Reducing Energy Penalty in Wastewater Treatment: Fe-Cu-Modified MWCNT Electrodes for Low-Voltage Electrofiltration of OMC
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrodes with “Sandwich” Structure
2.2. Material Morphology Testing
2.3. Electrochemical Property Testing
2.4. Degradation of Octyl Methoxycinnamate by Electrically Activated Persulfate at Sandwich Electrode
3. Results
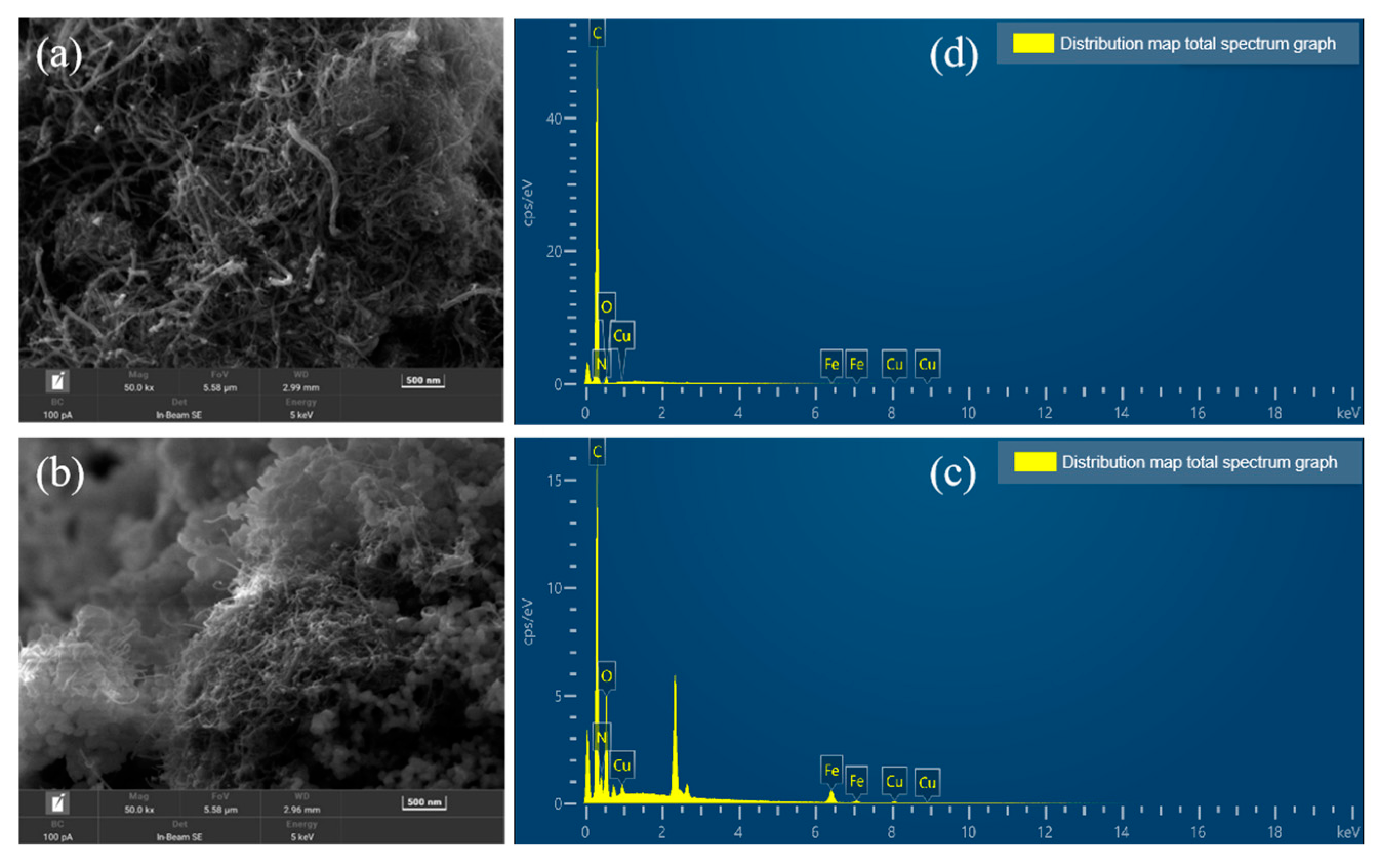
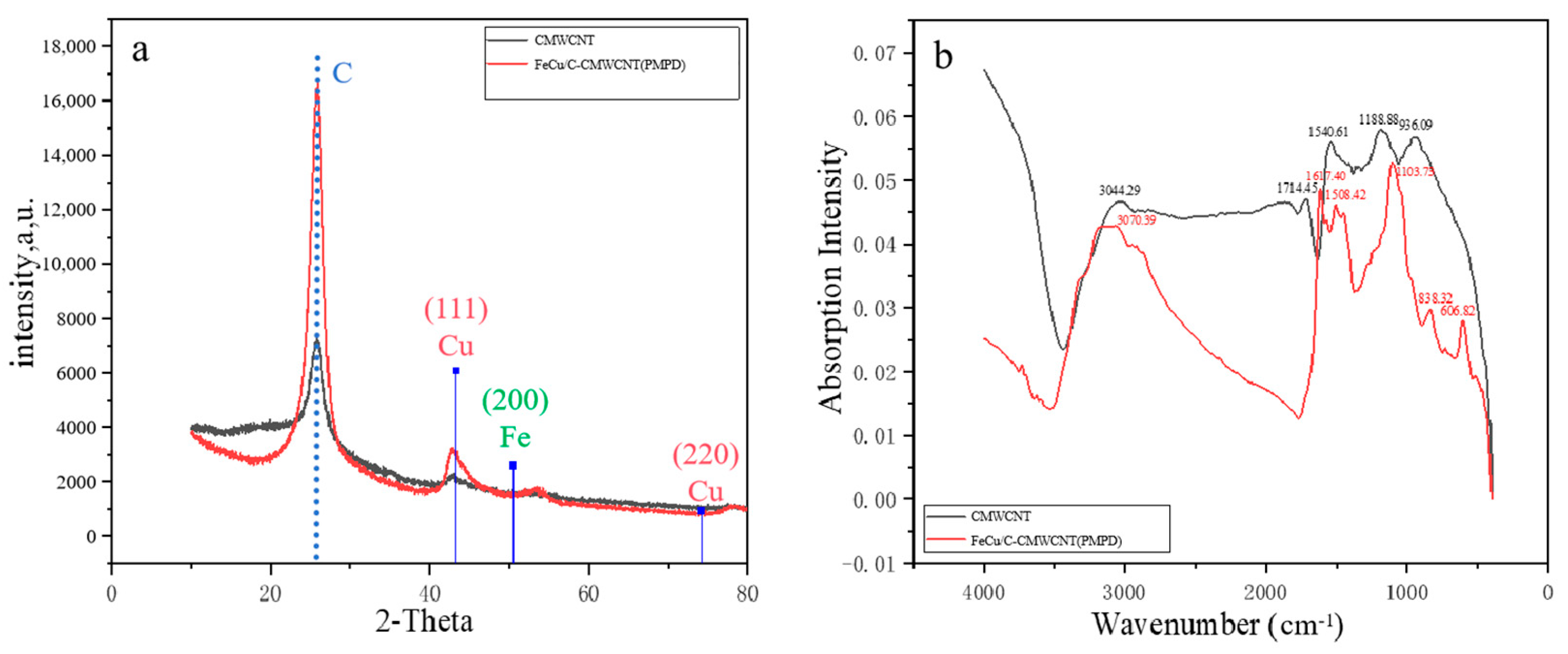
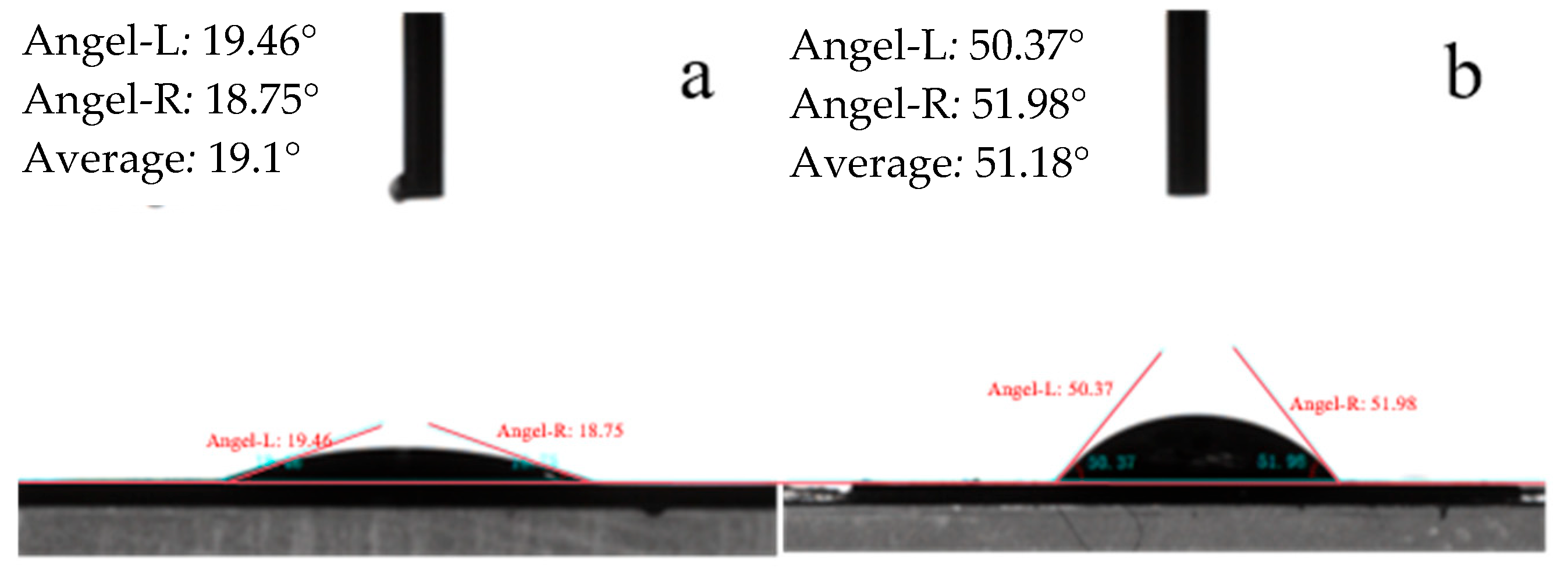
3.1. Analysis of Morphology Test Results
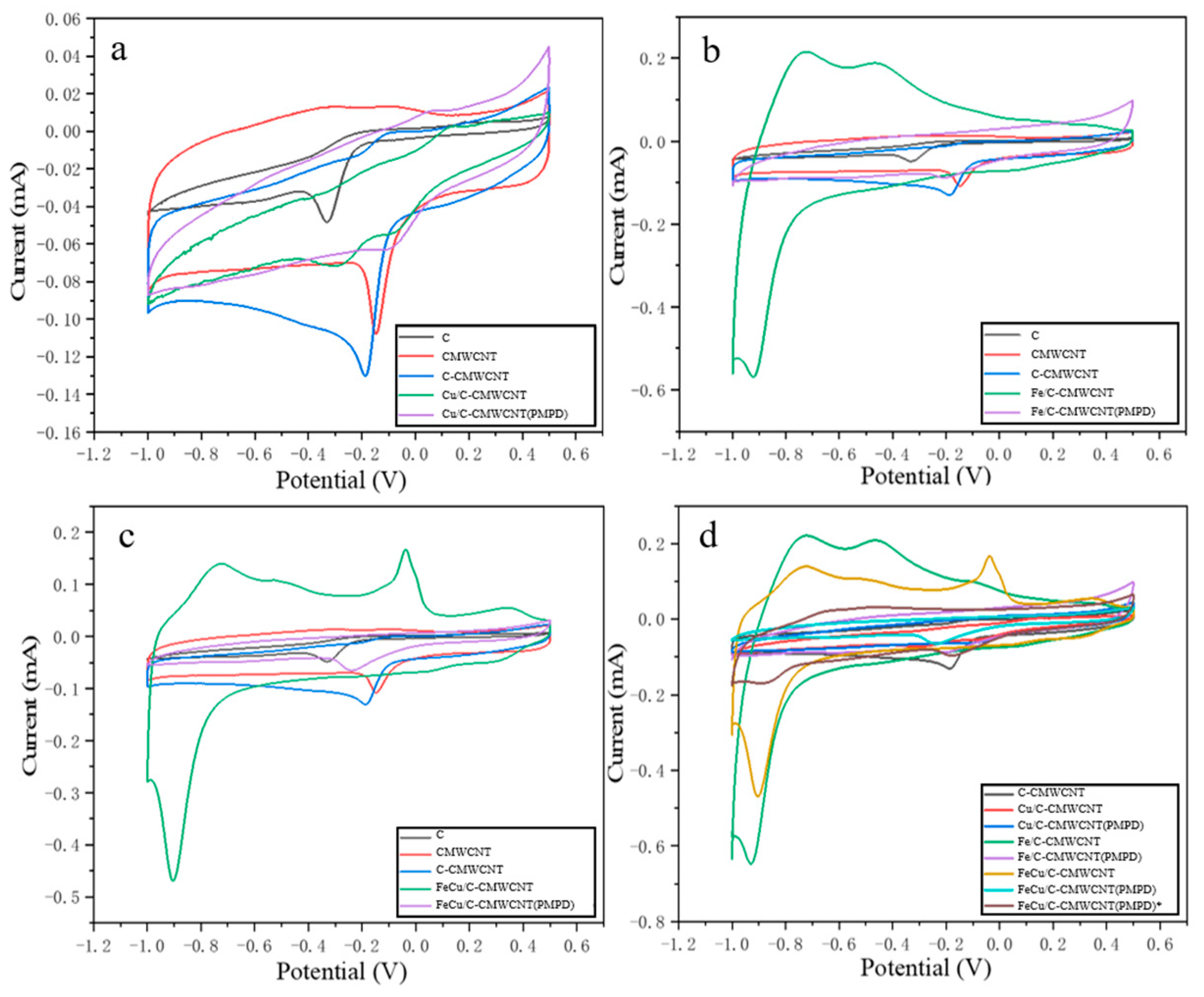
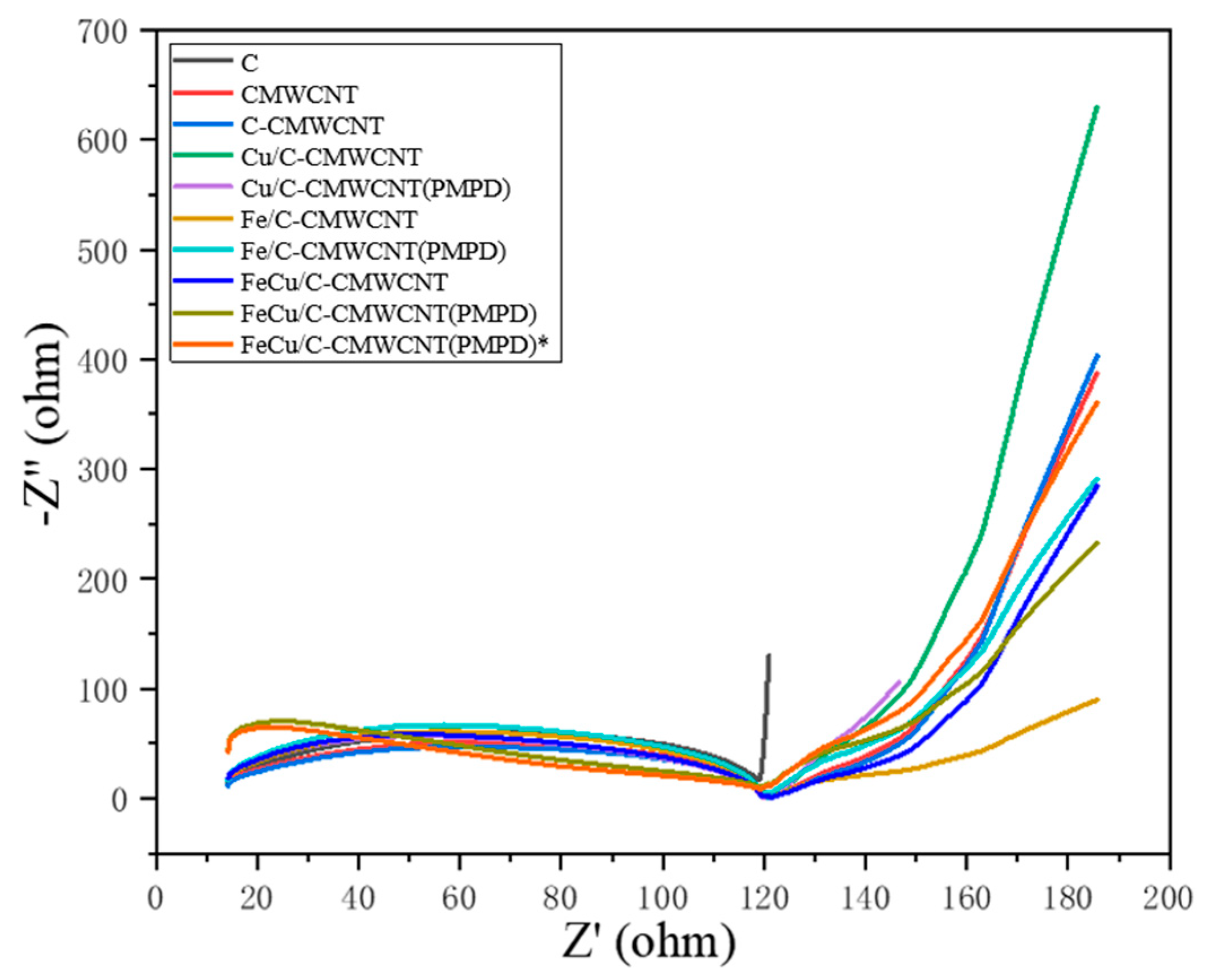
3.2. Modified Material Performance Test Analysis
3.3. Degradation of OMC by Electrically Activated Persulfate at Sandwich Electrodes
3.3.1. Principle of Electro-Activated Sodium Persulfate Oxidative Degradation of Organic Pollutants
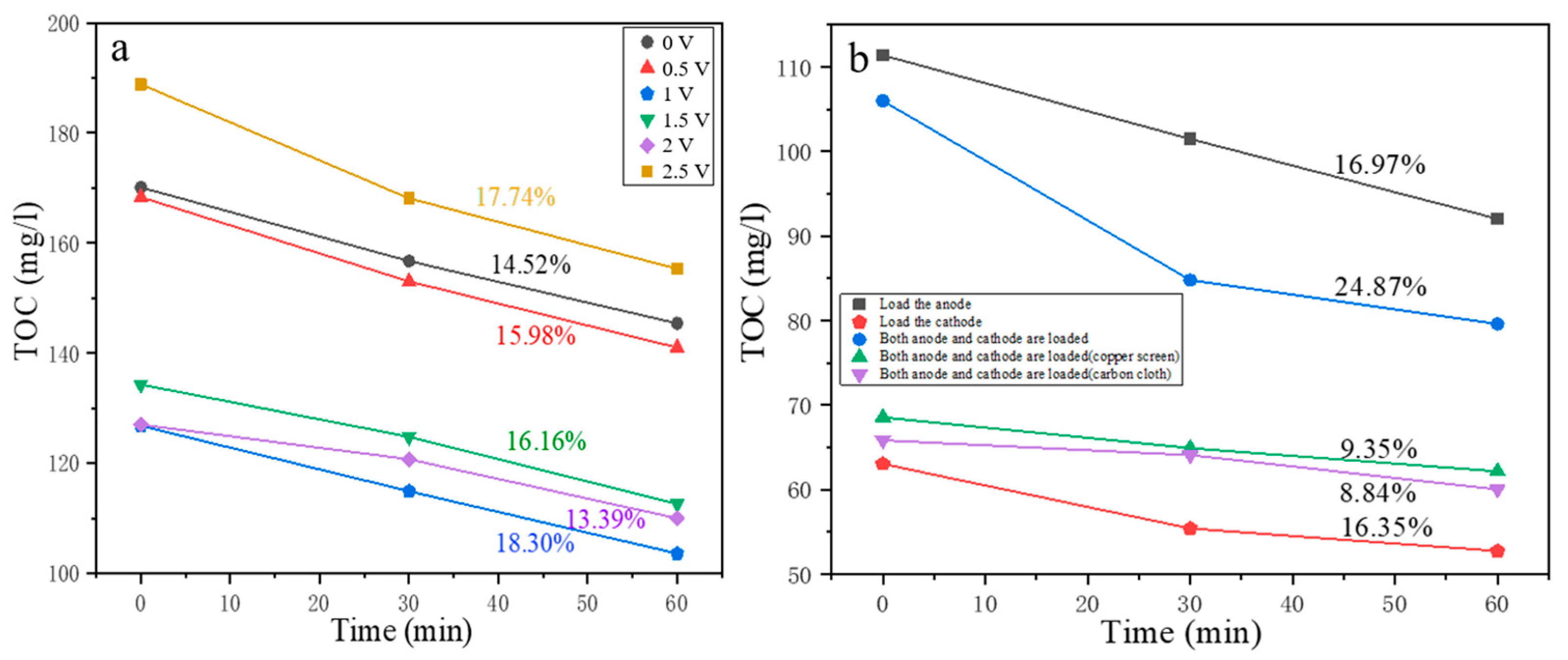
3.3.2. Degradation Effect of OMC by Electrically Activated Persulfate at Sandwich Electrodes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OMC | Octyl methoxycinnamate |
| PPCP | Personal care product |
| CNT | Carbon nanotube |
| MWCNT | Multi-walled carbon nanotube |
| EMR | Electrolytic manganese residue |
| ROX | Roxarsine |
| CMWCNT | Carboxylated multi-walled carbon nanotube |
| MPD | M-phenylenediamine |
| PMPD | Poly-m-phenylenediamine |
| PVDF | Polyvinylidene |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscope |
| XRD | X-ray diffraction |
| FTIR | Fourier infrared spectroscopy |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| DC | Direct current |
| TOC | Total organic carbon |
| EDX | Energy dispersive X-ray spectroscopy |
| NCs | Nanocrystals |
| PEC | Photoelectrochemical |
| NMP | N-methyl-2-pyrrolidone |
| N-GN | Nitrogen-doped graphene |
| APAP | Acetaminophen |
| NPs | Nanoparticles |
References
- Pei, J.; Hu, J.; Zhang, R.; Liu, N.; Yu, W.; Yan, A.; Han, M.; Liu, H.; Huang, X.; Yu, K. Occurrence, Bioaccumulation and Ecological Risk of Organic Ultraviolet Absorbers in Multiple Coastal and Offshore Coral Communities of the South China Sea. Sci. Total Environ. 2023, 868, 161611. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.A.; Hopkins, F.E.; Saha, M.; Jha, A.N. Ecotoxicological effects of sunscreen derived organic and inorganic UV filters on marine organisms: A critical review. Mar. Pollut. Bull. 2025, 213, 117627. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef]
- Nataraj, B.; Maharajan, K.; Hemalatha, D.; Rangasamy, B.; Arul, N.; Ramesh, M. Comparative toxicity of UV-filter octyl methoxycinnamate and its photoproducts on zebrafish development. Sci. Total Environ. 2020, 718, 134546. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Chen, Y.; Yao, B.; Chen, X.; Yu, Y.; Yang, J.; Zhou, Y. Pharmaceuticals and Personal Care Products (PPCPs) in the Aquatic Environment: Biotoxicity, Determination and Electrochemical Treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I. Contributions of Electrochemical Oxidation to Waste-Water Treatment: Fundamentals and Review of Applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Karim, M.A.H.; Hama, S. Biochar-Based Catalysts: An Efficient and Sustainable Approach for Water Remediation from Organic Pollutants via Advanced Oxidation Processes. J. Environ. Manag. 2025, 390, 126245. [Google Scholar] [CrossRef]
- Silva, J.A. Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review. Sustainability 2025, 17, 3439. [Google Scholar] [CrossRef]
- Ratola, N.; Cincinelli, A.; Alves, A.; Katsoyiannis, A. Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J. Hazard. Mater. 2012, 239, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Cui, T.; Huang, F.; Zhang, Y.; Han, W. Membrane separation coupled with electrochemical advanced oxidation processes for organic wastewater treatment: A short review. Membranes 2020, 10, 337. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.S.; Jiang, Y.X.; Sun, L.; Sun, X.H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef]
- Meskher, H.; Ragdi, T.; Thakur, A.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.; Pandey, A.K.; Saidur, R.; Singh, P.; et al. A review on CNTs-based electrochemical sensors and biosensors: Unique properties and potential applications. Crit. Rev. Anal. Chem. 2024, 54, 2398–2421. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Du, H.; Tuo, D.; Qi, X.; Wang, T.; Wu, J.; Li, G. UiO-66/carboxylated multiwalled carbon nanotube composites for highly efficient and stable voltammetric sensors for gatifloxacin. ACS Appl. Nano Mater. 2023, 6, 19403–19413. [Google Scholar] [CrossRef]
- O-Rak, K.; Ummartyotin, S.; Sain, M.; Manuspiya, H. Covalently grafted carbon nanotube on bacterial cellulose composite for flexible touch screen application. Mater. Lett. 2013, 107, 247–250. [Google Scholar] [CrossRef]
- Ören Varol, T.; Anik, Ü. Fabrication of multi-walled carbon nanotube–metallic nanoparticle hybrid nanostructure based electrochemical platforms for sensitive and practical colchicine detection. New J. Chem. 2019, 43, 13437–13446. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, S.; Sun, Z.; Zhang, Y.; Jin, W. Alkaline electrochemical advanced oxidation process for chromium oxidation at graphitized multi-walled carbon nanotubes. Chemosphere 2017, 183, 156–163. [Google Scholar] [CrossRef]
- Pan, G.; Sun, X.; Sun, Z. Fabrication of multi-walled carbon nanotubes and carbon black co-modified graphite felt cathode for amoxicillin removal by electrochemical advanced oxidation processes under mild pH condition. Environ. Sci. Pollut. Res. 2020, 27, 8231–8247. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; Zhong, H.; Hu, L.; Sun, W. Multi-walled carbon nanotubes facilitated roxarsone elimination in SR-AOPs by accelerating electron transfer in modified electrolytic manganese residue and forming surface activated-complexes. Water Res. 2021, 200, 117266. [Google Scholar] [CrossRef]
- Chang, C.F.; Chen, T.-Y.; Chin, C.J.M.; Kuo, Y.T. Enhanced electrochemical degradation of ibuprofen in aqueous solution by PtRu alloy catalyst. Chemosphere 2017, 175, 76–84. [Google Scholar] [CrossRef]
- Yu, G.; Wei, P.; Wang, F.; Liu, J. Doping copper ions into an Fe/N/C composite promotes catalyst performance for the oxygen reduction reaction. ChemElectroChem 2017, 4, 1509–1515. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Li, L.; Wang, X.; Duan, H.; Luo, C. Multiwalled carbon nanotubes-doped polymeric ionic liquids coating for multiple headspace solid-phase microextraction. Talanta 2014, 123, 18–24. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, T.; Li, X.; Cui, Q.; Wu, Q.; Wang, X.; Song, K.; Ge, D. Low-temperature hydrothermal synthesis of novel 3D hybrid nanostructures on titanium surface with mechano-bactericidal performance. ACS Biomater. Sci. Eng. 2021, 7, 2268–2278. [Google Scholar] [CrossRef]
- Tellam, J.; Zong, X.; Wang, L. Low temperature synthesis of visible light responsive rutile TiO2 nanorods from TiC precursor. Front. Chem. Sci. Eng. 2012, 6, 53–57. [Google Scholar] [CrossRef]
- Tian, X.; Wang, W.; Wang, Y.; Komarneni, S.; Yang, C. Polyethylenimine Functionalized Halloysite Nanotubes for Efficient Removal and Fixation of Cr (VI). Microporous Mesoporous Mater. 2015, 207, 46–52. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.X.; Cong, X.; Wu, S.S.; Wu, J.B.; Bao, Y.-F.; Cao, M.-F.; Wu, L.; Lin, M.-L.; Wang, X.; Tan, P.-H.; et al. Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction. Nat. Catal. 2024, 7, 646–654. [Google Scholar] [CrossRef]
- Pan, Y.; Shen, X.; Yao, L.; Bentalib, A.; Peng, Z. Active sites in heterogeneous catalytic reaction on metal and metal oxide: Theory and practice. Catalysts 2018, 8, 478. [Google Scholar] [CrossRef]
- Li, C.; Yan, S.; Fang, J. Construction of lattice strain in bimetallic nanostructures and its effectiveness in electrochemical applications. Small 2021, 17, 2102244. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, M.W.; Kim, H.J.; Choung, J.W.; Jung, C.; Kim, C.H.; Lee, K.Y. Effect of Ag doping on Pd/Ag-CeO2 catalysts for CO and C3H6 oxidation Ag. J. Hazard. Mater. 2021, 415, 125373. [Google Scholar] [CrossRef]
- Özbilen, S. Influence of atomising gas on particle characteristics of Al, Al–1 Wt-%Li, Mg, and Sn powders. Powder Metall. 2000, 43, 173–180. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, T.S.; Lee, J.K.; Kim, H.J.; Kim, D.H.; Bae, J.C. Effect of powder size on the consolidation of gas atomized Cu54Ni6Zr22Ti18 amorphous powders. Intermetallics 2006, 14, 1000–1004. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, H.Z.; Li, H.; Bian, L.I.; Lu, Y.Y. Synthesis and characterization of MWNTs coated with BaFe12O19 composites. Mater. Mech. Eng. 2010, 34, 88–91. [Google Scholar]
- Rajendran, D.; Ramalingame, R.; Adiraju, A.; Nouri, H.; Kanoun, O. Role of solvent polarity on dispersion quality and stability of functionalized carbon nanotubes. J. Compos. Sci. 2022, 6, 26. [Google Scholar] [CrossRef]
- Nahid, M.; Seyedeh, M.T. Synthesis of Mesoporous Antimicrobial Herbal Nanomaterial-Carrier for Silver Nanoparticles and Antimicrobial Sensing. Food Chem. Toxicol. 2022, 165, 113077. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, D.H.; Shin, B.J.; Kim, D.Y.; Lee, H.K.; Tae, H.S. Conductive polymer synthesis with single-crystallinity via a novel plasma polymerization technique for gas sensor applications. Materials 2016, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Kausar, A.; Siddiq, M. Polyvinylchloride intercalated poly (ethylene glycol)-modified-multi-walled carbon nanotube buckypaper composites via resin-infiltration technique. J. Plast. Film. Sheeting 2016, 32, 217–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, H.; Wang, J.; Chi, Q.; Zhang, T.; Zhang, C.; Feng, Y.; Zhang, Y.; Cao, D.; Zhu, K. PEO/Li1.25Al0.25Zr1.75(PO4)3 composite solid electrolytes for high-rate and ultra-stable all-solid-state lithium metal batteries with impregnated cathode modification. Inorg. Chem. Front. 2024, 11, 1289–1300. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.; Yu, K.; Chai, H.; Tian, M.; Qu, L.; Dong, H.; Zhang, X. Smartphone light-driven zinc porphyrinic MOF nanosheets-based enzyme-free wearable photoelectrochemical sensor for continuous sweat vitamin C detection. Chem. Eng. J. 2023, 455, 140779. [Google Scholar] [CrossRef]
- Niu, L.; Li, Q.; Wei, F.; Chen, X.; Wang, H. Electrochemical impedance and morphological characterization of platinum-modified polyaniline film electrodes and their electrocatalytic activity for methanol oxidation. J. Electroanal. Chem. 2003, 544, 121–128. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, H.W.; Park, J.M.; Park, H.-S.; Yoon, C. Oxidation of Polyvinyl Alcohol by Persulfate Activated with Heat, Fe2+, and Zero-Valent Iron. J. Hazard. Mater. 2009, 168, 346–351. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (OH/ O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhang, K.; Qi, C. Sodium Persulfate-Assisted Mechanochemical Degradation of Tetrabromobisphenol a: Efficacy, Products and Pathway. Chemosphere 2016, 150, 551–558. [Google Scholar] [CrossRef]
- Chen, W.S.; Jhou, Y.C.; Huang, C.P. Mineralization of Dinitrotoluenes in Industrial Wastewater by Electro-Activated Persulfate Oxidation. Chem. Eng. J. 2014, 252, 166–172. [Google Scholar] [CrossRef]
- Luo, H.; Li, C.; Sun, X.; Ding, B. Cathodic Indirect Oxidation of Organic Pollutant Paired to Anodic Persulfate Production. J. Electroanal. Chem. 2017, 792, 110–116. [Google Scholar] [CrossRef]
- Pattanaargson, S.; Limphong, P. Stability of octyl methoxycinnamate and identification of its photo-degradation product. Int. J. Cosmet. Sci. 2001, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Li, F.; Hu, X.; Xie, Z.; Hua, T. Activation of peroxymonosulfate in an electrochemical filter by MnFe2O4-rGO electro-assisted catalytic membrane for the degradation of oxytetracycline. J. Environ. Chem. Eng. 2022, 10, 107008. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Yu, Y.B.; Hong, J. Paracetamol Degradation and Disinfection via Electrocatalytic Oxidation by Using N-Doped Graphene as Anode. Catal. Lett. 2022, 152, 2607–2624. [Google Scholar] [CrossRef]
- Zohreh, A.J.; Abbas, R. Electrocatalytic Disinfection of E. Coli Using Ni-Fe/Fe3O4 Nanocomposite Cathode: Effect of Fe3O4 Nanoparticle, Humic Acid, and Nitrate. Sep. Purif. Technol. 2022, 294, 121140. [Google Scholar] [CrossRef]
- Song, Y.; Kim, D.; Hong, S.; Kim, T.S.; Kim, K.J.; Park, J.Y. Bimetallic Synergy from a Reaction-Driven Metal Oxide–Metal Interface of Pt–Co Bimetallic Nanoparticles. ACS Catal. 2023, 13, 13777–13785. [Google Scholar] [CrossRef]
- Chen, Q.F.; Xiao, Y.; Hua, K.; Zhang, H.T.; Zhang, M.T. Bimetallic Synergy in Oxygen Reduction: How Tailored Metal–Metal Interactions Amplify Cooperative Catalysis. J. Am. Chem. Soc. 2025, 147, 14504–14518. [Google Scholar] [CrossRef]
- Yan, N.; Liao, L.; Yuan, J.; Lin, Y.; Weng, L.; Yang, J.; Wu, Z. Bimetal Doping in Nanoclusters: Synergistic or Counteractive? Chem. Mater. 2016, 28, 8240–8247. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Li, S.; Tang, J.; Hua, T.; Li, F. Mechanism of the Application of Single-Atom Catalyst-Activated PMS/PDS to the Degradation of Organic Pollutants in Water Environment: A Review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Hu, J.; Yang, F.; Qu, J.; Cai, Y.; Yang, X.; Li, C.M. Synergetic Bimetallic Catalysts: A Remarkable Platform for Efficient Conversion of CO2 to High Value-Added Chemicals. J. Energy Chem. 2023, 87, 162–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zeng, J.; Fan, X.; Li, F.; Hua, T. Reducing Energy Penalty in Wastewater Treatment: Fe-Cu-Modified MWCNT Electrodes for Low-Voltage Electrofiltration of OMC. Energies 2025, 18, 4077. https://doi.org/10.3390/en18154077
Yu L, Zeng J, Fan X, Li F, Hua T. Reducing Energy Penalty in Wastewater Treatment: Fe-Cu-Modified MWCNT Electrodes for Low-Voltage Electrofiltration of OMC. Energies. 2025; 18(15):4077. https://doi.org/10.3390/en18154077
Chicago/Turabian StyleYu, Lu, Jun Zeng, Xiu Fan, Fengxiang Li, and Tao Hua. 2025. "Reducing Energy Penalty in Wastewater Treatment: Fe-Cu-Modified MWCNT Electrodes for Low-Voltage Electrofiltration of OMC" Energies 18, no. 15: 4077. https://doi.org/10.3390/en18154077
APA StyleYu, L., Zeng, J., Fan, X., Li, F., & Hua, T. (2025). Reducing Energy Penalty in Wastewater Treatment: Fe-Cu-Modified MWCNT Electrodes for Low-Voltage Electrofiltration of OMC. Energies, 18(15), 4077. https://doi.org/10.3390/en18154077





