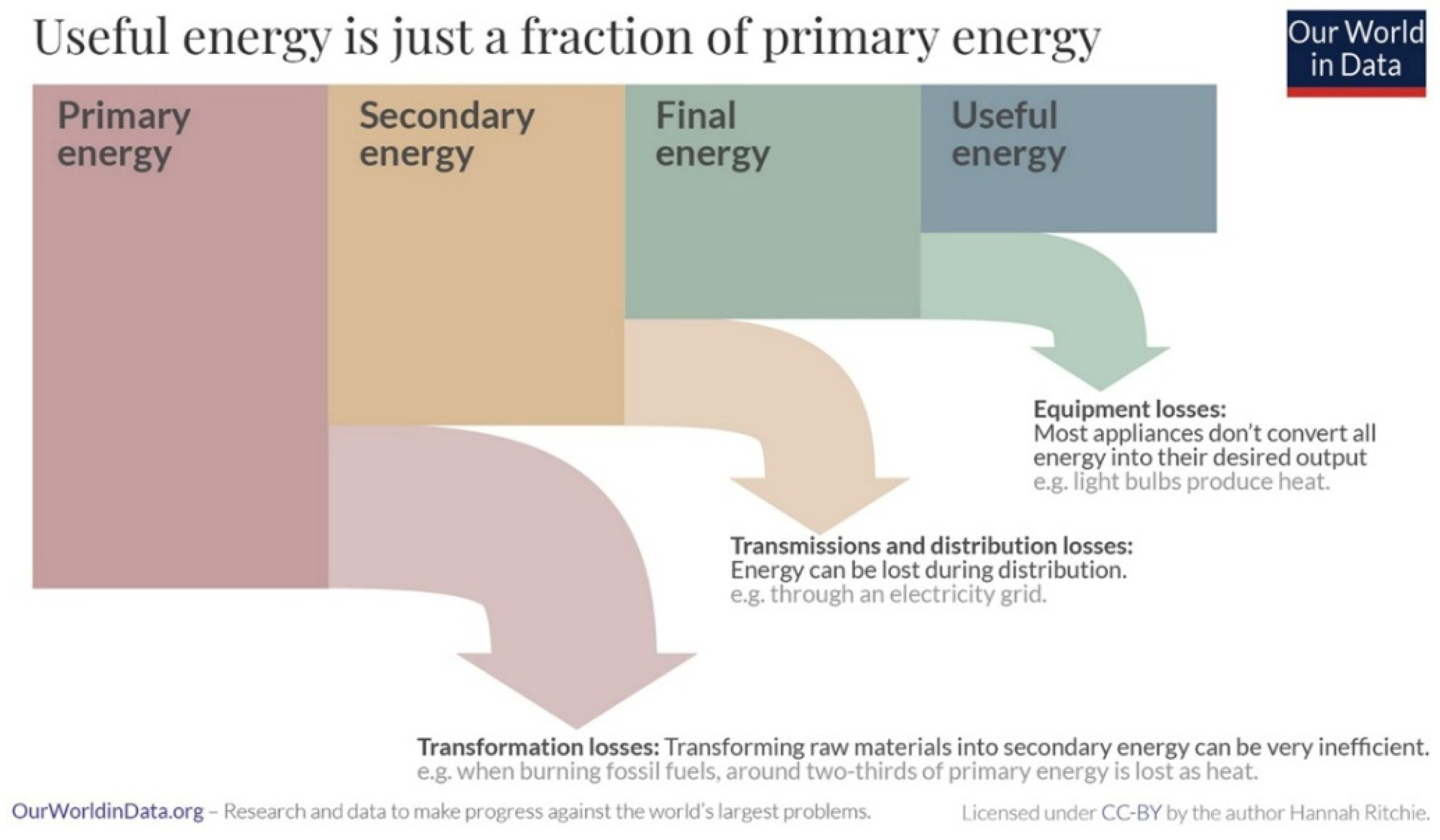
The use of various alternative, potentially low-carbon, transportation energy sources requires careful consideration of several factors, as have been discussed in detail by Dunlap [
15]. Cost, safety and environmental sustainability are important for all modes of transportation. Energy and power density are also important, although rail transportation can avoid the use of portable stored energy through the application of external electrical connections. Here we review the properties of three promising portable energy storage technologies for transportation use, batteries, hydrogen and electrofuels. It is interesting to begin with a comparison of the energy density and power density for these technologies.
Figure 12 shows a Ragone diagram for some common energy storage devices. In addition to the storage technologies mentioned above, other possibilities, such as supercapacitors, flywheels and compressed air, exist but are either in the early stages of development or are best suited for use as an additional energy source for hybrid vehicles. In most cases, other than some rail transportation systems, transportation energy sources must be portable. It is, therefore, essential that energy sources should have sufficient energy density. This is particularly important for road transportation, where there are limits to overall vehicle weight and size. In this case, energy density can be related to vehicle characteristics such as range. As well, transportation applications require energy sources that provide a suitable power level to ensure acceptable vehicle performance, such as acceleration and maximum speed.
3.1. Batteries
Battery electric vehicles have existed since the late 19th century. In fact, some of the first motorized road vehicles utilized batteries connected to an electric motor. Battery electric vehicles comprised the majority of road vehicles in many locations from the 1890s until the 1920s, when advances in internal combustion engines, as well as the need to travel longer distances, made gasoline-powered vehicles the dominant transportation technology. In recent years, battery electric vehicles have reappeared as a result of the transition away from fossil fuel use. These vehicles are either hybrid battery–gasoline or battery electric vehicles and use, almost exclusively, lithium-ion batteries. The increase in battery electric vehicle sales (including plug-in hybrids) is illustrated in
Figure 13, where, for 2023, it is seen that there were about 13 million plug-in electric road vehicles sold. This is in comparison to the total number of passenger vehicles sold worldwide of about 77 million. It is, however, seen in the figure that electric vehicle sales are largely concentrated in China and, to a lesser extent in Europe, with significantly fewer vehicles sold in North America and elsewhere.
In recent years, batteries have also been utilized for other modes of transportation. In the rail sector, the most common approach has been the use of traditional diesel technology in conjunction with battery power to produce a hybrid locomotive [
18]. In this case, fuel efficiency is improved through the use of energy recovery by regenerative braking, analogous to conventional hybrid gasoline–battery road vehicles.
In the case of maritime transportation, battery-operated ferries for crossings up to about 30 min (one way) have been successfully implemented in China, Norway and the United States. Such ferry routes typically provide sufficient time (e.g., 10 to 20 min) during loading to recharge the batteries for the subsequent crossing (see [
19,
20]).
Experimental aircraft have also been produced which utilize battery power. The first commercially available battery-powered aircraft became available in 2020. This was the Velos Electro produced by Pipistrel in Slovenia. The Velos Electro has a total permitted payload of 172 kg and a range of about 200 km. A prototype small battery electric commuter aircraft that can carry two pilots and nine passengers on flights up to 815 km is under development by the aviation company Eviation. Based on the low specific energy and power associated with batteries, as shown in
Figure 12, other options, as discussed below, may find more success for air transportation.
3.2. Hydrogen
Hydrogen has been promoted as a viable energy storage medium to replace fossil fuels. At present, about 95% of all hydrogen is produced by steam reforming fossil fuel methane. While the use of hydrogen as a fuel does not emit greenhouse gases, carbon dioxide released during steam reforming must be sequestered in order to make hydrogen a carbon-neutral fuel. More appropriately, hydrogen can be produced by a number of methods that do not utilize fossil fuel precursors. It is convenient to “color” code hydrogen according to its method of production.
Table 6 summarizes the definitions of the different colors of hydrogen in terms of their method of preparation. Green hydrogen, produced by electrolysis of water using renewable energy, is the most straightforward approach to the production of hydrogen without greenhouse gas emissions. Yellow hydrogen, produced using solar electricity, is a subset of green hydrogen. Electrolysis, in which hydrogen is produced from electricity by the process
using an electrolyzer, can be anywhere from about 62% to 82% efficient. The most common electrolyzers currently in use are the proton exchange membrane (PEM) electrolyzer and the alkaline electrolyzer.
The use of energy from nuclear reactors is another potentially viable approach to the production of low-carbon hydrogen. Pink hydrogen follows from the method of production for green hydrogen by using electricity from a nuclear reactor to produce hydrogen by electrolysis. Red hydrogen utilizes heat from a high-temperature nuclear reactor (see [
21]) to produce hydrogen using thermochemical reactions in the presence of a catalyst. This approach is in the fairly early stages of development (see [
22]). A more straightforward approach to using nuclear thermal energy for hydrogen production is high-temperature electrolysis [
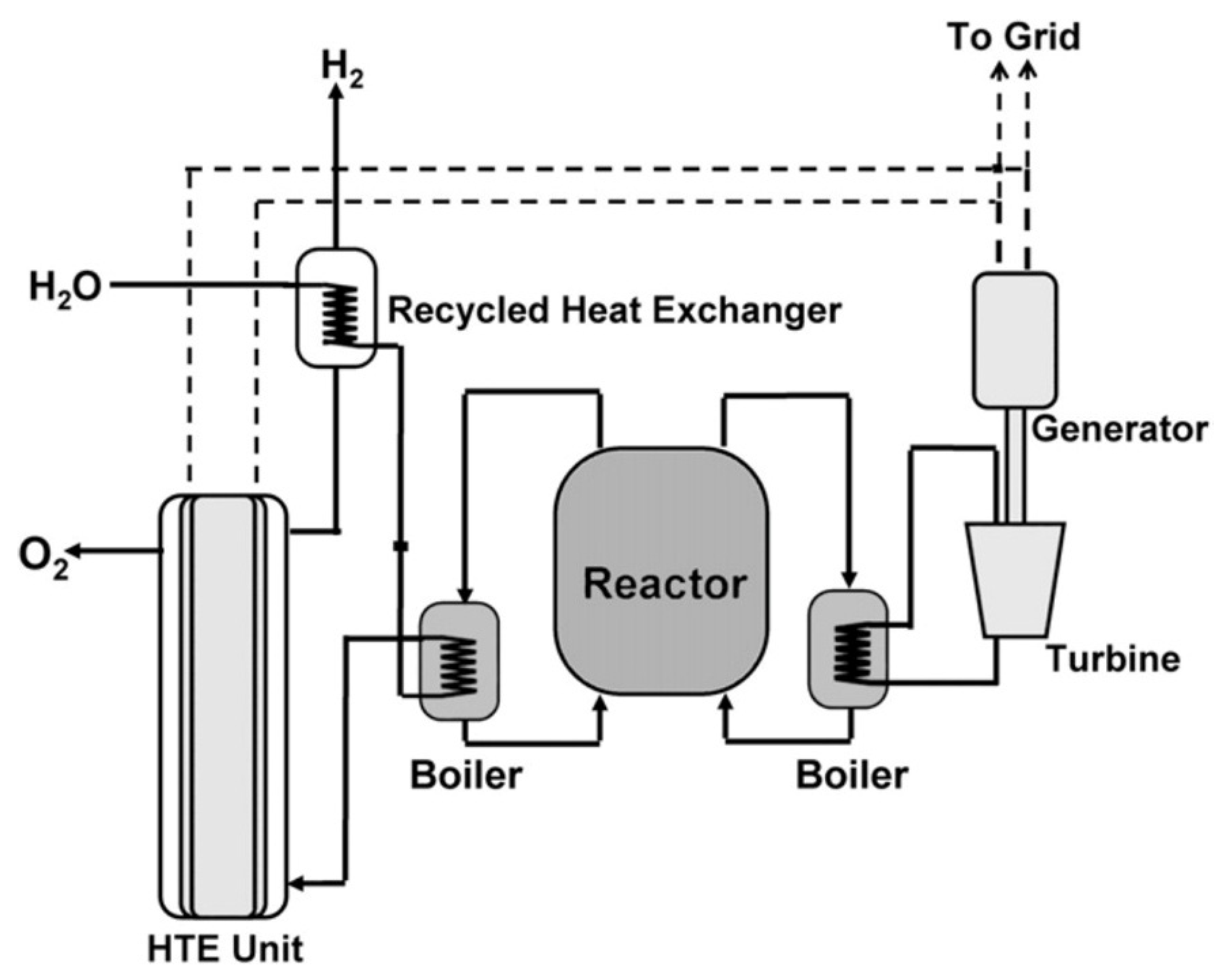
23]. A basic schematic for such a system is illustrated in
Figure 14. In this case, the electrical efficiency of electrolysis is improved through the use of surplus thermal energy from the reactor.
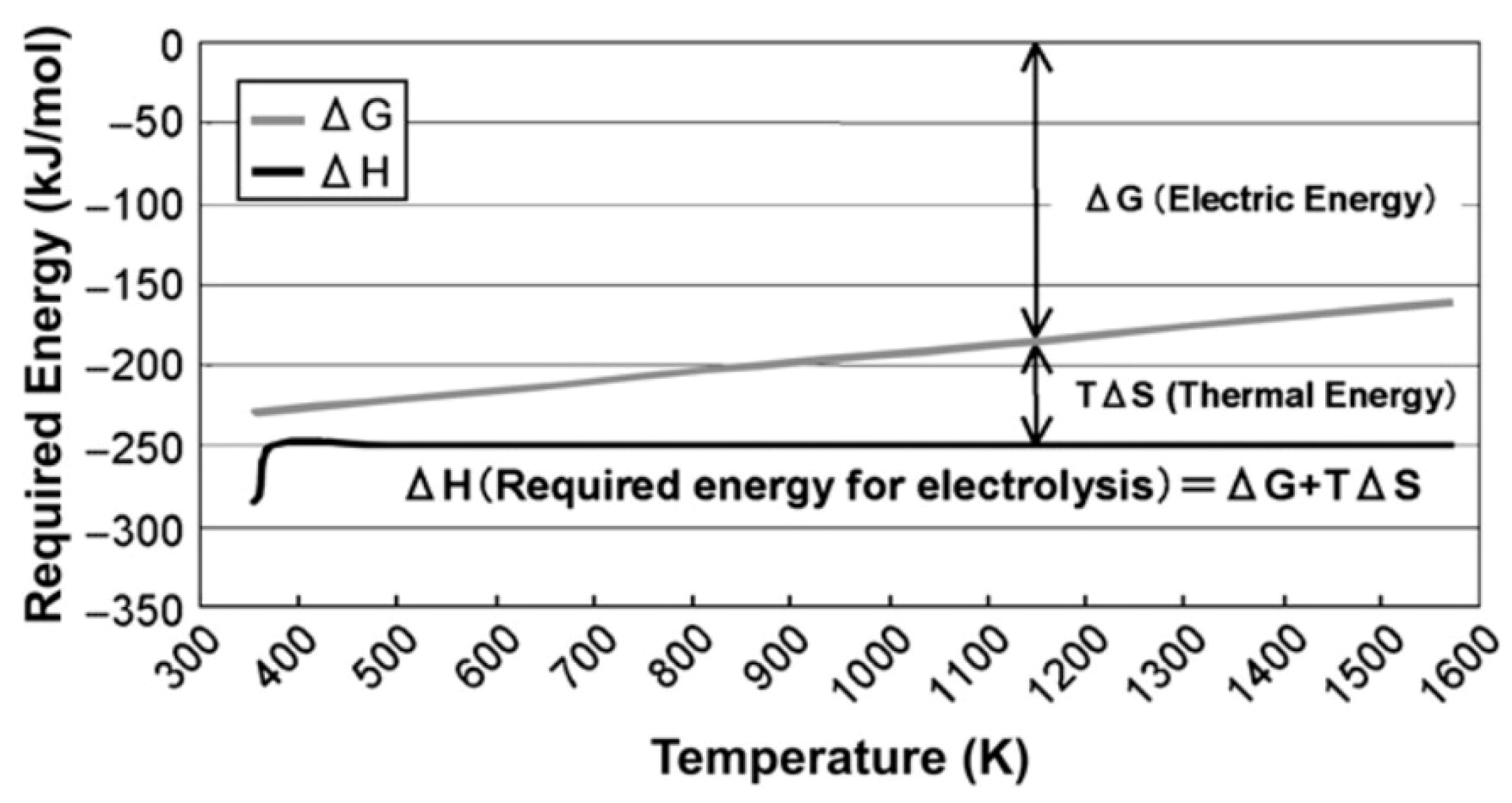
Figure 15 illustrates the typical reduction in required electrical energy for electrolysis that results from the addition of thermal energy. Dunlap [
21] has recently reviewed the properties of Generation IV nuclear reactors that are under development. The typical output temperature for the six classes of Generation IV reactors is summarized in
Table 7. Very high-temperature reactors and gas-cooled fast reactors are both cooled with helium gas and offer the highest available output temperatures.
Figure 15 shows that utilizing the surplus thermal energy from a very high-temperature reactor at 1000 °C reduces the electrical input for electrolysis by about 30%.
Table 8 provides a summary of some of the properties of hydrogen that are relevant to its use as a fuel. Major difficulties in the use of hydrogen as a fuel result from its very low density and its low boiling temperature. Practical hydrogen transport and storage, particularly for transportation applications, involves liquefaction or storage as a high-pressure gas and such approaches must be considered in the development of appropriate infrastructure and in the assessment of overall energy efficiencies. These factors are discussed in detail in
Section 4, below. Storing the energy content of hydrogen utilizing chemical reactions is considered in
Section 3.3 below. Techniques that involve hydrogen adsorption in a solid may become important in the future. As shown in the table, the energy density of hydrogen as a high-pressure gas or as a liquid is still substantially less than that of conventional fossil fuels, e.g., gasoline has an energy density of about 35 MJ/L compared to hydrogen at 10 MJ/L in the liquid state and 5.7 MJ/L as a gas at 70 MPa.
Hydrogen may be utilized as a fuel for transportation (or other applications) by either combustion to produce heat or in a fuel cell to produce electricity. In either case, the net reaction is the oxidation of hydrogen to produce water,
Experimental and commercially available road vehicles that utilize hydrogen as fuel have been produced. Some early examples of hydrogen-fueled road vehicles have used production internal combustion gasoline engine passenger automobiles with engines that have been modified to use dual fuels, hydrogen and gasoline. These include the 12-cylinder BMW Hydrogen 7 and the rotary engine Mazda RX-8 Hydrogen XE. More recently, hydrogen has been used in hybrid vehicles that use fuel cells in conjunction with lithium-ion batteries. Recent commercially available fuel cell vehicles include the Honda Clarity, Hyundai Nexo and Toyota Mirai. Other automobile manufacturers that are developing fuel cell vehicles that are expected to be available in the near future include Audi, BMW and Mercedes Benz. Using the Toyota Mirai as an example, it is possible to estimate the overall efficiency of hydrogen fuel cell vehicles. For comparison, we look at a similar size/weight gasoline vehicle (e.g., Toyota Camry) which has an overall EPA fuel consumption of 8.1 L/100 km. Using the energy content of gasoline (34.8 MJ/L) and an estimated internal combustion engine efficiency of 20% (see
Section 4.4), we obtain an energy at the wheels of 0.56 MJ/km. The Mirai has a hydrogen fuel capacity of 5.0 kg and a manufacturer’s estimated range of 647 km. The overall efficiency of the Mirai can be expressed as
where
H is the energy content per kg of the fuel. It is common to express the efficiency of a fuel cell using the lower heating value of the fuel [
24], which, in the case of hydrogen, is 120 MJ/kg. This gives the LHV efficiency as 60% and the energy lost as thermal energy of the water by-product is ignored. In the present case, it would be more appropriate to include the lost thermal energy by considering the HHV efficiency [
24], where
H = 142 MJ/kg, in order to analyze the overall renewable electricity to wheel efficiency. This gives the HHV efficiency as 52%. While this example provides information concerning a specific production hydrogen fuel cell vehicle, a more general discussion of efficiency is provided in
Section 4.4.
Hydrogen fuel cells have also been used for hybrid fuel cell-battery rail transportation. In the early 2000s, the Japan East Railway Company developed an experimental hydrogen fuel cell-lithium-ion battery railcar [
15]. In 2009, the BNSF Railway Co. and Vehicle Projects Inc. in the United States unveiled an experimental hydrogen fuel cell locomotive that was to be used to evaluate the viability of fuel cell locomotives as a possible replacement for diesel locomotives. A more significant development occurred in 2018 when two hydrogen fuel cell trains became operational on a 100 km section of rail line between Buxtehude and Cuxhaven, Germany. The trains were Coradia iLint trains manufactured by the French multinational corporation Alstom SA [
25].
Around 2000, a small experimental hydrogen fuel cell boat, the 12 m long
Hydra, was tested in Leipzig, Germany [
26]. Work to consider the viability of hydrogen fuel cells for the propulsion of larger maritime vessels is still in the very early stages.
While the high specific energy of hydrogen (142 MJ/kg) makes it an attractive fuel for air transportation, the low density of hydrogen, even in the compressed or liquefied states, makes its application less than straightforward. In fact, as noted above, the energy and power requirements for aircraft make the implementation of alternative low-carbon technologies particularly challenging. An inspection of
Figure 12 shows that the specific energy that is available from both hydrogen internal combustion engines and hydrogen fuel cells is similar. However, as the figure indicates, available specific power is typically greater for hydrogen internal combustion engines than for hydrogen fuel cells.
The most notable aircraft to utilize a hydrogen internal combustion engine was the Tupolev Tu-155 constructed by Aviakor in Samara in the former Soviet Union. The Tu-154 was a modified version of the commercial Tu-154 aircraft that was adapted to use alternative aviation fuels in one of its three turbofan engines. It was first flown in 1988, and its initial test flights included the use of liquid hydrogen as a fuel. It was operational until 1991, although later flights concentrated on the use of liquefied natural gas as an alternative fuel.
The first hydrogen fuel cell aircraft that was capable of carrying a person was the Antares DLR-H2 [
27]. A larger fuel cell aircraft was tested in 2020. This was the six-seat ZeroAvia HyFlyer I [
28]. Both of these aircraft were fuel cell-lithium-ion battery hybrids. A careful analysis of power sources and power requirements for the ZeroAvia indicates that under heavy load conditions, such as during take-off, power is provided primarily by the batteries [
29]. While hydrogen aircraft have not progressed beyond the experimental stage, Airbus has recently indicated an interest in developing this technology for commercial aircraft [
30].
3.3. Electrofuels
Electrofuels, also called e-fuels, are produced from hydrogen and, if the hydrogen has been obtained using low-carbon technology, then the e-fuel can also be produced in a manner that does not contribute to greenhouse gases. Some possible electrofuels and their basic properties are summarized in
Table 9. The method of preparation of these, along with a description of their use, follows.
3.3.1. Methane
The electrofuel methane (e-methane) can be produced from low-carbon hydrogen by the Sabatier reaction in the presence of a catalyst such as alumina or nickel at a temperature of 300 °C to 400 °C,
This process is typically about 92% efficient. Energy may be recovered from the e-methane either by combustion or in the use of certain types of fuel cells, such as solid oxide fuel cells. In either case, the overall reaction is
The process is carbon-neutral provided the hydrogen production does not produce greenhouse gases.
The properties of e-methane are very similar to those of natural gas, which is typically about 90% methane. Extensive infrastructure exists for the storage, transport and utilization of natural gas. As methane is a gas at room temperature, it can be compressed to reduce its volume or it can be liquified, as is completed for the production of liquified natural gas (LNG).
There has been some use of natural gas as a fuel for road transportation and a few such vehicles have been commercially available. Most notably, the Honda Civic GX, available in the United States from 1998 to 2015, utilized a 4-cylinder gasoline engine that was modified to run on compressed natural gas. Several natural gas vehicles have been available in Brazil where they have been offered as multi-fuel vehicles capable of running on compressed natural gas as well as ethanol and ethanol–gasoline mixtures.
Methane (or natural gas) is a suitable fuel for use in a dual-fuel diesel locomotive engine. Such development is in its early stages [
31].
Marine transportation has, perhaps, made the greatest use of methane as a fuel. In recent years, tankers, cargo ships and cruise ships have utilized liquified natural gas as an engine fuel [
32,
33]. Initial use of LNG as a fuel was implemented on LNG tankers, where LNG boil-off was utilized for ship propulsion. More recently, ships with engines designed for LNG or dual-fuel LNG-marine diesel have been in use. In particular, Carnival Corporation has been proactive in the construction of new ships that use LNG as fuel [
34]. Natural gas (or methane) produces less CO
2 per unit energy generated than heavier fossil fuels such as diesel. However, it is important to guard against the release of methane into the atmosphere. Since methane is a much more effective greenhouse gas than carbon dioxide, even small methane emissions can negate the positive effects of lower CO
2 emissions. Such methane release can result from leaks in the fuel system but can also be the result of methane slips, where unburned methane escapes through the engine exhaust.
The use of methane or natural gas as an aviation fuel has been minimal. The Tupolev Tu-155, which has been used as a testbed for a hydrogen-fueled jet engine, has also been used to study the use of liquefied natural gas as an aviation fuel.
3.3.2. Ammonia
Ammonia may be prepared from hydrogen by the Haber–Bosch process,
This reaction is most efficient at a temperature of around 450 °C and a pressure of 10 MPa. It is the only fuel, other than hydrogen, that can undergo oxidation, either by combustion or in a fuel cell, and not release carbon dioxide. The oxidation process is
In addition to the lack of greenhouse gas emissions, ammonia is also relatively easy to liquefy due to its fairly high boiling temperature. On the negative side, however, ammonia is highly toxic and corrosive, so that leaks to the atmosphere must be strictly avoided and equipment must be designed from suitably corrosion-resistant materials. From a practical standpoint, the construction of a suitable ammonia internal combustion engine must deal with the relatively low flammability of the fuel. For this reason, it is important to include a combustion promoter in the fuel mix. This can be completed by including a second fuel supply or onboard cracking on the vehicle with appropriate mixing of the fuel delivered to the engine. Onboard cracking consists of the inclusion of an appropriate component in the fuel system that breaks down some of the ammonia to produce hydrogen (and nitrogen) by the reaction
The hydrogen is then mixed with the ammonia to act as a combustion promoter. Details of recent developments in ammonia internal combustion engines have been reviewed by Kobayashi et al. [
35] and Qi et al. [
36].
In 2023, the Chinese vehicle manufacturer Guangzhou Automobile Group Co. (GAC), of which Toyota owns 50%, announced the development of an ammonia-fueled passenger vehicle. The vehicle uses a four-cylinder ammonia engine that produces 120 kW [
37]. Ammonia has also been considered as fuel for heavy-duty trucks. In 2019, the Canadian company Hydrofuel Inc announced that it would be undertaking a project to convert diesel generators and heavy-duty truck engines to operate on ammonia fuel [
38]. More recently, in 2023, the United States company Amogy Inc. reported that they had constructed and tested a heavy-duty semi-truck powered by a 300 kW ammonia-fueled engine [
39].
Perhaps the most suitable use of ammonia as a fuel has been in the maritime transportation sector. In this case, the cost and space required for additional equipment, as well as a suitable distribution network, may be less problematic than for road vehicles. A basic diagram of a marine ammonia engine with an onboard cracking system is illustrated in
Figure 16. For such applications, ammonia engines may be either reciprocating (i.e., piston) engines or turbines. Recent examples of ammonia engines for marine applications include a two-stroke ammonia-fueled engine being developed by MAN Energy Solutions [
40] and a four-stroke ammonia engine being developed by the Wärtsilä Corporation [
41].
Reformed ammonia fuel cells utilize an onboard cracking system to convert ammonia fuel to hydrogen for use in a conventional hydrogen fuel cell. While this approach is fairly straightforward, the hydrogen that is produced by the cracking process contains some quantity of residual ammonia and this is inconsistent with the operational fuel requirements of hydrogen fuel cells.
Direct ammonia fuel cells, where ammonia fuel is fed directly into the fuel cell, are in the fairly early stages of development. Progress in this area has been reviewed by Afif et al. [
43] and Jeerh et al. [
44]. Amogy Inc. has recently entered into an agreement with the Canadian fuel cell manufacturer Ballard Power Systems to develop a 200 kW ammonia fuel cell system for marine applications.
3.3.3. Methanol
As noted in
Table 9, methanol is the only simple electrofuel that is a liquid at room temperature and atmospheric pressure. It is also a fairly direct substitute for gasoline in spark ignition internal combustion engines. Although methanol’s energy density is only about half that of gasoline, methanol engines have the advantage of higher thermodynamic efficiency than gasoline engines. As well, methanol is more biodegradable than gasoline and much of the current fossil fuel infrastructure could be readily adapted to methanol.
Methanol is produced from hydrogen at elevated temperatures in the presence of a catalyst by the reaction
Methanol can be used to produce heat by combustion or electricity by means of a methanol fuel cell by the reaction
Thus, if hydrogen is produced in a carbon-free manner, then the use of methanol is carbon-neutral.
The earliest use of methanol as a fuel in road vehicles dates from the 1980s. Experimental studies were conducted in a number of countries including Canada, Norway, Sweden, the United Kingdon and the United States. In the early 2010s, extensive development of methanol road vehicles, primarily taxis, began in China. By around 2019, there were more than 10,000 methanol vehicles operating in China. This development was aimed primarily at the use of coal resources to produce methanol and not with the aim of reducing greenhouse gas emissions.
In 2016, a methanol program was initiated in Iceland. Methanol is produced at the George Olah Renewable Methanol Plant in Svartsengi Grindavik from electrolytic hydrogen generated using renewable energy (geothermal or hydroelectric) and carbon dioxide, which is a by-product of the geothermal energy infrastructure. Vehicles are sourced from China and are based on the methanol road vehicles developed there as taxis. A small tank of gasoline is incorporated in the vehicle as an aid to starting in cold weather.
Methanol (although not low-carbon methanol) has been used as a fuel for the internal combustion engines in the ferry
Stena Germanica. This is a 240 m ferry that services the route from Kiel, Germany to Gothenburg, Sweden. The original marine diesel engines were converted to dual-fuel use in 2015. The ferry is capable of running on either conventional marine gas oil or methanol. As of the early 2020s, several major ocean transport companies including A. P. Moller-Maersk, CMA CGM, Cosco Shipping Lines and Cargill Ocean Transportation have been involved in the development of methanol maritime engines (see, e.g., [
45]).
Methanol fuel cells may be either direct methanol fuel cells or reformed methanol fuel cells (often referred to as indirect methanol fuel cells). In the latter case, a reformer converts methanol into hydrogen, which is then utilized in a conventional (typically proton exchange membrane) hydrogen fuel cell. The basic design of a reformed methanol fuel cell is illustrated in
Figure 17. A 2:1 mixture of methanol and water is input into the reformer and hydrogen is produced by the reaction
followed by
Reformed methanol fuel cells have been used in road vehicles. Mercedes-Benz has produced several experimental methanol fuel cell vehicles constructed on an A-Class platform. The most recent, the Necar 5, was produced in 2000 and utilizes a single stack reformed methanol fuel cell that produces 75 kW. The vehicle has a top speed of 150 km/h [
47]. German automobile manufacturer Gumpert Aiways Automobile produces a limited-edition methanol fuel cell vehicle named the Gumpert Nathalie [
48]. This vehicle is a fuel cell-battery hybrid that uses a 15 kW reformed methanol fuel cell in conjunction with a 60 kWh Li-ion battery. Four independent drive motors provide a total output of 400 kW to give a top speed of 300 km/h. The price is around USD 450,000.
3.3.4. Dimethyl Ether
Dimethyl ether is most easily prepared by the dehydration of methanol by the reaction
The energy content of dimethyl ether is recovered by the combustion process
A straightforward analysis based on Equations (9), (13) and (14) shows that if the hydrogen used to produce the methanol is carbon-free, then the use of dimethyl ether as a fuel is carbon-neutral.
Dimethyl ether, as shown in
Table 9, is a gas at room temperature and pressure. However, its boiling point at −24 °C means that it can be easily liquified. In fact, its characteristics are fairly similar to those of propane. Propane has a boiling temperature of –42.2 °C and can be liquified at room temperature under a pressure of about 1 MPa (i.e., 10 atm). Dimethyl ether can be liquified at room temperature under a pressure of about 0.5 MPa (5 atm). Thus, the extensive existing infrastructure that is used for the distribution of propane can also be largely utilized for dimethyl ether.
Dimethyl ether is a suitable replacement for diesel fuel in compression ignition internal combustion engines with only fairly minor engine modifications. Therefore, it has applications for both light-duty and heavy-duty road vehicles. At present, however, the development of dimethyl ether as a transportation fuel is in its very early stages. Volvo developed a dimethyl ether-fueled heavy-duty vehicle engine and constructed several prototype vehicles in the early 2010s. These have been tested on roadways in Europe and in the laboratory in the United States as part of a collaborative project involving Oak Ridge National Laboratory, Volvo North America and Pennsylvania State University [
49]. Further road tests of dimethyl ether-powered trucks have been conducted in Canada and the United States [
50,
51]. Thus far, results have been encouraging.