1. Introduction
Municipal Solid Waste Incinerators (MSWI) play a crucial role in waste management through reduction, energy recovery, and greenhouse gas emission mitigation [
1]. The by-products of this process include bottom ash and fly ash, which contain a wide range of elements—from macronutrients (Ca, Si, Al) to heavy metals and trace elements (Pb, Cd, Zn, Cu, As, Se, Sb). Their diverse composition and potential environmental mobility require detailed analysis.
Critical Raw Materials (CRMs) are strategic and irreplaceable materials essential for modern technologies, such as renewable energy, electronics, and electromobility. The term was introduced by the US Committee on Critical Mineral Impacts in 2008 [
2]. In 2023, the EU identified 40 such materials, including lithium, barite, and tantalum [
3]. Their recovery has become necessary due to China’s dominance in global production, particularly of Rare Earth Elements (REEs) (98%) [
4]. The search for CRMs includes not only natural deposits but also anthropogenic sources, such as industrial and municipal waste. Recovering them from waste, e.g., MSWI, is a rapidly developing research area and part of the circular economy.
The composition of ash from incinerators depends on the type and conditions of combustion. It contains macronutrients (Ca, Si, Al, Fe, Mg, Na, K) as well as heavy metals and trace elements (Zn, Cu, Pb, Cd, Ni, Cr, As, Sb, Hg, Se, Tl, Mo, REEs, Au, Pt) [
5,
6]. The distribution of elements in ash is strongly dependent on their physicochemical properties. Volatile elements (Hg, Cd) are mainly present in fly ash, while lithophilic elements (Cr, Cu) are found in bottom ash. Heavy metals often form stable complexes with organic matter, affecting their mobility and bioavailability [
7,
8].
This study presents a series of results. The objective of the research was to conduct a detailed identification and description of the content of selected critical raw materials and determine the mutual relationships between elements present in bottom ash generated during municipal solid waste incineration (MSWI). In particular, the focus was on analyzing the content and co-occurrence of trace elements and macroelements such as Ag, Al, Au, Ba, Co, Cr, Cu, Fe, Li, Ni, Mn, Pt, Sb, Sn, Sr, V, Zn, HREEs, and LREEs. This analysis aimed to identify characteristic correlation relationships that could indicate chemical affinity between elements, resulting from their physicochemical properties, source of origin, and binding and stabilization mechanisms in ash. The analysis included statistical analysis, which comprised descriptive statistics, correlation analysis, and cluster analysis. The obtained results not only contribute to a better understanding of the chemical composition of ash but also can help plan more effective strategies for recovering and utilizing secondary raw materials within the circular economy.
Publications by Denafas et al., Mendes et al., and Aslani and Taghipour have highlighted the phenomenon of seasonal variability in both the quantity and composition of generated waste [
9,
10,
11]. In particular, Aslani and Taghipour emphasized not only the seasonal dynamics of waste composition but also the percentage of materials recoverable through recycling from mixed waste streams, as well as the variability of non-recyclable waste fractions [
11]. Studies conducted in Eastern Europe further underscore the intra-year variability in the amount and composition of municipal waste across European countries [
9]. This observed variability in waste composition is expected to be reflected in fluctuations in the elemental composition of incineration bottom ash. Given that sample collection in the present study was carried out over the course of a full calendar year, it was possible to evaluate intra-annual variability in the ash’s chemical characteristics. Within the framework of this study, an attempt was made to evaluate the seasonal variability of elemental content in bottom ash, which enables the identification of potential influences of changes in waste composition over the annual cycle. The analysis also included an assessment of correlations between individual elements, which may indicate common origins, geochemical affinity, or similar retention mechanisms in mineral phases of ash. Furthermore, the application of statistical methods allowed for the differentiation of groups of elements with similar chemical and technological properties, representing a crucial step towards developing strategies for selective recovery of secondary raw materials and assessing potential environmental risks. The presented issues are key in the context of implementing a circular economy and rational utilization of critical raw materials derived from anthropogenic sources.
Numerous studies have focused on the overall chemical composition of bottom ash (IBA) and fly ash generated from municipal solid waste incineration (MSWI), with a particular emphasis on the complex mineral phases and amorphous compounds present in IBA, including oxides (SiO
2, Al
2O
3, CaO, Fe
2O
3) and heavy metals such as Zn, Cu, Pb, Cr, and Ni [
1,
5,
6]. In recent years, the utilization of IBA as a secondary raw material has gained significant attention, aligning with the principles of a circular economy. Advances in technologies such as physical separation, seasoning, washing, carbonation, and vitrification have improved the environmental properties of IBA and enhanced its potential for metal recovery [
12,
13].
The significance of this study extends beyond the scientific context, directly relating to the strategic objectives of the European Union’s raw materials policy. As demand for raw materials used in renewable energy, electromobility, and advanced digital technologies continues to grow, it is essential to explore alternative sources of critical raw materials (CRMs), such as lithium, cobalt, rare earth elements (REEs), and antimony [
12,
14]. One key direction is “urban mining”, which involves the recovery of elements from industrial and municipal waste, including MSWI ash.
This study is particularly important because it provides unique data for Central Europe, a region where systematic analyses of IBA chemical composition are scarce. Furthermore, the presented methodology, which includes seasonal analysis and correlation between elements, enables not only a better understanding of IBA’s chemical variability but also the formulation of data-driven recovery strategies that consider the relationships between elements, seasonal variations, and potential co-recovery [
15].
The objective of this study is to address these knowledge gaps through a comprehensive analysis of IBA’s chemical composition, taking into account seasonality, element correlations, and co-recovery potential. The obtained data have significant implications for planning recovery strategies and integrating IBA into the European circular economy.
2. Materials and Methods
2.1. Incineration Bottom Ash
The Thermal Waste Treatment Plant (ZTPO) in southern Poland is a modern facility designed for the disposal of unsegregated municipal waste. The plant employs a grate-fired combustion technology with a heat recovery boiler and a comprehensive flue gas cleaning system, including selective non-catalytic reduction of NOx, semi-dry absorption using lime milk, and heavy metal adsorption using activated carbon. This process takes place under high-temperature conditions—the temperature in the combustion chamber reaches values of 850–1000 °C, ensuring effective oxidation of organic substances and reduction of gaseous pollutants.
The combustion process generates residues in the form of bottom ash (IBA), fly ash, and waste from flue gas cleaning. Particular attention was paid to bottom ash, which is the largest mass flow of secondary waste generated at ZTPO. After cooling in a water quenching system, IBA undergoes a stabilization process to stabilize its moisture content, followed by valorization, which involves the use of magnetic separators, electrostatic separators, impact crushers, and sorting equipment. These operations enable the recovery of significant amounts of ferrous and non-ferrous metals and prepare the bottom ash for further utilization.
To characterize the chemical composition of secondary waste, a planned sampling of bottom ash was carried out in 2021. Samples were collected weekly over 52 consecutive weeks, in accordance with the BS-EN 14899:2005 standard [
16], ensuring representativeness and reliability of the results. Based on the principles of composite sampling, 10 kg laboratory samples were collected and subsequently subsampled for chemical analysis.
A total of 52 IBA samples were analyzed. The samples were analyzed for the content of elements such as Ag, Al, Au, Ba, Co, Cr, Cu, Fe, Li, Ni, Mn, Pt, Sb, Sn, Sr, V, Zn, HREE, and LREE. The heavy rare earth elements (HREE) fraction included elements such as Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, while the light rare earth elements (LREE) fraction included elements such as La, Sc, Y, Ce, Pr, Nd, Sm.
The preparation of the bottom ash analytical sample involved weighing and drying of the samples, followed by crushing to ensure that more than 70% of the sample passed through a 2 mm sieve. The crushing was performed using a Terminator Jaw Crusher TM Engineering (TM Engineering Ltd., Burnaby, BC, Canada). The crushed material (250 g) was then pulverized and sieved through a 75 μm sieve. The grinding was carried out using an LM201 ESSA FLSmidth mill (FLSmidth A/S, Valby, Copenhagen, Denmark). Subsequently, the analytical samples were digested with a modified aqua regia solution consisting of equal parts of concentrated HCl, HNO3, and distilled water for one hour in a heating block. Finally, the sample was made up to volume with diluted HCl.
The determination of the content of elements such as Ag, Al, Au, Co, Cr, Cu, Dy, Er, Eu, Fe, Gd, Ho, La, Li, Lu, Mn, Nd, Ni, Pr, Pt, Sb, Sn, Tm, V, Yb, and Zn was carried out using inductively coupled plasma mass spectrometry (ICP-MS) with an ELAN 9000 spectrometer (PerkinElmer SCIEX, Wellesley, MA, USA). In contrast, the determination of other elements such as Ba, Ni, Sr, Y, Nb, Sc was performed by mixing the bottom ash samples with a lithium metaborate-lithium tetraborate flux in a crucible, followed by fusion in a furnace. The cooled melt was then digested in nitric acid and analyzed using inductively coupled plasma mass spectrometry with a Ciros Vision spectrometer.
Quality control was performed using reference standards STD BVGEO01 [
17] and OREAS 262 [
18]. The analytical procedure involved careful attention to detail, including sample preparation, digestion, and analysis, to ensure the accuracy and precision of the results. Laboratory tests were carried out by Bureau Veritas Commodities Canada Ltd. (Vancouver, BC, Canada), using certified reference materials, ensuring high-quality data. The obtained results of chemical analyses formed a database that was subject to further statistical analysis.
2.2. Data
The analyzed data consisted of a dataset comprising 17 elements and 2 sums of elements with 52 measurements taken over the course of a year, at weekly intervals. The analyzed elements contents in the ash were expressed in parts per million (ppm). Gold (Au), platinum (Pt), nickel (Ni), manganese (Mn), chromium (Cr), aluminum (Al), iron (Fe), cobalt (Co), antimony (Sb), vanadium (V), barium (Ba), lithium (Li), and strontium (Sr) had no censored values. Censored observations above the upper detection limit were found in silver (Ag): 1, copper (Cu): 5–9.6%, tin (Sn): 15–28.8%, and zinc (Zn): 3–5.8%. In the HREE group, europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), and ytterbium (Yb) had no censored values. Only lutetium (Lu) had 2 observations below the lower detection limit, accounting for 3.8% of the total data. In the LREE group, lanthanum (La), scandium (Sc), yttrium (Y), cerium (Ce), praseodymium (Pr), neodymium (Nd), and samarium (Sm) had no censored values.
Censored values above the detection limit for three elements (Cu, Sn, Zn) were replaced using Tobit regression method [
19]. Tobit regression extends standard regression models to accommodate situations where the dependent variable is subject to censoring by incorporating both observed and censored values. Under the assumption that the latent variable follows a normal distribution, model parameters are obtained via maximum likelihood estimation, which properly accounts for the censoring process. This approach is especially advantageous for datasets with many censored observations, as it yields unbiased estimates of the relationships among variables [
19,
20].
In cases where censored values below the detection limit accounted for less than 4% of the data, as was the case for lutetium, they were replaced using a classical approach according to guidelines, which involves replacing them with half of the detection limit value [
21]. Similarly, a single censored value above the detection limit for silver was replaced with a random value from the distribution of this element, in accordance with guidelines for environmental data analysts [
22].
Additionally, to evaluate whether there are differences in elemental content over the course of a year, the data were divided into groups. The data were divided into 4 seasons, according to the metrological division for the northern hemisphere, which is used to compare periods of the same length and calculate statistics. According to this division: spring (1.03–31.05), summer (1.06–31.08), autumn (1.09–30.11), and winter (1.12–28.02).
2.3. Methods
The objective of the correlation analysis was to evaluate the presence of relationships between elements, their strength (
Table 1), direction, and shape. To this end, the linear Pearson correlation coefficient and non-parametric Spearman rank correlation were used for each pair of elements [
23]. The results are presented in the form of a correlogram, and the strength of the correlation was assessed based on
Table 1. A statistical significance level of
p-value = 0.05 was adopted. For a given
p-value and 50 degrees of freedom, the linear Pearson correlation coefficient will be statistically significant for correlations with values higher than 0.274 and lower than −0.274.
One-way analysis of variance (ANOVA) is a widely employed statistical technique utilized to determine the significance of differences between data subsets. This method is applicable when there are at least three mutually independent groups exhibiting normal distribution and homogeneous variance. Prior to conducting ANOVA, the key assumptions of normality, homogeneity of variances, and independence of cases must be verified. Normality of each group can be assessed using the Shapiro–Wilk test or Kolmogorov–Smirnov or using histograms with the skewness values and kurtosis. Equality of group variances can be evaluated with Levene’s test or Brown-Forsythe test. The assumption of independence is determined from the design of the study [
24,
25]. The ANOVA procedure employs the F-statistic to evaluate statistical significance, wherein the F-test compares the variance of each group mean to the overall group variance. If the within-group variance is smaller than the between-group variance, the F-test yields a higher F-value, indicating a greater likelihood that the observed difference is statistically significant. The null hypothesis posits that there are no statistically significant differences between group means, whereas the alternative hypothesis suggests that at least two groups exhibit statistically significant differences from one another [
25,
26].
One-way ANOVA is inherently designed to test a global alternative to the null hypothesis and may, therefore, be suboptimal if the research focus lies on a single specific contrast. It is statistically optimal under a squared-error loss framework but not when losses are better represented by absolute differences, in which case tests based on absolute deviations (e.g., median-based methods) can yield lower expected loss. Moreover, because ANOVA assumes normally distributed residuals, its
p-values may be imprecise and its power reduced when applied to data from heavy-tailed or markedly non-normal distributions, where permutation or nonparametric alternatives often perform better [
25,
27].
In the context of analysis of variance (ANOVA), post hoc tests are utilized to identify which specific groups differ significantly from one another, following the detection of an overall difference between groups in the ANOVA test. While ANOVA itself indicates whether there are differences among means across multiple groups, it does not specify which particular groups differ. Post hoc tests enable pairwise comparisons (two groups at a time) to determine which of these comparisons are statistically significant. The most commonly employed post hoc tests include the Newman–Keuls test, Bonferroni test, SIDAK test, Tukey’s HSD test, Tukey’s test for unequal sample sizes, and Scheffe test. For this analysis, we opted to use Tukey’s HSD test, which is more liberal than the Scheffe test and more conservative than the Newman–Keuls test [
28].
2.4. Workflow
The overall analytical workflow is summarized in
Figure 1, which outlines the main stages of the study:
Weekly sampling of bottom ash over 52 weeks;
Sample preparation (drying, crushing, grinding, digestion), instrumental measurements (ICP-MS, ICP-OES);
Data preprocessing (Tobit regression, ½ LOD substitution for censored data);
Statistical analyses (descriptive statistics, linear Pearson correlation coefficient and non-parametric Spearman rank correlation, one-way ANOVA with post hoc Tukey’s HSD);
Result visualization;
Comparative analysis with MSWI from Europe, Japan, and Korea;
Interpretation in the context of circular economy strategies.
2.5. Tools
The analyses were performed using R version 4.4.2 “Pile of Leaves” [
29] with RStudio 1 September 2024 “Cranberry Hibiscus”, utilizing the following packages: corrplot v. 0.95 (for correlation plotting), dplyr v. 1.1.4 (for data manipulation), ggplot v. 3.5.1 (for data visualization), and tidyr v. 1.3.1 (for data manipulation) [
30,
31].
3. Results
A characteristic feature of the elemental composition in Incineration Bottom Ash (IBA) is its high variability associated with the specificity of waste fed into the incinerator. As shown in
Table 2 and
Table S1, there is a significant range between minimum and maximum values, as well as high standard deviation values. Additionally, for some elements such as chromium, copper, manganese, nickel, vanadium, zinc, HREE (Heavy Rare Earth Elements), and LREE (Light Rare Earth Elements), the difference between the mean and median values exceeds 20% of the mean value. The maximum values are often multiple times higher than the median values. For example, the maximum value for LREE is as much as 74 times higher than the median value (28 ppm). Only for aluminum is the maximum value less than twice the median. A tenfold difference between the maximum and median values occurs for chromium, nickel, HREE, and LREE (
Table 2).
The variability of elements composition in IBA is presented in
Figure 2 and
Table S1. It is worth noting that there are no clear trends, patterns, or seasonality. The characteristic feature is the significant fluctuations in elemental composition that occur even in adjacent samples, which are only one week apart. Notably, the concentration of selected elements exceeds 100,000 ppm for 50 analyzed IBA samples, and in more than half of the cases, it exceeds 125,000 ppm.
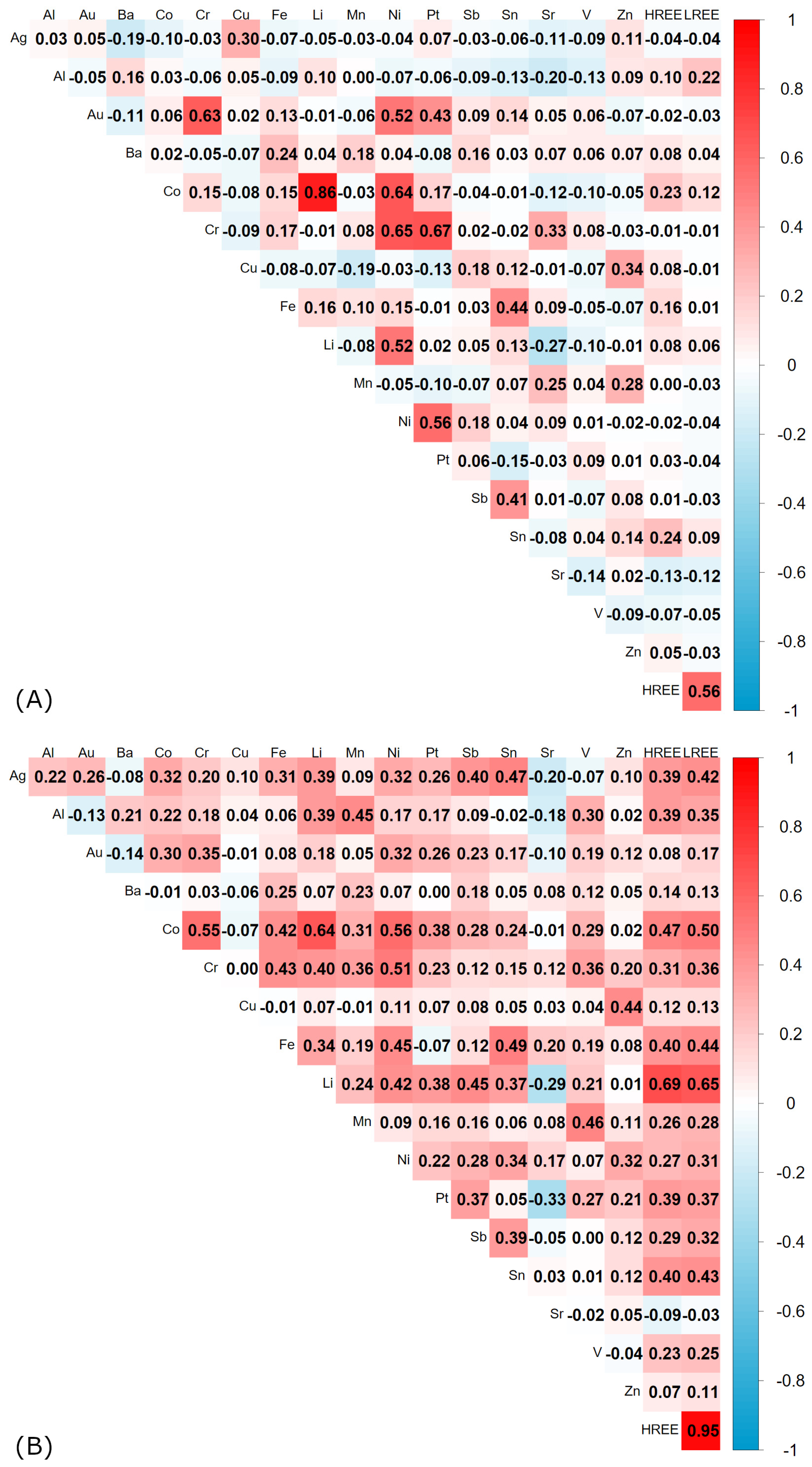
The correlation relationships between elements in Incineration Bottom Ash (IBA) from the investigated incinerator are typically nonlinear, with higher values of Spearman’s rank correlation coefficient (
Figure 3). Except for one correlation, all statistically significant correlations have a positive character, both for linear Pearson and Spearman’s rank correlations. The highest value of Spearman’s correlation coefficient was calculated for the pair of element groups HREE (Heavy Rare Earth Elements) and LREE (Light Rare Earth Elements), with a value of 0.95 (
Figure 3B). These two element groups are also strongly correlated with lithium, with values of 0.69 and 0.65, respectively. Interestingly, in the case of linear Pearson correlation, the correlation with lithium is 0.08 and is statistically insignificant. Both HREE and LREE exhibit nonlinear correlations with chromium, tin, aluminum, iron, cobalt, antimony, and vanadium. These correlations are classified as low-to-moderate. Additionally, LREE is lowly correlated with nickel and manganese (
Figure 3).
In addition to these correlations, silver exhibits a statistically significant relationship with copper in the case of Pearson’s linear correlation. When analyzing non-parametric relationships, it becomes apparent that correlations are more numerous and can be classified as low-to-moderate. For instance, silver is correlated with cobalt, iron, lithium, nickel, tin, antimony, HREE, and LREE, which can be observed in
Figure 3. Moreover, aluminum is correlated non-parametrically with five elements, of which only the correlation with manganese has a moderate strength. The remaining correlations with vanadium, HREE, LREE, and lithium are low (
Figure 3B).
It is also worth noting that gold exhibits a linear correlation with chromium, nickel, and platinum, with the correlation with chromium being moderately high. Gold also exhibits a statistically significant non-parametric correlation with cobalt of low strength (
Figure 3). In contrast, barium does not exhibit any statistically significant correlations. On the other hand, cobalt is one of the elements with numerous and stronger correlations, including five correlations with moderate strength: nickel, chromium, iron, HREE, and LREE. The correlation with lithium is moderately high, while the correlations with silver, gold, manganese, tin, and vanadium are low (
Figure 3).
Chromium exhibits statistically significant correlations with nine elements, of which the correlations with nickel, iron, cobalt, and lithium have moderate strength, while the correlations with gold, manganese, vanadium, HREE, and LREE are low, as can be observed in
Figure 3. Similarly, copper is correlated only with zinc, with a moderate strength (see
Figure 3B). Iron exhibits eight correlations, of which six have moderate strength: nickel, chromium, tin, cobalt, HREE, and LREE. The correlations with silver and lithium are low (
Figure 3B).
Lithium is an element for which some correlations have a linear character. Specifically, the correlation between lithium and nickel is higher for Pearson’s linear correlation and has moderate strength. The correlation between lithium and cobalt has high strength. The remaining correlations occur with silver, chromium, tin, aluminum, iron, antimony, and have a nonlinear character, which can be classified as low-to-moderate. Additionally, lithium exhibits a statistically significant negative correlation with strontium (
Figure 3).
Furthermore, manganese exhibits five statistically significant correlations, of which two with aluminum and vanadium have moderate strength. The remaining correlations with chromium, cobalt, and LREE have low strength (
Figure 3B). Nickel exhibits 11 correlations, including four with moderate strength: chromium, iron, cobalt, and lithium. The remaining correlations with silver, gold, tin, antimony, HREE, LREE, and zinc are low (see
Figure 3B).
In a similar manner, platinum exhibits a linear correlation with chromium and nickel, with the correlation with chromium being moderately high. Platinum also exhibits statistically significant non-parametric correlations with cobalt, antimony, HREE, and LREE, which have low strength. Additionally, platinum is significantly correlated non-parametrically with strontium, with a low negative relationship (
Figure 3). Antimony exhibits seven statistically significant correlations, of which the correlations with silver and lithium can be classified as moderate. The remaining correlations are low: nickel, tin, cobalt, HREE, and LREE (
Figure 3B).
Tin exhibits six statistically significant correlations, of which four can be classified as moderate: silver, iron, HREE, and LREE. The remaining correlations with nickel, antimony, and lithium are low (
Figure 3B). Vanadium is statistically significantly correlated with four elements: manganese, chromium, aluminum, and cobalt, with low strength, except for the correlation with manganese, where the correlation strength is moderate (see
Figure 3B). Finally, zinc is statistically significantly correlated only with two elements: copper and nickel, which are correlations of moderate and low strength, respectively (
Figure 3B).
In conclusion, strontium exhibits a low positive correlation with chromium and two low negative non-parametric correlations with lithium and platinum (
Figure 3B). These findings highlight the complex relationships between elements in Incineration Bottom Ash, emphasizing the need for further research to fully understand these interactions.
Pearson and Spearman correlation analyses revealed strong relationships between several element pairs. Moderately high and high correlation coefficients were observed for Li-Co, Cr-Co, Cr-Ni, Cr-Pt, Ni-Co, and LREE–HREE across both methods, indicating common sources or co-behavior during combustion.
Prior to conducting the ANOVA analysis, the assumption of normal distribution within each seasonal group was evaluated using the Shapiro–Wilk test. For aluminum (Al), the data from spring, summer, and winter followed a normal distribution, whereas the autumn group did not meet the normality assumption. In the case of barium (Ba), strontium (Sr), and heavy rare earth elements (HREE), the normality assumption was satisfied for spring, summer, and autumn, but not for winter. Levene’s test indicated homogeneity of variances across all seasonal groups for each of the analyzed elements.
Although the Shapiro–Wilk test indicated that one of the seasonal groups did not follow a normal distribution, the ANOVA procedure was still applied based on the robustness of the method. One-way ANOVA is generally considered robust to moderate deviations from normality, particularly when the sample sizes across groups are equal or comparable and when the assumption of homogeneity of variances is met. In this study, Levene’s test confirmed equal variances across all groups. Moreover, visual inspection of Q-Q plots and distribution histograms suggested no severe skewness. Therefore, despite the violation of normality in a single group, the ANOVA test was deemed appropriate for detecting differences in elemental concentrations between seasons.
A one-way ANOVA was conducted to examine whether the elemental content in IBA varies by season. The results indicated a significant difference between seasons for barium, F (3, 48) = 3.69, p = 0.019, and strontium, F (3, 48) = 8.915, p < 0.001. Additionally, marginally significant differences were observed for aluminum, F (3, 48) = 2.66, p = 0.059, and HREE, F (3, 48) = 2.221, p = 0.098, although these effects did not reach the conventional threshold of p < 0.05.
A post hoc Tukey’s Honest Significant Difference (HSD) test was conducted to identify which seasons exhibited significant differences from one another. Specifically, this analysis aimed to determine whether certain elements varied significantly across seasons. For barium, Tukey’s HSD test revealed that significant seasonal differences were observed between summer and spring (mean difference = 128.30), p.adj = 0.045, as well as between winter and spring (mean difference = 138.86), p.adj = 0.026. Additionally, a trend towards significance was noted for the difference between fall and spring (mean difference = 112.32), p.adj = 0.097.
Notably, similar seasonal patterns were observed for strontium, where Tukey’s HSD test revealed significant seasonal differences between fall and spring (mean difference = 140.55), p.adj < 0.001, as well as between fall and summer (mean difference = 92.98), p.adj = 0.008. Furthermore, a significant difference was noted between winter and fall (mean difference = −78.31), p.adj = 0.033.
In contrast, the elements that did not exhibit significant seasonal differences, as indicated by ANOVA effects with p-values greater than 0.05, and lower than 0.1 included aluminum and HREE. For aluminum, Tukey’s HSD test revealed that the largest seasonal differences were observed between summer and spring (mean difference = 8930.77), although this difference was not statistically significant, p.adj = 0.091. A similarly high difference was noted between winter and spring (mean difference = 8892.31), p.adj = 0.093. For HREE, Tukey’s HSD test revealed that the largest seasonal difference was observed between winter and fall (mean difference = 7.81), although this difference was not statistically significant, p.adj = 0.14.
The between-season variability for these elements, as well as the observations, are also illustrated in
Figure 4. Box-and-whisker plots and bar charts provide a visual representation of the seasonal patterns and differences. Despite the fact that the ANOVA test results were not significant at the adopted level of significance for aluminum, differences between seasons can be observed in
Figure 4. Over the analyzed time period, aluminum exhibits a notable trend where the average values for the spring and autumn seasons, as well as for the summer and winter seasons, are similar However, they differ substantially in terms of the interquartile range (IQR) (
Figure 4B). Furthermore,
Figure 4A show that higher concentrations of aluminum were more frequently recorded during the summer season. The maximum value for this element was also detected during this period. This is particularly evident when compared to the lower aluminum contents observed during the spring season (
Figure 4A). In the fall season, the average aluminum content decreased to a level comparable to that of the spring. However, isolated observations with values significantly higher than the mean continued to occur (
Figure 4A,B).
Figure 4C,D confirm the results of the ANOVA analysis and Tukey’s HSD test for barium. The average value for the spring season is lower than that of the other seasons, which have similar values (
Figure 4D). Additionally, a higher IQR value is observed during the spring season. Furthermore, within the designated spring period, aside from a pronounced decrease, a sudden increase in barium content in IBA is noted in the last two samples of this period (
Figure 4C). Outlier values are only present in the winter (maximum) and autumn seasons.
For strontium, an interesting variability in the content of this element in IBA is observed, despite the absence of changes in the technological process of the incinerator and the type of waste burned. In the second half of the year from 31 sample number, the strontium content in IBA increases (
Figure 4E) from 170 ppm to 320 ppm. As a result, the lowest average content was observed in the spring season, and the highest in the fall season. These two seasons are characterized by relatively low IQR values (
Figure 4F). In contrast, the summer and winter periods included both low and high observations, resulting in a high interquartile range (
Figure 4E,F).
For heavy rare earth elements, a slight decrease in the average content of this sum of elements in IBA is observed during the autumn season, followed by an increase during the winter season (
Figure 4H). Outlier values occur during the winter season (
Figure 4G). The interquartile range for all seasons except winter is low and similar across the board (
Figure 4H).
Seasonal decomposition showed observable intra-annual fluctuations in the concentrations of barium and strontium, while aluminum and heavy rare earth elements displayed trends close to statistical significance. These patterns suggest that seasonal variation in waste input may influence the elemental makeup of IBA. Such findings are consistent with literature on waste seasonality in other regions.
4. Discussion
The strong correlation between Cu and Zn (r > 0.8) suggests their affinity for similar organic or mineral fractions [
7]. Similarly, Zn may be associated with sulfide or oxide fractions.
Iron (Fe) correlates with Ni, Sr, Sn, and Co, which is likely due to the presence of their oxides and spinels, common in incineration ashes [
32]. Their co-occurrence results from their similar chemical structure and affinity. During combustion, iron oxidizes, forming oxides and hydroxides that can adsorb other elements, leading to their fixation [
33]. A significant portion of iron is recovered during the secondary waste enrichment stage.
Cobalt, due to its low volatility, remains in the bottom ash during combustion. It exhibits moderate to high linear correlations with Ni and Li, indicating geochemical affinity. However, low correlations with Sn and HREE suggest that other factors may influence its content [
34].
Nickel remains in the bottom ash during combustion, often forming stable oxides [
35]. Correlation analysis revealed that nickel exhibits moderate to high linear correlations with Cr and Co, reflecting their proximity in the periodic table and geochemical affinity [
36].
Rare earth elements (HREE, LREE) form a distinct group with high mutual correlation (r > 0.9), indicating a common source and low mobility in ash [
37]. Previous studies have shown that rare earth elements are primarily bound to mineral phases such as phosphates or oxides [
38]. HREE and LREE are introduced into the waste stream through electronics, magnets, and industrial applications. During combustion, they remain in the bottom ash, forming stable compounds, and their distribution depends on specific mineralogical and geochemical factors, requiring targeted research to understand their mobility and potential environmental impact [
39].
The Sn-Sb correlation is also noteworthy. These elements often occur together in technological wastes, and their presence in ashes suggests similar anthropogenic sources and affinity for mineral phases with low solubility [
40]. Sn and Sb have similar atomic radii due to their proximity in the periodic table, resulting in mutual substitution in mineral crystal lattices [
41].
Chromium occurs mainly in two oxidation states: Cr(III), which is relatively stable and poorly soluble, and Cr(VI), which is toxic, highly mobile, and hazardous to the environment. Cr(VI) forms at high temperatures [
36]. Studies have shown that Cr correlates moderately to strongly with other metals such as Ni and Co, which often co-occur in alloys and electronic waste. Both Cr and Ni typically form oxides or spinel structures in slag.
The lack of correlation between Ba content and other elements may be due to its specific geochemical characteristics. Ba is a lithophile element associated with aluminosilicate minerals [
42], leading to uneven distribution in samples and absence of significant correlations with other elements. The presence of Ba in ashes can result from various industrial processes, making it challenging to model its concentration based on other variables.
Aluminum in municipal waste originates primarily from packaging (cans, foils), electronic devices, and construction materials. During combustion, aluminum mostly remains in the form of oxides in the bottom ash. Research has shown that metallic aluminum in bottom ash can react with water, producing hydrogen, raising concerns about safety and affecting the stability of ash used in building materials [
13].
Lithium enters the waste stream mainly through spent batteries and electronic devices. During combustion, Li may become volatile, especially at higher temperatures, but a significant portion can remain in the ash. The form in which it passes into secondary waste depends on specific combustion conditions. Studies have demonstrated that during battery combustion, water-soluble lithium compounds such as lithium carbonate (Li2CO3) are formed, which are more mobile [
43]. Lithium correlations with Co and HREE suggest potential co-occurrence in common minerals or alloys, particularly evident in the context of lithium-ion battery materials, where Li, Co, and HREE are integral components of cathode materials [
12]. Proper interpretation of this correlation is essential for developing effective recovery strategies. Moderate-to-high correlations with Co and HREE indicate that recovery processes targeting these elements may also facilitate.
The analysis of seasonal variability in the elemental content of IBA using one-way ANOVA revealed statistically significant differences for barium (Ba) and strontium (Sr) (p = 0.019 and p < 0.001, respectively), confirming a pronounced seasonality of these elements. In both cases, the lowest average values occurred in spring, while significantly higher concentrations were observed in summer and winter, as confirmed by post hoc Tukey’s test. This suggests the possibility of seasonal changes in waste composition.
Although the content of aluminum (Al) and heavy rare earth elements (HREE) did not reach classical significance levels (
p < 0.05), the observed borderline values (Al:
p = 0.059; HREE:
p = 0.098) indicate a trend towards seasonal differentiation. Relaxing the significance threshold to an exploratory level (e.g.,
p < 0.1), which is acceptable in environmental studies characterized by high variability [
28], allows for cautious interpretation of these results as potentially significant. The distribution of values on box plots supports this interpretation—the contents of Al, Ba, and Sr are lowest in spring, while interquartile range for Al and Sr is highest in summer. Outliers occur in winter—low for Al and high for Ba, Sr, and HREE. This may reflect seasonal differences in the type of waste burned, such as a greater proportion of electronic waste or metal waste in winter due to the heating season and holidays. The observed seasonality of two elements—Ba and Sr—as well as the borderline-significant seasonal trend in HREE and Al, appears to preliminarily confirm the presence of intra-annual variability in Polish municipal solid waste, consistent with findings reported in the recent literature [
9,
10,
11].
Similar seasonal dependencies of metal content in waste have been confirmed by other studies [
5], which observed variability in elemental concentrations depending on the time of year and type of waste burned. In the case of Ba and Sr, it can be assumed that their presence may be related to seasonal sources (e.g., fireworks, consumption of certain building materials). For HREE and Al, the presence of outliers in winter may result from a greater proportion of electronic waste and aluminum cans in the winter waste stream.
The obtained results have significant implications not only for the environment but also for the economy. The identified variability in the content of critical elements in bottom ash indicates their potential recovery as secondary raw materials, which is in line with the principles of a circular economy. Bottom ash, previously treated mainly as waste, may gain the status of a valuable material, especially in the context of growing demand for elements such as lithium, cobalt, HREE, and aluminum [
4].
Statistical data, particularly correlations and seasonal fluctuations in concentrations, can be used in decision-making processes related to the design and optimization of metal separation and recovery technologies from IBA. For example, high correlation between rare earth elements and lithium may suggest the possibility of simultaneous recovery of these components using similar extraction processes [
12]. Seasonal changes in elemental content may, in turn, provide a basis for flexible management of processing lines, e.g., by increasing the intensity of recovery during periods of higher concentration of these elements [
5].
A comparative analysis of the results obtained in this study with those from other regions revealed both similarities and significant differences, thereby enhancing the scientific value of the presented findings. For instance, the strong correlation between Cu and Zn (r > 0.8) confirms the results of studies conducted in Singapore, Japan, and Switzerland, where these elements also co-occurred, often in the form of oxide or sulfide phases [
5,
6,
13].
Similarly, the high mutual correlation between HREE and LREE (r = 0.95) is consistent with observations from China and the Netherlands, where REE were primarily associated with low-mobility oxide and phosphate mineral phases [
7,
38]. However, our study revealed a stronger-than-usual correlation between REE and Li, which may be attributed to the regional prevalence of lithium-ion batteries in the waste stream [
12,
43].
The seasonal variability of Ba and Sr content in ashes is more pronounced than in studies from countries such as Italy or Japan, where a stable waste collection system results in smaller seasonal fluctuations [
37,
40]. This may be due to local socio-economic factors such as heating seasons or holidays.
To place the observed elemental concentrations into a broader context, we compared the mean values from our MSWI bottom ash with data from seven other facilities—three located in Europe [
44,
45,
46], three in Japan [
47,
48], and one in Korea [
49]. This comparison revealed substantial variability across plants (see
Tables S2 and S3 in the
Supplementary Materials). Reported sample sizes ranged from approximately 20 to 85, with Denmark and Korea contributing the largest datasets (>80 samples), compared to 52 samples analyzed in this study [
45,
49].
Our Polish MSWI exhibits similar or slightly lower average concentrations of most elements compared to other European MSWI but shows markedly higher standard deviations, reflecting greater feedstock heterogeneity [
44,
45,
46]. Among European facilities, the German MSWI consistently records the highest levels of Al, Ba, Co, Cr, Cu, Fe, Mn, Ni, Sb, Sr, and Zn—up to three times greater than in other European bottom ashes [
46].
The Danish bottom ash often exceeds ours in Ba, Co, Cr, Ni, Sb, Sn, and V. Notably, nickel and barium concentrations in the Danish ash are several-fold higher than those observed in our samples [
45]. Bottom ash from Polish MWSI contains comparable or higher Au, Cu, Pt, and Zn (
Table S2).
Relative to the Swiss facility, our bottom ash has elevated Al, Co, Cr, Cu, Ni, Sn, Sr, V, and Zn, but marginally lower Au, Fe, and Li, and lower Pt.
Rare earth element (REE) concentrations likewise vary widely: the German ash shows the highest REE content overall, whereas our plant surpasses others only in Pr and Nd—though these values carry large standard deviations due to outliers—while other REEs are lower than in European MWSI (
Table S3) [
45,
46].
Comparing to East Asian MWSI, the Korean bottom ash is notable for low Ba, Cu, Fe, and Zn but high Co, Cr, and Sb, whereas Japanese plants exhibit higher Al, Au, Fe, Sn, and Zn than ours. Our bottom ash nonetheless contains more Ba and Cu and a similar total REE content to the Japanese MSWI, though it falls below Korean REE levels (
Tables S2 and S3) [
47,
48,
49]. This cross-facility comparison underscores the influence of regional waste streams and operational practices on ash chemistry.
In contrast to most previous studies, which focused on leachability tests and mineralogical analyses [
32], this study offers an integrated approach: correlation analysis, seasonal analysis, and statistical analysis. This methodology allows for the capture of temporal variability and may be useful in designing flexible strategies for resource recovery from IBA. Notably, the obtained results have potential direct industrial applications. The confirmed correlation between critical elements such as Li, Co, and REE suggests the possibility of their co-recovery using technologies like hydrometallurgy, pyrometallurgy, or thermally chemical selective processes [
12,
14,
15]. These technologies are being developed in the context of recycling spent lithium-ion batteries and REE-containing ashes.
The application of the obtained data in industrial practice can contribute to reducing dependence on imports of critical elements and increasing the EU’s raw material sovereignty. Moreover, effective recovery from IBA supports the achievement of European Green Deal goals, increasing resource efficiency and reducing environmental pressure by limiting the need for primary deposit exploitation [
3,
4].
The integration of the obtained data with technological solutions will enable not only the improvement of recovery efficiency but also a reduction in the EU’s dependence on imports of critical raw materials.
The main limitation of the study is the number of samples, which totals 52 and is restricted to a single calendar year. As a result, a comprehensive assessment of seasonality and long-term variability could not be performed. Additionally, a detailed analysis of the composition of the municipal waste input to the incineration plant would be necessary to fully evaluate the drivers of elemental variability in the bottom ash.
Subsequent stages of the project will focus on mineralogical and phase composition analysis of the bottom ash, which will enable the interpretation of elemental associations and potential speciation affecting metal mobility or recovery. In addition, an assessment of the technological recovery potential is planned.
5. Conclusions
This study provides comprehensive insights into the chemical composition of bottom ash samples, revealing a high degree of heterogeneity that reflects the variability of waste streams and combustion processes. This variability is associated with the specificity of wastes entering incineration facilities, resulting in significant fluctuations in elemental concentrations in bottom ashes. Statistical analyses revealed strong correlations between certain elements, including Cu-Zn, Co-Ni, and HREE-LREE, indicating common sources and similar geochemical properties. These results confirm existing literature on the co-occurrence of these elements in mineral or organic fractions and their affinity for solid phases such as oxides, spinel, or organic complexes.
The correlation analysis between elements in IBA samples from incineration facilities reveals complex relationships between individual elements. The most interesting correlations are those between rare earth elements (HREE and LREE), which form a distinct group with high mutual correlation, indicating a common source and low mobility in ash. These results are consistent with previous studies that have shown that rare earth elements are primarily bound to mineral phases such as phosphates or oxides. The study also highlights significant correlations between other elements, such as copper (Cu) and zinc (Zn), which suggest their affinity for similar organic or mineral fractions. Additionally, iron (Fe) exhibits correlation with nickel (Ni), strontium (Sr), tin (Sn), and cobalt (Co), which may be related to the presence of oxides and spinels in ashes.
The results of the analysis show that the elemental composition of IBA is highly variable, making it challenging to predict their concentrations. The lack of clear trends, patterns, and seasonality in elemental variability indicates that municipal waste incineration processes are unpredictable and complex. The study reveals significant variability in elemental concentrations in IBA, which may be caused by the specificity of wastes entering incineration facilities and processes occurring during combustion. This variability is particularly evident for elements such as chromium, copper, manganese, nickel, vanadium, zinc, HREE, and LREE, where maximum values are several times higher than the median.
The application of correlation analysis enabled the identification of elements with significant relationships between them. The study’s results have important practical implications for environmental safety, ash management, and element recovery. The high variability in elemental concentrations between IBA samples collected at weekly intervals highlights the need for long-term monitoring and average sampling for quality assessment purposes. The study found that only for aluminum is the maximum value lower than twice the median, while for other elements, there are significant differences between minimum and maximum values. The lack of clear seasonal trends and numerous outliers indicates a dynamic character of ash composition, emphasizing the importance of flexible statistical analysis methods.
The obtained results highlight the importance of understanding complex relationships between elements in ash samples and developing effective strategies for ash management and element recovery. These findings can serve as a basis for developing more efficient waste management and resource recovery strategies, ultimately contributing to a circular economy. Furthermore, this study emphasizes the need for further research on geochemical modeling of ash and integrating chemical data with process data related to combustion conditions. The results have significant implications for environmental safety, ash management, and element recovery, and can serve as a basis for developing more efficient waste management and resource recovery strategies.
Furthermore, the one-way ANOVA results indicate significant seasonal variability in the content of Ba and Sr in bottom ash, which may be directly related to differences in the type of waste burned during different seasons. Lower average values of these elements were observed during the spring season, while greater variability was noted during summer and winter. Although Al and HREE did not reach classical significance levels, their distributions suggest possible seasonal differentiation, particularly with distinct outliers during winter. These findings underscore the need for long-term monitoring and seasonal analysis of IBA composition to optimize resource recovery processes and assess environmental risk. Integrating chemical data with operational data on waste composition and combustion parameters may contribute to a better understanding of the variability and sources of individual elements in IBA.
The comparative analysis with international datasets confirmed that the concentrations of key recoverable metals in Polish incineration bottom ash (IBA), particularly aluminum, copper, iron and zinc, are comparable to those reported in Western European. Incineration plants in Korea and Japan exhibit markedly different elemental composition profiles in their bottom ash, with substantial variability even between facilities within the same country. Compared to these, the Polish facility analyzed in this study shows lower concentrations of certain elements relative to Japanese plants, but frequently higher levels than those reported for Korean facilities. Notably, with the exception of the German incinerator, the Polish ash demonstrates a composition closely resembling that of Swiss and Danish incineration plants. For instance, This suggests that Polish IBA constitutes a viable secondary source of these metals and may meet or surpass typical recovery thresholds.