Abstract
In this study, a selection of active materials were coated onto commercially available intermediate modulus carbon fibers to form and analyze the performance of novel composite cathodes for structural power composites. Various slurries containing polyvinylidene fluoride (PVDF), active material powders, 1-methyl-2-pyrrolidone (NMP) and carbon black (CB) were used to coat carbon fiber tows by immersion. Four active materials—lithium cobalt oxide (LCO), lithium iron phosphate (LFP), lithium nickel manganese cobalt oxide (NMC), and lithium nickel cobalt aluminum oxide (NCA)—were individually tested to assess their electrochemical reversibility. The cells were prepared with a polymer separator and liquid electrolytes and assembled in 2025-coin cells. Electrochemical analysis of the cathode materials showed that at C/5 and room temperature the measured capacities ranged from 39.8 Ah kg−1 to 64.7 Ah kg−1 for the LFP and NCA active materials, respectively. The full cells exhibited capacities of 18.1, 23.5, 27.2, and 28.2 Ah kg−1 after 55 cycles for LFP, LCO, NCA, and NMC811, respectively. Finally, visual and elemental analysis were performed via scanning electron microscope (SEM) and energy-dispersive x-ray (EDX) confirming desirable surface coverage and successful transfer of the active materials onto the carbon fiber tows.
1. Introduction
As advancements in technology continue to revolutionize various industries, there is an increasing demand for more efficient and sustainable energy storage solutions [1]. Traditional batteries, though efficient in their primary function of energy storage, often present limitations in terms of space utilization and overall system weight. EVs and electric aircraft, such as NASA’s X-57 Maxwell which was Empirical Systems Aerospace (Pismo Beach, CA, USA) and Joby Aviation (Santa Cruz, CA, USA), rely on separate systems that occupy a considerable amount of space. In total, 16 battery packs are installed into the mid-section of the fuselage contributing to 26% of the overall aircraft weight while performing a single energy storage function [2]. This has sparked a growing interest in developing innovative technologies that can perform multiple functions simultaneously, combining the roles of the battery and structure itself. This approach results in a multifunctional system that eliminates the need for individual components like battery packs, leading to a higher overall energy density [3,4].
Two approaches are described in the literature for integrating both electrical and mechanical performance in structures. The first approach involves adding electrical storage devices to existing structural components, for instance by embedding individual cells or batteries into the wings of UAVs [5] or creating sandwich structures with active materials [6]. This approach requires the assembly of separate components, resulting in a heterogeneous structure formed with noticeable distinctions between the electrical and mechanical components [7]. Another strategy centers on creating a uniform composite in which individual components serve multiple purposes simultaneously. For example, carbon fibers can provide both mechanical reinforcement and act as the electrode, while the polymer phase may function as the structural matrix and the electrolyte [3,7]. This concept—often referred to as a structural battery composite or, more precisely, a structural power composite—enables the seamless incorporation of energy storage directly into the load-bearing elements of systems. As shown in Figure 1, such integration can be applied to structures like an aircraft fuselage, resulting in space and mass reduction [7].
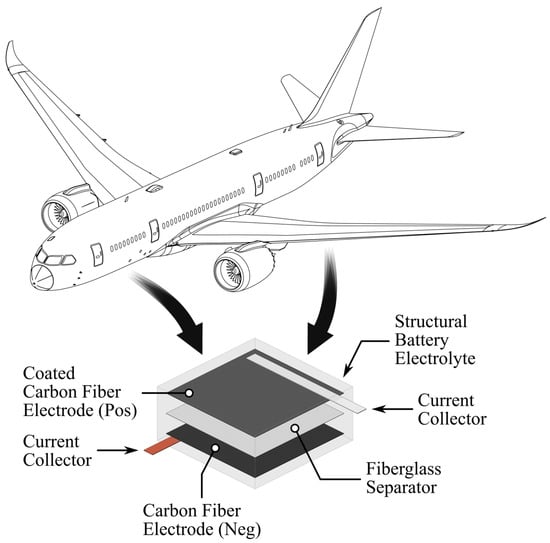
Figure 1.
Example of integrating a structural battery within the fuselage or wing structures of an aircraft, such as those used in commercial aviation. Beyond serving as a load-bearing element, the structural battery also provides capacity for storing electrical energy during operation.
The US Army Research Laboratory (ARL) was an early pioneer in creating a functioning structural battery from composite materials. Wetzel et al. investigated multiple composite reinforcement materials that could function as structural electrodes. These were combined with a glass-fiber weave serving as the separator and embedded within a polymer-based electrolyte matrix to assemble a complete cell [8,9,10]. Although the resulting demonstrator showed promising mechanical characteristics, it lacked sufficient electrical insulation rendering the cell non-viable. Another study conducted by Liu et al. [11] examined electrodes reinforced with short fibers embedded within a rigid polymer matrix. Their functioning prototype, however, suffered from manufacturing issues with the reinforced electrodes. Performance was further hindered by the lack of a sufficiently ion-permeable polymer electrolyte matrix, leading to the use of a robust gel electrolyte in place of a polymer-based one, which in turn reduced the cell’s mechanical integrity [11]. Building on these findings, Ekstedt et al. successfully developed a working structural battery laminate capable of producing a constant voltage of 3.3 V [12].
Laminated power composites encounter numerous challenges, requiring some innovation prior to becoming a viable technology. Several studies have highlighted the implementation of thick layers of separator materials as a significant challenge, as they restrict both power and energy densities. Thick separators increase internal resistances resulting in considerable capacity loss; however, some studies suggest for the elimination of the separator if a suitable rigid matrix material can maintain a physical separation between adjacent electrodes [13].
The lack of polymer electrolytes with both high ionic conductivity and desirable mechanical properties, which is critical for structural power composites, presents a separate challenge. Snyder et al. introduced employing electrolyte systems using block copolymers incorporating a monomer that forms cross-links to enhance the mechanical durability and a monomer with a high ethylene oxide content to promote the diffusion properties or the polymer [10]. A different approach, suggested by Shirshova et al. for structural supercapacitors, involves a bi-continuous polymer-ionic liquid system, enabling ions to diffuse through a porous layer of polymer matrix [14]. Some recent achievements in structural batteries with LFP active material include an energy density between 23.6 Wh kg−1 and 58 Wh kg−1, based on the total cell mass, with an elastic modulus of 25 GPa and tensile strength over 300 MPa are summarized in Table 1 [15,16].

Table 1.
Table summarizing selected properties of cell characteristics achieved by [15,16].
Compared with traditional stand-alone Li-ion battery cells with LFP cathodes, which are in the range of 90–160 Wh/kg, the state-of-the-art energy density for structural batteries is significantly lower. However, since structural batteries are embedded directly in vehicles and replace load-bearing structures, the structural batteries are often referred to a “massless” battery. Moreover, considering that carbon fibers are electrically conductive, they replace a large part of the otherwise necessary current collectors, significantly reducing the overall weight. For the development of more performant structural cells, 58 Wh/kg is set as a baseline.
The performance characteristics from various studies to date illustrate the great potential of structural power composites for on-board energy storage in applications such as aeronautic or astronautic vehicles [17].
Carbon fibers are regarded as highly effective reinforcement materials in composites offering high moduli of elasticity and relatively favorable densities compared to materials like aluminum or steel. Furthermore, carbon fibers exhibit electrochemical properties, cost-effectiveness, and non-toxicity comparable to those of graphite and other carbon-based anode materials utilized in lithium-ion batteries since 1991. Research has indicated that by comparing traditional designs to structural batteries (with equivalent energy storage), up to 60% weight savings can be achieved [13]. Current and former studies continue to demonstrate that carbon fibers can serve as an anode, current collector, and structural reinforcement, simultaneously, eliminating the need for separate components [18,19,20]. Various studies have successfully demonstrated that coating carbon fibers with active materials also makes them suitable to be used as cathodes [11,18,21,22]. The energy densities of structural power composites vary based on the type of active materials used and the method of application. Polyacrylonitrile (PAN)-based carbon fibers with intermediate modulus have demonstrated an appropriate balance between stiffness, mechanical strength and electrochemical efficiency maintained at charge/discharge rates up to 1 C over numerous cycles [23,24,25]. E. Jacques et al. investigated the impact of electrochemical cycling on the tensile properties of carbon fibers [26] and discovered that the CF’s tensile stiffness remains unchanged for over one thousand cycles. During lithiation, carbon fibers experienced an approximate 20% reduction in ultimate tensile strength, with only partial recovery observed after delithiation. It is also worth noting that carbon fibers have an electrical conductivity of 1000 S cm−1 intrinsically, enabling their use without the need for separate current collectors.
For the preparation of cathodes, various methods have been utilized to deposit a layer of active material onto carbon fiber tows. A common method noted in the literature is the application of electrophoretic deposition (EPD) to coat carbon fibers with olivine LiFePO4 (LFP), chosen for its relatively high theoretical specific capacity of 170 mAh g−1 (per gram of LFP active material) as well as its structural and chemical stability, or LiCoO2 [27,28,29]. By utilizing the natural electrical conductivity of carbon fibers, EPD enables the production of cathodes that offer high specific capacity, competitive rate capabilities, and excellent coating adhesion to the fiber surface [21]. A drawback of the described EPD technique is the batch process requiring the use of equipment that can be expensive (such as platinum rods) limiting the formation of cathode specimens to ~10 cm in length. At present, the technique does not seem readily scalable to perform on-demand reel-to-reel coating without requiring a complex and potentially expensive system for coating large quantities of carbon fiber. Moreover, the deposition of cathode slurry constituents onto substrates via physical processes, such as dip-coating, can be preferential from an economy at scale perspective much like physical deposition of slurry is used in creating electrodes for commercially available 18650 and 21700 format cylindrical cells as opposed to electrochemical processes such as EPD. Coating can also be performed using an incremental layering approach which necessitates carbonizing the organic binders present in the coating solution once the deposition is complete [30]. This process involves heating the system to temperatures of up to 450 °C in an inert atmosphere, thereby increasing both complexity and cost.
Dip-coating is another cathode-preparation method offering several advantages, such as a lower operating temperature and a less complex setup [31]. Moyer et al. [16] demonstrated the practicality of dip-coating carbon fibers by applying an epoxy layer to the carbon-fiber weave, achieving a uniform coating on the layered electrode. Results showed that mechanically applying LFP to the fibers yielded an average energy density of 52 Wh kg−1 for over 100 cycles, which is lower than the baseline energy density. A study by Yadav et al. [32] compared electrophoretic deposition to dip-coating on a single-filament battery and found that, while dip-coating provided strong electrochemical and structural performance, the single-filament approach is impractical for fabricating cells on the order of amp-hours.
A recent study by Petrushenko et al. describes a simple dip-coating process developed for preparing cathodes using carbon fiber tows for use in structural batteries [22]. The study details a process for preparing electrodes, installing them into 2025-type coin cells, and the results obtained from electrochemical analysis. However, most of the previous studies were performed on cathodes made with active materials that have a demonstrated low energy density, such as LFP, compared to other commercially available active materials, such as NCA or NMC. Dip-coating provides a simple-to-use process for evaluating cathode materials without requiring specialized equipment, such as an ultrasonic coating machine, to build electrodes. Furthermore, this process allows the active materials to fully permeate through the carbon fiber tow during submersion to more fully utilize the available surface area of the carbon fiber filaments, unlike spray coatings where the active material is deposited primarily on the outer surfaces of the carbon fiber tow.
The goal of this study is to demonstrate the benefits of the dip-coating process to evaluate other potential high energy density active material candidates for implementation in structural power composites. More specifically, cathodes were prepared with lithium iron phosphate (LFP) for determining a base-line, lithium cobalt oxide (LCO), lithium nickel cobalt aluminum oxide (NCA), and lithium manganese cobalt oxide (NMC811), cycled and compared to provide an energy density comparison and capacity retention for direct comparison of the materials. All cells were constructed with commercially available separators and LiPF6-based salt organic liquid electrolytes.
2. Materials and Methods
As received T800S-12K-50C carbon fiber (CF), provided by Toray Composites North America (Decatur, AL, USA), was utilized throughout the entire duration of the study. Selected fiber and functional properties are provided in Table 2.

Table 2.
Selected fiber and functional properties of Toray T800S-12K-50C carbon fiber.
Each carbon fiber tow was individually coated by immersion in a slurry bath containing one of four active materials—lithium cobalt oxide (LCO), lithium iron phosphate (LFP), lithium manganese cobalt oxide (NMC811), or lithium nickel cobalt aluminum oxide (NCA). After drying, the coated tows were cut into electrodes and assembled into cathode half-cells. Full cells were then assembled for electrochemical testing and analysis by combining as-received carbon fiber anodes with coated fiber cathodes, following the characterization of the half-cells. 2025-type coin cells were utilized throughout this study with the primary goal of evaluating the electrochemical characteristics of the electrodes. Furthermore, cells were able to be produced expeditiously with multiple cells of each type produced to verify the replicability of results.
2.1. Carbon Fiber Cathode Preparation and Cell Assembly
The reader is encouraged to review Sections 2.1 and 2.2 in Reference [22], a related study that provides a more comprehensive explanation of electrode preparation and the specialized tools created to aid in the coating and handling process. An abridged version is provided here for completeness.
To create CF-based cathodes, a slurry was prepared by mixing one of four active materials, polyvinylidene fluoride (PVDF), 1-methyl-2-pyrrolidone (NMP) solvent, and carbon black (CB) for 24 h at 500 RPM using a benchtop magnetic stirrer (Corning PC-410D) at room temperature. Formulation of the slurry mixture is listed in Table 3 with supporting details of the active materials listed in Table 4. The final weights of individual mixtures ranged between 6 and 8 g. All materials were used as received without modification. The particle sizes of LFP and LCO were not provided in the manufacturer’s documentation and were not measured directly. NCA and NMC were supplied by MTI Corporation (Richmond, CA, USA) with reported particle size ranges of D50 of 13.6 µm and 10.8 µm, respectively.

Table 3.
Mixture ratios for cathode slurry mixture contents weight.

Table 4.
Cathode material used in the slurry mixture.
A set of CF specimens was prepared by cutting and clasping CF tows using 3M 2216 epoxy to create loops measuring approximately 65 mm [2.5 inches] in diameter. After curing, the carbon fiber loops were placed in a tensioning tool applying approximately 2–3 pounds of force to keep the tows taut, preventing tangling and deformation during exposure to the slurry mixture. Just before coating, the cathode slurry was moved from the stirring plate into an aluminum trough, ensuring enough volume to fully immerse the fiber tow. Each carbon fiber sample was then dipped into the slurry for three minutes, removed, and set on a drying rack. The specimens were allowed to pre-dry for three hours at room temperature, followed by transfer to a benchtop vacuum drying oven (Yamato ADP-210C (Santa Clara, CA, USA)) set to 50 °C for a period of 12 h, using the house vacuum. Throughout the drying and pre-drying process, the CF specimens were oriented horizontally.
Once dried, the carbon fibers were removed released from the tensioning tools and trimmed to the desired length. The uncoated portions of the specimens, as well as the epoxy clasps, were then discarded. Each CF tow measures approximately 4.5 mm wide and at least four tows need to be aligned to create the necessary width for punching a 16 mm disk electrode (using the MTI Corporation MSK-T-10 Electrode punch (Richmond, CA, USA)). The punched electrode is then moved to the lower cell can and both are placed inside an argon-filled glovebox (M.Braun UNIlab Pro (Stratham, NH, USA), <1 ppm O2 and H2O) for final assembly. Inside the glovebox, 50 µL of electrolyte (LBC3051C—1M LiPF6 EC/DMC/DEC, 4:2:4 by volume) was applied onto the electrode. Then, a Celgard (Charlotte, NC, USA) 2325 separator (Ø19 mm) and a polypropylene cell gasket are installed. Another 50 µL of electrolyte was added on top of the separator, after which a disk electrode consisting of lithium metal (Sigma-Aldrich (Saint-Louis, MO, USA), Ø16 mm, 0.38 mm thick, 99.9% purity) was installed. The cell assembly was finalized by adding a spacer disk, wave washer, and positive cell case, which were then secured using a manual coin cell crimping tool (Hohsen Corporation, (Osaka, Osaka Prefecture, Japan)). After removal from the glovebox, the cell was allowed to rest for at least 24 h before being placed in the battery tester (Arbin Instruments BT2000 (College Station, TX, USA)). Figure 2 provides a visual summary of cell assembly.
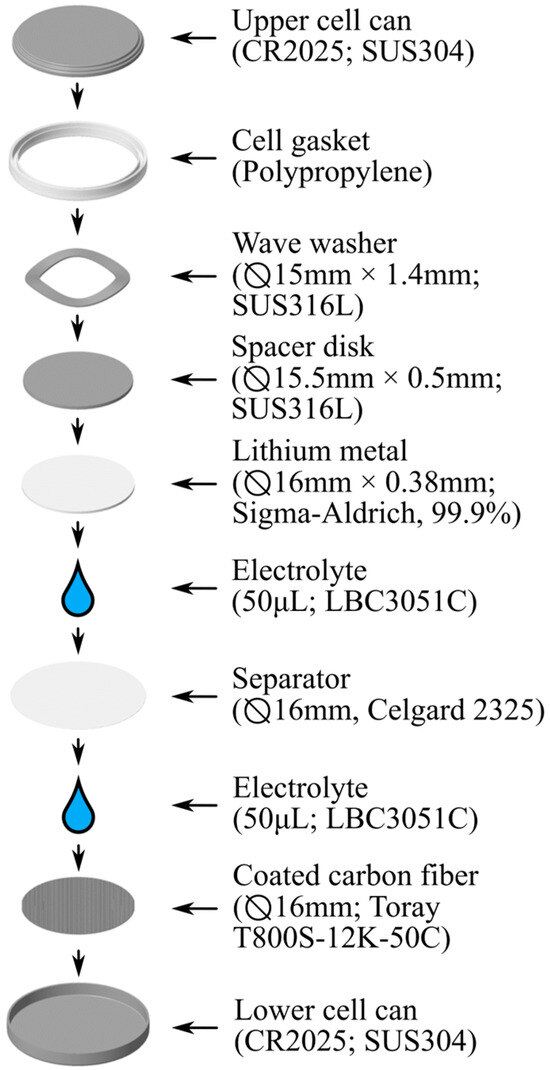
Figure 2.
A flowchart depicting the coin cell assembly for evaluating coated carbon fiber half-cells. Cells are assembled with a lithium metal disk as the counter electrode.
Finally, the four active material coatings underwent examination using a scanning electron microscope (SEM) to visually assess the quality of the coating. Specimen micrographs were captured using secondary electron (SE) detection at 5 kV and a working distance of 10.00 mm. To verify the presence of individual elements in the active materials, energy-dispersive X-ray spectroscopy (EDS) was used.
2.2. Carbon Fiber Full Cell Assembly
To assemble the full cells, the procedure in Section 2.1 of Reference [22] was followed to create a CF anode. The anode was then transferred into the glovebox. 50 μL of electrolyte was then dispensed onto the electrode and a separator was carefully placed above it. Using the transfer jig shown in Figure 3e [22], a carbon fiber cathode was then introduced inside the glovebox and positioned over the separator. An additional 50 μL of electrolyte was placed onto the cathode. The assembly was finalized by installing the remaining components: the cell gasket, spacer disk, wave washer, and upper cell case. The cell was sealed using a crimping tool. After being taken out of the glovebox, the cell was allowed to rest for at least 24 h before being transferred to a battery tester.
2.3. Electrochemical Testing
After assembling the coin cells, they were placed into the coin cell fixtures used with the Arbin MSTAT battery tester (Arbin Instruments, (College Station, TX, USA), 23 mm Coin Cell Battery Holder, (College Station, TX, USA)). Next, cyclic voltammetry (CV) scans were performed on each cell type to determine the suitable range of redox potentials. Starting from the cell’s open circuit potential (OCP) to prevent exposure to high currents, the CV sweeps proceeded toward an upper switching potential at a constant rate, , before reversing direction and sweeping back to a lower switching potential at the same rate. To evaluate the response of the cell after the solid electrolyte interface (SEI) formation, four additional sweeps were performed. The table below summarizes the sweep rate and switching potentials for the four cathode materials in both half-cells and full-cells (Table 5). Following the completion of the CV, the cells were removed and stored.

Table 5.
Potential limits and scan rates used in cyclic voltammetry for different cell types. Each cell’s forward and reverse scans were performed at identical sweep rates.
The CV data was used to establish an appropriate initial range for cycling the different cell types. Subsequent cells were constructed and cycled, with cycling potentials gradually adjusted until satisfactory cell capacity retention was achieved. Table 6 provides a summary of the established potentials as well as the rest period between consecutive charge and discharge cycles.

Table 6.
Summary of cell cycling potentials. A rest period was added to the cycling schedule before each charge and discharge cycle.
The newly born cells underwent cycling based on the limits outlined in Table 6. A galvanostatic cycling profile was employed for five cycles at an initial pilot current. An initial pilot current was applied to the cells using a galvanostatic cycling profile for five cycles. After the fifth cycle was completed, the cycling data was analyzed in real-time to adjust the current and achieve a target rate of C/5 for each individual cell. Cell cycling then proceeded uninterrupted for an additional 50 cycles. After completing all 55 cycles, the cells were collected and set aside. The following Results and Discussion section presents and analyzes the CV and cycling data.
3. Results
3.1. Dip-Coated Fiber-Based Half-Cells
Slurries were prepared by combining conductive carbon black filler, 1-methyl-2-pyrrolidone solvent, an active material, and polyvinylidene fluoride binder in the proportions listed in Table 3. To ensure uniformity, the slurry was thoroughly mixed at room temperature before application. Coating of CF specimens was carried out following the procedure outlined in Section 2, repeating the process until no fewer than five specimens were acquired. One of the four specimens was used for SEM and EDX analysis while the remaining ones were used for producing electrodes.
The carbon fibers, along with their coatings, were inspected via scanning electron microscopy. Figure 3 illustrates the cross-section and surface features of each active material: LFP (Figure 3a,b), LCO (Figure 3c,d), NCA (Figure 3e,f), and NMC (Figure 3g,h). Variations in coating coverage are evident in the micrographs, which reveal exposed fibers in certain sections of the CF tow across its length and width. A noticeable difference in homogeneity can be observed among the coatings, with LFP displaying more uniformity compared to LCO, NCA, and NMC811. This difference may be attributed to the larger particle size distributions of LCO, NCA, and NMC811, as evidenced by the fine granularity of the LFP coating when compared to the others. Different formations are also evident in the micrographs, including fiber-to-fiber bridging and continuous layers firmly attached to individual carbon fibers, covering a large part of their exposed areas. This uneven deposition, including areas where the carbon fiber remains exposed, may reduce effective electrochemical surface area and increase susceptibility to parasitic reactions. Although this study does not directly address the load-bearing capabilities of structural power composites, it is important to recognize that, beyond coating coverage, retaining some exposed carbon fibers is crucial. The solid electrolyte must bond not only to the active material but also to the carbon fibers themselves in order to effectively transfer stresses within the resulting structure. If the active material covers the CF surfaces entirely, the electrolyte will solely adhere to the active material, potentially leading to exfoliation or slippage without leveraging the structural properties of the carbon fibers. Moreover, Figure 3a,c,e,g indicate good penetration into the depth of the CF by the various slurry materials. The three-dimensional distribution of the materials within the carbon fiber tow suggests that dip coating effectively covers the fibers both on the surface and within the carbon fiber tow, ensuring comprehensive coating coverage.
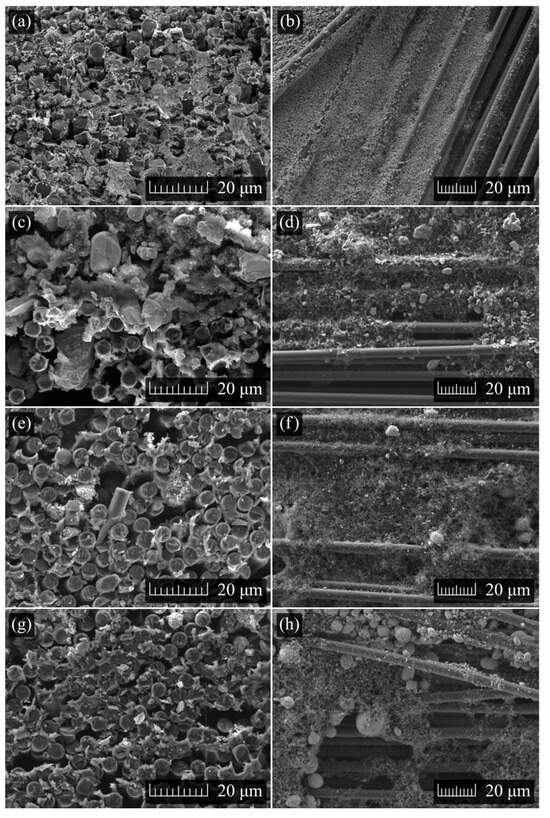
Figure 3.
SEM images: (a,b) cross-sectional and longitudinal LFP-coated fiber sample; (c,d) cross-sectional and longitudinal LCO-coated fiber sample; (e,f) cross-sectional and longitudinal NCA-coated fiber sample; (g,h) cross-sectional and longitudinal NMC-coated fiber sample. 2000× and 3000× zoom were used for all longitudinal and cross-sectional micrographs, respectively.
An energy-dispersive X-ray spectroscopy (EDS) scan was conducted on the surfaces of the samples depicted in Figure 3b,d,f,h to validate the occurrence of the respective elements in the active material. The comprehensive elemental analysis findings, along with the corresponding error margins, are presented in Table 7. It should be noted that the EDS instrument cannot detect elements occurring prior to beryllium on the periodic table, therefore the weight percentage contribution is not provided. The results strongly suggest that the individual constituents incorporated into the slurry were effectively transferred onto the fiber samples for all four active materials. For instance, the EDS analysis of the LFP-coated sample confirmed the presence of oxygen, phosphorus, and iron, as expected. Similar findings were observed for the anticipated constituents of the LCO-, NCA-, and NMC-coated samples.

Table 7.
Energy-dispersive X-ray spectroscopy was conducted on the longitudinal samples shown in Figure 3. The abbreviation ND indicates the absence of an element despite its expected presence, while a dash (-) denotes that an element was not specifically targeted during the search.
After a resting period post-construction, cyclic voltammetry was conducted on the newly assembled half-cells. The cells were swept between 2.5 V and 4.5 V, versus Li/Li+, at a scan rate of = 0.001 V s−1. Figure 4 displays the results of five consecutive cycles obtained from freshly prepared cells, with subfigures a, b, c, and d representing LFP, LCO, NCA, and NMC811 cells, respectively. The symmetry in oxidation and reduction peaks, along with significantly lower redox peaks, immediately distinguishes LFP cells from the others. This discrepancy can be attributed to the olivine crystal structure of LFP, which imparts a unique cyclic voltammetry shape compared to the layered crystalline structure of LCO and the spinel structures of NCA and NMC. Among the four cells, LCO demonstrates a notably high redox peak. Although NCA and NMC exhibit similar profiles, likely due to their shared crystalline structure, they display different redox peaks with NMC exhibiting at a higher potential. Overall, the cyclic voltammetry data suggests favorable reversibility of the intercalation process for the coated CF tows.
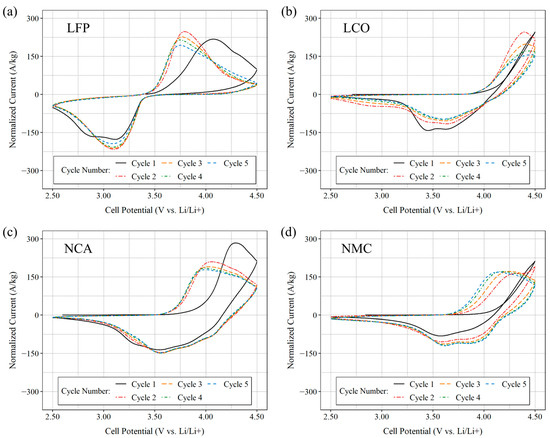
Figure 4.
Cyclic voltammogram plots from half-cells with (a) LFP coating, (b) LCO coating, (c) NCA coating, and (d) NMC coating at a scan rate of = 0.001 V s−1 was applied from 2.500 V to 4.500 V vs. Li/Li+. The half-cells were assembled from coated CF tows and employed a lithium disk as the reference electrode.
Newly born half-cells underwent cycling based on the schedule outlined in Table 5, utilizing a pilot current of I = 350 µA. The first five formation cycles provided data used to calculate the correct current needed to reach a nominal C/5 rate for each individual cell. In Figure 5, the lithiation and delithiation profiles are shown for selected cycles. Cycles one and five represent the first and last cycles of formation that were conducted at the pilot current. From cycle six onwards, each of the four materials (LFP, LCO, NCA, and NMC811) were cycled at a rate of C/5, as depicted in Figure 5a, Figure 5b, Figure 5c, and Figure 5d, respectively.
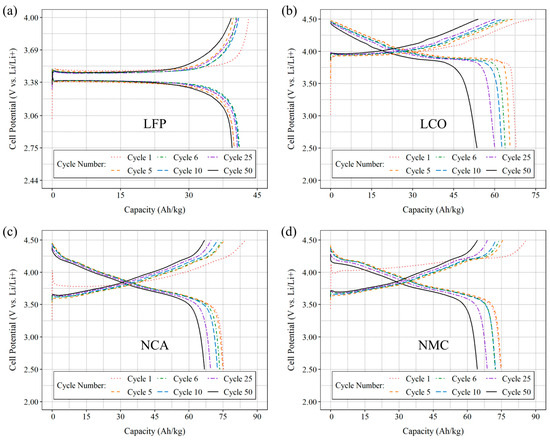
Figure 5.
Lithiation and delithiation cycles were selected and plotted as a function of capacity for half cells consisting of (a) LFP, (b) LCO), (c) NCA, and (d) NMC811. The first five cycles were carried out at a pilot current of 350 µA, followed by an additional 50 cycles at a rate of C/5. The cells were assembled using CF-coated electrodes and a lithium metal counter electrode.
Similar behaviors are observed in the cycling profiles of the electrodes, consistent with the results obtained from the CV sweeps. Specifically, the behaviors of NCA and NMC are similar, contrasting with the behaviors of LCO and LFP. The lithiation and delithiation profiles of the LFP electrode display a flat potential plateau, which is characteristic of the olivine-type structure. This suggests a consistent insertion and extraction of lithium ions across a wide range of capacities [33]. In contrast, the discharge curve of LCO displays a sloping pattern, indicating lattice expansion in the crystal structure as lithium ions intercalate into the material. As more lithium ions are intercalated, the voltage decreases further during the charging process. The curve exhibits an opposite slope during the extraction of lithium ions and eventually plateaus, indicating that most of the lithium ions have been removed. Among the four active materials studied, LCO exhibits the most significant degradation in discharge capacity, likely due to stability issues, resulting in capacity reduction over repeated cycles [34]. In comparison, NCA and NMC exhibit similar responses (Figure 5c,d) during charging and discharging, which can be attributed, in part, to their comparable spinel crystalline structure. Similarly to LCO, during the lithiation process, both NCA and NMC exhibit sloping curves due to the intercalation of lithium ions into the material. NCA contains both nickel and cobalt, both of which participate in the redox reaction [35]. Similarly, in NMC cathodes, nickel, manganese, and cobalt participate in reversible redox reactions. The slopes and plateaus represent the various transition states of the material as lithium ions intercalate and de-intercalate within the crystalline structure. The observed electrochemical behavior of the electrodes during the cycling process serves as compelling evidence supporting their potential for application in structural batteries.
The discharge capacity as a function of cycle number is presented in Figure 6a for each half-cell chemistry. It should be noted that in order to achieve a C/5 rate after five formation cycles, the current was increased for LCO-, NCA-, and NMC-coated electrodes. Conversely, for the LFP-coated cell, the current was decreased. This variation in current is believed to have contributed to the slight decrease in capacity between cycles five and six for LCO, NCA, and NMC, and an increase for LFP. Among the four chemistries, NCA half-cells showed the highest energy density at the end of 50 cycles at C/5, measuring 64.7 Ah kg−1. The NMC811 half-cell demonstrated a similar capacity to NCA, measuring 64.5 Ah kg−1. The LCO half-cell showed the highest capacity fade, measuring 63.9 Ah kg−1 and 50.7 Ah kg−1 at the first and last cycles at C/5, respectively. In contrast, the LFP half-cell demonstrated the least capacity degradation, with measurements of 41.1 Ah kg−1 and 39.8 Ah kg−1 for the same two cycles. Following the completion of the cycling process, cells were disassembled with cathodes removed and dried in a vacuum oven at 50 °C for a period of 24 h. The cell capacity was normalized by the mass of the dry electrode.
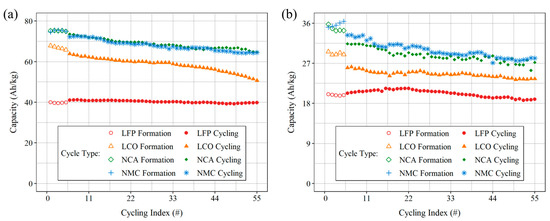
Figure 6.
Discharge capacity plotted as function of cycle index for LFP, LCO, NCA and NMC (a) half cells and (b) full cells. The first five cycles were performed at a pilot current of 350 µA for half cells and 250 µA for full cells followed by an additional 50 cycles at a rate of C/5.
The values for the first and last cycles at a rate of C/5 have been summarized in numerical form in Table 8 below. Experimental findings from half cells demonstrate that substituting NCA for LFP on the cathode side of a structural battery could potentially result in an increase of up to 62.6% in net energy density at 50 cycles. Further advancements are necessary to enhance the energy density and reduce capacity fade in the active material. This can be accomplished, for example, by reducing the size of the active material particles to maximize surface area minimizing macro instabilities within crystalline structures, optimizing the proportions of slurry constituents, and refining the dip-coating procedure through better process control and further refinement.
3.2. Full Cells with Dip-Coated Fibers
Cyclic voltammetry was performed on full cells following a rest period after their construction. The current response from each cell was determined by performing CV sweeps between potentials listed in Table 5. The results of five consecutive cycles are presented in Figure 7, with subfigures a, b, c, and d representing LFP, LCO, NCA, and NMC811 cells, respectively. When comparing the responses of the half cells in Figure 4 to the full cells in Figure 7 it is apparent that the shape of the cathodic reaction is generally maintained, although some phase changes occur at slightly different potentials. However, the anodic reactions show notable differences, as the lithium-ions intercalate into and adsorb to the CF rather than plating onto the lithium metal. In Figure 7a, the results demonstrate a considerable symmetry in the anodic and cathodic reactions, indicating good reversibility of the lithium ions as they transfer between electrodes in LFP cells. At higher potentials, there is a noticeable change in curvature, suggesting the occurrence of adsorption following the saturation of intercalation sites, as also demonstrated in Reference [19] other residual side-reactions. In the CV profiles of the LCO cell, the deposition of cobalt oxide during anodic reactions is evident [36]. This is supported by the blue color observed on the carbon fibers after cell disassembly and the similarity of CV observations of cobalt oxide mentioned in Reference [37]. NCA and NMC cells exhibited similar CV responses. Both cell types showed a slightly blue hue on the anode CF, though less pronounced than the LCO cell, indicating some plating had occurred during anodic reactions. In both NCA and NMC cells, the phase changes of the transition element were less pronounced compared to the respective half-cell plots in Figure 4, indicating a reduced intensity of the redox reaction when a CF anode is used instead of the lithium metal counter electrode. In summary, the cyclic voltammetry data indicates favorable reversibility of the intercalation process and confirms that the coated CFs can function alongside uncoated fibers as opposing electrodes.
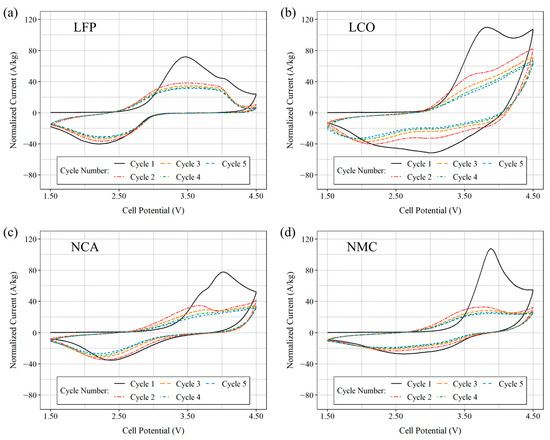
Figure 7.
Cyclic voltammogram plots from full cells with (a) LFP coating, (b) LCO coating, (c) NCA coating, and (d) NMC811 coating at a scan rate of = 0.001 V s−1 was applied from 1.500 V to 4.500 V. The full cells were assembled from coated CF tows on the cathode and bare CFs at the anode.
Full cells of each type were subjected to galvanostatic cycling between the potentials specified in Table 6, with 600 s relaxation periods between consecutive cycles. Figure 8 displays the normalized capacities of selected lithiation and delithiation cycles, with subfigures a, b, c, and d illustrating the behavior of LFP, LCO, NCA, and NMC811 cells, respectively. The first five cycles were performed at a current of I = 250 µA, after which the current was adjusted to achieve a rate of C/5. An additional 50 cycles were then conducted at the same rate.
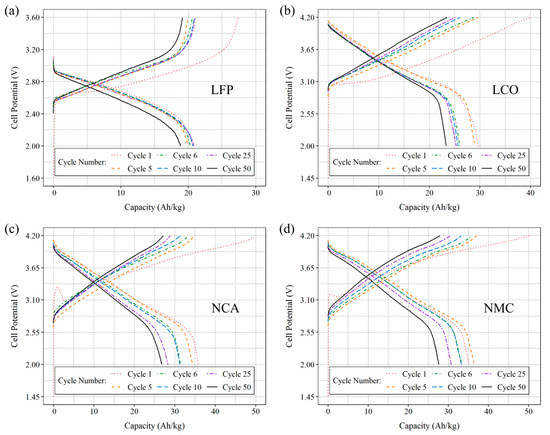
Figure 8.
Lithiation and delithiation cycles were selected and plotted as a function of capacity for full cells consisting of (a) LFP, (b) LCO), (c) NCA, and (d) NMC811. The first five cycles were carried out at a pilot current of 250 µA for formation, followed by an additional 50 cycles at a rate of C/5. The cells were assembled using a coated CF cathode and bare CF at the anode.
It is important to emphasize that full cells incorporating carbon fibers (CF) in both electrodes exhibit distinct profiles compared to the traditional graphite/LFP, LCO, NCA, and NMC811 cells. For instance, the expected flat plateau during the two-phase transition in the LFP electrode is absent. This absence is attributed to the presence of carbon fibers, which exhibit an amorphous hard-carbon behavior, resulting in a higher potential during the intercalation of lithium ions within their structure. This behavior has been previously observed in cells using CFs coated with LFP via the electrophoretic method, as mentioned in References [15,27], as well as in earlier work by the same researchers [22]. The distinction in behavior is likewise observed in LCO, NCA, and NMC811 cells when comparing their responses in Figure 8 to cells with graphite anodes.
Figure 6b presents a summary of the discharge capacity as a function of the cycle number. Among the four full-cell types, NMC811 exhibited the highest energy density at the end of 50 cycles at C/5, with a measurement of 28.2 Ah kg−1. The NCA cell demonstrated comparable performance to the NMC cell, with a capacity of 27.2 Ah kg−1. In contrast, the LFP cell exhibited the lowest capacity degradation, measuring 20.3 Ah kg−1 and 18.9 Ah kg−1 at the first and last cycles at C/5, respectively. Previous work by the same authors yielded a cell capacity of 24.7 Ah kg−1 cycled at C/20 after the completion of 20 cycles for a LFP full-cell [22]. The LCO cell exhibited a significantly lower degree of capacity degradation compared to that observed in the half-cell configuration (Figure 6a). Cells were disassembled following the completion of the cycling process with both electrodes removed and dried in a vacuum oven at 50 °C for a period of 24 h. The cell capacity was normalized by the combined dry mass of both electrodes. Table 8 provides a quantitative overview of the capacities of different chemistries, facilitating a direct comparison between half- and full-cell configurations.

Table 8.
Numerical summary of cell capacities at the first and last of 50 cycles performed at a rate of C/5 for each cell type. For purposes of energy density calculations, the mass of only the CF cathode was used in half cells and both the CF anode and CF cathode in full cells.
Table 8.
Numerical summary of cell capacities at the first and last of 50 cycles performed at a rate of C/5 for each cell type. For purposes of energy density calculations, the mass of only the CF cathode was used in half cells and both the CF anode and CF cathode in full cells.
| Cell Type and Cycle Number: | Lithium Iron Phosphate: | Lithium Cobalt Oxide: | Lithium Nickel Aluminum Cobalt Oxide: | Lithium Nickel Manganese Cobalt Oxide: |
|---|---|---|---|---|
| Half Cell: 1 | 41.1 Ah kg−1 | 63.9 Ah kg−1 | 73.5 Ah kg−1 | 72.2 Ah kg−1 |
| Half Cell: 50 | 39.8 Ah kg−1 | 50.7 Ah kg−1 | 64.7 Ah kg−1 | 64.5 Ah kg−1 |
| Full Cell: 1 | 20.3 Ah kg−1 | 26.0 Ah kg−1 | 31.4 Ah kg−1 | 33.3 Ah kg−1 |
| Full Cell: 50 | 18.9 Ah kg−1 | 23.5 Ah kg−1 | 27.2 Ah kg−1 | 28.2 Ah kg−1 |
By comparison, a commercially available LFP-cathode 18650 format cylindrical cell from a reputable supplier, with a nameplate capacity of 1100 mAh, supplies approximately 52.8 Ah kg−1, whereas the LFP cell in this study provides 18.9 Ah kg−1. It is important to note that in both cases, only the mass contribution of the electrodes and current collectors was considered for an accurate comparison. Similarly, a commercial NCA-cathode 18,650 cell featuring a nominal capacity of 3350 mAh, it delivers roughly 99.9 Ah kg−1 compared to 27.2 Ah kg−1 for the NCA cell in this study. The numerical comparison of the energy densities is summarized in Table 9. The masses of representative LFP and NCA cell components were taken from Golubkhov et al. [38] LFP cell components totaling 20.84 g (anode: 5.18 g, cathode: 9.66 g, aluminum foil: 2.14 g, copper foil: 3.86 g) and 33.52 g (anode: 11.67 g, cathode: 17.93 g, aluminum foil: 1.20 g, copper foil: 2.72 g) for an NCA cell.

Table 9.
Numerical summary comparing energy densities achieved in commercial cells based on cell data provided by Golubkhov et al. [38] to energy densities achieved in this study.
4. Conclusions
This study demonstrates the feasibility of incorporating four commercially available electrochemically active materials onto carbon fibers (CF) for use in structural power composites. Cells were built upon a preceding companion study wherein a custom suite of tools is developed for use in a mechanical dip-coating process for applying active material onto CFs [22]. Here, it was demonstrated that lithium cobalt oxide (LCO), lithium nickel cobalt aluminum oxide (NCA), and lithium nickel manganese cobalt oxide (NMC811) can be successfully applied to CFs using the previously introduced dip-coating method [22] in addition to previous studies that demonstrate application and feasibility of LFP [7,22].
To assess the performance of coated CF electrodes, cyclic voltammetry was conducted to measure the current response and establish suitable limits for galvanostatic cycling. Then, electrodes were subjected to five formation cycles at a pilot current, followed by 50 cycles at a rate of C/5, confirming the efficacy of the electrode materials. The resulting half-cells exhibited energy storage capacities of 39.8, 50.7, 64.7, and 64.5 Ah kg−1 for LFP, LCO, NCA, and NMC811 chemistries, respectively. Additionally, SEM analysis was employed to visually inspect the CF specimens and verify the quality of the coating. The dip-coating process was validated by EDX, which confirmed the transfer of active material onto the CF electrodes. Full cells were then assembled with dip-coated CF cathodes and bare CF anodes, achieving energy storage densities of 18.1, 23.5, 27.2, and 28.2 Ah kg−1 after 50 cycles at C/5.
Further refinement of the process is necessary to enhance energy density and reduce capacity fade in the cells. Suggestions for improvement include reducing the size of active material particles to maximize surface area, enhancing the uniformity of the active material coating through tuning of slurry composition and particle size distribution, optimizing the proportions of slurry constituents, and refining the dip-coating process. These refinements are expected to improve energy density and minimize side reactions associated with exposed carbon surfaces. Further improvements to the energy density can be made by adding a graphite coating to the CF anode with an optimized dip-coating process. Future studies would benefit from incorporating complementary characterization methods, such as X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), to verify the deposition, distribution, and phase composition of active materials on the carbon fiber tow.
Author Contributions
Conceptualization, D.P. and P.T.C.; methodology, D.P.; software, D.P.; validation, D.P., T.B., R.E.W. and P.T.C.; formal analysis, D.P.; investigation, D.P. and T.B.; resources, R.E.W.; data curation, D.P.; writing—original draft preparation, D.P.; writing—review and editing, P.Z., R.E.W., T.B. and P.T.C.; visualization, D.P.; supervision, P.T.C.; project administration, P.T.C.; funding acquisition, P.Z., R.E.W. and P.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
Support for this work was provided in part by the College of Charleston through the NASA South Carolina Space Grant (Proposal Nos. #10010606 and #155100-22-59775), the SmartState Center for Multifunctional Materials and Structures, and internal funding from the University of South Carolina Office of the Vice President for Research (ASPIRE-II) (#155100-21-57397).
Data Availability Statement
The data can be made available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CB | Carbon Black |
| PVDF | Polyvinylidene Fluoride |
| NMP | 1-Methyl-2-Pyrrolidone |
| LFP | Lithium Iron Phosphate |
| LCO | Lithium Cobalt Oxide |
| NCA | Lithium Nickel Cobalt Aluminum Oxide |
| NMC | Lithium Nickel Manganese Cobalt Oxide |
| NMC811 | Lithium Nickel Manganese Cobalt Oxide (8:1:1 composition) |
| SEM | Scanning Electron Microscope |
| EDX or EDS | Energy-Dispersive X-ray (Spectroscopy) |
| EV | Electric Vehicle |
| UAV | Unmanned Aerial Vehicle |
| ARL | Army Research Laboratory |
| PAN | Polyacrylonitrile |
| EPD | Electrophoretic Deposition |
| CV | Cyclic Voltammetry |
| OCP | Open Circuit Potential |
| SEI | Solid Electrolyte Interface |
| CF | Carbon Fiber |
| RPM | Revolutions Per Minute |
| V | Volt |
| GPa | Gigapascal |
| MPa | Megapascal |
| Ω-cm | Ohm-centimeter |
| ND | Not Detected |
References
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Chin, J.; Schnulo, S.L.; Miller, T.; Prokopius, K.; Gray, J.S. Battery Performance Modeling on Sceptor X-57 Subject to Thermal and Transient Considerations. In Proceedings of the AIAA Scitech 2019 Forum, San Diego, CA, USA, 7–11 January 2019. [Google Scholar]
- Asp, L.E.; Mats, J.; Lindbergh, G.; Xu, J.; Zenkert, D. Structural Battery Composites: A Review. Funct. Compos. Struct. 2019, 1, 042001. [Google Scholar] [CrossRef]
- Snyder, J.F.; Carter, R.H.; Wong, E.L.; Nguyen, P.A.; Xu, K.; Ngo, E.H.; Wetzel, E.D. Multifunctional Structural Composite Batteries. In Proceedings of the Society for the Advancement of Material and Process Engineering, Dallas, TX, USA, 6–8 December 2006. [Google Scholar]
- Thomas, J.P.; Qidwai, M.A. The Design and Application of Multifunctional Structure-Battery Materials Systems. JOM 2005, 57, 18–24. [Google Scholar] [CrossRef]
- Ladpli, P.; Nardari, R.; Kopsaftopoulos, F.; Chang, F.K. Multifunctional Energy Storage Composite Structures with Embedded Lithium-Ion Batteries. J. Power Sources 2019, 414, 517–529. [Google Scholar] [CrossRef]
- Asp, L.E.; Johansson, M.; Lindbergh, G.; Xu, J.; Zenkert, D. Structural Power Composites. Compos. Sci. Technol. 2014, 101, 41–61. [Google Scholar] [CrossRef]
- Wetzel, E.D. Reducing Weight: Multifunctional Composites Integrate Power, Communications, and Structure. AMPTIAC Q. 2004, 8, 91–95. [Google Scholar]
- Wetzel, E.D.; O’Brien, D.J.; Snyder, J.F.; Carter, R.H.; South, J.T. Multifunctional Structural Power and Energy Composites for U.S. Army Applications. In Proceedings of the AVT-141 Specialists’ Meeting, Vilnius, Lithuania, 2–4 October 2006. [Google Scholar]
- Snyder, J.F.; Carter, R.H.; Wetzel, E.D. Electrochemical and Mechanical Behavior in Mechanically Robust Solid Polymer Electrolytes for Use in Multifunctional Structural Batteries. Chem. Mater. 2007, 19, 3793–3801. [Google Scholar] [CrossRef]
- Liu, P.; Sherman, E.; Jacobsen, A. Design and Fabrication of Multifunctional Structural Batteries. J. Power Sources 2009, 189, 646–650. [Google Scholar] [CrossRef]
- Ekstedt, S.; Wysocki, M.; Asp, L.E. Structural Batteries Made from Fibre Reinforced Composites. Plast. Rubber Compos. 2010, 39, 148–150. [Google Scholar] [CrossRef]
- Johannisson, W.; Zenkert, D.; Lindbergh, G. Model of a Structural Battery and Its Potential for System Level Mass Savings. Multifunct. Mater. 2019, 2, 035002. [Google Scholar] [CrossRef]
- Shirshova, N.; Qian, H.; Shaffer, M.S.; Steinke, J.H.; Greenhalgh, E.S.; Curtis, P.T.; Kucernak, A.; Bismarck, A. Structural Composite Supercapacitors. Compos. Part A Appl. Sci. Manuf. 2013, 46, 96–107. [Google Scholar] [CrossRef]
- Asp, L.E.; Bouton, K.; Carlstedt, D.; Duan, S.; Harnden, R.; Johannisson, W.; Johansen, M.; Johansson, M.K.G.; Lindbergh, G.; Liu, F.; et al. A Structural Battery and Its Multifunctional Performance. Adv. Energy Sustain. Res. 2021, 2, 2000093. [Google Scholar] [CrossRef]
- Moyer, K.; Boucherbil, N.A.; Zohair, M.; Eaves-Rathert, J.; Pint, C.L. Polymer Reinforced Carbon Fiber Interfaces for High Energy Density Structural Lithium-Ion Batteries. Sustain. Energy Fuels 2020, 4, 2661. [Google Scholar] [CrossRef]
- Kühnelt, H.; Beutl, A.; Mastropierro, F.; Laurin, F.; Willrodt, S.; Bismarck, A.; Guida, M.; Romano, F. Structural Batteries for Aeronautic Applications—State of the Art, Research Gaps and Technology Development Needs. Aerospace 2022, 9, 7. [Google Scholar] [CrossRef]
- Kjell, M.H.; Jacques, E.; Zenkert, D.; Behm, M.; Lindbergh, G. PAN-Based Carbon Fiber Negative Electrodes for Structural Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1455. [Google Scholar] [CrossRef]
- Shellikeri, A.; Watson, V.; Adams, D.; Kalu, E.; Read, J.; Jow, T.; Zheng, J.; Zheng, J. Investigation of Pre-Lithiation in Graphite and Hard-Carbon Anodes Using Different Lithium Source Structures. J. Electrochem. Soc. 2017, 14, A3914. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon Materials for Ion-Intercalation Involved Rechargeable Battery Technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef]
- Hagberg, J.; Maples, H.A.; Alvim, K.S.; Xu, J.; Johannisson, W.; Bismarck, A.; Zenkert, D.; Lindbergh, G. Lithium Iron Phosphate Coated Carbon Fiber Electrodes for Structural Lithium Ion Batteries. Compos. Sci. Technol. 2018, 162, 235–243. [Google Scholar] [CrossRef]
- Petrushenko, D.; Rahmati, Z.; Barazanchy, D.; De Backer, W.; Mustain, W.E.; White, R.E.; Ziehl, P.; Coman, P.T. Dip-Coating of Carbon Fibers for the Development of Lithium Iron Phosphate Electrodes for Structural Lithium-Ion Batteries. Energy Fuels 2022, 37, 711–723. [Google Scholar] [CrossRef]
- Snyder, J.F.; Wong, E.L.; Hubbard, C.W. Evaluation of Commercially Available Carbon Fibers, Fabrics, and Papers for Potential Use in Multifunctional Energy Storage Applications. J. Electrochem. Soc. 2009, 156, A215–A224. [Google Scholar] [CrossRef]
- Hagberg, J.; Leijonmarck, S.; Lindbergh, G. High Precision Coulometry of Commercial PAN-Based Carbon Fibers as Electrodes in Structural Batteries. J. Electrochem. Soc. 2016, 163, A1790–A1797. [Google Scholar] [CrossRef]
- Fredi, G.; Jeschke, S.; Boulaoued, A.; Wallenstein, J.; Rashidi, M.; Liu, F.; Harnden, R.; Zenkert, D.; Hagberg, J.; Lindbergh, G.; et al. Graphitic Microstructure and Performance of Carbon Fibre Li-Ion Structural Battery Electrodes. Multifunct. Mater. 2018, 1, 015003. [Google Scholar] [CrossRef]
- Jacques, E.; Kjell, M.H.; Zenkert, D.; Lindbergh, G.; Behm, M.; Willgert, M. Impact of Electrochemical Cycling on the Tensile Properties of Carbon Fibres for Structural Lithium-Ion Composite Batteries. Compos. Sci. Technol. 2012, 72, 792–798. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Xu, J.; Xia, Z.; Sun, J.; Asp, L.E.; Palermo, V. Electrophoretic Coating of LiFePO4/Graphene Oxide on Carbon Fibers as Cathode Electrodes for Structural Lithium Ion Batteries. Compos. Sci. Technol. 2021, 208, 108768. [Google Scholar] [CrossRef]
- Duygu, Y.; Yucel, D.; Lindbergh, G.; Zenkert, D. Carbon Fiber Based Positive Electrodes in Laminated Structural Li-Ion Batteries. ECS Meet. Abstr. 2020, 983, MA2020-02. [Google Scholar]
- Danzi, F.; Salgado, R.M.; Oliveira, J.E.; Arteiro, A.; Camanho, P.P.; Braga, M.H. Structural Batteries: A Review. Molecules 2021, 26, 2203. [Google Scholar] [CrossRef]
- Bouton, K.; Chen, B.; Zenkert, D.; Lindbergh, G. Structural Positive Electrodes for Multifunctional Composite Materials. In Proceedings of the 22nd International Conference on Composite Materials, Melbourne, Australia, 11–16 August 2019. [Google Scholar]
- Tang, X.; Yan, X. Dip-Coating for Fibrous Materials: Mechanism, Methods and Applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Yadav, A.; De, B.; Singh, S.K.; Sinha, P.; Kar, K.K. Facile Development Strategy of a Single Carbon-Fiber-Based All-Solid-State Flexible Lithium-Ion Battery for Wearable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 7974–7980. [Google Scholar] [CrossRef]
- Li, D.; Zhou, H. Two-Phase Transition of Li-Intercalation Compounds in Li-Ion Batteries. Mater. Today 2014, 17, 451–463. [Google Scholar] [CrossRef]
- Wang, H.; Jang, Y.-I.; Huang, B.; Sadoway, D.R.; Chiang, Y.-M. TEM Study of Electrochemical Cycling-Induced Damage and Disorder in LiCoO2 Cathodes for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1999, 146, 473. [Google Scholar] [CrossRef]
- Flores, E.; Vonruti, N.; Novak, P.; Aschauer, U.; Berg, E.J. Elucidation of LixNi0.8Co0.15Al0.05O2 Redox Chemistry by Operando Raman Spectroscopy. Chem. Mater. 2018, 30, 4694–4703. [Google Scholar] [CrossRef]
- Kumar, R. Review of Cobalt Oxide (CoO) Thin Films Prepared by Various Techniques. J. Phys. Conf. Ser. 2022, 2267, 012002. [Google Scholar] [CrossRef]
- Nakaoka, K.; Nakayama, M.; Ogura, K. Electrochemical Deposition of Spinel-Type Cobalt Oxide from Alkaline Solution of Co2+ with Glycine. J. Electrochem. Soc. 2002, 149, C159. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal Runaway of Commercial 18650 Li-Ion Batteries with LFP and NCA Cathodes—Impact of State of Charge and Overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).