Abstract
Al/AgO seawater-activated batteries with high specific energy and high specific power are widely used at present. The AgO electrode determines the performance of the battery, with its active material utilization rate having a significant impact on the specific capacity, energy density and discharge capacity of the battery. Therefore, this study briefly introduces the structure and working principle of Al/AgO seawater-activated batteries. Starting from the AgO material itself, common preparation methods for such positive electrode materials—including sintered silver oxide electrodes, pressed silver oxide electrodes and thin-film silver oxide electrodes—are introduced, and the factors influencing their electrochemical performance are analyzed in depth. We elaborate on the relevant research progress regarding AgO electrodes in terms of improving battery performance, detailing the effects of the silver powder’s morphology, porosity, purity, ordered structure, surface treatment and doping modification methods on silver oxide electrodes. Finally, various methods for improving the electrochemical performance of silver oxide electrodes are detailed. Current challenges and possible future research directions are analyzed, and prospects for the future development of high-specific-energy batteries based on AgO electrode materials are discussed. Overall, this review highlights the characteristics of Al/AgO batteries, providing a theoretical basis for the development of high-performance Al/AgO batteries.
1. Introduction
Al/AgO batteries were first developed in the 1970s in the United States as high-performance seawater-activated batteries, presenting desirable electrochemical performance advantages such as high energy density, high specific capacity and high specific power. While the theoretical specific energy of Al/AgO batteries is 1090 Wh/kg, the actual specific energy of the battery can reach 280 Wh/kg, which is about twice as high as the theoretical and actual specific energies of lithium/silver and zinc/silver seawater-activated batteries [1,2,3,4,5,6]. Al/AgO batteries use aluminum alloy as the negative electrode material, silver oxide as the positive electrode and react with seawater as the electrolyte to generate a current. Using AgO material as the active cathode in aqueous Al/AgO batteries allows for a high capacity of 394.7 mAh/g at a current density of 500 mA/cm2, reaching 91.4% of the theoretical specific capacity density and showing excellent rate performance. A maximum output power density of up to 1260.54 mW/cm2 can be achieved at 900 mA/cm2 [7,8,9,10]. During discharge, the negative electrode undergoes dissolution of Al metal, consuming OH− to generate a large amount of aluminum salts (AlO2−), while the positive electrode AgO is reduced to Ag. The basic working principle is:
Negative electrode 2Al + 8OH− → 2AlO2− + 4H2O + 6e−
Positive electrode 3AgO + 3H2O + 6e− → 6OH− + 3Ag
Overall reaction 2Al + 3AgO + 2OH− → 2AlO2− + 3Ag + H2O
Silver oxide batteries are efficient and environmentally friendly energy cells that can help to reduce reliance on traditional fossil fuels and lower carbon dioxide emissions [11,12]. The high-value metals they contain, such as silver, make their recycling economically meaningful. Recycling silver oxide batteries not only allows for the extraction of precious metals such as silver, but also reduces environmental heavy metal pollution. The recycling methods for silver oxide batteries include physical disassembly, chemical treatment and wet metallurgy [13]. Due to their high energy density, high capacity and environmental friendliness compared to lithium-ion batteries and zinc-based aqueous batteries [14,15], Al/AgO batteries are considered some of the most promising water-based batteries. As competition among countries becomes increasingly fierce, the performance requirements for salt-activated batteries are also becoming more and more stringent. Aluminum/silver oxide batteries with high specific energy are favored by various countries and are widely used in both national defense and civilian applications [16,17,18]. The most representative cases are the MU90 light torpedo and the Black Shark heavy torpedo, which are equipped with SAFT’s Al/AgO batteries. Meanwhile, the French Eel torpedo, the Italian A290 torpedo and MU90 torpedo, among others, all use silver aluminum-oxide batteries as a power source for propulsion. The MU90 torpedo has a high speed of 50 kn, a low speed of 29 kn, and a full high-speed range of up to 12 km. The “Sea Eel” torpedo has a high speed of 53 kn and a low speed of 38 kn. The A290 torpedo is a rocket-assisted torpedo with a maximum speed of up to 57 kn. Later, silver aluminum-oxide batteries were successively applied to some heavy torpedoes, such as the Black Shark torpedo produced by the Italian company Whitehead. The anti-submarine torpedoes independently developed in South Korea also adopted silver aluminum-oxide batteries as a power source. In recent years, countries such as Europe and the United States have successively intensified their research efforts relating to the application of silver aluminum oxide batteries in torpedoes [19,20,21,22]. Through the development and optimization of low-cost, miniaturized, and lightweight Al/AgO primary batteries—such as miniaturized Al/AgO coin-shaped and self-powered batteries featuring painted paper electrodes—for portable applications, they can be implemented as commercial portable power sources to power microelectronic devices with low power consumption requirements, such as sensors or wearable electronic devices [19]. The demand for power sources with long endurance and high power density has surged with the rapid development of underwater unmanned equipment and deep-sea exploration technology, as traditional batteries are unable to meet the requirements of complex underwater environments at present. Therefore, accelerating research on Al/AgO batteries is not only expected to promote the technological progress of high-performance water-based batteries [23,24], but also has important significance for promoting the development of the marine economy and ensuring the upgrading of national defense equipment. The necessity and urgency of such research are becoming increasingly prominent [25,26,27].
As the improvement of battery discharge performance mainly depends on the AgO material, the AgO cathode material has become an increasingly hot research topic in the context of Al/AgO batteries. However, AgO has disadvantages such as low porosity, low utilization of active material, poor thermal stability and large discharge polarization, which limit its high current discharge performance and utilization rate when used as a cathode active material, greatly reducing the advantages related to the high energy density of Al/AgO batteries. Therefore, further improving the electrode structure and production methods to obtain batteries with high energy density and high performance has become a development trend, with scholars at home and abroad having conducted extensive research on AgO cathode materials. This article elaborates on the progress of research focused on the impacts of AgO materials on battery performance, as well as prospects for the future development of AgO electrodes based on their potential for high energy density and performance. The content studied in this article is depicted in Figure 1.
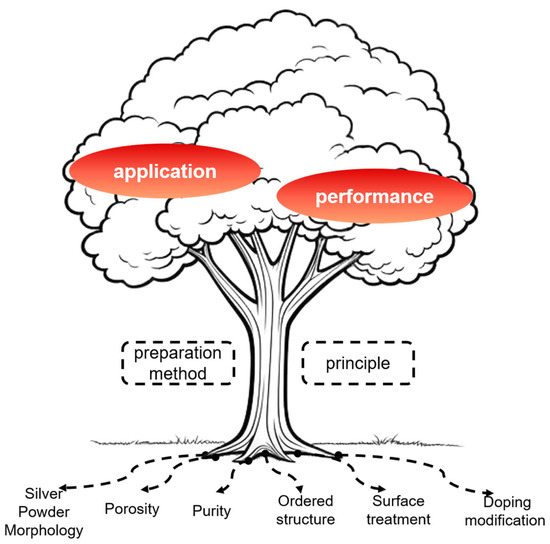
Figure 1.
Schematic diagram of the research content in this article.
2. Preparation Methods
The preparation methods for silver oxide (AgO) cathode materials can be classified into three types, including those for the preparation of sintered silver oxide electrodes, pressed silver oxide electrodes and thin-film silver oxide electrodes [28,29,30]. A comparison between these different preparation methods is provided in Table 1.

Table 1.
Comparison between methods for preparation of sintered silver oxide electrodes, pressed silver oxide electrodes and thin-film silver oxide electrodes.
2.1. Preparation of Sintered Silver Oxide Electrodes
Sintered silver oxide electrodes are silver-based electrode materials prepared using a high-temperature sintering process. The core preparation process of sintered silver oxide electrodes includes silver powder pressing, high-temperature sintering and electrochemical formation. Adopting a single formation process, using stainless steel plates as auxiliary electrodes, formation is carried out via suspension in potassium hydroxide electrolyte. Through the use of a two-stage constant current charging method (such as pre-charging with low current density and gradually increasing the current), the conversion of silver to silver oxide (AgO) can be promoted, reducing the generation of low conductivity by-products such as Ag2O and increasing the true surface area of the electrode while minimizing polarization. Mixing micro and nano silver powders in a certain proportion and sintering them to form a three-dimensional conductive network can improve the electrode’s specific surface area and electronic conductivity efficiency. The introduction of carbon nanotubes can significantly reduce the electrode’s ohmic resistance.
The preparation of sintered silver oxide electrodes mainly involves pressing silver powder and silver into silver electrodes and then oxidizing them into silver oxide electrodes. The preparation of silver electrodes can be carried out through roller pressing or mold pressing. The advantage of silver electrodes prepared via roller pressing lies in their high efficiency and suitability for mass production. Moreover, the distribution of silver powder on the silver electrodes produced using this method is generally uniform; however, the silver powder distribution may be uneven due to the vibration of the equipment. Silver electrodes prepared via molding are suitable for small-batch experiments. The size of the mold is designed according to the required size of the silver electrode, which saves the blanking process and reduces the waste generated during the development of the silver electrode at the same time. However, this process involves manually spreading the powder, and the different techniques of individuals can easily lead to the phenomenon of loose powder; that is, the silver powder being unevenly distributed on the electrode.
In the preparation of sintered silver oxide electrodes, high-purity silver powder is used as the core raw material, and the construction of high-performance electrodes is achieved through the three steps of molding, sintering and electrochemical activation. First, micron-scale silver powder is mixed with a small amount of nano silver powder, and conductive or pore-forming agents are added. A porous blank is then formed via mold pressing or roll pressing, followed by sintering to form metallurgical bonds between silver powder particles and obtain a silver matrix, providing a high specific surface area and ion transport channels for subsequent reactions. The formed silver matrix needs to be electrochemically transformed in potassium hydroxide as an electrolyte through staged constant-current oxidation (low-current pre-charging generates silver oxide, high-current completes conversion to silver oxide), efficiently converting silver into highly active silver oxide (AgO). After cleaning and drying, the electrode has a stable porous structure and high electrochemical activity. It is suitable for use in applications such as high-energy density batteries and special power sources, and can perform exceptionally well in scenarios characterized by low temperatures while maintaining high reliability.
2.2. Preparation of Pressed Silver Oxide Electrodes
The preparation method for pressed silver electrodes is similar to that for molded silver electrodes, with the silver powder in the mold being replaced with silver oxide powder. This silver oxide powder can be synthesized chemically (CP) or electrochemically (EP). The advantage of this method is that it eliminates the need for complex processes such as electrode formation and washing and drying, which can effectively improve the process efficiency and is thus significant for production at larger scales.
The preparation of pressed silver oxide electrodes requires pre-synthesized silver oxide powder as a raw material and achieves efficient production through direct cold-pressing molding. First, the silver oxide powder needs to be pre-treated: in the chemical synthesis (CP) method, silver oxide is precipitated by reacting silver nitrate with alkaline solution, which is then washed and dried to obtain micrometer-scale powder that is suitable for large-scale preparation. Meanwhile, the electrochemical synthesis (EP) method uses a silver anode, which is oxidized in an alkaline electrolyte, directly generating highly active nanoscale silver oxide with a larger specific surface area and reactivity. After pre-treatment, the powder is sieved to remove impurities, and the particle size ratio is adjusted according to the target performance. Then, it is cold-pressed in a mold to form the electrode sheet. Due to the presence of silver oxide as an active substance, there is no need for electrochemical conversion, water washing and high-temperature drying processes (as is the case for traditional sintered electrodes), which shortens the cycle from raw materials to finished products and significantly improves production efficiency.
This process allows for the flexible design of electrode structures through precise control of the pressing parameters: a porous structure is formed under low pressure (10–15 MPa), which is conducive to the rapid penetration of electrolytes and, thus, is suitable for micro batteries that require high capacity (such as watch button batteries); meanwhile, high pressure (20–30 MPa) results in a dense electrode with a mechanical strength greater than 10 MPa, which is suitable for high current discharge scenarios (such as emergency start-up power). The differences in synthesis methods directly affect the resulting electrodes’ performance: the CP method has a lower powder cost but lower crystallinity, while the EP method yields a nanostructured powder, consequently improving the utilization rate of active substances. Compared with sintered silver oxide electrodes, the pressing process avoids the problem of silver particle agglomeration caused by high-temperature sintering and does not require complex equipment, thus reducing energy consumption. It exhibits unique advantages in fields requiring cost- and production-efficiency-sensitive civilian batteries (such as hearing aid batteries and tire pressure monitoring sensor power supplies). At the same time, through the optimization of powder surface modifications, the stability of such electrodes in high-temperature environments can be further improved.
2.3. Preparation of Thin-Film Silver Oxide Electrodes
Compared with traditional electrodes, thin-film electrodes have a lower thickness, a larger contact area between the active material and the electrolyte, a higher liquid absorption rate of the electrode and, to a certain extent, a more uniform distribution of active material. This is very beneficial for improving the discharge power of the battery and the utilization rate of the active material. Therefore, thin-film electrodes are considered to have excellent electrochemical performance. Thin-film electrodes are generally prepared using methods such as magnetron sputtering or chemical vapor deposition.
Thin-film silver oxide electrodes have attracted much attention in the field of micro-energy storage due to their ultra-thin characteristics and excellent electrochemical performance. Their preparation process integrates nanomaterial processing and precision film-forming technologies, and the core process can be summarized in terms of three key steps: raw material synthesis, film-forming processing and post-treatment.
First, the preparation of the silver oxide precursor and its slurry is performed. The chemical precipitation method is usually used, which involves slowly dropping silver nitrate solution into a sodium hydroxide solution, controlling the reaction temperature via an ice water bath environment to promote the formation of nano silver oxide particles. After the reaction is complete, impurities are removed via multiple centrifugation and washing with deionized water. Then, pure silver oxide powder is dispersed in N-methylpyrrolidone (NMP) solvent and carbon nanotubes are added to enhance conductivity. After ultrasonic treatment, a uniform and stable slurry is formed.
In the film-forming process, an appropriate process is selected based on the characteristics of the substrate material. If a rigid substrate (such as ITO glass) is used, spin coating is commonly employed; while, for flexible substrates (such as PET), inkjet printing technology is used. After film-formation, the solvent is removed and annealed to tightly bond the film with the substrate and optimize the internal structure.
3. Factors Affecting AgO Cathodes
Ag and Cu compounds, such as halides and oxides, are the most commonly used cathode materials for seawater-activated batteries. Table 2 lists some cathode materials that are used (or likely to be used) in seawater-activated batteries [31]. Compared with other cathode electrode materials, the core advantages of AgO include its high specific capacity, high conductivity, high voltage and strong environmental adaptability, making it the preferred material for high-energy batteries—especially seawater-activated batteries.

Table 2.
Cathode materials used (or likely to be used) in seawater-activated batteries.
The AgO cathode material is an important part of a silver battery. As the active material of the cathode, the performance of the silver oxide cathode determines the electrochemical properties of the battery, such as its voltage, discharge rate, electrochemical utilization and so on. The cathode active material is also a key factor restricting the development of Al/AgO batteries. The performance parameters of a battery (e.g., voltage, operating time and discharge power) are directly related to its quality. The voltage platform, electrochemical activity and material utilization of the cathode material are greatly affected by factors such as the silver powder’s morphology, porosity, microstructure and surface treatments. Therefore, studying the factors that affect the discharge performance of silver batteries is considered to be of constructive significance for subsequent research on cathode materials.
3.1. Silver Powder Morphology
When using an AgO electrode as the cathode material, the morphology of the used silver powder is a key factor affecting the electrochemical performance of the battery. The characteristics affecting silver powder morphology mainly include the shape, internal and external surface area, volume and defects of the silver powder, which directly determine the particle distribution and chemical activity of the material. Generally speaking, the higher the sphericity of silver powder particles, the better the flowability; however, this may also reduce cross-linking between particles, decrease the electrode conductivity, and increase ohmic polarization. Nano silver powders have a unique volume effect, surface effect and quantum size effect due to being a nanoscale material, which can effectively reduce electrochemical polarization, ohmic polarization, improve the working voltage and high-rate output performance of batteries and provide a higher specific capacity. Research has shown that an AgO electrode material using micron-sized silver powder as the active substance had a utilization rate of about 70%. The low utilization rate of the active substance restricts the ability to improve the performance of silver oxide batteries. In recent years, researchers have found that, compared to granular nano silver powder, silver nanowires have special nano effects, low resistance, high specific surface area and other excellent physicochemical properties. As such, using them as the active substance in AgO electrodes can greatly improve the electrochemical performance of silver oxide batteries. To date, scholars at home and abroad have conducted numerous studies on the relationship between the morphology of silver powder and the discharge performance of the resulting batteries [32,33,34,35].
Pan et al. [32] assessed the electrochemical properties of granular nanostructured AgO cathode materials they synthesized, resulting in a high purity of 98.6% and particle size of about 65 nm. Even at a high charge/discharge current of up to 10,000 mA/g, the nanostructured AgO electrode still exhibited good electrochemical characteristics. The discharge voltage of this electrode was 1.4 V, with a discharge capacity of 360 mAh/g and a specific power density of up to 14 kW/kg. Mostafa Najaf [33] and others have studied the discharge performance of nanometer-thick silver battery cathode material AgO particles at different current densities. Their results showed that, under a constant current discharge condition of 80 mA/cm2, the capacity of the submicron/μm AgO particle mixture was the highest (400 mAh/g) with the smallest potential drop. At a high current density (600 mA/cm2), cathodes prepared with a high proportion of micron-sized AgO exhibited the best discharge performance. Wang et al. [34] prepared electrodes using silver nanowires with a length-to-diameter ratio of 398 at different molding pressures and sintering temperatures. The discharge specific capacity was found to reach 420 mAh/g, with an active material utilization rate of 85%. This is more than a 10% increase compared to conventional micron-sized silver powder electrodes, while maintaining good discharge performance. Wang et al. [35] and others have used silver nanowires (AgNWs) to prepare AgO electrodes based on their structure, with details of the microstructure shown in Figure 2. The developed electrodes had a large specific surface area, small thickness, low bulk resistance and stable structure, presenting significant advantages in terms of specific surface area and bulk resistance. Batteries developed based on these electrodes showed improved performance in terms of specific energy, specific power and active material utilization rate.
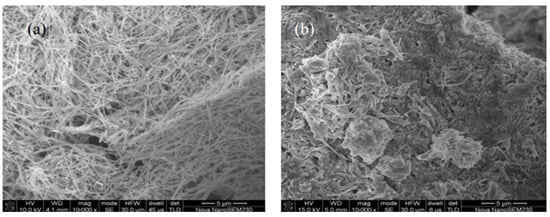
Figure 2.
SEM images of silver nanowire electrode: (a) pre-formation; and (b) after formation [35].
3.2. Porosity
Porosity directly affects the electrochemical activity of a positive electrode material, as well as the electrode’s potential and the material utilization rate. Regarding the active material of the AgO positive electrode, low porosity and low utilization rate of the active substance are disadvantages that greatly reduce the high-specific-energy-related advantages of AgO batteries. The low utilization rate of the active substance leads to an increase in silver powder content which, in turn, leads to thicker electrodes or lower porosity. This further reduces the utilization rate of the active material, creating a vicious cycle that ultimately significantly reduces a battery’s performance, while also increasing its weight and cost. The electrochemical activity of silver powder with low porosity is relatively low, and silver powder with suitable porosity usually has a larger specific surface area and higher activity; the utilization rate of the positive electrode material can also be improved in this way. While high-porosity electrodes have a larger reaction surface area, can provide more active sites, reduce current density and increase voltage platform, excessive porosity can lead to poor electrode formability, difficulties in process control and low yield. Therefore, further improving the electrode and its structure to increase the porosity and material utilization rate—thus improving manufacturing processes to achieve high energy density and high-performance batteries—has become a key area of research. It has been shown that using a porous electrode structure can significantly increase the electrode’s porosity and specific surface area, greatly reducing the actual current density of the battery, improving the utilization of active materials and enhancing the battery’s performance.
The inherent conductivity of AgO at room temperature is about 10−1~1 S/cm—much lower than that of metallic silver (about 6.3 × 105 S/cm). To improve the conductivity of AgO electrodes, many scholars have conducted research. Masoud Sabzi et al. [7] have studied the effects of carbon composite AgO nanoparticles on the electrochemical behavior and electronic performance of silver batteries. In this experiment, they prepared four silver oxide electrodes containing 5, 10, 15 and 20 wt% carbon powder. The chemical compositions of the prepared electrodes are presented in Table 3. In Figure 3, TEM and FE-SEM images of the silver oxide powder prepared in this study are shown. As can be seen from the XRD pattern presented in Figure 3C, the silver oxide used in this study presents both AgO and Ag2O allotropies. In Figure 4, SEM images prepared from all four electrodes before and after the sintering process are shown, from which it can be seen that the porosity of the electrode’s surface is very low and its uniformity is very suitable before sintering. Due to an increase in carbon content in the silver oxide electrode after the sintering process, the SEM images show signs of an increase in the number and size of apparent pores. Figure 5 depicts the mechanism by which carbon dioxide and carbon monoxide gases are formed through the decomposition of silver oxide, resulting in the formation of pores. The results show that increasing the C content in the AgO electrode can increase the electrode’s porosity, reduce the content of Ag2O and AgO phases in the oxidized silver electrode, increase the degree of pure silver formation and promote a decrease in the corrosion resistance of the oxidized silver electrode. In addition, discharge test results demonstrated that the AgO electrode with a carbon content of 10 wt% had the highest energy efficiency when used in silver batteries.

Table 3.
Chemical composition of the prepared electrodes (in weight percentage).
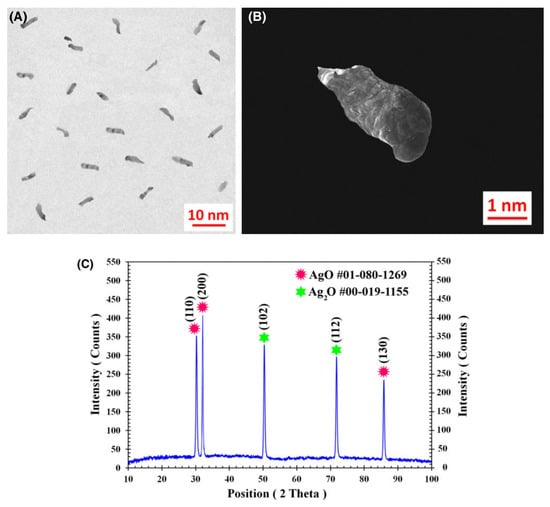
Figure 3.
Analysis of silver oxide powders prepared in [7]: (A) TEM; (B) FE-SEM; and (C) XRD.
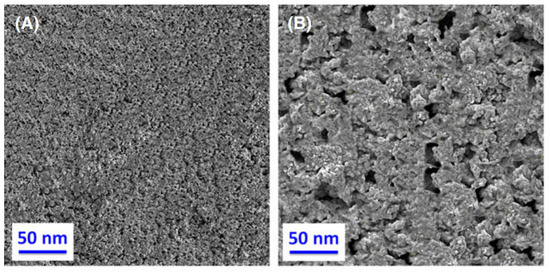
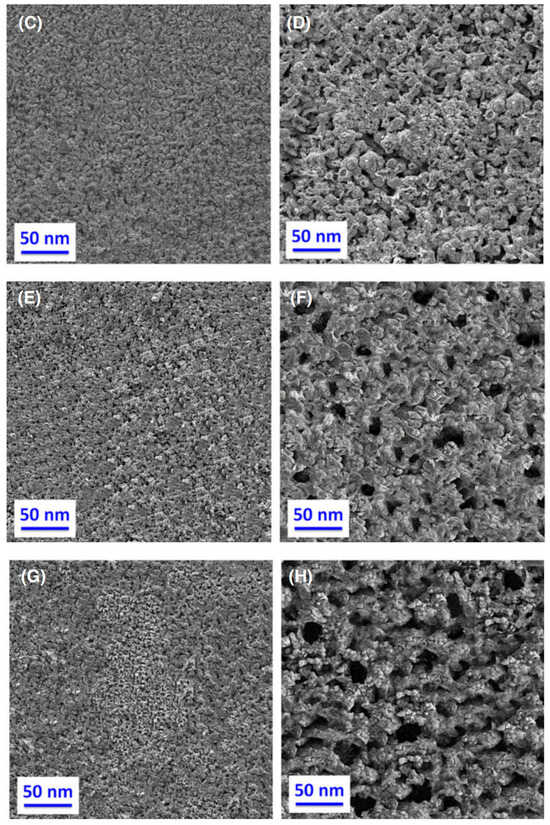
Figure 4.
SEM images of (A) electrode A before sintering; (B) electrode A after sintering; (C) electrode B before sintering; (D) electrode B after sintering; (E) electrode C before sintering; (F) electrode C after sintering; (G) electrode D before sintering; (H) electrode D after sintering [7].
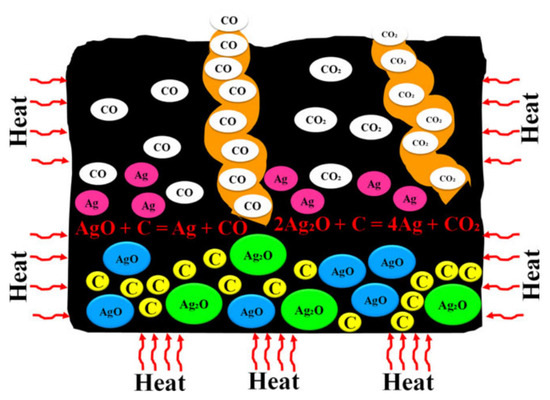
Figure 5.
The formation process of CO and CO2 gases during sintering of silver oxide electrodes and the effects of these gases on the porosity of the formed silver oxide electrodes [7].
Mostafa Najaf et al. [36] found that the porosity of AgO cathode materials bonded with polyvinylpyrrolidone (PVP) was high under high-rate discharge conditions compared to the case where carboxymethyl cellulose (CMC) was used as the binder. Furthermore, the PVP-based AgO cathode (Figure 6b) presented a uniform particle distribution; that is, the PVP-based cathode maintained a uniform particle distribution after discharge, with no cracks appearing, indicating its good discharge performance. In addition, the discharge capacity of the PVP-based cathode was much higher than that of the CMC-based cathode, with the specific capacity of the PVP cathode at 100 mA/cm2 reaching 391 mAh/g (Figure 7c,d).
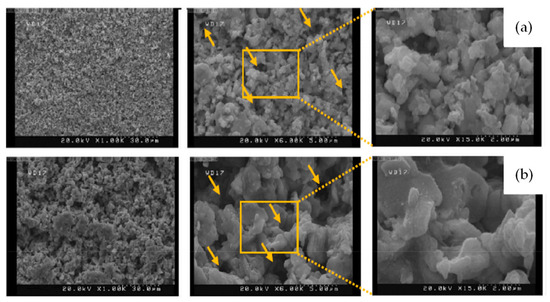
Figure 6.
(a) FESEM images of CMC-based AgO cathode; and (b) FESEM images of PVP-based AgO cathode [36]. The yellow arrows indicate pores with different sizes.
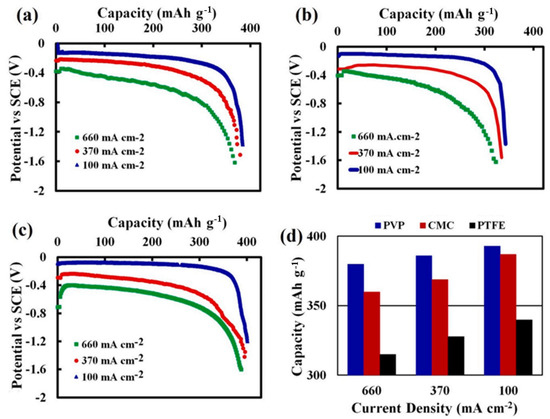
Figure 7.
(a) Rate performance of AGO cathodes using CMC, (b) PTFE, and (c) PVP as binders at different current densities; and (d) discharge capacity of cathodes with different binders at different current densities [36].
Xu et al. [37] first used reactive magnetron sputtering to deposit a NiO-oxidized Ag film to prepare layered porous AgO thin-films with abundant porosity and potential for large-scale production. It was found that an approximately 300 nm-thick Ag film can gradually be oxidized into a layered porous AgO nanorod array with a length of 4.2 mm. The layered porous AgO film contained at least two levels of porosity from mesopores to nanopores, which were generated through three different stages: oxidation of Ag film, formation of AgO nanorods and formation of layered porosity. The layered porous AgO film had a large specific surface area. Furthermore, utilizing assembled solid-state thin-film batteries with oxidized silver, it was demonstrated that the layered porous AgO film had potential for use in energy storage applications, with a specific capacity of up to 390 mAh/g. Jin et al. [38] immersed silver foil in 0.1 mol/L hydrochloric acid solution and prepared a pulse battery ultra-thin porous silver electrode with controllable thickness, excellent electrochemical performance and high power density using the redox method. The porosity of the active layer formed was about 65%. The thickness of the active layer can be controlled via the charge passed during the oxidation process. A 50 um-thick silver foil is used as the raw material, and the process resulted in an 80 um electrode with a nominal capacity of 14 C/cm2 on each side. These electrodes can charge and discharge at very high rates (typically 10 C charge and 102 C discharge), with an approximate utilization rate of 90% of the active material.
3.3. Purity
The purity of a battery’s cathode material AgO has a significant impact on its storage life. Low-purity AgO will greatly shorten the storage life of a battery, reduce its capacity and, thus, lead to a decrease in its discharge performance. Therefore, the purity of AgO is also an important factor that cannot be ignored in terms of battery performance. High-purity AgO materials have higher current density and good charge–discharge performance.
He et al. [39] have reported a high-purity (above 95%) AgO cathode material for use in a high-performance Al/AgO aqueous battery. Through research, it was found that the synthesized AgO cathode has a high specific capacity of 394.7 mAh/g. At a current density of 500 mA/cm2, the discharge voltage platform is close to 1.7 V, showing good charge transfer kinetics and electronic conductivity. Furthermore, a maximum output power density of 1260 mW/cm2 was also achieved at a current density of 900 mA/cm2. Yang et al. [40] have synthesized AgO powder using a chemical synthesis method, and obtained AgO powder with a purity of more than 96% by controlling the reactant concentrations and reaction temperatures at appropriate levels. Different electrodes were prepared using different binders and different structural conductive frameworks, which were further assembled into seawater-activated batteries in order to test the electrochemical performance of batteries prepared using different methods. The results showed that, when 1% PVDF content was added as a binder and a multi-layer honeycomb silver mesh was used as the conductive framework, the electrochemical performance of the AgO electrode was significantly improved. Yin et al. [41] performed chemical liquid phase deposition to prepare nano silver peroxide, with AgNO3 and Na2S2O8 as raw materials, adding surfactant NP20 and reacting at 60 °C for 30 min to prepare ultrafine AgO powder. The purity of the resulting AgO reached 99.9%, with a grain size of 32 nm and good charge–discharge performance. Zhang et al. [42] performed experiments and discovered a new method for the synthesis of high-purity nano AgO, using a NaCl–NaOH system to oxidize AgNO3 and prepare AgO with purity up to 98%, characterized by complete crystal structure, high purity and small particle size (65 nm). In addition, the assembled silver electrode with prepared AgO was subjected to cyclic voltammetry and charge–discharge tests. The results indicate that such AgO facilitates high current discharge with good electrochemical reversibility and charge–discharge performance.
3.4. Ordered Structure
To date, the most widely studied AgO electrode material is that with a disordered grain-dense stacking structure, with the AgO grains in the electrode arranged in a disorderly manner and with few gaps between grains. This structure is not conducive to diffusion of the electrolyte within the electrode, leading to a significant decrease in the reaction rate. During high current discharge, the diffusion of OH− generated by the AgO electrode is slow, easily leading to its aggregation and resulting in low utilization of the AgO electrode material during high-rate discharge. According to related research, when constructing electrode materials with a directionally ordered structure and large-sized AgO—that is, with an internal straight-through hole structure—the diffusion rate of the electrolyte solution in the electrode can be increased, which is beneficial for reducing various polarization processes in the electrochemical reaction system and reducing solution concentration polarization. This increases the electrochemical reaction rate and the utilization efficiency of the electrode’s active materials, ultimately improving the high current discharge capacity of the battery and resulting in better discharge performance.
The introduction of an electric field during the preparation of microcrystalline materials using electrochemical methods has a significant impact on the growth and self-assembly of molecules (e.g., the adsorption sites, conformation, and surface chemical reactions), thereby affecting the morphology and structure of the products. It is envisaged that the size and direction of the current can be adjusted to prepare electrode materials with straight-through hole structures, which will greatly facilitate the diffusion of electrolytes and reduce the resistance between electrodes and electrolytes, thereby improving the speed of electrochemical reactions and the utilization rate of active substances. The maintenance of straight-through hole structures will also have a positive impact on the improvement of cycling performance. Therefore, electrode materials with ordered AgO array structures are expected to possess significantly improved cycling performance as well as increased energy density [20,41,43,44].
Zhang et al. [44] used electrochemical methods to prepare AgO electrode materials with large-sized ordered structures, and studied the crystal structure, phase composition, microstructure, formation mechanism and discharge performance of the samples. Their results showed that the prepared AgO material has a strong (002) orientation, with no other impurities. Under the combined action of an electric field force and gas evolution, the AgO material formed an ordered columnar structure through oriented self-assembly, presenting a column diameter of 1–3 μm. This structure is conducive to the diffusion of electrolyte solution in the electrode, reducing polarization effects and significantly improving the discharge performance of large-sized AgO electrodes. At a discharge current density of 770 mA/cm2, the average voltage at a discharge cutoff voltage of 1.5 V for the AgO electrode was 1.67 V, with an electrochemical utilization rate of 75.6%, demonstrating improved high-rate discharge performance and utilization rate.
Tian et al. [45] prepared AgO electrode materials with an ordered array structure by electrochemically oxidizing Ag nanoparticles in concentrated sodium hydroxide solution, which significantly improved the resulting battery discharge performance. The microstructure is shown in Figure 8, from which it can be seen that the prepared electrode material has a unique pore array structure, which is beneficial for improving the capacity, discharge rate and cycle life of batteries, while reducing polarization to a certain extent. At a 3 C rate, the specific capacity of the battery reached 422.6 mAh/g (see Figure 9), with an electrode material utilization rate of 97.8%. At a 7 C rate, the specific capacity reached 387.8 mAh/g, with a battery active material utilization rate of 89.7%. Subsequently, in the study [46], the oxidation of silver nanoparticles in an aqueous solution was first induced, using glucose to reduce AgNO3 to prepare nano silver powder and, thereby, constructing a highly ordered needle-like array of silver oxide crystals. According to electrochemical testing research, the highly ordered needle-like AgO electrode prepared using the flat contact electro-oxidation method has good reduction capability and high rate discharge capability, with a specific capacity of 426.9 mAh/g, thus reaching 98.8% of the theoretical value (432 mAh/g). Ultimately, the structure and orientation of AgO crystals are closely related to the direction of current and density. Highly ordered needle-like arrays of silver oxide crystals can effectively accelerate the transport of electrolytes and electrons, thereby achieving better discharge capabilities.
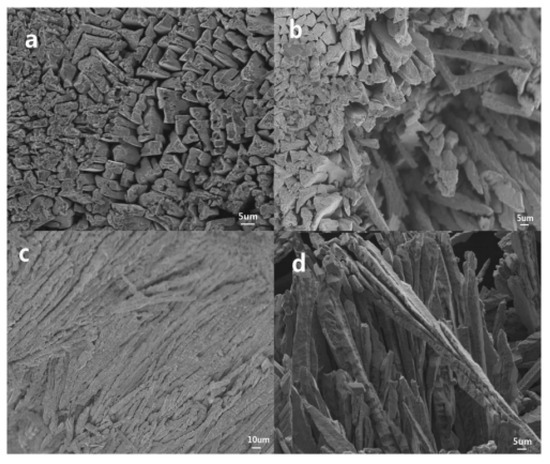
Figure 8.
SEM image of needle-like structured AgO crystals obtained through planar contact: (a,b) top view; (c) side view of fracture surface; and (d) magnified image of needle-like structure [45].
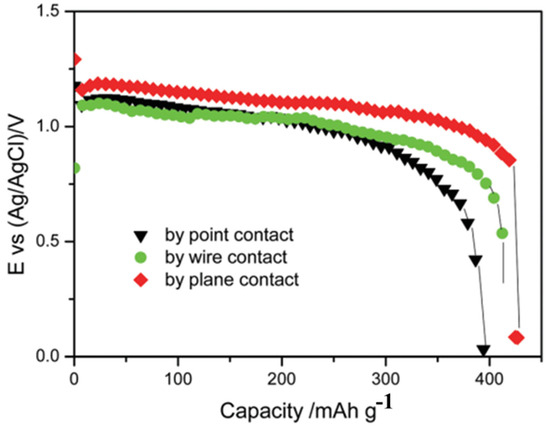
Figure 9.
Discharge curve of Al/AgO battery with highly ordered needle-like AgO as the positive electrode at 3 C rate and 60 °C [45].
3.5. Surface Treatment
Many positive electrode materials used in batteries undergo treatment before electrode sheet fabrication, mainly including heat and surface treatments. For example, Li-MnO2 battery cathode materials may undergo heat treatment on MnO2 powder before pressing into electrodes, thus transforming MnO2 into an ideal crystal structure, which facilitates the insertion and extraction of lithium. It is well-known that AgO exhibits thermodynamic instability and is prone to thermal decomposition—typically starting at 100 °C—and, hence, is generally not subjected to heat treatment. During storage, decomposition of AgO often leads to a decrease in working voltage, capacity loss and even battery failure. Reportedly, at room temperature, AgO will decompose significantly within 5 to 10 years. There are also reports that, in sealed batteries, the content of AgO will decrease by 10% within the first three years of storage at room temperature, while it will take another 30 years to decompose by another 10%. While opinions vary, one thing is certain: the issue of decomposition of AgO under storage conditions. In particular, when in the same room with reducing agents such as aluminum/zinc powder, its decomposition will be accelerated. Practical research has shown that surface treatment of AgO can effectively slow down the rate of AgO decomposition, and may also improve its electrochemical performance.
One common method of surface treatment is coating. Meng [47] treated AgO prepared using a chemical method with a surface coating, and then conducted TG-DSC tests to study its thermodynamic stability, allowing for the calculation of its activation energy and storage life. The test results showed that the stability of AgO without surface treatment is comparable to that previously reported, and it can be stored for about 20 years at room temperature with a decomposition rate of around 10%. After treatment with fluoroelastomer or sodium silicate, the decomposition activation energy of AgO was found to be approximately 128–130 kJ·mol−1. AgO treated with sodium silicate or fluoroelastomer can be stored at room temperature for 44 years with AgO content remaining above 80%. Li [48] used sodium silicate as a thermal stabilizer coated on the surface of chemically synthesized AgO particles, which can effectively inhibit the thermal decomposition of the AgO material. The effects of the relative content of sodium silicate on the constant current polarization performance of a chemically synthesized AgO cathode material were studied: the lower the relative content of sodium silicate, the smaller the constant current polarization of the AgO cathode material.
Mehdi et al. [49] successfully increased the discharge capacity of a AgO cathode material via surface treatment using fluorite-type La2Pb2O7 (LPO) nanoparticles. When the current density was 250 mA/g, the performance of the surface-coated AgO cathode active material was significantly improved compared to that of pure AgO. The discharge capacity of the AgO + 5%LPO cathode increased to 431 mAh/g from 371 mAh/g for the pure AgO cathode (as shown in Figure 10), while the highest power density increased from 583 mW/cm2 (for pure AgO) to 664 mW/cm2. Furthermore, electrochemical impedance spectroscopy (EIS) analysis revealed that the charge transfer resistance of the AgO + 5%LPO cathode (2.88 Ω) was much lower than that of the pure AgO cathode (6.13 Ω). The results suggested that the good performance of the LPO-coated AgO materials is due to the addition of LPO nanoparticles, which enhance their intergranular conductivity.
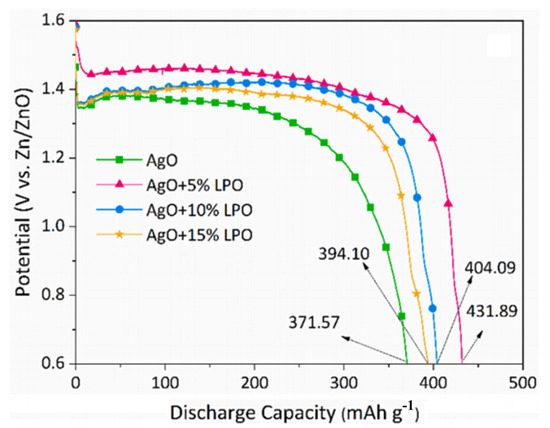
Figure 10.
Effects of different contents of LPO nanoparticles (5 wt%, 10 wt% and 15 wt%) on constant current discharge curves of AgO cathode electrodes at a current density of 250 mA g−1 [49].
Additionally, Tvarusko [50] studied the effects of transition and non-transition elements on the conductivity of chemically prepared AgO, concluding that Pb and Hg can be potentially used as additives to improve the conductivity of AgO materials. In addition to the type of additives, the ways in which they are added have a significant impact on the electrochemical performance of AgO materials. Additives can be introduced into AgO active materials through co-precipitation or physical mixing methods. In physical mixtures, the AgO active material includes a conductive surface coating composed of metal oxides, which can improve inter-particle conductivity and discharge potential, significantly increasing the utilization of its full capacity.
3.6. Doping Modification
Doping is a method that is commonly used for electrode material modification, which involves adding a small amount of other elements or compounds to the electrode material in order to improve aspects such as the discharge capacity or storage time of the battery electrode material. The concentration of doped atoms or ions has a significant impact on the properties of the resulting materials, and needs to be strictly controlled in experiments. Different doping methods are adopted for different systems and compounds. There have also been many studies on AgO doping, and some achievements have been made in this context [51,52,53].
Takeda [51] used solutions of nitrates of Al, Pb, Cd, Te and Tl in the process of preparing AgO, adding them to the silver nitrate solution such that they were formed simultaneously with AgO in the form of Al(OH)3, PbO, CdO, TeO2 or Tl2O3. The influence of doping with these elements on the resulting silver electrode was investigated. The results showed that, although adding 0.3% Cd, 0.1% Te and 0.1% Tl improved the stability of the silver electrode, the introduction of additives increased the electrode’s resistance. When treating the synthesized AgO with a reducing solution, the surface of the AgO particles undergoes a reduction reaction to generate Ag, which reacts with the internal AgO to form Ag2O, thereby eliminating the high voltage plateau. Gu [52] prepared AgO as a positive electrode material using a chemical oxidation method, with aluminum as the negative electrode material. Different additives were added to the alkaline electrolyte to investigate their effects on the discharge performance of the resulting Al/AgO batteries. Through adding NaAlO2 to the alkaline electrolyte, the low-rate discharge performance of the Al/AgO battery was improved. At a 0.1 C rate, the specific capacity of the battery increased from 282 mAh/g to 337 mAh/g; after adding NaCl to alkaline electrolyte, the specific capacity of the battery increased from 225 mAh/g to 260 mAh/g at a 3 C rate, such that the high-rate discharge performance of the Al/AgO battery was improved. Liu [53] added nano Ag2O particles to a silver electrode and tested its discharge performance. The results revealed that the addition of nano Ag2O particles in the range of 10% to 35% resulted in the best discharge performance of the silver electrode, leading to a capacity increase of 20% to 30%.
4. Summary and Outlook
Al/AgO batteries based on AgO electrodes have outstanding advantages, including high discharge specific energy, high output power and stable discharge. These electrodes currently hold an irreplaceable position in the field of batteries, and AgO has been widely researched and applied as a positive electrode material. This article briefly introduced the advantages and working principles of Al/AgO seawater-activated battery. Then, it elaborated on the impacts of AgO cathode materials on battery performance, highlighting that factors such as the silver powder’s morphology, porosity, structure, surface treatment and doping modifications all play crucial roles in improving the performance of the resulting batteries.
Future research on AgO cathode materials is expected to mainly focus on improving the utilization and purity of the AgO electrode active materials, finding suitable porosity values and regular organizational structures, and determining the effects of certain surface or doping treatments on the cathode materials. Based on the comprehensive factors mentioned above, there is still a great possibility to obtain ideal electrode materials. In addition, continuous innovation is needed in research on material processing technologies, in order to achieve constantly innovative processes and production technologies. This can lead to the development of electrodes based on AgO electrode materials with better structure and performance, as well as addressing cost reduction issues.
Al/AgO batteries are one of the most promising water-based batteries. AgO-positive electrode materials are one of the key materials used in Al/AgO batteries, and their performance directly affects many electrical properties (e.g., voltage and usage time) of Al/AgO batteries. Therefore, improving the electrochemical performance of silver oxide electrodes is a challenge that urgently needs to be addressed. Methods to improve the electrochemical performance of silver oxide electrodes include preparing highly active silver powders and optimizing their surface morphology and particle size parameters; adopting a porous electrode structure to increase the porosity and specific surface area of the electrode, thus greatly reducing the true current density of the battery, improving the utilization rate of active materials and enhancing the performance of the battery; using high-purity AgO materials to improve the current density and charge discharge performance; constructing ordered structures to increase the rate of chemical reactions; performing surface treatment of AgO to decrease the decomposition rate of AgO; and doping the synthesized AgO with small amounts of other elements or compounds. While the type of battery considered in this article has significant advantages in terms of cost, toxicity and other factors, the electrode materials used in this type of battery utilize precious metals and, thus, their manufacturing costs are relatively high. Therefore, more in-depth research should be conducted to optimize the performance of such electrode materials in the future. At the same time, the electrolyte involved in the reaction has some chemical components that may affect the seawater environment and the health of marine organisms. Therefore, in future research, the electrolyte components and other materials used can be optimized accordingly.
Author Contributions
Writing—original draft and Methodology, P.C.; writing—review and editing and Data curation, Q.Z.; data curation and Methodology, C.W.; data curation and Writing—review & editing, P.D.; formal analysis and Investigation, Y.Y.; methodology and Investigation, J.C.; resources and Formal analysis, X.W.; supervision and Data curation, W.X.; supervision, Software and Writing—review & editing, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate the constructive feedback from the anonymous reviewers, which greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vatsalarani, J.; Kalaiselvi, N.; Karthikeyan, R. Effect of mixed cations in synergizing the performance characteristics of PVA-based polymer electrolytes for novel category Zn/AgO polymer batteries-a preliminary study. Ionics 2009, 15, 97–105. [Google Scholar] [CrossRef]
- Hashim, M.G.; Hu, C.; Wang, X.; Wan, B.Y.; Xu, J. Room temperature synthesis and photocatalytic property of AgO/Ag2Mo2O7 heterojunction nanowires. Mater. Res. Bull. 2012, 47, 3383–3389. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wang, X.; Yang, S.; Xiong, C. Progress and applications of seawater-activated batteries. Sustainability 2023, 15, 1635. [Google Scholar] [CrossRef]
- Ozgit, D.; Hiralal, P.; Amaratunga, G.A.J. Improving performance and cyclability of zinc-silver oxide batteries by using graphene as a two dimensional conductive additive. ACS Appl. Mater. Interfaces 2014, 6, 20752–20757. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carpenter, R.; Buttry, D. Electrochemical cycling of polycrystalline silver nanoparticles produces single-crystal silver nanocrystals. Langmuir 2017, 33, 13490–13495. [Google Scholar] [CrossRef] [PubMed]
- Srimuk, P.; Husmann, S.; Presser, V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. RSC Adv. 2019, 9, 14849–14858. [Google Scholar] [CrossRef]
- Sabzi, M.; Dezfuli, S.M. A study on the effect of compositing silver oxide nanoparticles by carbon on the electrochemical behavior and electronic properties of zinc-silver oxide batteries. Int. J. Appl. Ceram. Technol. 2018, 15, 1446–1458. [Google Scholar] [CrossRef]
- Leng, P.S.; Wang, H.B.; Wu, B.F.; Zhao, L.; Deng, Y.J.; Cui, J.T. Ag Decorated Co3O4-Nitrogen Doped Porous Carbon as the Bifunctional Cathodic Catalysts for Rechargeable Zinc-Air Batteries. Sustainability 2022, 14, 13417. [Google Scholar] [CrossRef]
- Kong, M.X.; Bu, L.X.; Wang, W. Investigations on the discharge/charge process of a novel AgCl/Ag/carbon felt composite electrode used for seawater batteries. J. Power Sources 2021, 506, 230210. [Google Scholar] [CrossRef]
- Hwang, S.M.; Park, J.S.; Kim, Y.; Go, W.; Han, J.; Kim, Y.; Kim, Y. Rechargeable seawater batteries-from concept to applications. Adv. Mater. 2019, 31, e1804936. [Google Scholar] [CrossRef]
- Li, C.; Hu, L.; Ren, X.; Lin, L.; Zhao, C.; Weng, Q.; Sun, X.; Yu, X. Asymmetric Charge Distribution of Active Centers in Small Molecule Quinone Cathode Boosts High-Energy and High-Rate Aqueous Zinc-Organic Batteries. Adv. Funct. Mater. 2024, 34, 2313241. [Google Scholar] [CrossRef]
- Rashmi, B.N.; Harlapur, S.F.; Avinash, B.; Ravikumar, C.R.; Nagaswarupa, H.P.; Kumar, M.A.; Gurushantha, K.; Santosh, M.S. Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorg. Chem. Commun. 2020, 111, 107580. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, C.; Yliniemi, K.; Lundstrom, M. Recovery of high-purity silver from spent silver oxide batteries by sulfuric acid leaching and electrowinning. ACS Sustain. Chem. Eng. 2020, 8, 15573–15583. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Park, J.B.; Sun, Y.K.; Wu, F.; Amine, K. Aprotic and Aqueous Li–O2 Batteries. Chem. Rev. 2014, 114, 5611–5640. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Challenges and potential advantages of membranes in lithium air batteries: A review. Renew. Sustain. Energy Rev. 2017, 77, 1114–1129. [Google Scholar] [CrossRef]
- Meng, Z.S.; Li, R.L.; Wu, Q.B.; Qin, T.; Bai, Y.; Sun, K. Qualifying the Cathode Aging Process for Storage Life Prediction for Primary AgO-Zn Batteries. Electrochim. Acta 2024, 492, 144264. [Google Scholar] [CrossRef]
- Yu, K.; Xiong, H.Q.; Wen, L.; Dai, Y.L.; Yang, S.H.; Fan, S.F.; Teng, F.; Qiao, X.Y. Discharge behavior and electrochemical properties of Mg-Al-Sn alloy anode for seawater activated battery. Trans. Nonferrous Met. Soc. China 2015, 25, 1234–1240. [Google Scholar] [CrossRef]
- Shafiq, U.; Fiaz, A.; Amin, B.; Ataf, A.A.; Ramsha, R.; Bhajan, L.; Rizwan, H.; Bing, X. Solvothermal preparation of ZnO nanorods as anode material for improved cycle life Zn/AgO batteries. PLoS ONE 2013, 8, e75999. [Google Scholar]
- Gonzalez-Guerrero, M.J.; Gomez, F.A. Miniaturized Al/AgO coin shape and self-powered battery featuring painted paper electrodes for portable applications. Sens. Actuators B Chem. 2018, 273, 101–107. [Google Scholar] [CrossRef]
- Ma, Z.; Li, X. The study on microstructure and electrochemical properties of Al-Mg-Sn-Ga-Pb alloy anode material for Al/AgO battery. J. Solid State Electrochem. 2011, 15, 2601–2610. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Cui, M.; Wang, J.; Kang, W.; Foo, C.; Lee, P.S. Stretchable silver-zinc batteries based on embedded nanowire elastic conductors. Adv. Energy Mater. 2014, 4, 1301396. [Google Scholar] [CrossRef]
- Lal Bahadur, G.; Kumaresh babu, S.P.; Srinivasan, S.A.; Pathanjali, G.A. Study of passivation treatment on SS321 electrolyte reservoir used in AgO-Zn reserve battery, for defence applications. Mater. Today Proc. 2020, 27, A1–A7. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, Q.; Du, Z.; Du, Y.; Li, S.; Liu, F. Design and Implementation of an Electrolyte Temperature Control System for AgO-Al Batteries. Batteries 2025, 11, 244. [Google Scholar] [CrossRef]
- Zheng, L.I.; Zhang, H.T.; Zhang, Q.; Kun, Y.U. Effect of binders on electrochemical properties of AgO cathode material for aqueous AgO–Al batteries. Trans. Nonferrous Met. Soc. China 2025, 35, 1648–1661. [Google Scholar]
- Ipadeola, A.K.; Abdullah, A.M.; Eid, K. Recent advances in porous multimetallic alloy-based anodes for rechargeable alkali metal-ion batteries. Energy Mater. 2024, 4, 400079. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Eid, K.; Abdullah, A.M. Porous transition metal-based nanostructures as efficient cathodes for aluminium-air batteries. Curr. Opin. Electrochem. 2023, 37, 101198. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Y.; Huang, T.; Du, X.; Lu, Q.; Eid, K. Facile in situ polymerization synthesis of poly(ionic liquid)-based polymer electrolyte for high-performance solid-state batteries. Energy Convers. Manag. X 2024, 22, 100570. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Yua, H.; Kim, Y. High energy density rechargeable metal-free seawater batteries: A phosphorus/carbon composite as a promising anode material. J. Mater. Chem. A 2018, 6, 3046–3054. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, H.; Hwang, D.Y.; Park, J.; Kim, K.S.; Ahn, S.; Kim, Y.; Kwak, S.K.; Yu, Y.; Kang, S.J. Reliable seawater battery anode: Controlled sodium nucleation via deactivation of the current collector surface. J. Mater. Chem. A 2018, 6, 19672–19680. [Google Scholar] [CrossRef]
- Wu, M.L.; Wang, D.; Wan, L.J. Directed block copolymer self-assembly implemented via surface-embedded electrets. Nat. Commun. 2016, 7, 10752. [Google Scholar] [CrossRef]
- Li, S.; Tian, X. Progress of seawater batteries: From mechanisms, materials to applications. J. Power Sources 2024, 617, 235161. [Google Scholar] [CrossRef]
- Pan, J.Q.; Sun, Y.Z.; Wang, Z.H.; Wan, P.Y.; Liu, X.G.; Fan, M.H. Nano silver oxide (AgO) as a super high charge/discharge rate cathode material for rechargeable alkaline batteries. J. Mater. Chem. 2007, 17, 4820–4825. [Google Scholar] [CrossRef]
- Najafi, M.; Abedini, A. The effect of dimensional ratio and proportion of micron-nanoparticles on discharge performance of silver (II) oxide cathode. Ionics 2019, 25, 3269–3276. [Google Scholar] [CrossRef]
- Li, H.Q.; Wang, Y.G.; He, P.; Zhou, H.S. A novel rechargeable Li-AgO battery with hybrid electrolytes. Chem. Commun. 2010, 46, 2055–2057. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, D.; Ghugal, S.G.; Kulkarni, A.; Mishra, P.; Shende, A.G.; Umare, S.S.; Sasikala, R. Silver/Silver(II) oxide (Ag/AgO) loaded graphitic carbon nitride microspheres: An effective visible light active photocatalyst for degradation of acidic dyes and bacterial inactivation. Appl. Catal. B Environ. 2018, 221, 339–348. [Google Scholar] [CrossRef]
- Najafi, M.; Abedini, A. The critical role of polymeric binders on AgO cathodes in high rate batteries. Thin Solid Film. 2021, 721, 138532. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.Q.; Zhang, Q.Y.; Ma, C.Y.; Wang, Q.; Wen, D.H.; Li, X.N. Hierarchically structured AgO films with nano-porosity for photocatalyst and all solid-state thin film battery. J. Alloys Compd. 2019, 802, 210–216. [Google Scholar] [CrossRef]
- Jin, X.B.; Lu, J.T.; Xia, Y.; Liu, P.F.; Tong, H. Ultra-thin silver electrodes for high power density pulse batteries. J. Power Sources 2001, 102, 124–129. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Wang, Y.; Xu, W.; Zhang, Q.; Wang, X.; Liu, H.; Yang, G.; Zhang, H.; Song, J.; et al. A high-purity AgO cathode active material for high-performance aqueous AgO-Al batteries. J. Power Sources 2022, 551, 232151. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Yang, P.; He, Y.; Wang, S.; Zhao, P.; Wang, H. Recovery of Valuable Metals from Spent LiNi0.8Co0.1Mn0.1O2 Cathode Materials Using Compound Leaching Agents of Sulfuric Acid and Oxalic Acid. Sustainability 2022, 14, 14169. [Google Scholar] [CrossRef]
- Chen, T.; Dai, P.X.; Wu, J.Y.; Wang, D.; Wan, L.J. Disorder-order transformation of trithiocyanuric acid adlayer on a Au(111) surface induced by electrode potential. J. Phys. Chem. C 2011, 115, 16583–16589. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, H.; Li, Z. Enhancement of CO2 adsorption on high surface area activated carbon modified by N2, H2 and ammonia. Chem. Eng. J. 2010, 160, 571–577. [Google Scholar] [CrossRef]
- Wen, R.; Pan, G.B.; Wan, L.J. Oriented Organic Islands and One-Dimensional Chains on a Au(111) Surface Fabricated by Electrodeposition: An STM Study. J. Am. Chem. Soc. 2008, 130, 12123–12127. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.N.; Liu, X.; Liu, X.R.; Chen, T.; Yan, H.; Zhong, Y.; Wang, D.; Wan, L. Bilayer molecular assembly at a solid/liquid interface as triggered by a mild electric field. Angew. Chem. 2014, 53, 13395–13399. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, Z.Y.; Liu, C.Y. Construction of needle-like crystalline AgO ordered structures from Ag nanoparticles and their properties. New J. Chem. 2018, 42, 5376–5381. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.Q.; Zhang, Q.Y.; Ma, C.Y.; Li, X.N.; Wang, Q.; Wen, D.H. Abnormal Oxidation of Ag Films and Its Application to Fabrication of Photocatalytic Films with a-TiO2/h-Ag2O Heterostructure. J. Phys. Chem. C 2017, 121, 9901–9909. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Behpour, M. Synthesis and characterization of AgO nanostructures by precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2016, 27, 1191–1196. [Google Scholar] [CrossRef]
- Moghanni-Bavil-Olyaei, H.; Arjomandi, J. Performance of Al-1Mg-1Zn-0.1Bi-0.02In as anode for the Al-AgO battery. RSC Adv. 2015, 5, 91273–91279. [Google Scholar] [CrossRef]
- Rahbar, M.; Behpour, M. Fluorite type La2Pb2O7 nanoparticles coated onto AgO as enhanced performance cathode active material for alkaline primary cell. J. Power Sources 2022, 521, 230887. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Takehera, Z. Elecrode phenomena of silver-silver oxide system in alkaline battery. J. Electrochem. Soc. Jpn. 1963, 31, 91–104. [Google Scholar] [CrossRef]
- Takeda, K.; Hattori, T. Optimization of the Amount of Additives to AgO Cathodes on High-Drain Pulse Performance of Zn/AgO Cells. J. Electrochem. Soc. 2001, 148, A44. [Google Scholar] [CrossRef]
- Gu, J.Y.; Cai, Z.F.; Wang, D.; Wan, L.J. Single-Molecule Imaging of Iron-Phthalocyanine-Catalyzed Oxygen Reduction Reaction by in Situ Scanning Tunneling Microscopy. ACS Nano 2016, 10, 8746–8750. [Google Scholar] [CrossRef]
- Liu, H.T.; Xia, X.; Guo, Z.P. A novel silver oxide electrode and its charge–discharge performance. J. Appl. Electrochem. 2002, 32, 275–279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).