1. Introduction
In response to the climate crisis, many countries are actively investing in the development of sustainable, carbon-free, and affordable energy technologies. In this context, high-temperature molten salt energy systems are attracting increasing interest due to their advantageous thermal properties and flexibility in high-temperature operations.
High-temperature molten salt energy systems (HT-MSESs) utilize molten salts primarily as heat-transfer fluids (HTFs) and thermal storage media to effectively transfer and store thermal energy. Such systems share several distinct advantages, including high heat capacity and excellent heat-transfer characteristics, enabling efficient storage and transfer of thermal energy at elevated temperatures. Additionally, molten salts exhibit superior thermal stability and are operable over wide temperature ranges (typically from 300 °C up to above 700 °C), allowing for stable and robust operation under diverse thermal conditions. The flexible utilization of stored thermal energy, such as on-demand electricity production or industrial processes, is another significant benefit. Furthermore, molten salts provide a stable long-duration energy storage capability, maintaining thermal energy with minimal heat loss over extended periods. High compatibility with conventional power generation technologies, particularly steam Rankine cycles, facilitates their integration into existing energy infrastructures.
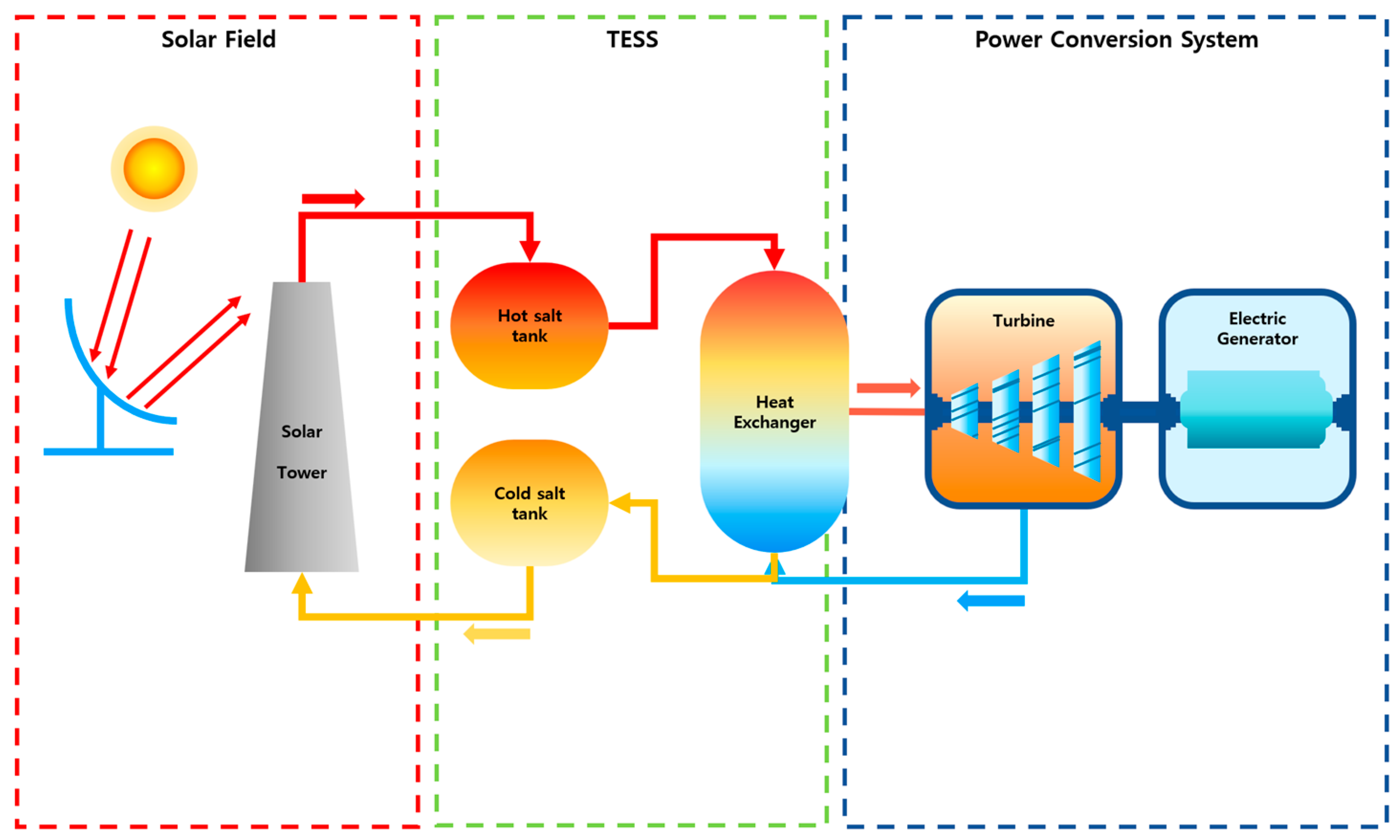
Representative examples of HT-MSESs include Concentrated Solar Power-integrated Thermal Energy Storage Systems (CSP-TESSs) and Molten Salt Reactors (MSRs). CSP-TESSs capture and store solar thermal energy during sunlight hours, subsequently using this stored heat to generate electricity during non-sunny periods, thereby enhancing grid stability and renewable energy integration [
1]. MSRs are advanced nuclear reactor systems that dissolve nuclear fuel in high-temperature molten salts, serving simultaneously as fuel and coolant. This unique design offers enhanced safety features and superior thermal efficiency compared to conventional solid-fuel nuclear reactors.
Recent interest in chloride-based molten salts, in particular, stems from their favorable characteristics such as high thermal stability, relatively low melting points, and minimal corrosivity toward structural alloys. Chloride salts are increasingly recognized as promising candidate materials for advanced HT-MSESs. However, despite these benefits, current knowledge regarding the thermophysical properties of chloride salts remains limited, particularly their melting points, eutectic compositions, and thermal stability at elevated temperatures.
Recently, rapid growth in electricity demand driven by advances in artificial intelligence (AI) and the electric vehicle industry has heightened the need for improved thermal efficiency in HT-MSESs. This has motivated extensive research efforts aimed at developing novel heat-transfer fluids (HTFs) that can operate reliably and efficiently at elevated temperatures. Among the various candidate materials being explored, ternary chloride salts have attracted significant attention due to their desirable characteristics, including high thermal stability, low corrosivity, and comparatively low melting points. Although previous studies have investigated the melting points and thermal stability of certain compositions of these ternary chloride salts, further validation remains necessary. In particular, additional measurements and validations are required to assess thermal stability under high-temperature conditions exceeding 600 °C and to analyze melting point variations across various compositions. A detailed discussion of prior research on thermophysical property measurements of ternary chloride salts is provided in the next section.
Figure 1 illustrates the layout of a CSP-TESS power generation system. In molten salt-based energy systems, partial solidification of salts within piping can obstruct fluid circulation, potentially leading to localized overheating, structural degradation, or salt leakage incidents. To prevent solidification-related accidents, it is recommended to maintain the minimum operational temperature at least 100 °C above the melting point of the salt [
2,
3,
4]. Therefore, precise knowledge of the molten salt’s melting point is essential to ensure thermal and operational safety of the system.
The importance of maintaining the molten salt composition is explained in Romero-Serrano et al.’s study, which analyzed the melting point sensitivity to composition changes in LiF-BeF
2(FLiBe). The melting point of FLiBe varies significantly with its composition change, demonstrating that it is crucial to maintain its composition to reduce uncertainties in heat-transfer performance and to prevent accidents.
Figure 2 shows the phase diagram of FLiBe salt and the melting points with respect to specific compositions as reported by Romero-Serrano et al. [
5].
Table 1 presents the experimentally reported melting points near the eutectic region, as indicated by the red circle in
Figure 2, based on the data from Romero-Serrano et al. [
5]. The melting point of the FLiBe mixture reaches the minimum at a BeF
2 mole fraction of about 0.53 and then increases, indicating a nonlinear behavior [
5].
These results underline the importance of maintaining precise composition to ensure consistent heat-transfer performance and safety. This rationale applies to chloride salts as well, making it essential to conduct analyses on the melting point and thermal stability of various chloride salt compositions. Such analyses are crucial for designing chloride salt-based HT-MSESs, ensuring that changes in salt composition do not compromise the system’s operational stability and safety.
In a binary chloride salt system, the presence of only two components results in relatively simple chemical interactions. Consequently, the phase diagram can be represented using two variables—the mole fraction of one component and temperature—leading to the formation of a single, fixed eutectic point.
In contrast, ternary chloride salt systems involve three components, introducing more complex chemical interactions. This complexity allows for multiple eutectic points across different compositions, thereby forming a broader eutectic line or eutectic region rather than a single, fixed eutectic point. Thus, it is essential to examine how the melting point of the salt varies with composition within this eutectic region in ternary molten salt systems. Such an examination is crucial for verifying the thermochemical stability of salts within the eutectic region and for developing effective strategies for utilizing ternary chloride salts in HT-MSESs. Therefore, after identifying suitable chloride-based HTF candidates for HT-MSESs, it is necessary to investigate melting point variations and thermal stability near the eutectic points and eutectic regions of these binary and ternary chloride salts. This represents the main objective of the present study.
In this paper, binary and ternary molten chloride salts were selected as HTF candidates. Their expected eutectic points and eutectic regions were reported in previous work employing the CALPHAD (CALculation of PHAse Diagrams) method, which only provides the theoretical prediction. Based on these earlier studies, specific compositions were selected for melting point measurements and thermal stability analyses. Common thermal characterization techniques, such as DSC (differential scanning calorimetry) and TGA (thermogravimetric analysis), were employed to experimentally verify the melting points and thermal stability of the selected compositions. Finally, based on the measured thermal properties, the most suitable molten chloride salt mixtures and compositions for use as HTFs in HT-MSESs were recommended.
3. Results and Discussion
3.1. Melting Point Measurements
This section analyzes the melting point measurement results obtained from the DSC analysis for the samples with the various compositions. The melting points of NaCl-MgCl2 predicted using the CALPHAD method are compared with the melting points measured experimentally in this study. For NaCl-KCl-MgCl2 and NaCl-KCl-ZnCl2, the melting points measured in this study are compared with the data from the previous studies for certain compositions. In the case of NaCl-KCl-ZnCl2, two new compositions were additionally measured. Thermal analyses were conducted using a STA 449 F3 Jupiter®® (NETZSCH, Selb, Germany), a simultaneous TGA-DSC instrument. All melting point measurements were conducted with a heating rate of 5 K/min, and argon was used as the purge gas at a flow rate of 50 mL/min. Additionally, lids were placed on the crucibles to prevent interaction between the sample and the atmosphere, minimize evaporation, and ensure uniform heat distribution, thereby improving the accuracy of the melting point measurements.
3.1.1. Melting Point of NaCl-MgCl2
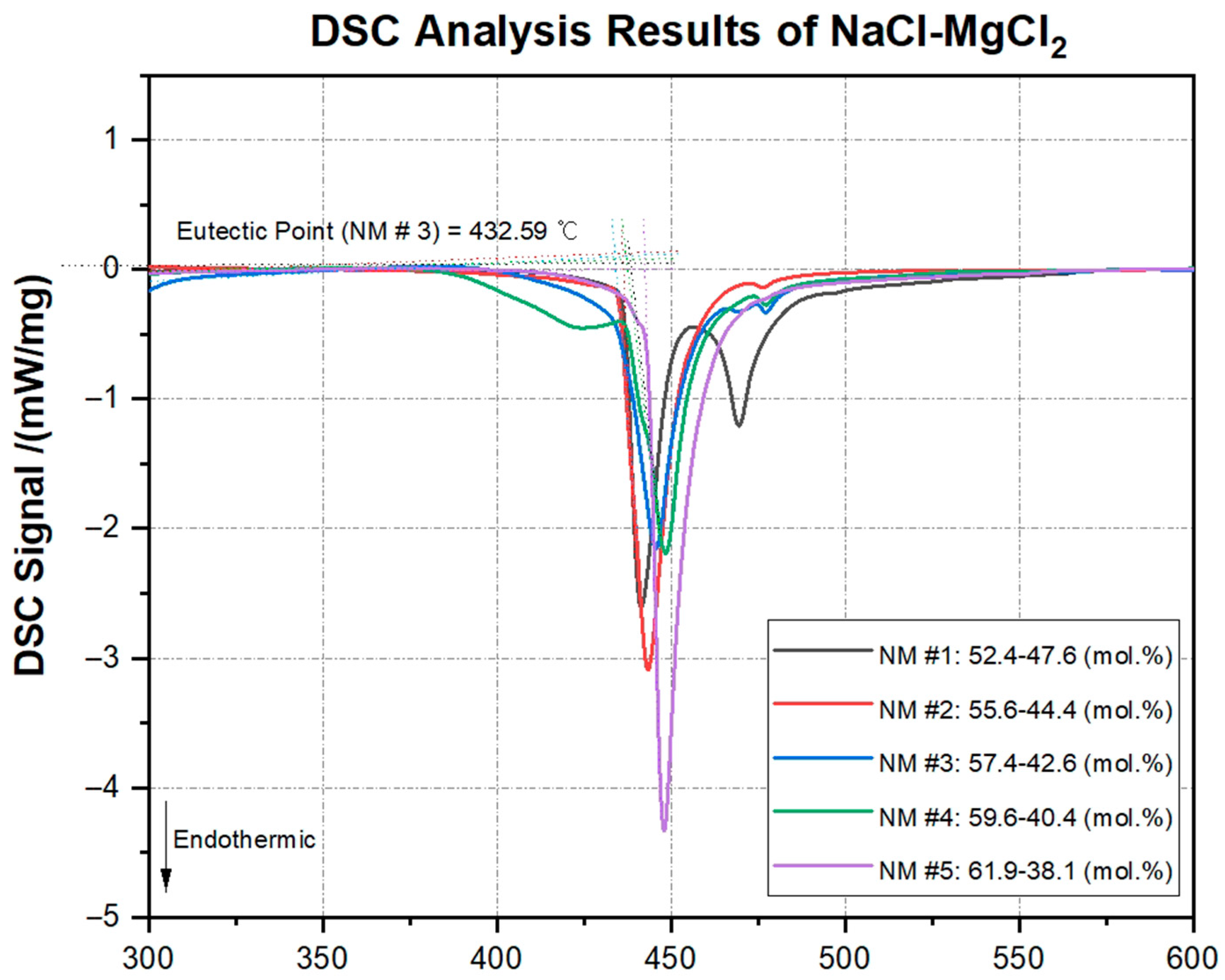
The NaCl-MgCl2 system was analyzed across multiple compositions. The measured melting points consistently show minor deviations from the predicted values. The predicted eutectic composition of NaCl-MgCl2 with a mole fraction of 57.9–42.1 (mol.%) has a predicted melting point of 459.13 °C, whereas the eutectic point measured in this experiment was 432.59 °C, 26.54 °C lower. This pattern was similarly observed in the other four compositions. Considering that all measured compositions showed differences of 25.57 °C to 29.79 °C from the predicted melting points, it can be concluded that the melting points near the eutectic composition of NaCl-MgCl2 are significantly lower than those predicted by the CALPHAD method. This finding is supported by the results obtained in this study.
Additionally, the composition with 52.4–47.6 (mol.%) clearly shows a secondary peak, indicating that it deviates from the eutectic composition. The DSC signals measured for other compositions show slight oscillations toward the end rather than secondary peaks. This phenomenon occurs because the salt samples were prepared by grinding them in a mortar, leading to some portions of the salt not melting uniformly in the crucible. The 61.9–38.1 (mol.%) salt composition produced the clearest DSC curve because it melted uniformly when heated in the equipment. Nevertheless, since distinct endothermic peaks were generated in all DSC curves, all measured samples can be considered chemically balanced.
Figure 6 presents the DSC signal curves of NaCl-MgCl
2 mixtures for all compositions whose melting points were measured in this experiment. The comparative analysis results of the predicted and measured melting points for NaCl-MgCl
2 are summarized in
Table 8.
3.1.2. Melting Point of NaCl-KCl-MgCl2
The NaCl-KCl-MgCl
2 mixtures were compared with data from previous studies by Villada et al. and Xu et al., as well as with melting points predicted by the CALPHAD method and those measured in this study [
6,
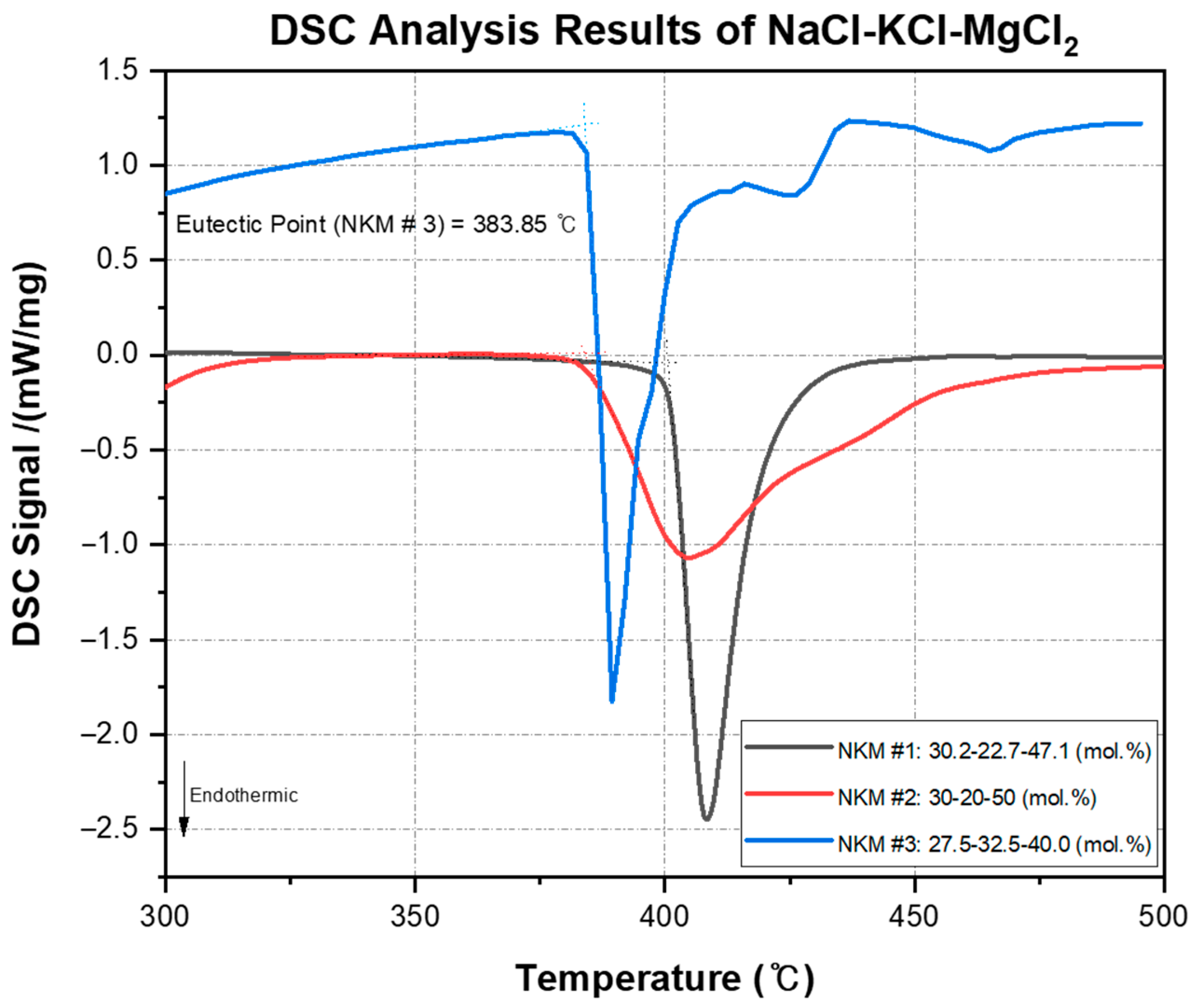
7]. Three compositions were examined: (1) 30.2–22.7–47.1 (mol.%), (2) 30–20–50 (mol.%), and (3) 27.5–32.5–40 (mol.%). The expected melting points for these compositions were 385.4 °C, 396 °C, and 383 °C, respectively. In contrast, the reported melting points from the previous studies were 388.55 °C, 390.5 °C, and 404.9 °C. In this study, the experimentally measured melting points were found to be 399 °C, 384.45 °C, and 383.85 °C, respectively.
For the 30.2–22.7–47.1 (mol.%) mixture, although there was little difference between the expected melting point (385.4 °C) and the reported melting point (388.55 °C), the experimentally measured melting point (399 °C) showed a significant discrepancy. Several factors could account for this difference. First, variations in experimental conditions, such as DSC equipment calibration or sample preparation, can influence the results. Second, differences in the purity of the materials can impact melting points, with higher purity components typically resulting in values closer to predictions, while impurities can cause deviations. The observed discrepancy in this mixture may be related to the fact that it contained the lowest amount of KCl, the component with the highest purity among the samples, leading to greater deviation from the predicted melting point. Third, the phase equilibrium of the NaCl-KCl-MgCl2 ternary mixture can exhibit very complex behavior due to interactions between the different components. Particularly, slight variations in the molar fractions of each chloride in the mixture can result in non-linear changes in ionic interactions and phase transitions. These interactions alter the thermodynamic properties of the mixture, leading to discrepancies between the predicted and experimentally measured melting points.
In contrast, the 27.5–32.5–40 (mol.%) mixture’s measured melting point (383.85 °C) closely aligned with the predicted value (383 °C), indicating better agreement with theory. This suggests that this composition behaves more stably as a eutectic mixture, with lower sensitivity to compositional changes.
Figure 7 illustrates the DSC analysis results of NaCl-KCl-MgCl
2 mixtures for all analyzed compositions.
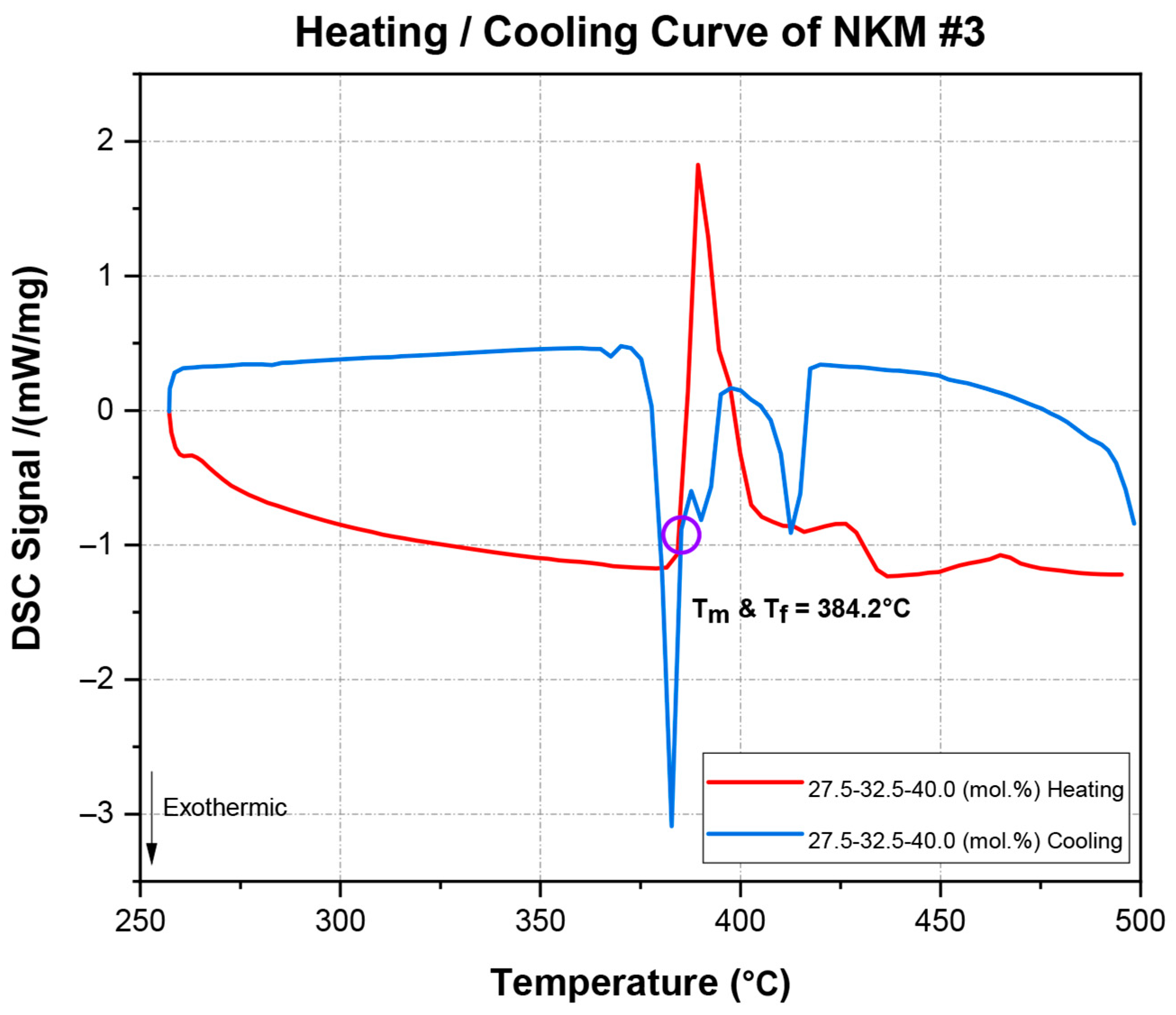
Figure 8 illustrates the heating and cooling curve of the NaCl-KCl-MgCl
2 (27.5–32.5–40.0 mol.%) mixture, as determined by DSC. The red curve corresponds to the heating process, while the blue curve represents the cooling process. Notably, both the melting and freezing points are observed to coincide precisely at 384.2 °C (T
m and T
f), indicating that the material transitions between solid and liquid phases without any observable supercooling or hysteresis during the thermal cycle. A summary of the comparative melting point analysis data for NaCl-KCl-MgCl
2 is provided in
Table 9.
3.1.3. Melting Point of NaCl-KCl-ZnCl2
For NaCl-KCl-ZnCl
2, the study investigated three compositions measured by Xu et al. and two new compositions that had not been measured previously: (1) 13.8–41.9–44.3 (mol.%), (2) 18.6–21.9–59.5 (mol.%), (3) 13.4–33.7–52.9 (mol.%), (4) 16.2–28.2–55.6 (mol.%), and (5) 13.8–38.0–48.2 (mol.%). While there were slight differences between the predicted melting points and the experimentally measured melting points for these compositions, they closely matched the melting points measured in the study by Xu et al. These results indicate that while the CALPHAD method can provide a rough estimate of melting points, its accuracy is significantly limited, and the reliability of predicting the trend of melting point changes with compositional variation bears substantial error.
Figure 9 shows the heating and cooling curves for the five measured compositions of NaCl-KCl-ZnCl
2. The measured data are summarized in
Table 10.
It is interesting to observe that the heating and cooling curves in
Figure 9 show the temperature difference between the melting points and freezing points ranging from a minimum of 27.86 °C to a maximum of 39.57 °C. The previously explained
Figure 8 demonstrates a consistent thermal transition for NaCl-KCl-MgCl
2, where the melting and freezing points are identical at 384.2 °C, indicating no supercooling. This reflects stable and predictable phase behavior, with the heating and cooling curves overlapping perfectly.
In contrast, in
Figure 9, NaCl-KCl-ZnCl
2 reveals significant supercooling. The average melting point (T
m) is recorded at 206 °C, while the average freezing point (T
f) is notably lower at 172 °C. This disparity is primarily attributed to the phenomenon of supercooling, where a substance remains in its liquid state even below its freezing point.
One of the main causes of supercooling is the delay in nucleation, which is closely related to the cooling rate. During the phase transition process, nucleation is essential for the substance to convert to solid state. Rapid cooling rates can prevent the formation of stable nuclei, leading to a greater degree of supercooling as the liquid maintains its state below the freezing point. Conversely, slower cooling rates may allow sufficient time for nucleation to occur, thereby reducing supercooling. For the NaCl-KCl-ZnCl2 mixtures, such delays in nucleation due to rapid cooling lead to supercooling, thereby lowering the observed freezing point. The supercooling effect can also be influenced by the uniformity of the sample. However, in this case, the cooling curves for NaCl-KCl-ZnCl2 show a single peak, suggesting that sample non-uniformity was not a significant factor in supercooling for these mixtures.
In conclusion, the temperature difference between the melting points and freezing points of NaCl-KCl-ZnCl2 mixtures is mainly due to supercooling, influenced by factors such as nucleation delay caused by rapid cooling rates. In this experiment, a cooling rate of 5 K/min was sufficient for uniform cooling of NaCl-KCl-MgCl2. However, for NaCl-KCl-ZnCl2, discrepancies between the freezing and melting points were observed under the same cooling conditions, indicating the need for additional experiments under various cooling conditions.
The melting point data obtained in this study not only support compositional optimization of HTFs, but also contribute to the development of operating strategies for high-temperature molten salt energy systems. As noted in the introduction, inaccurate melting point estimation may lead to solidification-related incidents such as local overheating, pipe rupture, or salt leakage during operation. Therefore, selecting compositions with lower and more stable melting behavior near the eutectic region can reduce these risks and enhance both thermal efficiency and operational safety.
3.2. Thermal Stability Evaluation
The thermal stability of salts is the most important indicator when evaluating their suitability as HTFs for HT-MSESs. Poor thermal stability can cause the salt to evaporate at high temperatures or chlorine gas to volatilize, resulting in significant weight loss. Such evaporation or gas volatilization can increase pressure within pipes or heat exchangers, raising the risk of damage to system components. Therefore, high thermal stability is a critical requirement for HTFs in HT-MSESs. Generally, thermal stability is assessed by verifying that the cumulative weight loss remains within 3–5% under high-temperature conditions.
During TGA measurements, experiments were conducted in an argon atmosphere with a flow rate of 50 mL/min and a heating rate of 5 K/min. To ensure accurate thermogravimetric measurements, Pt-Rh crucibles equipped with lids made of the same material were used. Due to the potential for equipment contamination caused by the high vaporization rates of certain salt mixtures, the temperature range for each sample was varied accordingly. The thermal stability results obtained by TGA analysis in this study are presented in
Figure 10 and
Table 11. The salts analyzed include the following: (1) NaCl-MgCl
2 (57.9–42.1 mol.%), (2) NaCl-KCl-MgCl
2 (30.2–22.7–47.1 mol.%), and (3) NaCl-KCl-ZnCl
2 (13.8–41.9–44.3 mol.%). For the NaCl-KCl-ZnCl
2 system, to assess the impact of moisture on thermal stability, samples prepared under ambient air conditions were compared with those produced inside a glovebox, where moisture and oxygen levels were maintained below 5 ppm.
The two NaCl-KCl-ZnCl2 samples exhibited weight loss below 200 °C, whereas NaCl-KCl-MgCl2 and NaCl-MgCl2 showed weight loss around 230 °C. This phenomenon is attributed to hydrolysis reactions caused by the highly hygroscopic nature of ZnCl2 and MgCl2. It was observed that hydrolysis reactions occur at lower temperatures in mixtures containing ZnCl2 compared to those containing MgCl2. This is because ZnCl2 exhibits higher hygroscopicity than MgCl2, allowing it to absorb moisture and undergo hydrolysis at lower temperatures. ZnCl2 has weaker ionic bonds and a larger ionic radius, facilitating interactions with water molecules. Additionally, the crystal structure of ZnCl2 is less stable, making it more susceptible to moisture absorption.
The NaCl-MgCl2 sample exhibited high thermal stability up to approximately 700 °C, with negligible weight loss below 5%. This indicates that the mixture maintains structural integrity without significant thermal decomposition or evaporation within the tested temperature range. Among the chloride salts examined, the NaCl-KCl-MgCl2 sample demonstrated the highest thermal stability, showing weight loss below 4% when heated up to 700 °C. This superior stability can be attributed to the optimized composition of the ternary mixture, resulting in stronger ionic bonds and reduced volatility.
The NaCl-KCl-ZnCl2 sample analyzed in a glovebox, minimizing moisture and atmospheric contamination, showed improved thermal stability compared to the air-exposed sample. However, noticeable weight loss still occurred starting around 500 °C, although less severely than the air-exposed sample. The controlled glovebox environment reduces but does not entirely eliminate decomposition of NaCl-KCl-ZnCl2. This indicates that exposure to moisture significantly accelerates the decomposition process, making NaCl-KCl-ZnCl2 considerably less stable at high temperatures compared to other salt candidates.
The NaCl-KCl-MgCl2 sample exhibited better thermal stability compared to the NaCl-MgCl2 sample. Although both contain hygroscopic MgCl2, and NaCl-KCl-MgCl2 even has a higher MgCl2 content, the ternary mixture displayed superior thermal stability. This can be explained by differences in ionic bonding strength between binary and ternary salts. The ternary salt can form stronger ionic interactions, stabilizing its overall structure and reducing the volatility of its constituents.
In conclusion, the NaCl-KCl-MgCl2 mixture exhibits the best thermal stability among the tested samples, making it the most suitable HTF candidate for HT-MSESs operating at high temperatures. Conversely, due to the high hygroscopicity and thermal instability of ZnCl2, the NaCl-KCl-ZnCl2 mixture was found unsuitable as an HTF for HT-MSESs operating above 600 °C. Its instability, particularly in high-humidity environments, would significantly increase the cost and effort required to maintain salt integrity during production, transportation, and system operation. These disadvantages render NaCl-KCl-ZnCl2 less competitive as an HTF candidate compared to NaCl-MgCl2 and NaCl-KCl-MgCl2.
4. Summary and Conclusions
The primary objective of this study was to analyze the thermal stability and melting behavior of chloride-based molten salts in order to identify suitable HTF candidates for HT-MSESs. While chloride salts have attracted attention due to their low melting points and minimal corrosivity toward stainless steel, experimental data on their thermophysical properties remain limited. This study addresses this gap by experimentally assessing key thermal characteristics relevant to HT-MSES design and operation.
Three chloride mixtures—NaCl-MgCl2, NaCl-KCl-MgCl2, and NaCl-KCl-ZnCl2—were investigated using DSC and TGA. In the NaCl-MgCl2 system, the measured melting points were consistently 25–30 °C lower than those predicted by the computational method, indicating the method’s limitation in accurately estimating melting temperatures near eutectic regions. Among the tested compositions, NaCl-KCl-MgCl2 exhibited the most promising properties, with a melting point around 384 °C and stable behavior up to 700 °C. NaCl–MgCl2 also showed favorable thermal stability, albeit with slightly higher melting points. Although NaCl-KCl-ZnCl2 demonstrated the lowest melting point, its high hygroscopicity and thermal instability above 500 °C rendered it unsuitable for high-temperature operation. These trends were confirmed under both ambient and moisture-controlled conditions.
DSC measurements showed strong reproducibility and agreement with previous studies. Thermal stability tests revealed that ZnCl2-containing salts began decomposing above 500 °C, while MgCl2-based salts—NaCl-MgCl2 and NaCl-KCl-MgCl2—maintained mass loss below 5% up to 700 °C. Notably, both MgCl2-based salts remained stable under ambient conditions, and their performance is expected to improve further under enhanced moisture control.
Compared with FLiBe, a well-studied fluoride-based molten salt, the chloride salts exhibited lower sensitivity to compositional variation in melting point. NaCl-MgCl2 and NaCl-KCl-MgCl2 also maintained high thermal stability up to 700 °C, supporting their viability as reliable HTFs.
In conclusion, this study demonstrates the potential of chloride-based molten salts for HT-MSESs and identifies NaCl-KCl-MgCl2 as the most promising candidate due to its optimal combination of low melting point and thermal stability. Future work should focus on evaluating the long-term performance of this mixture under operational conditions and exploring alternative chloride formulations with enhanced thermal characteristics.