Abstract
The aim of the study was to evaluate the chemical composition and biogas potential of selected ruderal and expansive plant species: Heracleum sosnowskyi, Aegopodium podagraria, Chaerophyllum bulbosum, Acer negundo, and Urtica dioica. Plant material was collected from a 19th-century park in the village of Niegolewo (Greater Poland Voivodship) and analyzed for cellulose, lignin, hemicellulose, extractives, and ash content before and after methane fermentation. Fermentation followed DIN 38 414-S8, and chemical analyses used standardized methods (TAPPI, Seifert, and DIN). Statistical analyses included ANOVA, CVA, and hierarchical clustering. The highest biogas yield was obtained from A. podagraria, which is associated with low lignin and high hemicellulose degradation. The results confirm the potential of ruderal biomass as a diverse source for biogas production.
1. Introduction
Intensive economic development and the resulting transformations of the natural environment have a significant, and often adverse, impact on both habitats and the plant species growing within them. These changes frequently lead to the disturbance or even complete destruction of natural habitats, which are subsequently replaced by ruderal habitats that facilitate the establishment and growth of ruderal plant species. Such plants most commonly colonize areas that have been altered to varying degrees by human activity, especially urban environments, roadsides, industrial areas, spoil heaps, and similar sites. The dominance of ruderal species in a given area typically correlates with the degree of habitat degradation.
Relatively often, in such ecologically weakened or degraded habitats, expansive plant species emerge. Due to their adaptive traits, they are able to colonize these environments with ease. Such plants share a number of characteristics, notably the production of a large quantity of seeds that are resistant to unfavorable environmental conditions and capable of germinating even under suboptimal circumstances. They usually spread either through deliberate or unintentional human assistance, but also via natural mechanisms, posing a threat to native species that lack comparable adaptations to dynamic environmental conditions [1]. Particularly dangerous are invasive species that are both expansive and alien to local ecosystems. They pose a substantial threat to native flora by outcompeting and displacing indigenous species, eventually contributing to their extinction. These adaptive traits make invasive plants a serious threat to native plant communities, especially considering that effective methods to curb their spread and prevent further invasions have not yet been developed [2].
In recent years, numerous fast-growing species capable of easily colonizing new areas have appeared across Europe, primarily due to human activity and climate change, including global warming and an extended growing season. The introduction and expansion of such species often lead to the transformation of diverse plant communities into monocultures dominated by expansive or invasive species. This results in a loss of local biodiversity and damage to ecosystems [3]. However, the mass occurrence of these species in a given area may potentially offer opportunities for the industrial utilization of their biomass, for instance, in biogas production, particularly through the use of plant material that is left over from mowing. Currently, the mowed biomass is typically only shredded and left to decompose on-site.
This group of plants included, among others, Sosnowski’s hogweed Heracleum sosnovskyi Manden (Apiaceae), ground elder Aegopodium podagraria L. (Apiaceae), bulbous chervil Chaerophyllum bulbosum L. (Apiaceae), box elder Acer negundo L. (Sapindaceae), and stinging nettle Urtica dioica (Urticaceae). All the studied taxa are expansive species, with two of them, H. sosnovskyi and A. negundo, listed as invasive species in Poland. The remaining three species, however, are considered useful plants—either medicinal herbs or edible species.
To provide a better understanding of the ecological challenges posed by the studied species in the research area, as well as their potential for practical use, a brief overview of each species is presented below.
H. sosnovskyi originates from the Caucasus, Transcaucasia, and Turkey, where it occurs in mountainous areas, forests, and meadows [4,5]. It was also cultivated as an ornamental and highly melliferous plant due to its accessible nectaries and was grown in botanical and home gardens [6,7]. Initially used as silage for cattle, it was later abandoned as a forage crop. However, as the remaining plants were not fully eradicated, this expansive species began to spread rapidly into surrounding areas, such as riparian forests (e.g., alder and ash woods), riverbanks, urban zones, illegal dumps, roadsides, and railway areas. Its competitive and reproductive capabilities, rapid germination, dense growth, large size (reaching up to 300 cm), and ability to form a large seed bank in the soil pose a significant threat to the species composition of invaded plant communities. It suppresses natural biocoenoses, resulting in a marked decline in species diversity. Moreover, this plant may affect other species through allelopathy, inhibiting their growth. Its sap [8] contains phototoxic compounds that cause severe skin irritation and burns [9]. In Poland, it has been recorded in many locations and is steadily expanding its range across the country, primarily colonizing semi-natural and ruderal habitats. It is a highly invasive alien species, spreading rapidly and threatening various natural habitats.
A. podagraria is a perennial plant native to Europe and Western Asia, although introduced populations also occur in Northwestern Europe. It is widespread in the Polish flora. Typically found in shaded, moist habitats, such as thickets, deciduous forest floors, along fences and buildings, wastelands, and gardens, it is a synanthropic species often observed in ruderal sites. It spreads vigorously through vegetative means via rhizomes and is considered a troublesome weed despite is poorly germinating seeds. However, its strong regenerative ability from small rhizome fragments makes it highly effective at colonizing and dominating new areas. It forms dense, monospecific stands that significantly compete with the germinating seeds of other species, thus altering and impoverishing plant communities. Sometimes used as a ground cover plant in heavily shaded areas, it is also valued as a medicinal and edible plant. It contains vitamin C, minerals, carotenoids, proteins, and essential oils. In folk medicine, it has been used to treat gout and rheumatism. This is a very expansive species that poses a threat to many natural habitats.
C. bulbosum is commonly found in the wild across Europe, occurring in forests, nitrophilous fringes, and shrublands, both in lowlands and mountainous regions. It is a diagnostic species of the Central European oak-hornbeam forest Gallio-Carpinetum and characteristic of the Alliario-Chaerophylettum temuli community. It prefers moist, shaded locations, where it grows rapidly and forms dance patches. It is native to the Polish flora and is often observed in ruderal habitats, within communities dominated by weeds, or on rubble heaps. Due to its fast growth, prolific seed production, and environmental tolerance, particularly plasticity regarding light availability, it demonstrates clear expansiveness. It primarily reproduces through seeds, and its robust root system enables it to effectively colonize new areas. It is a toxic plant as its sap can irritate the skin, and accidental ingestion may cause gastrointestinal and even neurological symptoms.
U. dioica occurs naturally across Europe and is a cosmopolitan species that is commonly and widely distributed in temperate zones. It forms plant communities in moist, fertile habitats, including river valleys, alluvial forests, and wetland margins. It is a characteristic species of Poo-Arabidetum [10]. As a nitrophilous apophyte, it frequently occurs in ruderal habitats directly shaped by human activity, such as near houses, fences, spoil heaps, and landfills. It is also found as a weed in gardens and orchards. Thanks to its dual reproduction strategy—via rapidly germinating seeds (which do not require dormancy) and vegetative spread through extensive clones—it can expand aggressively even within a single growing season [11]. Its rhizomes are hardy, rapidly elongate, and become woody over time. This enables the plant to form compact monospecific stands that displace previously occurring species. This effect is especially visible in areas where nettle dominates en masse. It thrives in well-lit sites, where it flowers and sets seed abundantly. The plant produces large quantities of seeds rich in valuable oil containing essential unsaturated fatty acids (EFAs). It is both edible and medicinal, being rich in micro-and macroelements, antioxidants, proteins, and phytohormones. Its sap has stinging properties. Although U. doica is highly expansive and threatens many natural habitats, it also serves an important ecological role, being a host plant for butterflies such as the small tortoiseshell (Aglais urticae) and the peacock butterfly (Aglais io) [12].
A. negundo is a large, often multi-stemmed tree reaching 15–20 m in height, with a broad, irregular crown [13]. It is native to North and Central America. In Poland, it is an introduced and fully naturalized species. It is not native and is classified as a kenophyte. Introduced in the 18th–19th centuries, it later escaped cultivation and began to spread spontaneously, initially along large river valleys. It exhibits wide ecological tolerance, and although it prefers riparian and mixed deciduous forests, it easily adapts to both natural and anthropogenically transformed environments, including degraded or abandoned agricultural lands. As one of the few woody species to appear in ruderal habitats, it poses a significant threat to native biodiversity. It disperses naturally by wind and water, producing a large number of lightweight seeds. It grows quickly and regenerates efficiently. Though short-lived and prone to breakage, it is highly effective at forming numerous adventitious shoots. These traits enable it to outcompete native species, altering both the species composition and population structure of local ecosystems, including forests. It can displace both herbaceous and woody plants. A. negundo is an expansive, invasive species that presents a serious threat to native flora and biodiversity.
The aim of this study was to demonstrate the energy potential of five plant species originating from ruderal plant communities for biogas production.
2. Materials and Methods
2.1. Material Collection
Floristic surveys of ruderal vegetation were conducted during two growing seasons in 2024. However, the plant material for the present study (Figure 1) was collected in July 2024 in a 19th-century manor park located in the village of Niegolewo (N 52°22′03” E 16°25′50”) in the Greater Poland Voivodship. The sampled plants were in full vegetative development, with well-developed leaves and inflorescences (U. dioica, A. podagraria, C. bulbosum, and H. sosnowskyi), whereas in the case of Acer negundo, several samplings were cut, ranging in height from approximately 1.5 to 2.5 m.

Figure 1.
Habitats of the plant species used in this study: (1). U. dioica, (2). A. negundo, (3). A. podagraria, (4). H. sosnowskyi, and (5). C. bulbosum.
The study area is characterized by a mean annual temperature of 9.7 °C and an average annual precipitation of approximately 658 mm (based on multi-year data from 1991–2021). Analysis of long-term data indicates that July is both the warmest month (19.8 °C) and the one with the highest precipitation (92 mm), while January is the coldest month (0.5 °C) and April (39 mm) and February (40 mm) are the driest [14]. The region’s moderately warm climate creates ideal conditions for the development of the studied species.
Samples for analysis were collected from plant communities in which the examined taxa occurred with the greatest constancy and frequency, occasionally acting as dominants in specific vegetation patches. The phytocoenoses containing the analyzed species were located on land classified as recreational (manor park area) and arable land of soil quality class IIIa. The predominant soil types are proper brown soils, with a smaller proportion of podzolized and pdeudopodzolic soils. The prevailing soil textures are light loamy sands and light loams [15].
2.2. Chemical Composition
For the determination of structural and secondary components, commonly used standardized methods were applied, as described in the work by [16]. The chemical composition analysis included the determination of cellulose content [17], holocellulose [18], lignin content [19], and extractives [20]. Additionally, ash content was assessed using the DIN 51731 standard. The arithmetic difference between holocellulose and cellulose values allowed for the calculation of hemicellulose content.
2.3. Batch Test
Methane fermentation was performed in 2 dm3 glass reactors according to DIN 38 414-S8 and the guidance of VDI 4630 published by the Association of German Engineers in Dresden.
All aspects of the biogas production process, including the schematic design of the three-section biofermenter, have been described in the works of Lewicki et al. [21], Cieślik et al. [22], and Waliszewska et al. [16].
2.4. Statistical Analysis
In order to determine the separation among plant species before and after fermentation in relation to the variability within the analyzed experimental objects, canonical variate analysis (CVA) was applied [23]. In CVA, the original variables are transformed into orthogonal variates, taking into account the structure of the experimental objects [24,25]. The use of CVA enabled the visualization of relationships between the studied plant species and the contents of extractives, lignin, cellulose, holocellulose, and hemicellulose. The resulting canonical coordinates were used to generate separate dendrograms based on Euclidean distances [26] for pre- and post-fermentation samples. For this purpose, hierarchical clustering with the single linkage method was applied.
To assess differences in the contents of extractives, lignin, cellulose, holocellulose, hemicellulose, and ash in the analyzed plant species, both before and after fermentation, one-way analysis of variance and Tukey’s test (p < 0.05) were used. These statistical methods were also applied to evaluate differences in dry matter content, methane yield, biogas production, and the degree of organic matter degradation among the studied plants. Visualizations were generated using Python 3.13.2 and the following packages: matplotlib [27], numpy [28], pandas [29], and oraz scipy. Statistical analyses were performed using R 4.4.1 and the following packages: dplyr [30], stats [31], tidyr [32], agricolae [33], and morpho [34].
The arithmetic difference between the holocellulose and cellulose values enabled the calculation of hemicellulose content.
3. Results and Discussion
The content of individual components in plants depends on many factors, including the species, plant age, growth conditions, anatomical part, and others. Therefore, variations in the amounts of cellulose, lignin, hemicellulose, and even ash can be significant. For the selected plant species, Table 1 presents the percentage contents of chemical components before and after anaerobic fermentation, with ash content determined only prior to fermentation. Statistically significant differences were observed in the levels of extractives, lignin, cellulose, holocellulose, and hemicellulose among the analyzed species, both before and after fermentation. In terms of the content of individual components, significant statistical variation was found between most of the studied species. An exception was noted in the percentage of hemicellulose before fermentation, where no statistically significant differences occurred between Acer negundo and Chaerophyllum bulbosum, and between Heracleum sosnowskyi, Urtica dioica, and Aegopodium podagraria, which allowed the formation of two homogeneous groups. For the other components, more than two statistically distinct groups were identified, both in pre- and post-fermentation conditions. Mean values and confidence intervals for each chemical component are presented in Figure 2.

Table 1.
Main components of selected plant species with statistically significant differences before and after methane fermentation (Tukey’s test). Mean values with standard deviation for each species are presented (statistically significant at p < 0.05).
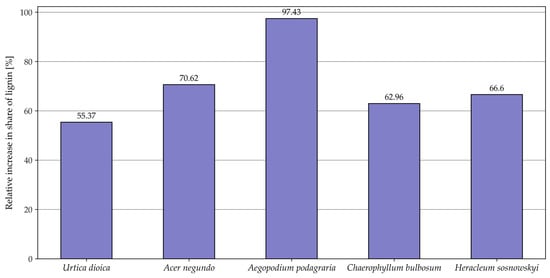
Figure 2.
Apparent increase in lignin content as a result of the fermentation process [%].
No significant changes were observed in holocellulose content before and after fermentation for U. dioica and A. negundo, or in hemicellulose content for C. bulbosum and H. sosnowskyi. In general, the fermentation process led to a decrease in the percentage of extractives and an apparent increase in lignin and cellulose content. A true increase in holocellulose content was observed only in A. podagraria, C. bulbosum, and H. sosnowskyi. Changes in hemicellulose levels following fermentation were species-dependent: a decrease was recorded for U. dioica and A. negundo, while A. podagraria showed an increase.
All plant species analyzed in this study were characterized by a high content of ethanol-extractable compounds. These values ranged from 10.78% in the case of stringing nettle to 15.10% for box elder (Table 1).
Similar results for extractive content before fermentation in grasses were reported by Waliszewska et al. (2024) [16]. However, the examined monocotyledonous species showed a higher proportion of extractives in the digestate after fermentation compared to the dicotyledonous species analyzed in this study. The content of extractive substances in wood ranges from 0.3% to 9.8%, depending on the species [35]. According to Pereira et al. (2013) [36], eucalyptus contains between 3.1% and 5.0% of these compounds. Similar amounts, ranging from 4.25% to 5.04%, were found in eucalyptus by Mancini et al. [37]. The fermentation process clearly reduced the amount of extractive substances. In the case of nettles, this represented more than a 4.5-fold decrease. After fermentation, the content of ethanol-soluble compounds ranged from 2.34% to 5.69%. A reduction in extractive content following biomass modification was also observed by Esteves et al. (2022) [38].
Wood contains small amounts of ash, typically between 0.2 and 1.0% [35]; however, cereal straw, grasses, and other annual plants contain significantly higher levels of mineral substances. For example, timoty grass and meadow fescue were found to contain between 4.99% and 7.02% of ash [39]. Gizińska-Górna et al. (2016) [40] reported an ash content of 2.2% for willow, 2.4% for giant miscanthus and Jerusalem artichoke, and 10.4% for common reeds. In the fresh raw material analyzed in this study, high ash contents were also observed. Stinding nettle, ground elder, and Sosnowski’s hogweed had comparable ash contents of 12.94%, 13.02%, and 12.35%, respectively. Similar ash levels (18.6% D.M.) in Silphium L. leaves were reported by Kowalska et al. [41]. In contrast, box elder and bulbous chervil contained nearly half as much ash at 7.64% and 6.82%, respectively. Godin et al. [42] reported 10.9% ash in Jerusalem artichoke, 14.2% in fescue, and only 3.5% in miscanthus.
Cellulose and lignin are structural components of plant biomass. Cellulose provides the plant with strength and flexibility, whereas lignin reinforces the cell wall, providing it with hardness and resistance to decomposition. The cellulose content in the studied plants was relatively low, ranging from 27.21% and 27.35% in box elder and bulbous chervil, 32.78% in Sosnowski’s hogweed, and 31.82% in nettle. Ground elder contained 28.73% cellulose. In Silphium leaves, Kowalska et al. [41] reported cellulose levels between 22.5% and 33.2%, while Acazacei et al. [43] found values of 29.96% in Silphium perfoliatum and only 21.29% in Helianthus tuberosus. Similar levels were reported by Titei (2014) [44]. Godin et al. [42] observed significant variability in cellulose content in annual plants, ranging from 26.3% to 48.4%. Meserszmit et al. (2024) [45] also reported a low cellulose content for both mown and abandoned (non-managed) grasslands, amounting to 25.28% and 25.61%, respectively. In a previous study, Meserszmit et al. (2021) [46] recorded an average cellulose content of 25.86% in plant communities dominated by grasses and herbs. Similarly, Melts et al. (2014) [47] reported cellulose levels ranging from 25.97% in herbs to 33.61% in grasses. Anaerobic digestion had a substantial impact on cellulose content in the digestate. In all cases, an apparent increase in cellulose content was observed, which ranged from 34.35% for bulbous chervil to 40.87% for nettle. The cellulose content in the digestates of the remaining species was similar (39.07%, 38.03%, and 39.60%) (Table 1).
Lignin, a component of the plant cell wall, is relatively resistant to fermentation and is difficult to degrade. In the fresh biomass, lignin content ranged from 17.26% in ground elder to 23.56% in box elder. Nettle, Sosnowski’s hogweed, and bulbous chervil contained comparable levels of lignin at 19.37%, 19.00%, and 20.19%. Meserszmit et al. (2024) [45] reported a lignin content of 12% in grasses, while in an earlier study, Meserszmit et al. (2021) [46] determined its level to be 11.89%. Godin et al. [42] reported much lower lignin contents, ranging from 3.2% to 10.1%, likely due to the use of the Van Soest method for lignin determination. A similar range of values, oscillating between 5.05% (sedges) and 9.55% (legumes and other herbaceous plants), was reported by Melts et al. (2014) [47]. Such a low content of this structural component in plants is most likely due to the method of lignin determination, according to Van Soest. Using the Tappi method [19] produced lignin contents of 28.8–31.4% in eucalyptus wood [36]. After fermentation, significantly higher lignin contents were measured in the digestates (Figure 2). This increase is only apparent as it reflects the proportion of lignin relative to the reduced post-fermentation mass. Lignin contents in the digestates ranged from 30.10% for nettle to 40.21% for box elder. For the other digestates, the values were similar: 34.06%, 31.66%, and 32.91% (Table 1).
Hemicelluloses, as lowly polymerized carbohydrates, are the most rapidly degraded during the fermentation process, leading to the formation of simple sugars [48].
Wood typically contains about 18–25% hemicellulose [35,36,49]. Annual plants can contain significantly more of this component. For example, oil palm mesocarp fiber contains 33.1%, rice straw contains 24.4%, corncob contains 35.8%, sugarcane bagasse contains 30.6%, and sugarcane straw contains 27.0% [48]. Wang et al. [50] reported a hemicellulose content of approximately 25% in wheat straw, while Meserszmit et al. (2024) [45] recorded values of around 19.5% and 18.51% in an earlier study [46]. Similar results were also reported by Melts et al. (2014) [47].
Among the plants studied in this work, the highest hemicellulose content in fresh biomass was found in bulbous chervil (34.35%) and box elder (32.03%) (Table 1). Nettle, Sosnowski’s hogweed, and ground elder contained 26.98%, 24.22%, and 25.75% hemicellulose, respectively. The fermentation process led to a decrease in hemicellulose content in the cases of nettles and box elder. In the corresponding digestates, hemicellulose content was 19.89% and 20.05%, respectively. In the digestate of ground elder, however, an increase in hemicellulose content of more than 6% was observed compared to the raw material. This is, of course, an apparent increase, likely due to the more-than-fourfold reduction in extractive substances. In the case of Sisnowski’s hogweed and bulbous chervil, the hemicellulose content in the digestates was similar to that of fresh biomass (Table 1) (Figure 3).
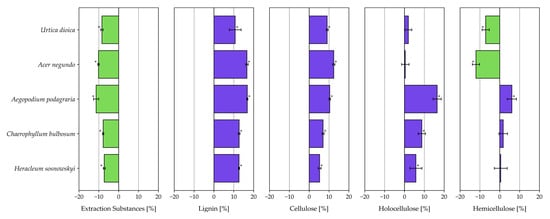
Figure 3.
Changes in the chemical composition of the analyzed plant species [%] before and after fermentation (statistically significant at p < 0.05). * (To the left of the zero value on the X-axis—values before fermentation; right side—values after fermentation).
Based on the data presented in Table 1, canonical variate analysis (CVA) was performed, the results of which allowed for a graphical comparison of the studied plant species in terms of the content of the analyzed components before and after fermentation. A clear differentiation is observed between the samples subjected to fermentation and those that were not, resulting from differences in chemical composition. The distribution of coordinates along the first two canonical axes explains 89.39% and 7.23% of the variance, respectively (Figure 4).
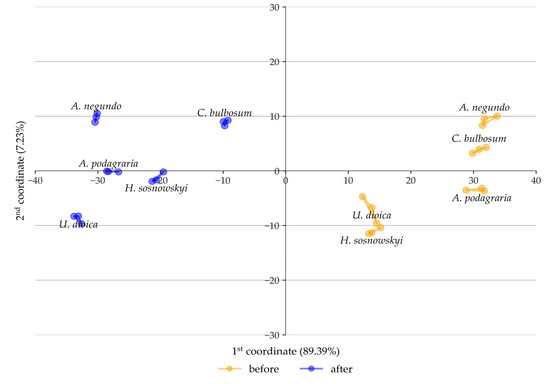
Figure 4.
CVA analysis of the set of analyzed chemical composition components before and after fermentation along the coordinates exhibiting the highest degree of variance.
This indicates that the percentage content of the analyzed substances before and after the fermentation process differentiates the studied plant species to a greater extent than interspecific differences in the content of these components. The dendrograms (Figure 5 and Figure 6) illustrate the similarities between the studied plants before and after fermentation in terms of the content of extractives, lignin, cellulose, holocellulose, and hemicellulose. Among the non-fermented plant samples, the greatest similarity was observed between A. negundo and C. bulbosum, followed by U. dioica and H. sosnowskyi. It was noted that the two resulting groups showed no significant internal similarity between members of the same group (Figure 5). In the case of fermented samples, A. negundo differed the most from the other species (Figure 6).
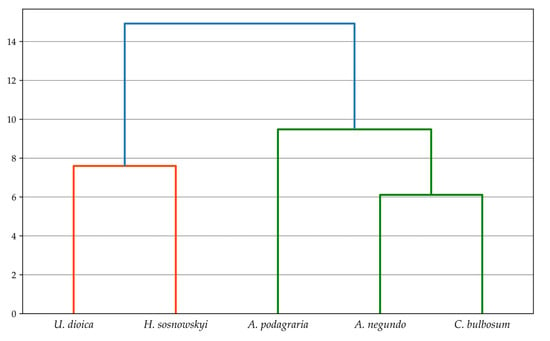
Figure 5.
Classification dendrogram of the combined set of analyzed species before fermentation based on chemical composition values. Hierarchical clustering using the single linkage method and Euclidean distance was applied.
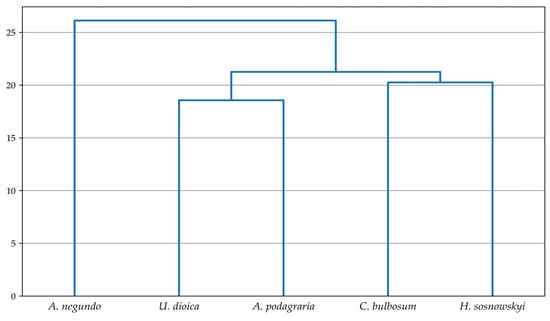
Figure 6.
Classification dendrogram of the combined set of analyzed species after fermentation based on chemical composition values. Hierarchical clustering using the single linkage method and Euclidean distance was applied.
To summarize the analysis of changes in chemical composition before and after anaerobic digestion, it can be concluded that the composition of the digestate depends on the plant species or, more precisely, on the proportion and structures of structural and nonstructural components. During fermentation, lowly polymerized carbohydrates are degraded, and there is a significant reduction in extractive substances. When analyzing the relationship between the content of individual components and the amount of biogas produced, it was found that only in the case of ground elder, which was characterized by the lowest lignin content, was the highest biogas yield recorded. For the other species, no clear correlation was found. Explaining these patterns requires further research, particularly in relation to molecular structures, chemical bonding, and other physicochemical factors.
Table 2 presents data on dry matter (DM) and organic dry matter (oDM) content. The dry matter content ranged from 15.61% in A. podagraria to 39.01% in A. negundo, with statistically significant differences dividing the species into three homogeneous groups. For comparison, Waliszewska et al. [16] reported much higher dry matter contents for grass species, ranging between 89.85% and 92.98%. However, these were not the highest DM values observed for monocotyledons reported by the same author [51]. Czubaszek et al. [52], in a study comparing grasses and grass silage across three seasons, observed substantial variation in dry matter percentages depending on the season, while the differences between the components remained at a similar level. Only the values obtained during the spring season (38.00% and 37.37%) were higher than those recorded for U. dioica and were lower than for A. negundo and C. bulbosum. Czekała et al. [53] reported a dry matter content of 93.37% for coffee husks, and between 41.27% and 45.72% for three different types of spent coffee grounds. In turn, Gizińska-Górna [40], in a comparison of four different plant species (grasses, trees/shrubs, and herbaceous plants), reported DM contents of 50.8% for the common reed, 55% for willow, 33.9% for giant miscanthus, and 50.1% for the Jerusalem artichoke. With regard to organic dry matter content, statistically significant differences were also observed, with values ranging from 86.98% to 93.18%, forming four homogeneous groups. The organic matter share in the dry mass of A. podagraria, U. dioica, and H. sosnowskyi (86.98%, 87.06%, and 87.65%, respectively) was similar to the concentrations reported for Lolium perenne by Waliszewska et al. [16]. Czekała et al. [53] reported higher values, ranging from 93.34% to 98.42%. In contrast, Czubaszek et al. [52] reported oDM values for spring and summer that were lower than those observed in the present study. Gizińska-Górna et al. [40] found values ranging from 89.6% (common reed) to 97.8% (willow).

Table 2.
The content of dry matter and organic dry matter in plant samples with statistically significant differences (Tukey’s test). Mean values with standard deviations for each species are presented (statistically significant at p < 0.05).
Statistically significant differences (p < 0.05) in methane and biogas production were observed among the analyzed plant species (Table 3). The percentage of methane content ranged from 47.27% (A. podagraria) to 50.25% (H. sosnowskyi), forming two statistically homogeneous groups. Czubaszek et al. [52] reported methane concentrations in grasses ranging from 55.7% to 59.7%, and in grass silage from 55.0% to 60.0%. Gizińska-Górna et al. [40] found methane contents for four plant species as follows: 50.9% for willow, 51.0% for Jerusalem artichoke, 53.0% for common reed, and 54.9% for giant miscanthus. In comparison, Czekała et al. [53] reported methane yields ranging from 52.68% to 54.50%.

Table 3.
The content of methane, biogas yield, and degree of decomposition of organic matter in plant samples with statistically significant differences (Tukey’s test). Mean values with standard deviations for each species are presented (statistically significant at p < 0.05).
For the amount of methane and biogas produced from fresh mass (Table 3), a less distinct division into two homogeneous groups was observed among the analyzed plant species. Methane yields ranged from 23.45 m3∙Mg−1 for A. podagraria to 52.69 m3∙Mg−1 for C. bulbosum. Gizińska-Górna et al. [40], in terms of methane yield from fresh biomass, reported results with a wide range of variation (e.g., 66.0 m3∙Mg−1 for Jerusalem artichoke and 108.0 m3∙Mg−1 for the common reed), where even the lowest value was significantly higher than those obtained in the present study. Czekała et al. [53] reported substantially higher cumulative methane production from coffee husks and spent coffee grounds, ranging from 122.86 to 173.59 m3∙Mg−1. Likewise, the total biogas production values were considerably higher than those reported by the authors of this study. When analyzing methane production (Table 3, Figure 7) and biogas yield from dry matter (Figure 8), the grouping of species was again less distinct. Methane yields ranged from 110.8 m3∙Mg−1 for H. sosnowskyi to 150.23 m3∙Mg−1 for A. podagraria (Figure 9). In contrast, Meserszmit et al. [46] reported significantly higher yields of methane and biogas at 245.78 m3∙Mg−1 and 464.85 m3∙Mg−1, respectively. In terms of the percentage of organic matter degradation, A. podagraria showed a clearly distinct value compared to the other species, reaching 53.97%.
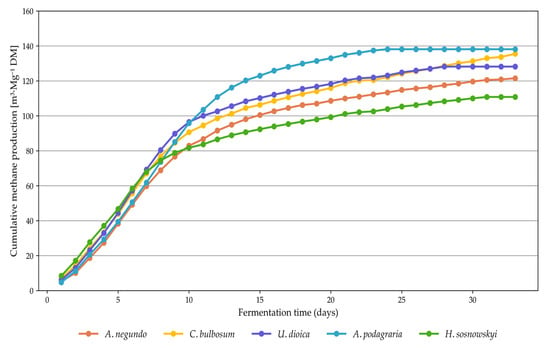
Figure 7.
Daily methane production from ruderal plant species.
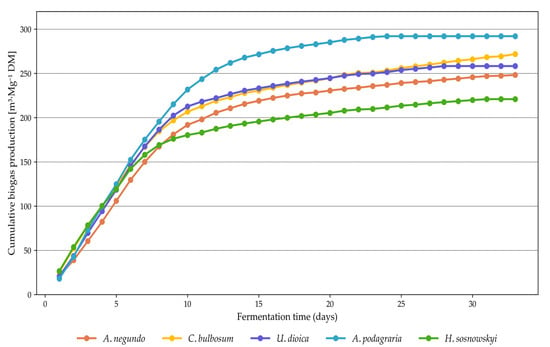
Figure 8.
Daily biogas production from ruderal plant species.
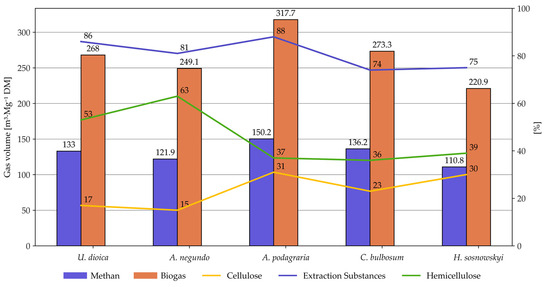
Figure 9.
Daily biogas and methane production from ruderal plant species.
4. Conclusions
This study showed that all analyzed ruderal plants were characterized by the high values recorded for Acer negundo (15.10%) and Urtica dioica (10.78%). The fermentation process significantly reduced extractive substances, by more than fourfold in some cases, as well as hemicelluloses, particularly in Urtica dioica and Acer negundo. The apparent increase in cellulose and lignin content after fermentation was the result of the proportion of soluble fractions. Among the tested species, Aegopodium podagraria produced the highest amount of biogas, largely due to its low lignin content, which facilitated hemicellulose degradation. For the remaining plants, no clear correlation was observed between chemical composition and biogas yield, suggesting that both quantitative characteristics and structural characteristics play a key role. Overall, ruderal plant biomass presents a promising but species-diverse raw material for biogas production. Despite its potential, further research is needed to optimize fermentation speed and assess its energy efficiency to enhance sustainable bioenergy solutions. The low amount of methane and biogas produced from H. sosnowskyi was due to the use of the entire plant, with the highest weight contribution coming from the inflorescences and stems. The authors of this study believe that better results for biogas production could be achieved by using only the leaves, which constitute a substantial amount of biomass in the early growth stages of the plant. In contrast, the fully developed plant should be used for combustion purposes. This, of course, requires more in-depth analyses using the aforementioned species. A similar situation may also occur with species such as A. podagraria and C. bulbosum, as well as other members of the Apiaceae family. The results confirm the potential of ruderal biomass as a feedstock for biogas production, although a high degree of interspecies variability was observed. A limitation of this study is the lack of comprehensive knowledge regarding the influence of molecular structures and chemical bonding on fermentation efficiency. Therefore, future research should focus on analyzing the ultrastructure of polymers in relation to the rate and yield of fermentation, optimizing process conditions for individual species, evaluating the energy efficiency of fermentation using ruderal biomass, and exploring the potential application of these substrates in co-fermentation with other organic wastes. Such an approach will enable a more complete utilization of the biodiversity found in ruderal habitats for sustainable bioenergy production.
Author Contributions
Conceptualization, M.M., W.C. and B.W.; methodology, A.S. and M.C.; software, A.L. and M.C.; validation, M.Z., J.D. and M.M.; formal analysis, L.M.; investigation, M.J.-S.; resources, M.M., B.W. and W.C.; data curation, B.W., M.C. and A.L.; writing—original draft preparation, M.J.-S., M.M., A.L. and B.W.; writing—review and editing, L.M., J.D. and W.C.; visualization, A.L.; supervision, B.W. and M.Z.; project administration, M.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted with a budget from the Department of Chemical Wood Technology, the Department of Biosystems Engineering, and the Department of Grassland and Natural Landscape at the Poznan University of Life Sciences.
Data Availability Statement
Available upon reasonable request.
Acknowledgments
The authors are grateful for support provided by the Poznan University of Life Sciences, Poland, including technical support and materials used for field experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cerevkova, A.; Ivashchenko, K.; Miklisova, D.; Ananyeva, N.; Renco, M. Influence of invasion by Sosnowsky’s hogweed on nematode communities and microbial activity in forest and grassland ecosystems. Glob. Ecol. Conserv. 2020, 21, e00851. [Google Scholar]
- Nielsen, C.; Ravn, H.P.; Nentwig, W.; Wade, M. (Eds.) The Giant Hogweed Best Practice Manual; Guidelines for the Management and Control of an Invasive Weed in Europe; Forest and Landscape Denmark: Hørsholm, Denmark, 2005. [Google Scholar]
- Thiele, J.; Otte, A. Analysis of habitats and communities invaded by Heracleum mantegazzianum Somm. et Lev. (Giant hogweed) in Germany. Phytocoenologia 2006, 36, 281–320. [Google Scholar] [CrossRef]
- Jahodova, S.; Froberg, L.; Pysek, P.; Geltman, D.; Trybush, S.; Karp, A. Taxonomy, identification, genetic relationships and distribution of large Heracleum species in Europe. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pysek, P., Cock, M.J.W., Nentwig, W., Ravn, H.P., Eds.; CAB International: Wallingford, UK, 2007; p. 119. [Google Scholar]
- Jahodova, S.; Trybush, S.; Pysek, P.; Wade, M.; Karp, A. Invasive species of Heracleum in Europe: An insight into genetic relationships and invasion history. Divers. Distrib. 2007, 13, 99–114. [Google Scholar] [CrossRef]
- Grigorievskaya, A.Y.; Starodubtseva, E.A.; Khlyzova, N.Y.; Agafonov, V.A. Adventive Flora of the Voronezh Region: Historical, Biogeographical, Ecological Aspects; Publishing House of Voronezh State University: Voronezh, Russia, 2004. [Google Scholar]
- Geltman, D.V. Composition and ecological-phytocenotic features of communities with the participation of the invasive species Heracleum Sosnowskyi (Apiaceae) in north-west European Russia. Rastit. Resur. (Plant Res.) 2009, 3, 68–75. [Google Scholar]
- Mironova, D.Y.; Varadarajan, V.; Timakhovich, I.V.; Barakova, N.V.; Tokbaeva, A.A.; Rumiantceva, O.N.; Pomazkova, E.E.; Baranov, I.V.; Tishchenko, L.I. Methods of Commercialization and Usage of Sosnovsky hogweed Processing. Recycling 2022, 7, 77. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Aghbabayi, M.; Rashidi-Monfared, S.; Falahi Charkhabi, N.; Gharanjik, S.; Ahmadi, N. Furanocoumarins from Heracleum persicum L.: Unveiling their biosynthesis and gene expression. Ind. Crops Prod. 2023, 203, 117160. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Taylor, K. Biological flora of British Isles: Urtica dioica L. J. Ecol. 2009, 97, 1436–1458. [Google Scholar] [CrossRef]
- Vogl, C.R.; Hardtl, A. Production and processing of organically grown fiber nettle (Urtica dioica L.) and its potential use in the natural textile industry. A review. Am. J. Altern. Agric. 2003, 18, 119–128. [Google Scholar] [CrossRef]
- Johnson, O.; More, D. Drzewa; Multico Oficyna Wydawnicza: Warszawa, Poland, 2009. [Google Scholar]
- Available online: https://pl.climate-data.org/europa/polska/greater-poland-voivodeship/niegolewo-146939/ (accessed on 14 March 2025).
- Available online: https://mapy.geoportal.gov.pl/imapnext/imap/index.html?moduleId=modulRol&mapview=52.366627%2C16.428465%2C10000s (accessed on 14 March 2025).
- Waliszewska, B.; Waliszewska, H.; Grzelak, M.; Majchrzak, L.; Gaweł, E.; Murawski, M.; Sieradzka, A.; Vaskina, I.; Spek-Dźwigała, A. Evaluation of Changes in the Chemical Composition of Grasses as a Result of the Methane Fermentation Process and Biogas Production Efficiency. Energies 2024, 17, 4100. [Google Scholar] [CrossRef]
- Seifert, K. Zur Frage der Cellulose-Schnellbestimmung nach der Acetylaceton-Methode. Das Pap. 1960, 14, 104–106. [Google Scholar]
- TAPPI T 9 wd-75; Holocellulose in Wood. Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 2006.
- TAPPI T 222 om-06; Acid-Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 2006.
- TAPPI T 204 cm-07; Solvent Extractives of Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 2007.
- Lewicki, A.; Pilarski, K.; Janczak, D.; Czekała, W.; Rodríguez Carmona, P.C.; Cieślik, M.; Witaszek, K.; Zbytek, Z. The biogas production from herbs and waste from the herbal industry. J. Res. Appl. Agric. Eng. 2013, 58, 114–117. [Google Scholar]
- Cieślik, M.; Dach, J.; Lewicki, A.; Smurzyńska, A.; Janczak, D.; Pawlicka-Kaczorowska, J.; Boniecki, P.; Cyplik, P.; Czekała, W.; Jóźwiakowski, K. Methane fermentation of the maize straw silage under meso- and thermophilic conditions. Energy 2016, 115, 1495–1502. [Google Scholar] [CrossRef]
- Krzanowski, W.J. Principles of Multivariate Analysis: A User’s Perspective, 2nd ed.; Oxford University Press: New York, NY, USA, 2000; p. 608. [Google Scholar]
- Kayzer, D.; Czerwińska-Kayzer, D.; Florek, J.; Staniszewski, R. Financial Security as a Basis for the Sustainable Development of Small and Medium-Sized Renewable Energy Companies—A Polish Perspective. Sustainability 2024, 16, 5926. [Google Scholar] [CrossRef]
- Florek, J.; Staniszewski, R.; Czerwińska-Kayzer, D.; Kayzer, D. Functioning of the Energy Sector Under Crisis Conditions—A Polish Perspective. Energies 2024, 17, 6161. [Google Scholar] [CrossRef]
- Lejeune, M.; Caliński, T. Canonical analysis applied to multivariate analysis of variance. J. Multivar. Anal. 2000, 72, 100–119. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- The Pandas Development Team. Pandas-Dev/Pandas: Pandas, version 2.2.3; Zenodo: Genève, Switzerland, 2024. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation, R package version 1.1.4; 2023. Available online: https://dplyr.tidyverse.org (accessed on 30 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 30 March 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data, R Package Version 1.3.1. 2024. Available online: https://tidyr.tidyverse.org (accessed on 30 March 2025).
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research, R package version 1.3-7; 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 30 March 2025).
- Schlager, S. Morpho and Rvcg—Shape Analysis in R. In Statistical Shape and Deformation Analysis; Zheng, G., Li, S., Szekely, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 217–256. ISBN 9780128104934. [Google Scholar]
- Prosiński, S. Chemia Drewna; PWRiL: Warszawa, Poland, 1984. [Google Scholar]
- Pereira, B.L.C.; Carneiro, A.d.C.O.; Carvalho, A.M.M.L.; Colodette, J.L.; Oliveira, A.C.; Fontes, M.P.F. Influence of Chemical Composition of Eucalyptus Wood on Gravimetric Yield and Charcoal Properties. BioResources 2013, 8, 4574–4592. [Google Scholar] [CrossRef]
- Mancini, T.L.; Ramalho, G.F.M.; Trugilho, P.F.; Hein, G.P.R. Estimation of total extractive content of wood from planted and native forests by near infrared spectroscopy. iForest 2021, 14, 18–25. [Google Scholar] [CrossRef]
- Esteves, B.; Ayata, U.; Cruz-Lopez, L.; Bras, I.; Ferreira, J.; Domingos, I. Changes in the content and composition of the extractives in thermally modified tropical hardwoods. Maderas Cienc. Tecnol. 2022, 24, 22. [Google Scholar] [CrossRef]
- Platače, R.; Adamovičs, A. The evaluation of ash content in grass biomass used for energy production. WIT Trans. Ecol. Environ. 2014, 2, 1057–1065. [Google Scholar] [CrossRef]
- Gizińska-Górna, M.; Czekała, W.; Jóźwiakowski, K.; Lewicki, A.; Dach, J.; Marzec, M.; Pytka, A.; Janczak, D.; Kowalczyk-Juśko, A.; Listosz, A. The possibility of using plants from hybrid constructed wetland wastewater treatment plant for energy purposes. Ecol. Eng. 2016, 95, 534–541. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Evaluation of Chemical Composition of Some Silphium L. Species as Alternative Raw Materials. Agriculture 2020, 10, 132. [Google Scholar] [CrossRef]
- Godin, B.; Ghysel, F.; Agneessens, R.; Schmit, T.; Gofflot, S.; Lamaudiere, S.; Sinnaeve, G.; Goffart, J.-P.; Gerin, P.; Stilmant, D.; et al. Determination de la cellulose, des hemicelluloses, de la lignine et des cendres dans diverses cultures lignocellulosiques dediees a la production de bioethanol de deuxieme generation. Biotechnol. Agron. Soc. Environ. 2010, 14, 549–560. [Google Scholar]
- Acazacei, V.; Teleuaca, A.; Muntean, A. The Perspective of Cultivation and Utilization of the Species Silphium perfoliatum L. and Helianthus tuberosus L. in Moldova. Bull. Univ. Agric. Sci. Vet. Med. 2013, 70, 160–166. [Google Scholar] [CrossRef]
- Titei, V. Biological Peculiarities of Cup Plant (Silphium perfoliatum L.) and Utilization Possibilities in the Republic of Moldova; Lucrări Ştiinţifice. 2014, Volume 57, p. 1. Available online: http://193.231.26.26/handle/20.500.12811/2213 (accessed on 30 March 2025).
- Meserszmit, M.; Swacha, G.; Pavlů, L.; Pavlů, V.; Titěra, J.; Jabłoński, S.; Łukaszewicz, M.; Kącki, Z. Effect of mowing versus abandonment of mesic grasslands in Central Europe on biomass use for biogas production: Implications for semi-natural ecosystem conservation. J. Environ. Manag. 2024, 368, 122132. [Google Scholar] [CrossRef]
- Meserszmit, M.; Swacha, G.; Pavlů, L.; Pavlů, V.; Trojanowska-Olichwer, A.; Kącki, Z. Species composition of semi-natural mesic grasslands as a factor influencing the methane yield of plant biomass (Central Europe). GCB Bioenergy 2021, 14, 54–64. [Google Scholar] [CrossRef]
- Melts, I.; Normak, A.; Nurk, L.; Heinsoo, K. Chemical characteristics of biomass from nature conservation management for methane production. Bioresour. Technol. 2014, 167, 226–231. [Google Scholar] [CrossRef]
- Harahap, B.M. Degradation Techniques of Hemicellulose Fraction from Biomass Feedstock for Optimum Xylose Production: A Review. J. Keteknikan Pertan. Trop. Dan Biosist. 2020, 8, 107–124. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Sienkiewicz, A.; Piotrowska-Niczyporuk, A.; Wassen, M.J.; Bajguz, A. Possibilities of Utilising Biomass Collected from Road Verges to Produce Biogas and Biodiesel. Energies 2024, 17, 1751. [Google Scholar] [CrossRef]
- Czekała, W.; Łukomska, A.; Pulka, J.; Bojarski, W.; Pochwatka, P.; Kowalczyk-Juśko, A.; Oniszczuk, A.; Dach, J. Waste-to-energy: Biogas potential of waste from coffee production and consumption. Energy 2023, 276, 127604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).