Abstract
The presence of various inhibitory compounds in lignocellulosic hydrolysates poses a significant challenge for bioethanol production, requiring yeasts with exceptional multistress tolerance. This study introduces the novel application and demonstrates the robust performance of the nonconventional yeast Saccharomycodes ludwigii APRE2 for efficient bioethanol production directly from undetoxified sugarcane bagasse hydrolysate (SBH) at 37 °C. This approach critically eliminates the need for the costly detoxification pretreatments often required in industrial processes. Initial experiments confirmed S. ludwigii APRE2’s capability to ferment undetoxified SBH. To optimize fermentation efficiency, a central composite design (CCD) approach was implemented. This statistical method identified the following precise optimal parameters: sugar concentration (143.95 g/L), diammonium phosphate (4.99 g/L), pH (4.98), yeast extract (8.94 g/L), and magnesium sulfate (2.22 g/L). Under these optimized conditions, impressive results were achieved: a maximum ethanol concentration of 38.11 g/L, productivity of 1.59 g/L·h, and yield of 0.45 g/g. Notably, the ethanol productivity and theoretical yield achieved by S. ludwigii APRE2 using this inhibitor-rich, undetoxified SBH (containing acetic acid, formic acid, furfural, and 5-(hydroxymethyl)furfural) were superior to those previously reported for other ethanologenic yeasts under similar challenging conditions. This research establishes S. ludwigii APRE2 as a highly promising and industrially viable candidate for sustainable bioethanol production from lignocellulosic biomass, with its key novelty being its superior performance on undetoxified feedstocks, potentially reducing overall production costs.
1. Introduction
Sugarcane (Saccharum officinarum) stands as one of the most economically important crops grown worldwide. According to the Food and Agriculture Organization of the United Nations [1], global sugarcane production reached approximately 2 billion tons in 2023, with Brazil leading production at 782.59 million tons (mt), followed by India (490.53 mt), China (104.57 mt), and Thailand (93.98 mt).
The sugar production process generates sugarcane bagasse as its major byproduct, accounting for approximately 37 to 40% of the raw material [2]. While some bagasse serves as fuel for heat generation in sugar factories, a considerable portion remains unutilized. This byproduct is particularly valuable due to its high content of insoluble carbohydrates, primarily cellulose and hemicellulose. Multiple studies have documented the composition of sugarcane bagasse, with Pereira et al. [3] reporting 36–40% cellulose and 28–32% hemicellulose on a dry weight basis. Subsequent research by de Souza et al. [4] found similar concentrations of 39.2% cellulose and 37.9% hemicellulose, while de Araujo Guilherme et al. [5] documented slightly different proportions at 38.6% cellulose and 27.9% hemicellulose. More recent studies have indicated even higher cellulose contents, with Chamnipa et al. [6] reporting 47.9% cellulose and 31.4% hemicellulose, and Dolpatcha et al. [7] finding 49.09% cellulose and 29.30% hemicellulose. These carbohydrates can be converted into fermentable sugars to produce high-value products. Its substantial cellulose and hemicellulose content makes sugarcane bagasse a promising feedstock for various bioproducts, particularly second-generation bioethanol, which has gained recognition as an important alternative biofuel used worldwide.
The pretreatment and hydrolysis of sugarcane bagasse into fermentable sugars represent critical processes for utilizing this agricultural waste as a feedstock for bioethanol production. Several pretreatment platforms have been developed and employed, including chemical, physical, physico-chemical, and biological approaches [8]. Among these techniques, chemical processes—particularly dilute acid pretreatment—are commonly utilized due to their operational advantages. These advantages include ease of handling, low-cost operation resulting from lower temperature requirements, high efficiency in separating plant cell wall components, a substantial yield of fermentable sugars, and broad applicability across diverse biomass sources [6,9,10,11,12,13].
Despite these benefits, a significant drawback of dilute acid pretreatment is the generation of inhibitory compounds during the process. These inhibitors include organic acids (such as acetic acid and formic acid), furan derivatives (furfural and 5-hydroxymethyl furfural or 5-HMF), and various phenolic compounds [11,14]. The concentrations of these inhibitors vary depending on the raw materials and pretreatment conditions; however, their accumulation can adversely affect microbial growth and metabolic activity during ethanol fermentation, thereby reducing overall conversion efficiency and ethanol yield. To overcome this challenge, researchers have developed several mitigation strategies. These include implementing detoxification processes to remove or reduce inhibitors in the hydrolysate and developing multistress-tolerant ethanol-producing microorganisms. Among these approaches, the application of multistress-tolerant ethanologenic microbes has emerged as particularly promising. These specialized microorganisms can withstand the presence of inhibitory compounds while maintaining efficient fermentation performance, thereby maximizing the potential of sugarcane bagasse as a sustainable feedstock for second-generation bioethanol production. This biological approach not only addresses the inhibitor challenge but also potentially reduces processing costs by eliminating the need for separate detoxification steps, ultimately enhancing the economic viability of the overall bioethanol production process.
Multistress-tolerant yeasts have gained significant attention in bioethanol production due to their ability to withstand various stress conditions during fermentation processes. Numerous studies have successfully isolated and identified multistress-tolerant yeasts capable of efficient bioethanol production [12,15,16]. However, research has predominantly focused on specific genera such as Pichia kudriavzevii [12,15,16,17,18,19], Kluyveromyces marxianus [16,20,21], and Candida tropicalis [12,16]. In contrast, relatively limited information exists regarding other potentially valuable multistress-tolerant yeasts, particularly Saccharomycodes ludwigii [22]. This nonconventional yeast species has been isolated from diverse ecological niches, including sweet wine [23], soil [24], and coconut inflorescence sap [25]. S. ludwigii demonstrates considerable potential for various fermentation applications, including biofuel production, winemaking, and low-alcohol beer brewing [26]. Most notably, recent studies have established that this yeast exhibits remarkable multistress tolerance toward critical fermentation inhibitors, including heat (up to 43 °C), ethanol (up to 8% v/v), acetic acid (up to 10 g/L), furfural (up to 1.5 g/L), and 5-(hydroxymethyl) furfural (up to 2.5 g/L) [22].
While S. ludwigii possesses promising characteristics relevant to bioethanol production, such as inherent stress tolerance, a significant research gap persists concerning its practical application and performance with challenging lignocellulosic feedstocks. Specifically, the capability of S. ludwigii to efficiently produce ethanol from undetoxified sugarcane bagasse hydrolysate (SBH), a substrate rich in various microbial inhibitors, remains largely unexplored. To the best of our knowledge, no prior studies have systematically documented the ethanol production efficiency of this nonconventional yeast using raw, undetoxified SBH.
The primary novelty of this work, therefore, lies in addressing this gap by providing the first comprehensive evaluation of S. ludwigii APRE2’s ethanol fermentation performance using undetoxified SBH, containing a mixture of inhibitors including acetic acid, formic acid, furfural, and 5-HMF, as the sole carbon source. Furthermore, this study innovatively employs statistical experimental design approaches to determine the optimal fermentation conditions that maximize the ethanol content, yield, and productivity for this specific yeast–substrate combination. By demonstrating the potential of S. ludwigii to effectively ferment undetoxified SBH, this research aims to contribute valuable insights towards expanding the range of robust yeast strains suitable for economically viable lignocellulosic bioethanol production. Ultimately, these findings could establish S. ludwigii as a compelling candidate for industrial applications, particularly by mitigating the costs and complexities associated with hydrolysate detoxification.
2. Materials and Methods
2.1. Chemicals
Chemicals for culture medium preparation, including yeast extract, malt extract, and peptone (bacteriological grade), were purchased from TM media (Titan Biotech Ltd., Delhi, India). Other chemicals, such as isopropanol and ethanol (HPLC grade), glucose, and magnesium sulfate (MgSO4), were obtained from KemAusTM (Sydney, New South Wales, Australia). Sulfuric acid was purchased from Merck (Merck Ltd., Darmstadt, Germany), while phenol was obtained from Sigma-Aldrich (St. Louis, MI, USA).
2.2. Microbial Strain and Culture Conditions
In this study, the multistress-tolerant yeast Saccharomycodes ludwigii APRE2, previously isolated from tropical acidic fruits [22], was utilized. The strain was maintained on yeast malt (YM) agar slants (3 g/L yeast extract, 3 g/L malt extract, 5 g/L peptone, 10 g/L glucose, and 20 g/L agar) at 4 °C. For inoculum preparation, a loopful of yeast cells was transferred to YM broth and incubated in a controlled incubator shaker (JSR, Gongju, Republic of Korea) at 35 °C and 150 rpm for 16 h. These cells were then transferred to fresh YM broth at an initial concentration of approximately 105 cells/mL and incubated under identical conditions for 9 h. The resulting yeast culture served as the starter culture for all subsequent experiments.
2.3. Sugarcane Bagasse Hydrolysate (SCH) Preparation
Sugarcane bagasse was obtained from the Mitr Phu Wiang Sugar Factory in Khon Kaen, Thailand. The bagasse was dried in a hot air oven at 60 °C until it reached a constant weight, then was pulverized into small pieces of approximately 0.5 × 0.5 cm. The prepared bagasse was subjected to acid pretreatment following the method described by Sritrakul et al. [11]. This pretreatment involved transferring 10% (w/v) of the dried sugarcane bagasse into a 3% (v/v) sulfuric acid solution and keeping it at room temperature for 16 h. The mixture was subsequently heated at 121 °C and 0.1 MPa for 1 h. After pretreatment, the liquid fraction (acid hydrolysate) was collected by filtration through a muslin cloth and used directly as a feedstock for ethanol production without detoxification.
The remaining solid fraction of the acid-pretreated sugarcane bagasse was washed with running tap water and dried at 60 °C until constant weight before enzymatic hydrolysis. For this process, 5% (w/v) of the acid-pretreated solid fraction was transferred into 50 mM sodium citrate buffer (pH 4.8) with cellulase (Cellic® CTec2, Sigma-Aldrich, St. Louis, MI, USA) added at a concentration of 15 filter paper units (FPU)/g dry solid. After incubation at 50 °C and 200 rpm for 24 h, the enzymatic reaction was terminated by heating the reaction mixture in boiling water for 5 min. The liquid fraction (enzymatic hydrolysate) was collected by centrifugation at 8000 rpm for 10 min and used as feedstock for ethanol production in subsequent experiments.
The concentrations of the sugars and lignocellulosic inhibitors in both the acid and enzymatic hydrolysates were determined using high-performance liquid chromatography (HPLC).
2.4. Ethanol Production from Acid and Enzymatic Hydrolysate of Sugarcane Bagasse
A comparative analysis of the ethanol production by S. ludwigii was conducted using acid and enzymatic hydrolysates as feedstocks in a batch fermentation process. The fermentations were performed in 250 mL Erlenmeyer flasks, each containing 100 mL of the respective hydrolysate supplemented with 1.5 g/L of yeast extract and 3.0 g/L of peptone [7]. Prior to fermentation, each medium was inoculated with a starter culture of S. ludwigii at an initial concentration of approximately 1 × 107 cells/mL. The process was carried out in a controlled incubator shaker at 37 °C and 150 rpm. Samples were collected at predetermined time intervals throughout the fermentation period to monitor ethanol concentration using gas chromatography (GC) and sugar concentration using the phenol–sulfuric acid method.
2.5. Optimization Conditions for Ethanol Production from SBH
For this experiment, the enzymatic hydrolysate of sugarcane bagasse was used as a feedstock for ethanol production. Response surface methodology (RSM) based on central composite design (CCD) was employed to determine the optimal conditions for ethanol production from SBH by S. ludwigii. Based on a literature review, various independent variables that may affect ethanol production were tested, including sugar concentration [27,28], diammonium phosphate (DAP) [12,29], pH [29,30], yeast extract [27,28,29], and magnesium sulfate (MgSO4) [29,30,31]. The experimental codes and actual values (low and high) of each variable are summarized in Table 1.

Table 1.
Codes and actual values of the independent variables for the optimization experiment using central composite design (CCD).
Regression analysis was performed on the data generated from the experimental design using Design Expert version 13 (Demo version) (Stat-Ease Inc., Minneapolis, MN, USA), with ethanol concentration as the response variable. The predictive model of the design is presented as a second-order polynomial function in Equation (1):
where Y is the predicted response, xᵢ and xⱼ are the independent variables, β0 is a constant term, βᵢ represents the linear coefficient, βᵢᵢ represents the squared coefficient, and βᵢⱼ is the interaction coefficient.
Analysis of variance (ANOVA) was performed using Design-Expert version 13 (Demo version) to evaluate the statistical significance of the independent variables. The coefficient of determination (R2) was determined to monitor the reliability of the experimental design. The optimized fermentation conditions obtained from the response surface plots and the prediction equation established from the statistical experimental model were verified through batch mode fermentation in a flask scale, as previously described.
2.6. Analytical Methods and Statistical Analysis
Total sugar was determined by the phenol–sulfuric acid method using glucose as a standard [32]. Ethanol concentration (P, g/L) was measured by GC (GC-14B, Shimadzu, Kyoto, Japan) following the procedure described by Laopaiboon et al. [33]. Volumetric ethanol productivity (Qp, g/L·h) and ethanol yield (Yp/s, g ethanol produced/g glucose consumed) were calculated according to the method of Nuanpeng et al. [27]. Sugar compositions (glucose, xylose, and arabinose) and inhibitors (acetic acid, formic acid, furfural, 5-hydroxymethyl furfural (5-HMF), and vanillin) in the SBH were analyzed using HPLC (Shimadzu, Kyoto, Japan) equipped with an Aminex HPX-87H column. Sugars were detected using a refractive index detector, while inhibitors were detected using a UV-VIS detector (Shimadzu, Kyoto, Japan) at a 210 nm wavelength. A 5 mM sulfuric acid (H2SO4) mobile phase was used at a flow rate of 0.6 mL/min at 40 °C. All experiments were performed twice, each with two replications, with their results expressed as means ± standard deviations (SDs).
3. Results and Discussion
3.1. Ethanol Production Potential of S. ludwigii Using Acid and Enzymatic Sugarcane Bagasse Hydrolysate as Feedstock
The chemical compositions of sugarcane bagasse hydrolysates used in the current study were comprehensively analyzed by Dolpatcha et al. [7], who reported detailed findings on sugar content and inhibitory compounds. The acid hydrolysate contained 25.81 g/L of total sugar, with xylose as the predominant component (20.32 g/L), followed by arabinose (2.90 g/L) and glucose (2.59 g/L). In contrast, the enzymatic hydrolysate yielded a significantly higher total sugar content at 63.88 g/L, with glucose being the major constituent (43.54 g/L), followed by xylose (19.29 g/L) and arabinose (1.05 g/L) (Supplement Materials Table S1).
Marked differences were also observed in their lignocellulosic inhibitor profiles. Acetic acid was the principal inhibitor in the acid hydrolysate (3.77 g/L), substantially higher than in the enzymatic hydrolysate (0.89 g/L). Conversely, formic acid (2.80 g/L) and furfural (1.57 g/L) were the predominant inhibitors in the enzymatic hydrolysate, whereas these were found at much lower concentrations in the acid hydrolysate (0.26 g/L and 0.08 g/L, respectively). 5-HMF was detected in minimal amounts in both, while vanillin was present exclusively in the acid hydrolysate and absent in the enzymatic preparation (Supplementary Materials Table S1). These compositional variations underscore the distinct chemical outcomes of acid versus enzymatic hydrolysis techniques [7].
A primary objective of this research was to evaluate the direct fermentability of these hydrolysates without detoxification. The fermentation of the acid hydrolysate yielded no detectable ethanol (Figure 1). This outcome is primarily attributed to an insufficient glucose concentration (2.59 g/L), as this is an essential carbon source for cellular growth and ethanol biosynthesis for the investigated yeast strain. The high concentration of acetic acid (3.77 g/L) in this hydrolysate would also pose a significant challenge to fermentation, had sufficient glucose been available. Throughout this fermentation, xylose and arabinose concentrations remained constant, confirming the inability of this nonconventional yeast to metabolize pentose sugars, consistent with previous reports [22,26]. In contrast, when the enzymatic hydrolysate was used as the substrate, its higher glucose concentration (43.54 g/L) facilitated substantial ethanol production, reaching 20.54 g/L after 24 h, with a conversion efficiency of 0.48 g ethanol per gram of glucose consumed. This yield approaches the theoretical maximum (0.51 g/g). Notably, this robust fermentation occurred despite the presence of 0.89 g/L acetic acid, 2.80 g/L formic acid, and 1.57 g/L furfural. As with the acid hydrolysate, pentose sugars remained unconsumed.
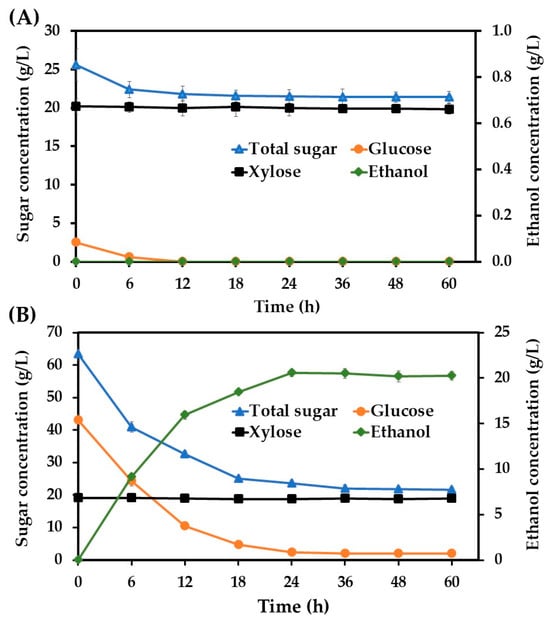
Figure 1.
The ethanol production from acid (A) and enzymatic hydrolysates (B) of sugarcane bagasse without detoxification at 37 °C using Saccharomycodes ludwigii.
It should be highlighted in this study that based on our mass balance calculations, an initial 100 g of dry sugarcane bagasse (determined herein to contain 49% cellulose) yielded approximately 43.54 g of glucose through enzymatic hydrolysis. The subsequent fermentation of this glucose then produced approximately 20.54 g of ethanol from the same initial 100 g of sugarcane bagasse. These overall yields correspond to an approximate 80.8% conversion efficiency of cellulose to glucose, and an approximate 92.5% efficiency for the conversion of glucose to ethanol during fermentation.
The successful fermentation of the enzymatic hydrolysate, despite its inhibitor load, highlights the remarkable tolerance of the nonconventional yeast strain employed. Lignocellulosic inhibitors, including weak acids, furan derivatives, and phenolic compounds, are well documented for their capacity to impede the microbial growth and fermentation of several ethanologenic microorganisms, such as Saccharomyces cerevisiae, P. kudriavzevii, and K. marxianus [7,16,34,35,36,37,38]. Weak acids, such as the acetic and formic acid found in our hydrolysates, arise from hemicellulose deacetylation (acetic acid) or HMF/furfural degradation (formic, levulinic acid) [34,39]. They primarily inhibit by diffusing across the plasma membrane in their undissociated form, then dissociate in the higher-pH cytoplasm, which lowers the intracellular pH [40]. This acidification disrupts homeostasis, reduces viability, and diverts ATP from growth to proton extrusion via H+-ATPases, while also potentially damaging cell membranes and denaturing enzymes [40,41,42,43,44,45]. Furan derivatives, such as the furfural present in our enzymatic hydrolysate, are generated from either pentose (furfural) or hexose (5-HMF) degradation. Even at low concentrations (0.5–2.0 g/L), these aldehydes can damage DNA, RNA, proteins, and cell membranes, reduce the activity of key glycolytic and ethanol-producing enzymes like pyruvate dehydrogenase and alcohol dehydrogenase, and induce reactive oxygen species (ROS), leading to oxidative stress and cellular damage [46,47,48,49]. Phenolic compounds, exemplified by the vanillin detected in the acid hydrolysate, typically damage cell membranes, impairing their barrier and enzyme matrix functions [50]. Certain phenolics can also act as protonophores, dissipating mitochondrial electrochemical gradients [40], though the full scope of phenolic inhibition is complex due to their heterogeneity [51].
The observed fermentation efficiency with the enzymatic hydrolysate, despite levels of formic acid (2.80 g/L) and furfural (1.57 g/L) that are known to be inhibitory, underscores the strain’s robustness. Previous research by Pilap et al. [23] established the capacity of this yeast to withstand acetic acid (up to 10 g/L), furfural (up to 1.5 g/L), and 5-HMF (up to 2.5 g/L) without significant metabolic impairment, which aligns with our current findings.
Given these results, the enzymatic hydrolysate was selected as the superior feedstock for subsequent ethanol production experiments. Before further use, this hydrolysate underwent evaporation to concentrate its total sugar content to approximately 300 g/L. This specific concentration was targeted for two primary reasons, with significant implications for both laboratory handling and potential industrial applications. Firstly, a high sugar concentration, such as 300 g/L, creates an osmotically challenging environment that inhibits the growth of many undesirable microorganisms. This inherent preservative effect is crucial for maintaining the quality and integrity of the hydrolysate, especially if it needs to be stored for any period before its utilization in fermentation, thereby minimizing substrate loss and the formation of inhibitory byproducts by contaminants. Secondly, working with a concentrated sugar stock significantly simplifies the logistics of preparing fermentation media, particularly at larger scales. Such a concentrated hydrolysate allows for a reduced storage volume, as less physical space is required compared to a dilute solution. Furthermore, it facilitates more efficient handling and transportation, as moving and managing smaller, concentrated volumes is more practical and potentially cost-effective. This approach also enables flexible and accurate medium formulation, whereby the fermentation medium can be easily and precisely prepared to the desired operational sugar concentration through simple dilution with water or other nutrient solutions, providing operational flexibility and consistency in initiating fermentation batches. Therefore, the concentration step is justified not only for enhancing the stability of the sugar feedstock against contamination but also for streamlining subsequent process operations, which are key considerations for transitioning to and operating under industrial conditions.
3.2. Optimization Conditions for Ethanol Production from SBH
Based on a comprehensive literature review, several key factors were identified that significantly influence ethanol fermentation efficiency and yield, particularly under high-temperature conditions. These factors include sugar concentration [27,28], DAP [12,29], the pH of the fermentation medium [29,30], yeast extract [27,28,29], and MgSO4 [29,30,31]. To investigate these parameters, their impact on ethanol fermentation from the enzymatic hydrolysate of sugarcane bagasse at 37 °C using S. ludwigii was systematically optimized. The optimization process employed CCD in the Design-Expert program, which generated 47 experimental runs with varying combinations of the selected factors. The actual values for each variable in these experimental runs are summarized in Table 2.

Table 2.
Codes and actual values of each independent variable, as well as the predicted and actual values of the ethanol concentrations from the central composite design (CCD) experiment.
The fermentation experiments were conducted on a flask scale using 100 mL of the fermentation medium. The actual ethanol concentrations obtained from the experimental runs ranged from 7.87 to 37.03 g/L, which aligned closely with the predicted values (6.87–36.26 g/L). The highest ethanol concentration of 37.03 g/L was achieved in run 35, which utilized the following specific parameter values: 150 g/L sugar concentration, 5.0 g/L DAP, pH 5.0, 8.5 g/L yeast extract, and 2.5 g/L MgSO4. In contrast, the lowest ethanol yield of 7.87 g/L was observed in run 29, which employed a significantly higher sugar concentration of 268.92 g/L while maintaining the same levels of the other parameters (5.0 g/L DAP, pH 5.0, 8.5 g/L yeast extract, and 2.5 g/L MgSO4) (Table 2). This substantial reduction in ethanol production can be attributed to the excessive sugar concentration creating high osmotic pressure, which previous studies have shown adversely affects microbial growth and metabolic activity [52,53]. This finding underscores the critical importance of optimizing sugar concentration in ethanol fermentation processes, as concentrations that are too high can paradoxically reduce overall yield despite providing more of a fermentable substrate.
A quadratic polynomial equation was developed to predict ethanol concentration based on the experimental data from the CCD experiment. The equation is
where P is the ethanol concentration (g/L) and A, B, C, D, and E are the code factors for sugar concentration, DAP, pH, yeast extract, and MgSO4, respectively.
P (g/L) = 36.26 − 1.08A − 0.10B − 0.15C + 0.42D − 0.89E − 0.2AB + 0.24AC + 0.11AD +
0.24AE − 0.48BC + 0.21BD − 0.19BE − 0.15CD − 0.10CE − 0.003DE − 4.74A2 − 0.97B2 −
3.93C2 − 1.62D2 − 1.61E2
0.24AE − 0.48BC + 0.21BD − 0.19BE − 0.15CD − 0.10CE − 0.003DE − 4.74A2 − 0.97B2 −
3.93C2 − 1.62D2 − 1.61E2
The statistical analysis of variance (ANOVA) of the experimental data obtained from the CCD is summarized in Table 3. The results indicate that the established model was statistically significant, while the lack of fit was not, implying that the model is reliable for predicting response values. The relatively high determination coefficient (R2 = 0.98) demonstrates a close alignment between the predicted and actual values of the response variable, suggesting that the established model could explain 98% of the ethanol concentration’s variance.

Table 3.
An analysis of variance (ANOVA) of the central composite design (CCD) for ethanol production from sugarcane bagasse hydrolysate (SBH) at 37 °C by Saccharomycodes ludwigii.
Based on the linear and quadratic terms shown in Table 3, three variables, specifically sugar concentration (A), yeast extract (D), and MgSO4 (E), significantly affected the ethanol production from SBH by S. ludwigii (p < 0.05). These findings align with the previous studies by Nuanpeng et al. [27], Nuanpeng et al. [28], Phong et al. [29], Pereira et al. [31], and Nguyen et al. [30]. Although no interaction effects were found among these significant variables, an interaction effect between DAP and pH (BC) was detected, suggesting these two factors are also important parameters for ethanol fermentation by this nonconventional yeast, particularly when their combined effect is considered, rather than as individual factors.
To examine the interactive effects of the five selected variables on ethanol production by S. ludwigii, three-dimensional (3-D) response surfaces and contour plots were generated using the established model and experimental data from the CCD experiment. As illustrated in Figure 2, each variable pair exhibited distinct interactive effects on ethanol production. When analyzing the interactive effect of sugar concentration with the other variables, ethanol content increased as sugar concentration rose to approximately 150 g/L, beyond which ethanol concentration decreased. This pattern suggests the existence of substrate inhibition at higher sugar concentrations, a phenomenon commonly observed in yeast fermentation [29,54,55]. Varying DAP concentrations showed no significant impact on the ethanol production by S. ludwigii (Figure 2A), indicating that this strain may efficiently utilize nitrogen sources already present in the medium.
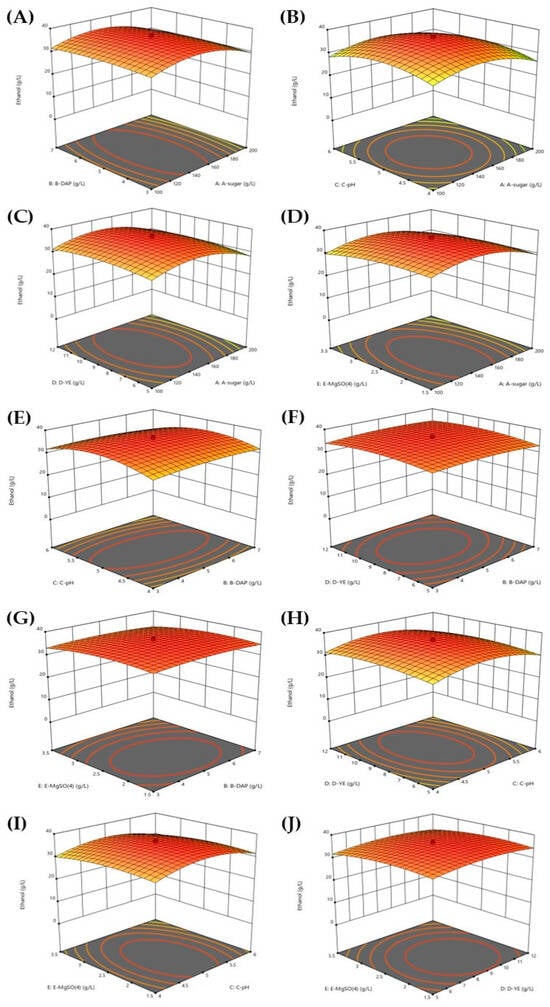
Figure 2.
Response surfaces and contour plots of interaction between sugar and DAP (A), sugar and pH (B), sugar and yeast extract (C), sugar and MgSO4 (D), DAP and pH (E), DAP and yeast extract (F), DAP and MgSO4 (G), pH and yeast extract (H), pH and MgSO4 (I), and yeast extract and MgSO4 (J) on ethanol production from SBH at 37 °C by S. ludwigii.
With respect to pH, a slight increase in ethanol content was observed when the initial pH of the fermentation medium was raised from 4.0 to 5.0, followed by a minor decrease at pH values exceeding 5.0 (Figure 2B). This optimal pH range aligns with previous studies [27]. Similar to DAP, minimal changes in ethanol concentration were detected when varying either yeast extract concentration (Figure 2C) or MgSO4 content (Figure 2D), while maintaining different sugar concentrations. These results collectively suggest that among the parameters studied, sugar concentration exerts the most significant influence on ethanol production by S. ludwigii, a finding consistent with the fundamental principles of fermentation stoichiometry, where sugar serves as the primary carbon source for conversion to ethanol.
With sugar concentration held constant at the middle point (150 g/L), most variable pairs showed no interactive effects on ethanol production, with one notable exception. No significant interaction was observed between DAP and yeast extract (Figure 2F) or between DAP and MgSO4 (Figure 2G). However, a clear interactive effect was detected between DAP and pH (Figure 2E). Ethanol production increased with pH, reaching its maximum at approximately pH 5.0, after which ethanol concentration declined as pH continued to increase. Similarly, when both sugar and DAP concentrations were fixed at their middle points, varying the concentrations of either yeast extract (Figure 2H) or MgSO4 (Figure 2I) resulted in minimal changes to ethanol production. This indicates that when other parameters are optimized, the individual effects of these nutrients become less pronounced. The interaction between yeast extract and MgSO4 (Figure 2J) also showed no significant impact on ethanol production when the other variables were held constant. Despite these minimal interaction effects, the highest ethanol content was achieved at specific concentrations of yeast extract and MgSO4, approximately 9.0 g/L and 2.5 g/L, respectively. These findings suggest that while these nutrients are necessary for optimal fermentation, their concentrations become less critical once they reach certain threshold levels, provided that other key parameters (sugar concentration, pH, and DAP) are appropriately regulated.
Based on the second-order polynomial model and response surface analysis, the predicted optimum conditions for ethanol production from SBH by S. ludwigii at 37 °C were determined to be the following: a sugar concentration of 143.95 g/L, DAP concentration of 4.99 g/L, pH of 4.98, yeast extract concentration of 8.94 g/L, and MgSO4 concentration of 2.22 g/L. To validate these theoretical predictions, a verification experiment was conducted in a 250 mL flask-scale fermentation using these optimized parameters. As shown in Figure 3, the ethanol concentration increased significantly during fermentation, reaching its maximum value at 24 h. This peak in ethanol production corresponded directly with the reduction in total sugar and glucose concentrations in the medium. Notably, the xylose concentration remained constant throughout the fermentation period, confirming that S. ludwigii lacks the metabolic capability to utilize this pentose sugar.
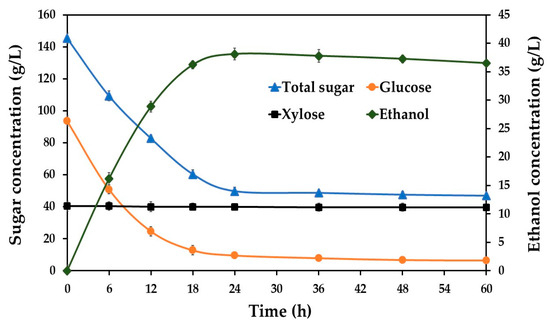
Figure 3.
The sugar utilization and ethanol production profiles of S. ludwigii at 37 °C using SBH as a feedstock.
Under these optimized conditions, the maximum ethanol concentration achieved was 38.11 g/L, with a volumetric ethanol productivity of 1.59 g/L·h and an ethanol yield of 0.45 g ethanol produced per g of glucose consumed. The experimental ethanol concentration (38.11 g/L) closely matched the model’s predicted value (36.48 g/L), with a difference of only 4.5%. This strong agreement between the predicted and experimental results validates the accuracy and reliability of the established model for predicting ethanol production from SBH using S. ludwigii. These findings demonstrate the effectiveness of response surface methodology for optimizing bioethanol production parameters in this nonconventional yeast system.
A notable observation from the fermentation profile presented in Figure 3 is the incomplete utilization of glucose by S. ludwigii APRE2. After 60 h of fermentation, a residual glucose concentration of approximately 5 g/L was detected in the medium. This phenomenon of incomplete substrate consumption is likely attributable to the combined inhibitory stresses exerted by the accumulating ethanol product and the inherent lignocellulosic-derived compounds within the sugarcane bagasse hydrolysate. It is well documented that such stressors can synergistically compromise yeast cell performance. Specifically, ethanol and lignocellulosic inhibitors can alter cell membrane structure and integrity, leading to reduced efficiency in glucose uptake systems [56,57]. Furthermore, these compounds can also impair the activity of critical enzymes in the glycolytic pathway, including hexokinase, triosephosphate dehydrogenase, and alcohol dehydrogenase, ultimately resulting in less efficient sugar metabolism and ethanol conversion [58,59].
During ethanol fermentation, yeast cells may synthesize various byproducts in addition to ethanol, particularly when subjected to stressful conditions such as high temperatures, elevated ethanol concentrations, or the presence of inhibitors. Among these, glycerol, ergosterol, and trehalose are recognized as major compounds synthesized under such stress conditions [60,61]. These compounds serve critical physiological functions, including stabilizing the yeast plasma membrane, protecting biological macromolecules, and maintaining cellular redox balance, thereby aiding yeast survival and activity [60,61,62,63].
In the present study, the primary analytical focus was on quantifying bioethanol content, productivity, and yield. Consequently, other potential byproducts, such as those mentioned above, were not directly measured. However, the observed ethanol yield in this study (0.45 g/g), while significant, suggests that a portion of the substrate was likely diverted towards the synthesis of these or other byproducts. This diversion is a common characteristic of fermentation processes, reflecting the partitioning of carbon flux among various metabolic pathways, which can be influenced by inherent process conditions. Therefore, we acknowledge the probable formation of such compounds, and the precise nature and quantity of these byproducts under our specific experimental conditions warrant further clarification. Further investigation, incorporating methodologies to detect and measure these compounds, would be necessary to fully elucidate the metabolic flux and to quantify the extent of byproduct formation.
To contextualize the ethanol production performance of S. ludwigii APRE2 using SBH, a comparative analysis was conducted against other ethanologenic yeasts reported in the literature utilizing diverse lignocellulosic feedstocks (Table 4). We acknowledge that direct, fully normalized comparisons across studies are inherently challenging. Variations in experimental conditions—such as medium compositions, cultivation parameters, and fermentation durations—are not always uniformly detailed in cited works and can significantly influence overall process evaluation. Despite these limitations, this benchmarking exercise provided valuable insights, indicating that S. ludwigii APRE2 demonstrates competitive ethanol production capabilities. Notably, its performance with SBH regarding ethanol concentration, productivity, and overall yield was comparable to values previously reported for this specific strain when using pineapple waste as a feedstock [22].
Specifically, the ethanol concentration achieved by S. ludwigii APRE2 from SBH surpassed those reported for P. kudriavzevii RGB3.2 and K. marxianus RGB4.5 using rice straw [16], K. marxianus CECT10875 using woody and herbaceous biomass [64], S. cerevisiae TC-5 using corncob residue [65], and S. cerevisiae PTCC5052 using wheat straw [66]. Furthermore, the ethanol concentration from S. ludwigii APRE2 was marginally higher than that from P. kudriavzevii RZ8-1 utilizing sugarcane bagasse [17] and S. cerevisiae employing pomelo peel waste [67]. Although the ethanol concentration produced by S. ludwigii APRE2 was lower than that reported for Pichia fermentans MTCC189 [68] and S. cerevisiae [69], its ethanol productivity and theoretical yield were remarkably greater in these instances.
A particularly noteworthy finding from this comparison is the ethanol productivity. The productivity of S. ludwigii APRE2 in the present study (1.59 g/L/h) was substantially higher than the values reported across the comparative works in [16,17,64,65,66,67]. This enhanced productivity is a critical parameter for industrial feasibility, suggesting a potentially more rapid and efficient bioconversion process with S. ludwigii APRE2, even when considering the aforementioned challenges in directly comparing all operational parameters.

Table 4.
The ethanol production from SBH by S. ludwigii and other ethanologenic yeasts using different lignocellulosic materials as feedstocks.
Table 4.
The ethanol production from SBH by S. ludwigii and other ethanologenic yeasts using different lignocellulosic materials as feedstocks.
| Microbe | Feedstock | Fermentation Parameter 1 | References | |||||
|---|---|---|---|---|---|---|---|---|
| S (g/L) | T (°C) | P (g/L) | Qp (g/L·h) | TY (%) | T (h) | |||
| P. kudriavzevii RZ8-1 | Sugarcane bagasse 2 | 85 | 37 | 35.51 | 1.48 | 81.75 | 24 | [17] |
| 85 | 40 | 33.84 | 1.41 | 77.91 | 24 | |||
| P. kudriavzevii RGB3.2 | Rice straw | 19.10 | 40 | 9.32 | 0.39 | 95.49 | 24 | [16] |
| K. marxianus RGB4.5 | Rice straw | 19.10 | 40 | 8.03 | 0.33 | 82.27 | 24 | |
| P. fermentans MTCC189 | Wheat straw | 172.00 | 30 | 92.00 | 0.55 | 62.1 | 168 | [68] |
| K. marxianus CECT10875 | Woody and herbaceous biomass | NR | 42 | 19.0 | NR | 71.2 | NR | [64] |
| S. cerevisiae | Coffee pulp | NR | 30 | 71.39 | 0.99 | NR | 72 | [69] |
| Wheat bran | NR | 30 | 68.91 | 0.96 | NR | 72 | ||
| S. cerevisiae | Pomelo peel waste | NR | 30 | 36.00 | 0.75 | 73.50 | 48 | [67] |
| S. cerevisiae TC-5 | Corncob residue 3 | NR | 40 | 31.96 | 0.22 | NR | 144 | [65] |
| S. cerevisiae PTCC5052 | Wheat straw | NR | 25 | 24.02 | 0.25 | NR | 96 | [66] |
| S. ludwigii APRE2 | Pineapple waste | 105.65 | 37 | 38.02 | 1.58 | 82.35 | 24 | [22] |
| S. ludwigii APRE2 | Sugarcane bagasse 4 | 143.95 | 37 | 38.11 | 1.59 | 88.24 | 24 | This study |
1 S, initial sugar concentration; T, fermentation temperature; P, ethanol concentration; Qp, volumetric ethanol productivity; TY, theoretical yield of ethanol; T, fermentation time; and NR, not reported. 2 The ethanol fermentation media were prepared from sugarcane bagasse hydrolysate supplemented with 2 g/L yeast extract, 2 g/L peptone, and 2 g/L MgSO4; 3 corncob hydrolysate supplemented with 4 g/L (NH4)2SO4, 1 g/L yeast extract, 1 g/L NH4H2PO4, and 0.1 g/L MgSO4·7H2O; and 4 sugarcane bagasse hydrolysate supplemented with 1.5 g/L yeast extract and 3.0 g/L peptone.
Regarding inhibitory compounds, although the concentration of lignocellulosic inhibitors in the concentrated enzymatic hydrolysate was not directly measured, we estimate their concentration likely increased approximately 2.2-fold compared to the original hydrolysate. This estimation is based on the proportional increase observed in sugar content during the concentration process. Despite this potential increase in inhibitory compounds, the glucose utilization profile and ethanol production efficiency of S. ludwigii remained robust. The fermentation performance was not significantly affected by these inhibitors, suggesting that S. ludwigii APRE2 possesses a notable tolerance to lignocellulosic inhibitors commonly present in hydrolysates. This inhibitor tolerance, combined with the competitive ethanol yields demonstrated in our comparative analysis, positions S. ludwigii APRE2 as a promising candidate for industrial-scale lignocellulosic ethanol production processes.
While this study successfully identified optimal conditions for bioethanol production from SBH using S. ludwigii in a batch fermentation mode, the observed substrate inhibition at sugar concentrations above 150 g/L, coupled with the known product inhibition by ethanol [22], highlights potential limitations for achieving even higher ethanol titers and productivities. A promising avenue for future research, therefore, involves the implementation and optimization of a fed-batch fermentation strategy. Fed-batch operations could alleviate the high initial substrate toxicity by maintaining lower, non-inhibitory sugar concentrations in the fermentation medium throughout the process. This approach could potentially allow for the utilization of higher total substrate loadings over time, leading to an increased final ethanol concentration and improved overall process efficiency. Future studies could investigate various feeding strategies (e.g., constant feed rate, exponential feeding, or feedback-controlled feeding based on residual sugar levels) to determine the most effective method for maximizing ethanol yield and productivity with S. ludwigii and SBH. Furthermore, comparing the performance of fed-batch fermentation directly against the optimized batch process established in this work would provide quantitative insights into the benefits of this alternative operational mode, potentially leading to a more industrially viable bioethanol production process from SBH.
The current study also demonstrates that S. ludwigii cannot metabolize pentose sugars into ethanol, a finding consistent with the observed unutilized xylose and arabinose [22,26]. Ethanol production in this nonconventional yeast from hexose sugars proceeds via the Embden–Meyerhoff–Parnas (EMP) pathway. In contrast, the efficient fermentation of pentose sugars, particularly xylose, necessitates the pentose phosphate pathway (PPP). This pathway involves several key enzymes, including xylose reductase (XR), xylitol dehydrogenase (XDH), ribulosephosphate-3-epimerase, transaldolase (TAL), and transketolase (TK) [70,71,72], which are evidently not effectively expressed or functional for the ethanolic fermentation of pentoses in this strain.
To enhance the valorization of lignocellulosic biomass by enabling pentose utilization in S. ludwigii, several strategies could be explored. One approach is co-fermentation, employing this strain alongside specialized pentose-utilizing yeasts, such as P. stipitis or Pachysolen tannophilus [72]. Alternatively, the direct strain improvement in S. ludwigii itself could be pursued through techniques such as random mutagenesis, protoplast fusion, adaptive laboratory evolution, or targeted genetic engineering. Successfully implementing such strategies could not only yield robust xylose-utilizing yeast strains but also significantly increase the overall economic viability of converting lignocellulosic feedstocks to biofuels and biochemicals.
Indeed, with several highly efficient distiller yeast strains now available, their inclusion in future research is noteworthy. Specifically, a comparative study on ethanol production from SBH by S. ludwigii using established distiller yeasts, such as Thermosacc Dry or Ethanol Red [73], as reference strains, would be highly beneficial for benchmarking performance and guiding further applications.
Various cellular mechanisms are involved in the stress response in yeast cells, including the regulation of cell wall integrity, remodeling of cellular structure and membrane transport, activation of antioxidative systems, and changes in signaling pathway and gene transcription under stress conditions [61,74,75,76,77]. Different species of yeasts respond differently to stress conditions. Thus, further study to elucidate the cellular mechanisms in response to stress conditions in the multistress-tolerant yeast S. ludwigii is needed.
Future research should focus on applying advanced transcriptomic and proteomic approaches to comprehensively map the stress response pathways in S. ludwigii under lignocellulosic hydrolysate conditions [78,79], while comparative genomic analyses with conventional bioethanol yeasts could reveal unique genetic elements responsible for its superior stress tolerance [75,78,80]. Investigating epigenetic regulation mechanisms [81], membrane lipid composition changes [82,83], and key transcription factors governing multistress responses [84,85] would provide crucial insights for developing robust industrial strains. The acquired information provides not only basic knowledge on how this yeast responds to stress but also gives useful information to develop a robust yeast with desirable traits, as well as about the modification of fermentation conditions for a highly efficient lignocellulosic ethanol fermentation process.
4. Conclusions
This study demonstrates that the multistress-tolerant yeast S. ludwigii APRE2 exhibits exceptional potential for ethanol production using undetoxified sugarcane bagasse hydrolysate as a feedstock. Initial testing under unoptimized conditions revealed that this nonconventional yeast produced 20.54 g/L of ethanol with a yield of 0.48 g/g from the enzymatic hydrolysate. Statistical analysis through linear and quadratic regression identified sugar concentration, yeast extract, and MgSO4 as the three critical variables significantly influencing ethanol production. Under optimized conditions through CCD (143.95 g/L sugar concentration, 4.99 g/L DAP, pH 4.98, 8.94 g/L yeast extract, and 2.22 g/L MgSO4), S. ludwigii APRE2 achieved a highest ethanol concentration of 38.11 g/L, along with a productivity of 1.59 g/L·h and yield of 0.45 g/g. These performance metrics are comparable to other leading ethanologenic yeasts. These results establish S. ludwigii as a promising multistress-tolerant yeast for sustainable bioethanol production from lignocellulosic biomass, offering significant advantages for industrial applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en18133428/s1, Table S1. Chemical compositions of the acid and enzymatic hydrolysates of sugarcane bagasse used in this study.
Author Contributions
Conceptualization, P.K., S.T., J.A., K.F., R.L., M.Y. and P.T.; methodology, P.K., S.T., W.P. and P.T.; software, P.T.; validation, P.K., S.T., W.P. and P.T.; formal analysis, P.K., S.T., W.P. and P.T.; investigation, P.K., S.T., W.P. and P.T.; resources, P.T.; data curation, P.K., S.T. and P.T.; writing—original draft preparation, P.K., S.T., W.P. and P.T.; writing—review and editing, P.K., S.T., M.Y. and P.T.; visualization, P.T.; supervision, J.A., M.Y. and P.T.; project administration, P.T.; funding acquisition, J.A. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fundamental Fund (FF) of Khon Kaen University and the National Science, Research, and Innovation Fund (NSRF), year 2025. A part of this work was also supported by the Ministry of Higher Education, Science, Research and Innovation through Reinventing University (2025), Khon Kaen University, Thailand.
Data Availability Statement
The experimental data are contained within the article.
Acknowledgments
The authors thank the Mitr Phu Wiang Sugar Factory, Khon Kaen, Thailand, for providing the sugarcane bagasse. The authors also thank Wutthichai Janmahong and Kannika Boonkert for their technical assistance in the chemical analysis, as well as the Department of Biotechnology, Faculty of Technology, and the Research Center for Value-Added Agricultural Products (FerVAAPs), Khon Kaen University, for all their research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations. Available online: https://ourworldindata.org/grapher/sugar-cane-production?country=USA~BRA~IND~CHN~European+Union~THA (accessed on 19 April 2025).
- Junpen, A.; Pansuk, J.; Garavait, S. Estimation of reduced air emissions as a result of the implementation of the measure to reduce burned sugarcane in Thailand. Atmosphere 2020, 11, 366. [Google Scholar] [CrossRef]
- Pereira, S.C.; Maehara, L.; Machado, C.M.M.; Farinas, C.S. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.F.R.; Dutra, E.D.; Leite, F.C.B.; Cadete, R.M.; Rosa, C.A.; Stambuk, B.U.; Stamford, T.L.M.; de Morais, M.A., Jr. Production of ethanol fuel from enzyme-treated sugarcane bagasse hydrolysate using d-xylose-fermenting wild yeast isolated from Brazilian biomes. 3 Biotech 2018, 8, 312. [Google Scholar]
- de Araujo Guilherme, A.; Dantas, P.V.F.; Padilha, C.E.A.; Dos Santos, E.S.; de Macedo, G.R. Ethanol production from sugarcane bagasse: Use of different fermentation strategies to enhance an environmental-friendly process. J. Environ. Manag. 2019, 234, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Chamnipa, N.; Klanrit, P.; Thanonkeo, S.; Thanonkeo, P. Sorbitol production from a mixture of sugarcane bagasse and cassava pulp hydrolysates using thermotolerant Zymomonas mobilis TISTR548. Ind. Crops Prod. 2022, 188, 115741. [Google Scholar] [CrossRef]
- Dolpatcha, S.; Phong, H.X.; Thanonkeo, S.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Adaptive laboratory evolution under acetic acid stress enhances the multistress tolerance and ethanol production efficiency of Pichia kudriavzevii from lignocellulosic biomass. Sci. Rep. 2023, 13, 21000. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L. Bioethanol production techniques from lignocellulose biomass as alternative fuel: A review. Int. J. Mech. Eng. Technol. 2019, 10, 34–71. [Google Scholar]
- Dussan, K.J.; Silva, D.D.V.; Moraes, E.J.C.; Arruda, P.V.; Felipe, M.G.A. Dilute-acid hydrolysis of cellulose to glucose from sugarcane bagasse. Chem. Eng. Trans. 2014, 38, 433–438. [Google Scholar]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Sritrakul, N.; Nitisinprasert, S.; Keawsompong, S. Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric. Nat. Resour. 2017, 51, 512–519. [Google Scholar] [CrossRef]
- Phong, H.X.; Klanrit, P.; Dung, N.T.P.; Yamada, M.; Thanonkeo, P. Isolation and characterization of thermotolerant yeasts for the production of second-generation bioethanol. Ann. Microbiol. 2019, 69, 765–776. [Google Scholar] [CrossRef]
- Sunkar, B.; Bhukya, B. Bi-phasic hydrolysis of corncobs for the extraction of total sugars and ethanol production using inhibitor resistant and thermotolerant yeast, Pichia kudriavzevii. Biomass Bioenergy 2021, 153, 106230. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Park, E.H.; Kim, M.D. Isolation of thermotolerant yeast Pichia kudriavzevii from nuruk. Food Sci. Biotechnol. 2017, 26, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Avchar, R.; Lanjekar, V.; Kshirsagar, P.; Dhakephalkar, P.K.; Dagar, S.S.; Baghela, A. Buffalo rumen harbours diverse thermotolerant yeasts capable of producing second-generation bioethanol from lignocellulosic biomass. Renew. Energy 2021, 173, 795–807. [Google Scholar] [CrossRef]
- Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production. Braz. J. Microbiol. 2018, 49, 378–391. [Google Scholar] [CrossRef]
- Aouine, M.; Elalami, D.; Koraichi, S.I.; Haggoud, A.; Barakat, A. Exploring natural fermented foods as a source for new efficient thermotolerant yeasts for the production of second-generation bioethanol. Energies 2022, 15, 4954. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, P.; Zhou, X.; Zheng, J.; Ma, Y.; Liu, C.; Wu, T.; Li, H.; Wang, X.; Wang, H.; et al. Isolation, identification, and characterization of an acid-tolerant Pichia kudriavzevii and exploration of its acetic acid tolerance mechanism. Fermentation 2023, 9, 540. [Google Scholar] [CrossRef]
- Charoensopharat, K.; Thanonkeo, P.; Thanonkeo, S.; Yamada, M. Ethanol production from Jerusalem artichoke tubers at high temperature by newly isolated thermotolerant inulin-utilizing yeast Kluyveromyces marxianus using consolidated bioprocessing. Antonie Van Leeuwenhoek 2015, 108, 173–190. [Google Scholar] [CrossRef]
- Hemansi; Himanshu; Patel, A.K.; Saini, J.K.; Singhania, R.R. Development of multiple inhibitor tolerant yeast via adaptive laboratory evolution for sustainable bioethanol production. Bioresour. Technol. 2022, 344, 126247. [Google Scholar] [CrossRef]
- Pilap, W.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of multistress tolerant yeast, Saccharomycodes ludwigii, for second-generation bioethanol production. Sci. Rep. 2022, 12, 22062. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Thammasittirong, S.N.; Chamduang, T.; Phonrod, U.; Sriroth, K. Ethanol production potential of ethanol-tolerant Saccharomyces and non-Saccharomyces yeasts. Pol. J. Microbiol. 2012, 61, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Udomsaksakul, N.; Kodama, K.; Tanasupawat, S.; Savarajara, A. Diversity of ethanol fermenting yeasts in coconut inflorescence sap and their application potential. Sci. Asia 2018, 44, 371–381. [Google Scholar] [CrossRef]
- Vejarano, R. Saccharomycodes ludwigii, control and potential uses in winemaking processes. Fermentation 2018, 4, 71. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Optimization conditions for ethanol production from sweet sorghum juice by thermotolerant yeast Saccharomyces cerevisiae: Using a statistical experimental design. Fermentation 2023, 9, 450. [Google Scholar] [CrossRef]
- Phong, H.X.; Klanrit, P.; Dung, N.T.P.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. High-temperature ethanol fermentation from pineapple waste hydrolysate and gene expression analysis of thermotolerant yeast Saccharomyces cerevisiae. Sci. Rep. 2022, 12, 13965. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Nguyen, K.H.V.; Nguyen, N.L.; Ho, X.T.T.; Truong, P.H.; Nguyen, K.C.T. Lychee-derived, thermotolerant yeasts for second-generation bioethanol production. Fermentation 2022, 8, 515. [Google Scholar] [CrossRef]
- Pereira, F.; Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs. Bioresour. Technol. 2010, 101, 7856–7863. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol−sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tang, J.; Chen, J.; Zhang, Q. Quantitative structure-toxicity relationship analysis of combined toxic effects of lignocellulose-derived inhibitors on bioethanol production. Bioresour. Technol. 2019, 289, 121724. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhao, Y.; Chang, J.S.; Lee, D.J. Inhibitor formation and detoxification during lignocellulose biorefinery: A review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.K.S.; Gupta, R.K.; Otari, S.V.; Gao, H.; Lee, J.K.; Zhang, L. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol. J. 2019, 14, 1800468. [Google Scholar] [CrossRef]
- Almeida, J.R.; Modig, T.; Petersson, A.; Hagerdal, B.H.; Liden, G.; Grauslund, F.G. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef]
- Casey, E.; Sedlak, M.; Ho, H.W.Y.; Mosier, N.S. Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Caspeta, L.; Castillo, T.; Nielsen, J. Modifying yeast tolerance to inhibitory conditions of ethanol production processes. Front. Bioeng. Biotechnol. 2015, 3, 184. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Hasunuma, T.; Sanda, T.; Yamada, R.; Yoshimura, K.; Ishii, J.; Kondo, A. Metabolic pathway engineering based on metabolomics confers acetic acid and formic acid tolerance to a recombinant xylose-fermenting strain of Saccharomyces cerevisiae. Microb. Cell Factories 2011, 10, 2. [Google Scholar] [CrossRef]
- Lin, F.M.; Qiao, B.; Yuan, Y.J. Comparative proteomic analysis of tolerant and adaptation of ethanologenic Saccharomyces cerevisiae to furfural, a lignocellulosic inhibitory compound. Appl. Environ. Microbiol. 2009, 11, 3765–3776. [Google Scholar] [CrossRef]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-hydroxymethylfurfural: Assessment of mutagenicity, DNA damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 9, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Modig, T.; Liden, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 32, 379–385. [Google Scholar] [CrossRef]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pretreatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Pilap, W.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of the newly isolated thermotolerant Kluyveromyces marxianus for high-temperature ethanol production using sweet sorghum juice. 3 Biotech 2018, 8, 126. [Google Scholar] [CrossRef]
- Bai, F.W.; Chen, L.J.; Zhang, Z.; Anderson, W.A.; Moo-Young, M. Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 2004, 110, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ozmihci, S.; Kargi, F. Comparison of yeast strains for batch ethanol fermentation of cheese-whey powder (CWP) solution. Lett. Appl. Microbiol. 2007, 44, 602–606. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahnhägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahnhägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Li, B.; Liu, N.; Zhao, X. Response mechanisms of Saccharomyces cerevisiae to the stress factors present in lignocellulose hydrolysate and strategies for constructing robust strains. Biotechnol. Biofuels Bioprod. 2022, 15, 28. [Google Scholar] [CrossRef]
- Walker, G.M.; Basso, T.O. Mitigating stress in industrial yeasts. Fungal Biol. 2020, 124, 387–397. [Google Scholar] [CrossRef]
- Eardley, J.; Timson, D.J. Yeast cellular stress: Impacts on bioethanol production. Fermentation 2020, 6, 109. [Google Scholar] [CrossRef]
- Hohmann, S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 300–372. [Google Scholar] [CrossRef] [PubMed]
- Mager, W.H.; de Boer, A.H.; Siderius, M.H.; Voss, H.-P. Cellular responses to oxidative and osmotic stress. Cell Stress Chaperones 2000, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, M. Enzymic hydrolysis of steam exploded herbaceous agricultural waste (Brassica carinata) at different particule sizes. Process Biochem. 2002, 38, 187–192. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Chaiyaso, T. Bioethanol production from cellulose-rich corncob residue by the thermotolerant Saccharomyces cerevisiae TC-5. J. Fungi. 2021, 7, 547. [Google Scholar] [CrossRef]
- Valamonfared, J.; Javanmard, A.S.; Ghaedi, M.; Bagherinasab, M. Bioethanol production using lignocellulosic materials and thermophilic microbial hydrolysis. Biomass Convers. Biorefinery 2024, 14, 16589–16601. [Google Scholar] [CrossRef]
- Huang, R.; Cao, M.; Guo, H.; Qi, W.; Su, R.; He, Z. Enhanced ethanol production from pomelo peel waste by integrated hydrothermal treatment, multienzyme formulation, and fed-batch operation. J. Agric. Food Chem. 2014, 62, 4643–4651. [Google Scholar] [CrossRef]
- Shrivastava, A.; Sharma, R.K. Conversion of lignocellulosic biomass: Production of bioethanol and bioelectricity using wheat straw hydrolysate in electrochemical bioreactor. Heliyon 2023, 9, e12951. [Google Scholar] [CrossRef]
- Alabdalall, A.H.; Almutari, A.A.; Aldakeel, S.A.; Albarrag, A.M.; Aldakheel, L.A.; Alsoufi, M.H.; Alfuraih, L.Y.; Elkomy, H.M. Bioethanol production from lignocellulosic biomass using Aspergillus niger and Aspergillus flavus hydrolysis enzymes through immobilized S. cerevisiae. Energies 2023, 16, 823. [Google Scholar] [CrossRef]
- Jeffries, T.W. Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol. 2006, 17, 320–326. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Khasa, Y.P.; Singh, A.; Zhang, Y.H.P. Bioethanol production from pentose sugars: Current status and future prospects. Renew. Sustain. Energy Rev. 2011, 15, 4950–4962. [Google Scholar] [CrossRef]
- Avanthi, A.; Kumar, S.; Sherpa, K.C.; Banerjee, R. Bioconversion of hemicelluloses of lignocellulosic biomass to ethanol: An attempt to utilize pentose sugars. Biofuels 2017, 8, 431–444. [Google Scholar] [CrossRef]
- Patelski, A.M.; Kobalczyk, A.; Dziekońska-Kubczak, U.; Januszewicz, B.; Domański, J. Effect of ultrasound on fermentation of thick molasses worts by distiller’s yeast. Appl. Sci. 2025, 15, 3811. [Google Scholar] [CrossRef]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Takagi, H. Molecular mechanisms and highly functional development for stress tolerance of the yeast Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2021, 85, 1017–1037. [Google Scholar] [CrossRef]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.I. Cellular stress impact on yeast activity in biotechnological processes-A short overview. Microorganisms 2023, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, W.B.; Wang, Y.T.; Zhao, X.Q. Regulatory mechanisms underlying yeast chemical stress response and development of robust strains for bioproduction. Curr. Opin. Biotechnol. 2024, 86, 103072. [Google Scholar] [CrossRef]
- Xu, J.R.; Mehmood, M.A.; Wang, L.; Ahmad, N.; Ma, H.J. Omics-based strategies to explore stress tolerance mechanisms of Saccharomyces cerevisiae for efficient fuel ethanol production. Front. Energy Res. 2022, 10, 884582. [Google Scholar] [CrossRef]
- Topaloğlu, A.; Esen, Ö.; Turanli-Yildiz, B.; Arslan, M.; Çakar, Z.P. From Saccharomyces cerevisiae to ethanol: Unlocking the power of evolutionary engineering in metabolic engineering applications. J. Fungi 2023, 9, 984. [Google Scholar] [CrossRef]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 2000, 11, 4241–4257. [Google Scholar] [CrossRef]
- Kubiak-Szymendera, M.; Pryszcz, L.P.; Bialas, W.; Celinska, E. Epigenetic response of Yarrowia lipolytica to stress: Tracking methylation level and search for methylation patterns via whole-genome sequencing. Microorganisms 2021, 9, 1798. [Google Scholar] [CrossRef]
- Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Beltran, G.; Torija, M.J. The role of the membrane lipid composition in the oxidative stress tolerance of different wine yeasts. Food Microbiol. 2019, 78, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Bourbon-Melo, N.; Sá-Correia, I. The cell wall and the response and tolerance to stresses of biotechnological relevance in yeasts. Front. Microbiol. 2022, 13, 953479. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Qi, X.; Lin, Y.; Guo, Y.; Zhang, Y.; Wang, Q. A hierarchical transcriptional regulatory network required for long-term thermal stress tolerance in an industrial Saccharomyces cerevisiae strain. Front. Bioeng. Biotechnol. 2022, 9, 826238. [Google Scholar] [CrossRef] [PubMed]
- Özel, A.; Topaloğlu, A.; Esen, Ö.; Holyavkin, C.; Baysan, M.; Çakar, Z.P. Transcriptomic and physiological meta-analysis of multiple stress-resistant Saccharomyces cerevisiae strains. Stresses 2024, 4, 714–733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).