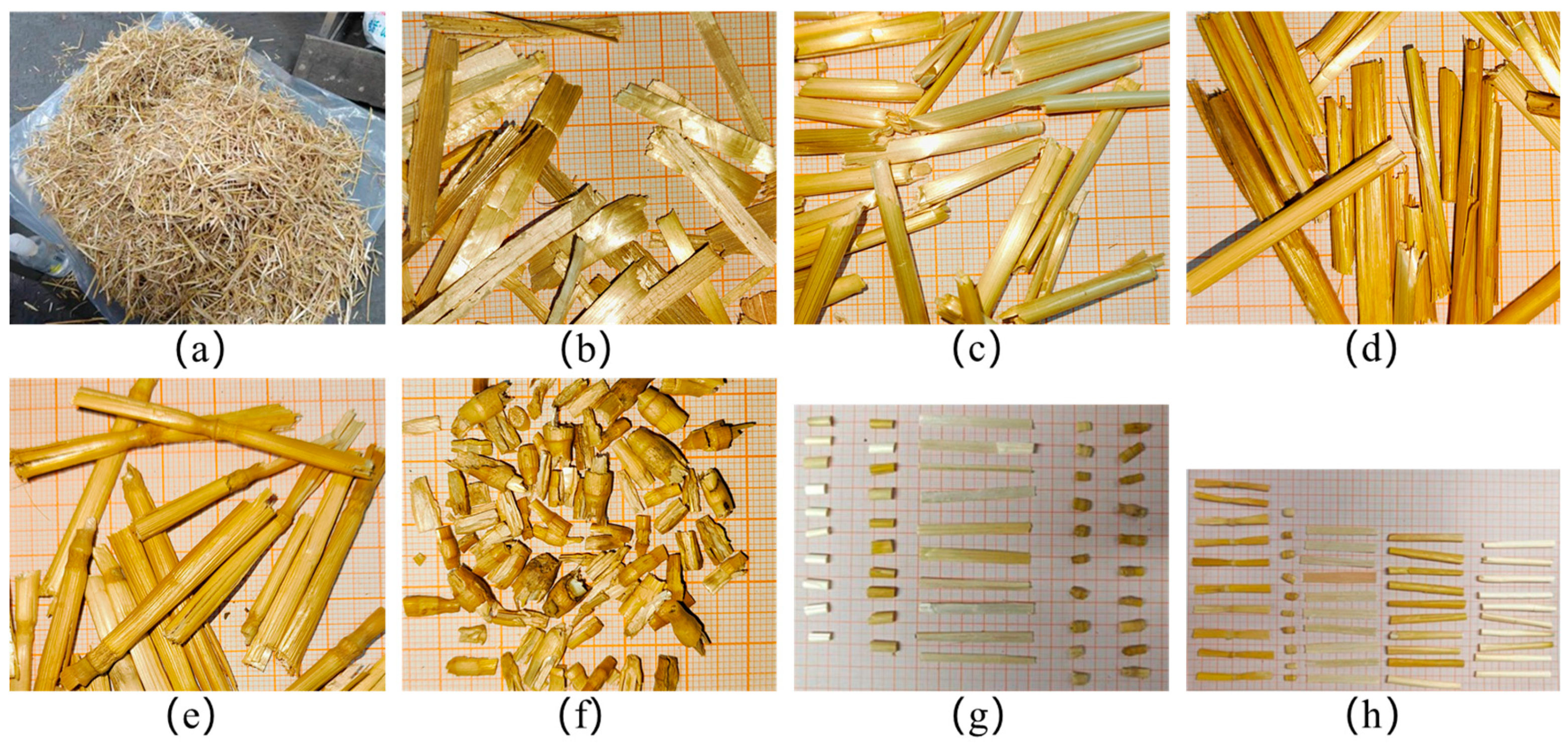
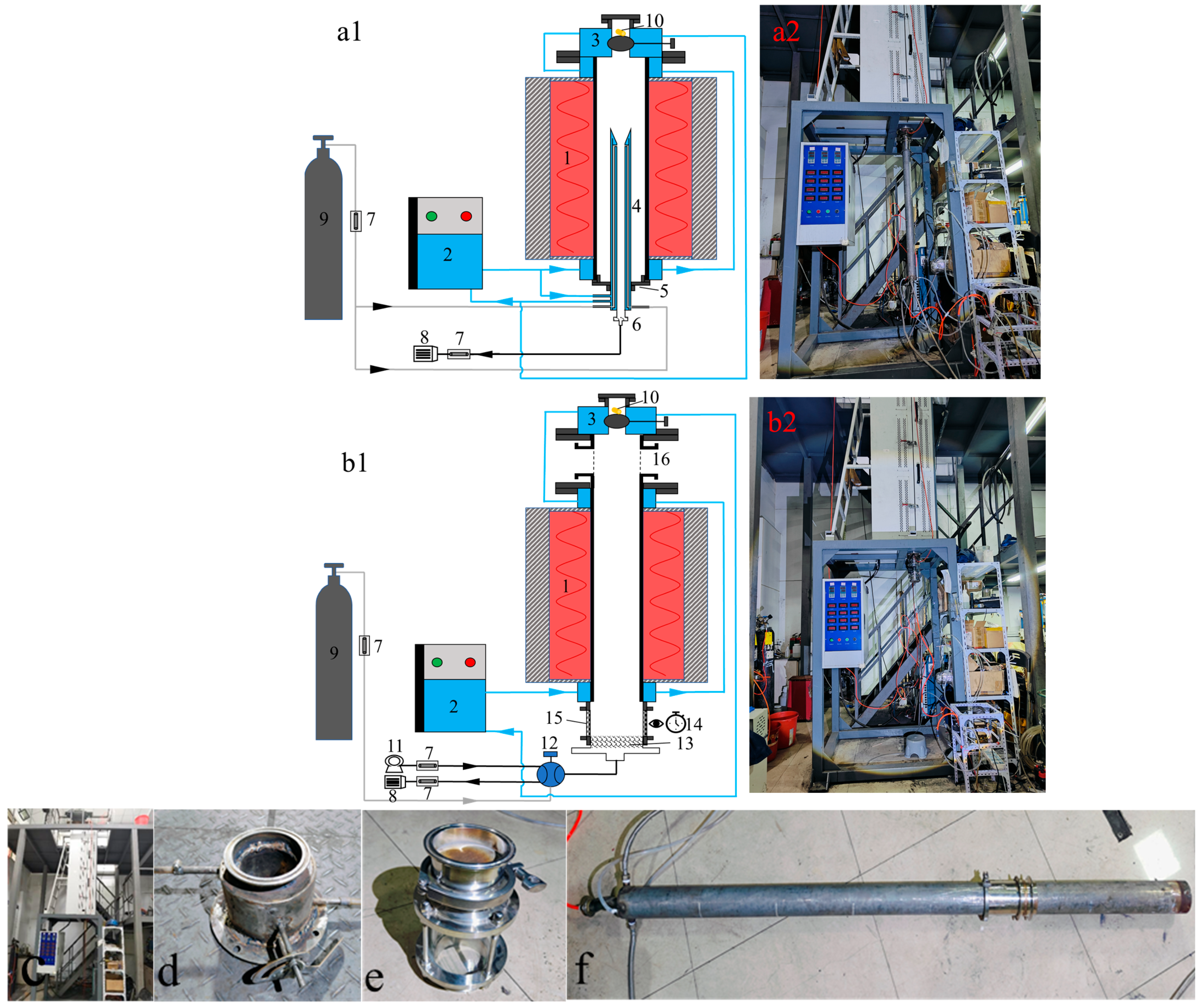
1. Introduction
Under the robust impetus of China’s “Achieving carbon neutrality by 2030” strategic goals, the transformation of China’s energy structure is accelerating. As coal-fired power generation remains the dominant source of electricity supply, there is an urgent need to develop practical and effective low-carbon technological pathways. Among these, the direct co-firing of biomass fuels within existing mature large-scale pulverized coal boiler systems has emerged as a key technological focus for both industry and academia, owing to its potential for rapid and substantial reduction in fossil fuel carbon emissions.
China’s biomass resources are predominantly agricultural residues such as crop straws (mainly rice, wheat, and corn straws), endowing this category of biomass with immense potential for energy utilization [
1]. Since such biomass is predominantly herbaceous in nature, it possesses a flexible fibrous texture. The coal mills (utilizing steel ball impact grinding) in existing coal-fired power plants are only suitable for processing rigid fuels (like coal and various minerals). In contrast, hammer mill shredders (employing gear-tearing mechanisms) are better suited for processing such flexible fibrous biomass. However, regardless of the crushing method used, energy consumption increases sharply as the target particle size decreases [
2,
3,
4]. Biomass like rice straw, wheat straw, and corn straw features a porous and hollow structure. It also has high combustion reactivity and a thin-walled structure. Therefore, within the combustion chamber of a coal-fired boiler, larger-sized biomass of these types may achieve combustion efficiency comparable to that of micron-sized coal powder.
Currently, experimental data on the combustion characteristics of large-sized straw biomass remain relatively scarce. Existing research predominantly focuses on micron- or millimeter-sized powdered biomass fuels and woody biomass fuels. For instance, Saastamoinen et al. [
5] investigated the combustion of 180–315 μm wood powder under pulverized coal boiler conditions using an entrained flow reactor and numerical simulations. Their results indicate that wood powder burns significantly faster than coal particles of the same size, suggesting the feasibility of utilizing larger wood particles in engineering applications. Riaza et al. [
6] tested four types of 75–150 μm biomass powders in a drop tube furnace at 1400 K using optical techniques, demonstrating minimal differences in combustion rates among different biomass powder types at identical sizes. Zhang et al. [
7] numerically modeled the combustion process of spherical and cylindrical wood particles. Their computational results reveal that increasing the particle diameter from 0.2 mm to 9.6 mm prolongs the volatile release time from 1.5 s to 40 s, with larger particle sizes reducing the volatile release rate per unit mass. Collectively, these studies demonstrate that particle size and biomass feedstock type have minimal impact on the combustion process at the micrometer scale. However, as particle size increases, the size effect exerts a substantially greater influence on the biomass conversion rate. As particle size increases further, the physicochemical reaction processes during fuel particle combustion undergo significant changes. Compared to powdered biomass fuels, larger biomass particles exhibit pronounced internal heat and mass transfer gradients, leading to marked differences in their heating process, pyrolysis volatile release pathways, and char formation pathways [
8,
9]. Simultaneously, the dominant factor controlling the char combustion rate of larger particles may shift from chemical reaction control (typical for powders) to diffusion control [
10,
11]. This size effect not only alters the reaction mechanisms of pyrolysis and char combustion (e.g., volatile flame structure, char combustion mode) but also correspondingly influences alkali metal release behavior [
12].
Fixed-bed reactors or flame furnaces are commonly used to study the thermochemical conversion characteristics of larger-sized fuel particles. Yu et al. [
9] studied the thermochemical conversion process of 0.5–5 mm pine wood particles in a fixed-bed reactor, demonstrating that larger particle sizes reduce carbon combustion reactivity. However, fixed beds cannot simulate the gas–solid contact conditions arising from the relative motion between particles and gas flow in pulverized coal boiler furnaces. Mason [
13] investigated the combustion of thirteen types of near-spherical 0.5–5 mm wood particles in a methane flame furnace at 1600 K, revealing that the combustion rates differed by up to a factor of three among different wood particle types. Flame furnaces facilitate the direct observation of particle ignition, flame propagation, and char combustion phenomena. Yet, they rely on fuel combustion (e.g., methane) for heat, making precise and independent control of the particle combustion atmosphere difficult. Furthermore, flame furnaces also fail to simulate the gas–solid contact conditions created by particle–gas relative motion. In contrast, drop tube furnaces (DTFs) are widely recognized as experimental facilities that can more comprehensively simulate pulverized coal boiler combustion conditions [
14,
15]. DTFs offer precise atmosphere control and can replicate the high temperatures (>1200 °C), high heating rates (>1000 °C/s), and gas–solid contact conditions of pulverized coal boiler furnaces.
However, DTFs are typically designed for powdered particles. Large-sized biomass particles have long burnout times and settle rapidly under gravity. Within the conventional furnace height and residence time, they often exit the furnace before achieving complete combustion, making it difficult to capture and study their full combustion process. For larger-sized fuel particles, some researchers have employed methods like suspending them using wire or placing them in wire mesh baskets within the DTF for thermochemical conversion [
16,
17]. However, these methods also suffer from the drawback of not fully simulating the gas–solid contact conditions resulting from particle–gas relative motion in pulverized coal boiler furnaces. Therefore, in existing research, experimental data on the combustion of large-sized biomass fuels under the high-temperature, high heating rate, and gas–solid contact conditions remain insufficient, particularly for the flexible straw biomass that will be studied in this paper.
Co-firing larger-sized straw biomass in pulverized coal boilers is a potential technological pathway for carbon emission reduction in China’s thermal power plants. However, existing research exhibits a severe lack of data on large-sized straw combustion under pulverized coal boiler combustion conditions. Therefore, this study selected typical large-sized (centimeter-scale) wheat straw particles that had undergone preliminary crushing and employed a two-mode experimental setup (sequential sampling during combustion and decoupled combustion experiments) in a DTF experimental system (simulating the high-temperature, high heating rate, and gas–solid contact conditions of a pulverized coal boiler furnace) to systematically investigate the combustion behavior and alkali metal release characteristics of this type of large-size straw biomass. The key parameters tracked include particle shape evolution, fragmentation, size reduction, dynamic changes in moisture/volatile matter/fixed carbon/ash content, burnout progression, and alkali metal (K) release. Based on detailed experimental data, the combustion progression of typical large-sized wheat straw particles in the DTF was summarized. This study obtained fundamental combustion characteristics and alkali metal release data for large-sized wheat straw particles under drop tube furnace (DTF), providing valuable experimental data and engineering application guidance for the direct co-firing of large-sized flexible straw biomass in pulverized coal boilers.
3. Results and Discussion
3.1. Combustion Characteristics
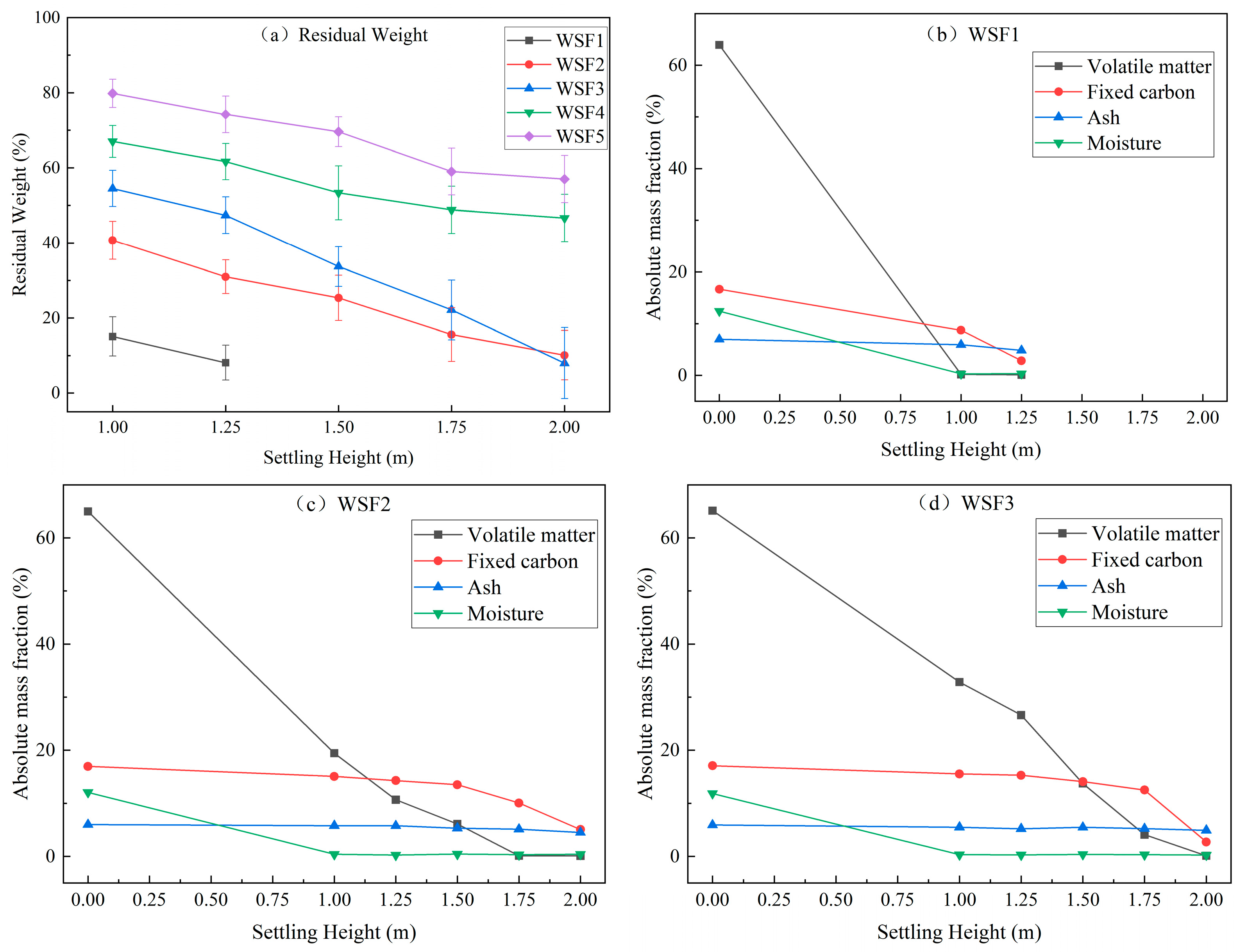
The typical shape changes, size changes, fragmentation probability, residual weight rate, and proximate analysis of the combustion process of five types of wheat straw particles are shown in
Figure 4,
Figure 5 and
Figure 6, respectively.
During combustion, WSF1 gradually bended, and its bundled fibers separated from each other, causing branching. As the settling height increased, it eventually formed into several needle-like particles. Fragmentation typically involved the radial fracture of these bundled fibers or their axial separation, producing shorter or narrower curved fragments or needle-like particles. WSF1 exhibited significantly higher size reduction, exceeding 60% in both axial and radial dimensions, compared to other particle types. This pronounced reduction likely resulted from the pre-separation of parallel fibers along the circumference of the originally tubular stem, reducing constraining forces. Due to its slow settling velocity and lower combustible content, moisture and volatiles were completely released by a 1 m settling height. Consequently, data for the early combustion stage were missing and will be obtained through decoupled combustion experiments.
For WSF2 and the inner tubular layer of WSF3, the circumferentially parallel-aligned bundled fibers in the inner tubular layer gradually separated or fractured, eventually forming needle-like and flake-like particles at greater heights. A key distinction was observed in WSF3, where the detachment of the outer leaf layer occurred before the volatile release from the inner tubular layer. This is likely because the outer leaf layer releases volatiles and contracts first, while the inner tubular layer has not yet initiated volatile release or begun contracting. Consequently, the outer leaf becomes mechanically constrained by the inner tubular layer during contraction, resulting in its fragmentation. Therefore, WSF3 fragmentation consisted of outer leaf detachment or breakage in the early combustion stage, while its inner tubular layer fragmented similarly to WSF2 in the later combustion stage. Volatile release was nearly complete for WSF2 at 1.5 m and for WSF3 at 1.75 m. The 1 cm size provided suitable settling velocity and combustible mass, enabling the capture of samples representing approximately 40% of the combustion process, primarily during the volatile release phase. Data for the remaining combustion information will be supplemented by decoupled combustion experiments.
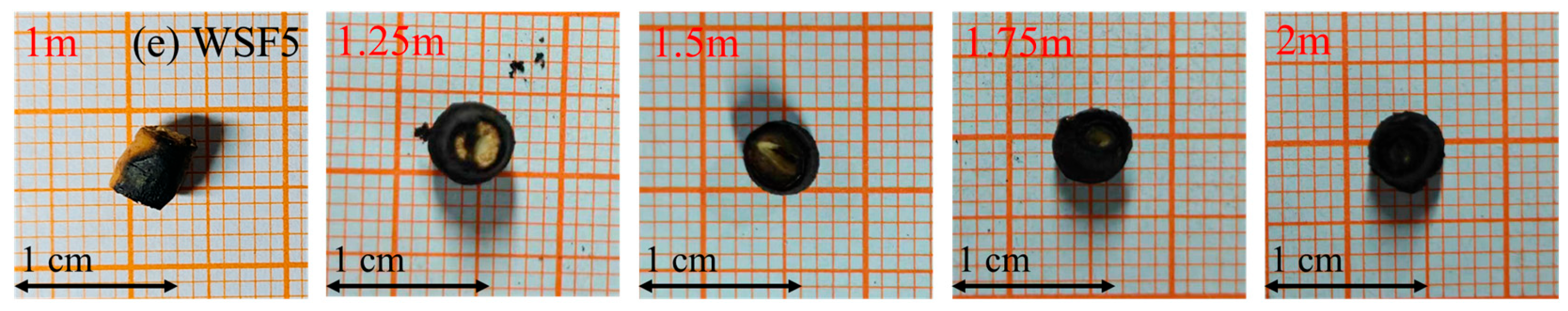
WSF4 and WSF5, characterized by faster settling velocity and higher combustible mass, yielded only early-stage samples, corresponding to approximately 20% mass loss. Notably, 25–35% of volatiles remained unreleased in both types even at the 2 m height. During this captured stage, their overall shape remained largely unchanged without significant fragmentation, though the minor spalling of needle-like char particles occurred occasionally. WSF4 showed less than 10% contraction in both axial and radial dimensions, while WSF5 developed concave end faces. The internal region of both WSF4 and WSF5 samples retrieved at all heights appeared yellow. Information regarding their later combustion stages will be acquired via decoupled combustion experiments.
Furthermore, apart from the inability to obtain early-stage samples for WSF1, an uneven yellow distribution was observed on the surfaces of all wheat straw particle types during the volatile release stage. This is because of the differential mass transfer and gas diffusion conditions on distinct surface regions during their settling, such as the windward side, leeward side, and inner walls of tubular particles, leading to non-uniform volatile release rates. This phenomenon demonstrates that during the combustion of large-sized biomass particles, the uneven gas–solid contact conditions on the surface result in asynchronous combustion processes at different locations on the surface.
3.2. Decoupling Combustion Characteristics
The typical shape changes in the five types of wheat straw particles after volatile release are shown in
Figure 7a. It can be observed that under a nitrogen atmosphere, after complete volatile release, WSF1 transformed into twisted narrow flakes; WSF2 became curved tubes with uneven diameters; WSF3 separated into its outer leaf layer and inner tubular layer, both bending, with the outer leaf layer exhibiting a higher length reduction rate than the inner tubular layer; WSF4 developed bent stems at both ends and its bundled fibers splayed apart; and some WSF5 particles exhibited concave end surfaces while others developed perforations through both ends.
The typical shape changes in the five types of wheat straw particles at the char combustion stage are shown in
Figure 7b. It can be observed that under an air atmosphere, at the char combustion stage, WSF1, WSF2, and WSF3 typically fragmented into several needle-like particles, indicating that the final combustion stage of centimeter-scale wheat straw particles involves the sustained burning of the carbon skeleton structure formed by bundled fibers. The stems at both ends of WSF4 usually fractured away from the central node, ultimately forming flake-like, needle-like, or blocky particles. This occurs because the central node exhibits a solid structure, while the leaf-wrapped stems at both ends are tubular with lower mechanical strength. Particularly after volatile release, the ends develop branched needle-like structures with significantly weakened mechanical strength, making them highly prone to fragmentation; the concentric carbon layers of WSF5 typically undergo layer-by-layer peeling or fragmentation, ultimately forming semi-tubular, flake-like, or blocky particles. This behavior originates from the nodular section of the raw sample, a solid cylinder formed by circumferential layer-by-layer stacking, causing the resulting char to easily delaminate circumferentially. Consequently, it can be inferred that the carbon combustion process of WSF4’s central node resembles that of WSF5. This indicates that none of the five types of wheat straw particles can maintain their original shape during char combustion. Fragmentation into needle-like, flake-like, or blocky particles dominates this stage. This finding differs from the observations made by Riaza et al. [
19] on the shape and size evolution of millimeter-sized woody biomass during combustion. Rod-like or needle-like millimeter-sized woody biomass particles tend to transform towards a more equidimensional (near-spherical) shape, whereas larger centimeter-scale straw biomass is more prone to bending, fiber bundle splaying, and fragmentation.
Notably, the char samples obtained after complete volatile release under nitrogen atmosphere remained intact, with no observed fragmentation or shedding of fine carbon particles. This contrasts with the observations made in the sequential sampling experiment, where wheat straw particles shed fine carbon particles or fragment during the volatile release stage. This difference in shedding/fragmentation behavior is likely related to the localized temperature increase caused by volatile combustion in the sequential sampling experiment, combined with the asynchronous combustion progress made at different locations on the particle surface (e.g., the windward side typically enters the char combustion stage earlier).
The residual weight and burnout degrees of the five types of wheat straw particles after volatile release and at the char combustion stage are shown in
Figure 8a. Here, WSF-V and WSF-C represent the samples after volatile release and at the char combustion stage, respectively, obtained from the decoupled combustion experiment. The char of WSF4 and WSF5 has larger masses and required higher counter-flow gas rates to extend their residence time in the furnace. However, high counter-flow rates tended to blow samples at the late stage of char combustion back into the furnace, so only samples at the mid-stage of char combustion could be collected.
The size reduction ratios of the five types of wheat straw particles after volatile release are shown in
Figure 8b. Compared to the size reduction ratios observed in the sequential sampling experiment (where volatiles were nearly fully released for WSF2 and WSF3 at drop heights of 1.5 m and 1.75 m, respectively), the size reduction ratios under nitrogen atmosphere were significantly higher. This indicated that the size reduction results under nitrogen atmosphere cannot reflect the size reduction behavior of wheat straw particles under actual combustion conditions. This discrepancy is likely because the particle residence time in the sequential sampling experiment was shorter, while the decoupled combustion experiment (nitrogen atmosphere) provided ample time for complete volatile release and size reduction. Particle size reduction during the char combustion stage primarily results from carbon consumption, whereas reduction during the volatile combustion stage is mainly caused by the release of internal matter. This study observed significantly lower reduction rates (5–65%) during pyrolysis for all wheat straw particle types compared to the 60–90% reported by Holmgren et al. [
20] for micrometer-scale near-spherical wheat straw particles. This contrast suggests that larger dimensions may constrain particle reduction through morphological and structural effects. Multiple studies on the reduction characteristics of large-sized (>1 cm) woody biomass particles during pyrolysis [citation needed] similarly demonstrate that biomass particle shrinkage is governed by particle size, geometric shape, and heating rate [
21,
22,
23,
24].
3.3. Release Characteristics of Alkali Metals (Potassium)
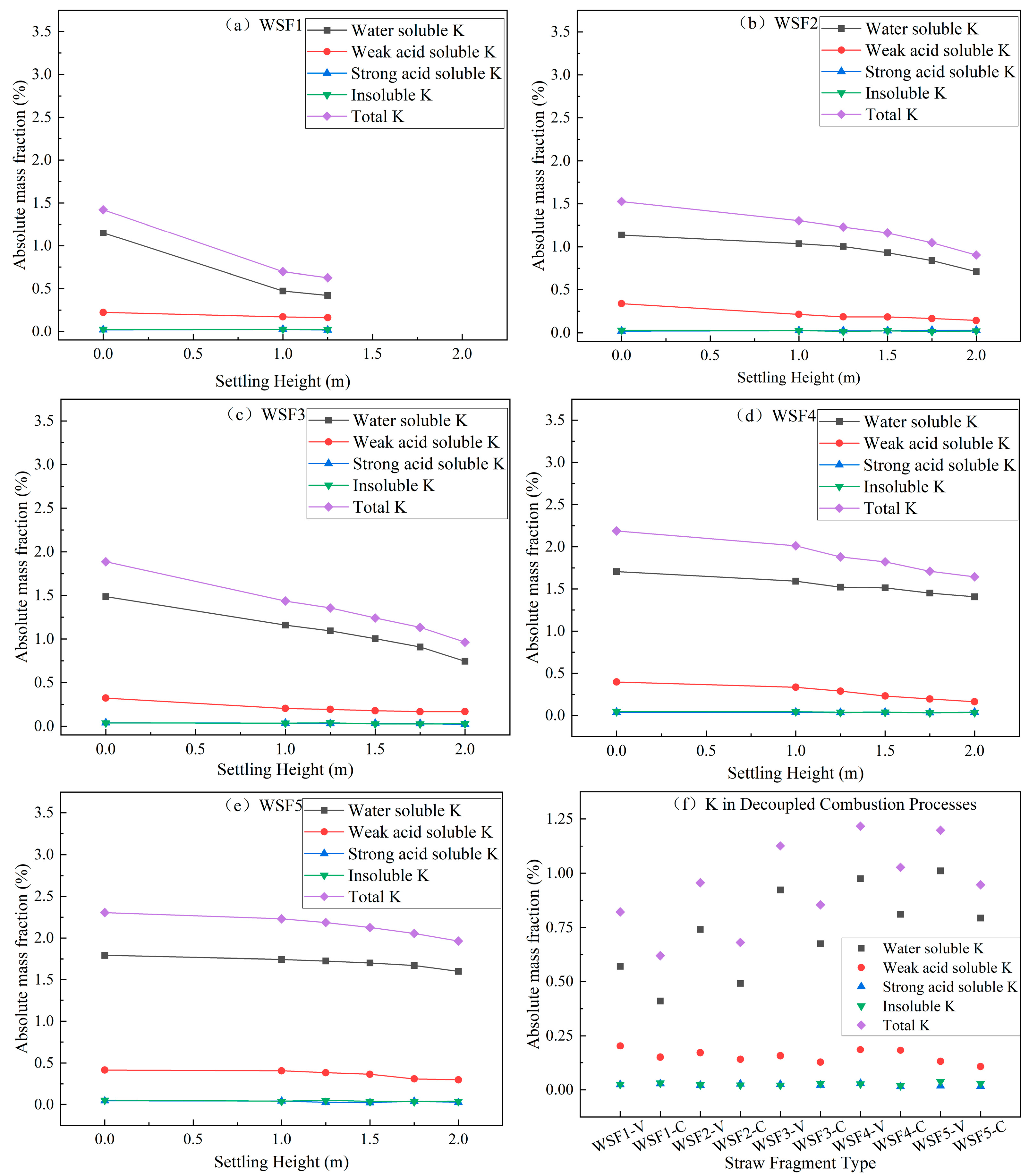
The potassium content during the combustion process of five types of wheat straw pellets is shown in
Figure 9. Specifically, the potassium content of samples obtained from the sequential sampling experiment is shown in
Figure 9a–e, while the potassium content of samples obtained from the decoupled combustion experiment is shown in
Figure 9f.
As the settling height increases, potassium was gradually released from all types of wheat straw particles. Among the released components, water-soluble potassium and weak acid-soluble potassium were the predominant fractions. In contrast, strong acid-soluble potassium and insoluble potassium were present at lower levels and exhibited relatively stable behavior during combustion. Water-soluble and weak acid-soluble potassium in biomass typically existed in organic/inorganic water-soluble or exchangeable ionic forms, whereas strong acid-soluble and insoluble potassium mostly belong to mineral salt potassium. Critically, water-soluble potassium released as KCl vapor crystallizes on high-temperature heat-transfer surfaces (e.g., boiler waterwalls and platen superheaters). These deposits induce the high-temperature corrosion of metal surfaces [
25,
26]. Meanwhile, the strong acid-soluble potassium and insoluble potassium retained in biomass ash, such as potassium silicates and aluminosilicates, typically serve as key contributors to adhesion, deposition, and slagging formation on convection pass heating surfaces in boilers [
27,
28].
Analysis combining the potassium content of the samples (
Figure 9f) and the burnout degree (
Figure 8a) revealed that after the complete release of volatiles from all types of wheat straw particles, the potassium release ratios exceeded 35%, and when the burnout degree exceeded 80%, the potassium release rate surpassed 50%. This aligns with the findings of Liu et al. [
29], who investigated the potassium release process during the combustion of compressed poplar wood and corn straw particles using a flame furnace and optical measurement techniques. Their results indicated that while the primary potassium release peak occurs during the volatile release stage, a smaller secondary peak is also observed during the char combustion stage. They attributed the potassium release peak in the char combustion stage to volatile–char interactions, char consumption, and temperature increase, which primarily results from char combustion. Previous studies have shown that a portion of potassium is retained in the ash during biomass combustion, and the potassium release rate is typically dependent on combustion conditions and biomass types [
30,
31,
32]. Given that wheat straw has a high potassium content of 1.9%, its potassium release rate can reach up to 50% under the simulated high-temperature, high-heating-rate and gas–solid contact conditions in this study. Consequently, when co-firing large-sized straw biomass fuels in pulverized coal boilers, significant attention must be paid to the risks of the high/low-temperature heating surface corrosion, slagging, and fouling caused by the substantial release of potassium from straw biomass fuels.
3.4. Combustion Process
Integrating the results from the sequential sampling experiments and the decoupled combustion experiments, the complete combustion process of five types of large-sized wheat straw particles can be summarized. In the original samples, most WSF1-4 particles were approximately 5 cm in length, while the cylindrical nodules in WSF4 or WSF5 measured about 0.5 cm. Thus, the 5 cm long WSF1-4 particles and the 0.5 cm long WSF5 nodules are taken as examples for the analysis.
WSF1 first undergoes approximately 0.9 s to complete the release of moisture and volatiles. During this stage, the particle experiences significant axial and radial size reduction (both >50%), forming bent, bifurcated slender flake coke particles, and releases about 42% of its potassium. Subsequently, the coke particle continues to burn for about 1.2 s to reach a 98% burnout degree. In this phase, the particle fragments into several shorter flakes or needle-like particles, achieving a potassium release ratio of 56%.
WSF2 first undergoes approximately 1.1 s to complete the release of moisture and volatiles. During this stage, the particle undergoes minor axial (~10%) and radial (>20%) size reduction, forming bent, bifurcated tubular coke particles, with a probability (<50%) of fragmenting into sub-particles larger than 3 mm, while releasing about 37% of its potassium. Subsequently, the coke particle burns for about 1.3 s to reach a 97% burnout degree. In this phase, the probability of fragmentation increases significantly (>50% and increasing as combustion progresses), primarily through circumferential separation or fracture of parallel-bundle fibers, forming flake or needle-like particles, with the potassium release ratio reaching 56%.
WSF3 first undergoes approximately 1.4 s to complete the release of moisture and volatiles. During this stage, the outer leaf layer burns and detaches or fragments first while the inner tubular layer undergoes minor axial and radial size reduction (both ~20%) and releases about 40% of its potassium. Subsequently, the coke particle burns for about 1.3 s to reach a 95% burnout degree. Due to the immediate detachment or fragmentation of the outer leaf layer upon entering the furnace, combined with fragmentation of the inner tubular layer, at least two particles burn simultaneously throughout the entire combustion process of WSF3. At a 95% burnout degree, the potassium release ratio reaches 55%.
WSF4 first undergoes approximately 3.1 s to complete the release of moisture and volatiles. During this stage, the particle undergoes minor axial and radial size reduction (<10%), forming coke particles with bent and bifurcated stems at both ends, with a low probability (<10%) of fragmenting, and releases about 44% of its potassium. Subsequently, the coke particle burns for about 3.5 s to reach an 85% burnout degree. In this phase, the stems at both ends fragment into needle-like or flake particles. Based on the combustion behavior of WSF5, the central node is inferred to undergo further fragmentation and burnout through circumferential layer-by-layer carbon exfoliation. At an 85% burnout degree, the potassium release ratio reaches 53%.
WSF5 first undergoes approximately 1.8 s to complete the release of moisture and volatiles. During this stage, the particle shape and dimensions remain largely stable, with a low probability (<6%) of exfoliation, yielding particles larger than 3 mm, but concavities or penetrations form at both end faces, and about 48% of its potassium is released. Subsequently, the coke particle burns for about 2.8 s to reach a 90% burnout degree. In this phase, the carbon layer around the circumference exfoliates in a layer-by-layer manner or breaks, forming semi-tubular, flake-like, or blocky particles, with the potassium release ratio reaching 59%.