Influence of Ni and Nb Addition in TiVCr-Based High Entropy Alloys for Room-Temperature Hydrogen Storage
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization Method
3. Results and Discussion
3.1. Structural Analysis
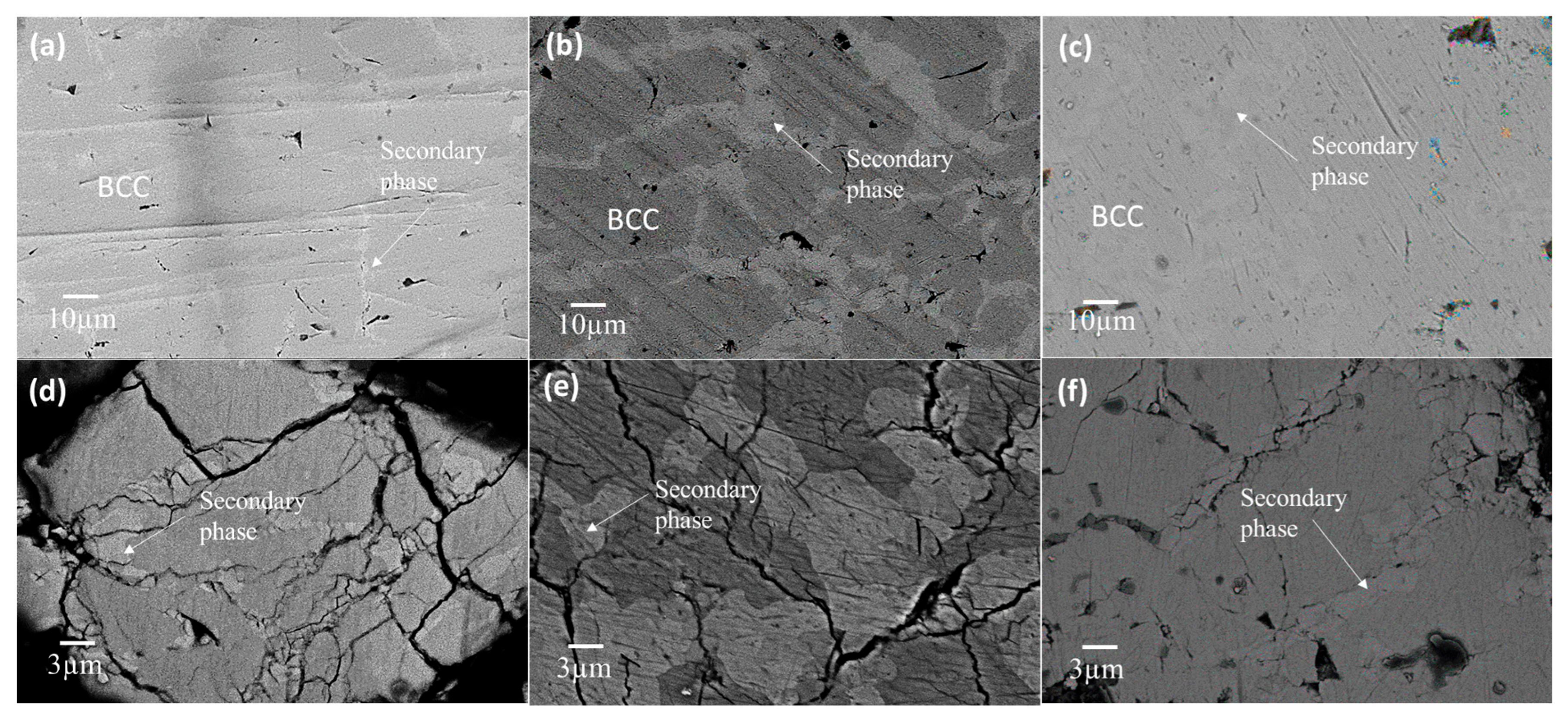
3.2. Microstructural Analysis
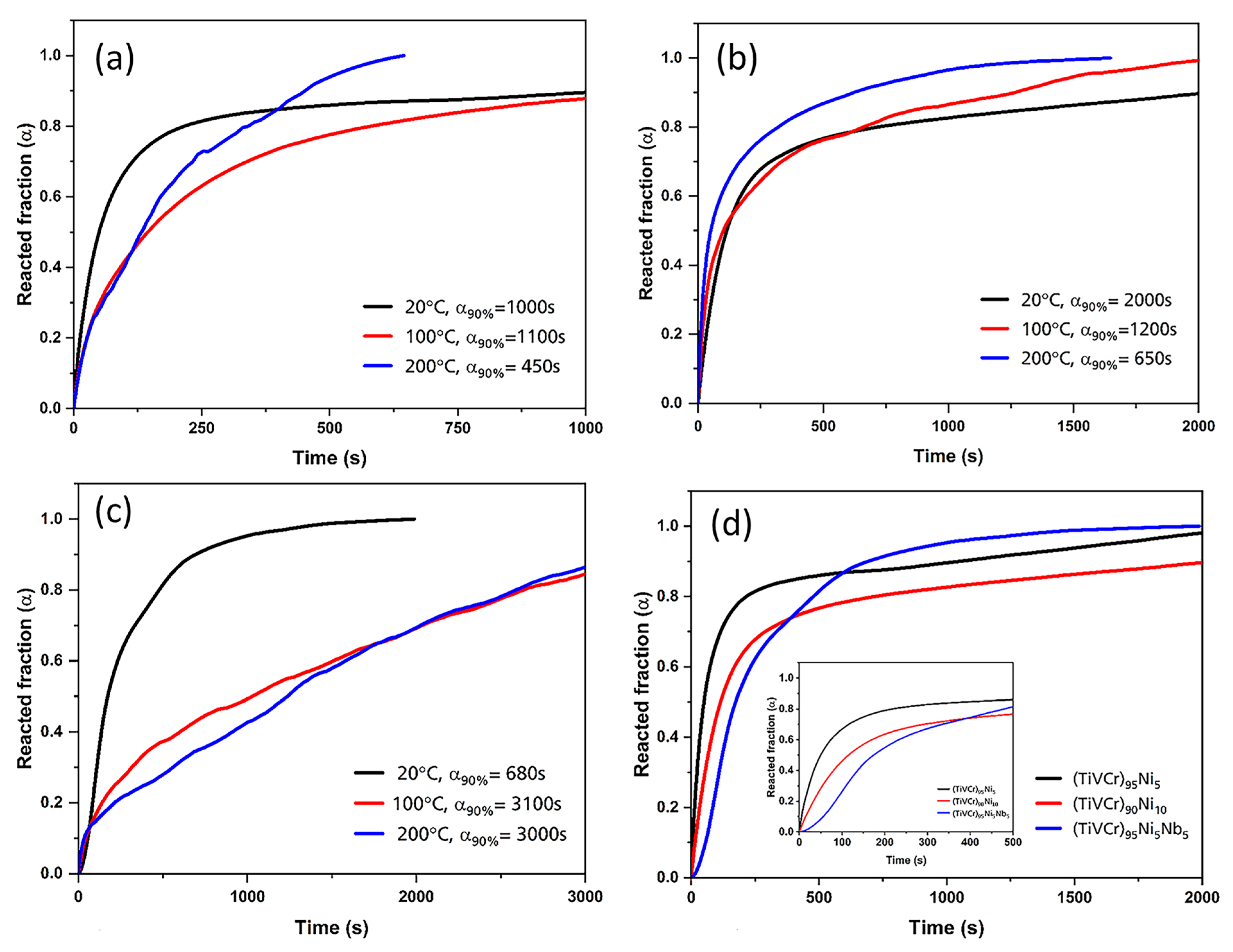
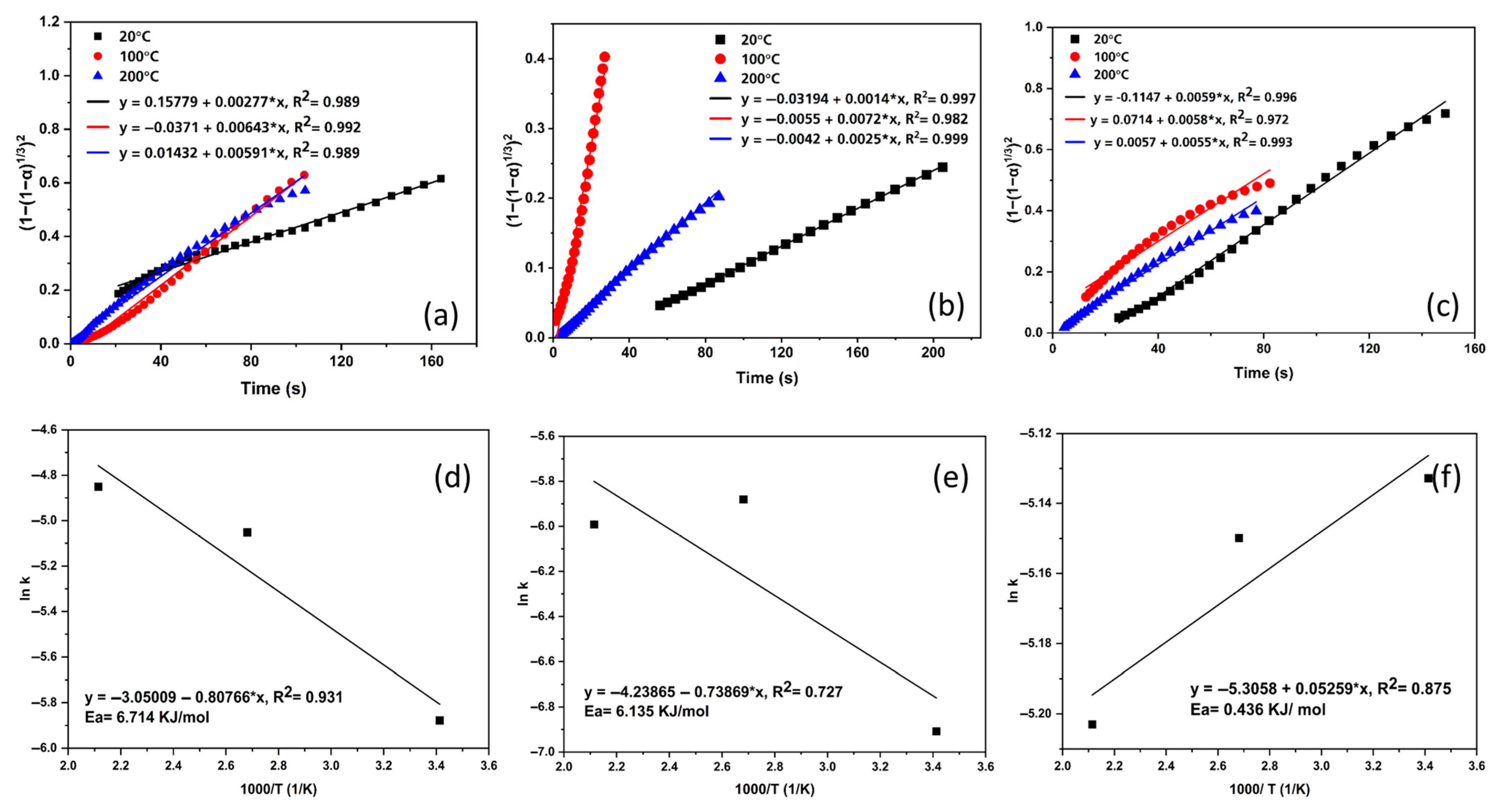
3.3. Kinetics Measurement
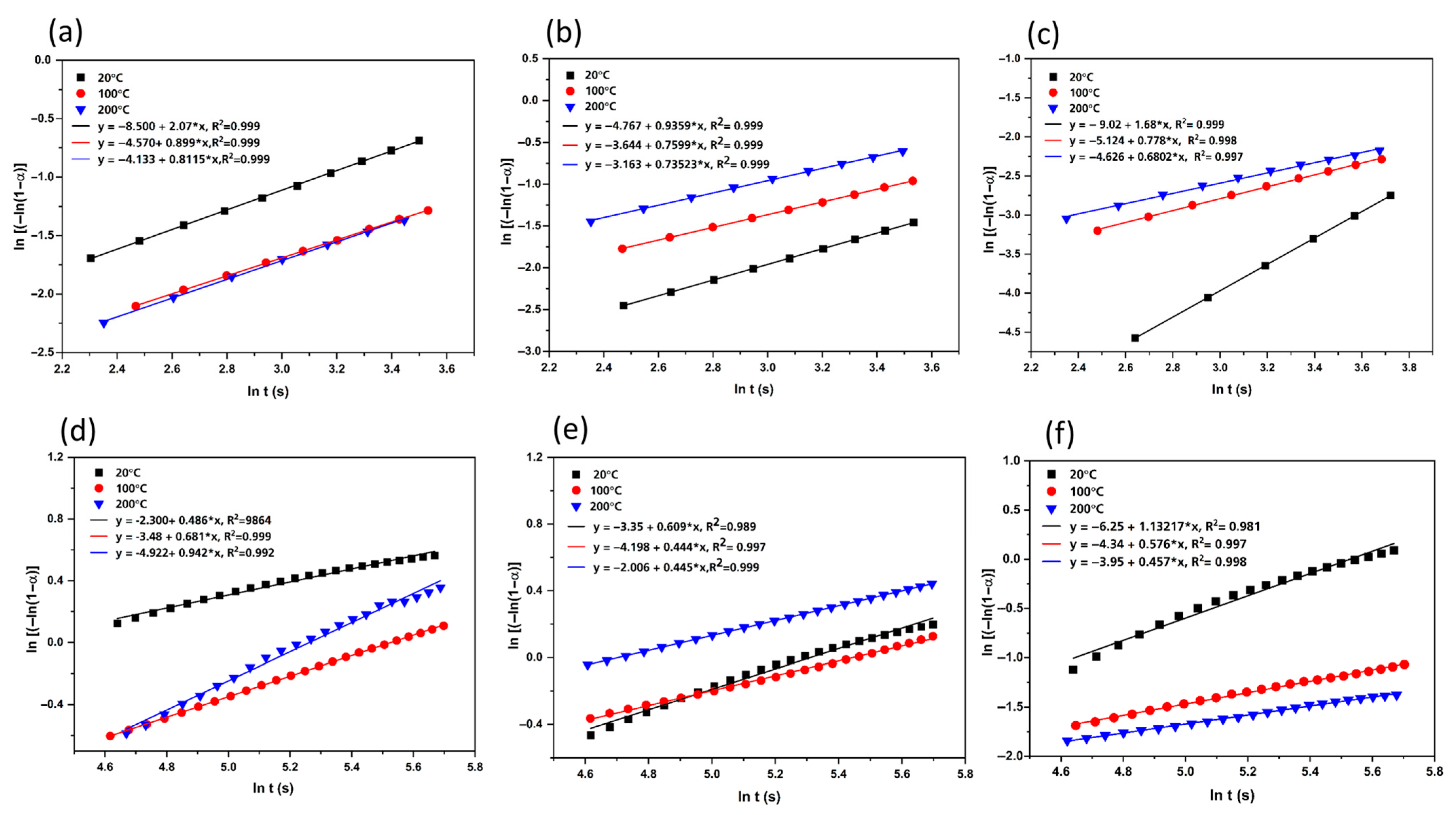
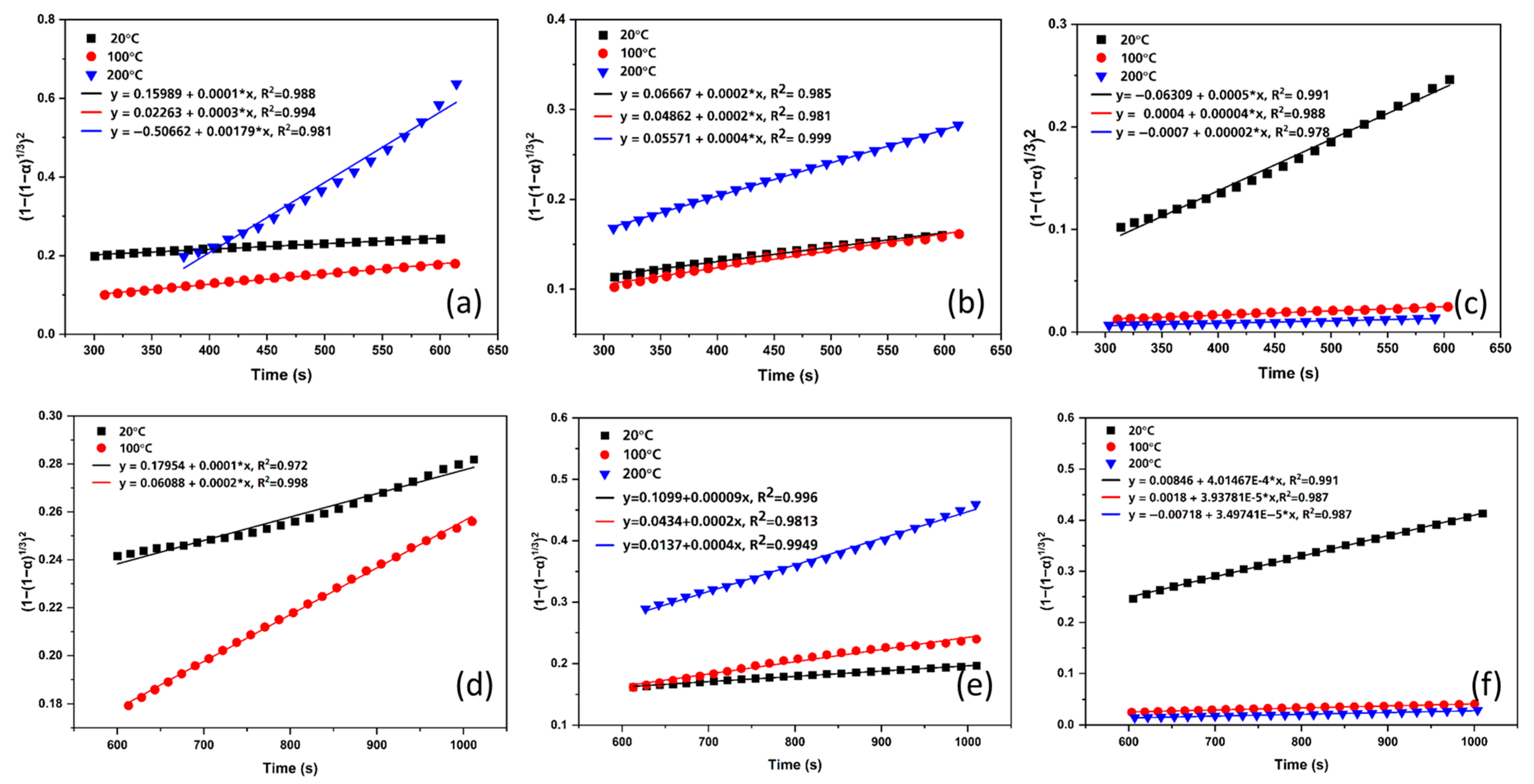
3.4. Kinetic Modeling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Krishnan, A.; Yoosuf, M.; Archana, K.; Arsha, A.S.; Viswam, A. Metal derivative (MD)/g-C3N4 association in hydrogen production: A study on the fascinating chemistry behind, current trend & future direction. J. Energy Chem. 2023, 80, 562–583. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Thangaraja, J.; Rajkumar, S.; Denis Ashok, S.; Sivaramakrishna, A.; Shamim, T. A review on green hydrogen production pathways and optimization techniques. Process Saf. Environ. Prot. 2025, 197, 107070. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Qureshi, T.; Khan, M.M.; Pali, H.S. The future of hydrogen economy: Role of high entropy alloys in hydrogen storage. J. Alloys Compd. 2024, 1004, 175668. [Google Scholar] [CrossRef]
- Yadav, T.P.; Kumar, A.; Verma, S.K.; Mukhopadhyay, N.K. High-Entropy Alloys for Solid Hydrogen Storage: Potentials and Prospects. Trans. Indian Natl. Acad. Eng. 2022, 7, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Krishna, S.A.; Noble, N.; Radhika, N.; Saleh, B. A comprehensive review on advances in high entropy alloys: Fabrication and surface modification methods, properties, applications, and future prospects. J. Manuf. Process. 2024, 109, 583–606. [Google Scholar] [CrossRef]
- Jeyaraman, S.; Manivasagam, T.G. Highly reversible Mg-containing multicomponent high-entropy alloys for electrochemical hydrogen storage applications. Int. J. Hydrogen Energy 2024, 83, 188–197. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. An approach to design single BCC Mg-containing high entropy alloys for hydrogen storage applications. Int. J. Hydrogen Energy 2021, 46, 25555–25561. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting electrons—A new approach to tailor the hydrogen sorption properties of high-entropy alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Ek, G.; Nygård, M.M.; Pavan, A.F.; Montero, J.; Henry, P.F.; Sørby, M.H.; Witman, M.; Stavila, V.; Zlotea, C.; Hauback, B.C.; et al. Elucidating the Effects of the Composition on Hydrogen Sorption in TiVZrNbHf-Based High-Entropy Alloys. Inorg. Chem. 2021, 60, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, T.G.; Iliksu, M.; Danilov, D.L.; Notten, P.H.L. Synthesis and electrochemical properties of binary MgTi and ternary MgTiX (X = Ni, Si) hydrogen storage alloys. Int. J. Hydrogen Energy 2017, 42, 23404–23415. [Google Scholar] [CrossRef][Green Version]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A Mater. Sci. Process. 2016, 122, 85. [Google Scholar] [CrossRef]
- Kohno, T.; Yoshida, H.; Kawashima, F.; Inaba, T.; Sakai, I.; Yamamoto, M.; Kanda, M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14. J. Alloys Compd. 2000, 311, 5–7. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Zheng, Z.; Cheng, H.; Yan, K.; Zhu, Z. Long-term hydrogen absorption/desorption properties and structural changes of LaNi4Co alloy with double desorption plateaus. J. Alloys Compd. 2019, 778, 681–690. [Google Scholar] [CrossRef]
- Balcerzak, M. Structure and hydrogen storage properties of mechanically alloyed Ti-V alloys. Int. J. Hydrogen Energy 2017, 42, 23698–23707. [Google Scholar] [CrossRef]
- Shen, S.; Li, Y.; Ouyang, L.; Zhang, L.; Zhu, M.; Liu, Z. V–Ti-Based Solid Solution Alloys for Solid-State Hydrogen Storage. Nano-Micro Lett. 2025, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, P.K.; Kojima, Y.; Kain, V. Cyclic hydrogen storage properties of V–Ti–Cr–Al alloy. Int. J. Hydrogen Energy 2018, 43, 7096–7101. [Google Scholar] [CrossRef]
- Kamble, A.; Sharma, P.; Huot, J. Effect of addition of Zr, Ni, and Zr-Ni alloy on the hydrogen absorption of Body Centred Cubic 52Ti-12V-36Cr alloy. Int. J. Hydrogen Energy 2018, 43, 7424–7429. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Laversenne, L.; Nassif, V.; Zepon, G.; Sahlberg, M.; Zlotea, C. Hydrogen storage properties of the refractory Ti–V–Zr–Nb–Ta multi-principal element alloy. J. Alloys Compd. 2020, 835, 155376. [Google Scholar] [CrossRef]
- Ma, X.F.; Ding, X.; Liu, E.L.; Chen, R.R.; Wang, X.X.; Zhang, Y.; Guo, J.J. Modification of BCC phase and the enhanced reversible hydrogen storage properties of Ti-V-Fe-Mn alloys with varied V/Fe ratios. China Foundry 2024, 21, 546–554. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kon, M.; Washio, K.; Shinozawa, T.; Ishikiriyama, M. TiCrVMo alloys with high dissociation pressure for high-pressure MH tank. Int. J. Hydrogen Energy 2009, 34, 1458–1462. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Xia, B.; Xu, N. Enhancement of hydrogen storage capacity of Ti–V–Cr–Mn BCC phase alloys. J. Alloys Compd. 2004, 372, 272–277. [Google Scholar] [CrossRef]
- Zareipour, F.; Shahmir, H.; Huang, Y.; Patel, A.K.; Dematteis, E.M.; Baricco, M. Hydrogen storage in TiVCr(Fe,Co)(Zr,Ta) multi-phase high-entropy alloys. Int. J. Hydrogen Energy 2024, 94, 639–649. [Google Scholar] [CrossRef]
- Towata, S.I.; Noritake, T.; Itoh, A.; Aoki, M.; Miwa, K. Cycle durability of Ti-Cr-V alloys partially substituted by Nb or Fe. J. Alloys Compd. 2013, 580 (Suppl. S1), S226–S228. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Jiang, L.; Li, Z.; Guo, X.; Ye, J.; Yuan, B.; Wang, S.; Hao, L. Effect of Fe and Al on hydrogen storage properties of 75 V-Ti-Cr alloys. J. Alloys Compd. 2021, 887, 161181. [Google Scholar] [CrossRef]
- Coluzzi, B.; Biscarini, A.; Mazzolai, G.; Mazzolai, F.M.; Tuissi, A.; Agresti, F.; Lo Russo, S.; Maddalena, A.; Palade, P.; Principi, G. Physical properties of hydrogen in TiVMnCr bcc alloys as deduced from hydrogen absorption/desorption and mechanical spectroscopy experiments. J. Alloys Compd. 2008, 456, 118–124. [Google Scholar] [CrossRef]
- Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.C.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Sleiman, S.; Chen, X.; Zhu, S.; Cheng, H.; Huot, J. Microstructure and hydrogen storage properties of Ti–V–Cr based BCC-type high entropy alloys. Int. J. Hydrogen Energy 2021, 46, 28709–28718. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. An overview of TiFe alloys for hydrogen storage: Structure, processes, properties, and applications. J. Energy Storage 2023, 68, 107772. [Google Scholar] [CrossRef]
- Sato, T.; Saitoh, H.; Utsumi, R.; Ito, J.; Nakahira, Y.; Obana, K.; Takagi, S.; Orimo, S.I. Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules 2023, 28, 1256. [Google Scholar] [CrossRef] [PubMed]
- Balcerzak, M.; Wagstaffe, M.; Robles, R.; Pruneda, M.; Noei, H. Effect of Cr on the hydrogen storage and electronic properties of BCC alloys: Experimental and first-principles study. Int. J. Hydrogen Energy 2020, 45, 28996–29008. [Google Scholar] [CrossRef]
- Silva, B.H.; Zlotea, C.; Vaughan, G.; Champion, Y.; Botta, W.J.; Zepon, G. Hydrogen absorption/desorption reactions of the (TiVNb)85Cr15 multicomponent alloy. J. Alloys Compd. 2022, 901, 163620. [Google Scholar] [CrossRef]
- Mazzolai, G.; Coluzzi, B.; Biscarini, A.; Mazzolai, F.M.; Tuissi, A.; Agresti, F.; Lo Russo, S.; Maddalena, A.; Palade, P.; Principi, G. Hydrogen-storage capacities and H diffusion in bcc TiVCr alloys. J. Alloys Compd. 2008, 466, 133–139. [Google Scholar] [CrossRef]
- Arashima, H.; Takahashi, F.; Ebisawa, T.; Itoh, H.; Kabutomori, T. Correlation between hydrogen absorption properties and homogeneity of Ti-Cr-V alloys. J. Alloys Compd. 2003, 356–357, 405–408. [Google Scholar] [CrossRef]
- Feng, Z.; Zhong, H.; Li, D.; Li, X.; Yang, B.; Li, S. Microstructure and hydrogen storage properties of Ti–V–Mn alloy with Zr, Ni, and Zr7Ni10 addition. J. Mater. Res. 2022, 37, 1591–1601. [Google Scholar] [CrossRef]
- Pericoli, E.; Ferretti, V.; Verna, D.; Pasquini, L. Tuning TiFe1−xNix Hydride Thermodynamics through Compositional Tailoring. ACS Appl. Energy Mater. 2025, 8, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional effects in TiFe for hydrogen storage: A comprehensive review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Soleimani, A.; Hosseini Dolatabadi, S.H.; Heidari, M.; Pinnarelli, A.; Mehdizadeh Khorrami, B.; Luo, Y.; Vizza, P.; Brusco, G. Progress in hydrogen fuel cell vehicles and up-and-coming technologies for eco-friendly transportation: An international assessment. Multiscale Multidiscip. Model. Exp. Des. 2024, 7, 3153–3172. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles; U.S. Drive, U.S. Department of Energy: Washington, DC, USA, 2017; pp. 1–29. Available online: https://www.energy.gov/sites/prod/files/2015/05/f22/fcto_myrdd_storage.pdf (accessed on 10 January 2025).
- Cho, S.W.; Han, C.S.; Park, C.N.; Akiba, E. Hydrogen storage characteristics of Ti-Cr-V alloys. J. Alloys Compd. 1999, 288, 294–298. [Google Scholar] [CrossRef]
- Kumar, A.; Banerjee, S.; Pillai, C.G.S.; Bharadwaj, S.R. Hydrogen storage properties of Ti2−xCrVMx (M = Fe, Co, Ni) alloys. Int. J. Hydrogen Energy 2013, 38, 13335–13342. [Google Scholar] [CrossRef]
- Kumar, A.; Banerjee, S.; Ruz, P.; Sudarsan, V. Hydrogen storage properties of Al-containing Ti2CrV alloys. Bull. Mater. Sci. 2024, 47, 8. [Google Scholar] [CrossRef]
- Kumar, A.; Shashikala, K.; Banerjee, S.; Nuwad, J.; Das, P.; Pillai, C.G.S. Effect of cycling on hydrogen storage properties of Ti2CrV alloy. Int. J. Hydrogen Energy 2012, 37, 3677–3682. [Google Scholar] [CrossRef]
- Kumar, A.; Banerjee, S.; Bharadwaj, S.R. Hydrogen storage properties of Ti0.32Cr0.43V0.25 alloy and its composite with TiMn2. J. Alloys Compd. 2015, 649, 801–808. [Google Scholar] [CrossRef]
- Singh, B.K.; Cho, S.W.; Bartwal, K.S. Effect on structure and hydrogen storage characteristics of composite alloys Ti0.32Cr0.43V0.25 with LaNi5 and rare-earth elements La, Ce, Y.J. Alloys Compd. 2009, 478, 785–788. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, A.; Ruz, P.; Sengupta, P. Influence of Laves phase on microstructure and hydrogen storage properties of Ti–Cr–V based alloy. Int. J. Hydrogen Energy 2016, 41, 18130–18140. [Google Scholar] [CrossRef]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1998, 6, 461–470. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Yang, X.S.; Chen, P.; Tsui, G.C.P.; Xu, Z.L.; Tang, R.; Xiao, F.; Chan, K. Compositionally complex doping for low-V Ti-Cr-V hydrogen storage alloys. Chem. Eng. J. 2023, 477, 146970. [Google Scholar] [CrossRef]
- Silva, B.H.; Zlotea, C.; Champion, Y.; Botta, W.J.; Zepon, G. Design of TiVNb-(Cr, Ni or Co) multicomponent alloys with the same valence electron concentration for hydrogen storage. J. Alloys Compd. 2021, 865, 158767. [Google Scholar] [CrossRef]
- Strozi, R.B.; Silva, B.H.; Leiva, D.R.; Zlotea, C.; Botta, W.J.; Zepon, G. Tuning the hydrogen storage properties of Ti-V-Nb-Cr alloys by controlling the Cr/(TiVNb) ratio. J. Alloys Compd. 2023, 932, 167609. [Google Scholar] [CrossRef]
- Bishnoi, A.; Sharma, P. Large-scale production of BCC solid solution hydrogen storage alloy. Int. J. Hydrogen Energy 2024, 75, 294–302. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Y.; Li, X.; Ke, H.; Wang, L.; Cao, T.; Wan, D.; Wang, B.; Xue, Y. Solid-State Hydrogen Storage Properties of Ti–V–Nb–Cr High-Entropy Alloys and the Associated Effects of Transitional Metals (M = Mn, Fe, Ni). Acta Metall. Sin. Engl. Lett. 2022, 36, 1113–1122. [Google Scholar] [CrossRef]
- Liang, J.; Li, G.; Ding, X.; Li, Y.; Wen, Z.; Zhang, T.; Qu, Y. The synergistic effect of Ni and C14 Laves phase on the hydrogen storage properties of TiVZrNbNi high entropy hydrogen storage alloy. Intermetallics 2024, 164, 108102. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Hessel Silva, B.; Montero, J.; Zlotea, C.; Champion, Y.; Botta, W.J.; Zepon, G. Structural characterization and hydrogen storage properties of the Ti31V26Nb26Zr12M5 (M = Fe, Co, or Ni) multi-phase multicomponent alloys. Int. J. Hydrogen Energy 2023, 48, 2247–2255. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Zhao, W.; Wang, D.; Zhang, Y.; Zhong, Y.; Zhang, X.; Wang, Z.; Hu, C.; Liu, J. Hydrogen transportation behaviour of V–Ni solid solution: A first-principles investigation. Materials 2021, 14, 2603. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Y.; de Paula Santos, W.; Moussa, M.; Serrano, L.B.; Bobet, J.L.; Santos, S.F.; Cardoso, K.R. Effect of Nb on hydrogen storage properties of Ti–V–Cr-based alloys. Mater. Chem. Phys. 2024, 328, 130011. [Google Scholar] [CrossRef]
- Takeuchi, A.; Akira, A.I. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Synthesis and hydrogen storage behavior of Mg–V–Al–Cr–Ni high entropy alloys. Int. J. Hydrogen Energy 2021, 46, 2351–2361. [Google Scholar] [CrossRef]
- Griessen, R.; Riesterer, T. Heat of formation models. In Hydrogen in Intermetallic Compounds I; Springer: Berlin/Heidelberg, Germany, 1988; pp. 219–284. [Google Scholar]
- Tominaga, Y.; Matsumoto, K.; Fuda, T.; Tamura, T.; Kuriiwa, T.; Kamegawa, A.; Takamura, H.; Okada, M. Protium absorption desorption properties of Ti-V-Cr-(Mn,Ni) Alloy. Mater. Trans. 2000, 41, 617–620. [Google Scholar] [CrossRef][Green Version]
- Wen, Z.; Li, G.; Wang, S.; Li, Y.; Zhang, T.; Ding, X.; Qu, Y. Effect of C15 Laves phase ratios on hydrogen absorption and desorption properties of Ti0.50−xV0.25CrxNb0.25 (x = 0.15, 0.25, 0.30, and 0.35) multicomponent alloys. J. Alloys Compd. 2025, 1018, 179159. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, T.P.; Mukhopadhyay, N.K. Notable hydrogen storage in Ti–Zr–V–Cr–Ni high entropy alloy. Int. J. Hydrogen Energy 2022, 47, 22893–22900. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, S.; Miao, H.; Liu, Y.; Pan, H. Pulverization mechanism of the multiphase Ti-V-based hydrogen storage electrode alloy during charge/discharge cycling. J. Alloys Compd. 2010, 489, 552–557. [Google Scholar] [CrossRef]
- Luo, L.; Yang, F.; Li, Y.; Li, L.; Li, Y. Investigation of the microstructure and hydrogen storage behavior of V48Fe12Ti15+xCr25−x (x = 0, 5, 10, 15) alloys. Int. J. Hydrogen Energy 2022, 47, 9653–9671. [Google Scholar] [CrossRef]
- Aranda, V.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Hydrogen storage properties of the TiVFeZr multicomponent alloy with C14-type laves phase structure. Intermetallics 2023, 162, 108020. [Google Scholar] [CrossRef]
- Ma, X.; Ding, X.; Chen, R.; Chen, X.; Song, Q.; Cui, H. Study on microstructure and the hydrogen storage behavior of a TiVZrNbFe high-entropy alloy. Intermetallics 2023, 157, 107885. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liang, J.; Wen, Z.; Zhang, T.; Ding, X.; Qu, Y. Influence of C14 Laves phase and Zr-rich phase interactions on the hydrogen storage properties of Ti32.5V27.5Zr7.5Nb32.5 high entropy hydrogen storage alloy. Intermetallics 2024, 168, 108239. [Google Scholar] [CrossRef]
- Ponsoni, J.B.; Aranda, V.; da Nascimento, T.S.; Strozi, R.B.; Botta, W.J.; Zepon, G. Design of multicomponent alloys with C14 laves phase structure for hydrogen storage assisted by computational thermodynamic. Acta Mater. 2022, 240, 118317. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V. Laves type intermetallic compounds as hydrogen storage materials: A review. J. Alloys Compd. 2022, 916, 165219. [Google Scholar] [CrossRef]
- Dangwal, S.; Edalati, K. Significance of interphase boundaries on activation of high-entropy alloys for room-temperature hydrogen storage. Int. J. Hydrogen Energy 2024, 50, 626–636. [Google Scholar] [CrossRef]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana, G.L.B.G.; Mohammadi, A.; Ma, Z.; Li, H.W.; Contieri, R.J. Hydrogen storage in TiZrNbFeNi high entropy alloys, designed by thermodynamic calculations. Int. J. Hydrogen Energy 2020, 45, 33759–33770. [Google Scholar] [CrossRef]
- Guéguen, A.; Joubert, J.M.; Latroche, M. Influence of the C14 Ti35.4V32.3Fe32.3 Laves phase on the hydrogenation properties of the body-centered cubic compound Ti24.5V59.3Fe16.2. J. Alloys Compd. 2011, 509, 3013–3018. [Google Scholar] [CrossRef]
- Hessel Silva, B.; Botta, W.J.; Zepon, G. Design of a Ti–V–Nb–Cr alloy with room temperature hydrogen absorption/desorption reversibility. Int. J. Hydrogen Energy 2023, 48, 32813–32825. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, A.; Laversenne, L.; Nassif, V.; Elkaim, E.; Zlotea, C. Hydrogen Storage Properties of a New Ti-V-Cr-Zr-Nb High Entropy Alloy. Hydrogen 2022, 3, 270–284. [Google Scholar] [CrossRef]
- Cho, S.W.; Yoo, J.H.; Chang, H.K.; Kim, W.B.; Kil, D.S.; Ahn, J.G. Changes in the microstructure and hydrogen storage properties of Ti-Cr-V alloys by ball milling and heat treatment. J. Alloys Compd. 2011, 509, 5545–5550. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Effect of particle size, pressure and temperature on the activation process of hydrogen absorption in TiVZrHfNb high entropy alloy. J. Alloys Compd. 2021, 861, 158615. [Google Scholar] [CrossRef]
- Zhai, Y.T.; Li, Y.M.; Wei, S.H.; Tolj, I.; Kennedy, J.; Yang, F. Progress in V-BCC based solid solution hydrogen storage alloys. J. Energy Storage 2025, 109, 115103. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Sleiman, S.; Ravalison, F.; Zhu, W.; Liu, H.; Cheng, H.; Huot, J. Hydrogen storage properties of V0.3Ti0.3Cr0.25Mn0.1Nb0.05 high entropy alloy. Int. J. Hydrogen Energy 2022, 47, 25724–25732. [Google Scholar] [CrossRef]
- Luo, L.; Han, H.; Feng, D.; Lv, W.; Chen, L.; Li, L.; Zhai, T.; Liu, S.; Sun, S.; Li, Y.; et al. Nanocrystalline High Entropy Alloys with Ultrafast Kinetics and High Storage Capacity for Large-Scale Room-Temperature-Applicable Hydrogen Storage. Renewables 2024, 2, 138–149. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhou, P.P.; Cao, Z.M.; Li, P.C.; Hu, J.T.; Xiao, H.Y.; Zhou, X.S.; Shen, H.H.; Zu, X.T. Composition and temperature influence on hydrogenation performance of TiZrHfMoxNb2−x high entropy alloys. J. Mater. Chem. A 2023, 11, 20623–20635. [Google Scholar] [CrossRef]
- Ma, X.; Ding, X.; Chen, R.; Cao, W.; Song, Q. Study on hydrogen storage property of (ZrTiVFe)xAly high-entropy alloys by modifying Al content. Int. J. Hydrogen Energy 2022, 47, 8409–8418. [Google Scholar] [CrossRef]
- Das, T.K.; Kumar, A.; Ruz, P.; Banerjee, S.; Sudarsan, V. Hydrogen storage properties of Ti2FeV BCC solid solution. J. Chem. Sci. 2019, 131, 98. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; Van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, X.; Chen, R.; Zhang, J.; Su, Y.; Wu, S.; Guo, J. Modification of nano-eutectic structure and the relation on hydrogen storage properties: A novel Ti–V–Zr medium entropy alloy. Int. J. Hydrogen Energy 2022, 47, 34533–34544. [Google Scholar] [CrossRef]
- Ruz, P.; Banerjee, S.; Halder, R.; Kumar, A.; Sudarsan, V. Thermodynamics, kinetics and microstructural evolution of Ti0.43Zr0.07Cr0.25V0.25 alloy upon hydrogenation. Int. J. Hydrogen Energy 2017, 42, 11482–11492. [Google Scholar] [CrossRef]
- Hara, M. Hydrogen-induced disproportionation of Zr2Co. J. Alloys Compd. 2003, 352, 218–225. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Jiang, X.; Li, X. Study on the hydrogen storage property of (TiZr0.1)xCr1.7−yFeyMn0.3 (1.05 < x < 1.2, 0.2 < y < 0.6) alloys. Prog. Nat. Sci. Mater. Int. 2018, 28, 470–477. [Google Scholar] [CrossRef]
- Li, T.; Li, Q.; Long, H.; Chou, K.C.; Luo, Q. Interpretation of negative temperature dependence of hydriding reaction in LaNi5-Mg alloys by modified Chou model. Catal. Today 2018, 318, 97–102. [Google Scholar] [CrossRef]
- Cheng, B.; Kong, L.; Cai, H.; Li, Y.; Zhao, Y.; Wan, D.; Xue, Y. Exploring microstructure variations and hydrogen storage characteristics in TiVNbCrNi high-entropy alloys with different Ni incorporation. Int. J. Hydrogen Energy 2024, 72, 29–40. [Google Scholar] [CrossRef]


| Composition | δ | Ω | VEC | ||||
|---|---|---|---|---|---|---|---|
| (TiVCr)95Ni5 | 10.33 | −8.16 | 6.68 | 2.62 | 5.24 | −17.51 | −37.44 |
| (TiVCr)90Ni10 | 10.92 | −11.16 | 6.69 | 2.02 | 5.50 | −16.1 | −35.70 |
| (TiVCr)90Ni5Nb5 | 11.50 | −8.22 | 6.65 | 2.96 | 5.25 | −18.5 | −37.45 |
| As-Synthesized | Hydride | ||||||
|---|---|---|---|---|---|---|---|
| Composition | Phase | Phase Fraction (%) | Lattice Parameter (Å) | * Cell Volume (Å3) | Phase | Lattice Parameter (Å) | * Cell Volume (Å3) |
| (TiVCr)95Ni5 | BCC | 82.2 | a = 3.048 | 28.31 | BCC Hydride | a = 3.159 | 31.52 |
| Secondary BCC | 17.8 | a = 2.881 | 23.91 | Secondary BCC | a = 2.884 | 23.98 | |
| (TiVCr)90Ni10 | BCC | 52.8 | a = 3.042 | 28.14 | BCC Hydride | a = 3.141 | 30.98 |
| Secondary BCC | 47.2 | a = 2.880 | 23.88 | Secondary BCC | a = 2.898 | 24.33 | |
| (TiVCr)95Ni5Nb5 | BCC | 89.6 | a = 3.038 | 28.04 | BCC Hydride | a = 3.142 | 31.01 |
| C15 Lave phase | 10.4 | a = 6.951 | 335.8 | C15 Lave phase | a = 6.992 | 341.8 | |
| Composition | 20 °C | 100 °C | 200 °C | |||
|---|---|---|---|---|---|---|
| abs | des | abs | des | abs | des | |
| (TiVCr)95Ni5 | 2.50 | 0.86 | 2.00 | 0.91 | 1.09 | 0.49 |
| (TiVCr)90Ni10 | 3.00 | 1.20 | 1.47 | 0.44 | 1.14 | 0.68 |
| (TiVCr)90Ni5Nb5 | 2.50 | 1.15 | 1.35 | 0.53 | 1.22 | 0.75 |
| Composition | Temperature (K) | Stage 1 (t = 0–35 s) | Stage 2 (t = 100–300 s) | ||||
|---|---|---|---|---|---|---|---|
| ƞ | k | R2 | ƞ | k | R2 | ||
| (TiVCr)95Ni5 | 293 | 2.07 | 0.0166 | 0.997 | 0.486 | 0.0088 | 0.986 |
| 373 | 0.899 | 0.0062 | 0.998 | 0.681 | 0.0059 | 0.999 | |
| 473 | 0.811 | 0.0061 | 0.999 | 0.940 | 0.0053 | 0.996 | |
| (TiVCr)90Ni10 | 293 | 0.935 | 0.0061 | 0.999 | 0.609 | 0.0040 | 0.989 |
| 373 | 0.759 | 0.0082 | 0.999 | 0.444 | 0.00431 | 0.997 | |
| 473 | 0.735 | 0.0135 | 0.999 | 0.445 | 0.011 | 0.999 | |
| (TiVCr)90Ni5Nb5 | 293 | 1.684 | 0.00472 | 0.999 | 1.132 | 0.00397 | 0.981 |
| 373 | 0.778 | 0.00138 | 0.999 | 0.576 | 0.00053 | 0.997 | |
| 473 | 0.680 | 0.0011 | 0.997 | 0.457 | 0.00017 | 0.998 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyaraman, S.; Danilov, D.L.; Notten, P.H.L.; Ragula, U.B.R.; Ramalingam, V.V.; Manivasagam, T.G. Influence of Ni and Nb Addition in TiVCr-Based High Entropy Alloys for Room-Temperature Hydrogen Storage. Energies 2025, 18, 3920. https://doi.org/10.3390/en18153920
Jeyaraman S, Danilov DL, Notten PHL, Ragula UBR, Ramalingam VV, Manivasagam TG. Influence of Ni and Nb Addition in TiVCr-Based High Entropy Alloys for Room-Temperature Hydrogen Storage. Energies. 2025; 18(15):3920. https://doi.org/10.3390/en18153920
Chicago/Turabian StyleJeyaraman, Srilakshmi, Dmitri L. Danilov, Peter H. L. Notten, Udaya Bhaskar Reddy Ragula, Vaira Vignesh Ramalingam, and Thirugnasambandam G. Manivasagam. 2025. "Influence of Ni and Nb Addition in TiVCr-Based High Entropy Alloys for Room-Temperature Hydrogen Storage" Energies 18, no. 15: 3920. https://doi.org/10.3390/en18153920
APA StyleJeyaraman, S., Danilov, D. L., Notten, P. H. L., Ragula, U. B. R., Ramalingam, V. V., & Manivasagam, T. G. (2025). Influence of Ni and Nb Addition in TiVCr-Based High Entropy Alloys for Room-Temperature Hydrogen Storage. Energies, 18(15), 3920. https://doi.org/10.3390/en18153920






