Dibenzyltoluene-Based Liquid Organic Hydrogen Carriers. Sustain. Energy Fuels 2020, 4, 4662–4670. [Google Scholar] [CrossRef]
- Lin, A.; Bagnato, G. Revolutionising Energy Storage: The Latest Breakthrough in Liquid Organic Hydrogen Carriers. Int. J. Hydrog. Energy 2024, 63, 315–329. [Google Scholar]
- Sekine, Y.; Higo, T. Recent Trends on the Dehydrogenation Catalysis of Liquid Organic Hydrogen Carrier (LOHC): A Review. Top. Catal. 2021, 64, 470–480. [Google Scholar] [CrossRef]
- Ramadhani, S.; Dao, Q.N.; Imanuel, Y.; Ridwan, M.; Sohn, H.; Jeong, H.; Kim, K.; Yoon, C.W.; Song, K.H.; Kim, Y. Advances in Catalytic Hydrogenation of Liquid Organic Hydrogen Carriers (LOHCs) Using High-Purity and Low-Purity Hydrogen. ChemCatChem 2024, 16, e202401278. [Google Scholar]
- Tardío, C.; Rodríguez, J.; Montes, C.; Martínez de Sarasa Buchaca, M.; López-Montenegro, S.; Esteban, C.; Gómez, F.; Campana, R. Activity and Degradation of Pt/Al2O3 Catalyst Assessment in Hydrogen Release from Perhydro-Dibenzyltoluene. Int. J. Hydrog. Energy 2024, 87, 1453–1460. [Google Scholar]
- Modisha, P.; Garidzirai, R.; Güneş, H.; Bozbag, S.E.; Rommel, S.; Uzunlar, E.; Aindow, M.; Erkey, C.; Bessarabov, D. A Promising Catalyst for the Dehydrogenation of Perhydro-Dibenzyltoluene: Pt/Al2O3 Prepared by Supercritical CO2 Deposition. Catalysts 2022, 12, 489. [Google Scholar]
- Modisha, P.; Gqogqa, P.; Garidzirai, R.; Ouma, C.N.M.; Bessarabov, D. Evaluation of Catalyst Activity for Release of Hydrogen from Liquid Organic Hydrogen Carriers. Int. J. Hydrog. Energy 2019, 44, 21926–21935. [Google Scholar]
- Feng, Z.; Chen, X.; Bai, X. Hydrogen Production from the Catalytic Dehydrogenation of Dodecahydro-N-Ethylcarbazole: Effect of Pd Precursor on the Catalytic Performance of Pd/C Catalysts. Environ. Sci. Pollut. Res. 2021, 28, 61623–61635. [Google Scholar]
- Shuang, H.; Chen, H.; Wu, F.; Li, J.; Cheng, C.; Li, H.; Fu, J. Catalytic Dehydrogenation of Hydrogen-Rich Liquid Organic Hydrogen Carriers by Palladium Oxide Supported on Activated Carbon. Fuel 2020, 275, 117896. [Google Scholar] [CrossRef]
- Cashel, J.; Yan, D.; Han, R.; Jeong, H.; Yoon, C.W.; Ambay, J.A.; Liu, Y.; Ung, A.T.; Yang, L.; Huang, Z. Chemical Bonds Containing Hydrogen: Choices for Hydrogen Carriers and Catalysts. Angew. Chem. Int. Ed. 2025, 64, e202423661. [Google Scholar]
- Zhang, J.; Yang, F.; Wang, B.; Li, D.; Wei, M.; Fang, T.; Zhang, Z.; Suib, S.L.; Zhang, J.; Yang, F.; et al. Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future. Materials 2023, 16, 3735. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, S.; Oh, J.; Shin, C.H.; Suh, Y.W. Hydrogenation of the LOHC Compound Monobenzyl Toluene over ZrO2-Supported Ru Nanoparticles: A Consequence of Zirconium Hydroxide’s Surface Hydroxyl Group and Surface Area. ChemCatChem 2018, 10, 3406–3410. [Google Scholar]
- Alconada, K.; Barrio, V.L. Enhancing Perhydrobenzyltoluene Dehydrogenation Performance with Co, Mo and Mn Metal Oxides: A Comparative Study with Pt/Al2O3 Catalyst. Appl. Catal. B Environ. Energy 2024, 357, 124349. [Google Scholar]
- Garidzirai, R.; Modisha, P.; Bessarabov, D. Assessment of Reaction Kinetics for the Dehydrogenation of Perhydro-Dibenzyltoluene Using Mg-and Zn-Modified Pt/Al2O3 Catalysts. Catalysts 2023, 14, 32. [Google Scholar]
- Chen, X.; Li, G.; Gao, M.; Dong, Y.; Yang, M.; Cheng, H. Wet-Impregnated Bimetallic Pd-Ni Catalysts with Enhanced Activity for Dehydrogenation of Perhydro-N-Propylcarbazole. Int. J. Hydrog. Energy 2020, 45, 32168–32178. [Google Scholar]
- Chen, X.; Gierlich, C.H.; Schötz, S.; Blaumeiser, D.; Bauer, T.; Libuda, J.; Palkovits, R. Hydrogen Production Based on Liquid Organic Hydrogen Carriers through Sulfur Doped Platinum Catalysts Supported on TiO2. ACS Sustain. Chem. Eng. 2021, 9, 6561–6573. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, T.W.; Kim, M.; Han, G.B.; Jeong, B.; Suh, Y.W. Mesoporous Acidic SiO2-Al2O3 Support Boosts Nickel Hydrogenation Catalysis for H2 Storage in Aromatic LOHC Compounds. ACS Sustain. Chem. Eng. 2022, 10, 15550–15563. [Google Scholar]
- Liu, L.; Gao, R.; Li, E.; Li, C.; Dong, Y.; Yang, M.; Zhu, T. Modulating Metal-Carrier Interaction of High-Loading Co-Al2O3-SiO2 Catalysts for Boosted N-Propylcarbazole Hydrogenation. J. Catal. 2024, 432, 115416. [Google Scholar]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Łuczak, J.; Lieder, M. Nickel-Based Catalysts for Electrolytic Decomposition of Ammonia towards Hydrogen Production. Adv. Colloid Interface Sci. 2023, 319, 102963. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-Precious-Metal Catalysts for Alkaline Water Electrolysis: Operando Characterizations, Theoretical Calculations, and Recent Advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [PubMed]
- Vermaak, V.; Vosloo, H.C.M.; Swarts, A.J. The Development and Application of Homogeneous Nickel Catalysts for Transfer Hydrogenation and Related Reactions. Coord. Chem. Rev. 2024, 507, 215716. [Google Scholar]
- Alconada, K.; Barrio, V.L. Evaluation of Bimetallic Pt–Co and Pt–Ni Catalysts in LOHC Dehydrogenation. Int. J. Hydrog. Energy 2024, 51, 243–255. [Google Scholar]
- Gao, X.; Chang, C.-R. Characterizing the Sequential Effects toward the Impregnations of Supported Bimetallic Catalysts. Mol. Catal. 2022, 527, 112411. [Google Scholar] [CrossRef]
- Shu, Y.; Murillo, L.E.; Bosco, J.P.; Huang, W.; Frenkel, A.I.; Chen, J.G. The Effect of Impregnation Sequence on the Hydrogenation Activity and Selectivity of Supported Pt/Ni Bimetallic Catalysts. Appl. Catal. A Gen. 2008, 339, 169–179. [Google Scholar]
- Zhang, Y.; Zhou, Y.; Zhang, S.; Zhou, S.; Sheng, X.; Wang, Q.; Zhang, C. Catalytic Structure and Reaction Performance of PtSnK/ZSM-5 Catalyst for Propane Dehydrogenation: Influence of Impregnation Strategy. J. Mater. Sci. 2015, 50, 6457–6468. [Google Scholar]
- Naicker, L.; Valand, J.; Govender, A.; Xaba, B.M.; Friedrich, H.B. Impact of the Impregnation Sequence on the Surface Characteristics on Cu-Ag Supported on γ-Alumina towards the Liquid Phase Competitive Hydrogenation of Octanal in the Presence of Octene. Mol. Catal. 2021, 515, 111879. [Google Scholar]
- Betti, C.; Badano, J.; MacCarrone, M.J.; Mazzieri, V.; Vera, C.; Quiroga, M. Effect of the Sequence of Impregnation on the Activity and Sulfur Resistance of Pt–Ni/γ-Al2O3 Bimetallic Catalysts for the Selective Hydrogenation of Styrene. Appl. Catal. A Gen. 2012, 435–436, 181–186. [Google Scholar]
- Bhogeswararao, S.; Srinivas, D. Catalytic Conversion of Furfural to Industrial Chemicals over Supported Pt and Pd Catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Dosso, L.A.; Vera, C.R.; Grau, J.M. Pt-Co and Pt-Ni Catalysts of Low Metal Content for H2 Production by Reforming of Oxygenated Hydrocarbons and Comparison with Reported Pt-Based Catalysts. Int. J. Chem. Eng. 2018, 2018, 4972070. [Google Scholar]
- Zhu, T.; Song, H.; Li, F.; Chen, Y. Hydrodeoxygenation of Benzofuran over Bimetallic Ni-Cu/γ-Al2O3 Catalysts. Catalysts 2020, 10, 274. [Google Scholar]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, G.; Lei, T.; Lv, Z.; Xiong, M.; Wang, L.; Xing, S.; Ma, J.; Jiang, Z.; Qin, Y. Enhanced Hydrogen Generation by Reverse Spillover Effects over Bicomponent Catalysts. Nat. Commun. 2022, 13, 118. [Google Scholar] [CrossRef]
- Qi, S.; Li, Y.; Yue, J.; Chen, H.; Yi, C.; Yang, B. Hydrogen Production from Decalin Dehydrogenation over Pt-Ni/C Bimetallic Catalysts. Chin. J. Catal. 2014, 35, 1833–1839. [Google Scholar]
- Park, K.W.; Choi, J.H.; Kwon, B.K.; Lee, S.A.; Sung, Y.E.; Ha, H.Y.; Hong, S.A.; Kim, H.; Wieckowski, A. Chemical and Electronic Effects of Ni in Pt/Ni and Pt/Ru/Ni Alloy Nanoparticles in Methanol Electrooxidation. J. Phys. Chem. B 2002, 106, 1869–1877. [Google Scholar]
- Qi, S.C.; Wei, X.Y.; Zong, Z.M.; Wang, Y.K. Application of Supported Metallic Catalysts in Catalytic Hydrogenation of Arenes. RSC Adv. 2013, 3, 14219–14232. [Google Scholar] [CrossRef]
- Zhang, S.; Johnson, D.D.; Shelton, W.A.; Xu, Y. Hydrogen Adsorption on Ordered and Disordered Pt-Ni Alloys. Top. Catal. 2020, 63, 714–727. [Google Scholar] [CrossRef]
- Alconada, K.; Agirre, I.; Barrio, V.L. Synergistic Effects of Pt-Doping in Ni/Al2O3 Catalysts for Hydrogen Storage Using Benzyltoluene as LOHC. Int. J. Hydrog. Energy 2025, 130, 631–643. [Google Scholar]
- Svintsitskiy, D.A.; Kibis, L.S.; Stadnichenko, A.I.; Slavinskaya, E.M.; Romanenko, A.V.; Fedorova, E.A.; Stonkus, O.A.; Doronkin, D.E.; Marchuk, V.; Zimina, A.; et al. Insight into the Nature of Active Species of Pt/Al2O3 Catalysts for Low Temperature NH3 Oxidation. ChemCatChem 2020, 12, 867–880. [Google Scholar]
- Wu, Y.; Yu, H.; Guo, Y.; Zhang, Y.; Jiang, X.; Sun, B.; Fu, K.; Chen, J.; Qi, Y.; Zheng, J.; et al. Promoting Hydrogen Absorption of Liquid Organic Hydrogen Carriers by Solid Metal Hydrides. J. Mater. Chem. A 2019, 7, 16677–16684. [Google Scholar] [CrossRef]
- Ye, X.; An, Y.; Xu, G. Kinetics of 9-Ethylcarbazole Hydrogenation over Raney-Ni Catalyst for Hydrogen Storage. J. Alloys Compd. 2011, 509, 152–156. [Google Scholar]
- Kot, M.; Kiderys, A.; Janiszewska, E.; Pietrowski, M.; Yang, C.M.; Zieliński, M. Hydrogenation of Toluene over Nickel Nanoparticles Supported on SBA-3 and AlSBA-3 Materials. Catal. Today 2020, 356, 64–72. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhang, J.; Gao, R.; Zhang, X.; Zou, J.J.; Pan, L. Intrinsic Kinetics of Dibenzyltoluene Hydrogenation over a Supported Ni Catalyst for Green Hydrogen Storage. Ind. Eng. Chem. Res. 2024, 63, 220–232. [Google Scholar]
- Ding, Y.; Dong, Y.; Zhang, H.; Zhao, Y.; Yang, M.; Cheng, H. A Highly Adaptable Ni Catalyst for Liquid Organic Hydrogen Carriers Hydrogenation. Int. J. Hydrog. Energy 2021, 46, 27026–27036. [Google Scholar] [CrossRef]

| Impregnation Method | Metal Content (Pt/Ni) (wt %) a | Metallic Area (m2/gPt) b | Particle Metal Size (nm) b | H2 Chemisorption (µmol/µmolPt) b |
|---|---|---|---|---|
| Pt-Nisimul | 0.23/0.25 | 116 | 2.01 | 0.24 |
| Pt-Niseq | 0.23/0.20 | 137 | 1.73 | 0.28 |
| Ni-Ptseq | 0.26/0.27 | 155 | 1.51 | 0.32 |
| Catalyst | Metal Content (Pt/Ni) (wt %) a | BET Surface Area (m2/g) b | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| Pt/Al2O3 | 0.22/- | 265 | 0.842 | 12.94 |
| Pt-Ni0.1/Al2O3 | 0.25/0.13 | 263 | 0.833 | 12.88 |
| Pt-Ni0.25/Al2O3 | 0.27/0.29 | 257 | 0.808 | 12.87 |
| Pt-Ni0.5/Al2O3 | 0.23/0.47 | 250 | 0.809 | 12.57 |
| Catalyst | Dispersion (%) | Metallic Area | Particle Size (nm) | H2 Chemisorbed | ||
|---|---|---|---|---|---|---|
| (m2/gsample) | (m2/gPt) | (µmol/gcat) | (µmol/molPt) | |||
| Pt/Al2O3 | 77.24 | 0.48 | 190.76 | 1.46 | 4.95 | 0.38 |
| Pt-Ni0.1/Al2O3 | 73.08 | 0.45 | 180.47 | 1.54 | 4.68 | 0.37 |
| Pt-Ni0.25/Al2O3 | 62.63 | 0.39 | 154.68 | 1.80 | 4.02 | 0.31 |
| Pt-Ni0.5/Al2O3 | 52.78 | 0.32 | 130.65 | 2.14 | 3.38 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, J.R.; García-Mancha, N.; Campana, R.; Tardío, C. Catalytic Role of Nickel in Hydrogen Storage and Release Using Dibenzyltoluene as a Liquid Organic Hydrogen Carrier. Energies 2025, 18, 4429. https://doi.org/10.3390/en18164429
Ruiz JR, García-Mancha N, Campana R, Tardío C. Catalytic Role of Nickel in Hydrogen Storage and Release Using Dibenzyltoluene as a Liquid Organic Hydrogen Carrier. Energies. 2025; 18(16):4429. https://doi.org/10.3390/en18164429
Chicago/Turabian StyleRuiz, Jesús Rodríguez, Nuria García-Mancha, Roberto Campana, and Carlos Tardío. 2025. "Catalytic Role of Nickel in Hydrogen Storage and Release Using Dibenzyltoluene as a Liquid Organic Hydrogen Carrier" Energies 18, no. 16: 4429. https://doi.org/10.3390/en18164429
APA StyleRuiz, J. R., García-Mancha, N., Campana, R., & Tardío, C. (2025). Catalytic Role of Nickel in Hydrogen Storage and Release Using Dibenzyltoluene as a Liquid Organic Hydrogen Carrier. Energies, 18(16), 4429. https://doi.org/10.3390/en18164429






