Abstract
Supercritical combustion is a promising technique for improving the efficiency and reducing the emissions of next-generation gas turbines. However, accurately modeling combustion under these conditions remains a challenge, particularly due to the complexity of chemical kinetics. This study aims to evaluate the applicability of a reduced global reaction mechanism compared to the detailed Foundational Fuel Chemistry Model 1.0 (FFCM-1) when performing hydrogen combustion with supercritical carbon dioxide and argon as diluents. Computational fluid dynamics simulations were conducted in two geometries: a simplified tube for isolating chemical effects and a combustor with cooling channels for practical evaluation. The analysis focuses on the evaluation of velocity, temperature, and the water vapor mass fraction distributions inside the combustion chamber. The results indicate good agreement between the global and detailed mechanisms, with average relative errors below 2% for supercritical argon and 4% for supercritical carbon dioxide. Both models captured key combustion behaviors, including buoyancy-driven flame asymmetry caused by the high density of supercritical fluids. The findings suggest that global chemistry models can serve as efficient tools for simulating supercritical combustion processes, making them valuable for the design and optimization of future supercritical gas turbine systems.
1. Introduction
In the face of escalating environmental concerns, climate change, global warming, and the unsustainable consumption of fossil fuels have become critical global challenges. The continued reliance on fossil fuels for energy production has led to rising carbon dioxide (CO2) emissions, contributing to a greenhouse effect that intensifies global warming. According to the Glasgow Climate Pact [1], if all currently announced climate pledges are fully implemented on schedule, global temperature rise could be limited to 1.8 °C by 2100. However, this still exceeds the Paris Agreement’s target of keeping global temperature increase well below 2 °C while striving to limit it to 1.5 °C. As a result, achieving rapid decarbonization is a critical priority for the energy sector, representing the most effective strategy for significantly reducing global carbon emissions over the next two decades. To combat this, innovative energy solutions are being explored, including the use of supercritical cycles and hydrogen combustion [2,3,4,5,6,7,8].
Supercritical cycles, particularly those utilizing supercritical CO2 and argon, offer a promising pathway for improving the efficiency of energy systems while minimizing environmental impact. These cycles operate at high temperatures and pressures, achieving greater thermal efficiency and reducing fuel consumption, which directly translates to lower emissions [9]. Among combustion-based supercritical CO2 (sCO2) power cycles, the Allam–Fetvedt cycle stands out as one of the most promising [2,3,10]. Operating at pressures of 300 bar or higher, the conditions within its combustor more closely resemble those of rocket engines than traditional gas turbine engines. This results in a significantly more complex combustor design compared to conventional gas turbine cycles, such as those using natural gas and air. The extremely high pressure, elevated power density, and substantial CO2 dilution notably influence both the combustion process and the combustor’s design. Consequently, expertise in combustion and combustor design from conventional gas turbine systems may not directly apply to sCO2 cycles. Extensive research has already been undertaken, and further efforts are essential to advance this technology toward widespread adoption. Current process modeling indicates that the target efficiencies for systems powered by natural gas and coal syngas are 59% and 52%, respectively. These values significantly exceed the efficiencies of conventional Rankine Cycle systems (approximately 35–50%) and traditional simple Brayton Cycle systems (around 30–40%) [9,11]. The turbine in the Allam cycle operates with an inlet pressure of approximately 300 bar and an inlet temperature of around 1150 °C. While this inlet temperature is relatively moderate for gas turbines, the inlet pressure is significantly higher than that of conventional gas turbines, though not exceptionally high for steam turbines. Consequently, the design of the Allam cycle turbine must integrate aspects of both gas turbine and steam turbine technologies. The maximum allowable temperature, limited by nickel-based alloys [12] (approximately 760 °C at the hot end of the regenerator), restricts the turbine outlet temperature. As a result, the turbine inlet temperature in the Allam cycle ranges between 1100 °C and 1300 °C. Although this is lower than the inlet temperatures of modern gas turbines, blade cooling technology remains necessary. According to a Toshiba report [13], conventional gas turbine cooling methods and thermal barrier coatings can be applied to the Allam cycle. Direct-fired oxy-fuel combustion is an effective heat source for supercritical carbon dioxide power cycles, offering a promising approach to delivering the required thermal energy input. This combustion method enables highly efficient power generation while seamlessly integrating carbon capture, achieving up to 99% CO2 capture from the generated emissions. Since combustion occurs at pressures exceeding 150 bar, the CO2 remains at a high pressure even after expanding through a turbine, making it nearly ready for pipeline transport. These benefits justify the significant effort invested in developing direct-fired oxy-combustors [3,14,15,16].
Supercritical CO2 turbines are not widely covered in the literature, particularly when it comes to technologically mature, operational sCO2 turbines. Similar to sCO2 turbines, argon-based gas turbine research in the literature is also extremely scarce. Recent studies on hydrogen-fueled argon power cycle engines highlight the growing interest in argon-based applications [17,18,19]. With regard to the challenges associated with supercritical gas turbines, with a focus on direct-fired supercritical combustion, maintaining flame stability is difficult due to the high velocities and density variations of supercritical fluids, increasing the risk of blowout or flashback, especially with hydrogen fuel. The high-pressure environment also accelerates combustion kinetics, leading to a higher risk of knocking or detonation. Efficient fuel injection and mixing are challenging due to the unique fluid properties of supercritical fluids, requiring advanced injector designs. Heat transfer management is critical because supercritical fluids exhibit high heat transfer coefficients, leading to the potential overheating of turbine components.
The aim of this study is to evaluate the applicability and accuracy of a global chemical reaction mechanism compared to a detailed chemistry model (Foundational Fuel Chemistry Model—FFCM-1) for simulating combustion under supercritical conditions, using carbon dioxide and argon as working fluids. The work, via providing computational fluid dynamics (CFD) simulations, enhances our understanding of supercritical combustion, specifically for its application in next-generation supercritical gas turbines. The novelty lies in the application of a global reaction mechanism that accurately reproduces the behavior of detailed kinetic radical-based FFCM-1 mechanisms under supercritical conditions. This work provides a foundation for accelerating the development and optimization of next-generation supercritical gas turbines working with supercritical fluids such as sCO2, sAr, etc., where detailed chemistry is often computationally prohibitive. These systems can work very efficiently due to the high density of supercritical fluids and reduced compression work. Additionally, the use of supercritical argon as a working fluid offers new insights into how alternative inert fluids influence flame shape, temperature fields, and flow behavior. The study assesses whether the global model can reliably reproduce combustion features while significantly reducing computational time, thereby offering a practical tool for the design and optimization of future high-efficiency supercritical gas turbine systems.
2. Materials and Methods
2.1. Study Geometries
Two different geometries were employed to analyze and compare the combustion behaviour under supercritical conditions. These are illustrated in Figure 1 and Figure 2.
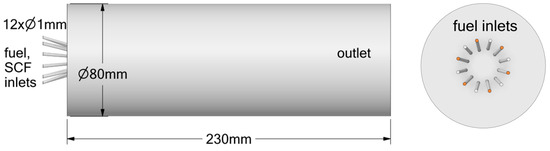
Figure 1.
Simplified tube geometry with injector, with 6 inlets of fuel (orange color) and 6 inlets of oxidizer.
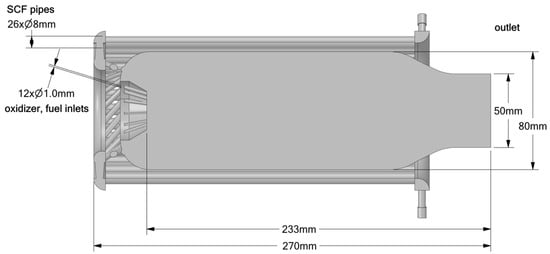
Figure 2.
Combustor geometry with supercritical fluid cooling tube and injection pattern the same as that for the tube.
The first geometry (Figure 1) consists of a simplified cylindrical tube used primarily to compare the performance of the detailed chemical mechanism (FFCM-1) and a reduced global reaction mechanism. The configuration includes a main combustion chamber with an axial injection system. Fuel is introduced through a central tube, where pure hydrogen is injected. The oxidizer, consisting of oxygen mixed with a supercritical fluid (either carbon dioxide or argon), is supplied through a surrounding coaxial inlet.
The second geometry (Figure 2) represents a more complex and realistic combustor design. While the injection system remains similar to that of the simplified tube, with separate inlets for hydrogen and oxidizer, the configuration includes additional features such as external cooling channels surrounding the combustion chamber. These channels circulate supercritical fluid to manage wall temperatures and replicate more accurately the thermal environment of modern gas turbine combustors.
This two-step geometric approach allows for an isolated analysis of chemical mechanism effects in the simple tube and a more integrated evaluation of fluid dynamics and thermal interactions in a practical combustor setting.
2.2. Models and Boundary Conditions
In the geometry used for this study, combustion stabilization is achieved through a swirling flow configuration, a widely adopted approach in gas turbine research [20,21]. Cameretti and Tuccillo [20] conducted RANS simulations of combustion in a tubular lean-premixed combustor using the standard k-ε turbulence model. Their study demonstrated the model’s limitations, particularly in predicting Reynolds stresses and flame speeds when compared with the Reynolds Stress Model. Conversely, Chakchak et al. [21] investigated diffusion flames under both rich and lean conditions using a combined experimental and numerical approach, also employing the k-ε model. Their results showed good agreement between simulation and experimental data. Based on the similarities in the flow configuration and combustion regime, the k-ε model was selected in the present study for turbulence modelling.
Chemical reactions play a central role in determining the flame structure, temperature distribution, and product species concentrations, all of which directly influence the flow field within the combustor. Hilbert et al. [22] highlighted the significance of detailed chemical mechanisms in achieving accurate combustion simulations, noting both the increased predictive capability and the computational challenges associated with such models. Similarly, Chen et al. [23] emphasized the role of detailed chemistry in their laminar combustion study, demonstrating that changes in initial pressure could shift dominant reaction pathways. The Foundational Fuel Chemistry Model 1.0 (FFCM-1) [24], a detailed reaction mechanism, has been validated for the accurate prediction of laminar burning velocities, even under high-pressure conditions and for rare diluents such as argon [25].
Despite their accuracy, detailed chemical mechanisms are associated with high computational costs and potential numerical instability [22]. Therefore, this study compares two chemical mechanisms: the experimentally validated detailed FFCM-1 mechanism, consisting of 38 species and 291 reactions, and a global mechanism. For simulations involving argon, the global model employs a single reaction (), while for carbon dioxide environments, a four-step global mechanism is used (; ; ; ), with reaction constants taken from [26].
The combustion–chemistry interaction was modelled using the Eddy Dissipation Concept (EDC) for the detailed mechanism and the Eddy Dissipation Model (EDM) for the global mechanism. Both models provide reasonable agreement with experimental data, with EDM offering better numerical stability and convergence in simplified scenarios.
Due to the non-ideal behaviour of fluids at supercritical conditions, the ideal gas law is inadequate [27,28]. Consequently, thermophysical properties were modelled using the Peng–Robinson equation of state, which has been validated in previous research on supercritical CO2 [29,30].
Radiative heat transfer was modelled using the Discrete Ordinates (DO) method, while the absorption coefficient was computed using the Weighted Sum of Gray Gases Model (WSGGM). These radiation models are well-established and widely used in combustion simulations. All governing equations and model formulations are documented in the ANSYS Fluent Theory Guide [31].
Numerical simulations were carried out using ANSYS Fluent 2024 R2 and R1, employing a pressure-based solver under steady-state assumptions. When studying the chemistry mechanism effect on combustion, two working fluid were used, i.e., argon (Case #3, 4) and carbon dioxide (Case #1, 2). The following conditions were applied to the carbon dioxide configuration: mass flow rate of H2 0.000333 kg/s; CO2 0.01275; and O2 0.00392 kg/s. Meanwhile, the following conditions were applied for argon: H2 0.00018 kg/s; Ar 0.01584 kg/s; and O2 0.00216 kg/s. The effect of the working fluid was further investigated using the geometry of a realistic combustor (Figure 2) in Case #5, 6 with a mass flow rate of H2 0.000333 kg/s; O2 0.00225 kg/s; SCF in oxidizer stream 0.014417 kg/s; and cooling SCF 0.03 kg/s. The inlet temperature of all gases was equal to 310 K. Table 1 show cases together with the most important parameters.

Table 1.
Cases studied in this paper including the geometry, mechanism, supercritical fluid and pressure in the combustion chamber.
2.3. Mesh Independence Study
Figure 3 presents a mesh independence study of the velocity and temperature profiles along the center axis of a simplified tube. The left plot shows the velocity profile, while the right plot displays the temperature distribution, each evaluated using four different mesh resolutions: 1 million, 2 million, 3 million, and 4.5 million cells. In the velocity plot, all meshes capture the general profile, which features a sharp rise, a peak around 0.08 m, a dip, and a secondary rise further downstream. As the mesh is refined, the curves converge, with the 3 million and 4.5 million cell results nearly overlapping, indicating that further mesh refinement has minimal impact on the velocity results.
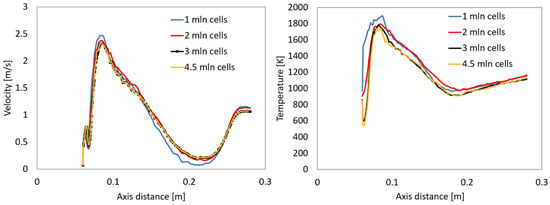
Figure 3.
Mesh independence study for velocity and temperature along the axis.
Similarly, the temperature plot reveals a steep increase to a peak near 0.08 m, followed by a gradual decline. While the 1 million cell mesh overpredicts the peak temperature, the results from 3 million and 4.5 million cells align closely, showing consistent behavior throughout the domain.
Overall, the results demonstrate that a mesh with approximately 3 million cells is sufficient to achieve mesh-independent solutions for both velocity and temperature in this simulation.
3. Results and Discussion
The results comparing the detailed chemical kinetics model (FFCM-1) with a global reaction mechanism are presented in Section 3.1. Simulations were conducted using carbon dioxide and argon as diluent inner gases. To isolate the influence of the chemical kinetics models, a simplified tube geometry (shown in Figure 1) was employed. This approach allows a clearer assessment of the chemical model’s impact, independent of the geometric complexity.
Section 3.2 extends the analysis to simulations performed on a realistic combustor geometry, depicted in Figure 2. In this case, different supercritical fluids were tested to evaluate their influence on combustor performance under identical design and operating conditions. In the real combustor configuration, argon and carbon dioxide serve not only as diluents in the oxidizer stream but also as coolants. The following sections present and discuss the corresponding results.
3.1. Comparison of Global and Detailed Chemistry Mechanism for Two Supercritical Working Fluids
Chemical reaction mechanisms play a fundamental role in combustion processes, as they dictate the rates of reactant consumption and product formation. These reactions ultimately determine the concentrations of intermediate and final species, which in turn influence the flame temperature depending on the dominant reaction pathways. Despite their importance, the implementation of detailed chemical mechanisms in CFD simulations remains computationally demanding and complex. Therefore, in this study, the performance of a detailed chemical mechanism (FFCM-1) is compared with that of a global reaction mechanism. As described previously, a simplified tube geometry was employed to isolate the effects of chemical kinetics without the added complexity of real combustor geometry. The analysis focused on two primary combustion parameters: velocity and temperature. These were examined across two or three different cross-sectional planes to capture the spatial behavior of the flame.
The results indicate that both the global and detailed (FFCM-1) mechanisms produce qualitatively similar velocity and temperature profiles, regardless of the cross-section. However, differences between the xz (Figure 4a,c and Figure 5a,d) and yz (Figure 4b,d and Figure 5b,e) planes are apparent, suggesting some directional dependence during flame development. The velocity and temperature fields (Figure 4) show the flame bending upward, opposite to the direction of gravity. This behavior is attributed to buoyancy effects, where lighter, hotter combustion gases rise while denser, cooler fluids are displaced downward. This gravitational influence is particularly pronounced when using supercritical fluids, which have much higher densities than gases under standard conditions. As a result, the effects of buoyancy are expected to be more significant in supercritical combustion compared to conventional gaseous combustion.
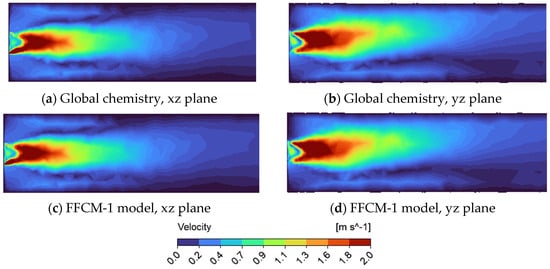
Figure 4.
Velocity profile in a tube using supercritical carbon dioxide as working fluid for the FFCM-1 and global model display on xz and yz cross—section. (a) Global chemistry model on xz cross—section; (b) Global chemistry model on yz cross—section; (c) FFCM-1 model on yz cross—section; (d) FFCM-1 model on yz cross—section.
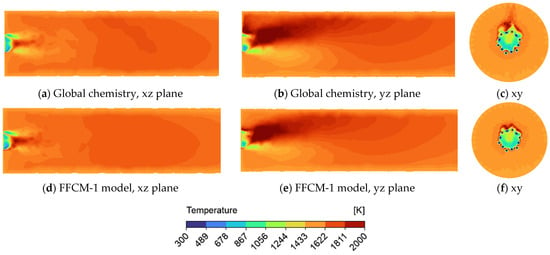
Figure 5.
Temperature profile in a tube using supercritical carbon dioxide as the working fluid for the FFCM-1 and global model display on the xz, yz, and xy cross-section plane. (a) Global chemistry model on xz cross—section plane; (b) Global chemistry model on yz cross—section plane; (c) Global chemistry model on xy plane; (d) FFCM-1 model on xz cross—section plane; (e) FFCM-1 model on yz cross—section plane; (f) FFCM-1 model on xy cross—section plane.
Temperature profiles (Figure 5) reveal that the flame exhibits asymmetry depending on the cross-sectional plane observed. This is evident despite the application of symmetric boundary conditions, reinforcing the conclusion that gravitational effects induce asymmetry. In particular, Figure 5b,e show the localized overheating of the tube wall on one side, while Figure 5c,f highlight clear asymmetric flame structures. Importantly, the temperature distributions are nearly identical between the global and FFCM-1 mechanisms, suggesting that for simplified geometries, global models may provide sufficiently accurate thermal predictions.
When argon is used as the diluent gas, both the velocity and temperature profiles exhibit slight differences compared to cases using carbon dioxide. The velocity fields (Figure 6) appear more ‘compact’ and concentrated near the inlet region, indicating reduced axial penetration and the stronger influence of inlet momentum. Despite these differences, a similar degree of asymmetry, as previously observed with CO2, is also present in the yz cross-sections (Figure 6b,d), further confirming the role of buoyancy-induced effects regardless of the diluent used. Quantitative analysis demonstrates a strong correlation between the global and detailed (FFCM-1) chemical models, with both yielding consistent results for key flow and thermal characteristics in the presence of argon.
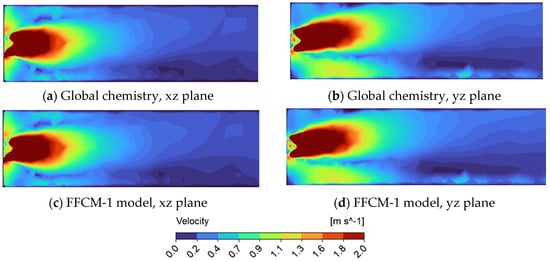
Figure 6.
Velocity profile in a tube using supercritical argon as the working fluid for the FFCM-1 and global model display on the xz and yz cross—section. (a) Global chemistry model on xz cross—section; (b) Global chemistry model on yz cross—section; (c) FFCM-1 model on yz cross—section; (d) FFCM-1 model on yz cross—section.
An analysis of Figure 7a,b reveals a noticeable asymmetry in the flame structure that is particularly evident in Figure 7c, where the high-temperature region (indicated in red) is deflected upward. This upward flame orientation highlights the influence of buoyancy effects. Furthermore, when comparing the temperature contours for argon and CO2, it is apparent that argon results in less cooling near the top region adjacent to the inlets (Figure 7c), whereas CO2 provides more effective thermal moderation in that area. This difference can be attributed to the distinct thermophysical properties of the two diluents, particularly their heat capacities and densities. Overall, the comparison between the FFCM-1 detailed mechanism and the global chemistry model shows excellent agreement, with both predicting nearly identical flame shapes and temperature distributions.
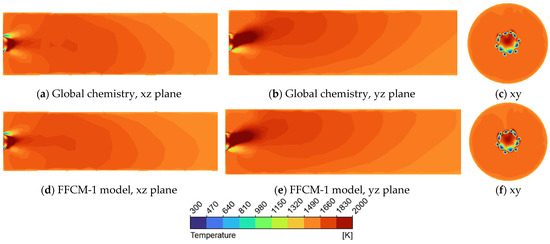
Figure 7.
Temperature profile in a tube using supercritical argon as the working fluid for the FFCM-1 and global model display on the xz, yz, and xy cross—section plane (a) Global chemistry model on xz cross—section plane; (b) Global chemistry model on yz cross—section plane; (c) Global chemistry model on xy cross—section plane; (d) FFCM-1 model on xz cross—section plane; (e) FFCM-1 model on yz cross—section plane; (f) FFCM-1 model on xy cross—section plane.
To enable a quantitative comparison between the global and detailed chemical mechanisms (FFCM-1), axial profiles of the water mass fraction, velocity, and temperature were extracted and plotted (Figure 8). For simulations using carbon dioxide, small discrepancies between the two models are observed near the outlet region, particularly in the water mass fraction and temperature fields. Despite these differences, the average relative error remains low, at 3.3% for the H2O mass fraction and 2.8% for temperature. Velocity predictions show excellent agreement, with an average relative error of only 2.1%. In the case of argon, the agreement between the global and detailed mechanisms is even stronger. The relative errors are reduced to 2.0% for the H2O mass fraction, 1.3% for temperature, and only 0.4% for velocity, indicating that both models provide nearly identical predictions for this diluent. Across all profiles, a noticeable jump in the parameters near the outlet is observed. This discontinuity is attributed to buoyancy effects, where gravitational forces act on the denser, cooler fluid regions, inducing variations in the flow and species distribution that become more pronounced toward the exit of the domain.
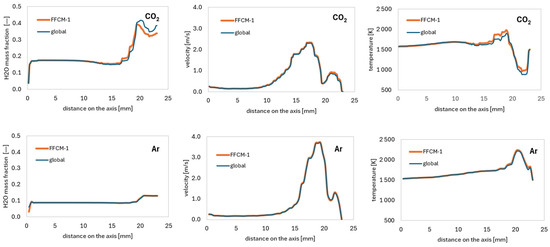
Figure 8.
Graphs comparing water mass fraction—(left), velocity—(middle), and temperature—(right) along the axis for carbon dioxide—(top), and argon—(bottom) for the FFCM-1 and global mechanism.
Eventually, the use of the global model allowed for a substantial reduction in computation time compared to the detailed radical-based model, with a reduction ratio of approximately 5:1.
3.2. Combustion Behaviour in the Real Combustor for Supercritical Carbon Dioxide and Argon
Building upon the simplified geometry analysis, Section 3.2 presents the results of simulations performed on a realistic combustor geometry (Figure 2). The objective here is to assess the influence of various supercritical fluids on combustion characteristics under identical design and operating conditions. In this configuration, both argon and carbon dioxide are used as diluents in the oxidizer stream and as coolants for the combustor walls, reflecting more practical operational scenarios.
Further analysis of the real combustor geometry shows distinct differences in combustion behaviour depending on the working fluid. As shown in Figure 9a,b, the flame temperature is higher when argon is used as the diluent compared to supercritical carbon dioxide. This can be attributed to the lower heat capacity of argon, which results in reduced thermal buffering and consequently higher peak temperatures. Due to the larger diameter of the combustion chamber compared to the simplified tube geometry, the flame exhibits a reflective behaviour in which it strikes the upper wall of the chamber. This interaction creates a recirculation zone near the location where the combustor transitions to a narrower exhaust tube. Such recirculation leads to the formation of localized hot spots in this corner region, which could pose a significant risk to the material integrity and durability of the system during long-term operation. These hot spots can be mitigated by redesigning the chamber geometry, increasing the diameter to prevent high-temperature regions from reaching the walls, or by reorienting the combustor to the vertical position, allowing buoyancy forces to aid the upward flow and reduce wall interactions. Velocity profiles further support these observations. While argon exhibits slightly higher overall velocities, both working fluids show a characteristic velocity increase near the geometric contraction leading to the exhaust section. This acceleration is a result of flow constriction and enhanced momentum near the outlet. The water vapor concentration also varies depending on the working fluid. With sCO2, the H2O mass fraction is higher near the inlet and tends to rise toward the top of the combustor, consistent with buoyancy effects. In contrast, for sAr, water vapor is more uniformly distributed. However, an additional recirculation zone is observed near the exhaust outlet in the sAr case, where part of the water vapor flow is redirected back into the combustion chamber. This reverse flow can contribute to flame instability and unsteady combustion dynamics, raising potential concerns for stable operation.
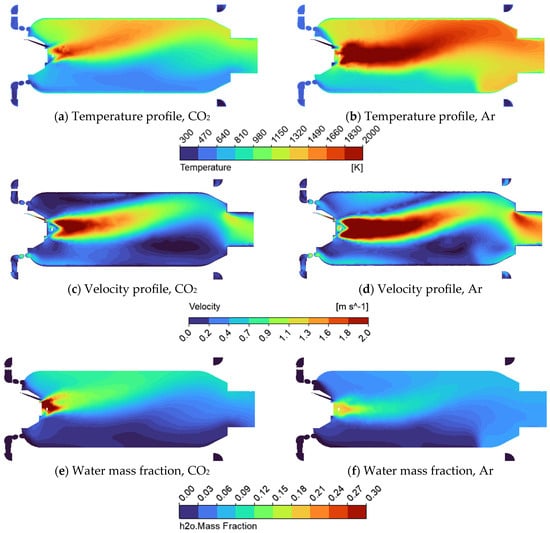
Figure 9.
Combustor geometry cross—section showing (a) Temperature profile for sCO2; (b) Temperature profile for sAr; (c) Velocity profile for sCO2; (d) Velocity profile for sAr; (e) Water mass fraction profile for sCO2; (f) Water mass fraction profile for sAr.
4. Conclusions
This study evaluated the performance of a detailed chemical mechanism (FFCM-1) and a global reaction mechanism for hydrogen combustion under supercritical conditions using two different working fluids: supercritical carbon dioxide and supercritical argon. Simulations were performed around 1.5 times the critical pressure to assess the model fidelity and flow behaviour in both simplified and realistic combustor geometries.
The comparison between the global and detailed chemical models demonstrated good agreement, with average relative errors below 2% for sAr and below 4% for sCO2 in key parameters such as the water vapor mass fraction, temperature, and velocity. This confirms that for supercritical conditions, the global mechanism, despite its simplicity (four reactions for CO2 and one for Ar), can reproduce results for the detailed model (291 reactions for Ar) with a significantly reduced computational cost. The computational advantage of the global model at 5× speed makes it suitable for multivariable optimization iterations such as combustion chamber shape and injection parameters. These findings suggest that global mechanisms may be reliably applied to a broader range of supercritical fluids, though case-specific validation is recommended due to fluid-dependent deviations in accuracy.
A key observation in this study is the substantial impact of buoyancy forces in supercritical combustion regimes. Unlike conventional combustion, where buoyancy plays a minor role, supercritical conditions lead to strong gravitational effects due to the higher fluid densities. This is manifested as upward flame deflection and asymmetric temperature profiles, even under symmetric boundary conditions.
Furthermore, the study shows that different supercritical fluids can be used as working media in the same combustor design, although certain operating parameters may need adjustment to maintain equivalent combustion behaviour. While trends in flow structure, temperature distribution, and product formation are similar between fluids, differences in magnitude highlight the importance of tailoring combustor designs to the specific thermophysical properties of the working fluid.
In conclusion, the use of global reaction mechanisms presents a promising path for efficient supercritical combustion modelling, and the careful consideration of buoyancy effects and fluid-specific properties is critical for accurate prediction and stable combustor operation.
Author Contributions
Conceptualization, S.O., J.M., H.P.-K., A.K.S. and A.P.; methodology, S.O., J.M. and A.P.; software, S.O. and J.M.; validation, S.O. and J.M.; formal analysis, S.O. and J.M.; investigation, S.O. and J.M.; resources, S.O. and J.M.; data curation, S.O. and J.M.; writing—original draft preparation, S.O. and J.M.; writing—review and editing, A.K.S. and A.P.; visualization, S.O. and J.M.; supervision, A.P., A.K.S. and H.P.-K.; project administration, A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union, grant number 101083748 within the HERMES project.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
The following nomenclature are used in this manuscript:
| Mass flow rate, | |
| T | Temperature, |
| w | mass fraction, |
| Subscription | |
| out | outlet |
| H2O | water |
| O2 | oxygen |
| Abbreviations | |
| CFD | Computational Fluid Dynamics |
| FFCM-1 | Foundational Fuel Chemistry Model 1.0 |
| SCF | Super Critical Fluid |
| sAr | Supercritical argon |
| sCO2 | Supercritical carbon dioxide |
References
- The Glasgow Climate Pact–Key Outcomes from COP26. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-glasgow-climate-pact-key-outcomes-from-cop26 (accessed on 1 June 2025).
- Reale, F. The Allam Cycle: A Review of Numerical Modeling Approaches. Energies 2023, 16, 7678. [Google Scholar] [CrossRef]
- Chan, W.; Morosuk, T.; Li, X.; Li, H. Allam Cycle: Review of Research and Development. Energy Convers. Manag. 2023, 294, 117607. [Google Scholar] [CrossRef]
- Xie, M.; Chen, X.; Chen, L.; Zhou, M.; Liu, Y.; Zeng, L.; Shi, H.; Zhang, F.; Xie, S.; Zhao, Y. Evaluating the Feasibility of a Novel Allam Cycle for Co-Generating Power and Water in Hot Regions. Energy Convers. Manag. 2024, 309, 118447. [Google Scholar] [CrossRef]
- Rodríguez Hervás, G.; Petrakopoulou, F. Exergoeconomic Analysis of the Allam Cycle. Energy Fuels 2019, 33, 7561–7568. [Google Scholar] [CrossRef]
- Shao, J.; Choudhary, R.; Davidson, D.F.; Hanson, R.K.; Barak, S.; Vasu, S. Ignition Delay Times of Methane and Hydrogen Highly Diluted in Carbon Dioxide at High Pressures up to 300 Atm. Proc. Combust. Inst. 2019, 37, 4555–4562. [Google Scholar] [CrossRef]
- Conaire, M.Ó.; Curran, H.J.; Simmie, J.M.; Pitz, W.J.; Westbrook, C.K. A Comprehensive Modeling Study of Hydrogen Oxidation. Int. J. Chem. Kinet. 2004, 36, 603–622. [Google Scholar] [CrossRef]
- Beigzadeh, A.; Alabbad, M.; Liu, D.; Aljohani, K.; Hakimov, K.; Kashif, T.A.; Zanganeh, K.; Croiset, E.; Farooq, A. Reaction Kinetics for High Pressure Hydrogen Oxy-Combustion in the Presence of High Levels of H2O and CO2. Combust. Flame 2023, 247, 112498. [Google Scholar] [CrossRef]
- Allam, R.J.; Fetvedt, J.E.; Forrest, B.A.; Freed, D.A. The Oxy-Fuel, Supercritical CO2 Allam Cycle: New Cycle Developments to Produce Even Lower-Cost Electricity from Fossil Fuels Without Atmospheric Emissions. In Proceedings of the ASME Turbo Expo 2014: Turbine Technical Conference and Exposition, Düsseldorf, Germany, 16–20 June 2014. Volume 3B: Oil and Gas Applications; Organic Rankine Cycle Power Systems; Supercritical CO2 Power Cycles; Wind Energy. [Google Scholar]
- Allam, R.J.; Palmer, M.R.; Brown, G.W.; Fetvedt, J.; Freed, D.; Nomoto, H.; Itoh, M.; Okita, N.; Jones, C. High Efficiency and Low Cost of Electricity Generation from Fossil Fuels While Eliminating Atmospheric Emissions, Including Carbon Dioxide. Energy Procedia 2013, 37, 1135–1149. [Google Scholar] [CrossRef]
- White, M.T.; Bianchi, G.; Chai, L.; Tassou, S.A.; Sayma, A.I. Review of Supercritical CO2 Technologies and Systems for Power Generation. Appl. Therm. Eng. 2021, 185, 116447. [Google Scholar] [CrossRef]
- Weiland, N.; Thimsen, D. A Practical Look at Assumptions and Constraints for Steady State Modeling of SCO2 Brayton Power Cycles. In Proceedings of the 5th International Symposium—Supercritical CO2 Power Cycles, San Antonio, TX, USA, 28–31 March 2016. NETL-PUB-20271. [Google Scholar]
- Sasaki, T.; Itoh, M.; Maeda, H.; Tominaga, J.; Saito, D.; Niizeki, Y. Development of Turbine and Combustor for a Semi-Closed Recuperated Brayton Cycle of Supercritical Carbon Dioxide. In Proceedings of the ASME 2017 Power Conference Joint with ICOPE-17, Charlotte, NC, USA, 26–30 June 2017. [Google Scholar] [CrossRef]
- Delimont, J.; Mcclung, A.; Portnoff, M. Direct Fired Oxy-Fuel Combustor for SCO2 Power Cycles: 1MW Scale Design and Preliminary Bench Top Testing. In Proceedings of the ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition, Charlotte, NC, USA, 26–30 June 2017. [Google Scholar] [CrossRef]
- Delimont, J.; Mcclung, A.; Portnoff, M. Simulation of a Direct Fired Oxy-Fuel Combustor for sCO2 Power Cycles. In Proceedings of the 5th International Symposium—Supercritical CO2 Power Cycles, San Antonio, TX, USA, 28–31 March 2016; Available online: https://sco2symposium.com/papers2016/OxyFuel/116paper.pdf (accessed on 1 March 2025).
- Coogan, S.; Gao, X.; Mcclung, A. Evaluation of Kinetic Mechanisms for Direct Fired Supercritical Oxy-Combustion of Natural Gas. In Proceedings of the ASME Turbo Expo, Seoul, Republic of Korea, 13–17 June 2016. [Google Scholar] [CrossRef]
- Jin, S.; Deng, J.; Xie, K.; Liang, X.; Wang, C.; Ding, W.; Li, L. Knock Control in Hydrogen-Fueled Argon Power Cycle Engine with Higher Compression Ratio by Water Port Injection. Appl. Energy 2023, 349, 121664. [Google Scholar] [CrossRef]
- Wang, C.; Deng, J.; Ding, W.; Huang, Y.; Tang, Y.; Li, L. Thermodynamic Analysis of Employing Argon as the Diluent and Adding Hydrogen in an HCCI Ammonia Engine: Ignition Characteristics and Performances of Combustion and NO Emissions. Int. J. Hydrogen Energy 2024, 49, 293–300. [Google Scholar] [CrossRef]
- Oleś, S.; Mularski, J.; Pyka, D.; Pawlak-Kruczek, H.; Pozarlik, A. Optimization of Hydrogen Supercritical Oxy-Combustion in Gas Turbines. Fuels 2025, 6, 6. [Google Scholar] [CrossRef]
- Cameretti, M.C.; Tuccillo, R. Combustion Features of a Bio-Fuelled Micro-Gas Turbine. Appl. Therm. Eng. 2015, 89, 280–290. [Google Scholar] [CrossRef]
- Chakchak, S.; Hidouri, A.; Ghabi, A.; Chrigui, M.; Boushaki, T. Numerical Study of Turbulent Swirling Diffusion Flame Under Lean and Rich Conditions Using Turbulence Realizable K-Epsilon Model. Combust. Sci. Technol. 2023, 195, 1461–1482. [Google Scholar] [CrossRef]
- Hilbert, R.; Tap, F.; El-Rabii, H.; Thévenin, D. Impact of Detailed Chemistry and Transport Models on Turbulent Combustion Simulations. Prog. Energy Combust. Sci. 2004, 30, 61–117. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Zhang, A.; Deng, H.; Wen, X.; Wang, F.; Mei, Y. Experimental and Numerical Study on the Effect of CO2 Dilution on the Laminar Combustion Characteristics of Premixed CH4/H2/Air Flame. J. Energy Inst. 2022, 102, 315–326. [Google Scholar] [CrossRef]
- Smith, G.P.; Tao, Y.; Wang, H. Foundational Fuel Chemistry Model Version 1.0 (FFCM-1). Available online: https://web.stanford.edu/group/haiwanglab/FFCM1/pages/FFCM1.html (accessed on 1 March 2025).
- Burke, M.P.; Chaos, M.; Dryer, F.L.; Ju, Y. Negative Pressure Dependence of Mass Burning Rates of H2/CO/O2/Diluent Flames at Low Flame Temperatures. Combust. Flame 2010, 157, 618–631. [Google Scholar] [CrossRef]
- Hjärtstam, S.; Normann, F.; Andersson, K.; Johnsson, F. Oxy-Fuel Combustion Modeling: Performance of Global Reaction Mechanisms. Ind. Eng. Chem. Res. 2012, 51, 10327–10337. [Google Scholar] [CrossRef]
- Ba, J.; Wei, W.; Zhao, L.; Gang, X.; Dong, W.; Zhou, T. Numerical Simulation of Trans-/near-/Supercritical Injection Characteristics Based on Real Fluid Properties. Energy 2023, 278, 127767. [Google Scholar] [CrossRef]
- Hickey, J.-P.; Ihme, M. Supercritical Mixing and Combustion in Rocket Propulsion. Available online: https://web.stanford.edu/group/ctr/ResBriefs/2013/02_hickey.pdf (accessed on 6 May 2025).
- Bartle, K.D.; Clifford, A.A.; Shilstone, G.F. Estimation of Solubilities in Supercritical Carbon Dioxide: A Correlation for the Peng-Robinson Interaction Parameters. J. Supercrit. Fluids 1992, 5, 220–225. [Google Scholar] [CrossRef]
- Sodeifian, G.; Hsieh, C.M.; Tabibzadeh, A.; Wang, H.C.; Arbab Nooshabadi, M. Solubility of Palbociclib in Supercritical Carbon Dioxide from Experimental Measurement and Peng–Robinson Equation of State. Sci. Rep. 2023, 13, 2172. [Google Scholar] [CrossRef] [PubMed]
- ANSYS Inc. Ansys Fluent Theory Guide. Available online: https://dl.cfdexperts.net/cfd_resources/Ansys_Documentation/Fluent/Ansys_Fluent_Theory_Guide.pdf (accessed on 1 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).