1. Introduction
The global water crisis affects around 3 billion people [
1]. This phenomenon is the result of a combination of anthropogenic and environmental factors. Rapid population growth, combined with increasing urbanization, significantly increases the water demand, particularly for agricultural needs [
2,
3,
4]. In addition, aging and inefficient infrastructure are causing significant losses during water delivery [
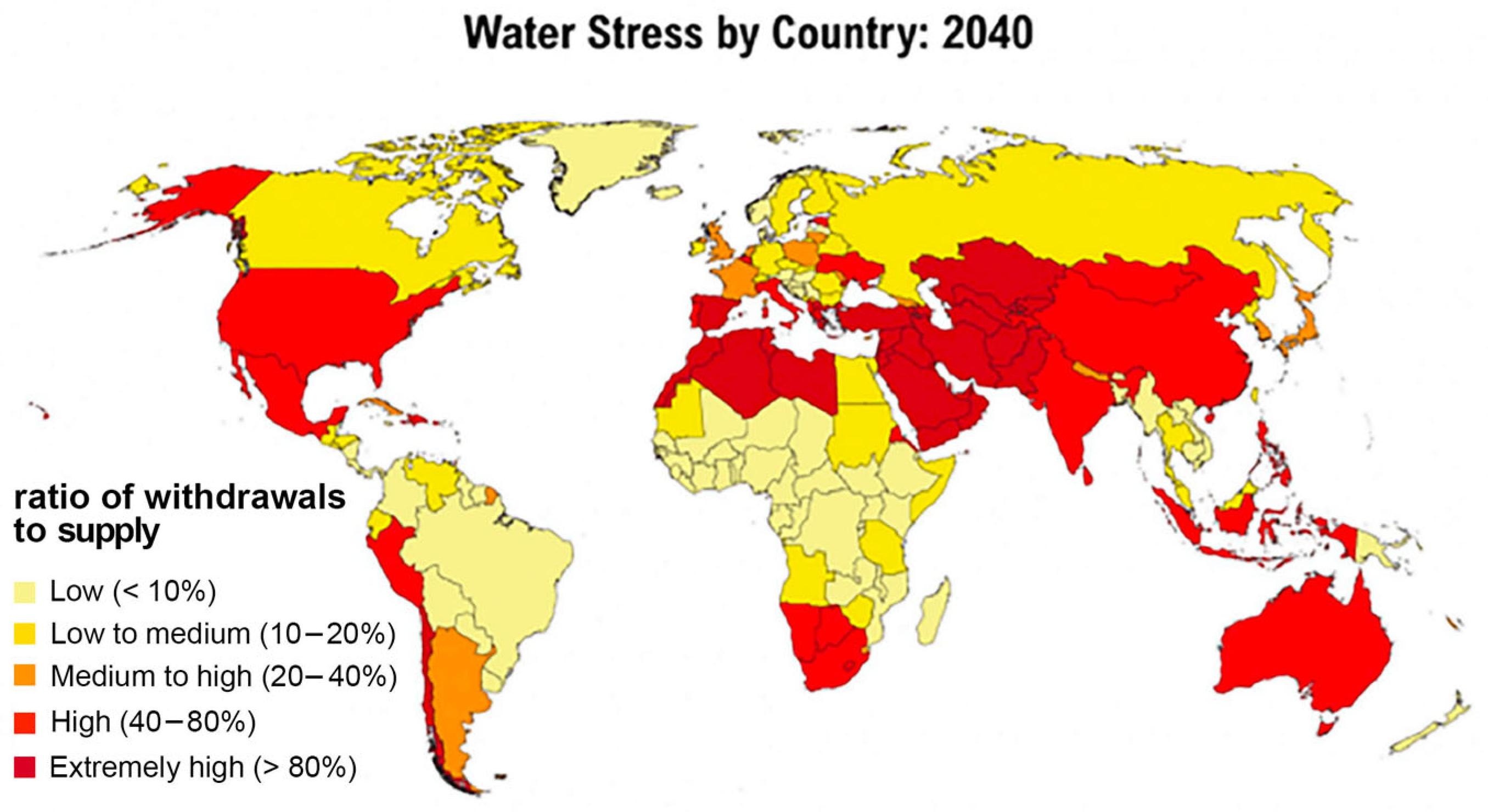
5]. Climate change is worsening the situation, with prolonged droughts and erratic rainfall reducing renewable freshwater resources. The outlook for 2040 is worrying: global water demand could increase by 20–30% due to pressure from population growth and industrialization [
5,
6,
7,
8,
9], as shown in
Figure 1. In many regions, freshwater resources may no longer be sufficient to meet basic needs. In addition, the intensification of extreme climate events could widen the disparities between areas with abundant resources and those already affected by severe water stress [
10]. These projections highlight the urgent need for sustainable, innovative solutions to preserve water resources.
Various techniques have been considered in response to this threat, including seawater desalination [
12,
13,
14,
15], reuse of treated wastewater [
15,
16], and rainwater harvesting. Although effective in producing large quantities of drinking water, desalination is energy-intensive, costly, and has a negative environmental impact due to brine discharge. Wastewater reuse is a sustainable solution, but it requires advanced infrastructure and remains negatively perceived by some populations. Climatic conditions limit rainwater harvesting and prevent its effectively deployed in arid regions.
In this context, atmospheric water harvesting (AWH) stands out as the most promising solution, due to its simplicity, rapid separation, low cost, and easy recyclability of adsorbents [
17]. This technology allows capturing air humidity, a ubiquitous resource, even in semi-arid areas [
18,
19]. AWH can be achieved through fog, dew, and sorbent-based AWH. Fog harvesting is limited by the need for a specific environment, such as mountainous areas where fog is common [
20,
21,
22]. In addition, this technology may be ineffective during dry seasons or when fog conditions are insufficient. Similarly, dew collection, although effective in regions with high temperature amplitude, depends on the temperature difference between day and night, which may make it less reliable in some areas or in summer [
23].
In contrast, AWH using adsorption methods represents a promising alternative to address the growing challenges of water scarcity [
24]. Although freshwater resources are limited and poorly distributed, the atmosphere contains a significant reserve of water in the form of vapor [
25,
26]. This renewable and ubiquitous resource offers a viable opportunity to develop innovative technologies.
Recent scientific advances have made it possible to optimize adsorption techniques by integrating adsorbent materials specially designed to capture moisture from the air efficiently. These materials, such as silica gels [
27], zeolites [
26], and MOFs (metal–organic frameworks) [
28], have porous structures and suitable thermodynamic properties that allow rapid adsorption and controlled release of water during desorption. Based on these different adsorbents, several studies have explored the design of small-scale AWH devices using various adsorbent materials. These systems have proven their efficiency in water vapor adsorption, showing benefits such as better portability, reduced heat and mass transfer losses, and increased water productivity [
29]. In typical cases, these units use sunlight for the desorption process during the day, while adsorption of water vapor from the ambient atmosphere occurs at night when relative humidity is higher [
30,
31,
32]. A pioneering prototype based on MOFs was presented by Kim et al. [
30] in 2017, in which their group developed a water collector that was tested in actual external conditions, achieving a water yield of 0.3 L
water/kg
sorbent. Subsequently, Fathieh et al. [
31] carried out experiments on a solar-powered AWH system in desert environments, making use of 0.825 kg of adsorbent to generate 55 g
water/day. However, these systems, which operate intermittently, face several limitations, notably regarding full utilization of the adsorbent material’s water production capacity.
To surmount these problems and achieve more efficient water harvesting, the researchers focused on several strategies, including optimizing overall system performance [
33,
34,
35]; incorporating solar-powered auxiliary equipment for heating, transporting, and condensing moist air [
36]; and introducing multi-cycle modes of operation. For instance, Li et al. [
34] incorporated a shelter to minimize exposure to solar irradiation during the adsorption process and used a transparent plastic film as a cover and condensation surface during desorption. This design approach enabled their setup to achieve water production of 1.6 kg
water/kg
sorbent during three adsorption/desorption cycles in a day. On the other hand, Lapotin et al. [
35] designed a two-stage unit that exploits the latent heat of condensation in the upper stage to facilitate desorption in the lower stage, yielding 0.77 kg
water/kg
sorbent.
This review aims to examine recent advances in the field of AWH materials and systems, focusing on adsorption mechanisms, adsorbent materials, and the technological challenges involved in maximizing the efficiency of these systems. Special emphasis is also placed on the thermodynamic study of adsorption mechanisms, including equilibrium and adsorption isotherms, which are essential for understanding and modelling material performance. Greater insight into adsorption processes, specific material properties, and their interaction with ambient conditions is fundamental to optimizing the performance of AWH technologies and making them a viable, durable, and scalable solution.
2. Classification of AWH Technologies
This review is structured to provide a comprehensive understanding of atmospheric water harvesting (AWH) using adsorption. It begins with a classification of AWH technologies, highlighting the principles and challenges of each approach. The following section focuses on adsorption-based AWH, detailing the cycle mechanisms and thermodynamic considerations critical to sorption processes. Subsequently, recent developments in adsorbent materials are presented, emphasizing their properties and performance for efficient water vapor capture. The review then addresses the component-level design of adsorption-based AWH systems, discussing engineering aspects that influence system efficiency. Finally, recent advances in solar-powered sorption-based AWH technologies are examined, showcasing innovative designs and operational improvements that enhance water harvesting performance.
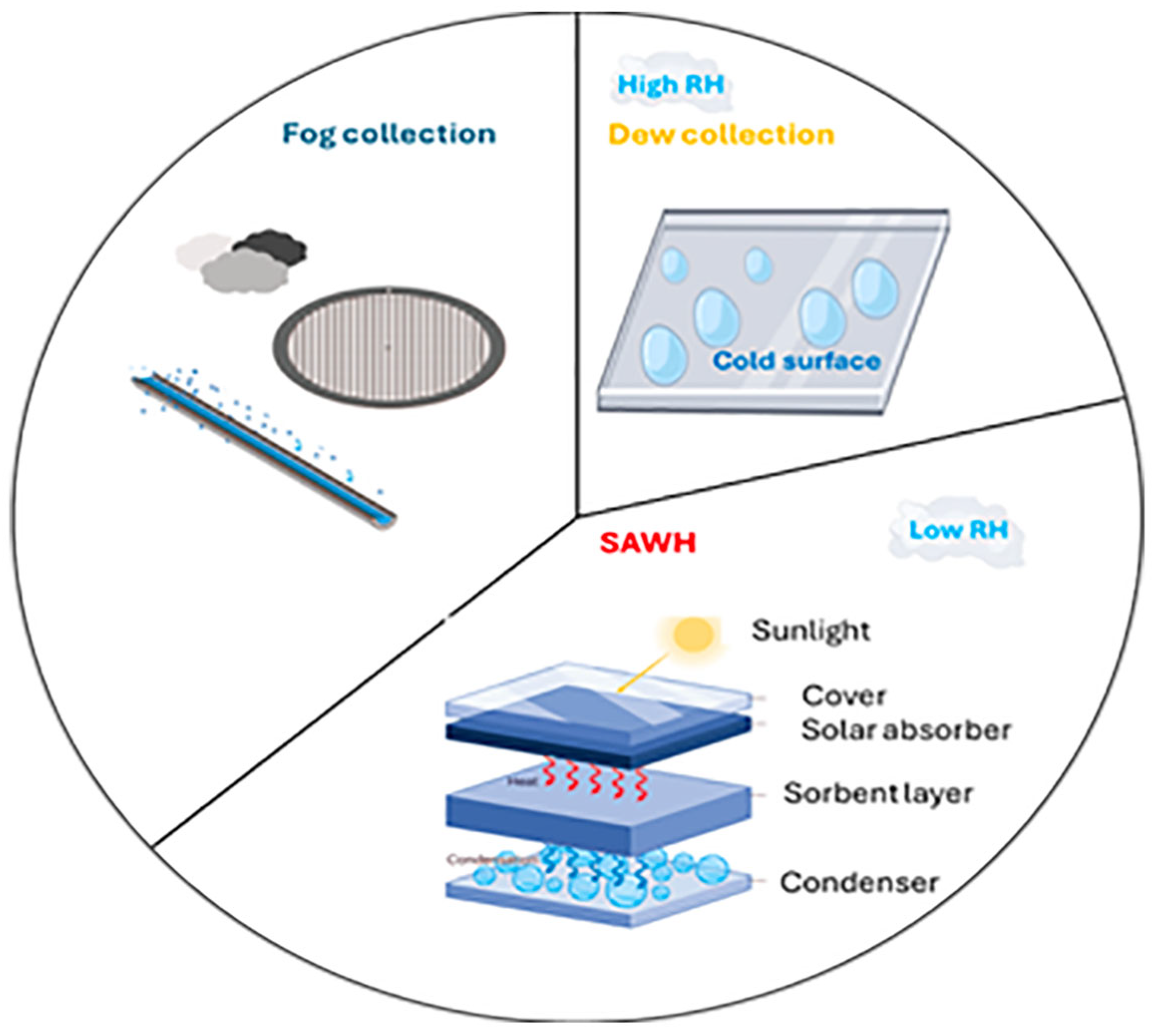
The capture of water from atmospheric vapor is primarily achieved using three distinct methods: fog collection, dew collection, and sorption (see
Figure 2).
The fog harvesting technique uses large nets to capture tiny droplets of water already present in the air in liquid form. The collection of dew water cools the air below its dew point, causing water vapor to condense into a liquid. Lastly, sorption atmospheric water harvesting (SAWH) is a more advanced process that separates the water vapor from the air, enabling various sorbents to operate even when the ambient dew point is below zero. After sorption, the adsorbent is heated, releasing the water vapor into a closed chamber where high local humidity conditions are created, allowing the vapor to condense.
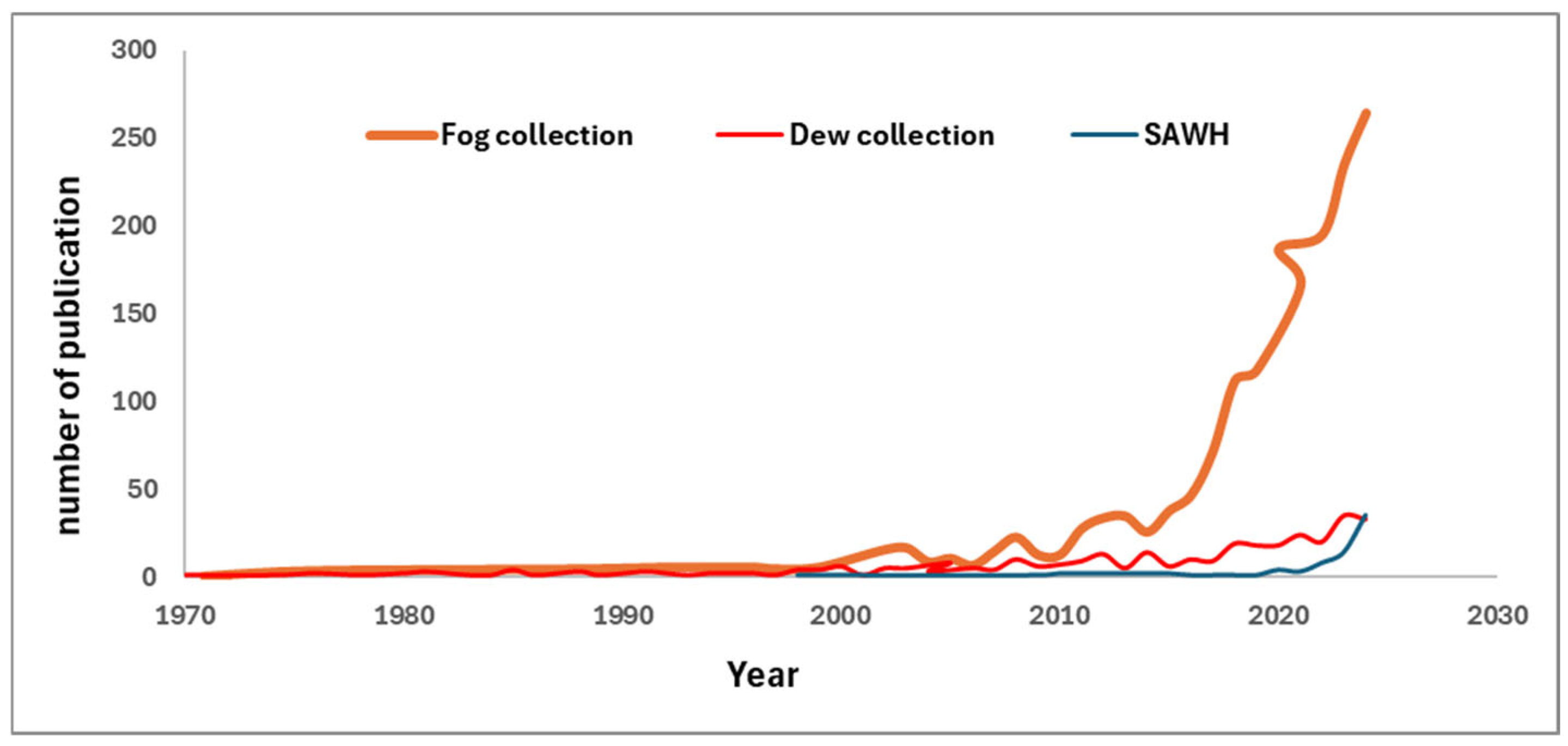
Figure 3 shows the number of papers published on the subjects in the last 30 years. It is possible to notice that there is a constant increase in the number of papers on the topic, indicating an increased interest in the issue. Among the various processes, fog collection has received the highest attention, but the number of papers on SAWH has steeply increased in the last 5 years, being now close to the works on dew collection.
The following sections provide an overview of the various techniques and their main features.
2.1. Fog Collection
Fog water harvesting is a practical and accessible solution to freshwater scarcity, especially in dry coastal climates. The most common approach is to use a rectangular grid perpendicular to the wind direction to capture water droplets in the fog. As the droplets are pushed against the grid by the wind, they coalesce and accumulate. Once they have reached a sufficient size, gravity causes them to fall into a gutter, which directs the collected water to a storage reservoir. The first recorded investigations of this technique were conducted in 1956 by the Catholic University of the North in Antofagasta, Chile [
37]. Subsequently, in 1987, Schemenauer with his group [
38] extended these studies to a larger scale in arid areas, including Namibia, Chile, Peru, Saudi Arabia, and Oman, during the monsoon season. The successful results of these projects have led to the expansion of similar fog-harvesting projects worldwide [
39].
2.2. Dew Water Collection
Dew collection relies on the principle of cooling a surface below the dew point of the surrounding air. When humid air meets this cold surface, the water vapor it contains condenses into droplets that can be collected. This technique is a more efficient way of collecting atmospheric water than fog capture, as it is less reliant on ambient conditions such as climate and terrain. It is also a cost-effective way of replacing precipitation in areas with minimal cloud cover. Initially, research in this field focused on passive radiative cooling as a means of collecting dew, as it operates without an external energy source [
40]. This technique is best adapted to the night, as the sun’s luminosity during the day increases the temperature of the condensation surface, thus reducing the cooling effect. The mechanism behind this relies on the ability of radiative cooling materials to emit thermal energy into deep space, thereby lowering their surface temperature below that of the surrounding air. When a sufficient temperature gradient is established, water vapor condenses on the cooled surface, creating liquid droplets that can then be collected [
41].
2.3. Sorption AWH
Atmospheric water harvesting by sorption is a promising solution to address water scarcity in arid and semi-arid regions [
42]. It relies on materials capable of capturing moisture present in the air and then releasing it in liquid form through controlled heating.
Two main sorption mechanisms enable this water capture: adsorption and absorption. Adsorption involves the adhesion of water molecules onto the surface of porous materials such as, MOFs, zeolites, or silica gels. These materials typically offer high water uptake while requiring relatively low energy for desorption. In contrast, absorption involves the penetration of water molecules into the bulk of the material. However, absorption-based systems often exhibit lower mechanical stability and durability, complicating their long-term application.
Among these two mechanisms, adsorption-based AWH has gained increasing attention due to its superior long-term material stability, mechanical robustness, and suitability for solid-state system integration. The performance of such systems depends on several factors: climatic conditions (temperature and humidity), material properties (water uptake capacity and sorption kinetics), and device design strategies (efficient air-material contact and integration of renewable energy sources for adsorbent regeneration).
Current research efforts focus on developing materials with enhanced selectivity for water vapor, improved durability, and low-energy regeneration, enabling broader adoption of this technology. In the following section, we will focus specifically on atmospheric water harvesting by adsorption, exploring its operating principles, thermodynamic fundamentals, and recent advances in materials and system design.
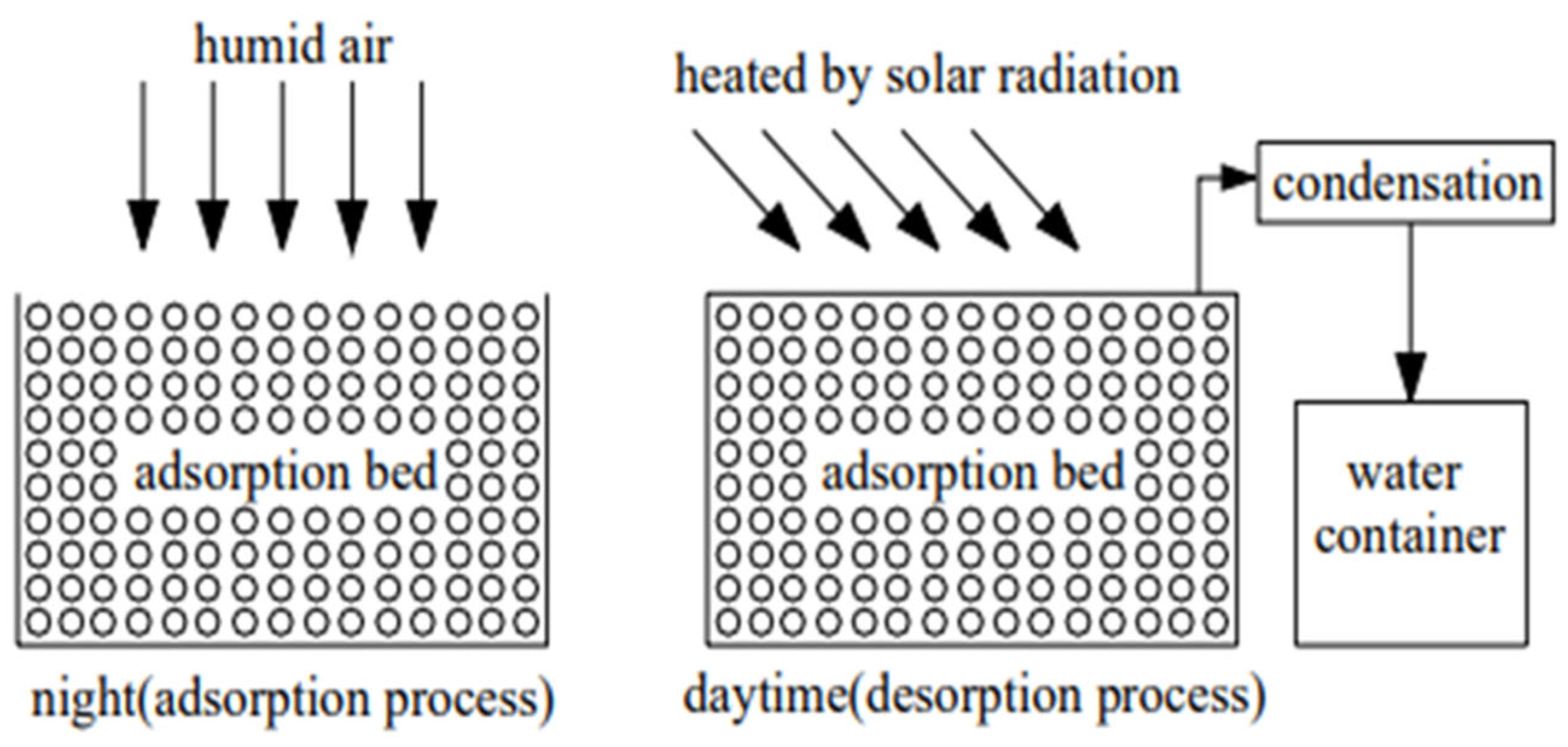
3. Adsorption AWH: Cycle Mechanisms and Thermodynamics Considerations [42]
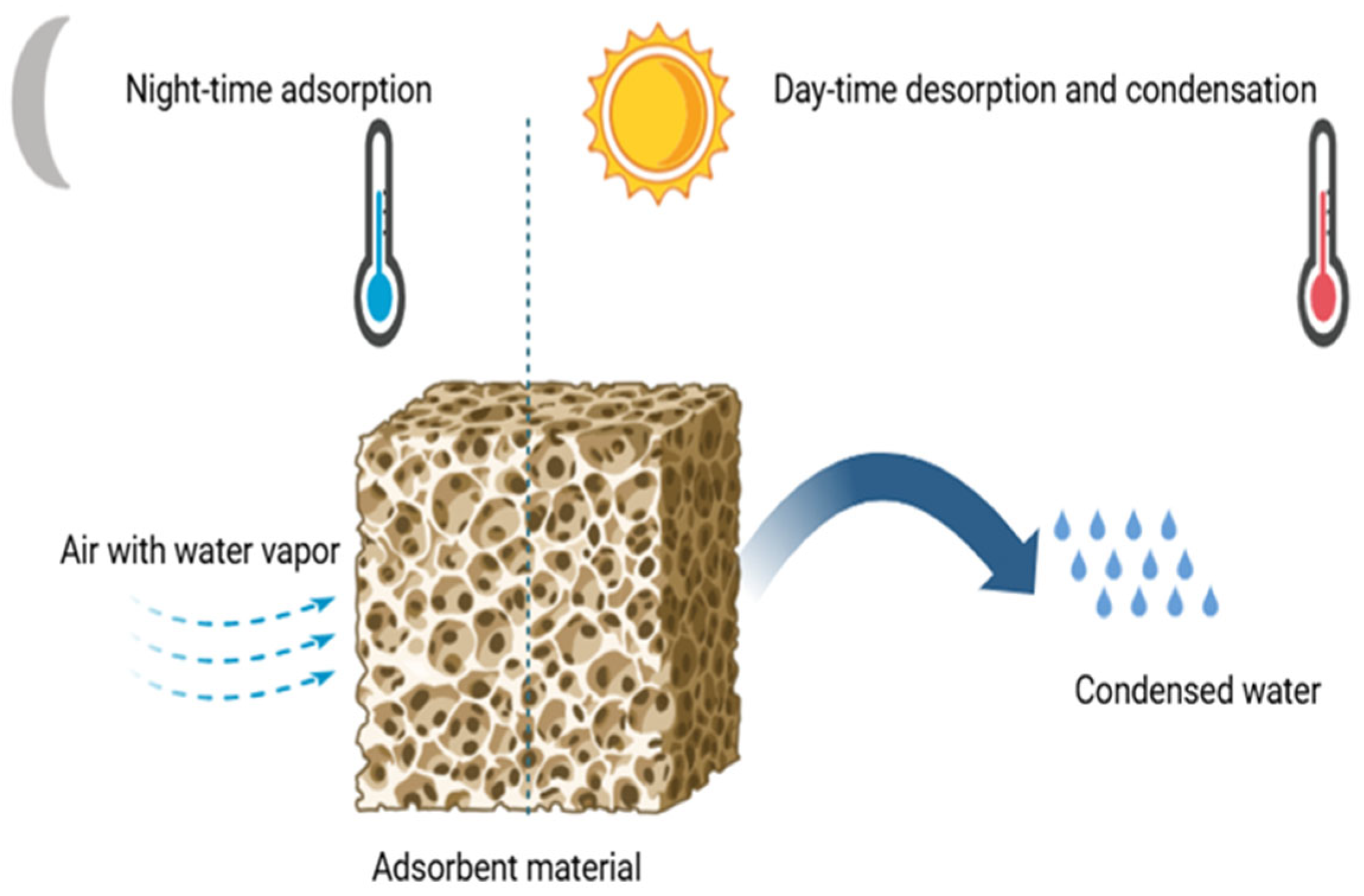
Atmospheric water harvesting by adsorption is essentially a process involving an adsorption–desorption cycling system, in which a sorbent material is used to capture water vapor from the atmosphere and release it by heating [
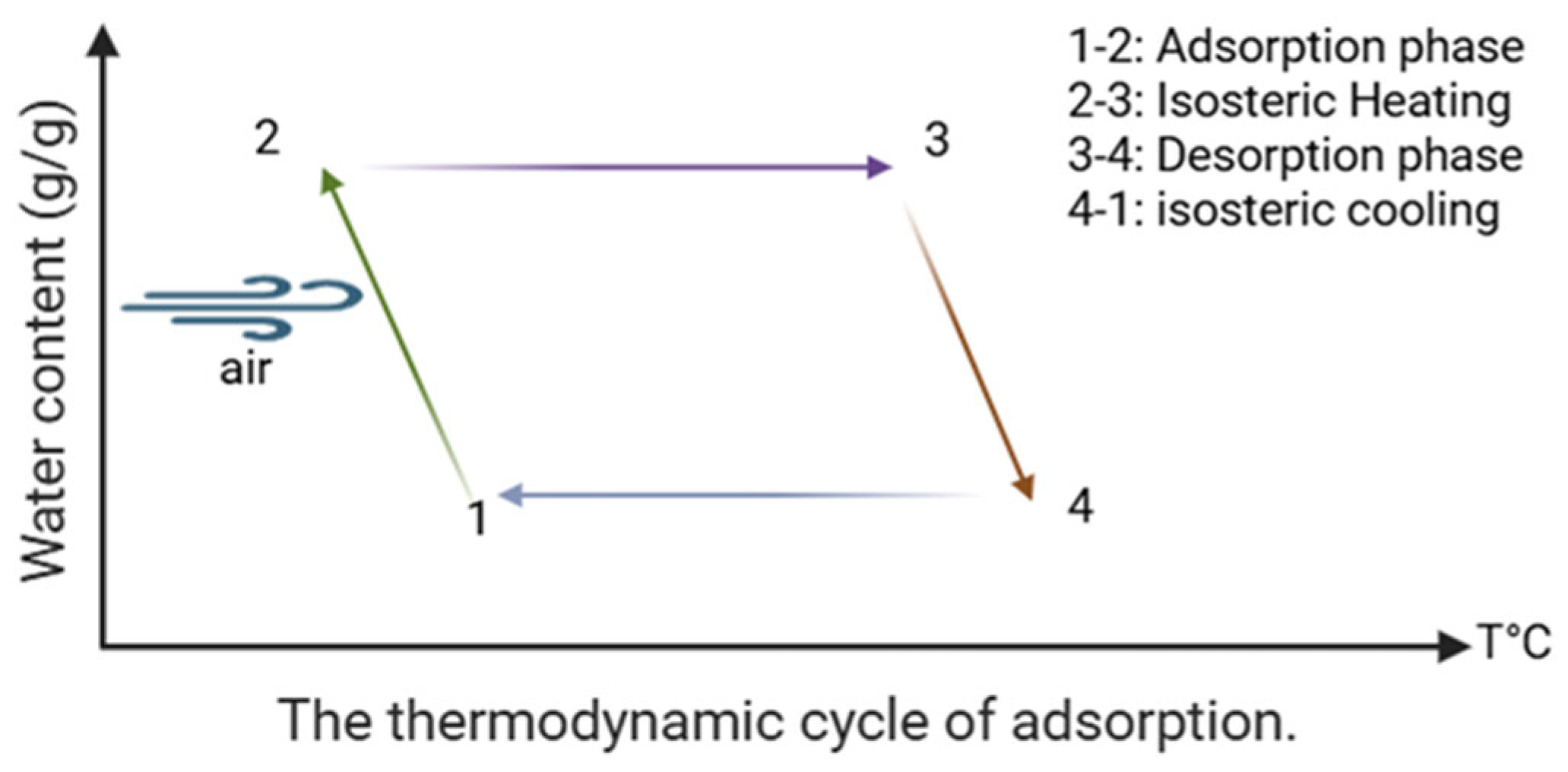
43]. During the adsorption phase, the system uses a cooling source to lower the temperature of the adsorbent material, thus promoting water vapor adsorption while rejecting the exothermic heat generated by the process. In the desorption phase, a heat source is utilized to regenerate the material, causing the release of adsorbed water vapor, which can then be condensed to obtain liquid water, as shown in
Figure 4. This process, relying on the cyclic alternation between cooling and heating phases, enables continuous atmospheric water recovery, even under low-humidity conditions. The system’s efficiency depends on the properties of the adsorbent material, particularly its adsorption capacity, sorption kinetics, and thermophysical properties, which influence the overall performance of the process.
Adsorption-based atmospheric water harvesting systems function through a sequence of thermodynamic transformations that are repeated cyclically. These transformations can be divided into four distinct stages, each contributing to the system’s overall water recovery performance.
Figure 5 offers a schematic representation of this thermodynamic cycle.
The theoretical framework for evaluating such cycles was presented by Kim et al. [
44], who applied the first and second laws of thermodynamics to define the fundamental energy requirements and performance metrics.
The cycle begins with the adsorption step (from state 1 to state 2), where the adsorbent captures water vapor from the surrounding air. As this occurs, the material cools from a higher initial temperature
T1 to a lower final temperature
T2, releasing the adsorption heat into the environment. The thermal energy exchanged during this phase, normalized per kilogram of water collected, is given as follows:
Here, W (kg/kg) refers to the mass ratio of adsorbed water per unit mass of adsorbent, mW (kg) is the quantity of harvested water, and Cp,ad (J/kg/K), Cp,bi (J/kg/K), and Cp,W (J/kg/K) denote the specific heat capacities of the adsorbent, its binder (if present), and the water phase inside the material, respectively. The term had (J/kg) accounts for the heat released during adsorption.
The second stage of the cycle (state 2 to state 3) involves heating the adsorbent while maintaining a constant moisture content in an isosteric process. This heating elevates the temperature from
T2 to
T3, preparing the material for desorption. The associated heat input per kilogram of water is calculated as follows:
Following this, from point 3 to point 4, the desorption phase occurs. The temperature further increases from
T3 to
T4, allowing water molecules to detach from the adsorbent and migrate toward the condenser. The energy consumed during this segment of the cycle includes sensible and latent components and can be modeled as follows:
In this expression, da and db are the specific humidity values at the outlet of the adsorber and condenser, respectively; ωb is the humidity ratio corresponding to point b; Cp,air and Cp,v represent the specific heat capacities of dry air and water vapor, respectively; and Tcond denotes the condensation temperature at which desorbed water vapor transitions back to liquid.
Once desorption is complete, the adsorbent undergoes cooling from point 4 to point 1. This final phase resets the material to its initial state, with its temperature falling from
T4 back to
T1. The heat released during this cooling stage is quantified as follows:
The total energy (
Qreg) required to regenerate the adsorbent across a full cycle excluding auxiliary energy inputs like air circulation is the sum of the energy used during heating and desorption:
To assess the efficiency of the AWH cycle, two key performance indicators are employed: thermal efficiency and second-law efficiency. The thermal efficiency (
ηthermal) expresses the ratio between the useful latent heat of the collected water and the energy required for regeneration:
Here,
hfg is the latent heat of vaporization of water. Second-law efficiency (
ηsecond law), in contrast, evaluates how closely the system approaches the ideal minimum energy required for water extraction:
The theoretical minimum heat input,
QH,min, corresponds to the exergy demand of a perfectly reversible process. Its value, per unit of water extracted, is obtained as follows:
In this final equation, g0, gi, and gW are the Gibbs free energies of the outlet air, inlet air, and liquid water, respectively. di and d0 are the inlet and reference specific humidities. The temperatures T0 and TH represent the ambient and heating source temperatures. The factor () corresponds to Carnot efficiency, setting a thermodynamic upper bound on the heat-to-work conversion.
For AWH applications, an external energy source, often solar or waste heat, is primarily used to initiate the desorption stage, during which adsorbed water vapor is released and subsequently directed to a condenser where it transitions to the liquid phase, mirroring principles observed in thermal desalination systems. The efficiency and viability of such AWH systems are typically evaluated through a set of thermodynamic and kinetic performance indicators. One critical parameter is the adsorption capacity per unit mass of adsorbent Δ
x (kg
water/kg
adsorbent), which is expressed as follows:
This quantifies the amount of water that can be captured and released per cycle and is directly influenced by operating temperatures and the sorbent’s affinity for water. The relative pressure (
RP), defined as the ratio between the partial pressure of water vapor and the saturation pressure at the sorbent temperature, is a dimensionless parameter that determines the driving potential for mass transfer; adsorption becomes favorable at lower RP values:
The water recovery capacity
Mwater (kg) represents the total mass of water extracted in a single cycle and depends on the air humidity, flow rate, and exposure time during sorption:
The recovery rate (
RR) is a normalized metric expressing the fraction of water removed from the incoming humid air stream, providing insight into the efficiency of mass transfer processes:
The specific energy consumption for heating
SECheat (J/kg
water) evaluates the thermal energy input required per unit of water harvested and is a vital metric for comparing energy efficiency across different system designs. It considers the enthalpy change needed to raise the air temperature from ambient to desorption conditions:
The heat required for air regeneration
Wheat (J) quantifies the total thermal load imposed during the desorption phase, which depends on the air’s specific heat capacity (
Cp), the volumetric flow rate, and the temperature difference between inlet and outlet streams.
From a thermodynamic perspective, the system’s overall performance hinges on the balance between the heat input for desorption and the heat released during adsorption, driven by the enthalpy of adsorption (had) and the system’s ability to exploit ambient vapor pressure differences.
Key influencing variables include temperature (T), relative humidity (RH), water vapor partial pressure (Pv), and saturation pressure (Psat), all of which determine the extent of sorption under varying atmospheric conditions. Additionally, the moisture content of the air at specific process points, namely, dcond, ddesorber,I, and dsorber,o, is essential for calculating RR and SEC, thereby characterizing the net productivity of the system. Time-dependent variables such as tadsorption and tdesorption further affect the system’s dynamic response and throughput. Understanding the interplay among these parameters allows for the rational optimization of AWH technologies aimed at maximizing water yield while minimizing energy consumption.
The performance of adsorption-based atmospheric water harvesting (AWH) systems strongly depends on the choice of adsorbent material. Indeed, the water vapor capture capacity, adsorption kinetics, and ease of thermal regeneration vary significantly from one material to another. This diversity in behavior makes the selection of the adsorbent crucial for adapting the system to specific climatic conditions and available energy constraints. In the following section, we review the most commonly used materials in adsorption-based AWH, with a focus on their dynamic performance, adsorption isotherms, and sorption equilibrium characteristics.
4. Recent Developments in Adsorbents for Use in AWH
In an atmospheric water harvesting system by adsorption, the selection of the adsorbent material is a key factor in determining performance [
45]. Various types of sorbents are commonly used for this purpose, including conventional nanoporous solids, advanced polymers, MOFs, hydrogels, salt-based composites, and liquid sorbents [
46]. A summary of their respective properties is provided in
Table 1. The choice of an appropriate adsorbent is guided by several critical factors (see
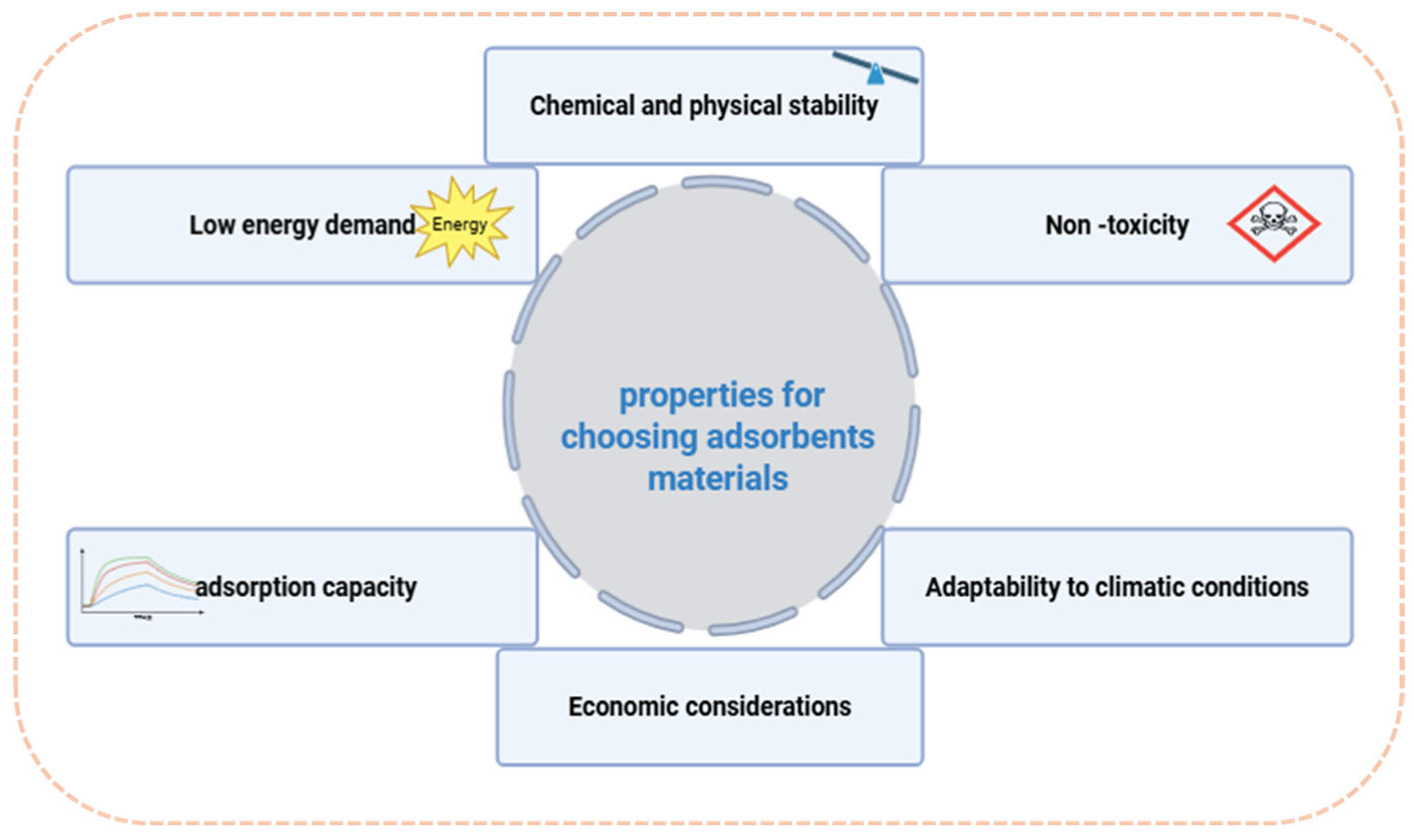
Figure 6). First, the material must exhibit a high water uptake capacity to effectively capture and retain moisture from ambient air. Second, the adsorption and desorption kinetics play a crucial role, as faster cycles enhance overall system efficiency. The chemical and structural stability of the material is also essential to ensure long-term durability without significant degradation. Additionally, the adsorbent must be non-toxic and safe for use in contact with potable water. Another key consideration is its ability to function efficiently under varying climatic conditions, adapting to fluctuations in temperature and humidity. Finally, economic factors, including production costs and material availability, influence large-scale feasibility and widespread adoption.
Nanoporous solids, such as silica gels, zeolites, and activated carbons, are extensively investigated for their vapor adsorption abilities because of their high specific surface area and porous structures. Silica gel, while low-cost and commercially available, has limited adsorption capacity, especially at low humidity. Zeolites, on the other hand, exhibit high water adsorption capacity but require high temperatures for regeneration. Activated carbons, despite their high porosity, suffer from low water absorption due to their hydrophobic nature.
More innovative materials, such as metal–organic frameworks (MOFs) and covalent organic structures (COFs), offer improved performance, particularly in terms of specific surface area and adaptability to a variety of conditions. However, their use is often constrained by issues related to hydrolytic stability and structural integrity. MOFs, for example, can demonstrate diverse adsorption mechanisms, but their high cost and poor thermal conductivity limit their practical applications.
Hydrogels, water-swollen polymers, present a competitive alternative with high water retention capacity and low energy requirements for regeneration. However, their slow water sorption kinetics and mechanical stability remain challenges. Hygroscopic salts, such as LiCl, while efficient in water adsorption, face issues like corrosion and leakage, leading to the development of composites incorporating these salts into porous matrices.
Lastly, liquid sorbents, such as saline solutions and ionic liquids (ILs), offer high sorption capacity with relatively low regeneration temperatures but suffer from slow kinetics, requiring large surface areas for practical applications. Developing biodegradable and high-performance sorbents for arid conditions is gaining more attention, with promising examples of new materials derived from biomass and agricultural waste. These new sorbents offer significant potential for atmospheric water harvesting (AWH) technologies while minimizing environmental impact.
The performance of adsorption-based atmospheric water harvesting (AWH) systems strongly depends on the choice of adsorbent material. Indeed, the water vapor capture capacity, adsorption kinetics, and ease of thermal regeneration vary significantly from one material to another. This diversity in behavior makes the selection of the adsorbent crucial for adapting the system to specific climatic conditions and available energy constraints. In the following section, we review the most commonly used materials in adsorption-based AWH, with a focus on their dynamic performance, adsorption isotherms, and sorption equilibrium characteristics.
4.1. Adsorbent Kinetic Performance
The term “adsorbent kinetic performance” refers to the rate at which adsorption and desorption processes occur, which has a significant impact on the performance of atmospheric water harvesting systems. A commonly adopted approach to enhance these processes involves increasing the desorption temperature, thereby promoting faster release of water vapor [
30]. However, this method also results in higher energy consumption. Consequently, enhancing the inherent kinetic properties of the adsorbent material itself becomes essential to improve system performance without raising energy demands.
As
Figure 7 shows, several parameters influence the dynamic process of the adsorbent itself and its performance.
This section discusses how to improve the kinetics of adsorption/desorption processes.
Enlarging the pore size of adsorbent materials is an effective strategy to facilitate the transport of water molecules, thereby enhancing both adsorption and desorption rates. For instance, hollow MIL-101(Cr) spheres exhibit improved water adsorption kinetics compared to the powdered form of the same material, as water molecules can more easily access the active sites [
56]. This hollow structure, characterized by the presence of macropores, led to an increase in adsorption and desorption rates by approximately 1.7 times (0.013 g·g
−1·min
−1) and 1.9 times (0.0098 g·g
−1·min
−1), respectively. Additionally, constructing a hierarchical pore architecture within the adsorbent has proven to be another promising approach to enhance the kinetic performance, taking advantage of the macropores’ ability to enable rapid mass transfer [
57].
Moreover, the kinetic process can be further accelerated by optimizing the packing density and crystal size of the adsorbent [
58]. For instance, low packing density and small crystal size promote faster molecular movement.
Recent studies have also successfully demonstrated the rapid transport of water molecules in nanoconfined channels, leading to a rapid kinetic process. Zhu et al. obtained porous carbon materials with nanochannels with a moderate pore size (0.85 nm) and an appropriate hydrophilic groups density (40%) for rapid water transport [
36]. Based on theoretical simulation, it was shown that 40% of the hydrophilic sites density has the lowest water diffusion barrier. In addition, ~1.1 nm pore size also has low barrier to water diffusion, ensuring appropriate RH for the application. Consequently, the customized porous carbon exhibited fast kinetics (adsorption rate constant is 0.071 min
−1).
While adsorption kinetics determine how rapidly a material can capture moisture from the air, this performance must be complemented by a thorough understanding of the material’s behavior at equilibrium. In fact, even a fast-responding adsorbent may prove inefficient if its equilibrium capacity is too low under typical ambient conditions. For this reason, adsorption isotherms and equilibrium properties must be examined in parallel with kinetic performance, as they define the maximum amount of water a material can retain at a given humidity and temperature. The following section explores these equilibrium aspects in detail.
4.2. Regeneration Temperature and Energy Demand
When selecting a sorbent for water vapor adsorption, several crucial properties must be considered. The material should exhibit a low saturation vapor pressure, a highly porous structure, and must be resistant to both thermal and chemical degradation, while also maintaining mechanical stability throughout its operational life. Silica gel is a commonly used example, with an adsorption heat close to 2600 kJ/kg, and it can be regenerated at relatively low temperatures starting around 55 °C [
59]. In contrast, zeolites demonstrate strong water uptake due to their high affinity for water molecules; however, they typically require regeneration temperatures around 200 °C [
60], which limits their use with low-temperature energy sources. Nevertheless, zeolites remain cost-effective as they can be produced using inexpensive processes.
A major challenge in atmospheric water harvesting (AWH) is finding sorbents that can undergo multiple regeneration cycles without significant performance loss, while minimizing the energy consumption required for regeneration. Traditional adsorbents tend to be more energy-intensive than more recent materials, such as metal–organic frameworks (MOFs), primarily due to stronger water-sorbent interactions that hinder easy desorption. As a result, considerable efforts have focused on developing materials that can be regenerated using low-grade heat or even solar energy. Bilal et al. [
61] reported that MOF-801 retained stable performance over five adsorption–desorption cycles, with regeneration temperatures ranging from 48 °C to 85 °C. Similarly, MOF-303 maintained its maximum adsorption capacity consistently across multiple cycles. These MOFs show great promise for AWH, as they allow water recovery at lower thermal input compared to conventional adsorbents like silica gel and zeolite.
However, some MOFs require very high regeneration temperatures due to the strong interactions between water molecules and the material’s internal structure. For example, MOF-74 features open metal sites that tightly bind water, necessitating regeneration temperatures as high as 190 °C [
62]. This high thermal demand makes MOF-74 less suitable for systems powered by low-grade or solar heat, thus limiting its applicability in energy-efficient and sustainable AWH technologies.
4.3. Adsorbent Cost
The economic viability of atmospheric water harvesting systems is closely tied to the cost of the sorbent materials used, as these costs can influence both capital investment and operating expenses. High-performance adsorbents like MOFs often come with high production costs, which can hinder their practical deployment, especially in large-scale or resource-limited settings [
63]. Moreover, while MOFs exhibit impressive water uptake capacities, their optimal performance is often restricted to specific relative humidity ranges, which may reduce their adaptability in diverse climates. In contrast, hybrid materials such as ACF–LiCl composites have demonstrated robust performance under various environmental conditions and offer a more financially sustainable option for AWH applications. Thus, when selecting materials, it is essential to weigh not only the adsorption efficiency but also the overall cost and scalability potential. Although MOFs are increasingly investigated for large-scale uses due to their structural versatility and sorption properties, their limited commercial availability remains a challenge. Chakraborty et al. [
64] examined strategies for the industrial production of MOFs, emphasizing the importance of cost reduction, environmental safety, and synthesis feasibility. Their study highlights the potential of aluminum-based MOFs for commercial production.
4.4. Stability and Durability
Ensuring that adsorbent materials maintain their performance over multiple cycles and under varying environmental conditions is fundamental for the sustainability of adsorption-based water harvesting systems. Although metal–organic frameworks (MOFs) stand out due to their tunable porosity, high surface area, and structural flexibility qualities that make them highly attractive for atmospheric water harvesting, their susceptibility to hydrolytic degradation presents a significant obstacle in real-world applications. As highlighted by An et al. [
65], the stability of MOFs in humid environments is largely influenced by factors such as the robustness of the metal–ligand coordination bonds and the chemical nature of the organic linkers. To address this, the study emphasizes the importance of developing frameworks with stronger coordination chemistry and selecting components that are more resistant to hydrolysis. Such approaches can significantly enhance the resilience of MOFs, enabling their reliable use across diverse conditions and repeated adsorption–desorption cycles, thereby improving the practicality and longevity of AWH technologies.
To conclude, selecting suitable sorbents for atmospheric water harvesting requires a careful balance between adsorption capacity, regeneration temperature, material stability, and cost. While advanced materials such as MOFs offer promising performance at low regeneration temperatures, their high production costs and sensitivity to moisture can limit their practical application. On the other hand, conventional materials like silica gel and zeolites are more affordable and durable, but often require higher energy input for regeneration. To facilitate comparison and highlight the strengths and limitations of different materials,
Table 2 provides a summary of key characteristics across some adsorbents examined in this review.
4.5. Environmental Safety and End-of-Life Considerations for Sorbents
Beyond adsorption performance and regeneration efficiency, recent studies emphasize the importance of environmental safety and end-of-life management of sorbent materials used in atmospheric water harvesting (AWH), particularly for advanced classes such as metal–organic frameworks (MOFs) and salt-based composites. These materials, although promising in terms of water uptake and low-temperature regeneration, may raise concerns related to toxicity, degradation, and recyclability. In terms of water quality, several experimental investigations have confirmed that specific sorbents can provide potable water that complies with World Health Organization (WHO) standards. Entezari et al. [
67], for instance, used inductively coupled plasma mass spectrometry (ICP-MS) to analyze water collected from an AWH system utilizing a CFA–LiCl hydrogel. The results revealed that the concentrations of key ions such as Na
+, K
+, Ca
2+, and Mg
2+ were significantly below the WHO thresholds, and lithium levels remained at a safe concentration of 0.2 mg/L. Similarly, Lyu et al. [
68] evaluated water collected using hydrogel-based sorbents and confirmed that both heavy metal concentrations (e.g., Pb
2+, Al
3+) and total organic carbon (TOC) levels were within acceptable limits. These findings support the short-term safety of certain sorbents under controlled regeneration conditions. However, environmental safety must also consider how operating parameters, particularly desorption temperature, influence the release of residual compounds. It has been observed that higher regeneration temperatures can accelerate water vaporization while increasing the risk of ionic or molecular carryover into the condensed water. For example, Lyu et al. [
68] reported that desorption at temperatures between 60 °C and 70 °C yielded safe water, whereas excessive temperatures led to a higher ionic concentration in the output. End-of-life considerations are especially relevant for MOFs and hygroscopic salts. While some MOFs like MOF-801 and MOF-303 demonstrate stability over multiple cycles with relatively low regeneration temperatures, others, such as MOF-74, require high desorption temperatures (up to 190 °C) [
62] and may release metal ions over time due to framework degradation. This raises concerns about the environmental impact of disposal or uncontrolled leaching. Salt-based sorbents, on the other hand, may dissolve or undergo deliquescence under high humidity, complicating their reuse and disposal.
4.6. Climatic Adaptation and Standardized Evaluation of Adsorption-Based AWH Systems
The selection of adsorbent materials for atmospheric water harvesting (AWH) systems is strongly influenced by ambient relative humidity (RH), owing to the diverse shapes of adsorption isotherms. These isotherm profiles, whether linear, exponential, or sigmoidal, determine the material’s adsorption behavior under fluctuating environmental conditions [
69]. Because no single adsorbent can respond optimally across the full RH spectrum, variations in humidity can significantly impact net water yield. Therefore, understanding the local climate, particularly air temperature, humidity, and theoretical atmospheric water availability, is a fundamental design step [
70].
Recently, materials featuring moderate stepwise isotherms (centered around 40% RH) or linear profiles have garnered interest due to their ability to operate efficiently in low-humidity environments with moderate energy consumption [
69]. For example, MOF-801(Zr) and MOF-303(Al), which exhibit adsorption steps at 0.05–0.15 and 0.1–0.25 P/P
0, respectively, demonstrate excellent performance in arid regions [
71]. However, certain adsorbents like MOF-801 are highly climate specific, showing optimal performance only under extremely hot and dry desert conditions [
72].
Conversely, other materials such as Cr-soc-MOF-1, MCM-41, and silica gel exhibit high adsorption capacities under more humid conditions, with maximum uptakes of 1.95, 0.8, and 0.5 g/g, respectively. Adsorbents that display stepwise isotherms at higher RH values have shown significant energy-saving potential in temperate climates, particularly at 25 °C with RH levels of 20–70% and at 30 °C with RH levels of 15–55% [
69]. Nevertheless, their performance tends to decline under harsher conditions, such as at 40 °C and RH between 20 and 30%. Additionally, mesoporous materials modified with hygroscopic salts, like MCM-41 + LiCl, present nearly linear isotherms and exhibit more consistent performance across a wide temperature and humidity range.
When equipped with appropriate thermal management systems, such as heat sinks or thermal reservoirs, active adsorption-based AWH systems can surpass the efficiency of conventional refrigeration-based methods. This advantage is particularly evident in low-humidity, low-temperature/high-humidity, and high-temperature/moderate-humidity regions [
69]. The inherent water-enrichment capability of advanced adsorbents further enhances system output, especially under high water demand scenarios. Consequently, except for tropical coastal zones, adsorption-based AWH appears to be a robust and energy-efficient solution for freshwater production across diverse climatic settings.
Given the strong influence of environmental parameters on adsorption system performance, there is a growing need to adopt standardized testing protocols and common key performance indicators (KPIs). Such measures would ensure consistency and reliability when comparing materials and system designs across studies. Moreover, integrating long-term and site-specific climate data into the design and evaluation process is essential. Tools such as the Photovoltaic Geographical Information System (PV-GIS) can provide detailed data on annual solar irradiance and long-term solar trends, while databases like the Renewable Atmospheric Parameters Statistics (RAPS) and the Integrated Precipitation and Temperature Analysis (IPTA) can be used to assess local air moisture, temperature fluctuations, and precipitation levels. Employing these resources is critical for ensuring that AWH systems are optimally tailored to local environmental conditions and for enabling reproducible and meaningful performance benchmarking at both material and system levels.
4.7. Adsorption Isotherms and Equilibrium Behavior
Over the years, extensive research has been conducted on a wide range of adsorbents, including silica gel [
73,
74], zeolite [
75,
76], activated alumina [
77,
78], polymer and carbon-based adsorbents [
79,
80,
81]. These materials exhibit different adsorption mechanisms, pore structures, and affinities toward water vapor, which influence their overall efficiency in capturing and releasing moisture.
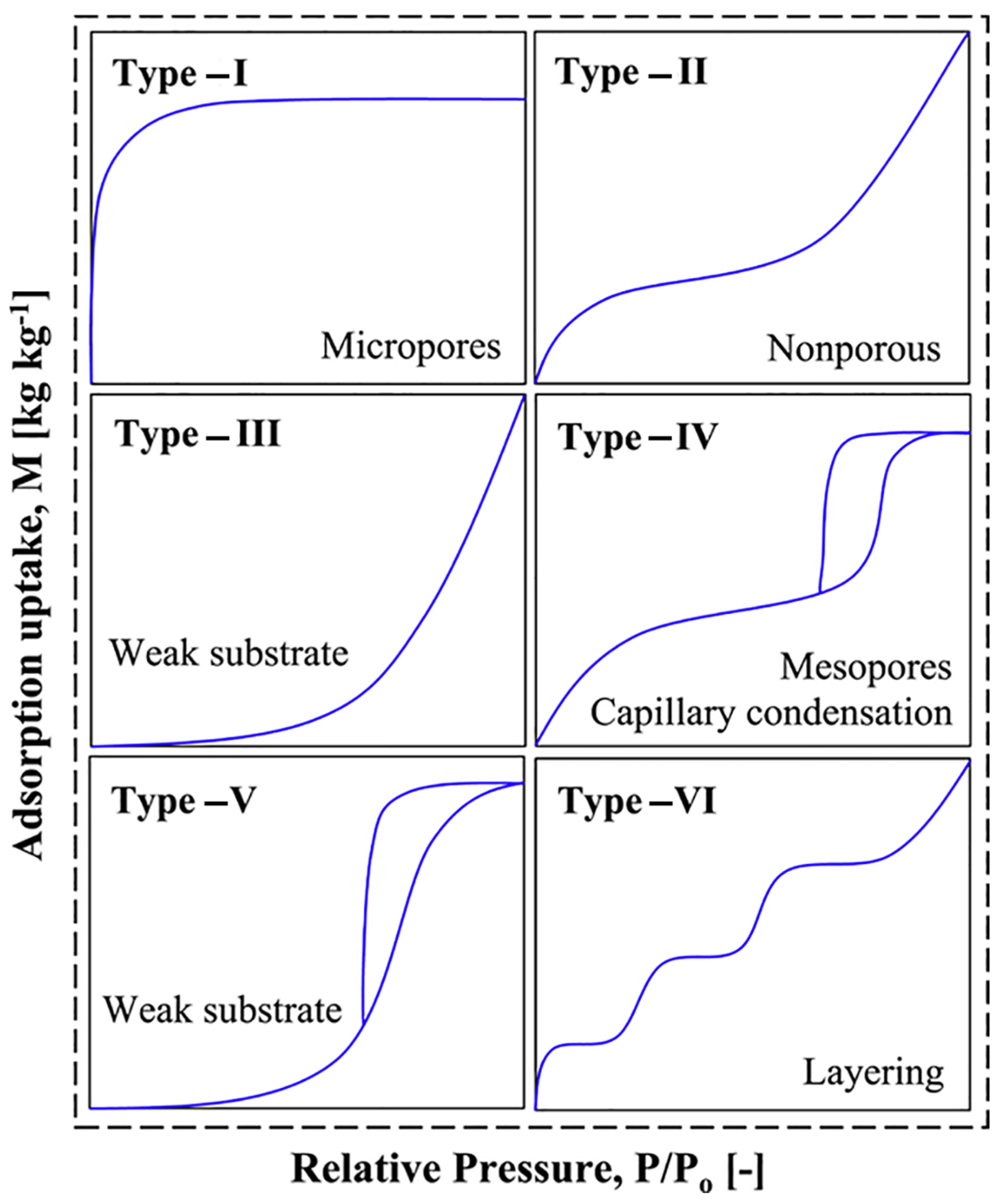
Although there are no standardized selection criteria for AWH adsorbents, certain key factors are generally considered when evaluating their suitability. Among these, a high water adsorption capacity and the presence of Type IV or Type V (S-shaped) adsorption isotherms are particularly desirable as IUPAC classification [
82]. These isotherm profiles indicate the presence of capillary condensation and multilayer adsorption, which are essential for efficient water uptake under varying humidity conditions. Additionally, adsorbents must demonstrate long-term stability, retaining their adsorption capacity over multiple adsorption–desorption cycles without significant structural degradation.
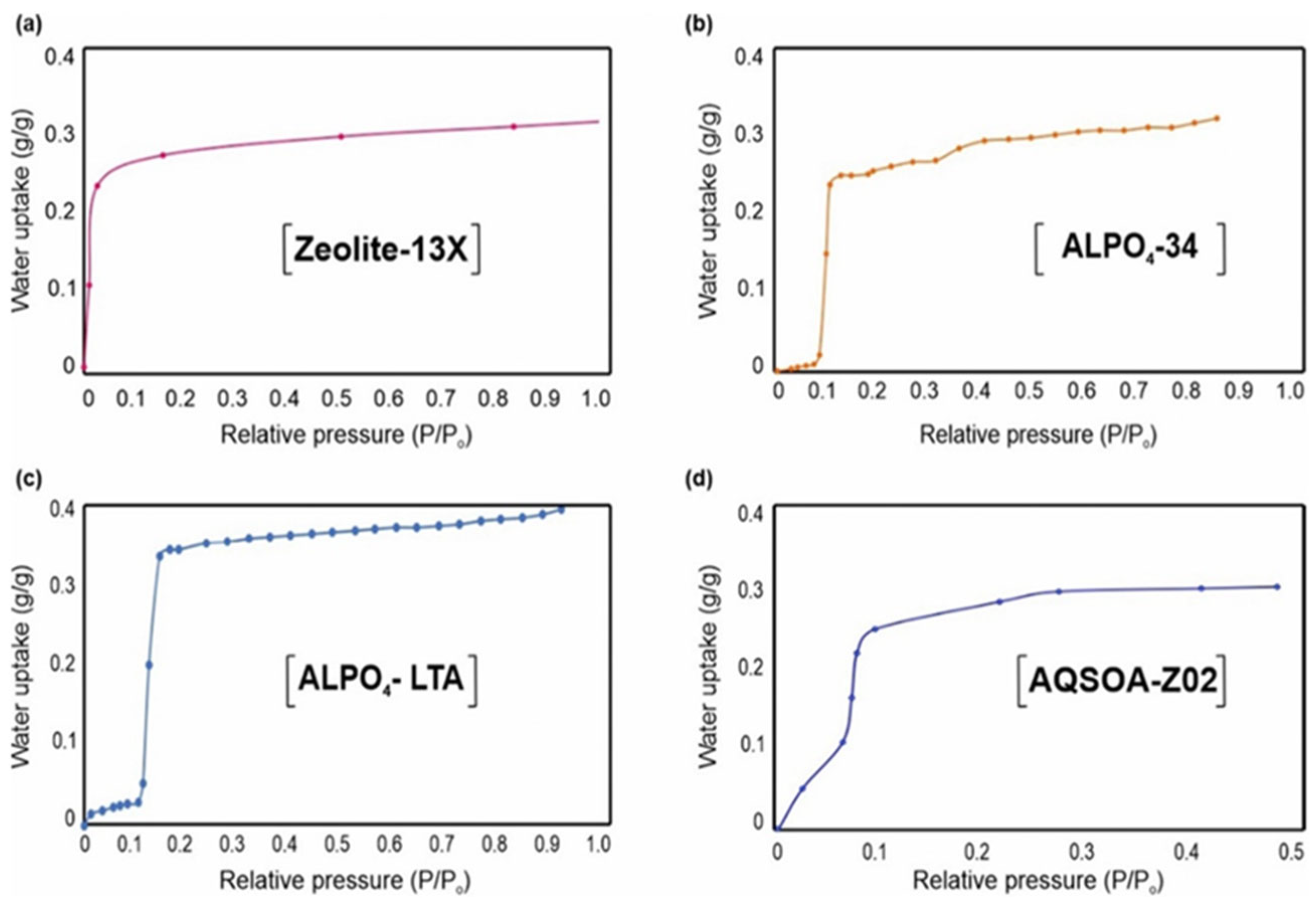
Figure 8 illustrates typical adsorption isotherms representing the moisture uptake behavior of different materials.
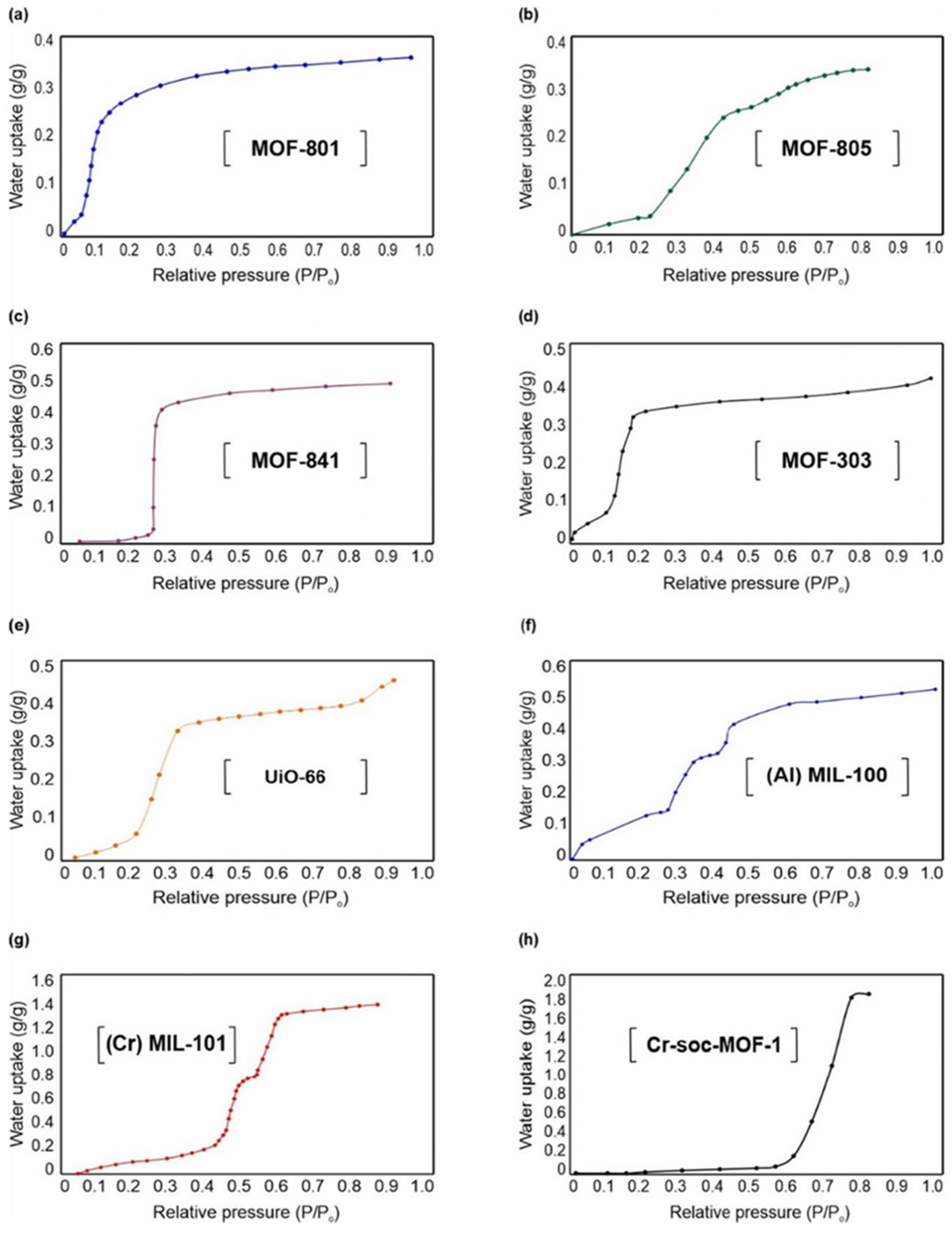
Figure 9 presents representative adsorption isotherms highlighting the water uptake behavior of different adsorbent materials. Among them, metal–organic frameworks (MOFs) stand out due to their tunable porous structure, surface chemistry, and hydrophilicity, which can be modified to optimize water adsorption performance. In particular, several MOFs exhibit S-shaped isotherms, where small variations in relative humidity or temperature cause significant changes in water uptake—an ideal characteristic for potable water harvesting applications. Compared to conventional materials such as silica gel and zeolites [
84], these MOFs not only offer superior water adsorption capacities but also operate efficiently at lower regeneration temperatures, making them ideal candidates for energy-efficient and climate-adaptable water recovery systems [
84].
Figure 10 shows the water adsorption isotherms of several microporous zeolites, including zeolite 13X, ALPO
4-34, and AQSOA-Z02. Among these, aluminophosphate-based materials such as ALPO
4-34 and AQSOA-Z02 are particularly noteworthy for their low regeneration temperatures, typically below 100 °C, as well as their reversible adsorption behavior and good hydrothermal stability. In contrast, zeolite 13X, while widely used for its high adsorption capacity and steep isotherm at low relative humidity, generally requires higher desorption temperatures, limiting its energy efficiency in low-temperature applications. Lapotin et al. [
58] conducted a comparative study on various microporous zeolites, highlighting that materials like AQSOA-Z02 can be regenerated at 75–95 °C and are capable of achieving significant water recovery rates (up to 0.77 L/m
2/day), making them promising candidates for solar-powered AWH systems.
To quantitatively describe these isotherms and extract thermodynamic information, various models can be employed. Among these models, the Dubinin–Astakhov (D–A) model is widely recognized for its ability to accurately represent the adsorption of gases and vapors onto microporous adsorbents. The D–A model, presented in Equation (14) [
91], is particularly useful for materials with a narrow pore size distribution, as it accounts for the heterogeneity of the adsorbent surface and the energy distribution of adsorption sites.
This model has been widely applied to evaluate adsorption equilibrium data for various adsorbent materials, including silica gel (RD SG) [
92], zeolite (AQSOA-Z01) [
93], ionogel (Syloid 72FP + 57 wt%, EMIM-Ac) [
94], polymer (PS-II) [
95], and nanofibrous membranes (GO-SSNF) [
96]. Each of these materials has distinct structural and surface properties, which influence their adsorption behavior. For example, zeolites, with their well-defined microporous frameworks, exhibit high adsorption capacities for water vapor, particularly at low relative humidity, making them excellent candidates for atmospheric water harvesting applications. On the other hand, ionogel and nanofibrous membranes, with their unique characteristics such as high surface area, tunable porosity, and selective water affinity, are particularly suited for specific adsorption tasks where high surface area and selective adsorption are required.
The versatility of the D–A model lies in its ability to capture the complex interactions between the adsorbent and the adsorbate, especially in systems where the adsorption process is driven by both physical and chemical forces. By applying the D–A model to these different materials, researchers gain a deeper understanding of the adsorption mechanisms at play, enabling them to select the most suitable adsorbent for a given application. Additionally, the model helps predicting the capacity of materials to adsorb specific compounds under varying environmental conditions, making it a powerful tool for the design and optimization of adsorption-based systems.
In this context, the variables represent the following parameters: W is the adsorption equilibrium uptake [kg/kg], Wmax is the maximum adsorption uptake [kg/kg], R0 is the specific gas constant [kJ/kg/K], T is the temperature [K], E is the energy characteristic [kJ/kg], psat refers to the saturation pressure [kPa], p is the vapor pressure [kPa], and n is the surface heterogeneity constant [-].
Similarly, the modified Langmuir (ML) model, as expressed in Equation (15) [
97], has been widely used to assess adsorption equilibrium data for different metal–organic frameworks (MOFs). This model is a refined version of the classical Langmuir model, accounting for surface heterogeneity and variations in adsorption energy. The ML model has been applied to evaluate a variety of MOFs, such as MOF-801 [
97], alum fumarate [
97], NH
2-UiO-66 [
98], MIL-100(Fe) [
97], and MIL-101(Cr) [
99]. These materials, known for their high surface area and tunable porosity, exhibit distinct adsorption characteristics, making the ML model an effective tool for their analysis. For example, MOF-801, with its large pores and high stability, shows significant adsorption potential for certain gases for example water vapor, while MIL-100(Fe) and MIL-101(Cr), with their metal centers and large surface areas, excel in gas adsorption tasks. The use of the ML model in these cases enhances the understanding of the adsorption processes and contributes to the optimization of MOF-based applications for AWH.
In this equation, the parameters α and
mML correspond to the pre-exponential factor and the heterogeneity parameter of the modified Langmuir (ML) model, respectively. The term
Q0st represents the isosteric heat of adsorption/desorption at zero coverage, expressed in [kJ/kg], while
hfg is the enthalpy of evaporation, also in [kJ/kg]. The values for the fitting parameters of both models are sourced from the literature and are summarized in
Table 3 and
Table 4.
5. Component-Level Design for Adsorption-Based AWH Systems
Beyond the choice of sorbent materials, the performance of adsorption-based atmospheric water harvesting systems is critically influenced by the design of their key components. An efficient component design enhances both heat and mass transfer processes, thereby improving the water harvesting capacity, energy efficiency, and overall functionality of the system.
Recent studies have increasingly focused on the optimization of adsorber geometries and material configurations to enhance system performance. By optimizing the design of adsorbent reactors, numerous studies have attempted to increase the water productivity of AWH systems. Kim et al. [
32] employed MOF-801 compressed in a copper metal foam to improve heat and mass transmission and overcome the limited thermal conductivity of sorbents. However, the peculiar interfacial air contact presented by this design limits production and uses a lot of energy. An ideal compromise, related to intracrystalline diffusivity, was found when the adsorbent layer’s thickness was optimized to 3 mm.
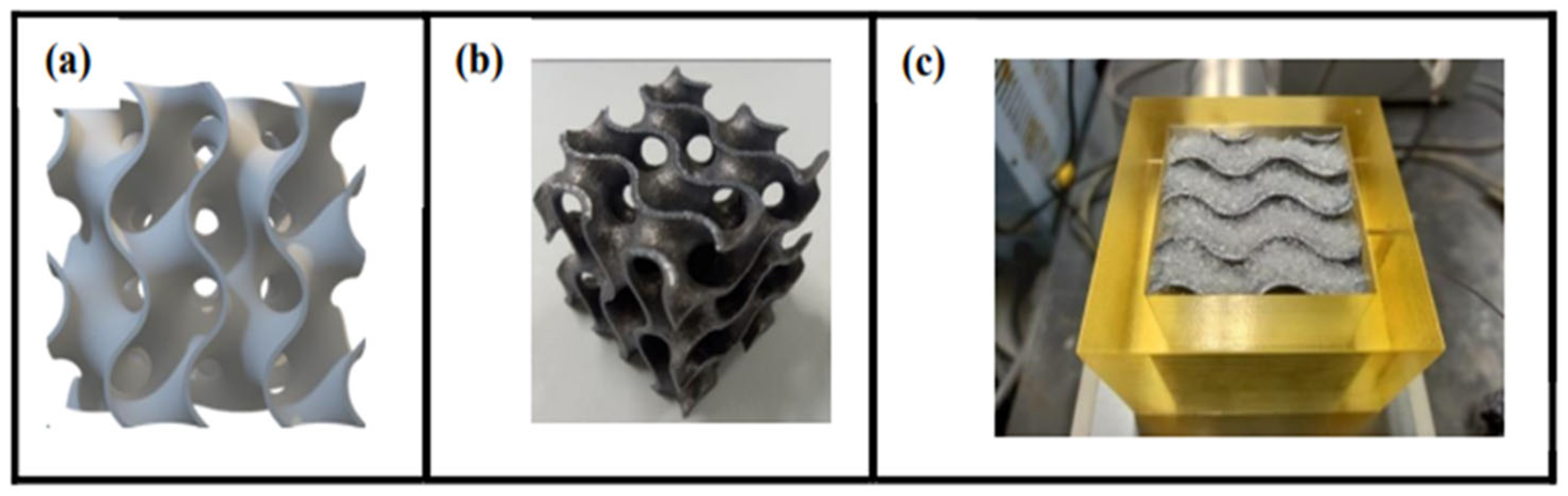
Additionally, cutting-edge structures like 3D-printable gyroids and triply periodic minimum surfaces (TPMS) (shown in
Figure 11) have proven their capacity to boost water output and system efficiency, particularly with 136 L/kg·m
2 and 6.8% efficiency at 70% RH, compared to 74 L/kg·m
2 and 3.7% without TPMS, thanks to better permeability and improved heat transfer. The thickness of the adsorbent layer constitutes an additional optimization lever. By reducing this thickness from 40 mm to 10 mm, water recovery almost doubled, reaching 391 L/kg·m
2, highlighting the importance of a synergy between geometric design and dimensional control [
100].
Tao et al. [
101] employed a nickel metal foam as a binder and a changing magnetic field for quick and uniform heating to speed up the regeneration of MIL-101(Cr) monoliths. This greatly enhanced the sorption kinetics, resulting in a daily production of 2.2 L/kg. LaPotin et al. [
58] also created a double-layer system of AQSOA Z01 zeolite, in which the metallic porosity (nickel foam) enhances the layer’s diffusivity and heat conductivity, reaching 0.77 L/m
2/day, which is higher than a single-layer system. These compact adsorbent bed designs demonstrate that improving adsorption capacity and water yield requires an effective bed design that promotes improved intracrystalline diffusivity and regulated temperature gradients (see
Figure 12). Additionally, the usage of structured structures seeks to maximize the movement of water vapor in the adsorbent layers. Wang et al. [
102] exploited a hexagonal honeycomb-shaped LiCl/CNTs hydrogel (5 mm thick) (see
Figure 12), increasing internal diffusion and reaching 3.97 g/cm
3 at 45 °C and 40% RH, for a production of 2.9 L/m
2.
Feng et al. [
103] used MIL-101(Cr) monoliths in finned structures with thermoelectric cooling (see
Figure 13), shifting the isotherms toward low humidities while maintaining a high adsorption capacity (0.98–1.05 g/g) at 25 °C and 30% RH, with a yield of 27 L/kg.
The development of compressible hierarchical monoliths of cellulose nanofibrils (
Figure 14) with graphene by Chen et al. [
104] finally opens the door for new ordered adsorbent architectures. These monoliths have a remarkable adsorption capacity of 2.60 kg/kg at 95% RH, surpassing that of conventional hydrogels and providing high elasticity and pressure sensitivity.
These advances in adsorbent reactors, whether compact or ordered, illustrate that the combination of innovative architecture, dimensional optimization, and thermal control makes it possible to improve the kinetics, adsorption capacity, and ultimately, the water productivity of AWH systems.
After examining in detail the component-level design of adsorption-based atmospheric water harvesting (AWH) systems with a particular focus on adsorbent materials, regeneration structures, and the mechanisms of mass and heat transfer, it becomes relevant to broaden the discussion toward more integrated and autonomous solutions. In this context, the integration of solar energy into adsorption-based AWH technologies represents a significant advancement. These approaches harness a renewable, clean, and abundant energy source to enhance the energy efficiency and sustainability of such systems. The following section will therefore focus on recent advances in solar-powered AWH technologies, highlighting innovations in system design, materials, and overall performance.
6. Recent Advances in Solar-Powered Sorption-Based AWH Technologies
In this section, the main advances at the system level for adsorption based AWH are presented.
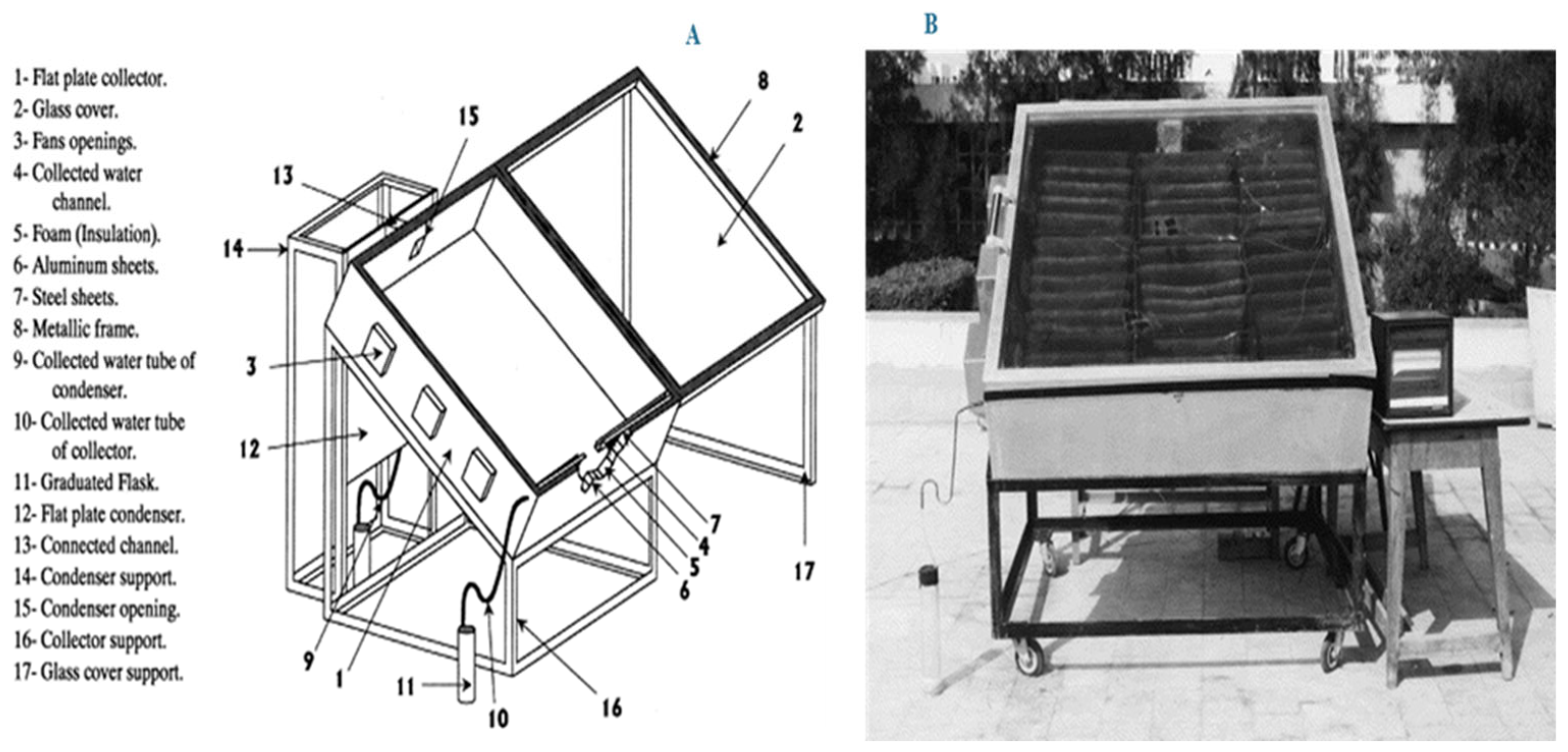
Gad et al. [
105] developed an integrated solar desiccant/collector system for atmospheric water harvesting, achieving a yield of approximately 1.5 L/m
2/day. The system operates in two distinct stages: during the night, it adsorbs atmospheric moisture, and during the day, solar energy is utilized to regenerate the desiccant and drive the condensation process. To enhance mass transfer, a thick corrugated fabric layer was incorporated, effectively increasing the surface area available for moisture absorption. Air is drawn into the desiccant bed through side-mounted fans, promoting efficient water vapor adsorption, which is subsequently released and condensed under solar radiation. Experimental tests were conducted across two seasons between October 1998 and June 1999, with the primary objective of evaluating system performance under Egyptian climatic conditions. CaCl
2, selected for its high availability and low cost, served as the working desiccant. Key operational parameters such as solar intensity and desiccant bed temperature were continuously monitored to assess and optimize system performance. A validated numerical model further supported the analysis by providing insights into the system’s water production efficiency.
Figure 15 illustrates the experimental setup, which includes a plate solar collector with a movable glass cover, a wave-shaped desiccant bed, and an air-cooled condenser featuring parallel plates.
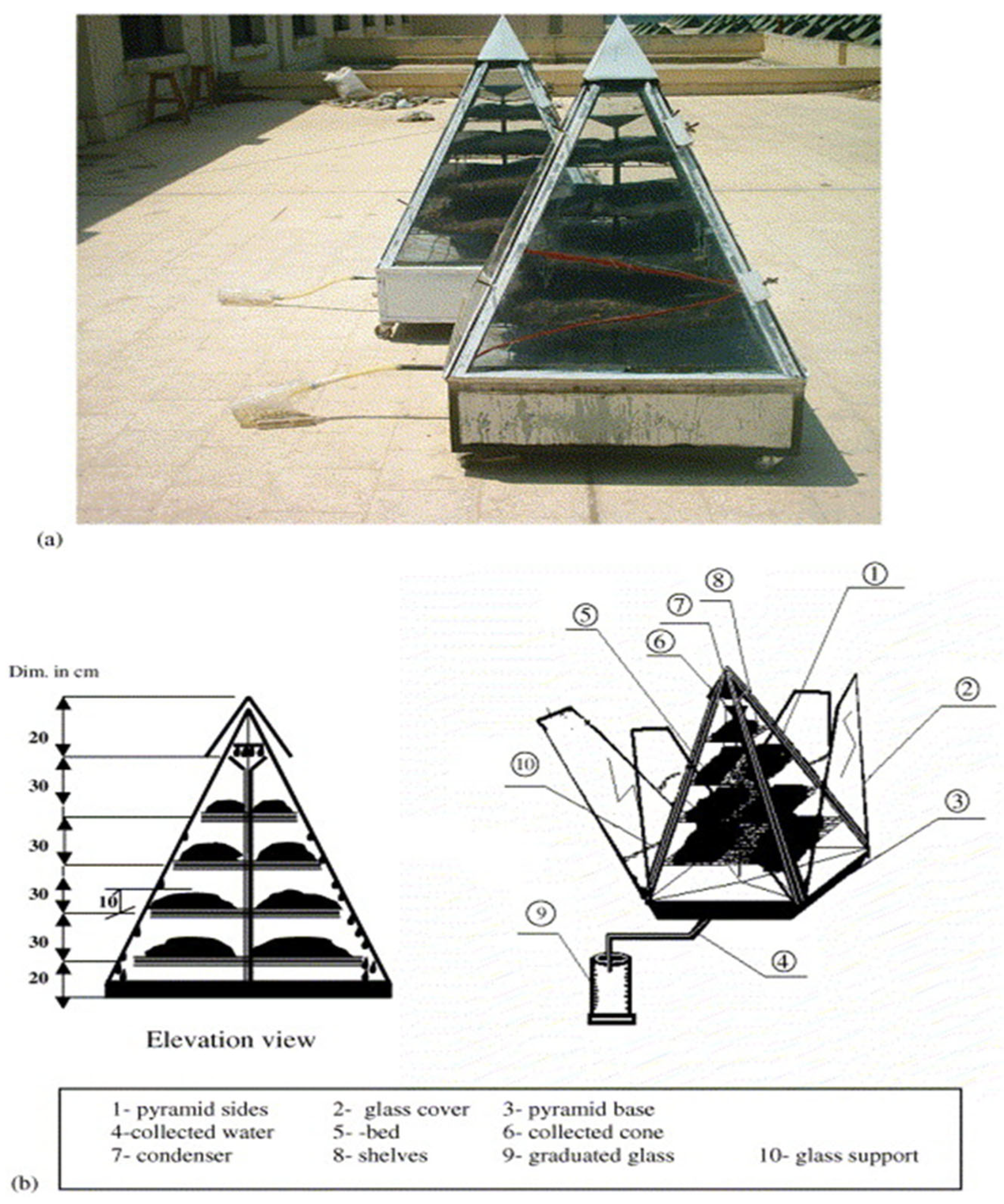
Kabeel et al. [
106] studied and designed a pyramidal solar collector system using desiccation for atmospheric water harvesting. Two prototype pyramids of identical dimensions were developed (
Figure 16), each containing a different type of bed material on the shelves: one made of sawdust and the other of fabric, both saturated with a 30% calcium chloride solution. The system operates in two distinct phases: during the night, the four sides of the pyramid remain open to allow moisture absorption from the air; during the day, the sides are closed to enable the regeneration of the desiccant using solar energy and the condensation of water vapor. Experimental tests were conducted in Egypt during the summer under varying outdoor climatic conditions to evaluate the effect of the pyramidal shape on the efficiency of the absorption and regeneration processes. The results showed that the cloth bed absorbed a greater amount of solution (9 kg) compared to the saw wood bed (8 kg), resulting in an approximately 5% increase in productivity. The system achieved a water production rate of about 2.5 L
water/m
2/day. This remarkable performance is mainly attributed to the optimized design of the pyramid, whose four glass surfaces and multi-shelf configuration led to a 90–95% improvement in water recovery compared to traditional horizontal and corrugated bed designs used in desiccant solar collector systems.
Ji et al. [
107] investigated a solar-driven water harvesting system incorporating an advanced composite adsorbent designed to enhance moisture capture and release efficiency, as shown in
Figure 17. The composite material consisted of MCM-41, a proprietary large-pore crystalline structure, serving as a host matrix, with calcium chloride integrated as the hygroscopic component to improve water uptake. This formulation achieved a high adsorption capacity of 1.75 kg
water/kg
adsorbent, demonstrating its strong affinity for atmospheric moisture. Furthermore, the system exhibited an impressive desorption efficiency, releasing over 90% of the absorbed water at a relatively low regeneration temperature of 80 °C, making it well-suited for solar-assisted applications. Experimental evaluations confirmed a fresh water yield surpassing 1.2 kg/m
2/day, emphasizing the system’s effectiveness in atmospheric water recovery. These findings highlight the potential of such composite adsorbents in enhancing water production efficiency, particularly in arid and semi-arid regions where conventional water sources are scarce.
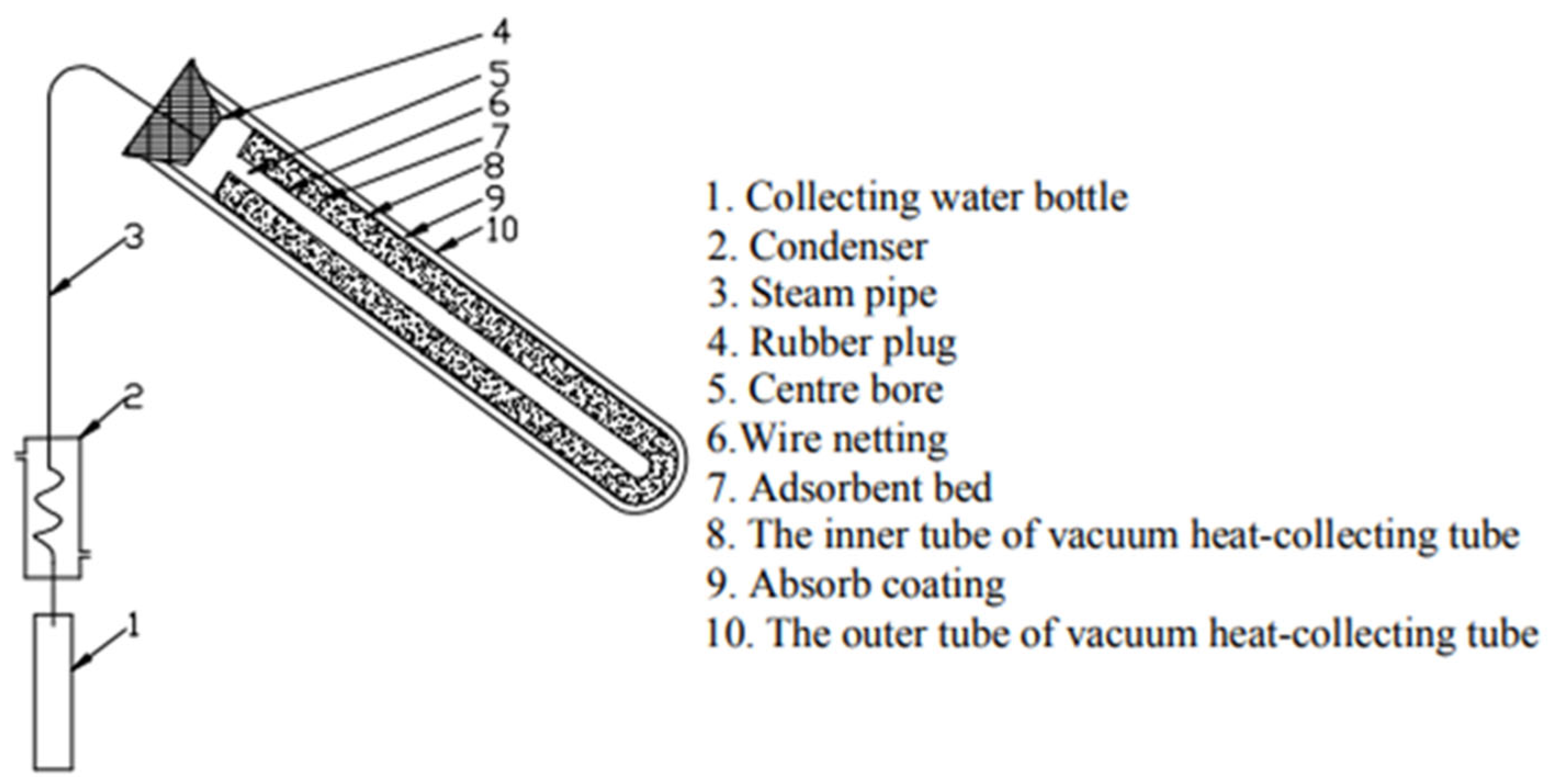
Liang and Zhao [
108] conducted an experimental analysis of a solar-powered adsorption system designed for atmospheric water harvesting, as depicted in
Figure 18. The setup featured a vacuum-insulated glass tube with a selective coating, serving as the adsorption bed, while composite adsorbent zeolite powder was selected as the adsorbent due to its high affinity for water vapor. The system was tested under varying environmental conditions in Shanghai in June and July 2011, with solar radiation levels ranging from 284 W/m
2 to 1007 W/m
2 and ambient temperatures fluctuating between 30.7 °C and 41.6 °C. After processing 10 m
3 of air, the system successfully produced 78 mL of condensed water at room temperature. These findings underscore the potential of combining solar energy with adsorption-based water harvesting techniques, offering a sustainable and efficient approach for extracting atmospheric moisture, particularly in arid regions with high solar exposure.
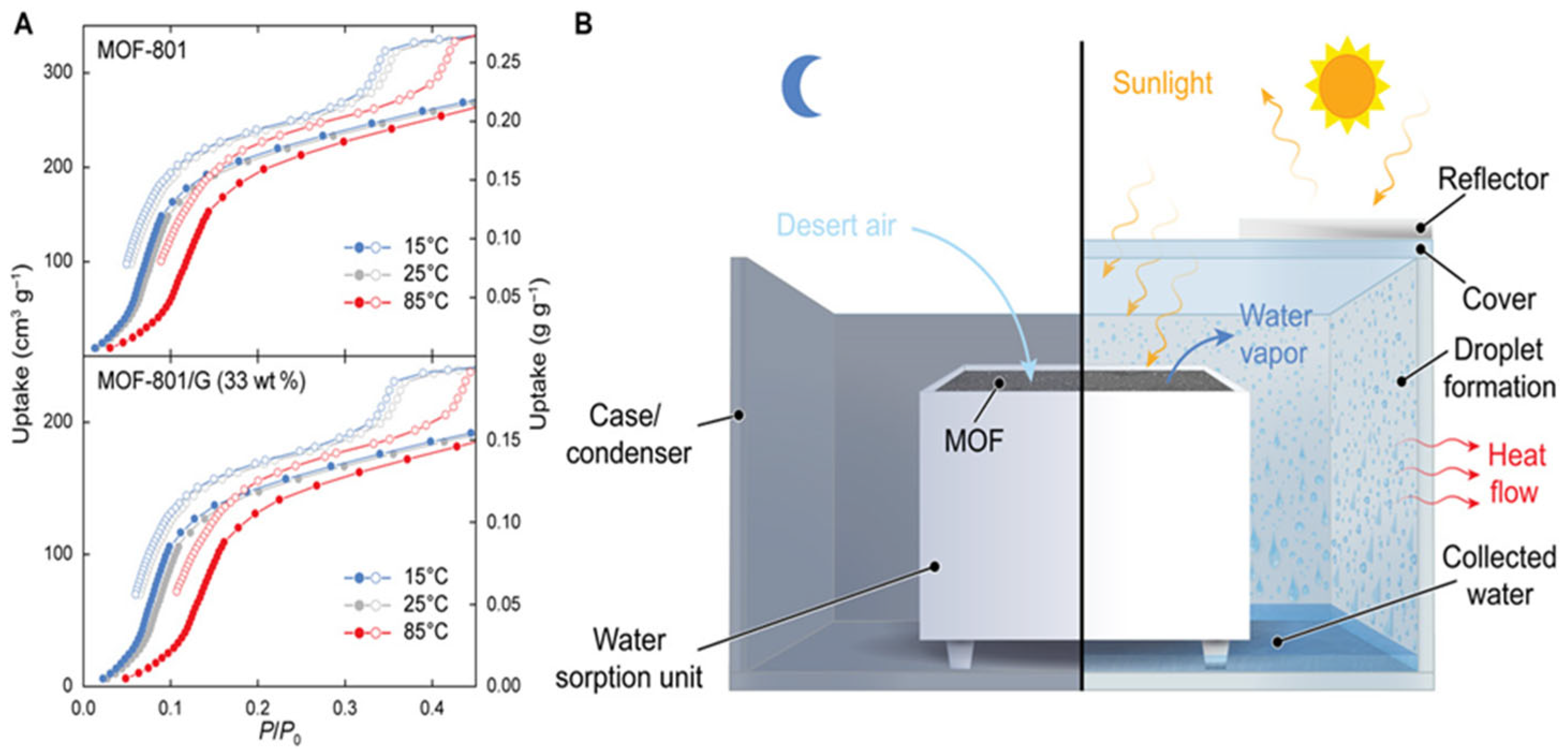
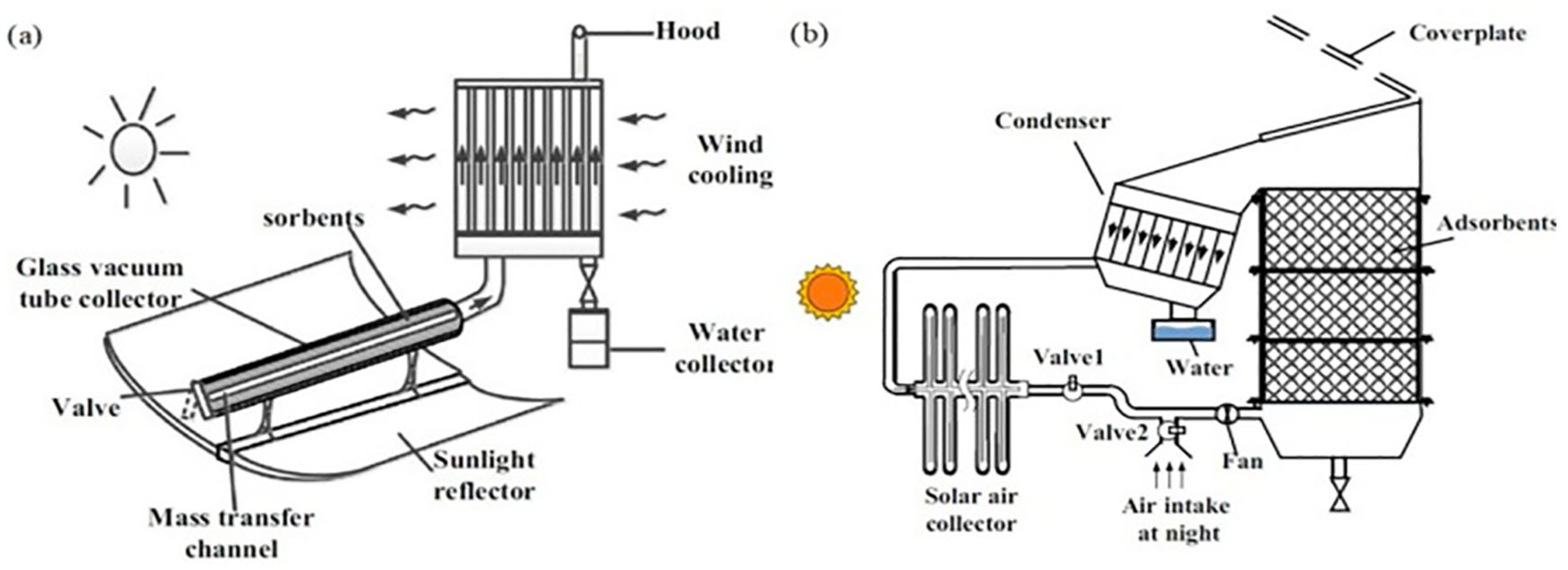
The idea of generating water from air in desert environments, particularly with low-energy consumption, is a promising but still developing concept. Fathieh et al. [
31] made significant strides by presenting a proof-of-concept system designed to capture water in areas with low relative humidity, which is typical of desert climates. The system they proposed operates based on natural cooling and solar energy, without the need for external power sources, making it highly sustainable. However, despite the promising design (see
Figure 19), this system relies on external cooling mechanisms, and its performance under actual desert conditions had not been thoroughly tested until their study. The research involved a two-phase approach to assess the feasibility of this technology. Initially, the prototype, utilizing up to 1.2 kg of the MOF-801, was tested in a controlled laboratory environment. This phase allowed the researchers to fine-tune the system’s operation and establish baseline data on its performance in a regulated setting. Following the laboratory tests, the prototype was moved to the Arizona desert, a location chosen for its arid climate and low humidity, which provided a realistic test environment for the technology’s effectiveness in real-world desert conditions.
The results of these field tests were promising. During the night cycle, the prototype successfully produced 100 gwater/kgMOF-801/day, relying solely on the natural cooling of the atmosphere and sunlight. This is a noteworthy achievement as it demonstrates the potential for harvesting water without needing additional energy sources, a crucial factor for the application of this technology in remote and off-grid desert regions where energy resources are limited.
Kim et al. [
30] developed and tested an atmospheric water harvesting (AWH) prototype incorporating a MOF-801 layer, designed to operate solely on solar energy, as shown in
Figure 20. The prototype, measuring 7 × 7 × 4.5 cm and weighing 1.34 g of activated MOF-801, uses the material’s high porosity to adsorb moisture during the night when relative humidity is high. During the day, sunlight heats the MOF layer through the glass cover, initiating the desorption of the captured water vapor. A condenser and heat sink manage the thermal process and help condense the vapor, recovering water. Field tests in low-humidity conditions (around 20% RH) showed the system could produce approximately 2.8 L
water/kg
MOF-801/day. The reflective enclosure enhances solar energy absorption, directing heat onto the MOF layer for efficient desorption. These findings demonstrate the viability of solar-powered AWH systems in arid regions, offering a sustainable solution for water production without relying on external energy sources. The study emphasizes the importance of optimizing material properties for improved performance in such low-humidity environments.
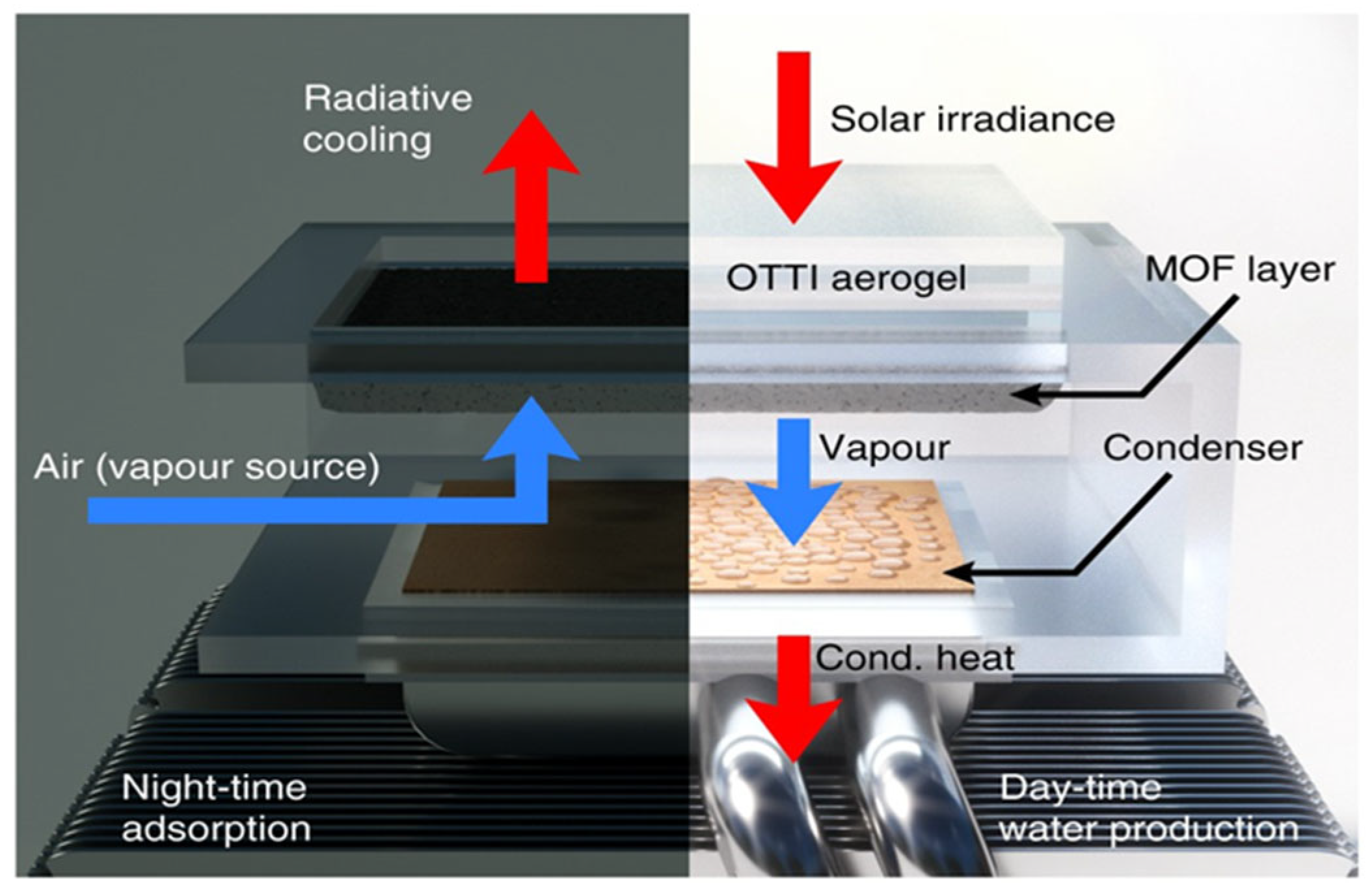
Inspired by the work of Kim et al. [
30], an atmospheric water harvesting system (as shown in
Figure 21) was tested in an arid environment characterized by low relative humidity (10–40%) and sub-zero dew points. This system, equipped with an air-cooled condenser and utilizing the same adsorbent material, was designed to operate efficiently under harsh, dry conditions. The testing revealed that the system successfully produced over 0.25 L
water/kg
adsorbent/day, demonstrating its capability to capture atmospheric moisture even in extreme conditions. The integration of the air-cooled condenser played a crucial role in maintaining the necessary temperature and facilitating effective condensation, while the choice of adsorbent allowed for substantial moisture adsorption, even with low humidity levels. These results underscore the potential of atmospheric water harvesting systems as a reliable solution for water production in arid regions, where traditional water sources are scarce and energy-efficient technologies are essential for sustainable water supply.
Wang et al. [
63] studied an atmospheric water harvesting system using ACF-LiCl composite sorbents with a sinusoidal honeycomb structure. Their research demonstrated that the water extraction process could be predicted using a thermodynamic diagram. A prototype was then tested under various temperature and humidity conditions throughout the year, confirming a daily water production of 38.5 kg in a humid climate (25 °C, 75% RH) with an energy consumption of 7.20 MJ/kg
water. These results highlight the feasibility of large-scale implementation.
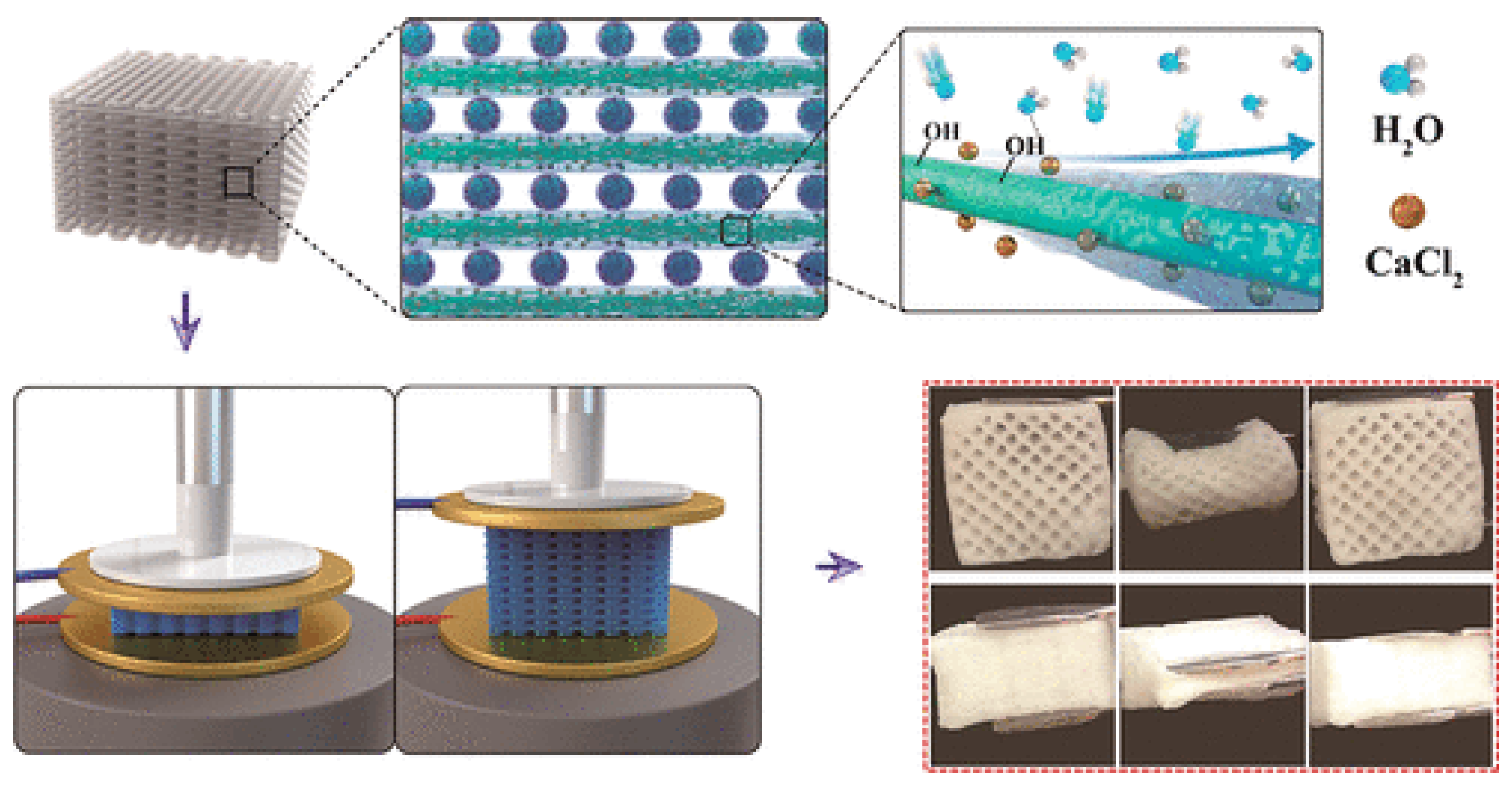
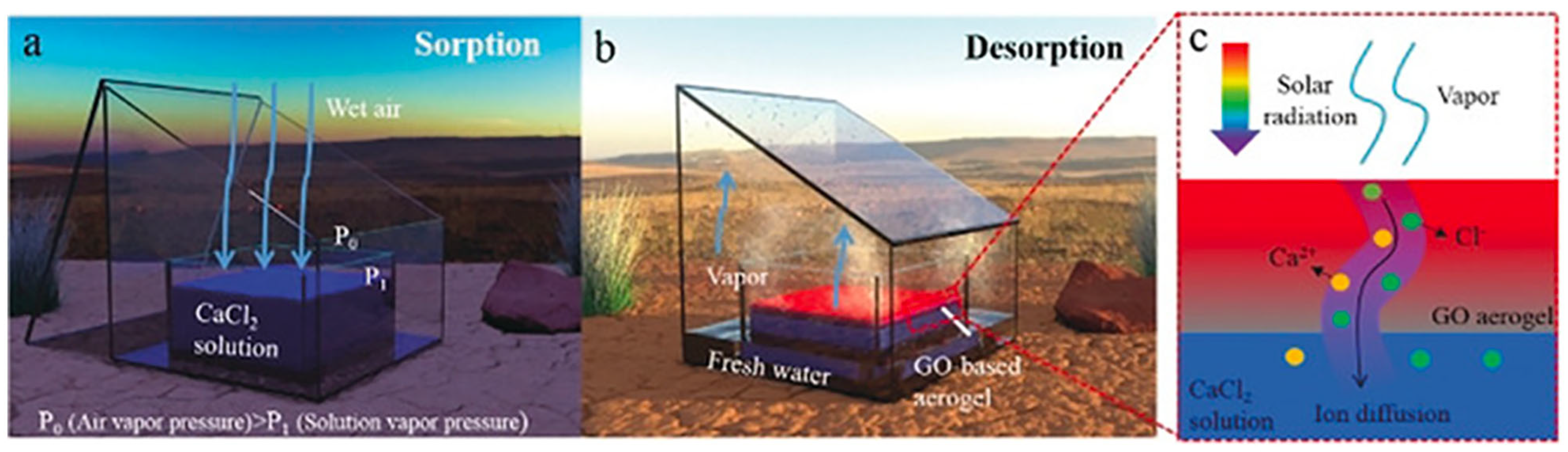
In another study, Wang et al. [
109] enhanced solar-driven water desorption using a GO-based aerogel with large pores, preventing salt accumulation. The system, utilizing a liquid CaCl
2 sorbent with a 50% concentration, operates in two phases: nighttime adsorption and daytime desorption (see
Figure 22). Experimental results showed a water production rate of 2.89 kg/m
2/day and desorption efficiency of 66.9% at 70% relative humidity. This cost-effective approach represents a significant advancement in making adsorption-based water harvesting technologies more accessible and efficient for freshwater production.
Wang et al. [
110] conducted an experimental study on two solar-powered adsorption-based devices for extracting water from the air, comparing an open system (see
Figure 23a) with an optimized semi-open system (see
Figure 23b). Both configurations utilized solar collectors operating between 70 and 80 °C to provide the thermal energy required for the desorption process. In the open system, a spiral structure containing 2.25 kg
ACF-CaCl2 was used as adsorbent material, allowing for the recovery of approximately 0.23 kg
water/cycle. However, this configuration showed certain limitations in terms of efficiency. In contrast, the semi-open system, designed to enhance heat and mass transfer, demonstrated significantly higher performance. By using 40.8 kg of consolidated ACF-LiCl sorbent and a solar collector area of 4 m
2, this device was able to produce up to 9 kg
water/cycle.
These findings highlight the importance of optimizing adsorbent materials and system design to improve the efficiency of AWH devices, particularly in arid environments where water access is limited. The integration of semi-open configurations appears to be a promising approach for maximizing water production while maintaining moderate energy consumption.
Lapotin et al. [
35] presented a groundbreaking two-stage AWH model using the AQSOA Z01 adsorbent, a material known for its high water adsorption capacity. The key innovation of this system lies in its two-stage design, which significantly enhances the water collection capacity. In the first stage, the adsorbent material adsorbs water from the humid air, utilizing the latent heat of condensation to support the desorption process in the second stage, as shown in
Figure 24. By recycling this heat, the system minimizes the energy required for desorption, leading to better efficiency and a higher overall water yield.
This two-stage configuration achieved a remarkable water adsorption rate of 0.77 L/m2/day, surpassing the performance of traditional single-stage AWH systems. This increased adsorption is due to the dual-phase process, where both adsorption and desorption are more efficient through heat recycling, making the system particularly advantageous for areas with high humidity. Furthermore, the model demonstrated that when combined with high-performance adsorbents such as AQSOA Z01, the system could be optimized to work seamlessly with solar-powered AWH units, offering a sustainable water harvesting solution.
In
Table 5 an overview of solar-powered sorption-based AWH systems is reported.
7. Limitations and Perspectives
Desiccant materials offer several promising advantages for AWH applications, notably their ability to passively capture water vapor from ambient air, even at low relative humidity. Materials such as MOFs, silica gels, zeolites, and hygroscopic salts have demonstrated considerable potential. For example, MOFs like MOF-801 or MIL-101(Cr) can reach water uptake values of over 1.2–1.4 g/g at 80% RH, while silica gel typically ranges between 0.3–0.4 g/g. These materials are advantageous due to their tunable porosity, selective adsorption properties, and low regeneration temperatures (often below 100 °C), which enable energy-efficient operation. However, there are also significant drawbacks. Many advanced materials, especially MOFs and salt-based composites, suffer from poor hydrothermal or mechanical stability, leading to degradation over repeated adsorption–desorption cycles. Some of them loose over 15–20% of their initial capacity after only 50–100 cycles. Additionally, safety concerns arise when desiccant materials contain toxic metals or leachable chemicals that could contaminate the harvested water if not properly encapsulated or filtered. Scalability is another key issue: while materials perform well under controlled lab conditions, the transition to real-world applications often reveals reduced efficiency due to inconsistent airflow, ambient fluctuations, and heat transfer limitations.
AWH systems that incorporate these desiccants also present both strengths and weaknesses. On the positive side, AWH devices offer decentralized, off-grid water solutions, making them attractive for arid and remote regions. Their ability to operate with renewable heat sources (e.g., solar thermal) makes them environmentally sustainable. Furthermore, their modularity allows for small-scale personal use or integration into larger systems. However, current systems face critical limitations. Daily productivity often remains low, ranging from 0.3 to 0.5 Lwater/kg of desiccant, whereas human consumption needs exceed 2 L/day. In terms of energy performance, the overall thermal efficiency is hindered by substantial losses during the desorption and condensation phases heat recovery efficiencies can fall below 30%. Long-term stability is also a challenge, with systems experiencing mechanical wear, performance drift, or fouling after prolonged use. Additionally, because AWH systems involve direct exposure of vapor to ambient air and adsorbents, the quality of the collected water can be compromised by airborne particulates, microorganisms, or volatile organic compounds. Thus, regular water quality monitoring and post-treatment using filters or UV sterilization are essential to meet WHO (World Health Organization) drinking water standards.
To overcome these challenges, a dual strategy is required. First, the development of next-generation desiccants must focus on combining high water uptake (>1.0 g/g), fast kinetics (<30 min to equilibrium), and excellent cyclability. Second, improving system-level design, particularly thermal management, air flow optimization, and condensation efficiency, would significantly enhance daily yield and energy performance. Integrating reliable filtration and purification modules will also be critical for safe water delivery. While AWH technologies hold strong potential, their deployment on a large scale will depend on how effectively these advantages can be preserved and these drawbacks addressed in real-world conditions.
8. Conclusions
This review explores the potential and challenges associated with various atmospheric water harvesting (AWH) technologies. The analysis of the existing literature reveals that these technologies face limitations, particularly in terms of their reliance on specific climatic conditions and high operational costs, which hinder the achievement of sufficient water yield. In this context, adsorption-based AWH systems have proven to be an effective solution, offering the ability to extract water from the atmosphere under a wide range of environmental conditions and at any time. The review delves deeply into the principles of adsorption equilibrium/isotherms and kinetics, demonstrating that adsorption-based systems exhibit superior performance compared to other AWH technologies. Moreover, the use of metal–organic frameworks (MOFs) has emerged as a promising advancement, offering enhanced water-harvesting capacities and lower regeneration temperature requirements compared to conventional desiccants. Despite the growing interest in adsorption-based AWH systems due to their environmentally sustainable nature, a significant knowledge gap persists among designers, developers, and industry stakeholders. This gap continues to hinder their commercial feasibility, underscoring the need for further research and development to bridge the gap and unlock the full potential of these systems.
To address this, future work should focus on translating laboratory-scale innovations into scalable and cost-effective prototypes, promoting the use of standardized performance metrics, and encouraging interdisciplinary collaboration to ensure practical deployment in diverse climatic regions.