After-Treatment Technologies for Emissions of Low-Carbon Fuel Internal Combustion Engines: Current Status and Prospects
Abstract
1. Introduction
2. Low-Carbon Fuels and Corresponding Emission After-Treatment Technologies
2.1. Methane Fuels
2.1.1. The Use of Methane Fuels
2.1.2. After-Treatment System for Methane Engines
2.2. Methanol Fuel
2.2.1. The Use of Methanol Fuel
2.2.2. After-Treatment System for Methanol Engines
2.3. Hydrogen Fuel
2.3.1. The Use of Hydrogen Fuel
2.3.2. After-Treatment System for Hydrogen Engines
2.4. Ammonia Fuel
2.4.1. The Use of Ammonia Fuel
2.4.2. After-Treatment System for Ammonia Engines
3. Conclusions
- Methane as a fuel results in relatively low CO2 and PM emissions, but tends to produce significant amounts of unburned hydrocarbons such as methane and formaldehyde. Due to the chemical stability and low reactivity of methane, traditional TWCs are generally ineffective at converting these compounds at low temperatures. Therefore, current strategies rely on integrating DOC with methane oxidation catalysts, implementing zoned catalyst designs, or applying ozone-assisted oxidation to improve low-temperature methane conversion efficiency.
- Methanol combustion under low-temperature conditions tends to generate unburned methanol and formaldehyde, yet no dedicated after-treatment systems have been developed specifically for methanol-fueled engines. As a result, general-purpose devices such as DOC, TWC, and POC are commonly used for emission control. Among them, POC has gained attention for its simple structure, low cost, and high purification efficiency. Furthermore, the combination of DOC and POC demonstrates significant potential for improving the removal efficiency of methanol-derived pollutants.
- Hydrogen combustion produces only water vapor, making it a zero-carbon fuel in terms of direct emissions. However, the high combustion temperature easily leads to the formation of thermal NOx. To achieve ultra-low emissions, hydrogen-fueled engines require an integrated approach combining optimized hydrogen injection/combustion strategies with advanced NOx after-treatment technologies such as SCR, to ensure low emissions.
- Ammonia has become a promising low-carbon alternative fuel due to its stable combustion performance, its ability to be produced from renewable energy sources, and its compatibility with existing storage and transportation infrastructure, and it offers significant advantages, including wide availability and ease of storage/transportation, positioning it as a promising low-carbon alternative. However, its practical application is hindered by inherent combustion challenges—notably low flame propagation speed and high minimum ignition energy—which often result in incomplete fuel oxidation and increased NOx emissions. Moreover, the toxic and corrosive nature of ammonia raises concerns over its unburned slip. SCR remains the dominant after-treatment technology for ammonia-fueled engines, and its combination with ASC and SDPF can significantly improve system stability and emission control. Electrochemical NOx decomposition, a novel reductant-free technology, also shows promise, though its high energy consumption currently limits its application to ammonia-based hybrid power systems.
- To enable the widespread application of low-carbon fuels in internal combustion engines, it is necessary to develop fuel-specific after-treatment routes that strike an optimal balance between emission reduction efficiency, thermal management, catalyst durability, and cost-effectiveness. And to provide a reference for future research, we have briefly presented information on some after-treatment devices, as shown in Table 1.
4. Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICE | Internal combustion engine |
| UHC | Unburned hydrocarbon |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CH4 | Methane |
| DP | Dual-fuel |
| BTE | Brake thermal efficiency |
| DOC | Diesel oxidation catalyst |
| POC | Particulate oxidation catalyst |
| TWC | Three-way catalyst |
| SCR | Selective catalytic reduction |
| SPDF | Selective catalytic reduction-coated diesel particulate filter |
| GHG | Greenhouse gas |
| NOx | Nitrogen oxides |
| PM | Particulate matter |
| NH3 | Ammonia |
| H2O | Water |
| H2 | Hydrogen |
References
- Wang, Y.; Xiao, G.; Li, B.; Tian, H.; Leng, X.; Dong, D.; Long, W. Study on the performance of diesel-methanol diffusion combustion with dual-direct injection system on a high-speed light-duty engine. Fuel 2022, 317, 123414. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, W.; Dong, P.; Tian, H.; Tian, J.; Li, B.; Wang, Y. Performance characteristics of a two-stroke low speed engine applying ammonia/diesel dual direct injection strategy. Fuel 2023, 332, 126086. [Google Scholar] [CrossRef]
- Kim, W.; Park, C.; Bae, C. Characterization of combustion process and emissions in amethane/diesel dual-fuel compression-ignition engine. Fuel 2021, 291, 120043. [Google Scholar] [CrossRef]
- Shere, A.; Subramanian, K.A. Experimental investigation on effects of equivalence ratio on combustion with knock, performance, and emission characteristics of dimethyl ether fueled CRDI compression ignition engine under homogeneous charge compression ignition mode. Fuel 2022, 322, 124048. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine emissions with air pollutants and greenhouse gases and their control technologies. J. Clean. Prod. 2022, 376, 134260. [Google Scholar] [CrossRef]
- Van Fan, Y.; Perry, S.; Klemeš, J.J.; Lee, C.T. A review on air emissions assessment: Transportation. J. Clean. Prod. 2018, 194, 673–684. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Pham, Q.; Park, S.; Agarwal, A.K.; Park, S. Review of dual-fuel combustion in the compression-ignition engine: Spray, combustion, and emission. Energy 2022, 250, 123778. [Google Scholar] [CrossRef]
- Macián, V.; Monsalve-Serrano, J.; Villalta, D.; Fogué-Robles, Á. Extending the potential of the dual-mode dual-fuel combustion towards the prospective EURO VII emissions limits using gasoline and OMEx. Energy Convers. Manag. 2021, 233, 113927. [Google Scholar] [CrossRef]
- China’s Stage VI Emission Standard for Heavy-Duty Vehicles (Final Rule); The International Council on Clean Transportation: Washington, DC, USA, 2018; 13p.
- Milojević, S.; Glišović, J.; Savić, S.; Bošković, G.; Bukvić, M.; Stojanović, B. Particulate matter emission and air pollution reduction by applying variable systems in tribologically optimized diesel engines for vehicles in road traffic. Atmosphere 2024, 15, 184. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Saleh, K.; Wandel, A.P.; Fattah, I.M.R.; Yusaf, T.; Alrazen, H.A. Influence of natural gas and hydrogen properties on internal combustion engine performance, combustion, and emissions: A review. Fuel 2024, 362, 130844. [Google Scholar] [CrossRef]
- Shuai, S.J.; Wang, Z.; Ma, X.; Xu, H.M.; He, X.; Wang, J.X. Low and zero-carbon technology pathways and key technologies for internal combustion engines under the carbon neutrality framework. J. Automot. Saf. Energy 2021, 12, 417–439. [Google Scholar]
- Eloffy, M.G.; Elgarahy, A.M.; Saber, A.N.; Hammad, A.; El-Sherif, D.M.; Shehata, M.; Elwakeel, K.Z. Biomass-to-sustainable biohydrogen: Insights into the production routes, and technical challenges. Chem. Eng. J. Adv. 2022, 12, 100410. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and practice of biomass fast pyrolysis processes for liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Energy Institute. Statistical Review of World Energy; Energy Institute: London, UK, 2024. [Google Scholar]
- Kakaee, A.H.; Paykani, A.; Ghajar, M. The influence of fuel composition on the combustion and emission characteristics of natural gas fueled engines. Renew. Sustain. Energy Rev. 2014, 38, 64–78. [Google Scholar] [CrossRef]
- Tavakoli, S.; Jensen, M.V.; Pedersen, E.; Schramm, J. Unburned hydrocarbon formation in a natural gas engine under sea wave load conditions. J. Mar. Sci. Technol. 2021, 26, 128–140. [Google Scholar] [CrossRef]
- Shi, W.; Wu, W.; Fan, H.; Sun, Q.; Niu, X.; Wang, S.; Yan, Z. Estimating CO2 and CH4 fluxes from reservoirs: Model development and site-level study. J. Hydrol. 2025, 654, 132794. [Google Scholar] [CrossRef]
- Soltic, P.; Hilfiker, T. Efficiency and raw emission benefits from hydrogen addition to methane in a prechamber–equipped engine. Int. J. Hydrogen Energy 2020, 45, 23638–23652. [Google Scholar] [CrossRef]
- Gremminger, A.; Pihl, J.; Casapu, M.; Grunwaldt, J.D.; Toops, T.J.; Deutschmann, O. PGM based catalysts for exhaust-gas after-treatment under typical diesel, gasoline and gas engine conditions with focus on methane and formaldehyde oxidation. Appl. Catal. B Environ. 2020, 265, 118571. [Google Scholar] [CrossRef]
- Shao, X.; Yang, S.; Yuan, Y.; Jia, H.; Zheng, L.; Liang, C. Study on the difference of dispersion behavior between hydrogen and methane in utility tunnel. Int. J. Hydrogen Energy 2022, 47, 8130–8144. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, Y.; Li, W. Review on the development of China’s methane industry in the background of “carbon neutrality”. Methane Ind. B 2022, 9, 132–140. [Google Scholar]
- Prasad, R.K.; Mustafi, N.; Agarwal, A.K. Effect of spark timing on laser ignition and spark ignition modes in a hydrogen enriched compressedmethane fuelled engine. Fuel 2020, 276, 118071. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Liu, X.; Wei, Z.; Tian, H.; Zhang, Q.; Li, Z. Numerical investigations on pilot ignited high pressure direct injectionmethane engines: A review. Renew. Sustain. Energy Rev. 2021, 150, 111390. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Hegab, A.H. Combustion and emission characteristics of a natural gas-fueled diesel engine with EGR. Energy Convers. Manag. 2012, 64, 301–312. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J. Experimental study of co-combustion ratio on fuel consumption and emissions of NG–diesel dual-fuel heavy-duty engine equipped with a common rail injection system. J. Energy Inst. 2016, 89, 578–585. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Sahoo, N.; Saha, U.K. Effect of engine parameters and type of gaseous fuel on the performance of dual-fuel gas diesel engines—A critical review. Renew. Sustain. Energy Rev. 2009, 13, 1151–1184. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zheng, Z.; Yao, M. Numerical investigation on the combustion and emission characteristics of a heavy-dutymethane-diesel dual-fuel engine. Fuel 2021, 300, 120998. [Google Scholar] [CrossRef]
- Winkler, A.; Dimopoulos, P.; Hauert, R.; Bach, C.; Aguirre, M. Catalytic activity and aging phenomena of three-way catalysts in a compressedmethane/gasoline powered passenger car. Appl. Catal. B Environ. 2008, 84, 162–169. [Google Scholar] [CrossRef]
- Lambert, C.K. Current state of the art and future needs for automotive exhaust catalysis. Nat. Catal. 2019, 2, 554–557. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Li, G.; Shao, S.; Li, P. Transient emission characteristics of a heavy-dutymethane engine at stoichiometric operation with EGR and TWC. Energy 2017, 132, 225–237. [Google Scholar] [CrossRef]
- Gong, J.; Pihl, J.; Wang, D.; Kim, M.Y.; Partridge, W.P.; Li, J.; Yezerets, A. O2 dosage as a descriptor of TWC performance under lean/rich dithering in stoichiometric natural gas engines. Catal. Today 2021, 360, 294–304. [Google Scholar] [CrossRef]
- Cho, H.M.; He, B.Q. Spark ignitionmethane engines—A review. Energy Convers. Manag. 2007, 48, 608–618. [Google Scholar] [CrossRef]
- Wahbi, A.; Tsolakis, A.; Herreros, J. Emissions control technologies for natural gas engines. In Natural Gas Engines: For TransPortation and Power Generation; Springer: Singapore, 2019; pp. 359–379. [Google Scholar]
- Einewall, P.; Tunestål, P.; Johansson, B. Lean Burnmethane Operation vs. Stoichiometric Operation with EGR and a Three Way Catalyst; SAE Technical Paper 2005-01-0250; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- Bauer, M.; Wachtmeister, G. Formation of formaldehyde in lean-burn gas engines; Entstehung von Formaldehyd in Mager-Gasmotoren. Mot. Z. 2009, 70, 50–57. [Google Scholar]
- Kim, K.H.; Jahan, S.A.; Lee, J.T. Exposure to formaldehyde and its potential human health hazards. J. Environ. Sci. Health Part C 2011, 29, 277–299. [Google Scholar] [CrossRef]
- Nylund, N.O.; Karvonen, V.; Kuutti, H.; Laurikko, J. Comparison of Diesel Andmethane Bus Performance; SAE Technical Paper 2014-01-2432; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Auvinen, P.; Nevalainen, P.; Suvanto, M.; Oliva, F.; Llamas, X.; Barciela, B.; Kinnunen, N.M. A detailed study on regeneration of SO2 poisoned exhaust gas after-treatment catalysts: In pursuance of high durability and low methane, NH3 and N2O emissions of heavy-duty vehicles. Fuel 2021, 291, 120223. [Google Scholar] [CrossRef]
- Hamedi, M.R.; Doustdar, O.; Tsolakis, A.; Hartland, J. Energy-efficient heating strategies of diesel oxidation catalyst for low emissions vehicles. Energy 2021, 230, 120819. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Cao, C.; Wang, S.; Lv, J.; Tan, D. The development of diesel oxidation catalysts and the effect of sulfur dioxide on catalysts of metal-based diesel oxidation catalysts: A review. Fuel Process. Technol. 2022, 233, 107317. [Google Scholar] [CrossRef]
- Kinnunen, N.M.; Keenan, M.; Kallinen, K.; Maunula, T.; Suvanto, M. Engineered Sulfur-Resistant Catalyst System with an Assisted Regeneration Strategy for Lean-Burn Methane Combustion. ChemCatChem 2018, 10, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.; Nicole, J.; Poojary, D. Ozone as an enabler for low temperature methane control over a current production Fe-BEA catalyst. Top. Catal. 2019, 62, 351–355. [Google Scholar] [CrossRef]
- Yasumura, S.; Saita, K.; Miyakage, T.; Nagai, K.; Kon, K.; Toyao, T.; Shimizu, K.I. Designing main-group catalysts for low-temperature methane combustion by ozone. Nat. Commun. 2023, 14, 3926. [Google Scholar] [CrossRef]
- Lott, P.; Casapu, M.; Grunwaldt, J.D.; Deutschmann, O. A review on exhaust gas after-treatment of lean-burn natural gas engines–From fundamentals to application. Appl. Catal. B Environ. 2024, 340, 123241. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Zhang, Z.; Tan, D.; Li, J.; Yin, Z.; Zhao, Z. Multi-objective optimization of the combustion chamber geometry for a highland diesel engine fueled with diesel/n-butanol/PODEn by ANN-NSGA III. Energy 2023, 282, 128793. [Google Scholar] [CrossRef]
- Chen, D.; Wang, T.; Yang, T.; Li, G.; Chen, Y.; Qiao, T. Effects of EGR combined with DOC on emission characteristics of a two-stage injected Fischer-Tropsch diesel/methanol dual-fuel engine. Fuel 2022, 329, 125451. [Google Scholar] [CrossRef]
- Liu, J.; Liang, W.; Ma, H.; Ji, Q.; Xiang, P.; Sun, P.; Ma, H. Effects of integrated aftertreatment system on regulated and unregulated emission characteristics of non-road methanol/diesel dual-fuel engine. Energy 2023, 282, 128819. [Google Scholar] [CrossRef]
- Huang, F.; Huang, D.; Wan, M.; Lei, J.; Shen, L. Experimental Investigation on Effects of Diesel Oxidation Catalysts on Emission Characteristics of a Methanol-Diesel Dual-Fuel Engine. J. Energy Eng. 2025, 151, 04024040. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Z.; Zhang, X.; Dong, F.; Yuan, W.; Chen, H. Overview for methanol and formaldehyde unregulated emissions of methanol fueled engines. J. Energy Inst. 2025, 120, 102089. [Google Scholar] [CrossRef]
- Wi, Y.J.; Shi, Z.H.; Zhang, Y.J.; Liu, S.H. Unregulated emission characteristics of a heavy-duty pure methanol engine. J. Tianjin Univ. (Sci. Technol.) 2024, 57, 1022–1029. [Google Scholar]
- Liu, J.; Gong, C.; Peng, L.; Liu, F.; Yu, X.; Li, Y. Numerical study of formaldehyde and unburned methanol emissions of direct injection spark ignition methanol engine under cold start and steady state operating conditions. Fuel 2017, 202, 405–413. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, H. Investigation on efficient and clean combustion pre-injection strategy of a diesel/methanol dual direct-injection marine engine under full load. Case Stud. Therm. Eng. 2024, 59, 104472. [Google Scholar] [CrossRef]
- Gong, C.; Liu, J.; Peng, L.; Liu, F. Numerical study of effect of injection and ignition timings on combustion and unregulated emissions of DISI methanol engine during cold start. Renew. Energy 2017, 112, 457–465. [Google Scholar] [CrossRef]
- Gong, C.; Sun, J.; Chen, Y.; Liu, F. Numerical study of cold-start performances of a medium compression ratio direct-injection twin-spark plug synchronous ignition engine fueled with methanol. Fuel 2021, 285, 119235. [Google Scholar] [CrossRef]
- Zhao, H.; Ge, Y.; Tan, J.; Yin, H.; Guo, J.; Zhao, W.; Dai, P. Effects of different mixing ratios on emissions from passenger cars fueled with methanol/gasoline blends. J. Environ. Sci. 2011, 23, 1831–1838. [Google Scholar] [CrossRef]
- Gong, C.; Peng, L.; Liu, F. Modeling of the overall equivalence ratio effects on combustion process and unregulated emissions of an SIDI methanol engine. Energy 2017, 125, 118–126. [Google Scholar] [CrossRef]
- Svensson, E.; Li, C.; Shamun, S.; Johansson, B.; Tuner, M.; Perlman, C.; Mauss, F. Potential Levels of Soot, NOx, HC and CO for Methanol Combustion; SAE Technical Paper 2016-01-0887; SAE International: Warrendale, PA, USA, 2016. [Google Scholar]
- Wei, L.J.; Yao, C.D.; Liu, J.H.; Wang, Q.G.; Yu, H.T. Intake and exhaust analysis and fuel efficiency of a diesel/methanol dual-fuel heavy-duty diesel engine. J. Eng. Thermophys. 2013, 34, 563–567. [Google Scholar]
- Cheung, C.S.; Zhang, Z.H.; Chan, T.L.; Yao, C. Investigation on the effect of port-injected methanol on the performance and emissions of a diesel engine at different engine speeds. Energy Fuels 2009, 23, 5684–5694. [Google Scholar] [CrossRef]
- Sileghem, L.; Huylebroeck, T.; Van Den Blucke, A. Performance and Emissions of a SI Engine Using Methano-Water Blends; SAE Technical Paper Detroi: SAE International 2013-01-1319; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Zhao, H.; Ge, Y.S.; Hao, C.; Han, X.; Fu, M.; Yu, L.; Shah, A.N. Carbonyl compound emissions from passenger cars fueled with methanol/gasoline blends. Total Environ. 2010, 408, 3607–3613. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.; Liu, F.; Liu, J.; Zhu, Z.; Li, G. Aldehydes and methanol emission mechanisms and characteristics from a methanol/gasoline-fueled spark-ignition (SI) engine. Energy Fuels 2009, 23, 6222–6230. [Google Scholar] [CrossRef]
- Vakkilainen, A.; Lylykangas, R. Particle Oxidation Catalyst (POC) for Diesel Vehicles; SAE Technical Paper 2004-28-0047; SAE International: Warrendale, PA, USA, 2004. [Google Scholar]
- Lu, Z.; Deng, S.; Gao, C.; Li, G.; Song, H.; Li, J. Emission characteristics and ozone formation potentials of gaseous pollutants from in-use methanol-, CNG-and gasoline-fueled vehicles. Environ. Monit. Assess. 2021, 193, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Liu, X.Y.; Bao, J.J.; Hu, J. Deposition Characteristics of Urea in Urea-SCR Systems and Mixer Optimization. Trans. CSICE 2023, 41, 86–95. [Google Scholar]
- Börnhorst, M.; Deutschmann, O. Advances and challenges of ammonia delivery by urea-water sprays in SCR systems. Prog. Energy Combust. Sci. 2021, 87, 100949. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, C.H.; Li, Y.Y.; Ye, R.L.; Sun, H.J. Research Progress on Selective Catalytic Reduction and Ammonia Slip Catalysts in Diesel Vehicles. J. Chang’an Univ. (Nat. Sci. Ed.) 2022, 42, 97–114. [Google Scholar]
- Shi, X.; Wang, Y.; Shan, Y.; Yu, Y.; He, H. Investigation of the common intermediates over Fe-ZSM-5 in NH3-SCR reaction at low temperature by in situ DRIFTS. J. Environ. Sci. 2020, 94, 32–39. [Google Scholar] [CrossRef]
- Zhang, W.B.; Chen, J.L.; Guo, L.; Zheng, W.; Wang, G.H.; Zheng, S.K.; Wu, X.Q. Research Progress on the NH3-SCR Mechanism of Metal-Loaded Zeolite Catalysts. J. Fuel Chem. Technol. 2021, 49, 1294–1315. [Google Scholar] [CrossRef]
- Chen, D.; Yan, Y.; Guo, A.; Rizzotto, V.; Lei, H.; Qiao, Z.; Simon, U. Mechanistic insights into the promotion of low-temperature NH3-SCR catalysis by copper auto-reduction in Cu-zeolites. Appl. Catal. B Environ. 2023, 322, 122118. [Google Scholar] [CrossRef]
- Shan, Y.; Sun, Y.; Du, J.; Zhang, Y.; Shi, X.; Yu, Y.; He, H. Hydrothermal aging alleviates the inhibition effects of NO2 on Cu-SSZ-13 for NH3-SCR. Appl. Catal. B Environ. 2020, 275, 119105. [Google Scholar] [CrossRef]
- Peng, J.Q.; Wang, P.; Li, F.X.; Zhu, Z.Z.; Ao, C.C.; Lei, L.L. Study on the Mechanism of Ce Modification Enhancing Potassium Poisoning Resistance of Cu/SSZ-13 Catalysts. Chin. Intern. Combust. Engine Eng. 2023, 44, 82–87+94. [Google Scholar]
- Xi, Y.; Su, C.; Ottinger, N.A.; Liu, Z.G. Effects of hydrothermal aging on the sulfur poisoning of a Cu-SSZ-13 SCR catalyst. Appl. Catal. B Environ. 2021, 284, 119749. [Google Scholar] [CrossRef]
- Lee, H.; Song, I.; Jeon, S.W.; Kim, D.H. Mobility of Cu Ions in Cu-SSZ-13 Determines the Reactivity of Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. Lett. 2021, 12, 3210–3216. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.G.; Shi, W.J.; Xiao, H.R.; Yang, R.M.; Chen, G.S.; Bi, K.G. Experimental Study on the Effect of CDPF Active Regeneration on SCR Performance. Automot. Eng. 2022, 44, 1280–1288. [Google Scholar]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Li, T. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl. Catal. A Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Jung, Y.; Pyo, Y.; Jang, J.; Woo, Y.; Ko, A.; Kim, G.; Cho, C. Nitrous oxide in diesel aftertreatment systems including DOC, DPF and urea-SCR. Fuel 2022, 310, 122453. [Google Scholar] [CrossRef]
- Yu, D.; Wang, P.; Li, X.; Zhao, H.; Lv, X. Study on the role of Fe species and acid sites in NH3-SCR over the Fe-based zeolites. Fuel 2023, 336, 126759. [Google Scholar] [CrossRef]
- Wang, P.; Yu, D.; Zhang, L.; Ren, Y.; Jin, M.; Lei, L. Evolution mechanism of NOx in NH3-SCR reaction over Fe-ZSM-5 catalyst: Species-performance relationships. Appl. Catal. A Gen. 2020, 607, 117806. [Google Scholar] [CrossRef]
- Qiao, Y.J.; Gong, L.; Dong, S.C.; Wang, H. Effect of CO2 on the NH3-SCR DeNOx Performance of Fe2O3 Catalysts. J. Dalian Univ. Technol. 2023, 63, 479–485. [Google Scholar]
- Farhan, S.M.; Pan, W.; Zhijian, C.; JianJun, Y. Innovative catalysts for the selective catalytic reduction of NOx with H2: A systematic review. Fuel 2024, 355, 129364. [Google Scholar] [CrossRef]
- Liu, Y.; Tursun, M.; Yu, H.; Wang, X. Surface property and activity of Pt/Nb2O5-ZrO2 for selective catalytic reduction of NO by H2. Mol. Catal. 2019, 464, 22–28. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Savva, P.G.; Costa, C.N. Hydrogen lean-DeNOx as an alternative to the ammonia and hydrocarbon selective catalytic reduction (SCR). Catal. Rev. Sci. Eng. 2011, 53, 91–151. [Google Scholar] [CrossRef]
- Chen, R.; Guo, M.X.; Du, J.C.; An, Z.Y.; Yang, Y.; Zhang, A.M. Research progress on Pd-based catalysts for hydrogen selective catalytic reduction. Precious Met. 2024, 45, 76–85+95. [Google Scholar]
- Guan, Y.; Liu, Y.; Lv, Q.; Wang, B.; Che, D. Review on the selective catalytic reduction of NOx with H2 by using novel catalysts. J. Environ. Chem. Eng. 2021, 9, 106770. [Google Scholar] [CrossRef]
- Savva, Z.; Petallidou, K.C.; Damaskinos, C.M.; Olympiou, G.G.; Stathopoulos, V.N.; Efstathiou, A.M. H2-SCR of NOx on low-SSA CeO2-supported Pd: The effect of Pd particle size. Appl. Catal. A Gen. 2021, 615, 118062. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, H.; Jia, B.; Wang, Z.; Liu, Z. Selective catalytic reduction of NOx by H2 over Pd/TiO2 catalyst. Chin. J. Catal. 2019, 40, 849–855. [Google Scholar] [CrossRef]
- Eldridge, T.J.; Borchers, M.; Lott, P.; Grunwaldt, J.D.; Doronkin, D.E. Elucidating the role of the state of Pd in the H2-SCR of NOx by operando XANES and DRIFTS. Catal. Sci. Technol. 2024, 14, 4198–4210. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Z.; Shen, C.; Rui, N.; Liu, C.J. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol. Green Energy Environ. 2022, 7, 807–817. [Google Scholar] [CrossRef]
- Duan, K.; Chen, B.; Zhu, T.; Liu, Z. Mn promoted Pd/TiO2–Al2O3 catalyst for the selective catalytic reduction of NOx by H2. Appl. Catal. B Environ. 2015, 176, 618–626. [Google Scholar] [CrossRef]
- Xu, C.; Sun, W.; Cao, L.; Yang, J. Highly efficient Pd-doped ferrite spinel catalysts for the selective catalytic reduction of NO with H2 at low temperature. Chem. Eng. J. 2016, 289, 231–238. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Irhamna, A.R.; Aziz, M. Production of ammonia as potential hydrogen carrier: Review on thermochemical and electrochemical processes. Int. J. Hydrogen Energy 2021, 46, 14455–14477. [Google Scholar] [CrossRef]
- Aziz, M.; Juangsa, F.B.; Irhamna, A.R.; Irsyad, A.R.; Hariana, H.; Darmawan, A. Ammonia utilization technology for thermal power generation: A review. J. Energy Inst. 2023, 111, 101365. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Al-Murisi, M.; Shehata, N.; Alami, A.H.; Radwan, A.; Sayed, E.T. Recent progress in Green Ammonia: Production, applications, assessment; barriers, and its role in achieving the sustainable development goals. Energy Convers. Manag. 2023, 277, 116594. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, M.; An, Z.; Wang, J.; Huang, Z.; Tan, H. Large eddy simulation on flame topologies and the blow-off characteristics of ammonia/air flame in a model gas turbine combustor. Fuel 2021, 298, 120846. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Sun, J.; Ma, P.; Li, S. Reducing NOx emission of swirl-stabilized ammonia/methane tubular flames through a fuel-oxidizer mixing strategy. Energy Fuels 2022, 36, 2277–2287. [Google Scholar] [CrossRef]
- Okafor, E.C.; Tsukamoto, M.; Hayakawa, A.; Somarathne, K.K.A.; Kudo, T.; Tsujimura, T.; Kobayashi, H. Influence of wall heat loss on the emission characteristics of premixed ammonia-air swirling flames interacting with the combustor wall. Proc. Combust. Inst. 2021, 38, 5139–5146. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Chen, H.E.; Li, J.; Wang, L.; Hu, Y.; Zhang, F.; Lai, J.M.; Li, K.; Ge, F.; Du, X.Y.; Li, K. Current status and prospects of ammonia-fueled internal combustion engine research. Automot. Dig. 2023, 17–31. [Google Scholar] [CrossRef]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Van Blarigan, P. Advanced internal combustion engine research. In Proceedings of the 2000 Doe Hydrogen Program Review Nrel-Cp-570-28890, San Ramon, CA, USA, 9–11 May 2000; pp. 1–19. [Google Scholar]
- Bro, K.; Pedersen, P.S. Alternative Diesel Engine Fuels: An Experimental Investigation of Methanol, Ethanol, Methane and Ammonia in a DI Diesel Engine with Pilot Injection; SAE Technical Paper 770794; SAE International: Warrendale, PA, USA, 1977. [Google Scholar]
- Reiter, A.J.; Kong, S.C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy Fuels 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Hasegawa, J.-Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. ChemRev 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Suzuki, M.; Tsujimura, T.; Furutani, H. ICOPE-15-1139 Power generation by a micro gas turbine firing kerosene and ammonia. In Proceedings of the International Conference on Power Engineering (ICOPE), Yokohama, Japan, 30 November–4 December 2015. [Google Scholar]
- Mitsubishi Power. Mitsubishi Power Commences Development of World’s First Ammonia-Fired 40MW Class Gas Turbine System--Targets to Expand Lineup of Carbon-Free Power Generation Options, with Commercialization Around 2025. 1 March 2021. Available online: https://power.mhi.com/news/20210301.html (accessed on 28 July 2025).
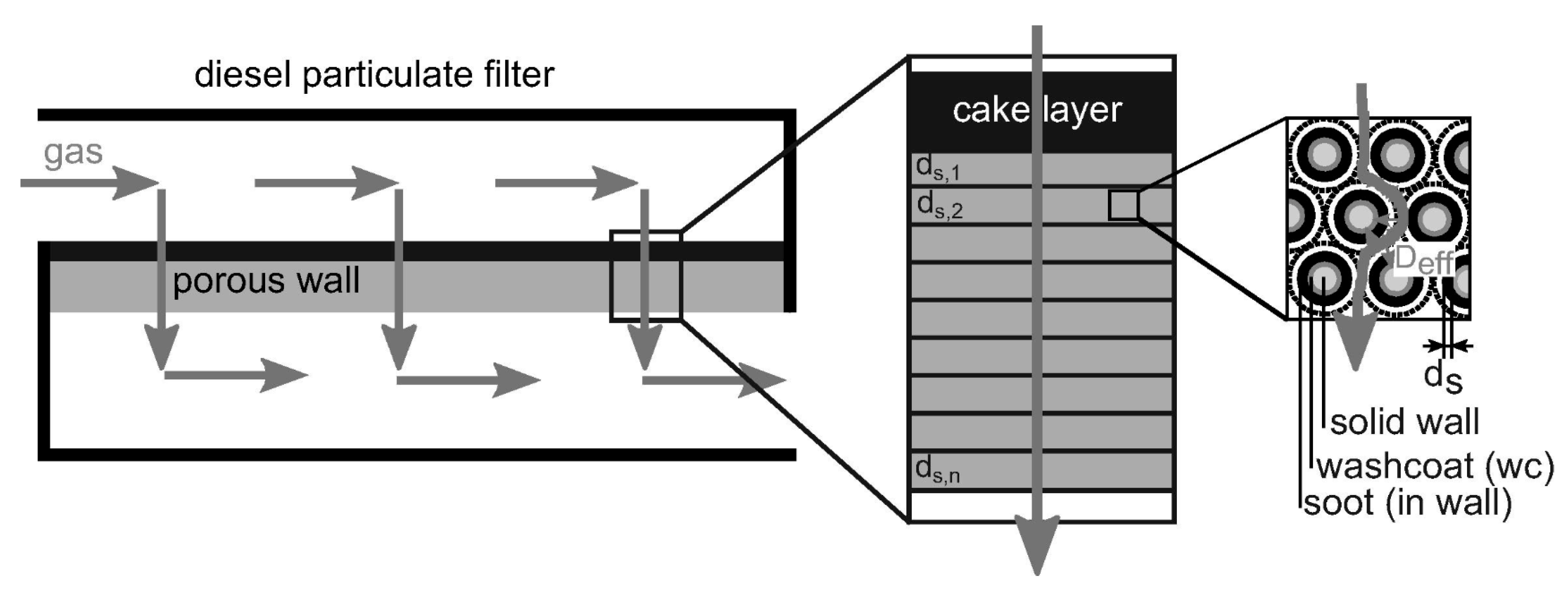
- Tan, P.Q.; Duan, L.S.; Lou, D.M.; Hu, Z.Y. Current Status and Development Trends of SDPF for Diesel Engines. China Environ. Sci. 2021, 41, 5495–5511. [Google Scholar]
- Purfürst, M.; Naumov, S.; Langeheinecke, K.J.; Gläser, R. Influence of soot on ammonia adsorption and catalytic DeNOx-properties of diesel particulate filters coated with SCR-catalysts. Chem. Eng. Sci. 2017, 168, 423–436. [Google Scholar] [CrossRef]
- Tan, P.; Chen, Y.; Wang, Z.; Duan, L.; Liu, Y.; Lou, D.; Zhang, Y. Experimental study of emission characteristics and performance of SCR coated on DPF with different catalyst washcoat loadings. Fuel 2023, 346, 128288. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, P.; Duan, L.; Liu, Y.; Lou, D.; Hu, Z. Emission characteristics and performance of SCR coated on DPF with different soot loads. Fuel 2022, 330, 125712. [Google Scholar] [CrossRef]
- Kim, H.J.; Jo, S.; Kwon, S.; Lee, J.T.; Park, S. NOX emission analysis according to after-treatment devices (SCR, LNT+SCR, SDPF), and control strategies in Euro-6 light-duty diesel vehicles. Fuel 2022, 310, 122297. [Google Scholar] [CrossRef]
- Karamitros, D.; Koltsakis, G. Model-based optimization of catalyst zoning on SCR-coated particulate filters. Chem. Eng. Sci. 2017, 173, 514–524. [Google Scholar] [CrossRef]
- Chigada, P.I.; Ahmadinejad, M.; Newman, A.D.; Ng, A.I.P.; Torbati, R.; Watling, T.C. Impact of SCR Activity on Soot Regeneration and the Converse Effects of Soot Regeneration on SCR Activity on a Vanadia-SCRF; SAE Technical Paper 2018-01-0962; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Zha, Y.; Cunningham, M.; Tang, Y.; Srinivasan, A.; Luo, J.; Heichelbech, J.; Szanyi, J. Sustained Low Temperature NOx Reduction; SAE Technical Paper 2018-01-0341; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Mihai, O.; Tamm, S.; Stenfeldt, M.; Wang, H.C.; Olsson, L. Evaluation of an integrated selective catalytic reduction-coated particulate filter. Ind. Eng. Chem. Res. 2015, 54, 11779–11791. [Google Scholar] [CrossRef]
- Hansen, K.K. Electrochemical Flue Gas Purification. SAE Int. J. Engines 2021, 14, 543–550. [Google Scholar] [CrossRef]
- Yang, R.; Yue, Z.; Zhang, S.; Yu, Z.; Wang, H.; Liu, H.; Yao, M. A novel approach of in-cylinder NOx control by inner selective non-catalytic reduction effect for high-pressure direct-injection ammonia engine. Fuel 2025, 381, 133349. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.S. Catalytic removal of nitrogen oxides (NO, NO2, N2O) from ammonia-fueled combustion exhaust: A review of applicable technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Ho, P.H.; Jabłońska, M.; Palkovits, R.; Rodríguez-Castellón, E.; Ospitali, F.; Fornasari, G.; Benito, P. N2O catalytic decomposition on electrodeposited Rh-based open-cell metallic foams. Chem. Eng. J. 2020, 379, 122259. [Google Scholar] [CrossRef]
- Pekridis, G.; Athanasiou, C.; Konsolakis, M.; Yentekakis, I.V.; Marnellos, G.E. N2O abatement over γ-Al2O3 supported catalysts: Effect of reducing agent and active phase nature. Top. Catal. 2009, 52, 1880–1887. [Google Scholar] [CrossRef]
- Haber, J.; Machej, T.; Janas, J.; Nattich, M. Catalytic decomposition of N2O. Catal. Today 2004, 90, 15–19. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent advances in catalytic decomposition of N2O on noble metal and metal oxide catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Huang, C.; Ma, Z.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Catalytic decomposition of N2O over Rh/Zn–Al2O3 catalysts. RSC Adv. 2017, 7, 4243–4252. [Google Scholar] [CrossRef]
- Guo, S.G.; Yang, F.Y.; Sun, N.N.; Lv, Z.H. Research on coordinated control technologies of N2O and NOx in dual-stage SCR systems. Trans. CSICE 2025, 43, 232–240. [Google Scholar]
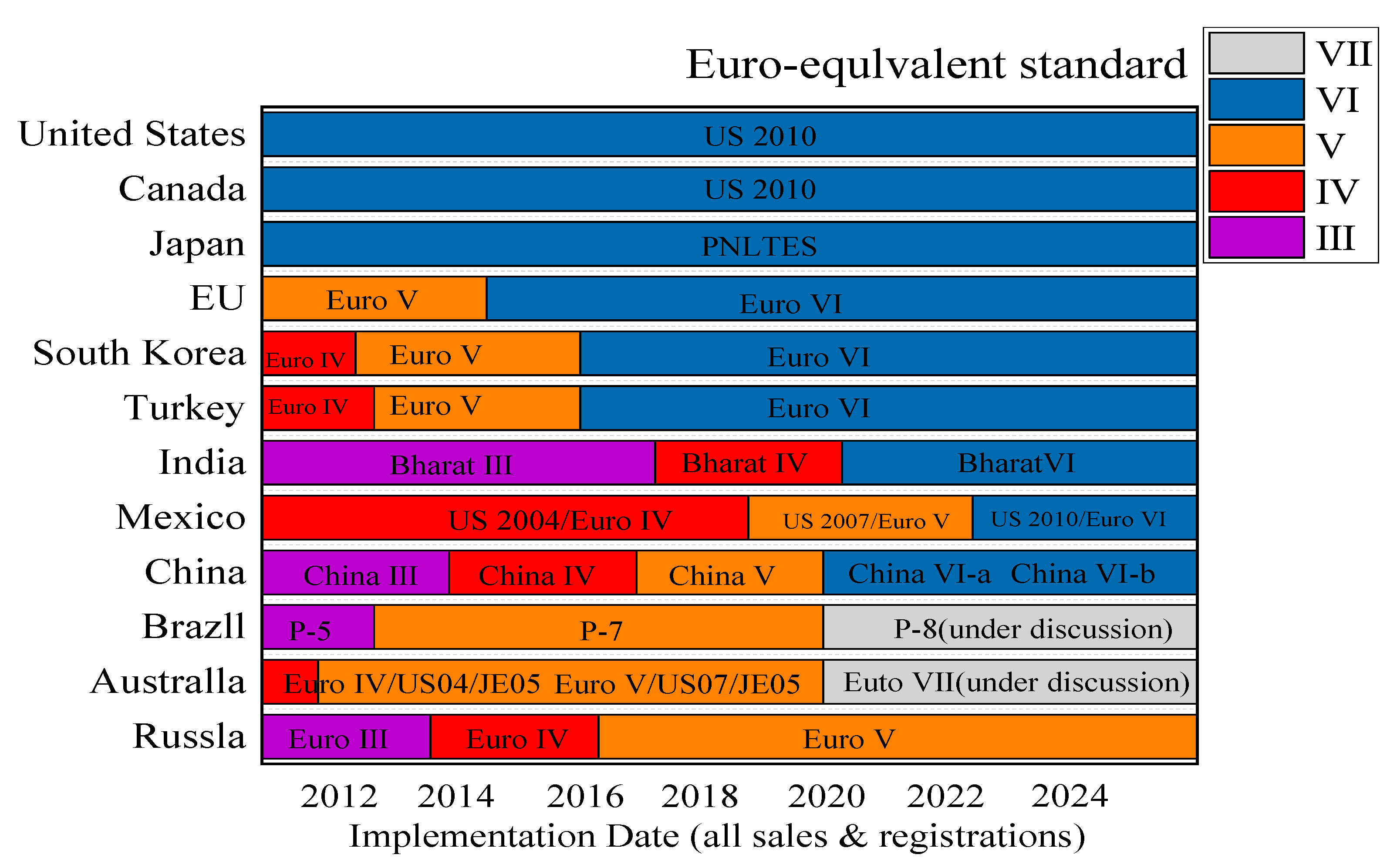
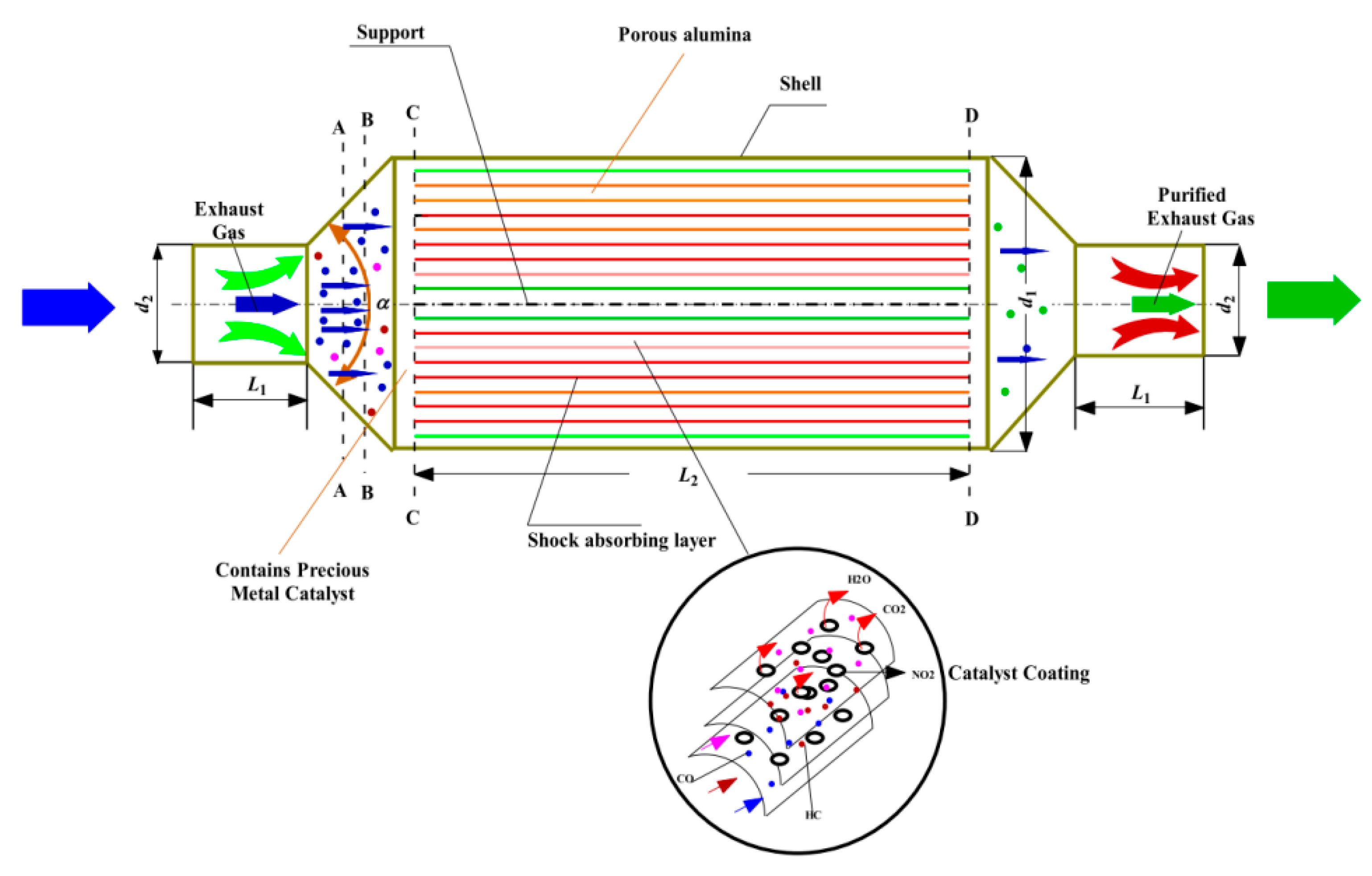
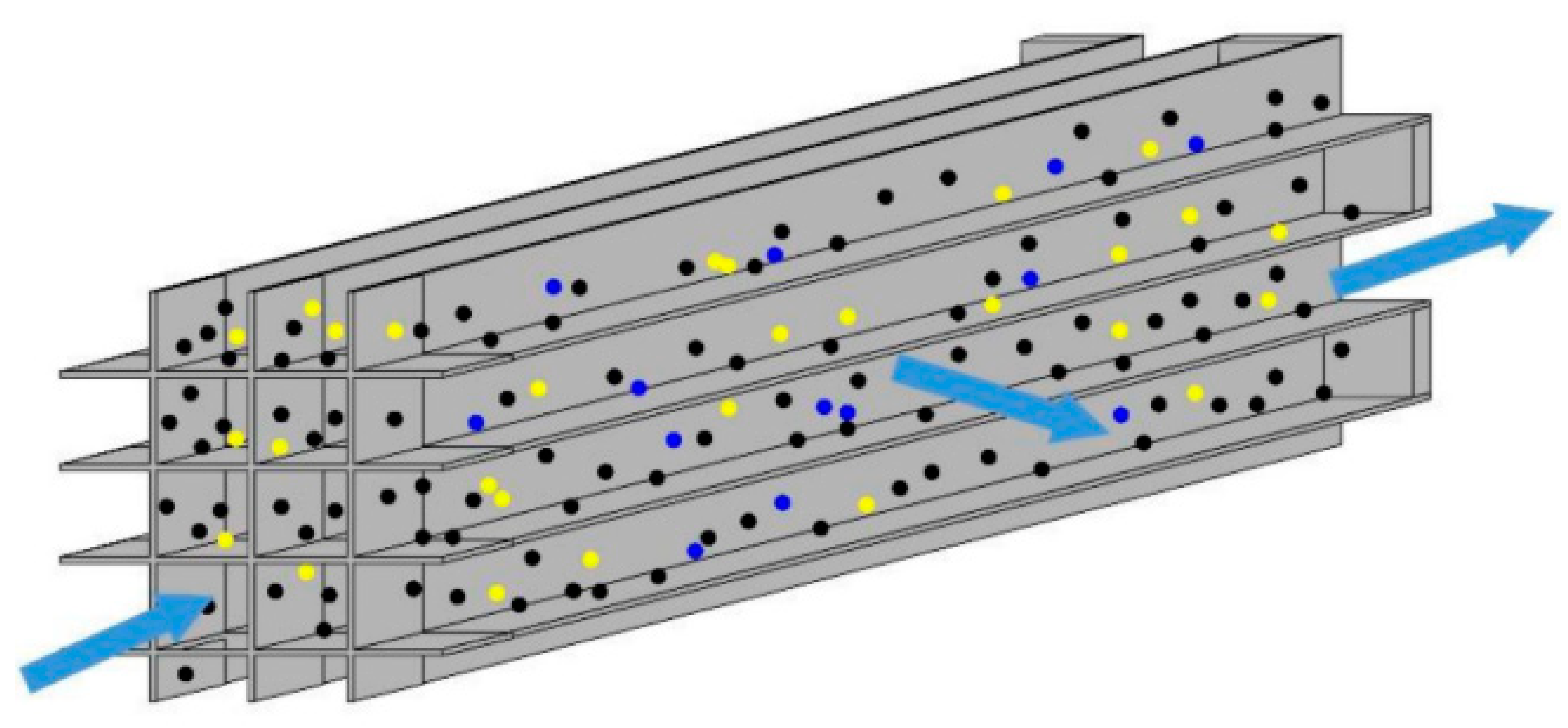
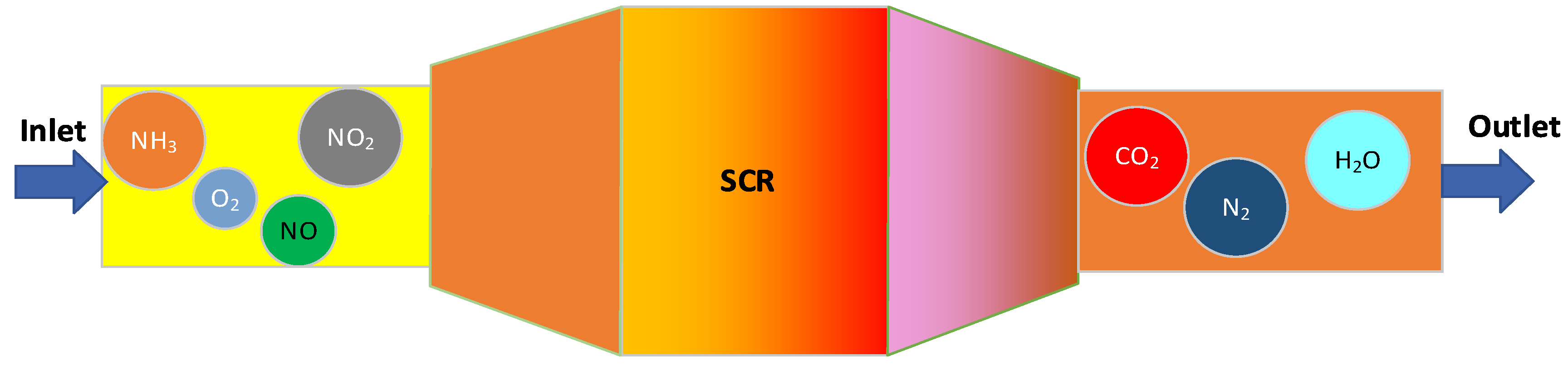
| Name | Primary Pollutants Treated | Purification Efficiency | Catalyst | Optimal Temperature Range |
|---|---|---|---|---|
| TWC | CO/HC | 80–90% | Pt\Pd\Rh | 250–500 °C |
| DOC | CO/HC | 90% | Pt\Pd | 220–350 °C |
| POC | CO/HC (Methane or methanol formation) | 40–70% | Pt\Pd | 250–400 °C |
| SCR | NOx | 90% | Cu-SSZ-13 (250–400 °C)\Fe-SSZ-13 (400–600 °C) | 200–500 °C (N2O tends to form at temperatures above 500 °C) |
| SDPF | NOx\PM | 90% (PM) 70–90% (NOx) | Cu/ZSM-5\Fe/ZSM-5 (The coating amount is approximately three times that of SCR) | 350–450 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, N.; Long, W.; Xie, C.; Tian, H. After-Treatment Technologies for Emissions of Low-Carbon Fuel Internal Combustion Engines: Current Status and Prospects. Energies 2025, 18, 4063. https://doi.org/10.3390/en18154063
Jin N, Long W, Xie C, Tian H. After-Treatment Technologies for Emissions of Low-Carbon Fuel Internal Combustion Engines: Current Status and Prospects. Energies. 2025; 18(15):4063. https://doi.org/10.3390/en18154063
Chicago/Turabian StyleJin, Najunzhe, Wuqiang Long, Chunyang Xie, and Hua Tian. 2025. "After-Treatment Technologies for Emissions of Low-Carbon Fuel Internal Combustion Engines: Current Status and Prospects" Energies 18, no. 15: 4063. https://doi.org/10.3390/en18154063
APA StyleJin, N., Long, W., Xie, C., & Tian, H. (2025). After-Treatment Technologies for Emissions of Low-Carbon Fuel Internal Combustion Engines: Current Status and Prospects. Energies, 18(15), 4063. https://doi.org/10.3390/en18154063







