Exploring Activated Carbons for Sustainable Biogas Upgrading: A Comprehensive Review
Abstract
1. Introduction
2. Carbonaceous Adsorbents for Biogas Upgrading
2.1. Activated Carbon Carbon Dioxide Adsorption Mechanism
2.2. Influence of Textural Properties
2.2.1. Specific Surface Area (SSA)
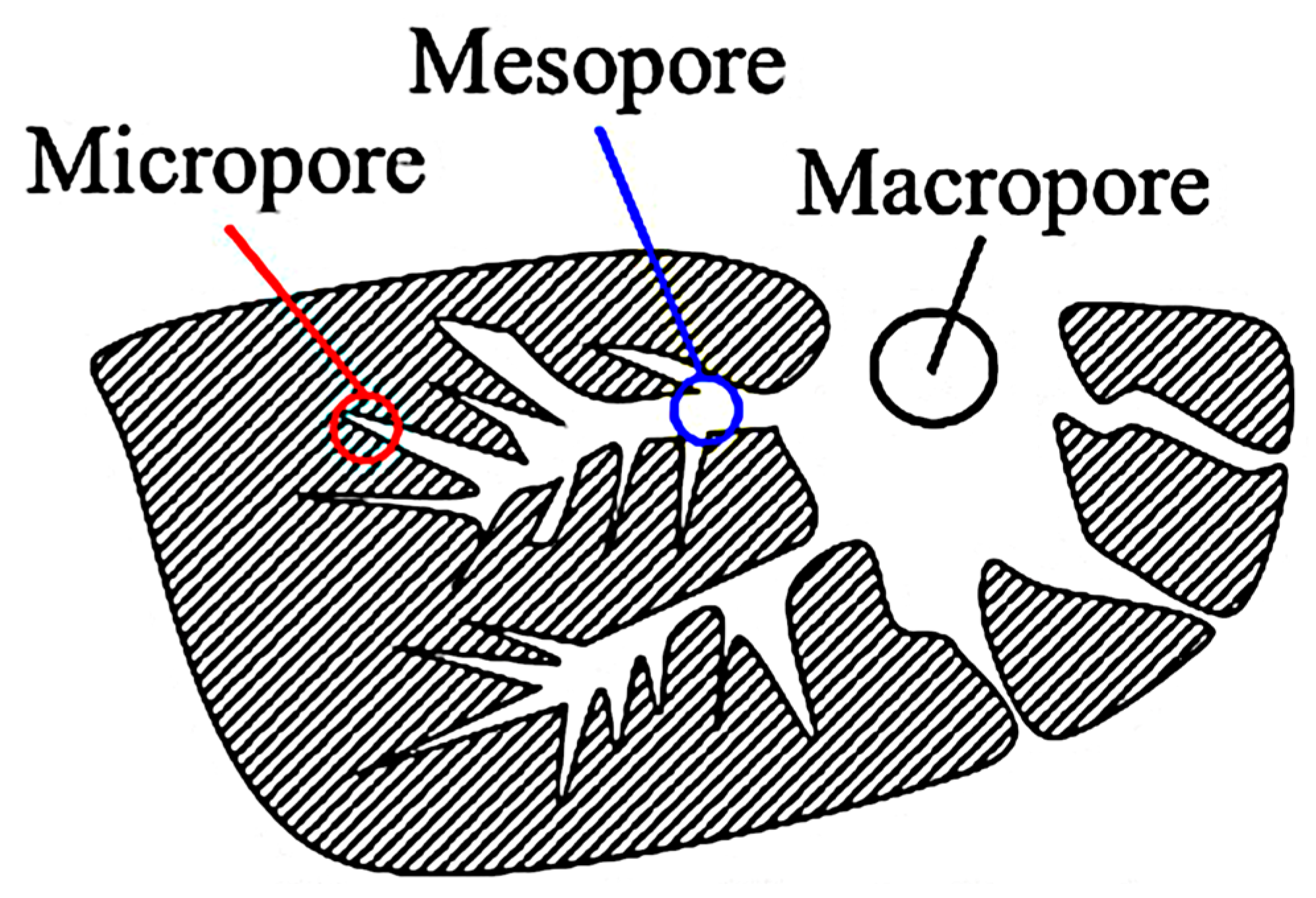
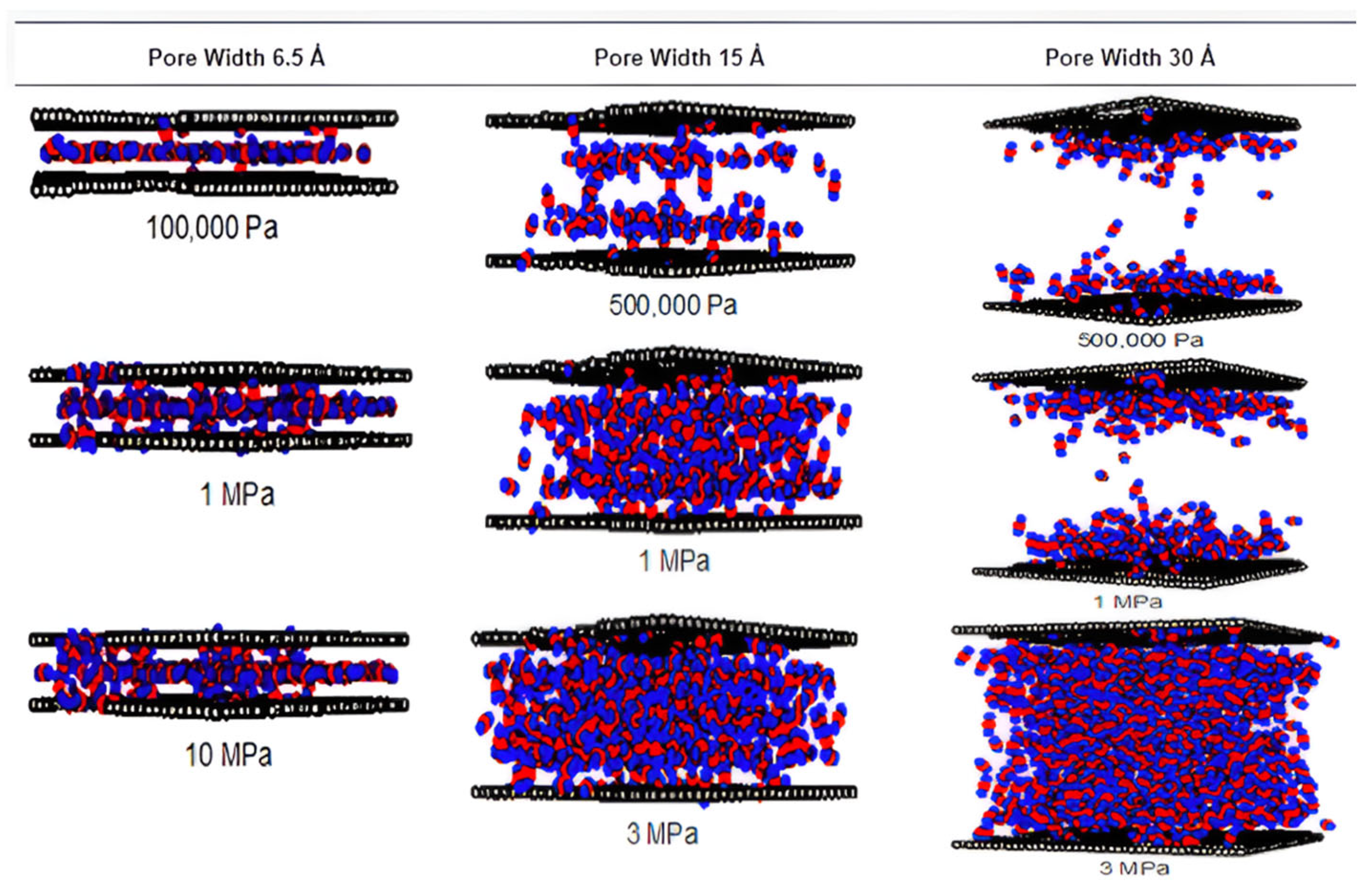
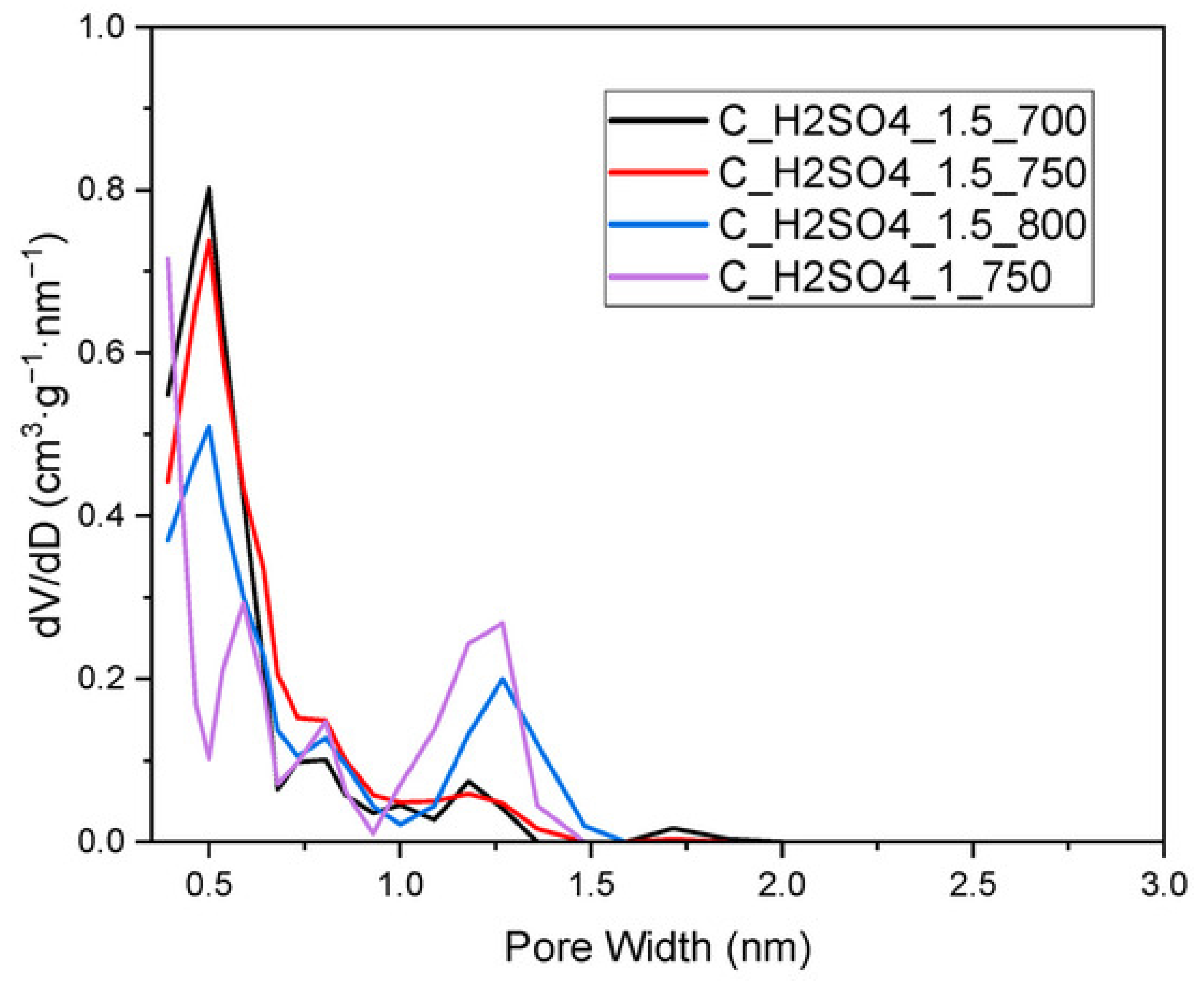
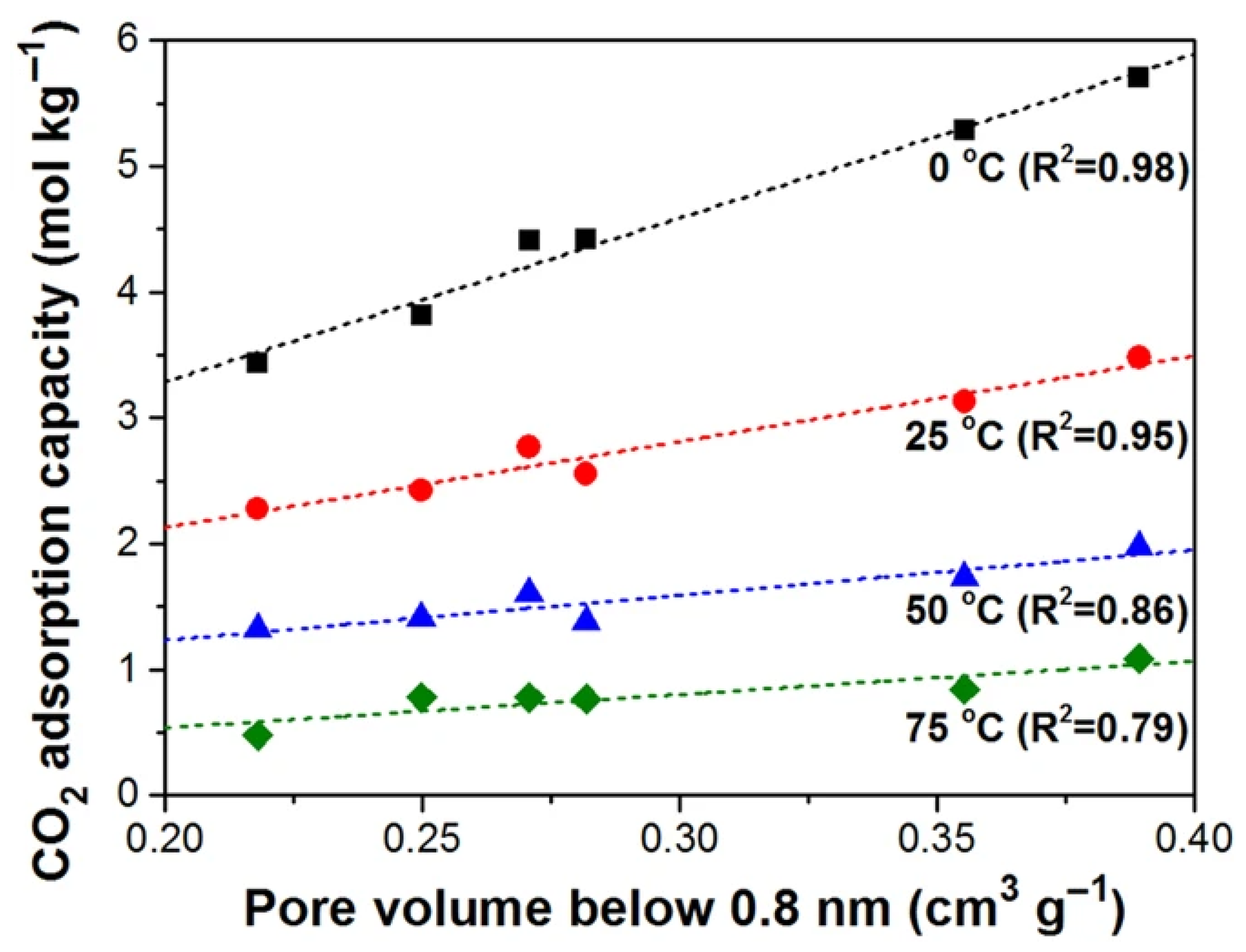
2.2.2. Pore Size Distribution (PSD) and Pore Volume
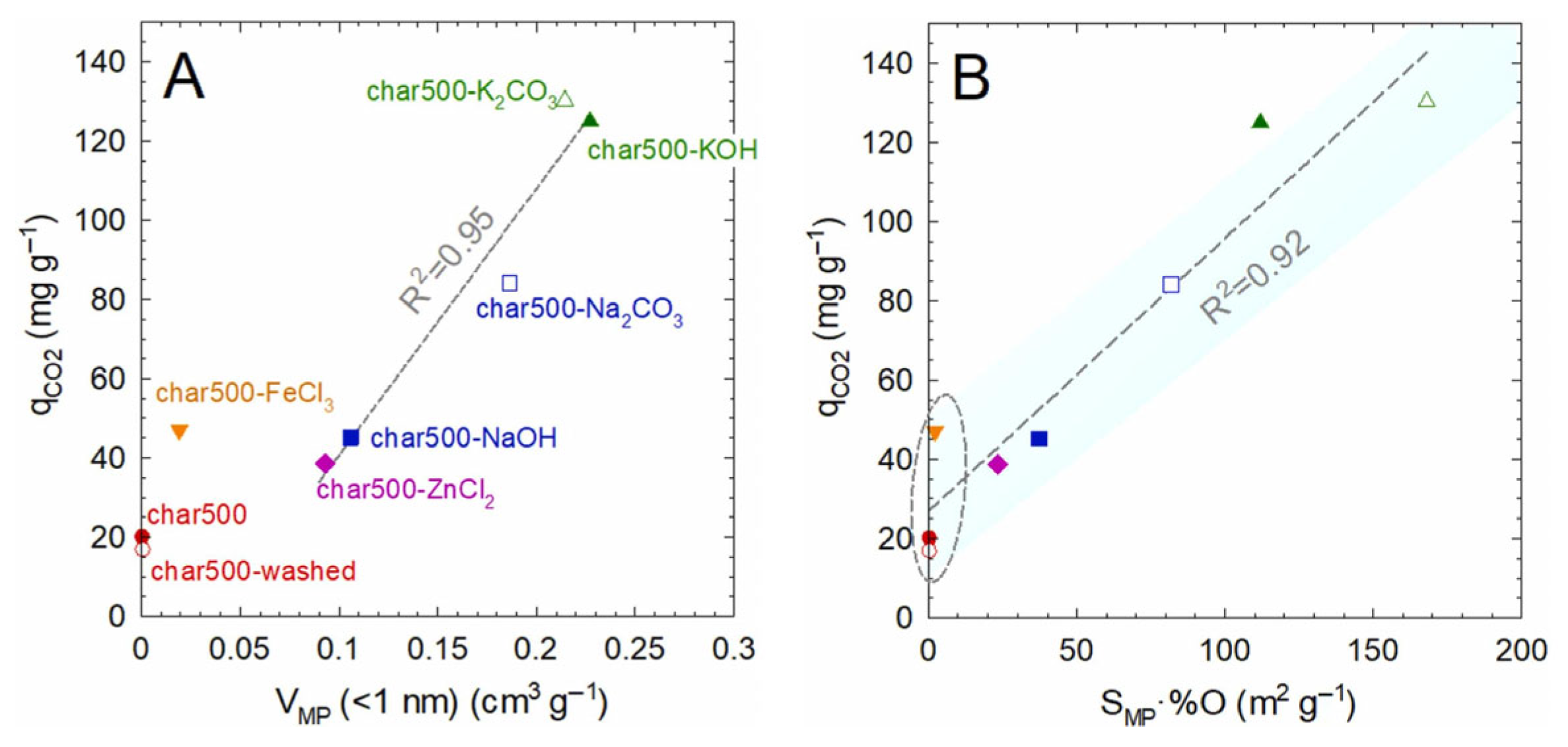
2.3. Influence of Chemical Composition and Surface Chemistry
2.4. Section Summary
3. Factors Influencing the Properties of Activated Carbons Relevant for Biogas Upgrading
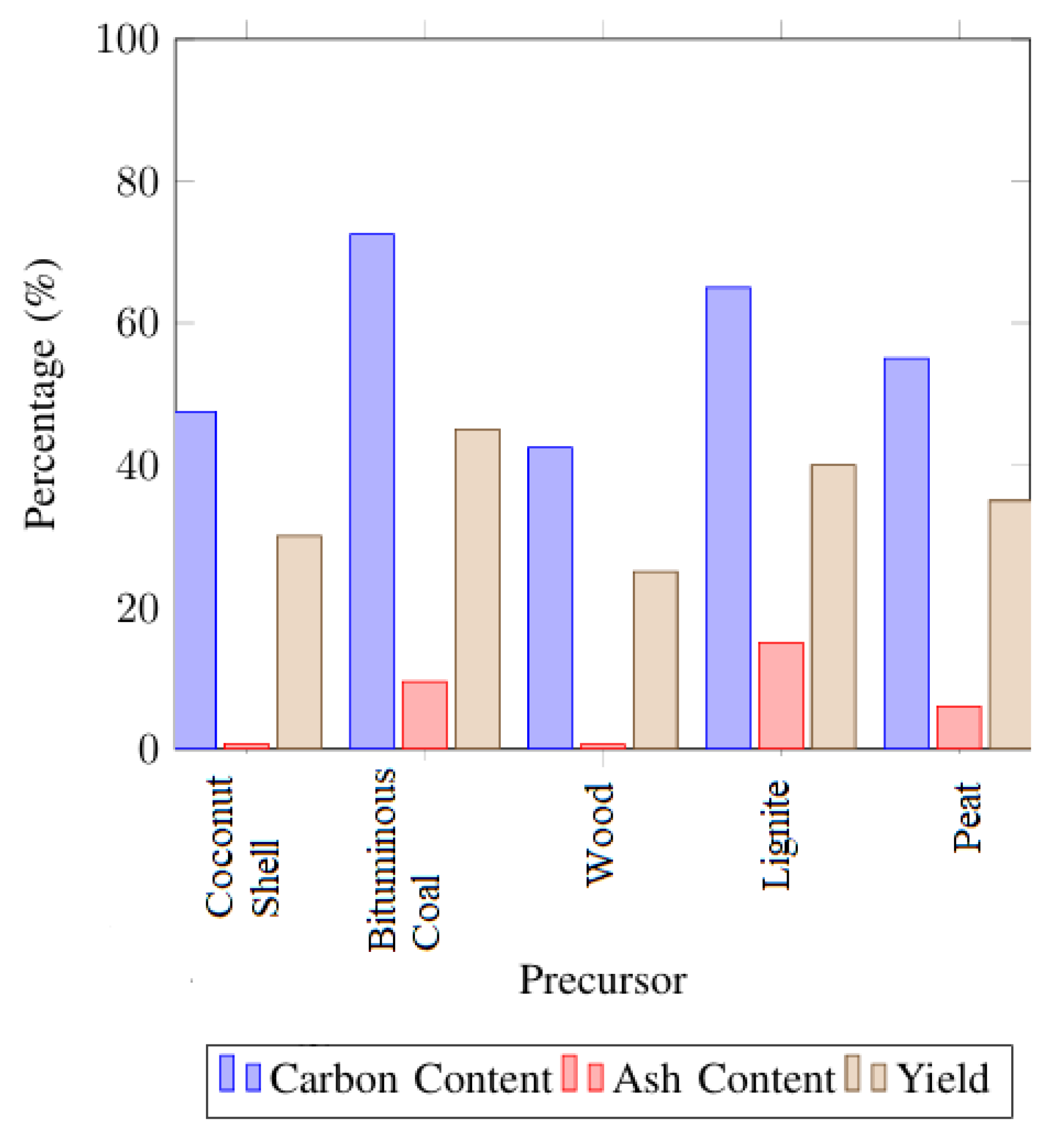
3.1. Substrate
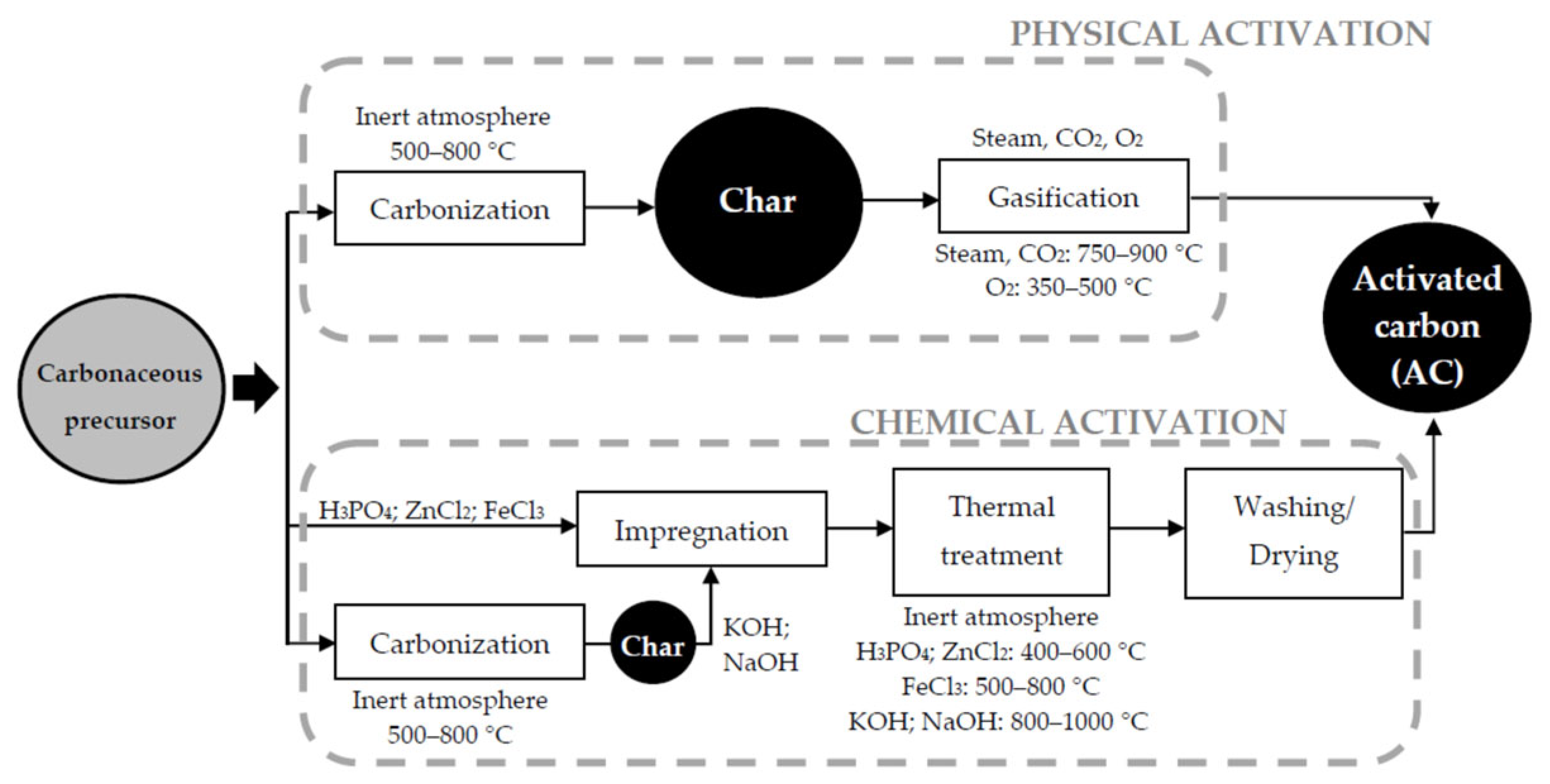
3.2. Influence of the Activation Method
3.3. Section Summary
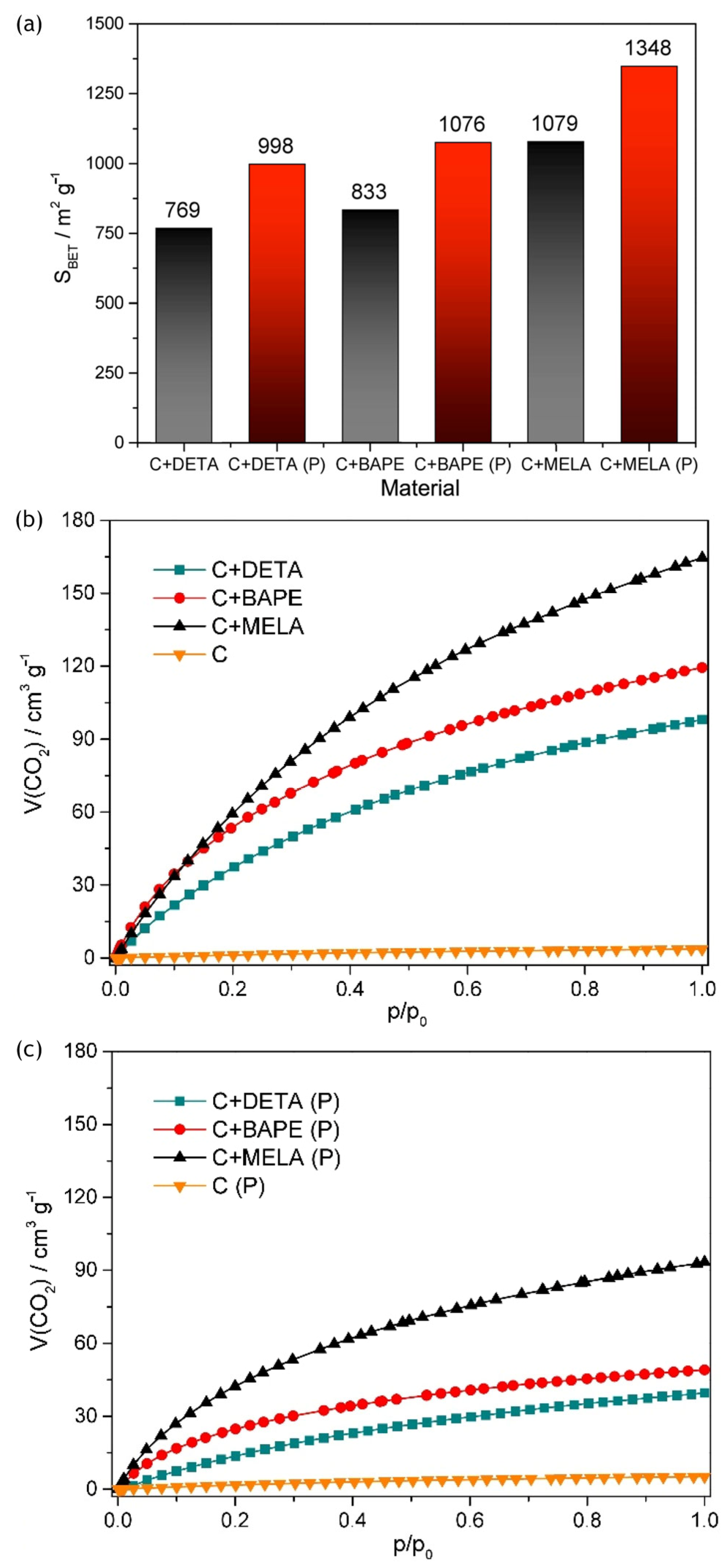
4. Post-Synthesis Methods for Improving Activated Carbon CO2 Adsorption and Separation for Biogas Upgrading
Section Summary
5. Additional Factors That Influence the Adsorption Performance of ACs
5.1. Comparison of ACs with Other Adsorbent Materials for Biogas Upgrading
5.2. Section Summary
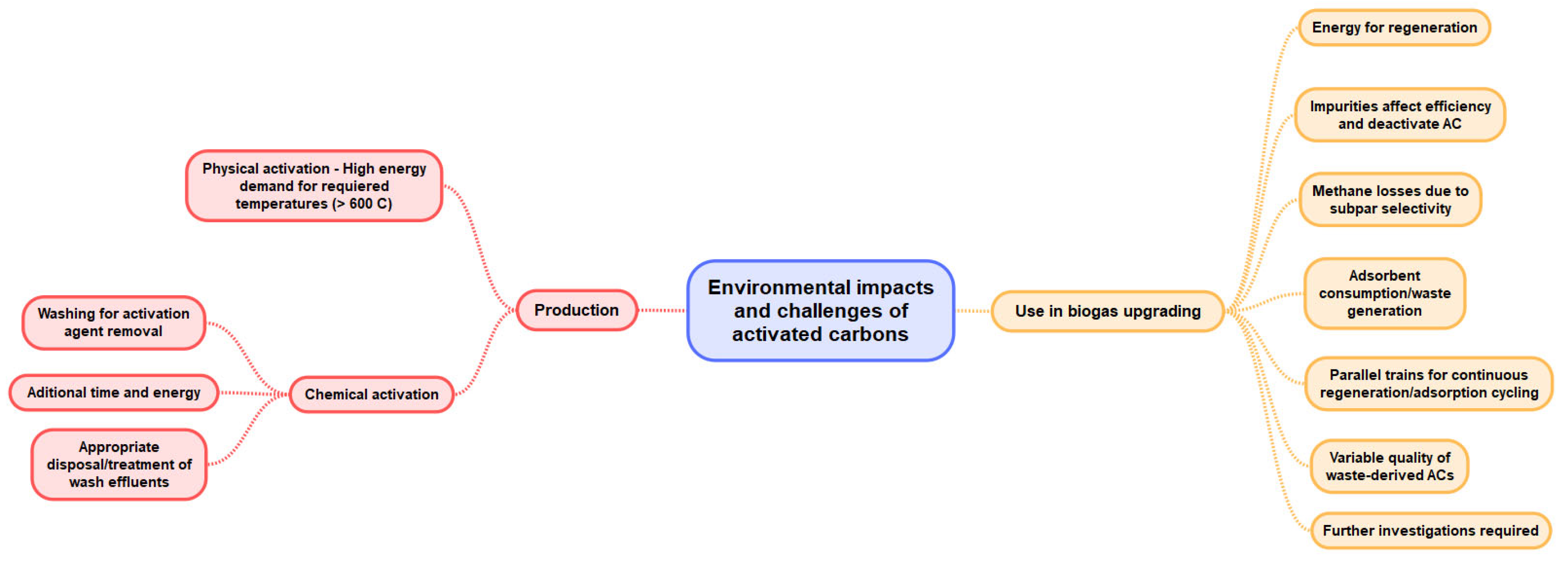
6. Implementation Barriers and Challenges
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UN. Sustainable Development Goals. 2015. Available online: https://sdgs.un.org/ (accessed on 1 April 2025).
- IEA. Renewables 2024. Paris. 2024. Available online: https://www.iea.org/reports/renewables-2024 (accessed on 1 April 2025).
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth; Paris. 2020. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth (accessed on 1 April 2025).
- Mihi, M.; Ouhammou, B.; Aggour, M.; Daouchi, B.; Naaim, S.; El Mers, E.M.; Kousksou, T. Modeling and forecasting biogas production from anaerobic digestion process for sustainable resource energy recovery. Heliyon 2024, 10, e38472. [Google Scholar] [CrossRef]
- Bernardo, M.; Lapa, N.; Fonseca, I.; Esteves, I.A.A.C. Biomass Valorization to Produce Porous Carbons: Applications in CO2 Capture and Biogas Upgrading to Biomethane—A Mini-Review. Front. Energy Res. 2021, 9, 625188. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; García-Depraect, O.; Santos-Beneit, F.; Bordel, S.; Lebrero, R.; Muñoz, R. Recent trends and advances in biogas upgrading and methanotrophs-based valorization. Chem. Eng. J. Adv. 2022, 11, 100325. [Google Scholar] [CrossRef]
- López, A.F.; Rodríguez, T.L.; Abdolmaleki, S.F.; Martínez, M.G.; Bugallo, P.M.B. From Biogas to Biomethane: An In-Depth Review of Upgrading Technologies That Enhance Sustainability and Reduce Greenhouse Gas Emissions. Appl. Sci. 2024, 14, 2342. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Cheng, K.Y.; Pivrikas, A.; Ho, G. A review of biogas upgrading technologies: Key emphasis on electrochemical systems. Water Sci. Technol. 2025, 91, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Sher, F.; Smječanin, N.; Hrnjić, H.; Karadža, A.; Omanović, R.; Šehović, E.; Sulejmanović, J. Emerging technologies for biogas production: A critical review on recent progress, challenges and future perspectives. Process Saf. Environ. Prot. 2024, 188, 834–859. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M.; Daniluk, M.; Żak, S. Biogas Upgrading Using a Single-Membrane System: A Review. Membranes 2024, 14, 80. [Google Scholar] [CrossRef]
- Alfa, K.A.; Saleh, N.A.; Beda, A.; Ghimbeu, C.M.; Dushime, G.I.; Marias, F.; Moynault, L.; Platel, V.; Hort, C. Approximate Adsorption Performance Indicator in Evaluating Sustainable Bamboo-Derived Adsorbents for Biogas Upgrading. Sustainability 2025, 17, 1445. [Google Scholar] [CrossRef]
- Thrän, D.; Adetona, A.; Borchers, M.; Cyffka, K.-F.; Daniel-Gromke, J.; Oehmichen, K. Potential contribution of biogas to net zero energy systems–A comparative study of Canada and Germany. Biomass Bioenergy 2025, 193, 107561. [Google Scholar] [CrossRef]
- Lawson, N.; Alvarado-Morales, M.; Tsapekos, P.; Angelidaki, I. Techno-economic assessment of biological biogas upgrading based on danish biogas plants. Energies 2021, 14, 8252. [Google Scholar] [CrossRef]
- Angelidaki, I.; Xie, L.; Luo, G.; Zhang, Y.; Oechsner, H.; Lemmer, A.; Munoz, R.; Kougias, P.G. Biogas upgrading: Current and emerging technologies. In Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Academic Press: Cambridge, MA, USA, 2019; pp. 817–843. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Wadi, B.; Manovic, V. Advances, challenges, and perspectives of biogas cleaning, upgrading, and utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Innovative Method for Biomethane Production Based on a Closed Cycle of Biogas Upgrading and Organic Substrate Pretreatment—Technical, Economic, and Technological Fundamentals. Energies 2025, 18, 1033. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel–A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Yang, M.; Baral, N.R.; Anastasopoulou, A.; Breunig, H.M.; Scown, C.D. Cost and Life-Cycle Greenhouse Gas Implications of Integrating Biogas Upgrading and Carbon Capture Technologies in Cellulosic Biorefineries. Environ. Sci. Technol. 2020, 54, 12810–12819. [Google Scholar] [CrossRef]
- Boer, D.G.; van de Bovenkamp, H.H.; Langerak, J.; Bakker, B.; Pescarmona, P.P. Evaluation of binderless LTA and SAPO-34 beads as CO 2 adsorbents for biogas upgrading in a vacuum pressure swing adsorption setup. Energy Adv. 2024, 3, 1581–1593. [Google Scholar] [CrossRef]
- Paz, L.; Gentil, S.; Fierro, V.; Celzard, A. Assessing the performance of adsorbents for CO2/CH4 separation in pressure swing adsorption units: A review. J. Environ. Chem. Eng. 2024, 12, 114870. [Google Scholar] [CrossRef]
- Li, Y.; Yi, H.; Tang, X.; Li, F.; Yuan, Q. Adsorption separation of CO2/CH4gas mixture on the commercial zeolites at atmospheric pressure. Chem. Eng. J. 2013, 229, 50–56. [Google Scholar] [CrossRef]
- Kapoor, R.; Subbarao, P.M.V.; Vijay, V.K.; Shah, G.; Sahota, S.; Singh, D.; Verma, M. Factors affecting methane loss from a water scrubbing based biogas upgrading system. Appl. Energy 2017, 208, 1379–1388. [Google Scholar] [CrossRef]
- Paolini, V.; Tratzi, P.; Torre, M.; Tomassetti, L.; Segreto, M.; Petracchini, F. Water scrubbing for biogas upgrading: Developments and innovations. In Emerging Technologies and Biological Systems for Biogas Upgrading; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–71. [Google Scholar] [CrossRef]
- Bo, C.; Guo, W.; Tang, C.; Li, J.; Lu, X. Dynamic Control Design and Simulation of Biogas Pressurized Water Scrubbing Process. IFAC-PapersOnLine 2018, 51, 560–565. [Google Scholar] [CrossRef]
- Jabraeelzadeh, A.; Gharehghani, A.; Saray, J.A.; Andwari, A.M.; Borhani, T.N. Techno-economic analysis of biogas upgrading through amine scrubbing: A comparative study of different single amines. Fuel 2025, 381, 133662. [Google Scholar] [CrossRef]
- Capra, F.; Fettarappa, F.; Magli, F.; Gatti, M.; Martelli, E. Biogas upgrading by amine scrubbing: Solvent comparison between MDEA and MDEA/MEA blend. Energy Procedia 2018, 148, 970–977. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Guo, T.; Tian, W.; Hao, J.; Guo, Q. The competitive adsorption mechanism of CO2, H2O and O2 on a solid amine adsorbent. Chem. Eng. J. 2021, 416, 129007. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies–Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Nishimura, A.; Takada, T.; Ohata, S.; Kolhe, M.L. Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure. Fuels 2021, 2, 194–209. [Google Scholar] [CrossRef]
- Micale, C. Bio-methane generation from biogas upgrading by semi-permeable membranes: An experimental, numerical and economic analysis. Energy Procedia 2015, 82, 971–977. [Google Scholar] [CrossRef]
- Naquash, A.; Qyyum, M.A.; Haider, J.; Bokhari, A.; Lim, H.; Lee, M. State-of-the-art assessment of cryogenic technologies for biogas upgrading: Energy, economic, and environmental perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111826. [Google Scholar] [CrossRef]
- Song, C.; Fan, Z.; Li, R.; Liu, Q.; Kitamura, Y. Efficient biogas upgrading by a novel membrane-cryogenic hybrid process: Experiment and simulation study. J. Membr. Sci. 2018, 565, 194–202. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Langé, S. Biogas to liquefied biomethane via cryogenic upgrading technologies. Renew. Energy 2018, 124, 75–83. [Google Scholar] [CrossRef]
- Biswas, R.; Ahmadi, V.; Ummethala, R.; Mozumder, M.S.I.; Aryal, N. Recent advances in electrochemical carbon dioxide reduction strategies in biogas upgrading and biomethane production. Chem. Eng. J. Adv. 2025, 22, 100722. [Google Scholar] [CrossRef]
- Pasternak, G. Electrochemical approach for biogas upgrading. In Emerging Technologies and Biological Systems for Biogas Upgrading; Elsevier: Amsterdam, The Netherlands, 2021; pp. 223–254. [Google Scholar] [CrossRef]
- Ray, S.; Kuppam, C.; Pandit, S.; Kumar, P. Biogas Upgrading by Hydrogenotrophic Methanogens: An Overview. Waste Biomass Valorization 2023, 14, 537–552. [Google Scholar] [CrossRef]
- Zhang, L.; Kuroki, A.; Tong, Y.W. A Mini-Review on In situ Biogas Upgrading Technologies via Enhanced Hydrogenotrophic Methanogenesis to Improve the Quality of Biogas From Anaerobic Digesters. Front. Energy Res. 2020, 8, 69. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Xie, T.; Seib, M.; Tale, V.P.; Zitomer, D. Anaerobic digester biogas upgrading using microalgae. In Integrated Wastewater Management and Valorization Using Algal Cultures; Elsevier: Amsterdam, The Netherlands, 2022; pp. 183–214. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kanai, D.; Sugiyama, K.; Fujii, K. Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology. Fermentation 2024, 10, 134. [Google Scholar] [CrossRef]
- Di Profio, P.; Ciulla, M.; Di Giacomo, S.; Barbacane, N.; Wolicki, R.D.; Fontana, A.; Moffa, S.; Pilato, S.; Siani, G. Emerging green strategies for biogas upgrading through CO2 capture: From unconventional organic solvents to clathrate and semi-clathrate hydrates. J. Mol. Liq. 2023, 391, 123196. [Google Scholar] [CrossRef]
- Moghaddam, E.A.; Larsolle, A.; Tidåker, P.; Nordberg, Å. Gas Hydrates as a Means for Biogas and Biomethane Distribution. Front. Energy Res. 2021, 9, 568879. [Google Scholar] [CrossRef]
- Gallego-Fernández, L.M.; Portillo, E.; Borrero, F.V.; Navarrete, B.; Vilches, L.F. CO2 capture for biogas upgrading using salts, hydroxides, and waste. In Circular Economy Processes for CO2 Capture and Utilization; Elsevier: Amsterdam, The Netherlands, 2024; pp. 7–24. [Google Scholar] [CrossRef]
- Kida, M.; Fujiwara, R.; Goda, H.; Sakagami, H.; Minami, H. Methane and Carbon Dioxide Separation Characteristics of Quaternary Ammonium Salt Hydrates for Biogas Upgrading under Static Conditions of Gas–Solid Contact. Energy Fuels 2023, 37, 9197–9206. [Google Scholar] [CrossRef]
- Obileke, K. Sustainability evaluation of current biogas upgrading techniques. In Innovations in the Global Biogas Industry; Elsevier: Amsterdam, The Netherlands, 2025; pp. 213–244. [Google Scholar] [CrossRef]
- Galloni, M.; Di Marcoberardino, G. Biogas Upgrading Technology: Conventional Processes and Emerging Solutions Analysis. Energies 2024, 17, 2907. [Google Scholar] [CrossRef]
- Aktar, K.; Yabar, H.; Mizunoya, T.; Islam, M.M. Application of GIS in Introducing Community-Based Biogas Plants from Dairy Farm Waste: Potential of Renewable Energy for Rural Areas in Bangladesh. Geomatics 2024, 4, 384–411. [Google Scholar] [CrossRef]
- Godfrey, O.U. Renewable Energy from Agricultural Waste: Biogas Potential for Sustainable Energy Generation in Nigeria’s Rural Agricultural Communities. J. Eng. Res. Rep. 2024, 26, 341–367. [Google Scholar] [CrossRef]
- Owusu, P.A.; Borkloe, J.K.; Mahamud, Y. Challenges towards Sustainable Energy as a Substitute for Fossil Fuels for the Case of Municipal Waste Management. J. Earth Energy Sci. Eng. Technol. 2024, 7, 1–14. [Google Scholar] [CrossRef]
- Selim, M.M.; Tounsi, A.; Gomaa, H.; Shenashen, M. Enhancing carbon capture efficiency in biogas upgrading: A comprehensive review on adsorbents and adsorption isotherms. AIP Adv. 2024, 14, 040703. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Nie, Z.; Lin, Y.; Jin, X. Research on the theory and application of adsorbed natural gas used in new energy vehicles: A review. Front. Mech. Eng. 2016, 11, 258–274. [Google Scholar] [CrossRef]
- Bałys, M.; Brodawka, E.; Jodłowski, G.S.; Szczurowski, J.; Wójcik, M. Alternative Materials for the Enrichment of Biogas with Methane. Materials 2021, 14, 7759. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Denayer, J.F. Biogas upgrading by adsorption processes: Mathematical modeling, simulation and optimization approach—A review. J. Environ. Chem. Eng. 2022, 10, 107483. [Google Scholar] [CrossRef]
- Karne, H.; Mahajan, U.; Ketkar, U.; Kohade, A.; Khadilkar, P.; Mishra, A. A review on biogas upgradation systems. Mater. Today. Proc. 2023, 72, 775–786. [Google Scholar] [CrossRef]
- Mrosso, R.; Mecha, A.C.; Kiplagat, J. Carbon Dioxide Removal Using a Novel Adsorbent Derived from Calcined Eggshell Waste for Biogas Upgrading. S. Afr. J. Chem. Eng. 2024, 47, 150–158. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Qi, P. Upgrading of Biogas to Methane Based on Adsorption. Processes 2020, 8, 941. [Google Scholar] [CrossRef]
- Solís, R.R.; Calero, M.; Pereira, L.; Ramírez, S.; Blázquez, G.; Martín-Lara, M.Á. Transforming a mixture of real post-consumer plastic waste into activated carbon for biogas upgrading. Process Saf. Environ. Prot. 2024, 190, 298–315. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, H.; Wen, S.; Zhang, W.; Hu, S.; Sun, K.; Jiang, J.; Ji, X. Biogas upgrading using aqueous bamboo-derived activated carbons. Bioresour. Technol. 2025, 419, 132055. [Google Scholar] [CrossRef]
- Yadavalli, G.; Lei, H.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yan, D. Carbon dioxide capture using ammonium sulfate surface modified activated biomass carbon. Biomass Bioenergy 2017, 98, 53–60. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Canevesi, R.L.S.; Schaefer, S.; Izquierdo, M.T.; Celzard, A.; Fierro, V. Roles of Surface Chemistry and Texture of Nanoporous Activated Carbons in CO2 Capture. ACS Appl. Nano Mater. 2022, 5, 3843–3854. [Google Scholar] [CrossRef]
- Gao, X.; Yang, S.; Hu, L.; Cai, S.; Wu, L.; Kawi, S. Carbonaceous materials as adsorbents for CO2 capture: Synthesis and modification. Carbon Capture Sci. Technol. 2022, 3, 100039. [Google Scholar] [CrossRef]
- Sosa, J.A.; Laines, J.R.; García, D.S.; Hernández, R.; Zappi, M.; de los Monteros, A.E.E. Activated Carbon: A Review of Residual Precursors, Synthesis Processes, Characterization Techniques, and Applications in the Improvement of Biogas. Environ. Eng. Res. 2022, 28, 220100. [Google Scholar] [CrossRef]
- Surra, E.; Ribeiro, R.P.P.L.; Santos, T.; Bernardo, M.; Mota, J.P.B.; Lapa, N. Evaluation of activated carbons produced from Maize Cob Waste for adsorption-based CO2 separation and biogas upgrading. J. Environ. Chem. Eng. 2022, 10, 107065. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Fazzino, F.; Fotia, A.; Malara, A.; Pedullà, A.; Calabrò, P.S. Comparison at laboratory scale of different types and configurations of commercial molecular sieves for one-step direct upgrading of biogas produced by anaerobic digestion. Fuel 2024, 365, 131292. [Google Scholar] [CrossRef]
- Maulidaturahma, E.; Ariyanto, T.; Lestari, R.A.S.; Purnomo, C.W. Biogas purification by using zeolite packing columns. AIP Conf. Proc. 2024, 3116, 060001. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, J.; Xiao, P.; He, Y.; Zhao, Q.; Chu, Z.; Liu, Y.; Li, Z.; Webley, P.A. Simultaneous biogas purification and CO2 capture by vacuum swing adsorption using zeolite NaUSY. Chem. Eng. J. 2018, 334, 2593–2602. [Google Scholar] [CrossRef]
- Duma, Z.; Makgwane, P.R.; Masukume, M.; Swartbooi, A.; Rambau, K.; Mehlo, T.; Mavhungu, T. A comprehensive review of metal-organic frameworks sorbents and their mixed-matrix membranes composites for biogas cleaning and CO2/CH4 separation. Mater. Today Sustain. 2024, 27, 100812. [Google Scholar] [CrossRef]
- Abd, A.A.; Kim, J.; Jasim, D.J.; Othman, M.R. Production of green methane by carbon dioxide capture from landfill biogas via pressure swing adsorption mediated by MOF ZIF-8. Energy Convers. Manag. 2024, 321, 119090. [Google Scholar] [CrossRef]
- Lee, M.S.; Park, M.; Kim, H.Y.; Park, S.J. Effects of Microporosity and Surface Chemistry on Separation Performances of N-Containing Pitch-Based Activated Carbons for CO2/N2 Binary Mixture. Sci. Rep. 2016, 6, 23224. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Yang, Y.; Kong, X.-M.; Li, P.; Yu, J.-G.; Ribeiro, A.M.; Rodrigues, A.E. Adsorption of Pure and Binary CO2, CH4, and N2 Gas Components on Activated Carbon Beads. J. Chem. Eng. Data 2015, 60, 2684–2693. [Google Scholar] [CrossRef]
- Durán, I.; Álvarez-Gutiérrez, N.; Rubiera, F.; Pevida, C. Biogas purification by means of adsorption on pine sawdust-based activated carbon: Impact of water vapor. Chem. Eng. J. 2018, 353, 197–207. [Google Scholar] [CrossRef]
- Buczek, B. Methane Recovery from Gaseous Mixtures Using Carbonaceous Adsorbents. Arch. Min. Sci. 2016, 61, 285–292. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. Microporous Carbon Adsorbents Prepared by Activating Reagent-Free Pyrolysis for Upgrading Low-Quality Natural Gas. ACS Sustain. Chem. Eng. 2020, 8, 977–985. [Google Scholar] [CrossRef]
- Całus-Makowska, K.; Grosser, A.; Grobelak, A.; Białek, H.; Siedlecka, E. Kinetic study of the simultaneous removal of ibuprofen, carbamazepine, sulfamethoxazole, and diclofenac from water using biochar and activated carbon adsorption, and TiO2 photocatalysis. Desalination Water Treat. 2024, 320, 100817. [Google Scholar] [CrossRef]
- Arenas, L.R.; Le Coustumer, P.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Removal efficiency and adsorption mechanisms of CeO2 nanoparticles onto granular activated carbon used in drinking water treatment plants. Sci. Total Environ. 2023, 856, 159261. [Google Scholar] [CrossRef]
- Paparo, R.; Trifuoggi, M.; Uggeri, F.; Nicolais, L.; Russo, V.; Di Serio, M. Intensification of the adsorption process to remove Iopamidol from water using granular activated carbon: Use of Rotating Packed Reactor and ultrasound technique in continuous flow technology. Chem. Eng. Process. Process Intensif. 2025, 209, 110166. [Google Scholar] [CrossRef]
- Salas, M.A.G.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Jiménez-Junca, C. Functionalized activated carbon with whey protein amyloid fibrils for adsorption of arsenic from water. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100956. [Google Scholar] [CrossRef]
- Sriprom, P.; Assawasaengrat, P.; Kraijan, P.; Laonork, S.; Rodmee, A.; Manamoongmongkol, K.; Permana, L.; Phumjan, L.; Kerdpiboon, S.; Puttongsiri, T. Activated carbon derived from Mahachanok mango seeds as a potential material to delay the ripening of mangoes. J. Agric. Food Res. 2024, 18, 101432. [Google Scholar] [CrossRef]
- Regadera-Macías, A.M.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Maldonado-Hódar, F.J. Optimizing filters of activated carbons obtained from biomass residues for ethylene removal in agro-food industry devices. Environ. Res. 2024, 248, 118247. [Google Scholar] [CrossRef] [PubMed]
- Priya, D.S.; Kennedy, L.J.; Anand, G.T. Porous activated carbon electrodes derived from Eleusine coracana biowaste in electric double layer capacitors: Nanoarchitectonics and performance. Results Surf. Interfaces 2024, 17, 100353. [Google Scholar] [CrossRef]
- Dhapola, P.S.; Kumar, S.; Karakoti, M.; Yahya, M.Z.A.; Punetha, V.D.; Pandey, S.; Chowdhury, F.I.; Savilov, S.V.; Singh, P.K. O, N co-doped porous activated carbon from polyvinyl chloride for super capacitors and solar cells application. Chem. Phys. Impact 2024, 9, 100721. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Alahakoon, A.M.B.S.; Mellander, B.E.; Albinsson, I. Activated carbon synthesized from Jack wood biochar for high performing biomass derived composite double layer supercapacitors. Carbon Trends 2024, 15, 100359. [Google Scholar] [CrossRef]
- Manimekala, T.; Sivasubramanian, R.; Dar, M.A.; Dharmalingam, G. Crafting the architecture of biomass-derived activated carbon via electrochemical insights for supercapacitors: A review. R. Soc. Chem. 2025, 15, 2490–2522. [Google Scholar] [CrossRef]
- Inthawong, S.; Wongkoblap, A.; Intomya, W.; Tangsathitkulchai, C. The Enhancement of CO2 and CH4 Capture on Activated Carbon with Different Degrees of Burn-Off and Surface Chemistry. Molecules 2023, 28, 5433. [Google Scholar] [CrossRef]
- Tahsin, M.; Shahinuzzaman, M.; Akter, T.; Abdur, R.; Bashar, M.S.; Kadir, M.R.; Hoque, S.; Jamal, M.S.; Hossain, M. Improved CO2 adsorption and desorption using chemically derived activated carbon from corn cob hard shell. Carbon Trends 2025, 19, 100495. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.H.; Kim, Y.H.; Rhee, K.Y.; Park, S.J. Roles of london dispersive and polar components of nano-metal-coated activated carbons for improving carbon dioxide uptake. Coatings 2021, 11, 691. [Google Scholar] [CrossRef]
- Muzarpar, M.S.; Leman, A.M. The Adsorption Mechanism of Activated Carbon and Its Application-A Review. Int. J. Adv. Technol. Mech. Mechatron. Mater. 2021, 1, 118–124. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, B.H.; Park, S.J.; An, K.H.; Kim, B.J. Pitch-derived activated carbon fibers for emission control of low-concentration hydrocarbon. Nanomaterials 2019, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Jepleting, A.; Mecha, A.C.; Sombei, D.; Moraa, D.; Chollom, M.N. Potential of low-cost materials for biogas purification, a review of recent developments. Renew. Sustain. Energy Rev. 2024, 210, 115152. [Google Scholar] [CrossRef]
- Karimi, M.; Silva, J.A.C.; Gonçalves, C.N.D.P.; de Tuesta, J.L.D.; Rodrigues, A.E.; Gomes, H.T. CO2 Capture in Chemically and Thermally Modified Activated Carbons Using Breakthrough Measurements: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2018, 57, 11154–11166. [Google Scholar] [CrossRef]
- Osterrieth, J.W.M.; Rampersad, J.; Madden, D.; Rampal, N.; Skoric, L.; Connolly, B.; Allendorf, M.D.; Stavila, V.; Snider, J.L.; Ameloot, R.; et al. How Reproducible are Surface Areas Calculated from the BET Equation? Adv. Mater. 2022, 34, e2201502. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Zhang, T.; Wang, B.; Wang, Y.; Wang, L.; Wei, J. Highly microporous nitrogen-doped carbons from anthracite for effective CO2 capture and CO2/CH4 separation. Energy 2020, 211, 118561. [Google Scholar] [CrossRef]
- Xing, L.A.; Yang, F.; Zhong, X.; Liu, Y.; Lu, H.; Guo, Z.; Lv, G.; Yang, J.; Yuan, A.; Pan, J. Ultra-microporous cotton fiber-derived activated carbon by a facile one-step chemical activation strategy for efficient CO2 adsorption. Sep. Purif. Technol. 2023, 324, 124470. [Google Scholar] [CrossRef]
- Ruiz, B.; Ferrera-Lorenzo, N.; Fuente, E. Valorisation of lignocellulosic wastes from the candied chestnut industry. Sustainable activated carbons for environmental applications. J. Environ. Chem. Eng. 2017, 5, 1504–1515. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Hong, S.M.; Jang, E.; Dysart, A.D.; Pol, V.G.; Lee, K.B. CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 2016, 6, 34590. [Google Scholar] [CrossRef]
- Cui, H.; Xu, J.; Shi, J.; You, S.; Zhang, C.; Yan, N.; Liu, Y.; Chen, G. Evaluation of different potassium salts as activators for hierarchically porous carbons and their applications in CO2 adsorption. J. Colloid. Interface Sci. 2021, 583, 40–49. [Google Scholar] [CrossRef]
- Siemak, J.; Michalkiewicz, B. Adsorption Equilibrium of CO2 on Microporous Activated Carbon Produced from Avocado Stone Using H2SO4 as an Activating Agent. Sustainability 2023, 15, 16881. [Google Scholar] [CrossRef]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Choi, P.-S.; Jeong, J.-M.; Choi, Y.-K.; Kim, M.-S.; Shin, G.-J.; Park, S.-J. A review: Methane capture by nanoporous carbon materials for automobiles. Carbon Lett. 2016, 17, 18–28. [Google Scholar] [CrossRef]
- Liu, B.; Ma, X.; Wei, D.; Yang, Y.; Zeng, Z.; Li, L. Development of ultramicropore-mesopore interconnected pore architectures for boosting carbon dioxide capture at low partial pressure. Carbon N. Y. 2022, 192, 41–49. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly Microporous Activated Carbons from Biomass for CO2 capture and Effective Micropores at Different Conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Hort, C.; Jeguirim, M.; Ghimbeu, C.M.; Limousy, L.; Bessieres, D. Experimental Determination of the CH4 and CO2 Pure Gas Adsorption Isotherms on Different Activated Carbons. J. Chem. Eng. Data 2018, 63, 3027–3034. [Google Scholar] [CrossRef]
- Mehrmohammadi, P.; Ghaemi, A. Investigating the effect of textural properties on CO2 adsorption in porous carbons via deep neural networks using various training algorithms. Sci. Rep. 2023, 13, 21264. [Google Scholar] [CrossRef]
- Zhu, B.; Shang, C.; Guo, Z. Naturally Nitrogen and Calcium-Doped Nanoporous Carbon from Pine Cone with Superior CO2 Capture Capacities. ACS Sustain. Chem. Eng. 2016, 4, 1050–1057. [Google Scholar] [CrossRef]
- Choma, J.; Osuchowski, L.; Marszewski, M.; Dziura, A.; Jaroniec, M. Developing microporosity in Kevlar®-derived carbon fibers by CO2activation for CO2adsorption. J. CO2 Util. 2016, 16, 17–22. [Google Scholar] [CrossRef]
- Sevilla, M.; Falco, C.; Titirici, M.-M.; Fuertes, A.B. High-performance CO2 sorbents from algae. RSC Adv. 2012, 2, 12792–12797. [Google Scholar] [CrossRef]
- Minagawa, H.; Nishikawa, Y.; Ikeda, I.; Miyazaki, K.; Takahara, N.; Sakamoto, Y.; Komai, T.; Narita, H. Characterization of sand sediment by pore size distribution and permeability using proton nuclear magnetic resonance measurement. J. Geophys. Res. Solid Earth 2008, 113, B07210. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Shen, Y.; Ji, W.; Li, Z. Insight into Water Occurrence and Pore Size Distribution by Nuclear Magnetic Resonance in Marine Shale Reservoirs, Southern China. Energy Fuels 2023, 37, 319–327. [Google Scholar] [CrossRef]
- Radlinski, A.P.; Mastalerz, M.; Hinde, A.L.; Hainbuchner, M.; Rauch, H.; Baron, M.; Lin, J.S.; Fan, L.; Thiyagarajan, P. Application of SAXS and SANS in evaluation of porosity, pore size distribution and surface area of coal. Int. J. Coal Geol. 2004, 59, 245–271. [Google Scholar] [CrossRef]
- Soto, C.; Cicuttin, N.; Carmona, F.J.; de la Viuda, M.; Tena, A.; Lozano, Á.E.; Hernández, A.; Palacio, L.; Prádanos, P. Gas adsorption isotherm, pore size distribution, and free volume fraction of polymer-polymer mixed matrix membranes before and after thermal rearrangement. J. Memb. Sci. 2023, 683, 121841. [Google Scholar] [CrossRef]
- Cornette, V.; Villarroel-Rocha, J.; Sapag, K.; Mons, R.D.; Toso, J.P.; López, R.H. Insensitivity in the pore size distribution of ultramicroporous carbon materials by CO2 adsorption. Carbon N. Y. 2020, 168, 508–514. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Xu, J.; Yan, N.; Zhang, C.; You, S. Fabrication of nitrogen doped and hierarchically porous carbon flowers for CO2 adsorption. J. CO2 Util. 2021, 51, 101617. [Google Scholar] [CrossRef]
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.G.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-Doped Carbon as CO2 Adsorbent with High CO2 Selectivity from Rationally Designed Polypyrrole Precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef]
- Vieillard, J.; Bouazizi, N.; Bargougui, R.; Brun, N.; Fotsing Nkuigue, P.; Oliviero, E.; Thoumire, O.; Couvrat, N.; Djoufac Woumfo, E.; Ladam, G.; et al. Cocoa shell-deriving hydrochar modified through aminosilane grafting and cobalt particle dispersion as potential carbon dioxide adsorbent. Chem. Eng. J. 2018, 342, 420–428. [Google Scholar] [CrossRef]
- Stevens, R.W.; Siriwardane, R.V.; Logan, J. In situ fourier transform infrared (FTIR) investigation of CO2 adsorption onto zeolite materials. Energy Fuels 2008, 22, 3070–3079. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Ghimbeu, C.M.; Ouederni, A. Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. Comptes Rendus Chim. 2015, 18, 63–74. [Google Scholar] [CrossRef]
- Staudt, J.; Musial, C.M.; Canevesi, R.; Fierro, V.; Ribeiro, C.; Alves, H.J.; Borba, C.E. Evaluation of the CH4/CO2 Separation by Adsorption on Coconut Shell Activated Carbon: Impact of the Gas Moisture on Equilibrium Selectivity and Adsorption Capacity. Heliyon 2024, 10, e30368. [Google Scholar] [CrossRef] [PubMed]
- Kamgang Maffo, E.; Clementine-Christelle, M.-E.; Kombou, V. Production of Activated Carbons: Processes, Applications, and Environmental Considerations. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5025307 (accessed on 1 April 2025). [CrossRef]
- Plaza, M.G.; Pevida, C.; Martín, C.F.; Fermoso, J.; Pis, J.J.; Rubiera, F. Developing almond shell-derived activated carbons as CO2 adsorbents. Sep. Purif. Technol. 2010, 71, 102–106. [Google Scholar] [CrossRef]
- Álvarez-Gutiérrez, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Adsorption Performance Indicators for the CO2/CH4 Separation: Application to Biomass-Based Activated Carbons. Fuel Process. Technol. 2016, 142, 361–369. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Ghouma, I.; Hort, C.; Ghimbeu, C.M.; Jeguirim, M.; Bessieres, D. CO2 and CH2 adsorption behavior of biomass-based activated carbons. Energies 2018, 11, 3136. [Google Scholar] [CrossRef]
- Ferrera-Lorenzo, N.; Fuente, E.; Suárez-Ruiz, I.; Ruiz, B. Sustainable activated carbons of macroalgae waste from the Agar-Agar industry. Prospects as adsorbent for gas storage at high pressures. Chem. Eng. J. 2014, 250, 128–136. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R.M. Physical synthesis and characterization of activated carbon from date seeds for CO2 capture. J. Environ. Chem. Eng. 2018, 6, 4245–4252. [Google Scholar] [CrossRef]
- Vilella, P.C.; Lira, J.A.; Azevedo, D.C.S.; Bastos-Neto, M.; Stefanutti, R. Preparation of biomass-based activated carbons and their evaluation for biogas upgrading purposes. Ind. Crops Prod. 2017, 109, 134–140. [Google Scholar] [CrossRef]
- Deng, S.; Hu, B.; Chen, T.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 2015, 21, 125–133. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, N. Activated Carbon Preparation from Eucalyptus Wood Chips using Continuous Carbonization-Steam Activation Process in a Batch Intermittent Rotary Kiln. Sci. Rep. 2020, 11, 13948. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. C 2020, 6, 21. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Sreńscek-Nazzal, J. An innovative and environmentally friendly bioorganic synthesis of activated carbon based on olive stones and its potential application for CO2 capture. Sustain. Mater. Technol. 2023, 38, e00717. [Google Scholar] [CrossRef]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Najar-Souissi, S.; Ouederni, A.; Fuente, E. From pomegranate peels waste to one-step alkaline carbonate activated carbons. Prospect as sustainable adsorbent for the renewable energy production. J. Environ. Chem. Eng. 2022, 10, 107010. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Oliveira, S.d.C.; Rodríguez-Reinoso, F. Tailoring Low-Cost Granular Activated Carbons Intended for CO2 Adsorption. Front. Chem. 2020, 8, 581133. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.G.; González, A.S.; Pis, J.J.; Rubiera, F.; Pevida, C. Production of Microporous Biochars by Single-Step Oxidation: Effect of Activation Conditions on CO2 Capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef]
- Krupšová, S.; Almáši, M. Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability. Molecules 2024, 29, 1158. [Google Scholar] [CrossRef]
- Álvarez-Gutiérrez, N.; García, S.; Gil, M.V.; Rubiera, F.; Pevida, C. Dynamic Performance of Biomass-Based Carbons for CO2/CH4 Separation. Approximation to a Pressure Swing Adsorption Process for Biogas Upgrading. Energy Fuels 2016, 30, 5005–5015. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Fonseca-Bermúdez, Ó.J.; Giraldo, L.; Sierra-Ramírez, R.; Bonillo, M.G.; Farid, G.; Moreno-Piraján, J.C. Bioorganic Activated Carbon from Cashew Nut Shells for H2 Adsorption and H2/CO2, H2/CH4, CO2/CH4, H2/CO2/CH4 Selectivity in Industrial Applications. Int. J. Hydrogen Energy 2024, 86, 662–676. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kiełbasa, K. Advances in modification of commercial activated carbon for enhancement of CO2 capture. Appl. Surf. Sci. 2019, 494, 137–151. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Foungchuen, J.; Pitakchon, T. Impregnation of Chitosan onto Activated Carbon for High Adsorption Selectivity towards CO2: CO2 Capture from Biohydrogen, Biogas and Flue Gas. J. Sustain. Energy Environ. 2012, 3, 153–157. [Google Scholar]
- Rattanaphan, S.; Rungrotmongkol, T.; Kongsune, P. Biogas improving by adsorption of CO2 on modified waste tea activated carbon. Renew. Energy 2020, 145, 622–631. [Google Scholar] [CrossRef]
- Reljic, S.; Martinez-Escandell, M.; Silvestre-Albero, J. Effect of Porosity and Surface Chemistry on CO2 and CH4 Adsorption in S-Doped and S-/O-co-Doped Porous Carbons. C-J. Carbon Res. 2022, 8, 41. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Ghimbeu, C.M.; Réty, B.; Ho, B.N.; Pino, D.; Vaulot, C.; Hort, C.; Bessieres, D. Surface-Modified Activated Carbon with a Superior CH4/CO2 Adsorption Selectivity for the Biogas Upgrading Process. Ind. Eng. Chem. Res. 2022, 61, 12710–12727. [Google Scholar] [CrossRef]
- Domínguez-Ramos, L.; Prieto-Estalrich, A.; Malucelli, G.; Gómez-Díaz, D.; Freire, M.S.; Lazzari, M.; González-álvarez, J. N-and S-Doped Carbons Derived from Polyacrylonitrile for Gases Separation. Sustainability 2022, 14, 3760. [Google Scholar] [CrossRef]
- Jang, D.-I.; Park, S.-J. Influence of nickel oxide on carbon dioxide adsorption behaviors of activated carbons. Fuel 2012, 102, 439–444. [Google Scholar] [CrossRef]
- Lu, X.; Jin, D.; Wei, S.; Zhang, M.; Zhu, Q.; Shi, X.; Deng, Z.; Guo, W.; Shen, W. Competitive adsorption of a binary CO2-CH4 mixture in nanoporous carbons: Effects of edge-functionalization. Nanoscale 2015, 7, 1002–1012. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Aguilar-Armenta, G.; Patiño-Iglesias, M.E.; Leyva-Ramos, R. Adsorption Kinetic Behaviour of Pure CO2, N2 and CH4 in Natural Clinoptilolite at Different Temperatures. Adsorpt. Sci. Technol. 2003, 21, 81–91. [Google Scholar] [CrossRef]
- Yu, H.-R.; Cho, S.; Bai, B.C.; Yi, K.B.; Lee, Y.-S. Effects of fluorination on carbon molecular sieves for CH4/CO2 gas separation behavior. Int. J. Greenh. Gas Control 2012, 10, 278–284. [Google Scholar] [CrossRef]
- Punpee, S.; Tongpadungrod, P.; Suttikul, T.; Phalakornkule, C. Characteristics of CO2 adsorption and desorption on activated carbon in comparison with zeolite 13X and carbon molecular sieve and applications in biogas upgrading using vacuum pressure swing adsorption. J. Chem. Technol. Biotechnol. 2023, 98, 2677–2690. [Google Scholar] [CrossRef]
- Rainone, F.; D’Agostino, O.; Erto, A.; Balsamo, M.; Lancia, A. Biogas upgrading by adsorption onto activated carbon and carbon molecular sieves: Experimental and modelling study in binary CO2/CH4 mixture. J. Environ. Chem. Eng. 2021, 9, 106256. [Google Scholar] [CrossRef]
- Gil, M.V.; Álvarez-Gutiérrez, N.; Martínez, M.; Rubiera, F.; Pevida, C.; Morán, A. Carbon adsorbents for CO2 capture from bio-hydrogen and biogas streams: Breakthrough adsorption study. Chem. Eng. J. 2015, 269, 148–158. [Google Scholar] [CrossRef]
- Rios, R.B.; Stragliotto, F.M.; Peixoto, H.R.; Torres, A.E.B.; Bastos-Neto, M.; Azevedo, D.C.S.; Cavalcante, C.L., Jr. Studies on the adsorption behavior of CO2-CH4 mixtures using activated carbon. Braz. J. Chem. Eng. 2013, 30, 939–951. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, P.; Huang, Z.; Liu, J. Adsorption Equilibrium and Diffusion of CH4, CO2, and N2 in Coal-Based Activated Carbon. ACS Omega 2023, 8, 10303–10313. [Google Scholar] [CrossRef]
- Chinea, L.; Slopiecka, K.; Bartocci, P.; Alissa Park, A.-H.; Wang, S.; Jiang, D.; Fantozzi, F. Methane enrichment of biogas using carbon capture materials. Fuel 2023, 334, 126428. [Google Scholar] [CrossRef]
- Bacsik, Z.; Cheung, O.; Vasiliev, P.; Hedin, N. Selective separation of CO2 and CH4 for biogas upgrading on zeolite NaKA and SAPO-56. Appl. Energy 2016, 162, 613–621. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5; MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Ghimbeu, C.M.; Ho, B.N.; Jeguirim, M.; Hort, C.; Bessieres, D. Comparative study of the CH4/CO2 adsorption selectivity of activated carbons for biogas upgrading. J. Environ. Chem. Eng. 2019, 7, 103368. [Google Scholar] [CrossRef]
- Dreisbach, F.; Staudt, R.; Keller, J.U. High pressure adsorption data of methane, nitrogen, carbon dioxide and their binary and ternary mixtures on activated carbon. Adsorption 1999, 5, 215–227. [Google Scholar] [CrossRef]
- Zaidi, S.T.H.; Ahmad, A.; Ismail, M.; Nordin, N.A.H.M.; Bustam, M.A.; Usman, M.; Asubonteng, D.; ul Hasnain, S.M.W. Enhanced CO2 adsorption and selectivity in CNT and piperazine modified Ni-MOF-74 nanocomposites. Solid State Sci. 2025, 161, 107855. [Google Scholar] [CrossRef]
- Amasha, H.; Ahmad, A.; Abdulazeez, I.; Al Hamouz, O.C.S. Microwave-synthesized heteroaromatic porous organic polymers for CO2 capture and hydrogen storage. Mater. Today Sustain. 2024, 27, 100879. [Google Scholar] [CrossRef]
- Bentivoglio, D.; Chiaraluce, G.; Finco, A. Economic assessment for vegetable waste valorization through the biogas-biomethane chain in Italy with a circular economy approach. Front. Sustain. Food Syst. 2022, 6, 1035357. [Google Scholar] [CrossRef]
- Rusanowska, P.; Zieliński, M.; Dębowski, M. Removal of CO2 from Biogas during Mineral Carbonation with Waste Materials. Int. J. Environ. Res. Public Health 2023, 20, 5687. [Google Scholar] [CrossRef]
- Baldinelli, A.; Desideri, U.; Fantozzi, F.; Cinti, G. Biogas-to-Power Systems Based on Solid Oxide Fuel Cells: Thermodynamic Analysis of Stack Integration Strategies. Energies 2024, 17, 3614. [Google Scholar] [CrossRef]



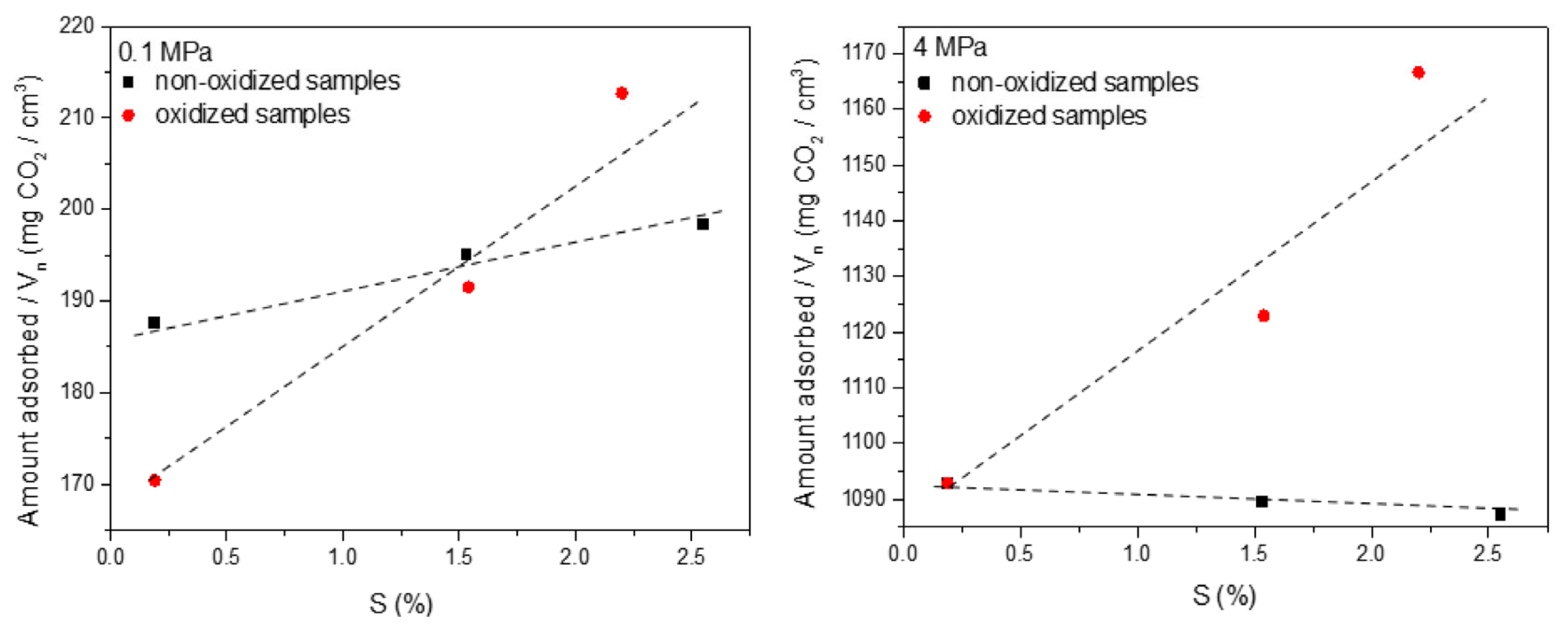
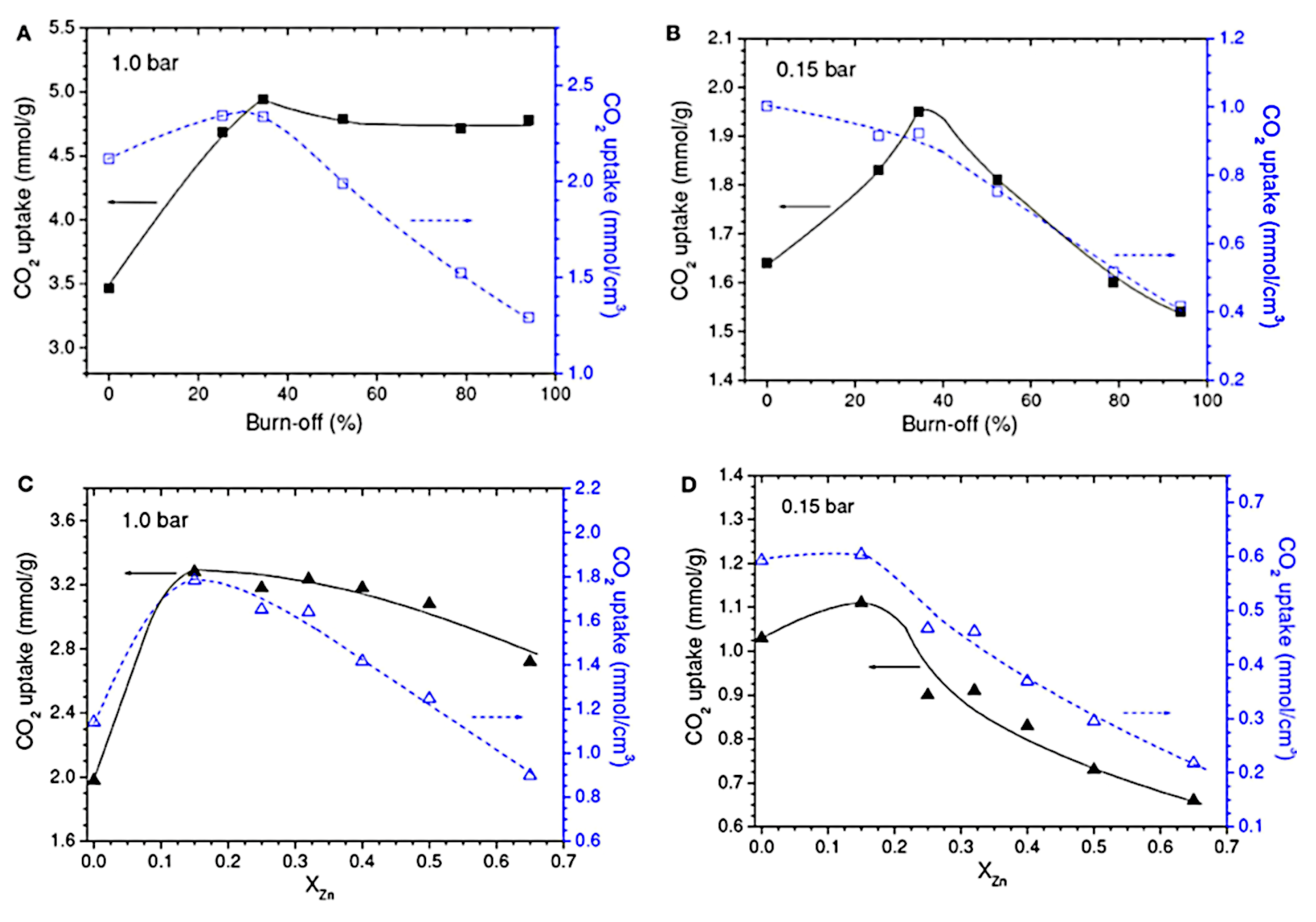
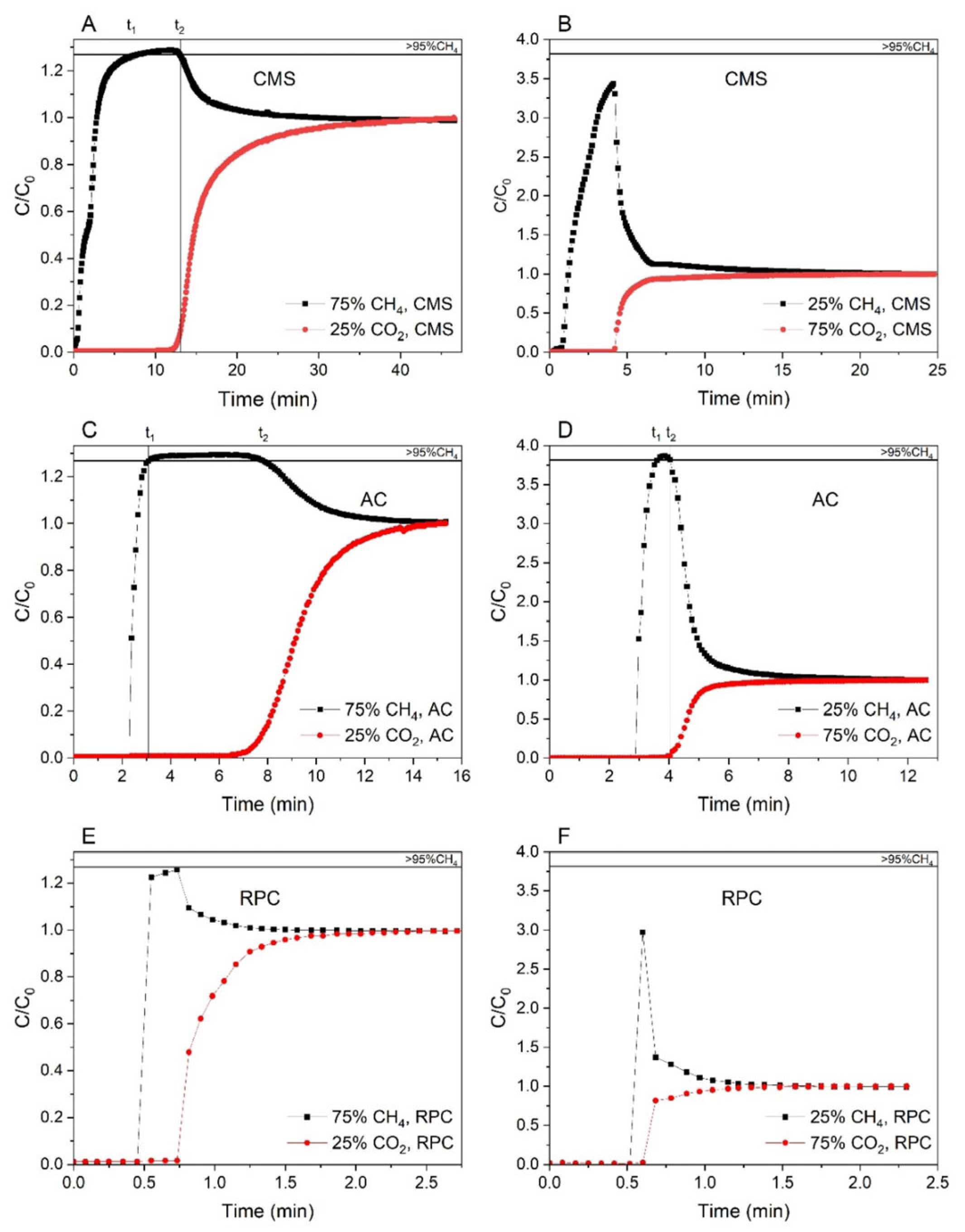
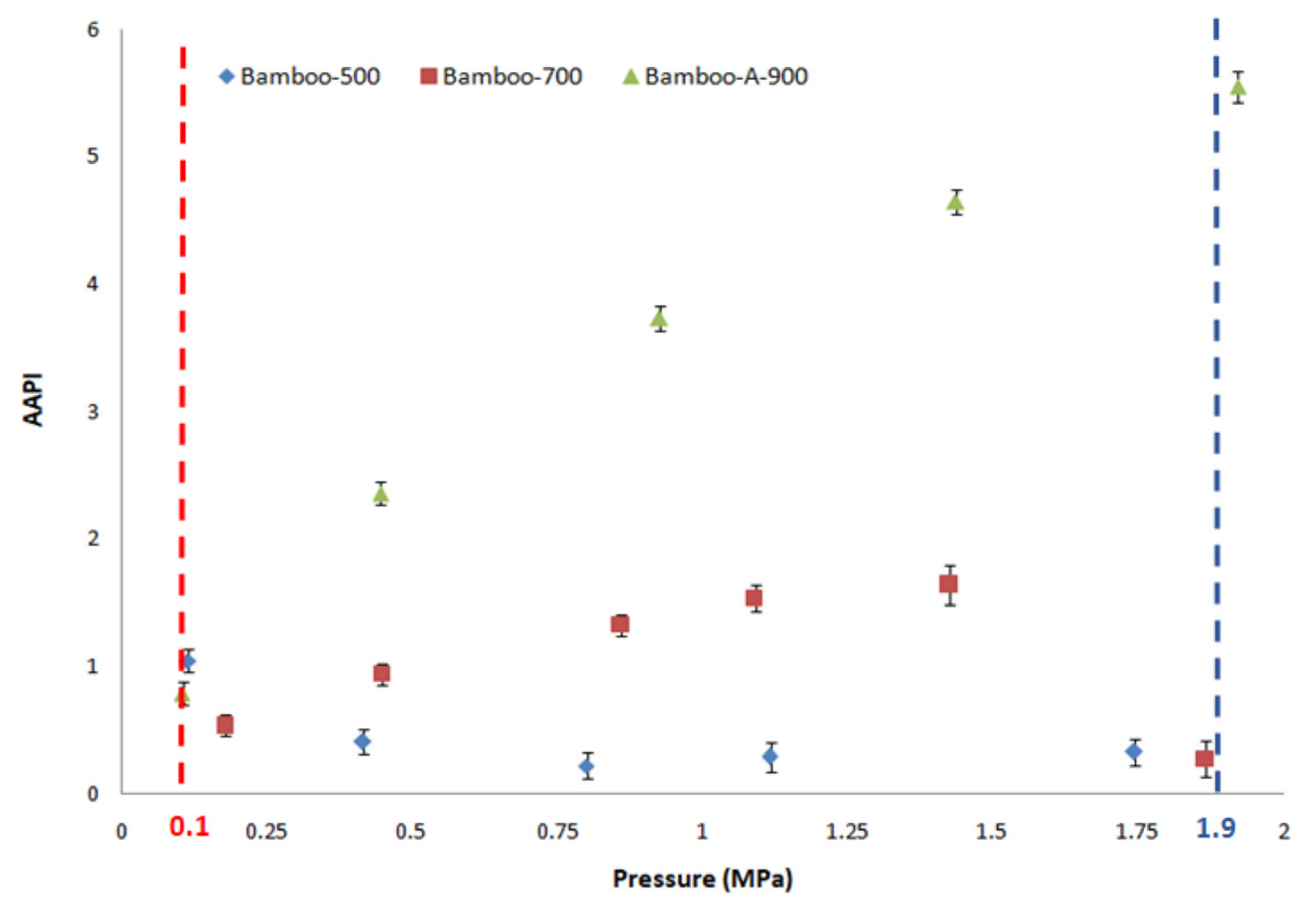
| Sample (Substrate) | SBET (m2 g−1) | VTot (cm3 g−1) | Vmicro (cm3 g−1) | V<0.7 nm (cm3 g−1) | CO2 Uptake (mmol g−1) at 298 K | References |
|---|---|---|---|---|---|---|
| AC-1 (anthracite) | 1004 | 0.48 | 0.23 a | 3.16 | [94] | |
| AC-4 (anthracite) | 1394 | 0.67 | 0.32 a | 3.49 | [94] | |
| AC-2 (anthracite) | 1615 | 0.80 | 0.22 a | 2.77 | [94] | |
| CC2-800 (cotton fiber) | 1371 | 0.609 | 0.3361 | 4.43 | [95] | |
| CC3-800 (cotton fiber) | 1343 | 0.600 | 0.2568 | 3.75 | [95] | |
| CHPA-2-750-f Chestnut shell) | 1792 | 0.729 | 0.293 | 0.297 | 13.36 | [96] |
| Bamboo-1-1073 | 1273 | 0.51 | 0.31 | 5.9 b | [58] | |
| Bamboo3-973 | 2332 | 1.00 | 0.37 | 7.0 b | [97] | |
| Bamboo-5-973 | 2980 | 1.41 | 0.28 | 5.3 b | [97] | |
| MCC | 648 | 0.299 | 0.266 | 0.218 c | 2.28 | [98] |
| MCC-K1 | 916 | 0.432 | 0.367 | 0.25 c | 2.42 | [98] |
| MCC-K2 | 1057 | 0.581 | 0.474 | 0.271 c | 2.77 | [98] |
| Precursor | SBET (m2 g−1) | VTot (cm3 g−1) | Vmicro (cm3 g−1) | Yield (%) | CO2 Max. Uptake (mol kg−1) at 298 K | SCH5/CO2 | References |
|---|---|---|---|---|---|---|---|
| Bamboo | 1846 | 0.78 | 0.36 | 21.7 | 7.0 | 8.5 | [97] |
| Coconut shell | 1105 | 0.35 | 0.34 | 7.59 | 6.5 | [120] | |
| Olive stones | 1178 | 0.49 | 0.45 | - | 10.873 | - | [124] |
| Avocado stones | 538 | 0.217 | 0.175 | 21 | 4.9142 (293 K) | - | [100] |
| Chestnut shell | 1792 | 0.729 | 0.295 | 34.14 | 13.36 | - | [96] |
| Defatted grape seeds | 1604 | 0.672 | 0.250 | - | 11.48 | - | [125] |
| Date seeds | 798.38 | - | 0.28 | 7.25 | 2.9 | - | [126] |
| Babassu coconut | 809 | 0.39 | 0.32 | 14.6 | 10.49 | 4.2 | [127] |
| Almond shell | 326 | 0.24 | 0.23 | 24 | 2.18 | - | [122] |
| Cherry stone | 1045 | 0.48 | 0.40 | - | 10.88 | 3.61 | [123] |
| Peanut shell | 956 | 0.77 | - | - | 4.03 | - | [128] |
| Sunflower seed | 1790 | 0.43 | - | - | 4.61 | - | [128] |
| Substrate | SBET (m2 g−1) | Vtot (cm3 g−1) | Vmic (N2) (cm3 g−1) | CO2 Uptake |
|---|---|---|---|---|
| PP | 585 | 0.28 | 0.2 | 4.11 |
| CP | 1379 | 0.58 | 0.51 | 4.18 |
| FL | 1593 | 0.74 | 0.54 | 4.12 |
| Sample (Precursor) | Activation Method/Conditions | CO2 Uptake (mol kg−1) | Refs. |
|---|---|---|---|
| Olive stones | 1. Physical: Carbonization at 873 K and H2O activation at 1023 K | (T = 303 K) 7.968 | [124] |
| 2. Physical: Carbonization at 873 K and H2O activation at 1023 K | 5.878 | ||
| 3. Chemical: H3PO4 impregnation at a weight ratio of 1:3 at 383 K. | 10.873 | ||
| Post-consumer plastic waste | Chemical: Pyrolysis at 450 or 500 °C and activation at 1:1 mass ratio with: | (T = 298 K) | [57] |
| 41.3/84.8 mg g−1 | ||
| 29.2/29.1 mg g−1 | ||
| 67.6/82.1 mg g−1 | ||
| 64.1/58.3 mg g−1 | ||
| 33.7/43.5 mg g−1 | ||
| 34.6/29.8 mg g−1 | ||
| Olive stones | Chemical: Carbonization at 500 °C and impregnation at a mass ratio of 1:1 with: | (T = 303 K) | [132] |
| saturated KOH, | 4.33 | ||
| 85% H3PO4, | 4.00 | ||
| KOH solution from banana peels extract | 3.58 | ||
| Pomegranate peel | Chemical: One-step activation with K2CO3 activating agent in proportions of 0.5:1 and 1:1 at varying temperatures: | (T = 298 K) | [133] |
| 11.8 | ||
| 13.0 | ||
| 15.1 | ||
| 12.4 | ||
| 13.7 | ||
| 16.3 | ||
| Chestnuts shells | Chemical: Carbonization at 750 °C followed by KOH impregnation in weight ratios of 1:1. and 2:1 and different temperatures: | % mass fraction | [96] |
| 35% | ||
| 42% | ||
| 51% | ||
| 58% | ||
| Coconut shell | Physical: Carbonization at 850 °C followed by CO2 activation at 750 °C until different burn-offs: | [134] | |
| 4.8/2.3 mmol cm−3 | ||
| 5.0/2.3 mmol cm−3 | ||
| 4.9/1.9 mmol cm−3 | ||
| 4.8/1.5 mmol cm−3 | ||
| 4.9/1.3 mmol cm−3 | ||
| Almond Shell | Physical: Activation with air at two temperatures and different reaction times: | (298 K) | [135] |
| 1.14 | ||
| 1.29 | ||
| 1.37 | ||
| 1.51 | ||
| 1.57 | ||
| 1.53 | ||
| Cellulose | 1. Hydrothermal activation with amines at 240 °C: | (273 K) | [136] |
| 4.38 | ||
| 5.33 | ||
| 7.34 | ||
| 2. Hydrothermal activation with amines, KOH activation and carbonization at 800 °C: | |||
| 1.76 | ||
| 2.19 | ||
| 4.16 | ||
| Cherry stones | Physical: single step activation with: | (303 K) | [137] |
| 5.14 | ||
| 4.48 | ||
| Sugarcane | Physical: carbonization at 750 °C and activation at carbonized at 850 °C with: | (298 K) | [138] |
| 1.6 | ||
| 2.6 | ||
| Chemical: direct pyrolysis and activation of the precursor impregnated with: | |||
| 2.7 | ||
| 4.3 |
| Precursor | Post-Treatment | References |
|---|---|---|
| Palm shell | Immersion for 3 days in chitosan solutions with initial concentrations of 0.1–2.0 g/L | [142] |
| Waste tea | Immersion in 5 mL of 3% w/v of EDA in methanol | [143] |
| Petroleum pitch | Treatment with H2S at elevated temperatures (600 °C and 800 °C) Pre-oxidation treatment with plasma followed by H2S treatment at elevated temperatures (600 °C and 800 °C) | [144] |
| Commercial AC | Air oxidation at 400 °C for 2 h, Pre-oxidation treatment followed by immersion in NH4OH for 36 h | [145] |
| Polyacrylonitrile | Mixing with sulfur in a 1:1 weight ratio for 1 h at 553 K | [146] |
| Commercial AC |
| [147] |
| Commercial AC |
| [92] |
| Commercial AC | Mixing with a saturated solution of ammonium sulfate salt at different AC/ammonium sulfate mass ratios (w(NH4)2SO4 = 4.76, 6.98 and 9.1%) | [59] |
| Sample | SCO2/CH4 | WC, CO2 (mol kg−1) | Qst, CO2 (KJ mol−1) | API |
|---|---|---|---|---|
| CS-CO2 | 4.35 | 2.83 | 21.15 | 1.27 |
| CS-H2O | 4.39 | 2.60 | 23.03 | 1.00 |
| Sample | Type | Selectivity (1 MPa, T = 303 K) | Max. Selectivity | Mol (%) CO2 | T (K) | Refs. |
|---|---|---|---|---|---|---|
| NaX * | Zeolite | 76 (0.1 MPa) | 50 | 303 | [21] | |
| CaX * | Zeolite | 22 (0.1 MPa) | 50 | 303 | [21] | |
| CaA * | Zeolite | 44 (0.1 MPa) | 50 | 303 | [21] | |
| 4A * | Zeolite | 200 | 50 | 293 | [158] | |
| 5A | Zeolite | 256.47 | 50 | [159] | ||
| CMS CT-350 CarboTech | CMS | 28.4 | 20 | [154] | ||
| CMS-240 (Xintao) | CMS | 34.9 | 10 | [154] | ||
| P-AC | AC | 38 | 50 | [20] | ||
| CNR-115-ox-am | AC | 129 | 50 | 303 | [146] | |
| CNR | AC | 1.8 | 50 | 303 | [160] | |
| GAC 1240 | AC | 2.7 | 50 | 303 | [160] | |
| AC WV1050 * | AC | 5.22 | 8.7 | 47 | 293 | [156] |
| Norit R1 * | AC | 2.7 | 42 | 298 | [161] | |
| HKUST-1-MOF * | MOF | 6 | 50 | 293 | [158] | |
| MOF-5 * | MOF | 15.53 | 50 | 298 | [159] | |
| MOF-177 * | MOF | 4.43 | 50 | 298 | [159] |
| AC | MOF | Zeolite | |
|---|---|---|---|
| CO2 adsorption capacity | High | Medium | High |
| CO2/CH4 selectivity | Low | Medium | High |
| PSA regeneration | Feasible | Not documented | Feasible (P < Patm) |
| Cost (USD kg−1) | 0.6–2.4 | 10.0–70.0 | 0.6–5.0 |
| Other considerations | Sustainable precursors | Low thermal and hydrolytic stability | High CO2 adsorption heat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peredo-Mancilla, D.; Bermúdez, A.; Hort, C.; Bessières, D. Exploring Activated Carbons for Sustainable Biogas Upgrading: A Comprehensive Review. Energies 2025, 18, 4010. https://doi.org/10.3390/en18154010
Peredo-Mancilla D, Bermúdez A, Hort C, Bessières D. Exploring Activated Carbons for Sustainable Biogas Upgrading: A Comprehensive Review. Energies. 2025; 18(15):4010. https://doi.org/10.3390/en18154010
Chicago/Turabian StylePeredo-Mancilla, Deneb, Alfredo Bermúdez, Cécile Hort, and David Bessières. 2025. "Exploring Activated Carbons for Sustainable Biogas Upgrading: A Comprehensive Review" Energies 18, no. 15: 4010. https://doi.org/10.3390/en18154010
APA StylePeredo-Mancilla, D., Bermúdez, A., Hort, C., & Bessières, D. (2025). Exploring Activated Carbons for Sustainable Biogas Upgrading: A Comprehensive Review. Energies, 18(15), 4010. https://doi.org/10.3390/en18154010






