Modification of Canola Oil Properties Using Ethyl Oleate and n-Hexane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
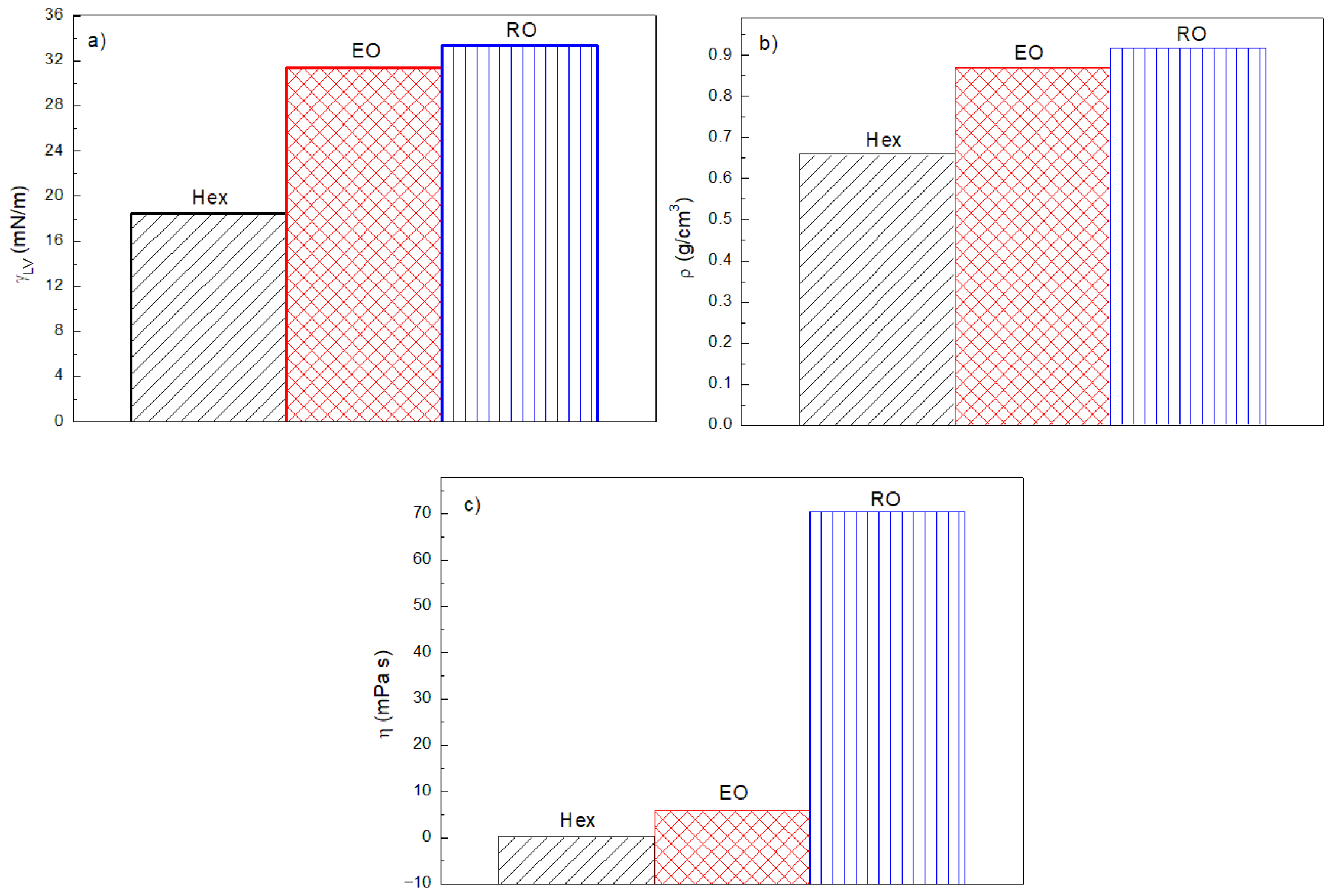
3.1. Some Physicochemical Properties of Canola Oil, n-Hexane, Ethyl Oleate, and Diesel Fuel
| Substance | % | M | ||||||
|---|---|---|---|---|---|---|---|---|
| Canola oil | 34.20 | 70.45 | 0.9161 | |||||
| Oleic acid | 62 | 282.46 | 31.92 | 26.91 | 0.895 | 0.8774 | 321.90 | 315.60 |
| Linoleic acid | 20 | 280.45 | 25.02 | 27.20 | 0.9012 | 0.8796 | 318.82 | 311.20 |
| -linolenic acid | 12 | 278.43 | 30.35 | 27.65 | 0.9134 | 0.8818 | 315.74 | 304.83 |
| Palmitic acid | 3 | 256.43 | 29.25 | 7.60 a | 0.9052 | 0.8755 | 292.87 | 283.29 |
| Stearic acid | 2 | 284.48 | 29.01 | 9.81 a | 0.842 | 0.8753 | 324.99 | 337.86 |
| Trans fatty | 0.5 | 358.57 | 27.51 | 9.12 b | 0.8521 | 0.8701 | 412.09 | 420.81 |
| Erucic acid | <0.1 | 338.58 | 28.12 | 8.75 a | 0.8571 | 0.8768 | 386.14 | 395.03 |
| n-hexane | 86.18 | 18.50 | 0.3102 | 0.6594 | 0.6668 | 129.23 | 130.69 | |
| Ethyl oleate | 310.51 | 31.00 | 5.81 | 0.8701 | 0.8504 | 365.11 | 356.87 | |
| Diesel fuel | 2.7 c | 0.8350 |
3.2. Surface Tension, Density, and Viscosity of Canola Oil Mixtures with n-Hexane and Ethyl Oleate
3.3. Surface Tension, Density, and Viscosity of RO + EO + Hex Mixture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Lu, J.; Ma, T.; Zhang, Y.; Gu, L.; Chen, X. Applications of vegetable oils and their derivatives as Bio-Additives for use in asphalt binders: A review. Const. Build. Mater. 2023, 383, 131. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Upadhhyaya, K.C. Vegetable Oil: Nutritional and Industrial Perspective. Curr. Genomics. 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, H.C.; Chauhan, R.; Smith, J.S. Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review. Foods 2024, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, I.; Olech, I.; Szymańska, E.J. The Use of Canola for Biofuel Production in the Context of Energy Security—A Systematic Literature Review. Energies 2025, 18, 2410. [Google Scholar] [CrossRef]
- Banga, S.M.; Pathak, V.V. Biodiesel production from waste cooking oil: A comprehensive review on the application of heterogenous catalysts. Energy Nexus 2023, 10, 100209. [Google Scholar] [CrossRef]
- Rajak, A.K.; Harikrishna, M.; Mahato, D.L.; Anandamma, A.U.; Pothu, R.; Sarangi, P.K.; Sahoo, U.K.; Vennu, V.; Boddula, R.; Karuna, M.S.L. Valorising orange and banana peels: Green catalysts for transesterification and biodiesel production in a circular bioeconomy. J. Taiwan Instit. Chem. Eng. 2024, 13, 105804. [Google Scholar] [CrossRef]
- Badsha, M.; Maheer, U.; Omar, F.M.; Ramli, A.; Balakrishnan, V.; Hamed, Y.; Arafath, A.; Hossain, S. Sustainable approaches on innovative utilization of household food waste as a renewable biomass for bioenergy production: Potential, challenges and social-environmental impacts. Biomass Bioenergy 2025, 200, 107995. [Google Scholar] [CrossRef]
- Lefebvre, A.H.; McDonell, V.G. Atomization and Sprays, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 300. [Google Scholar]
- Slavinskas, S. An Experimental Study on the Injection Characteristics and the Macroscopic Spray Characteristics of Rapeseed Oil-Diesel Fuel Blends. Appl. Sci. 2023, 13, 5944. [Google Scholar] [CrossRef]
- Khan, E.; Ozaltin, K.; Spagnuolo, D.; Bernal-Ballen, A.; Piskunov, M.V.; Di Martino, A. Biodiesel from Rapeseed and Sunflower Oil: Effect of the Transesterification Conditions and Oxidation Stability. Energies 2023, 16, 657. [Google Scholar] [CrossRef]
- Silva, L.E.; Santos, C.A.C.; Ribeiro, J.E.S.; Souza, C.C.; Sant’Ana, A.M.S. Rheological Analysis Of Vegetable Oils Used For Biodiesel Production In Brazil. Rev. Eng. Térmica 2015, 14, 31–36. [Google Scholar] [CrossRef]
- Maksimuk, Y.V.; Buglak, A.F.; Kruk, V.S.; Antonova, Z.A. Rheological properties of residual fuel oil containing fuel blends with bioadditives. Part 1. Rapeseed oil based bioadditives. Chem. Technol. Fuels Oils 2013, 49, 108–114. [Google Scholar] [CrossRef]
- Mawatari, T.; Fukuda, R.; Mori, H.; Mia, S.; Ohno, N. High Pressure Rheology of Environmentally Friendly Vegetable Oils. Tribol. Lett. 2013, 51, 273–280. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A Comprehensive Review on Properties of Edible and Non-Edible Vegetable Oil-Based Biodiesel: Composition, Specifications and Prediction Models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Mamedov, I.G.; Mamedova, G.; Azimova, N. Testing of Ethylene Glycol Ketal, Dioxane and Cyclopentanone as Components of B10, B20 Fuel Blends. Energy Environ. Storage 2022, 2, 9–12. [Google Scholar] [CrossRef]
- Binhweel, F.; Bahadi, M.; Elgamouz, A. A Comparative Review of Some Physicochemical Properties of Biodiesels Synthesized from Different Generations of Vegetative Oils. Mater. Today Proc. 2021, 47, 2217–2224. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P.; Zdziennicka, A.; Szymczyk, K.; Jańczuk, B. Changes of Some Physicochemical Properties of Canola Oil by Adding n-Hexane and Ethanol Regarding Its Application as Diesel Fuel. Appl. Sci. 2023, 13, 1108. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Somidi; A. K.R.; Dalai, A.K. Chemical/Structural Modification of Canola Oil and Canola Biodiesel: Kinetic Studies and Biodegradability of the Alkoxides. Lubricants 2017, 5, 11. [Google Scholar] [CrossRef]
- Gotovuša, M.; Medić, M.; Faraguna, F.; Šibalić, M.; Konjević, L.; Vuković, J.P.; Racar, M. Fatty acids propyl esters: Synthesis optimization and application properties of their blends with diesel and 1-propanol. Renew. Energy 2022, 185, 655–664. [Google Scholar] [CrossRef]
- Zeng, Y.; Shang, Z.; Zheng, Z.; Shi, N.; Yang, B.; Han, S.; Yan, J. A Review of Chemical Modification of Vegetable Oils and Their Applications. Lubricants 2024, 12, 180. [Google Scholar] [CrossRef]
- Wedler, C.; Trusler, J.P.M. Review of density and viscosity data of pure fatty acid methyl ester, ethyl ester and butyl ester. Fuel 2023, 339, 127466. [Google Scholar] [CrossRef]
- Xiao, H.-M.; Rao, D.; Zhao, S.; Hussain, D.; Chen, J.-L.; Luo, D.; Wang, D.; Lv, X.; Wei, F.; Chen, H. Formation of medium- and long-chain fatty alcohols in long-term stored oil and biodiesels revealed by chemical isotope labeling-liquid chromatography-high resolution mass spectrometry. Ind. Crops Prod. 2023, 193, 116171. [Google Scholar] [CrossRef]
- Rayapureddy, S.M.; Matijošius, J.; Rimkus, A.; Caban, J.; Słowik, T. Comparative Study of Combustion, Performance and Emission Characteristics of Hydrotreated Vegetable Oil–Biobutanol Fuel Blends and Diesel Fuel on a CI Engine. Sustainability 2022, 14, 7324. [Google Scholar] [CrossRef]
- Brahma, S.; Patir, B.N.K.; Basumatary, B.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Estevez, R.; López-Tenllado, F.J.; Aguado-Deblas, L.; Bautista, F.M.; Romero, A.A.; Luna, D. Evaluation of Advanced Biofuels in Internal Combustion Engines: Diesel/Fuel Oil/Vegetable Oil Triple Blends. Fuels 2024, 5, 660–672. [Google Scholar] [CrossRef]
- Sukjit, E.; Maneedaeng, A. Effect of mixed nonionic surfactants on microemulsion phase boundary, fuel property, and engine performance of biofuels. Energy Rep. 2022, 8, 722–730. [Google Scholar] [CrossRef]
- Phasukarratchai, N. Phase behavior and biofuel properties of waste cooking oil-alcohol-diesel blending in microemulsion form. Fuel 2019, 243, 125–132. [Google Scholar] [CrossRef]
- Arpornpong, N.; Attaphongc, C.; Charoensaengd, A.; Sabatinic, D.A.; Khaodhiar, S. Ethanol-in-palm oil/diesel microemulsion-based biofuel: Phase behavior, viscosity, and droplet size. Fuel 2014, 132, 101–106. [Google Scholar] [CrossRef]
- Najjar, R.; Zarei-Gharehbaba, L.; Tazerout, M. The exhaust emission characteristics of a water-emulsified diesel/sunflower oil blend fuel by ionic liquid surfactants. Inter. J. Environ. Sci. Technol. 2022, 19, 1175–1184. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P.; Jańczuk, B.; Zdziennicka, A.; Szymczyk, K. Modification of Canola Oil Physicochemical Properties by Hexane and Ethanol with Regards of Its Application in Diesel Engine. Energies 2021, 14, 4469. [Google Scholar] [CrossRef]
- Sander, P.; Longwic, R.; Jańczuk, B.; Zdziennicka, A.; Szymczyk, K. The Use of Canola Oil, n-Hexane, and Ethanol Mixtures in a Diesel Engine. SAE Int. J. Fuels Lubr. 2021, 14, 123–138. [Google Scholar] [CrossRef]
- Xin, H.; Hu, X.; Cai, C.; Wang, H.; Zhu, C.; Li, S.; Xiu, Z.; Zhang, X.; Liu, Q.; Ma, L. Catalytic Production of Oxygenated and Hydrocarbon Chemicals From Cellulose Hydrogenolysis in Aqueous Phase. Front. Chem. 2020, 5, 333. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, M.; Hudaya, T.; Soerawidjaja, T.H. Synthesis of Bio-hexane and Bio-Hexene from Sorbitol, Using Formic Acid as Reducing Agent. J. Eng. Technol. Sci. 2021, 53, 210106. [Google Scholar] [CrossRef]
- Liu, S.; Tamura, M.; Nakagawa, Y.; Tomishige, K. One-pot conversion of cellulose into n -hexane over the Ir-ReO x/SiO2 catalyst combined with HZSM-5. ACS Sustain. Chem. Eng. 2014, 2, 1819–1827. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Some physicochemical properties of ethyl oleate mixtures with ethanol and n-hexane. J. Mol. Liq. 2024, 408, 125318. [Google Scholar] [CrossRef]
- Suzara, R.; de Castro, S.; Eduardo, L.; de Barros, N.; Gambini, C. Effect of ethyl octanoate and ethyl oleate on the properties of gasoline fuel mixture. Energy Fuels 2019, 33, 9429–9436. [Google Scholar] [CrossRef]
- Senna, S.R.C.; Barros Neto, E.L.; Pereira, C.G. Evaluation of the lubrication of ethyl oleate and ethyl octanoate as gasoline additive. Braz. J. Petrol. Gas 2019, 13, 111–118. [Google Scholar] [CrossRef]
- Polish Standard PN-EN 590; Fuels for Motor Vehicles—Gas Oils—Requirements and Test Methods. European Committee: Brussels, Poland, 2011.
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Oss, C.J.V. Interfacial Forces in Aqueous Media, 1st ed.; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Van Oss, C.J.; Costanzo, P.M. Adhesion of anionic surfactants to polymer surfaces and low-energy materials. J. Adhes. Sci. Technol. 1992, 6, 477–487. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B.; Lubas, J.; Jaworski, A.; Kuszewski, H.; Woś, P.; Longwic, R.; Sander, P. N-hexane influence on canola oil adhesion and volumetric properties. Int. J. Adhes. Adhes. 2025, 140, 103990. [Google Scholar] [CrossRef]
- Klein, T.; Yan, S.; Cui, J.; Mangee, J.W.; Kroenlein, K.; Rausch, M.H.; Koller, T.M.; Fröba, A.P. Liquid Viscosity and Surface Tension of n-Hexane, n-Octane, n-Decane, and n-Hexadecane up to 573 K by Surface Light Scattering. J. Chem. Eng. Data 2019, 64, 4116–4131. [Google Scholar] [CrossRef] [PubMed]
- Oss, C.J.V.; Good, R.J. Surface tension and the solubility of polymers and biopolymers: The role of polar and apolar interfacial free energies. J. Macromol. Sci. 1989, 26, 1183–1203. [Google Scholar]
- Oss, C.J.V.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Oss, C.J.V.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Connors, K.A.; Wright, J.L. Dependence of surface tension on composition of binary aqueous-organic solutions. Anal. Chem. 1989, 61, 194–198. [Google Scholar] [CrossRef]
- Khossravi, D.; Connors, K.A. Solvent effects on chemical processes. 3. Surface tension of binary aqueous organic solvents. J. Sol. Chem. 1993, 22, 321–330. [Google Scholar] [CrossRef]
- Defay, R.; Prigogine, I.; Bellemas, A.; Everett, D.H. Surface Tension and Adsorption; John Wiley & Sons: New York, NY, USA, 1966. [Google Scholar]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Comparison of surface tension, density, viscosity and contact angle of ethyl oleate to those of ethanol and oleic acid. J. Mol. Liq. 2024, 400, 124525. [Google Scholar] [CrossRef]
- Gmehling, J.; Kolbe, B.; Kleiber, M.; Rarey, J. Chemical Thermodynamics for Process Simulation, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Modification of Canola Oil Properties Using Ethyl Oleate and n-Hexane. Energies 2025, 18, 3802. https://doi.org/10.3390/en18143802
Szymczyk K, Zdziennicka A, Jańczuk B. Modification of Canola Oil Properties Using Ethyl Oleate and n-Hexane. Energies. 2025; 18(14):3802. https://doi.org/10.3390/en18143802
Chicago/Turabian StyleSzymczyk, Katarzyna, Anna Zdziennicka, and Bronisław Jańczuk. 2025. "Modification of Canola Oil Properties Using Ethyl Oleate and n-Hexane" Energies 18, no. 14: 3802. https://doi.org/10.3390/en18143802
APA StyleSzymczyk, K., Zdziennicka, A., & Jańczuk, B. (2025). Modification of Canola Oil Properties Using Ethyl Oleate and n-Hexane. Energies, 18(14), 3802. https://doi.org/10.3390/en18143802








