Regenerative Oxidation Technology for VOC Treatment: A Review
Abstract
1. Introduction
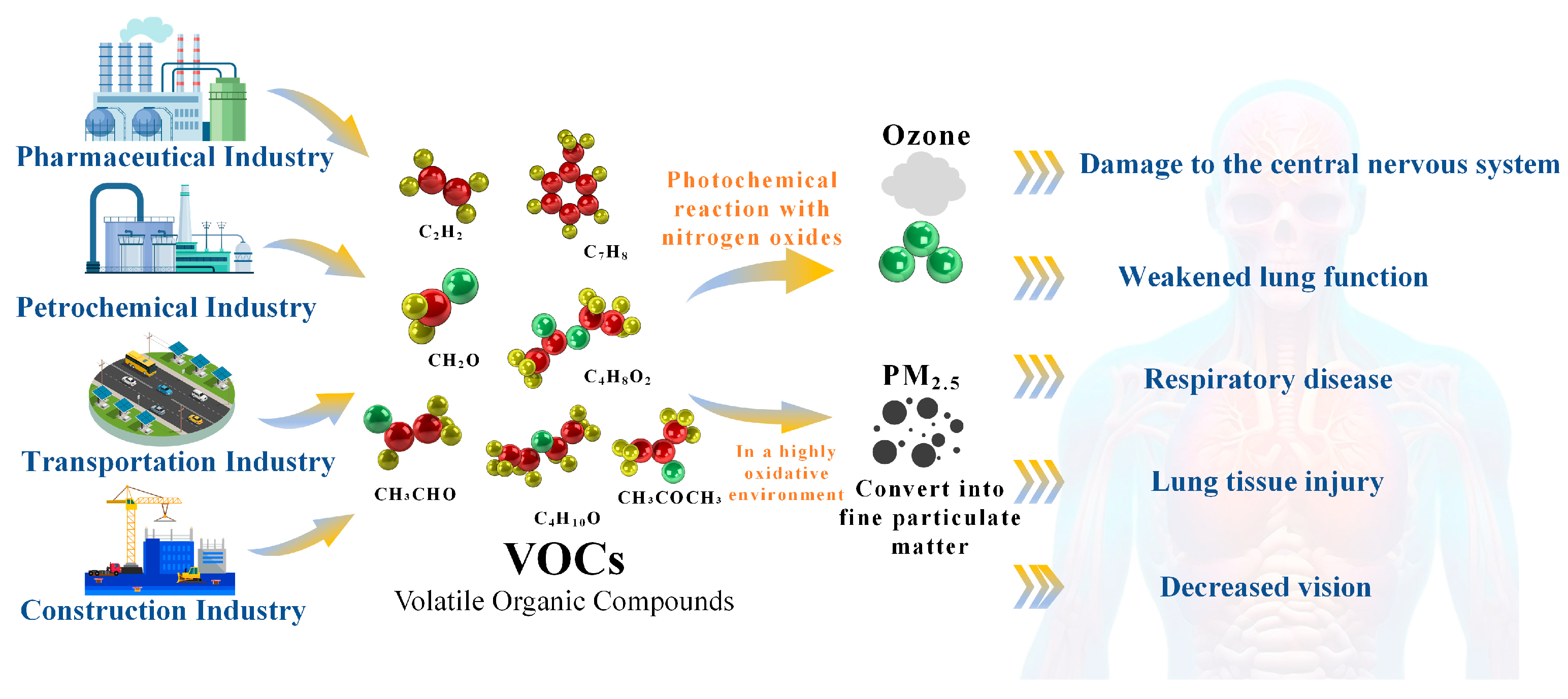
1.1. The Environmental and Human Health Impacts of VOC Emissions
1.2. VOC Treatment Technologies
2. Regenerative Thermal Oxidation
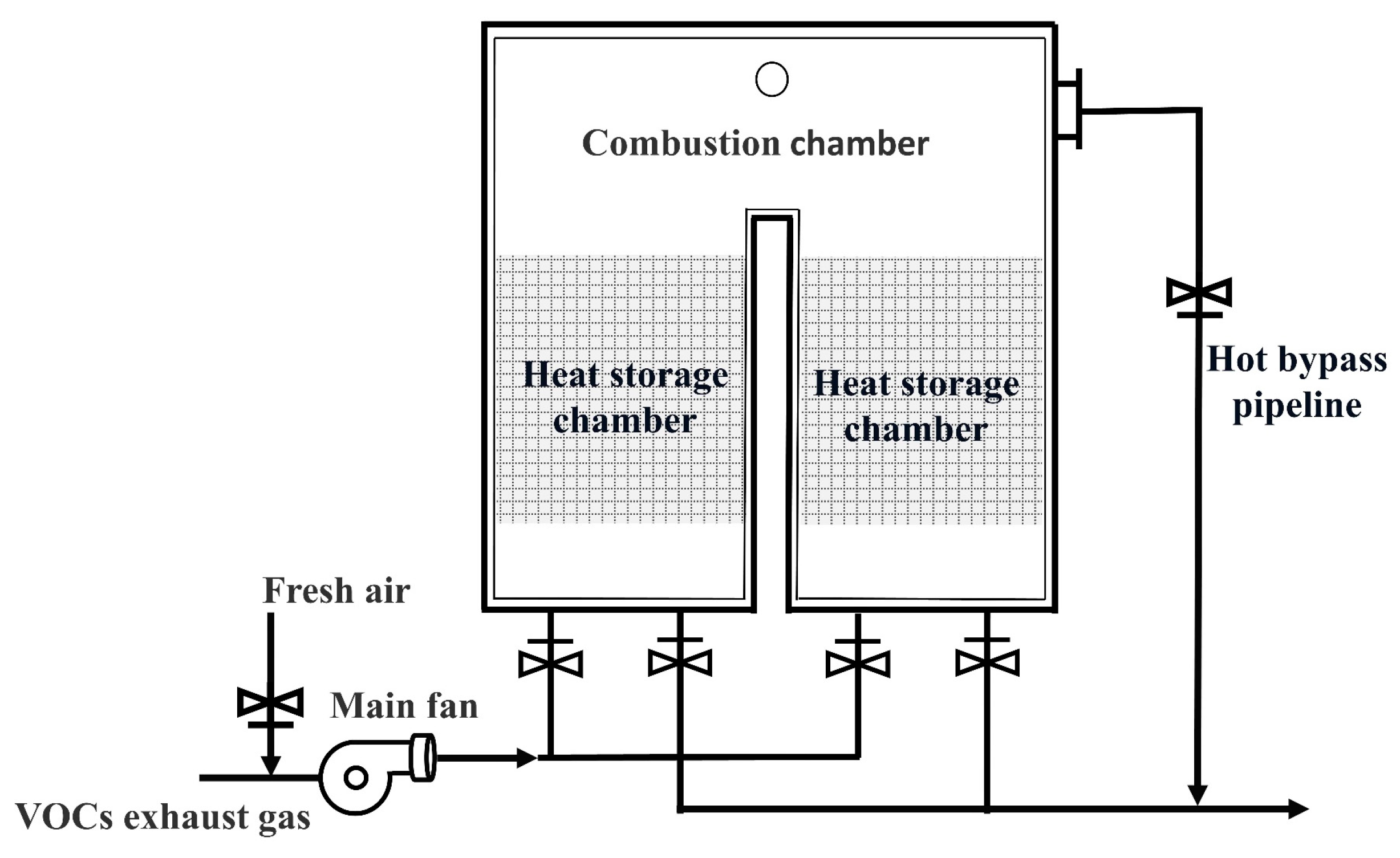
2.1. Working Principle of RTO
2.2. Types of RTO
2.2.1. Two-Chamber RTO
2.2.2. Three-Chamber RTO
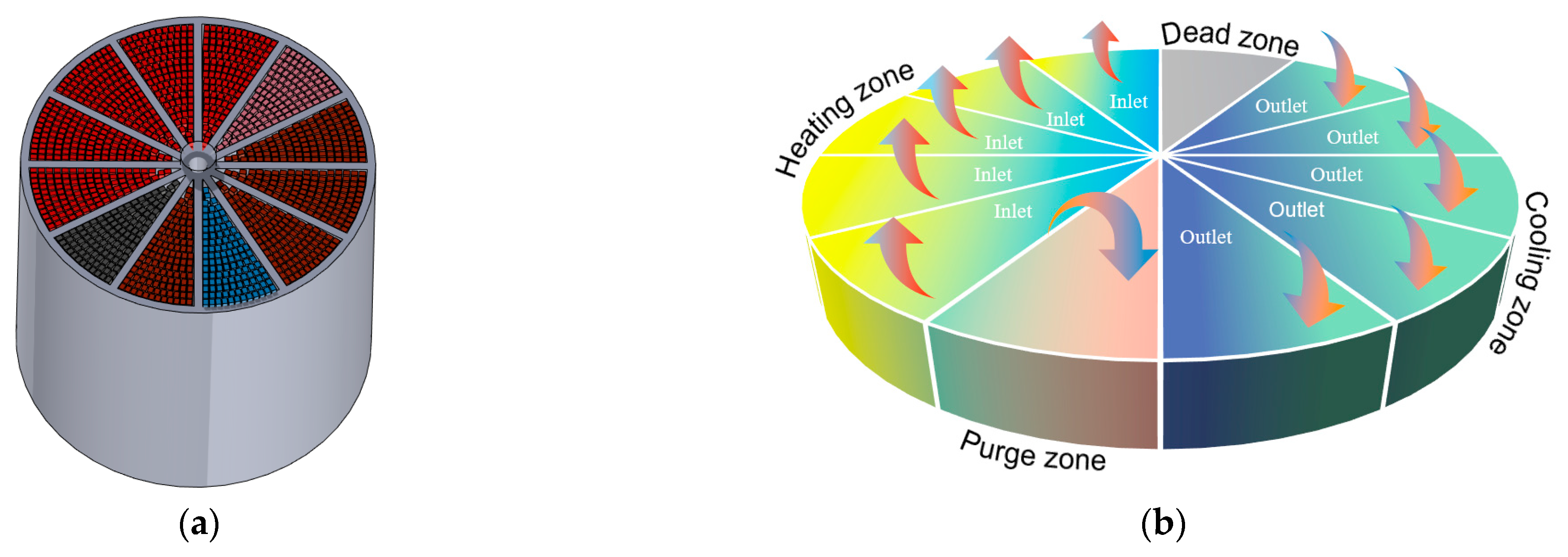
2.2.3. Rotary RTO
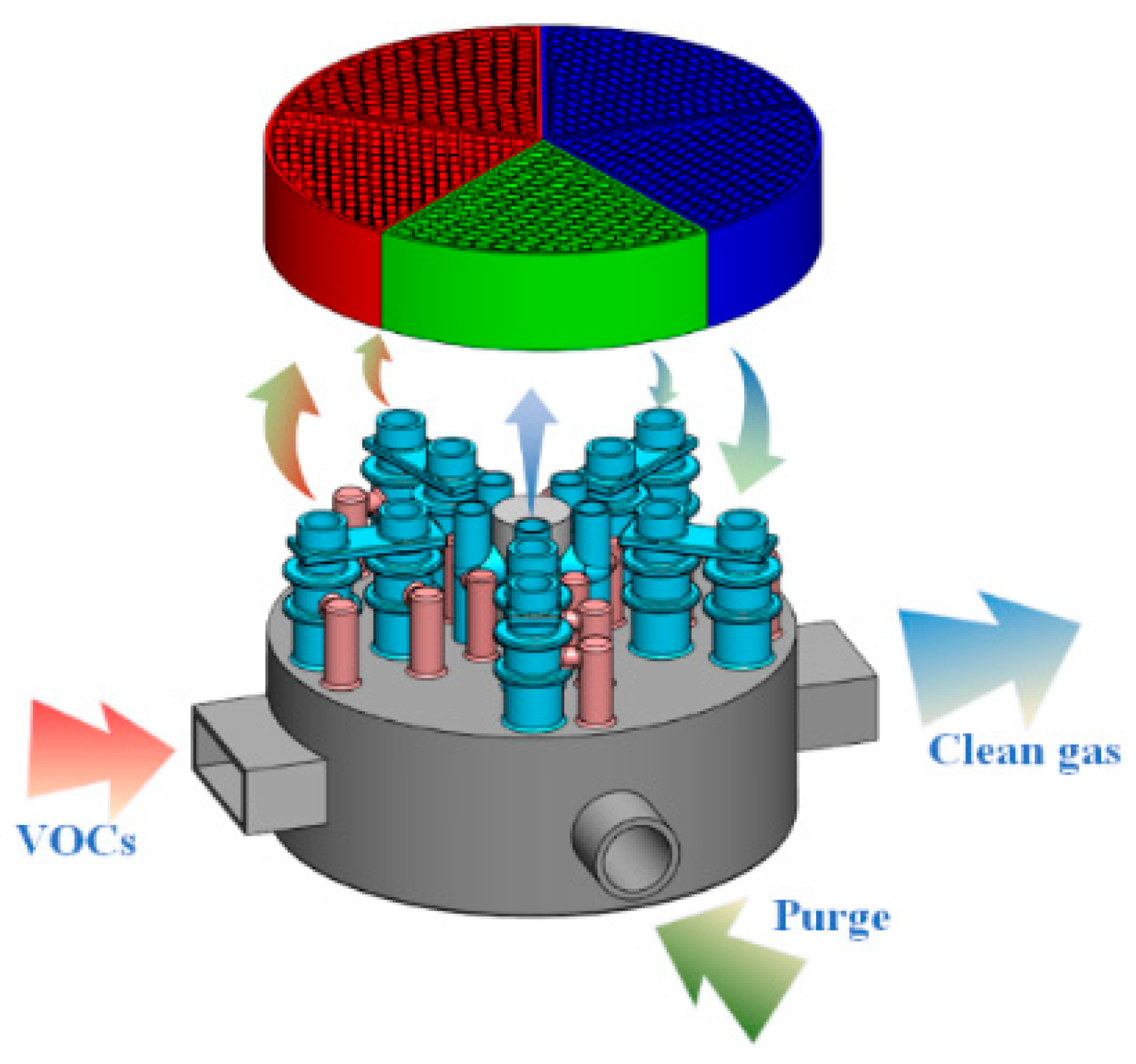
2.2.4. Single-Tube Multi-Valve RTO
3. Regenerative Catalytic Oxidizer
3.1. The Working Principle of RCO
3.2. Precious Metal Catalysts
3.3. Non-Precious Metal Oxide Catalysts
3.4. Mixed-Metal Catalysts
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anav, A.; De Marco, A.; Friedlingstein, P.; Savi, F.; Sicard, P.; Sitch, S.; Vitale, M.; Paoletti, E. Growing season extension affects ozone uptake by European forests. Sci. Total Environ. 2019, 669, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, X.; Ling, Z.; Zhao, J.; Guo, H.; Shao, M.; Wang, Z. Contributions of different anthropogenic volatile organic compound sources to ozone formation at a receptor site in the Pearl River Delta region and its policy implications. Atmos. Chem. Phys. 2019, 19, 8801–8816. [Google Scholar] [CrossRef]
- Bachheti, A.J.; Bhalla, P.; Bachheti, R.K.; Husen, A. Growth and development of medicinal plants, and production of secondary metabolites under ozone pollution. In Environmental Pollution and Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2022; pp. 25–38. [Google Scholar]
- Xu, J.; Hu, W.; Liang, D.; Gao, P. Photochemical impacts on the toxicity of PM2.5. Crit. Rev. Environ. Sci. Technol. 2022, 52, 130–156. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef]
- Ma, W.; Hu, J.; Li, J.; Gao, P.; Okoli, C.P.; Wang, P.; Li, J. Distribution, sources, and health risk assessment of VOCs/SVOCs in soils obtained from petrochemical-contaminated sites in Guangzhou, a subtropical coastal megacity in southern China. J. Clean. Prod. 2023, 426, 139198. [Google Scholar] [CrossRef]
- Hao, R.; Xue, S.; Sun, H.; Yang, T.; Wang, H. Emission Characteristics and the Environmental Impact of VOCs from Typical FRP Manufacture Industry. Atmosphere 2022, 13, 1274. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Li, G.; An, T. Using an integrated decontamination technique to remove VOCs and attenuate health risks from an e-waste dismantling workshop. Chem. Eng. J. 2017, 318, 57–63. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Chai, X.; Xu, L.; Zhang, L.; Ning, P.; Huang, J.; Tian, S. Interaction of inhalable volatile organic compounds and pulmonary surfactant: Potential hazards of VOCs exposure to lung. J. Hazard. Mater. 2019, 369, 512–520. [Google Scholar] [CrossRef]
- Mozaffar, A.; Zhang, Y.-L. Atmospheric volatile organic compounds (VOCs) in China: A review. Curr. Pollut. Rep. 2020, 6, 250–263. [Google Scholar] [CrossRef]
- Yoon, H.-I.; Hong, Y.-C.; Cho, S.-H.; Kim, H.; Kim, Y.H.; Sohn, J.R.; Kwon, M.; Park, S.-H.; Cho, M.-H.; Cheong, H.-K. Exposure to volatile organic compounds and loss of pulmonary function in the elderly. Eur. Respir. J. 2010, 36, 1270–1276. [Google Scholar] [CrossRef]
- Armenta-Reséndiz, M.; Ríos-Leal, E.; Rivera-García, M.T.; López-Rubalcava, C.; Cruz, S.L. Structure-activity study of acute neurobehavioral effects of cyclohexane, benzene, m-xylene, and toluene in rats. Toxicol. Appl. Pharmacol. 2019, 376, 38–45. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Dwivedi, P.; Gaur, V.; Sharma, A.; Verma, N. Comparative study of removal of volatile organic compounds by cryogenic condensation and adsorption by activated carbon fiber. Sep. Purif. Technol. 2004, 39, 23–37. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Davis, R.J.; Zeiss, R.F. Cryogenic condensation: A cost-effective technology for controlling VOC emissions. Environ. Prog. 2002, 21, 111–115. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, Y.; Yuan, J.; Guo, X.; Zhang, Q. Advances in Adsorption, Absorption, and Catalytic Materials for VOCs Generated in Typical Industries. Energies 2024, 17, 1861. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Yu, S.; Chen, H.; Guo, Y.; Zhou, C.; Zeng, Z.; Li, L. Designing activated carbon and porous carbon nanofibers for insight into their differences in adsorption affinity mechanisms of VOCs. Appl. Surf. Sci. 2024, 659, 159961. [Google Scholar] [CrossRef]
- Wang, X.; Chai, X.; Huang, W.; Li, X.; Zhu, B.; Li, X.; Zhou, Y.; Yang, Z.; Sun, X.; Fu, L. Green synthesis of biomass-derived porous carbon with hierarchical pores and enhanced surface area for superior VOCs adsorption. Mater. Today Commun. 2024, 39, 108906. [Google Scholar] [CrossRef]
- Zhang, L.; Weng, H.-X.; Gao, C.-J.; Chen, H.-L. Remove volatile organic compounds (VOCs) with membrane separation techniques. J. Environ. Sci. 2002, 14, 181–187. [Google Scholar]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Li, K.; Luo, X. Research Progress on Catalytic Combustion of Volatile Organic Compounds in Industrial Waste Gas. Catalysts 2023, 13, 268. [Google Scholar] [CrossRef]
- Wang, Q.; Yeung, K.L.; Bañares, M.A. Ceria and its related materials for VOC catalytic combustion: A review. Catal. Today 2020, 356, 141–154. [Google Scholar] [CrossRef]
- Wang, F.; Lei, X.; Hao, X. Key factors in the volatile organic compounds treatment by regenerative thermal oxidizer. J. Air Waste Manag. Assoc. 2020, 70, 557–567. [Google Scholar] [CrossRef]
- Morrone, P.; Di Maio, F.P.; Di Renzo, A.; Amelio, M. Modeling process characteristics and performance of fixed and fluidized bed regenerative thermal oxidizer. Ind. Eng. Chem. Res. 2006, 45, 4782–4790. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile organic compounds (VOCs) removal by photocatalysts: A review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of volatile organic compounds and their effects on biodegradability under co-existing conditions. Microbes Environ. 2017, 32, 188–200. [Google Scholar] [CrossRef]
- IIjima, S.; IIjima, S.; Nakayama, K.; Kubota, M.; Matsuda, H. Study on High-efficiency Heat Storage and Heat Release Conditions in a Pilot-scale Regenerative Thermal Oxidizer for Toluene Decomposition. J. CJpn. Soc. Mater. Cycles Waste Manag. 2009, 20, 252–261. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of Indoor Volatile Organic Compounds via Photocatalytic Oxidation: A Short Review and Prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, J.; Tian, C.; Zhang, Z.; Zhang, X.; Zhang, Z.; Li, D. Research on the progress of VOCs adsorption by biomass nanocomposites. J. Phys. Conf. Ser. 2022, 2022, 012023. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Favre, E. Energy e29fficiency of a hybrid membrane/condensation process for VOC (Volatile Organic Compounds) recovery from air: A generic approach. Energy 2016, 95, 291–302. [Google Scholar] [CrossRef]
- Strots, V.O.; Bunimovich, G.A.; Roach, C.R.; Matros, Y.S. Chapter 10—Regenerative catalytic oxidizer technology for VOC control. In Reaction Engineering for Pollution Prevention; Abraham, M.A., Hesketh, R.P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 113–126. [Google Scholar]
- Wu, L.; Zhu, Y.; Yuan, J.; Guo, X.; Zhang, Q. Volatile Organic Compounds (VOCs) in China: Progress and Prospects of Research on Treatment Technologies and Policy Provisions. J. Mater. Sci. Chem. Eng. 2024, 12, 1–43. [Google Scholar] [CrossRef]
- Lin, L.; Chai, Y.; Zhao, B.; Wei, W.; He, D.; He, B.; Tang, Q. Photocatalytic oxidation for degradation of VOCs. Open J. Inorg. Chem. 2013, 3, 14–25. [Google Scholar] [CrossRef]
- Datta, A.; Philip, L. Biodegradation of Volatile Organic Compounds from Paint Industries. Appl. Biochem. Biotechnol. 2012, 167, 564–580. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Z. Experimental and Numerical Investigations into Temperature Distributions and VOC Conversion Rate of RTO. IOP Conf. Ser. Earth Environ. Sci. 2021, 943, 012014. [Google Scholar] [CrossRef]
- Matros, Y.S.; Bunimovich, G.A.; Patterson, S.E.; Meyer, S.F. Is it economically feasible to use heterogeneous catalysts for VOC control in regenerative oxidizers? Catal. Today 1996, 27, 307–313. [Google Scholar] [CrossRef]
- Chou, M.-S.; Hei, C.-M.; Huang, Y.-W. Regenerative Thermal Oxidation of Airborne N, N-Dimethylformamide and Its Associated Nitrogen Oxides Formation Characteristics. J. Air Waste Manag. Assoc. 2007, 57, 991–999. [Google Scholar] [CrossRef]
- Iijima, S.; Nakayama, K.; Kuchar, D.; Kubota, M.; Matsuda, H. Optimum conditions for effective decomposition of toluene as VOC gas by pilot-scale regenerative thermal oxidizer. Int. J. Energy Power Eng. 2008, 2, 1589–1594. [Google Scholar][Green Version]
- Cannon, B.J. Dual-chamber RTO oxidizers provide cost-effective VOC compliance for metal finishers and coaters. Met. Finish. 2003, 101, 53–56. [Google Scholar] [CrossRef]
- Giuntini, L.; Bertei, A.; Tortorelli, S.; Percivale, M.; Paoletti, E.; Nicolella, C.; Galletti, C. Coupled CFD and 1-D dynamic modeling for the analysis of industrial Regenerative Thermal Oxidizers. Chem. Eng. Process.-Process Intensif. 2020, 157, 108117. [Google Scholar] [CrossRef]
- Hao, X.; Li, R.; Wang, J.; Yang, X. Numerical simulation of a regenerative thermal oxidizer for volatile organic compounds treatment. Environ. Eng. Res. 2018, 23, 397–405. [Google Scholar] [CrossRef]
- Amelio, M.; Florio, G.; Morrone, P.; Senatore, S. The influence of rotary valve distribution systems on the energetic efficiency of regenerative thermal oxidizers (RTO). Int. J. Energy Res. 2008, 32, 24–34. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Cao, L.; Guo, Y.; Jia, J. Development and Field-Scale Optimization of a Honeycomb Zeolite Rotor Concentrator/Recuperative Oxidizer for the Abatement of Volatile Organic Carbons from Semiconductor Industry. Environ. Sci. Technol. 2012, 46, 441–446. [Google Scholar] [CrossRef]
- Ruaud, M.; Loison, J.C.; Hickson, K.M.; Gratier, P.; Hersant, F.; Wakelam, V. Modelling complex organic molecules in dense regions: Eley–Rideal and complex induced reaction. Mon. Not. R. Astron. Soc. 2015, 447, 4004–4017. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Weinberg, W.H. Eley− Rideal surface chemistry: Direct reactivity of gas phase atomic hydrogen with adsorbed species. Acc. Chem. Res. 1996, 29, 479–487. [Google Scholar] [CrossRef]
- Vannice, M.A. An analysis of the Mars–van Krevelen rate expression. Catal. Today 2007, 123, 18–22. [Google Scholar] [CrossRef]
- Baxter, R.; Hu, P. Insight into why the Langmuir–Hinshelwood mechanism is generally preferred. J. Chem. Phys. 2002, 116, 4379–4381. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Lou, B.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.; Zhao, Y.; Zhao, W.; Jiang, Y.; Rui, Y. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Rooke, J.C.; Barakat, T.; Brunet, J.; Li, Y.; Finol, M.F.; Lamonier, J.-F.; Giraudon, J.-M.; Cousin, R.; Siffert, S.; Su, B.L. Hierarchically nanostructured porous group Vb metal oxides from alkoxide precursors and their role in the catalytic remediation of VOCs. Appl. Catal. B Environ. 2015, 162, 300–309. [Google Scholar] [CrossRef]
- Liu, L.; Song, Y.; Fu, Z.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of preparation method on the surface characteristics and activity of the Pd/OMS-2 catalysts for the oxidation of carbon monoxide, toluene, and ethyl acetate. Appl. Surf. Sci. 2017, 396, 599–608. [Google Scholar] [CrossRef]
- Gennequin, C.; Lamallem, M.; Cousin, R.; Siffert, S.; Aïssi, F.; Aboukaïs, A. Catalytic oxidation of VOCs on Au/Ce-Ti-O. Catal. Today 2007, 122, 301–306. [Google Scholar] [CrossRef]
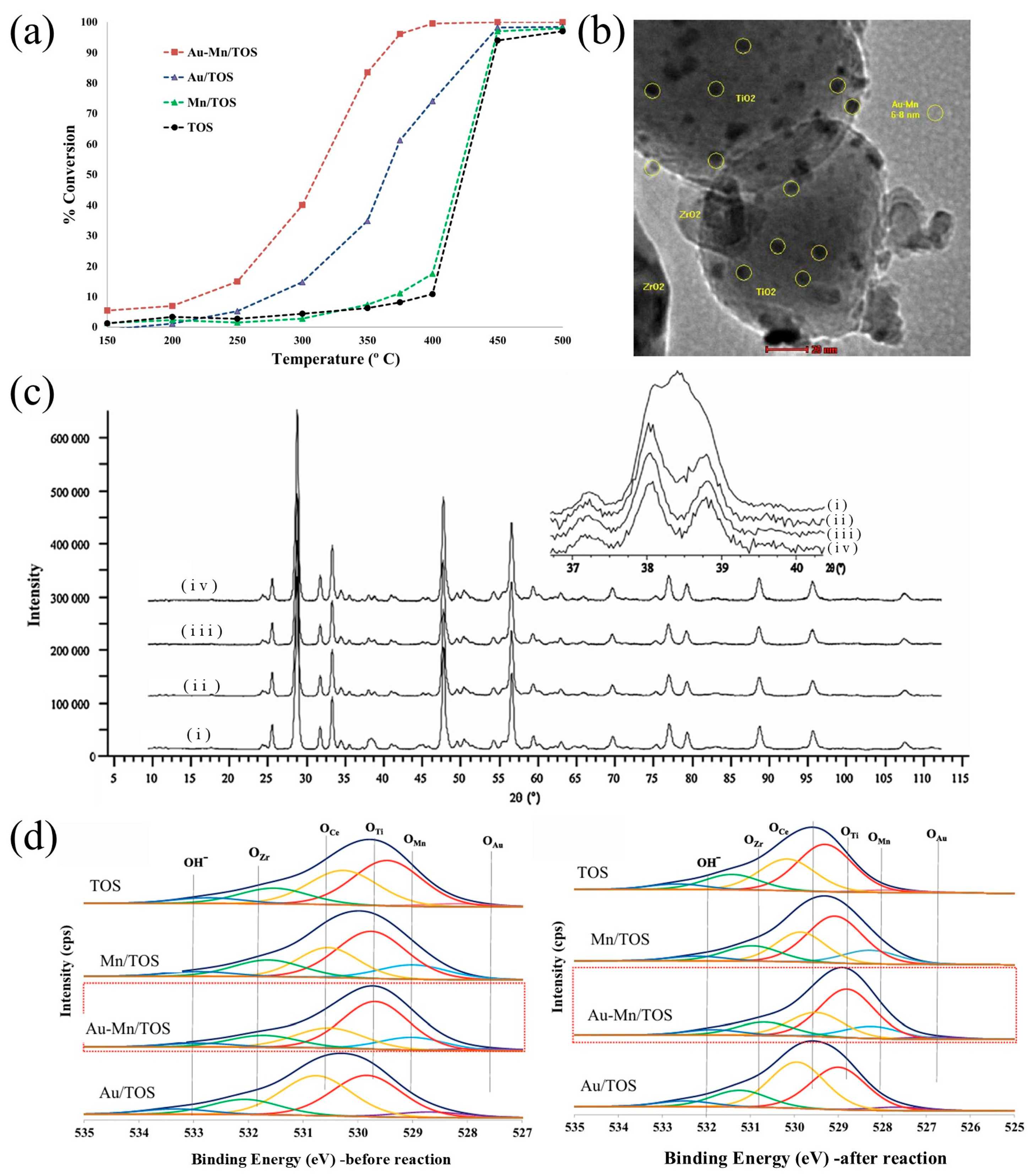
- Ali, A.M.; Daous, M.A.; Khamis, A.A.M.; Driss, H.; Burch, R.; Petrov, L.A. Strong synergism between gold and manganese in an Au–Mn/triple-oxide-support (TOS) oxidation catalyst. Appl. Catal. A Gen. 2015, 489, 24–31. [Google Scholar] [CrossRef]
- Ousmane, M.; Liotta, L.F.; Carlo, G.D.; Pantaleo, G.; Venezia, A.M.; Deganello, G.; Retailleau, L.; Boreave, A.; Giroir-Fendler, A. Supported Au catalysts for low-temperature abatement of propene and toluene, as model VOCs: Support effect. Appl. Catal. B Environ. 2011, 101, 629–637. [Google Scholar] [CrossRef]
- Tabakova, T.; Ilieva, L.; Petrova, P.; Venezia, A.M.; Avdeev, G.; Zanella, R.; Karakirova, Y. Complete benzene oxidation over mono and bimetallic Au–Pd catalysts supported on Fe-modified ceria. Chem. Eng. J. 2015, 260, 133–141. [Google Scholar] [CrossRef]
- Tabakova, T.; Kolentsova, E.; Dimitrov, D.; Ivanov, K.; Manzoli, M.; Venezia, A.; Karakirova, Y.; Petrova, P.; Nihtianova, D.; Avdeev, G. CO and VOCs catalytic oxidation over alumina supported Cu–Mn catalysts: Effect of Au or Ag deposition. Top. Catal. 2017, 60, 110–122. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. Performance of doped Pt/TiO2 (W6+) catalysts for combustion of volatile organic compounds (VOCs). Appl. Catal. B Environ. 1998, 15, 75–92. [Google Scholar] [CrossRef]
- Joung, H.-J.; Kim, J.-H.; Oh, J.-S.; You, D.-W.; Park, H.-O.; Jung, K.-W. Catalytic oxidation of VOCs over CNT-supported platinum nanoparticles. Appl. Surf. Sci. 2014, 290, 267–273. [Google Scholar] [CrossRef]
- Xu, Z.; Mo, S.; Li, Y.; Zhang, Y.; Wu, J.; Fu, M.; Niu, X.; Hu, Y.; Ye, D. Pt/MnOx for toluene mineralization via ozonation catalysis at low temperature: SMSI optimization of surface oxygen species. Chemosphere 2022, 286, 131754. [Google Scholar] [CrossRef]
- Gaálová, J.; Topka, P.; Kaluža, L.; Šolcová, O. Gold versus platinum on ceria–zirconia mixed oxides in oxidation of ethanol and toluene. Catal. Today 2011, 175, 231–237. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Morales-Torres, S.; Kapteijn, F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Moreno-Castilla, C.; Moulijn, J.A. Carbon-based monolithic supports for palladium catalysts: The role of the porosity in the gas-phase total combustion of m-xylene. Appl. Catal. B Environ. 2008, 77, 272–277. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Chen, J.; Yang, Y.; Wang, Y. Excellent catalytic activity and water resistance of UiO-66-supported highly dispersed Pd nanoparticles for toluene catalytic oxidation. Appl. Catal. B Environ. 2020, 269, 118767. [Google Scholar] [CrossRef]
- Hosseini, M.; Barakat, T.; Cousin, R.; Aboukaïs, A.; Su, B.L.; De Weireld, G.; Siffert, S. Catalytic performance of core–shell and alloy Pd–Au nanoparticles for total oxidation of VOC: The effect of metal deposition. Appl. Catal. B Environ. 2012, 111–112, 218–224. [Google Scholar] [CrossRef]
- Ren, S.; Liang, W.; Li, Q.; Zhu, Y. Effect of Pd/Ce loading on the performance of Pd–Ce/γ-Al2O3 catalysts for toluene abatement. Chemosphere 2020, 251, 126382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Liu, F.; He, H. Well-dispersed palladium supported on ordered mesoporous Co3O4 for catalytic oxidation of o-xylene. Appl. Catal. B Environ. 2013, 142–143, 72–79. [Google Scholar] [CrossRef]
- Musialik-Piotrowska, A.; Syczewska, K. Catalytic oxidation of trichloroethylene in two-component mixtures with selected volatile organic compounds. Catal. Today 2002, 73, 333–342. [Google Scholar] [CrossRef]
- Musialik-Piotrowska, A. Destruction of trichloroethylene (TCE) and trichloromethane (TCM) in the presence of selected VOCs over Pt-Pd-based catalyst. Catal. Today 2007, 119, 301–304. [Google Scholar] [CrossRef]
- He, C.; Xu, B.-T.; Shi, J.-W.; Qiao, N.-L.; Hao, Z.-P.; Zhao, J.-L. Catalytic destruction of chlorobenzene over mesoporous ACeOx (A=Co, Cu, Fe, Mn, or Zr) composites prepared by inorganic metal precursor spontaneous precipitation. Fuel Process. Technol. 2015, 130, 179–187. [Google Scholar] [CrossRef]
- García, T.; Solsona, B.; Taylor, S.H. Naphthalene total oxidation over metal oxide catalysts. Appl. Catal. B Environ. 2006, 66, 92–99. [Google Scholar] [CrossRef]
- Shie, J.-L.; Chang, C.-Y.; Chen, J.-H.; Tsai, W.-T.; Chen, Y.-H.; Chiou, C.-S.; Chang, C.-F. Catalytic oxidation of naphthalene using a Pt/Al2O3 catalyst. Appl. Catal. B Environ. 2005, 58, 289–297. [Google Scholar] [CrossRef]
- Aparicio, M.S.L.; Lick, I.D. Total oxidation of propane and naphthalene from emission sources with supported cobalt catalysts. React. Kinet. Mech. Catal. 2016, 119, 469–479. [Google Scholar] [CrossRef]
- Lv, X.; Cai, S.; Chen, J.; Yan, D.; Jiang, M.; Chen, J.; Jia, H. Tuning the degradation activity and pathways of chlorinated organic pollutants over CeO2 catalyst with acid sites: Synergistic effect of Lewis and Brønsted acid sites. Catal. Sci. Technol. 2021, 11, 4581–4595. [Google Scholar] [CrossRef]
- Ismail, A.; Zahid, M.; Hu, B.; Khan, A.; Ali, N.; Zhu, Y. Effect of Morphology-Dependent Oxygen Vacancies of CeO2 on the Catalytic Oxidation of Toluene. Catalysts 2022, 12, 1034. [Google Scholar] [CrossRef]
- Assebban, M.; Tian, Z.-Y.; El Kasmi, A.; Bahlawane, N.; Harti, S.; Chafik, T. Catalytic complete oxidation of acetylene and propene over clay versus cordierite honeycomb monoliths without and with chemical vapor deposited cobalt oxide. Chem. Eng. J. 2015, 262, 1252–1259. [Google Scholar] [CrossRef]
- de Rivas, B.; López-Fonseca, R.; Jiménez-González, C.; Gutiérrez-Ortiz, J.I. Highly active behaviour of nanocrystalline Co3O4 from oxalate nanorods in the oxidation of chlorinated short chain alkanes. Chem. Eng. J. 2012, 184, 184–192. [Google Scholar] [CrossRef]
- Ma, C.; Mu, Z.; He, C.; Li, P.; Li, J.; Hao, Z. Catalytic oxidation of benzene over nanostructured porous Co3O4-CeO2 composite catalysts. J. Environ. Sci. 2011, 23, 2078–2086. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vázquez, I.; Dejoz, A.; Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts. Appl. Catal. B Environ. 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.; Lu, G. Low-temperature catalytic combustion of trichloroethylene over cerium oxide and catalyst deactivation. Appl. Catal. B Environ. 2008, 81, 192–202. [Google Scholar] [CrossRef]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Behar, S.; Gonzalez, P.; Agulhon, P.; Quignard, F.; Świerczyński, D. New synthesis of nanosized Cu–Mn spinels as efficient oxidation catalysts. Catal. Today 2012, 189, 35–41. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhang, Y.; Yu, Y.; He, H.; Qin, X.; Wang, B. Shape dependence of nanoceria on complete catalytic oxidation of o-xylene. Catal. Sci. Technol. 2016, 6, 4840–4848. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Liu, J.; Tian, Z.; Jing, Y. Regulation of oxygen vacancies in cobalt-cerium oxide catalyst for boosting decontamination of VOCs by catalytic oxidation. Sep. Purif. Technol. 2021, 277, 119505. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Chen, X.; Konsolakis, M.; Psarras, A.C.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of toluene on Ce–Co and La–Co mixed oxides synthesized by exotemplating and evaporation methods. Catal. Today 2015, 244, 161–171. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Liu, G.; Li, S.; Li, D.; Li, W.; Chen, Y. Preparation of hierarchical layer-stacking Mn-Ce composite oxide for catalytic total oxidation of VOCs. J. Rare Earths 2015, 33, 62–69. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, L.; Guan, Q.; Fu, M.; Ye, D.; Wu, T. Catalytic oxidation of toluene over Au–Co supported on SBA-15. Mater. Res. Bull. 2015, 70, 567–572. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Solsona, B.; Agouram, S.; Sanchis, R.; López, J.M.; García, T.; Zanella, R. Low temperature total oxidation of toluene by bimetallic Au–Ir catalysts. Catal. Sci. Technol. 2017, 7, 2886–2896. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Liu, S. SnOx–MnOx–TiO2 catalysts with high resistance to chlorine poisoning for low-temperature chlorobenzene oxidation. Appl. Catal. A Gen. 2014, 482, 363–369. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Li, W.; Wu, X.; Tang, W.; Chen, Y. Effect of Cu substitution on promoted benzene oxidation over porous CuCo-based catalysts derived from layered double hydroxide with resistance of water vapor. Appl. Catal. B Environ. 2015, 166–167, 260–269. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, F.; Tong, J.; Qi, C. Complete oxidation of benzene with cobalt oxide and ceria using the mesoporous support SBA-16. Appl. Catal. A Gen. 2013, 467, 1–6. [Google Scholar] [CrossRef]
- de Rivas, B.; Sampedro, C.; García-Real, M.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I. Promoted activity of sulphated Ce/Zr mixed oxides for chlorinated VOC oxidative abatement. Appl. Catal. B Environ. 2013, 129, 225–235. [Google Scholar] [CrossRef]
- Zhou, G.; He, X.; Liu, S.; Xie, H.; Fu, M. Phenyl VOCs catalytic combustion on supported CoMn/AC oxide catalyst. J. Ind. Eng. Chem. 2015, 21, 932–941. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Li, W.; Chen, Y. Porous Mn–Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Chen, X.; Carabineiro, S.A.C.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of ethyl acetate over La-Co and La-Cu oxides. J. Environ. Chem. Eng. 2014, 2, 344–355. [Google Scholar] [CrossRef]
- Genty, E.; Cousin, R.; Capelle, S.; Gennequin, C.; Siffert, S. Catalytic Oxidation of Toluene and CO over Nanocatalysts Derived from Hydrotalcite-Like Compounds (X62+ Al23+): Effect of the Bivalent Cation. Eur. J. Inorg. Chem. 2012, 2012, 2802–2811. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Tian, P.; Zhou, J.; Tang, R.; Wu, S. A highly active and anti-coking Pd-Pt/SiO2 catalyst for catalytic combustion of toluene at low temperature. Appl. Catal. A Gen. 2017, 529, 60–67. [Google Scholar] [CrossRef]
- Liu, F.; He, H. Selective catalytic reduction of NO with NH3 over manganese substituted iron titanate catalyst: Reaction mechanism and H2O/SO2 inhibition mechanism study. Catal. Today 2010, 153, 70–76. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(χ)-CeO2 at low temperatures. Appl. Catal. B Environ. 2017, 203, 199–209. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.; Yu, Y.; He, C.; Shen, Z.; Liu, H. Regeneration and sulfur poisoning behavior of In/H-BEA catalyst for NOx reduction by CH4. Appl. Surf. Sci. 2017, 401, 120–126. [Google Scholar] [CrossRef]
- Oudar, J. Sulfur Adsorption and Poisoning of Metallic Catalysts. Catal. Rev. 1980, 22, 171–195. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Zhang, L.; Zhao, Z.; Li, X.; Wang, Y.; Xie, S.; Yang, H.; Guo, G. Controlled Generation of Uniform Spherical LaMnO3, LaCoO3, Mn2O3, and Co3O4 Nanoparticles and Their High Catalytic Performance for Carbon Monoxide and Toluene Oxidation. Inorg. Chem. 2013, 52, 8665–8676. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Dorin Popa, P.; Doroftei, C.; Ignat, M. Some nanograined ferrites and perovskites for catalytic combustion of acetone at low temperature. Ceram. Int. 2015, 41 Pt B, 4430–4437. [Google Scholar] [CrossRef]
- Tarjomannejad, A.; Farzi, A.; Niaei, A.; Salari, D. An experimental and kinetic study of toluene oxidation over LaMn1−xBxO3 and La0.8A0.2Mn0.3B0.7O3 (A=Sr, Ce and B=Cu, Fe) nano-perovskite catalysts. Korean J. Chem. Eng. 2016, 33, 2628–2637. [Google Scholar] [CrossRef]



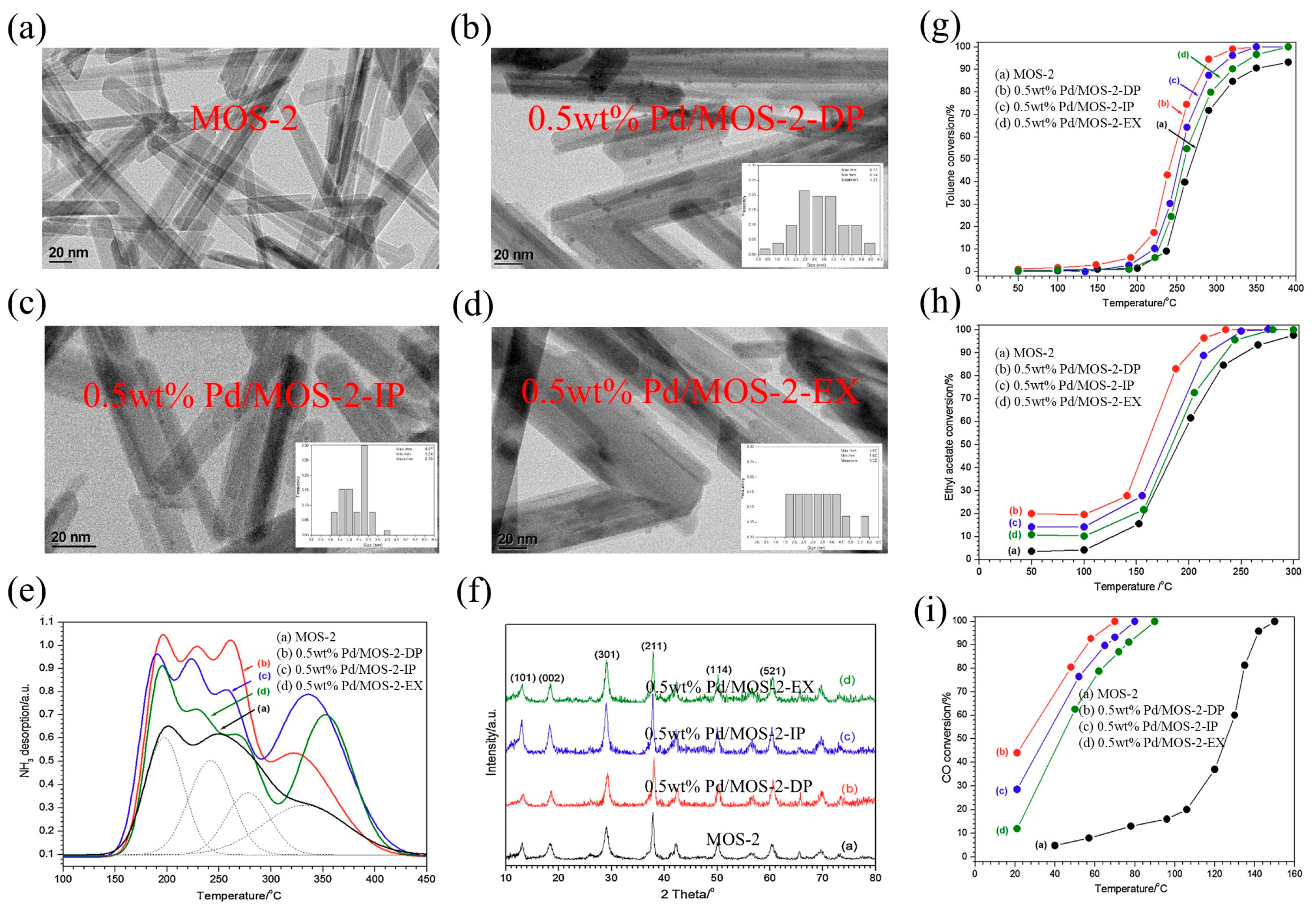
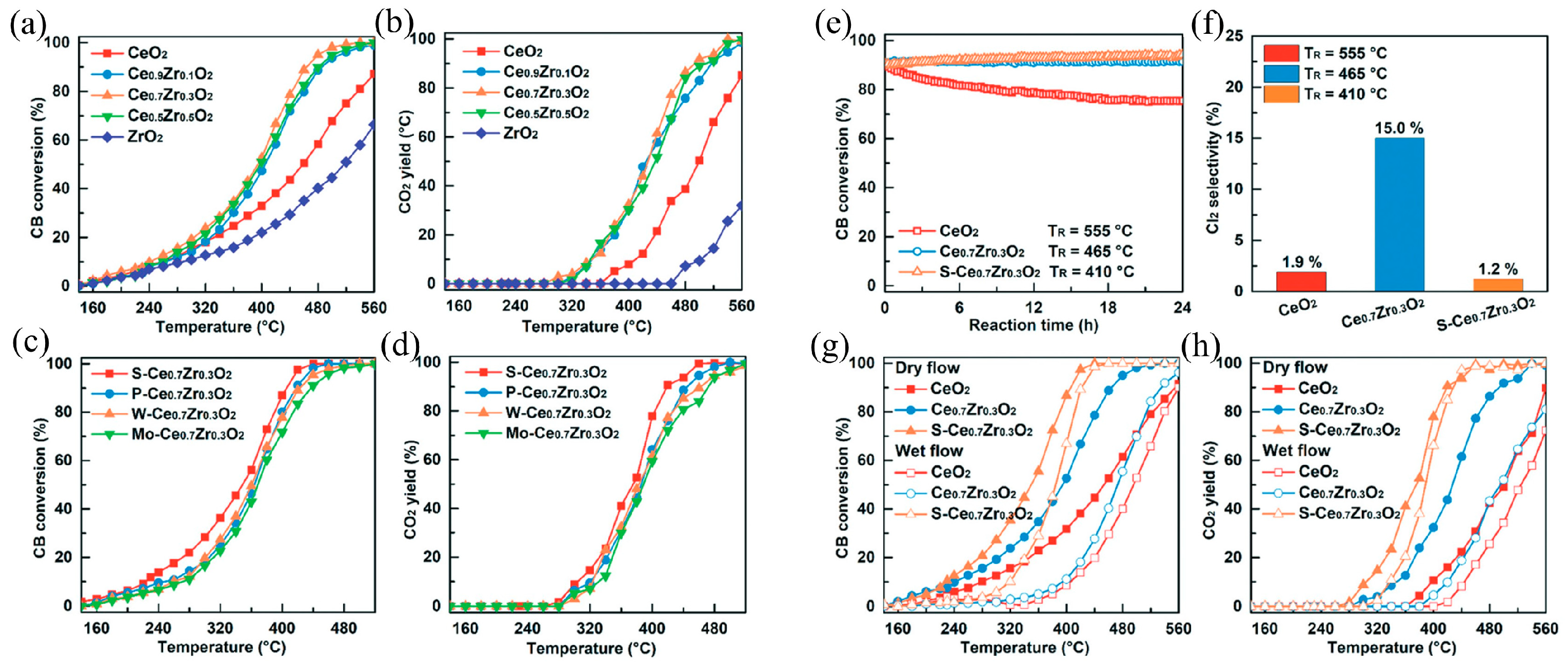
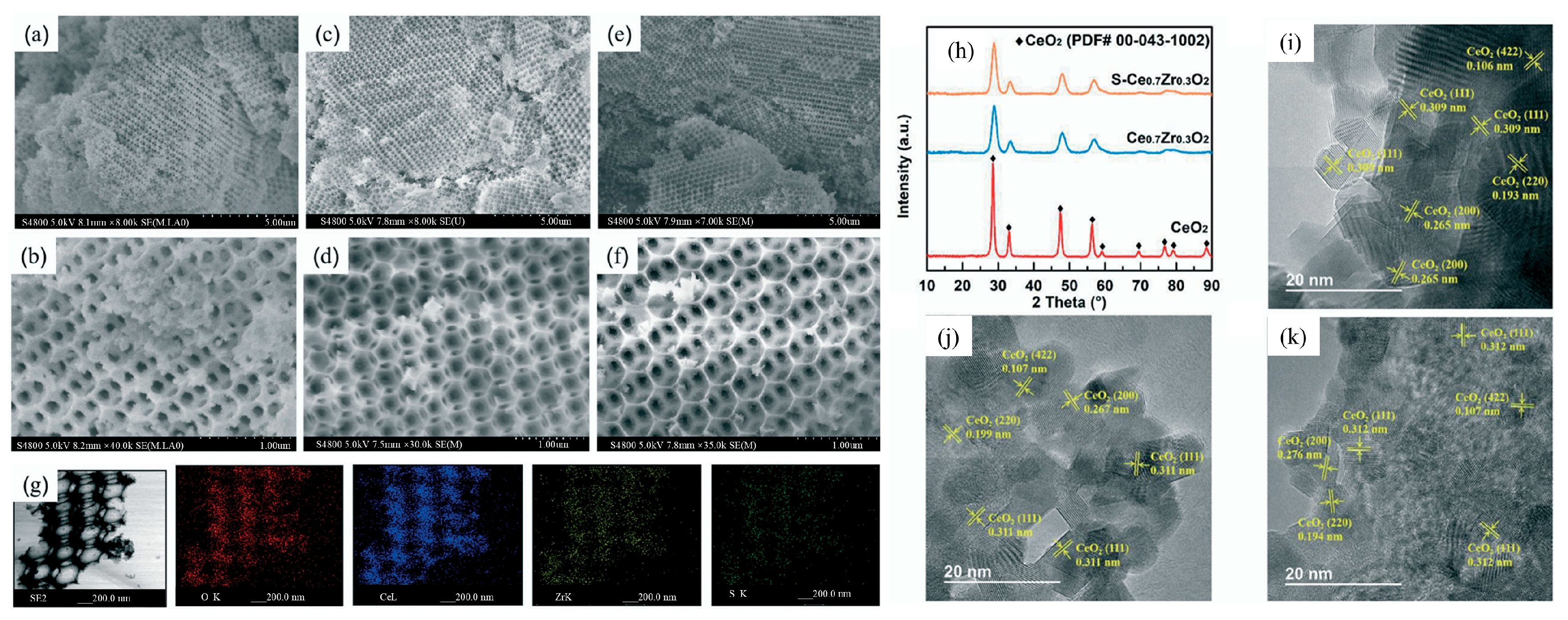

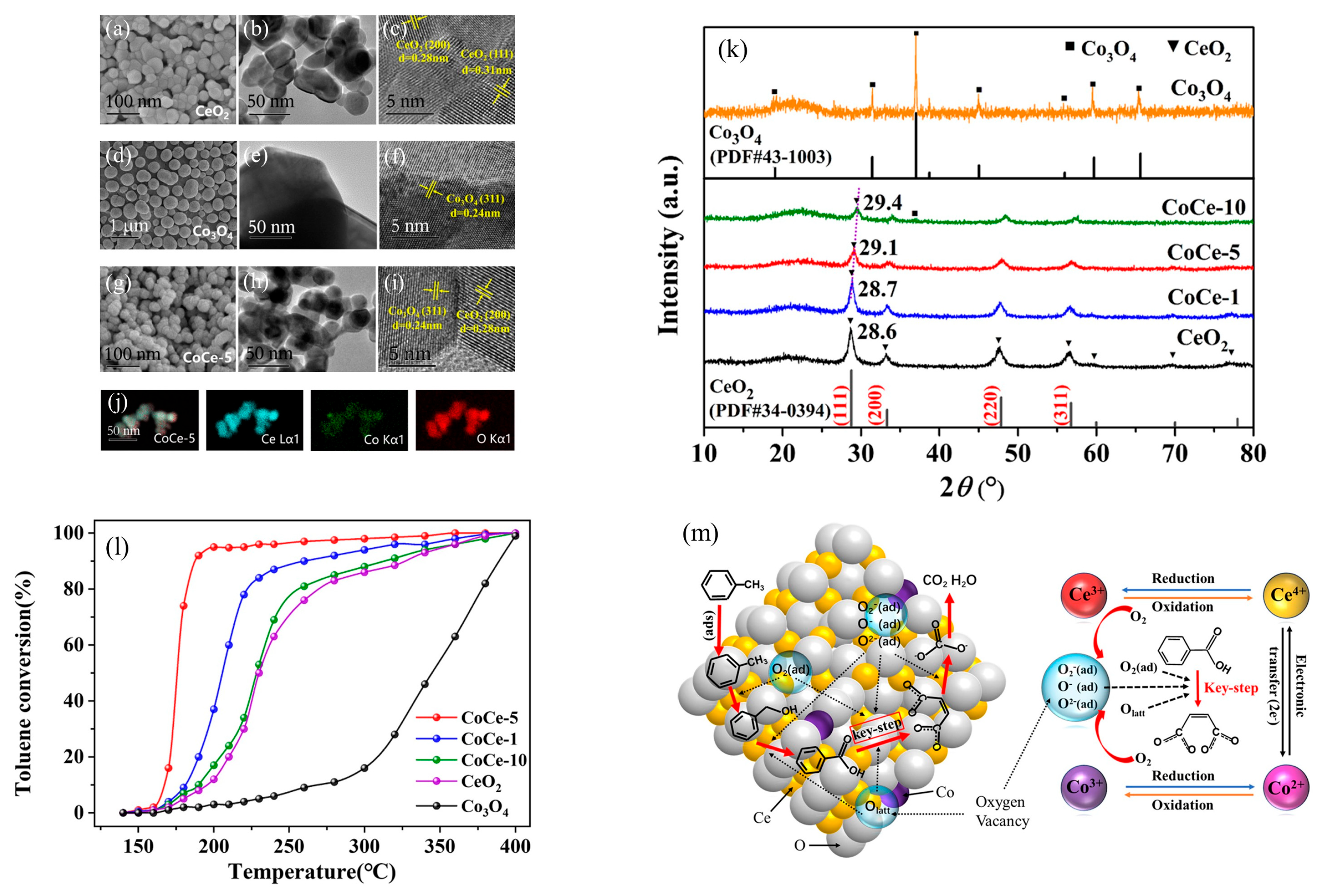

| Types of VOCs | Main Representative Objects |
|---|---|
| Hydrocarbons | Toluene, N-hexane, benzene, xylene, cyclohexane, etc. |
| Aldehydes and ketones | Formaldehyde, acetaldehyde, acetone, cyclohexanone, etc. |
| Ethers | Methyl ether, ethyl ether, dimethyl ether, benzyl ether, benzyl ether, epoxyethane, etc. |
| Esters | Ethyl acetate, butyl acetate, methyl acetate, ethyl formate, etc. |
| Cyanide | Hydrogen cyanide, acrylonitrile, acetonitrile, sodium cyanide, potassium cyanide, benzonitrile, etc. |
| Amide | Dimethylformamide, diethylformamide, methylacetamide, formamide, etc. |
| Halogenated hydrocarbon | Chloroethylene, dichloromethane, trichloroethylene, bromomethane, freon, etc. |
| Processing Technology | Processing Methods | Specificities |
|---|---|---|
| Separation and recycling technology | Absorption method | Simple equipment, easy to operate, low cost, but low processing efficiency, poor effect on non-polar or insoluble in water exhaust gas [19]. |
| Adsorption method | High processing efficiency, large equipment volume, no secondary pollution, low cost, easy to clog, and humidity and temperature affect the treatment effect [17]. | |
| Condensing method | Simple process, easy to operate, high energy consumption, large operating costs, high treatment costs [34]. | |
| Membrane separation method | Simple process, high processing efficiency, low energy consumption, no secondary pollution, high cost of membrane material [22], more suitable for high-concentration gas treatment. | |
| Decomposition and conversion technologies | RCO method | Low energy consumption, high purification efficiency, no secondary pollution, expensive catalyst cost, with a need to be replaced regularly [35]. |
| RTO method | Large initial investment, large footprint, high removal rate, applicable to various types and concentrations of VOCs [36]. | |
| Photocatalytic method | Photocatalytic device equipment investment is small, simple structure, easy operation and maintenance, high requirements for light source and catalyst, no secondary pollution [37]. | |
| Biodegradation method | Slow reaction speed, large floor space, processing efficiency greatly affected by environmental conditions, low operating costs [38]. |
| Type | Two-Chamber RTO | Three-Chamber RTO | Rotary RTO | Single-Tube Multi-Valve RTO | Notes |
|---|---|---|---|---|---|
| Removal efficiency | 95% | 99% | 99.5% | 99.5% | |
| Thermal efficiency | 90% | 95% | 95% | 95% | |
| Maximum concentration handling | <1 g/m3 | <5 g/m3 | <5 g/m3 | <10 g/m3 | 50 mg/m3 is emission standard |
| Number of valves | 4 | 9 | 1 | 15 | |
| Valve form | Push-down valve/lift valve | Push-down valve/lift valve | Rotary valve | Triple eccentric hard-sealing butterfly valve | |
| Valve life | 1–2 years | 1–2 years | 0.5–1 years | 3–5 years | |
| Number of heat storage chambers | 2 | 3 | 12 | 5 | |
| Land occupation | 100% | 130% | 65% | 65% | Using a two-chamber RTO as the baseline |
| Catalyst | Support | VOCs | Temperature (°C) | Conversion (%) | Reference |
|---|---|---|---|---|---|
| Au | CeZr | Ethanol | 222 | 90 | [55] |
| Au | CeZr | Toluene | 322 | 90 | |
| Au | Ce0.3Ti0.7O2 | Propylene | 293 | 100 | [57] |
| Au | Ce0.1Ti0.9O2 | Propylene | 300 | 100 | |
| Au | TiO2 | Propylene | 400 | 100 | |
| Au | CeO2/ZrO2/TiO2 | Propylene | 450 | 95 | [58] |
| Au | CeO2 | Propene | 230 | 100 | [59] |
| Au | Ce7.5/Al2O3 | Propene | 250 | 100 | |
| Au | TiO2 | Propene | 320 | 100 | |
| Au | Al2O3 | Propene | 341 | 100 | |
| Au | CeO2 | Toluene | 293 | 100 | |
| Au | Ce7.5/Al2O3 | Toluene | 330 | 100 | |
| Au | TiO2 | Toluene | 400 | 100 | |
| Au | Al2O3 | Toluene | 450 | 100 | |
| Au | CeO2/Fe2O | Benzene | 200 | 100 | [60] |
| Au | Cu:Mn 2:1 | Methanol | 180 | 92 | [61] |
| Au | Cu:Mn 2:1 | Dimethyl ether | 360 | 95 | |
| Au | CuO | Ethyl acetate | 289 | 100 | [62] |
| Au | Fe2O3 | Ethyl acetate | 354 | 100 | |
| Au | La2O3 | Ethyl acetate | 325 | 100 | |
| Au | MgO | Ethyl acetate | 290 | 100 | |
| Au | NiO | Ethyl acetate | 345 | 100 | |
| Au | CuO | Toluene | 315 | 100 | |
| Au | Fe2O3 | Toluene | 345 | 100 | |
| Au | La2O3 | Toluene | >400 | 100 | |
| Au | MgO | Toluene | 387 | 100 | |
| Au | NiO | Toluene | 320 | 100 | |
| Pt | γ-Al2O3 | Ethyl acetate | 310 | >90 | [63] |
| Pt | TiO2 | Ethyl acetate | 260 | >90 | |
| Pt | TiO2(0.45% W6+) | Ethyl acetate | 220 | >90 | |
| Pt | γ-Al2O3 | Benzene | 180 | 90 | |
| Pt | TiO2 | Benzene | 170 | 90 | |
| Pt | TiO2(0.45% W6+) | Benzene | 160 | 90 | |
| Pt | Carbon nanotubes | Benzene | 112 | 100 | [64] |
| Pt | Carbon nanotubes | Toluene | 109 | 100 | |
| Pt | Carbon nanotubes | Ethylbenzene | 106 | 100 | |
| Pt | Carbon nanotubes | Meta xylene | 104 | 100 | |
| Pt | MnOx-T | Toluene | 30 | 98 | [65] |
| Pt | MnOx-LT | Toluene | 70 | 99 | |
| Pt | MnOx-B | Toluene | 90 | 99 | |
| Pt | MnOx-HB | Toluene | 90 | 99 | |
| Pt | CeZr | Ethanol | 193 | 90 | [66] |
| Pt | CeZr | Toluene | 207 | 90 | |
| Pd | Carbon | m-Xylene | 170 | 100 | [67] |
| Pd | UiO-66 | Toluene | 200 | 100 | [68] |
| Pd | TiO2 | Toluene | 260 | 100 | [69] |
| Pd | γ-Al2O3 | Toluene | 200 | 90 | [70] |
| Pd | Nb2O5 | Toluene | 285 | 90 | [55] |
| Pd | Ta2O5 | Toluene | 281 | 90 | |
| Pd | Co3O4(3D) | O-xylene | 196 | 90 | [71] |
| Pd | Co3O4(3DL) | O-xylene | 254 | 90 |
| Catalyst | VOCs | T90 (°C) | T100 (°C) | Reference |
|---|---|---|---|---|
| CuO | Ethyl acetate | 290 | 311 | [62] |
| Fe2O3 | Ethyl acetate | 355 | 370 | |
| La2O3 | Ethyl acetate | 367 | 384 | |
| MgO | Ethyl acetate | >400 | >400 | |
| NiO | Ethyl acetate | 344 | 365 | |
| CuO | Toluene | 309 | 330 | |
| Fe2O3 | Toluene | 394 | - | |
| La2O3 | Toluene | >400 | >400 | |
| MgO | Toluene | >400 | >400 | |
| NiO | Toluene | 324 | 330 | |
| Co3O4 | Acetylene | - | 360 | [80] |
| Co3O4 | Propylene | - | 460 | |
| Co3O4 | 1,2-Dichloroethane | - | 350 | [81] |
| Co3O4 | Benzene | 263 | - | [82] |
| Nb2O5 | Toluene | 400 | [55] | |
| Mn3O4 | Toluene | 270 | - | [83] |
| Mn2O3 | Toluene | 295 | - | |
| MnO2 | Toluene | 375 | - | |
| Co3O4 | Propane | 250 | [84] | |
| CeO2 | Trichloroethylene | 205 | [85] | |
| Mn2O3 | Ethylbenzene | 374 | [86] | |
| Mn3O4 | Toluene | 325 | 100 | [87] |
| CuO | Toluene | - | 535 | |
| CeO2 | O-xylene | 240 | - | [88] |
| CeO2 | Naphthalene | 195 | - |
| Catalyst | VOCs | Temperature (°C) | Conversion (%) | Reference |
|---|---|---|---|---|
| Au-Pd | Toluene | 168 | 90 | [92] |
| Au-Co | Toluene | 300 | 100 | [93] |
| Au-Ir | Toluene | 250 | 100 | [94] |
| Mn-Ti | Chlorobenzene | 177 | 90 | [95] |
| Sn-Mn-Ti | Chlorobenzene | 225 | 97 | |
| Cu-Co | Benzene | 290 | 90 | [96] |
| Co-Ce (6:1) | Benzene | 295 | 100 | [97] |
| Co-Ce (12:1) | Benzene | 285 | 100 | |
| Co-Ce (18:1) | Benzene | 268 | 100 | |
| Co-Ce (24:1) | Benzene | 310 | 100 | |
| Ce-Zr | 1–2 Dichloroethane | 120 | 90 | [98] |
| Mn-Co | toluene | 250 | 98.7 | [99] |
| Mn-Co | Ethyl acetate | 194 | 90 | [100] |
| Mn-Co | n-hexane | 210 | 90 | |
| Mn-Ce | Benzene | 260 | 90 | [91] |
| Mn-Ce | Toluene | 245 | 90 | |
| Mn-Ce | Ethyl acetate | 180 | 90 | |
| La-Cu (1:1 CX) | Ethyl acetate | 310 | 100 | [101] |
| La-Cu (1:2 CX) | Ethyl acetate | 309 | 100 | |
| La-Cu (1:1 EM) | Ethyl acetate | 319 | 100 | |
| La-Cu (1:2 EM) | Ethyl acetate | 304 | 100 | |
| La-Co (1:1 CX) | Ethyl acetate | 245 | 100 | |
| La-Co (1:2 CX) | Ethyl acetate | 243 | 100 | |
| La-Co (1:1 EM) | Ethyl acetate | 242 | 100 | |
| La-Co (1:2 EM) | Ethyl acetate | 239 | 100 | |
| Cu-Ce | Chlorobenzene | 328 | 99 | [74] |
| Mn-Al | Toluene | 260 | 100 | [102] |
| Co-Al | Toluene | 310 | 100 | |
| Cu-Al | Toluene | 345 | 100 | |
| Fe-Al | Toluene | 385 | 100 | |
| Ni-Al | Toluene | 380 | 100 | |
| Pd-Pt | Toluene | 160 | 98 | [103] |
| Cu-Mn (8:2) | Toluene | 377 | 100 | [87] |
| Cu-Mn (86:14) | Toluene | 390 | 100 | |
| Cu-Mn (94:6) | Toluene | 380 | 100 | |
| Cu-Mn (98:2) | Toluene | 389 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Zhang, T.; Ling, Z.; Liu, M.; Zeng, X. Regenerative Oxidation Technology for VOC Treatment: A Review. Energies 2025, 18, 3430. https://doi.org/10.3390/en18133430
Yang P, Zhang T, Ling Z, Liu M, Zeng X. Regenerative Oxidation Technology for VOC Treatment: A Review. Energies. 2025; 18(13):3430. https://doi.org/10.3390/en18133430
Chicago/Turabian StyleYang, Peng, Tao Zhang, Zhongqian Ling, Maosheng Liu, and Xianyang Zeng. 2025. "Regenerative Oxidation Technology for VOC Treatment: A Review" Energies 18, no. 13: 3430. https://doi.org/10.3390/en18133430
APA StyleYang, P., Zhang, T., Ling, Z., Liu, M., & Zeng, X. (2025). Regenerative Oxidation Technology for VOC Treatment: A Review. Energies, 18(13), 3430. https://doi.org/10.3390/en18133430







