Thermogravimetric Assessment and Kinetic Analysis of Forestry Residues Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Feedstock
2.2. Experimental Conditions
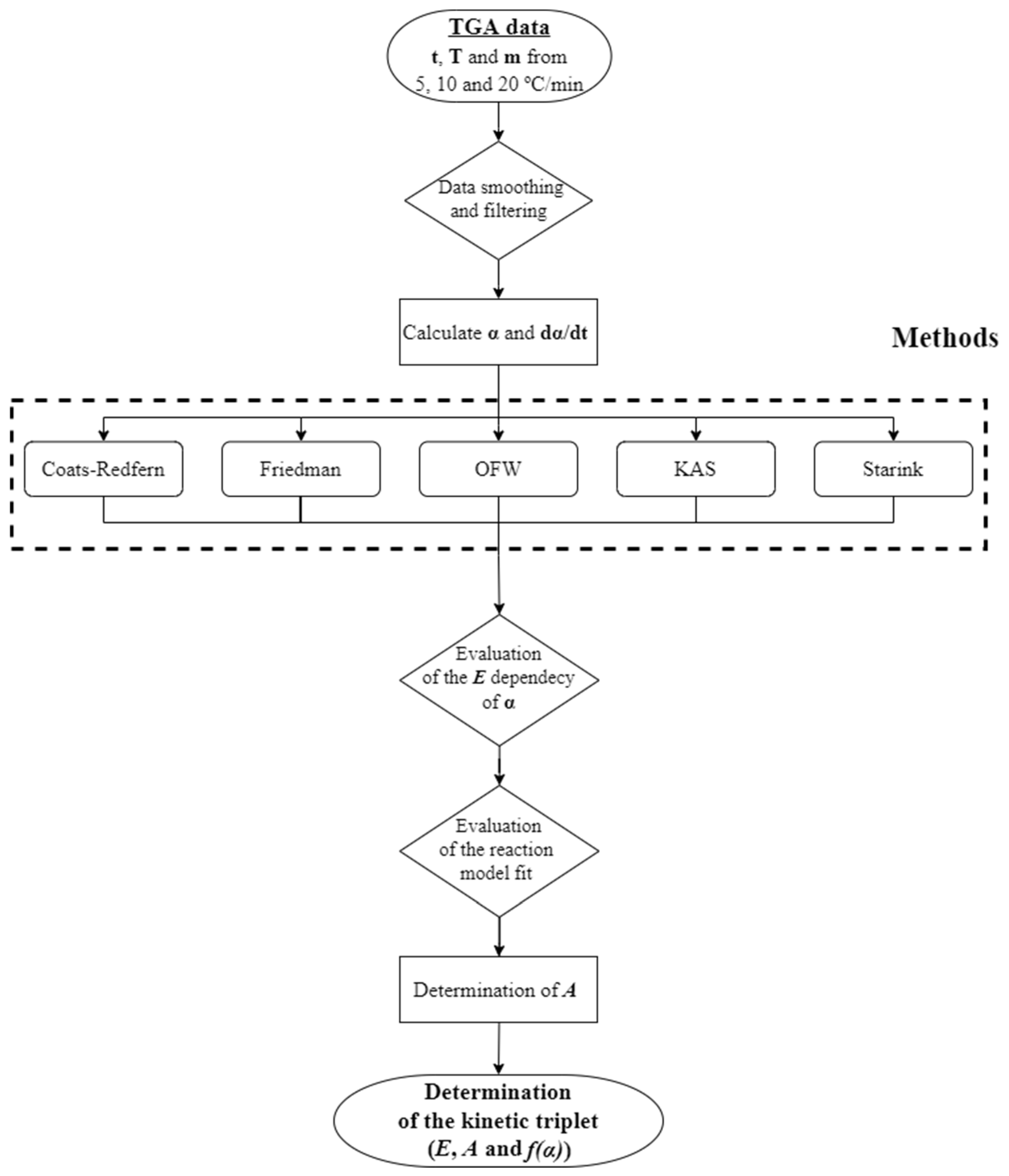
2.3. Kinetic Study
2.4. Data Analysis
3. Results and Discussion
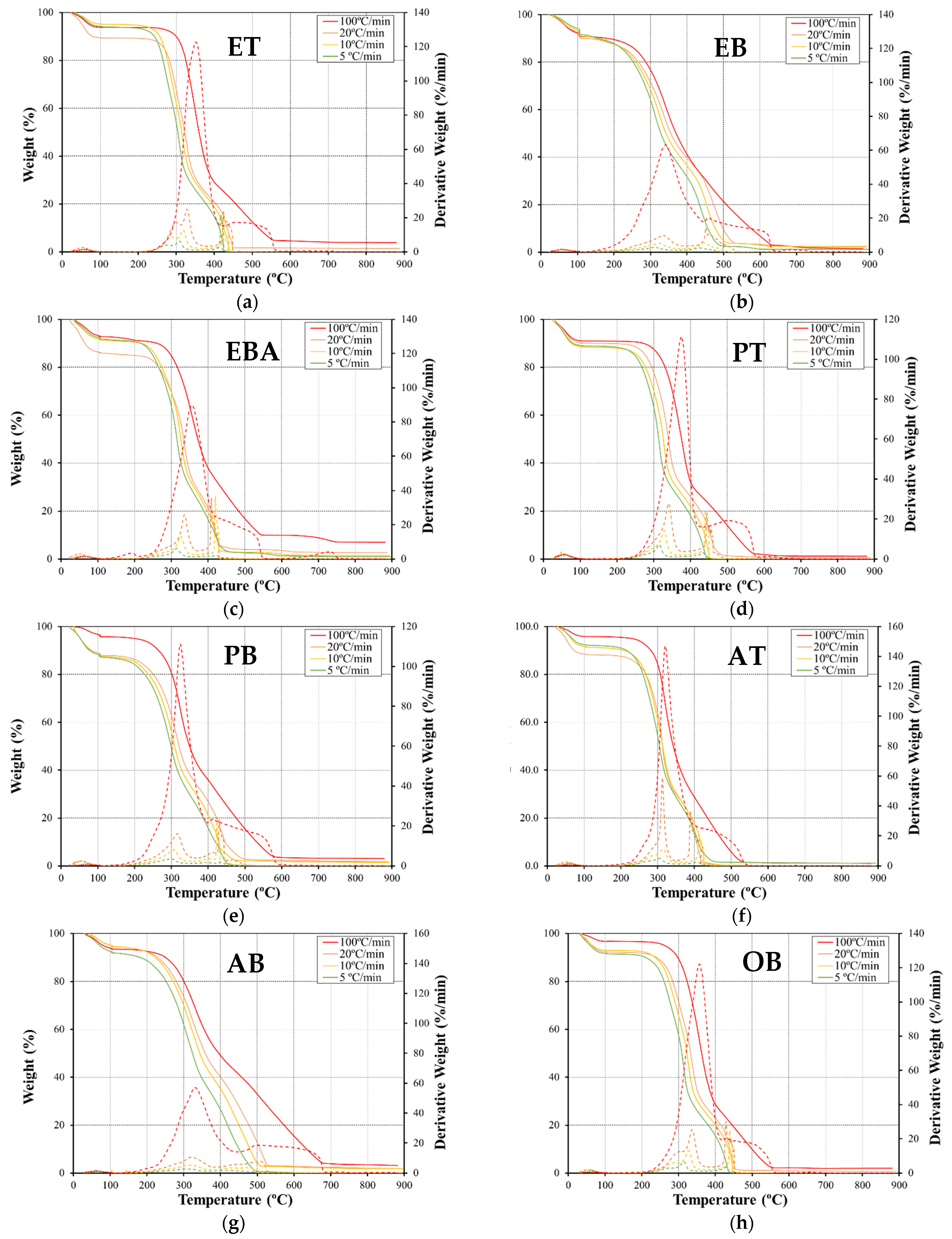
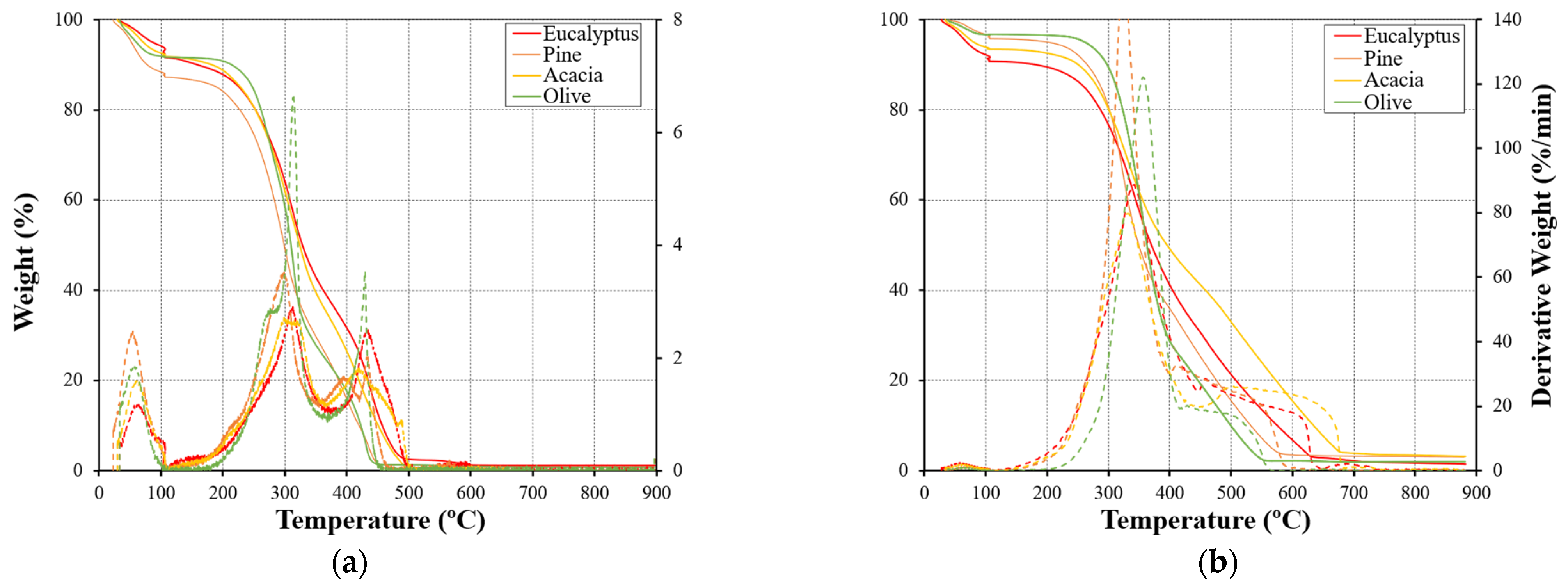
3.1. Thermal Decomposition
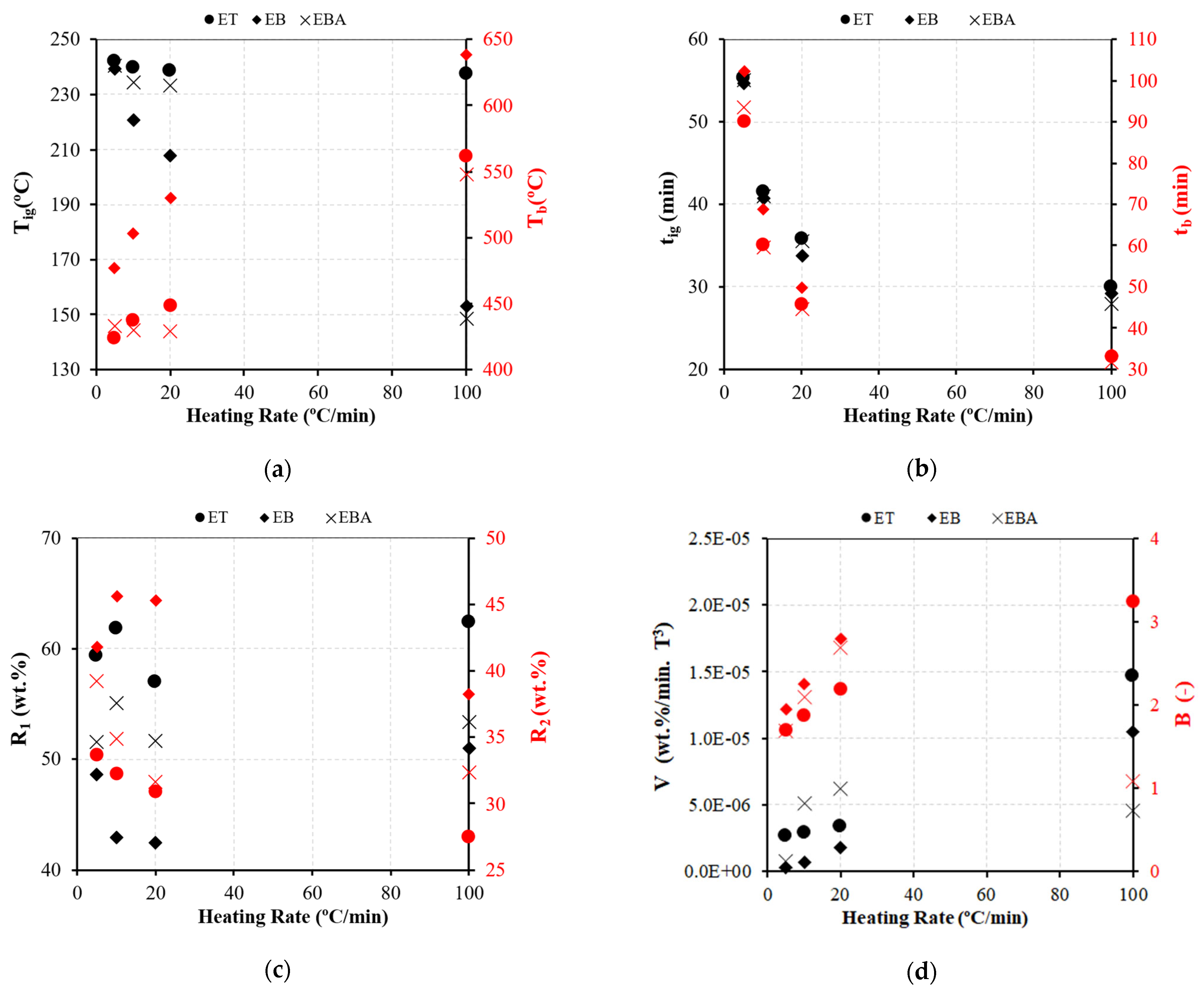
3.1.1. Influence of the Heating Rate
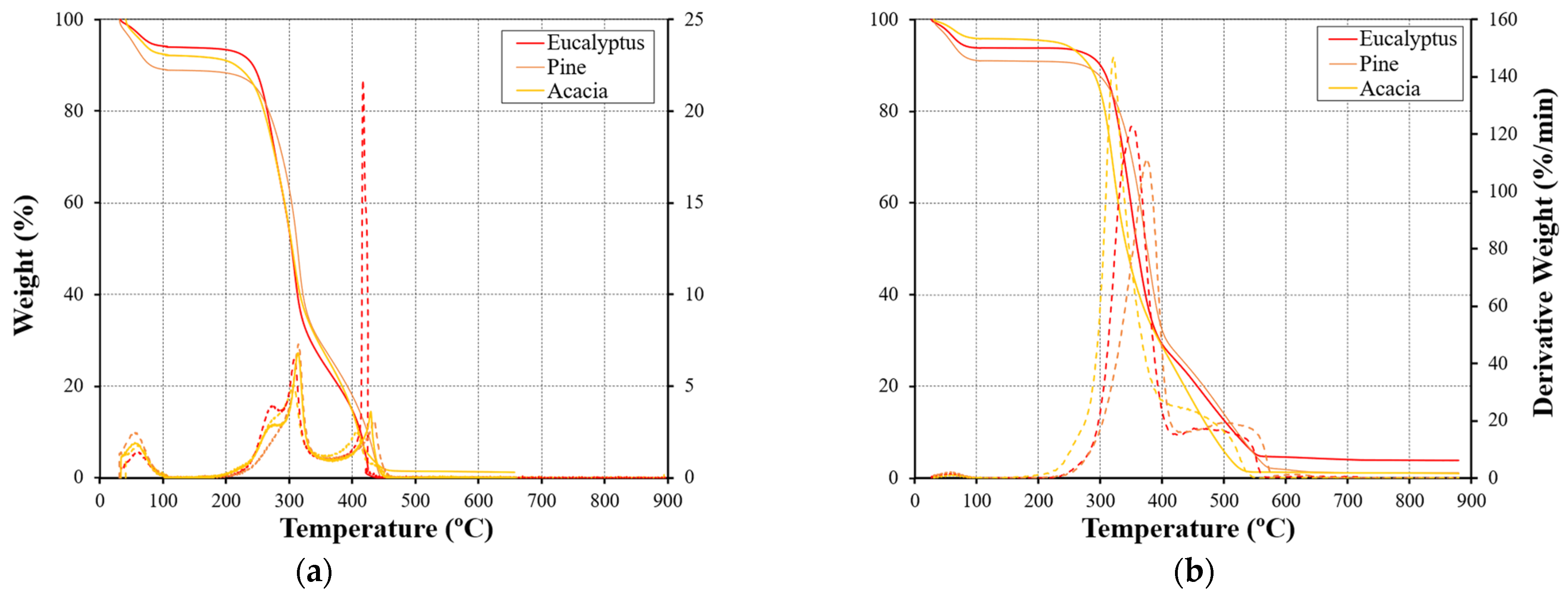
3.1.2. Comparative Analysis of Various Fuel Types
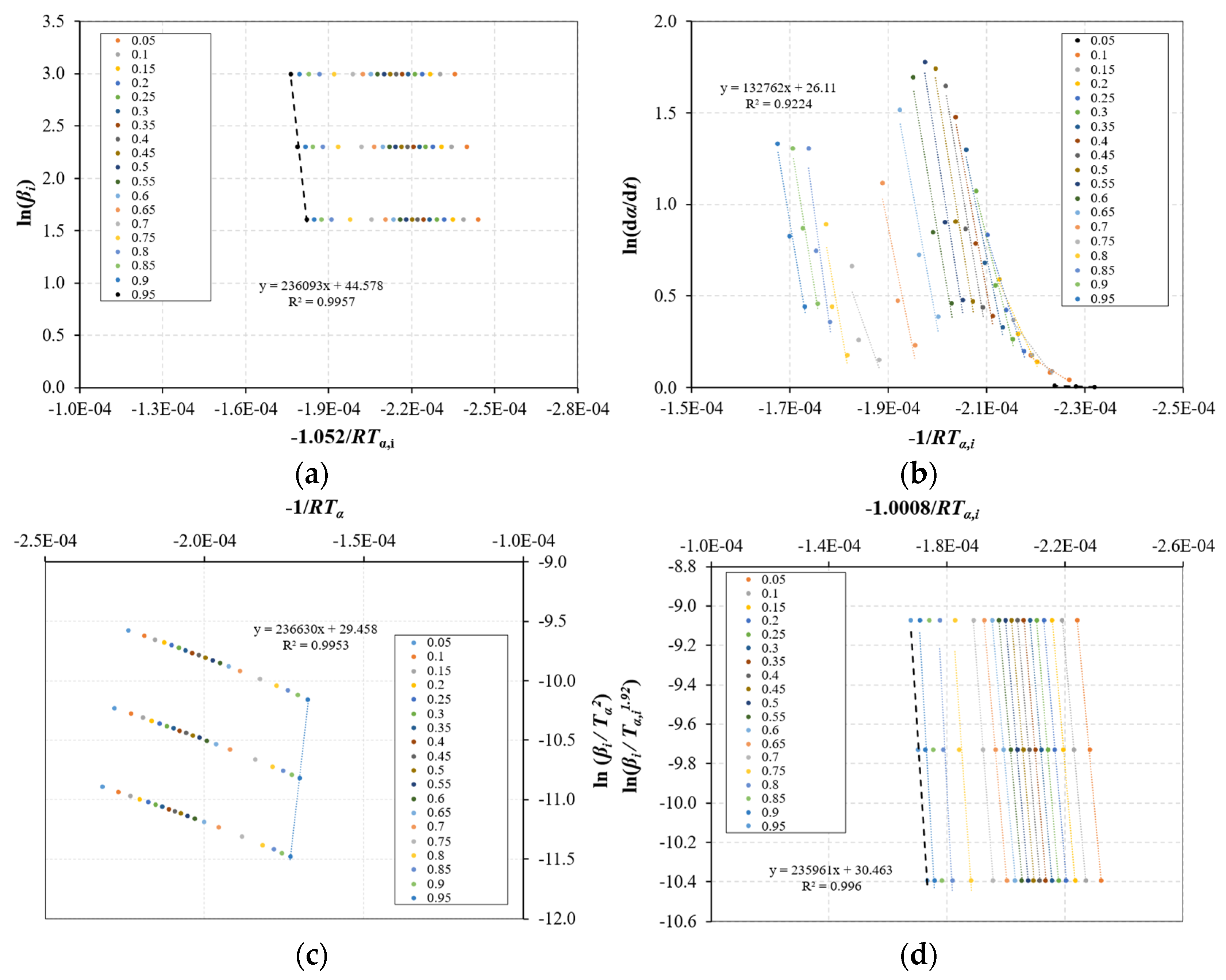
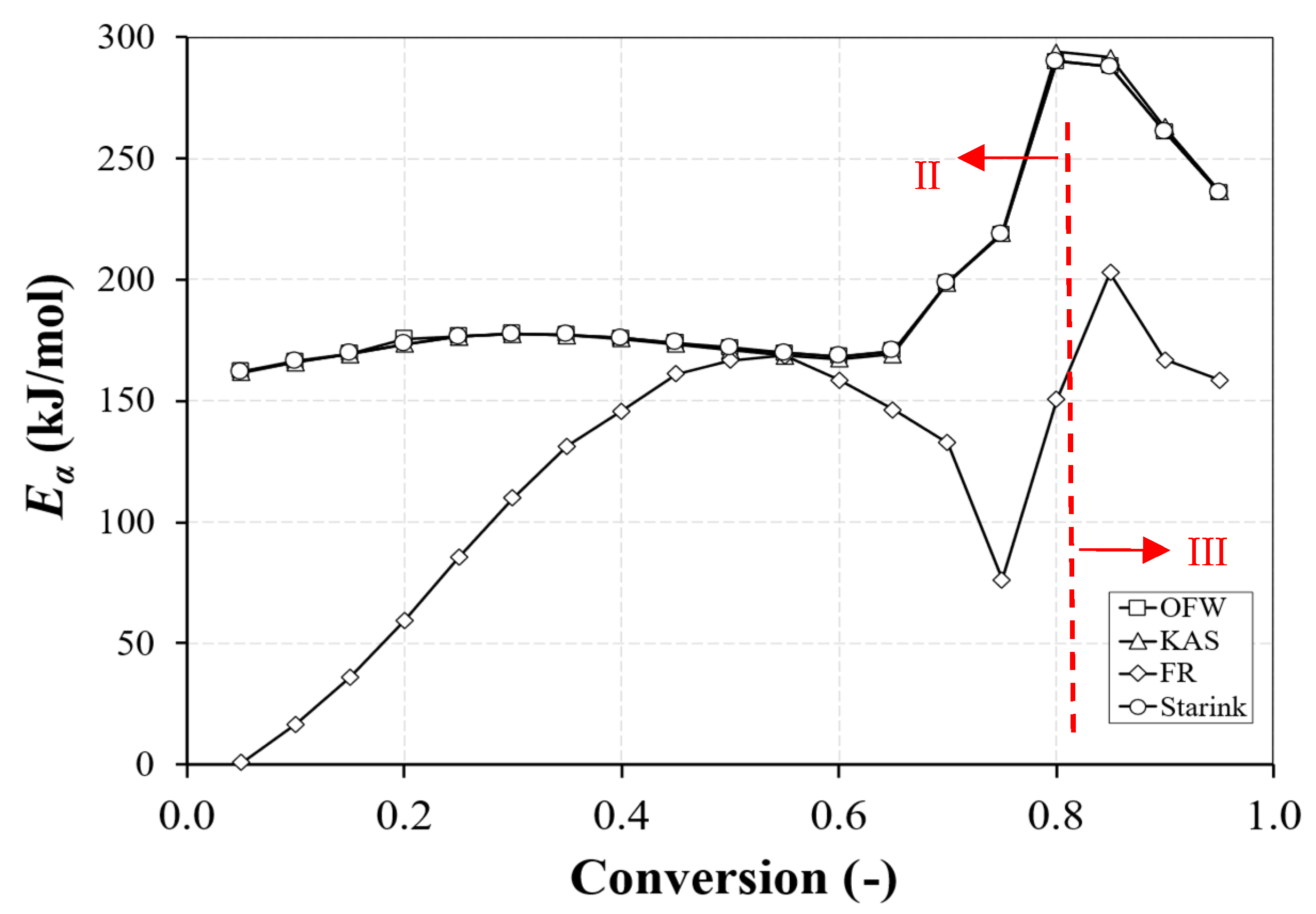
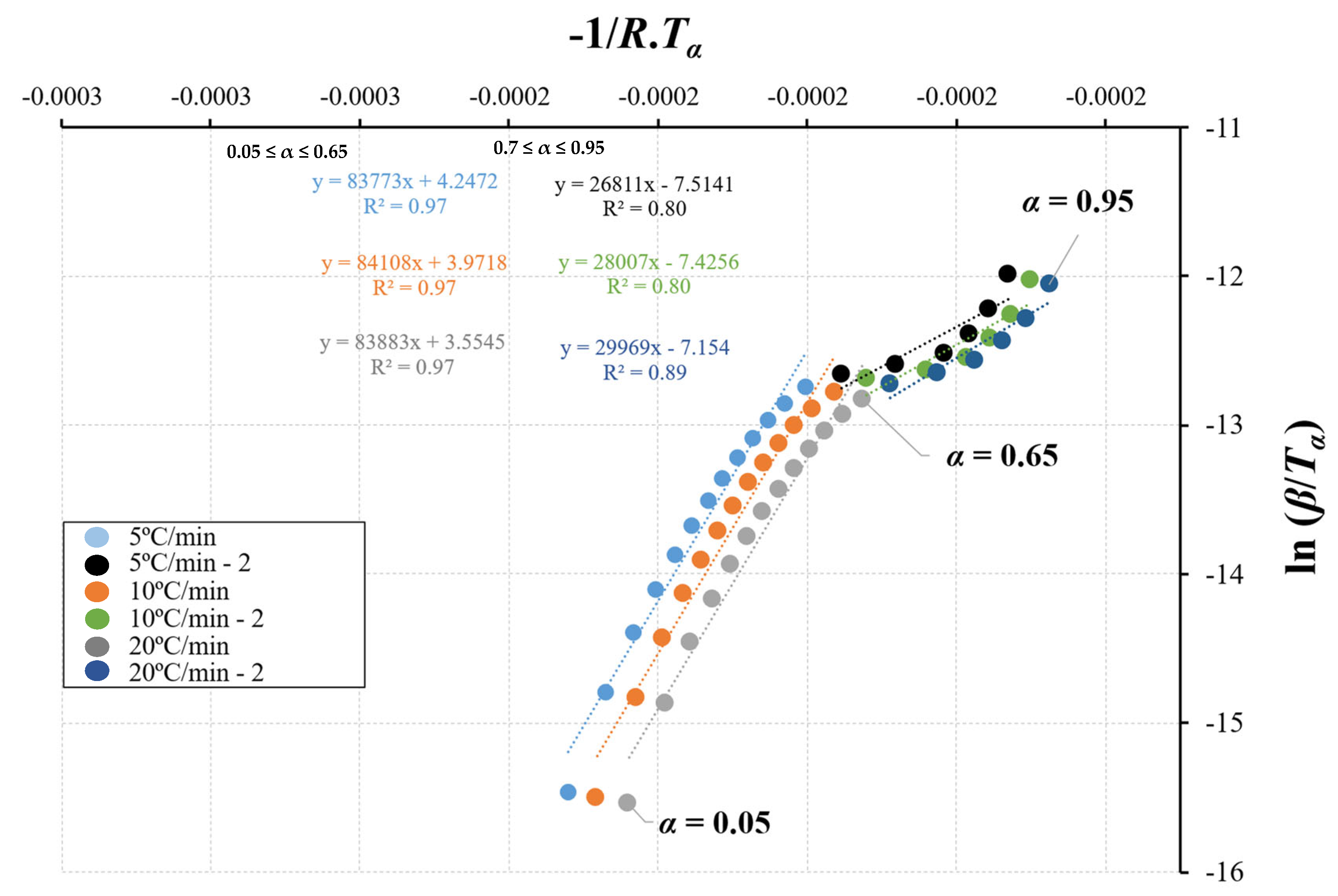
3.2. Kinetic Parameters
3.2.1. Evaluation of the Kinetic Method
3.2.2. Influence of the Type of Fuel
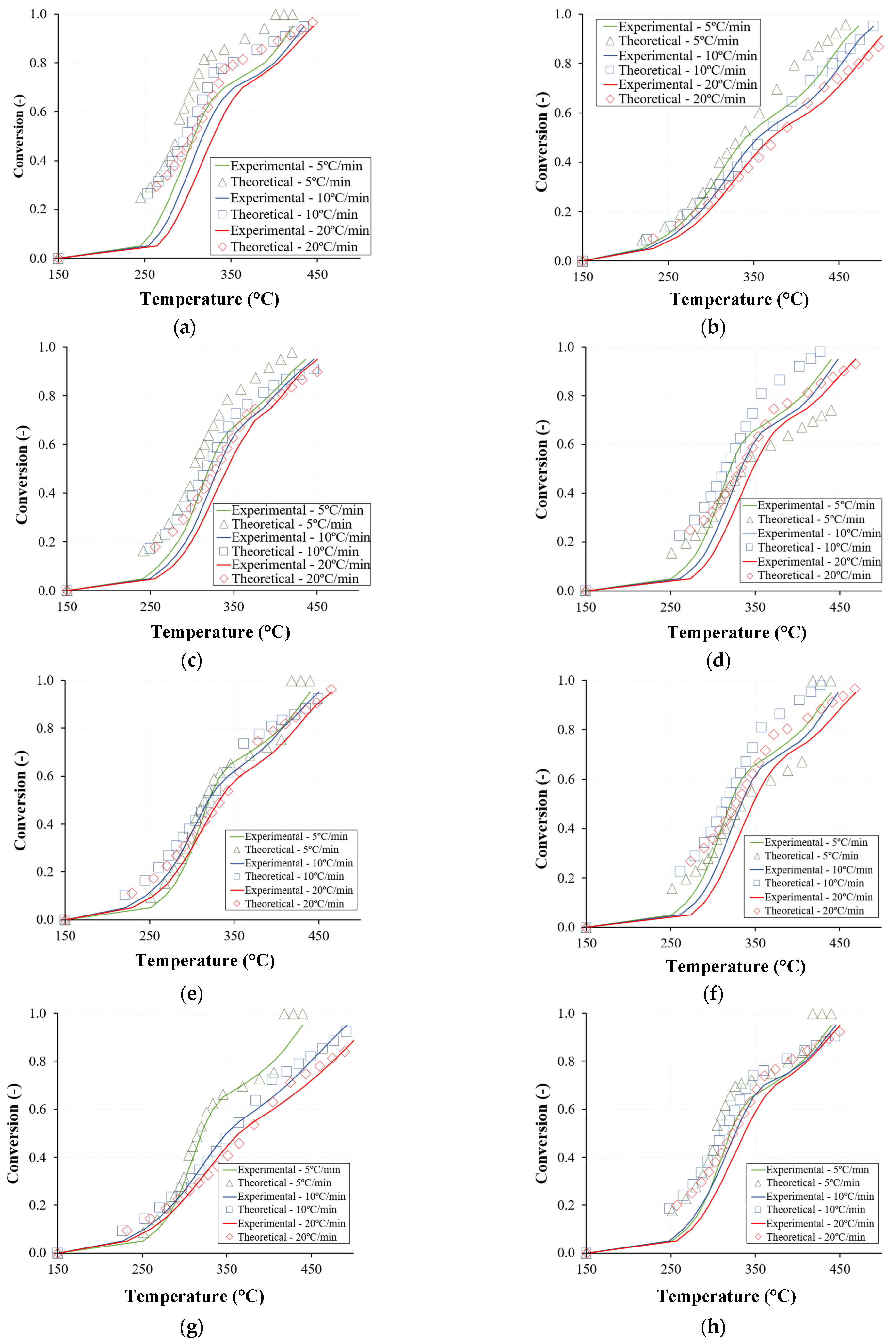
3.3. Reconstruction and Modeling
4. Conclusions
- The evaluation of combustion performance at a particle scale by TGA showed that the conversion of all biomass fuels takes place over a wide range of temperatures, which can be divided into three different zones: drying, devolatilization, and char combustion;
- Regarding the differences between the combustion behavior of the different fuels, a higher mass was observed for the inner part of the fuels, and there was a more effective conversion of the second-stage reaction. In its turn, the outer part of the eucalyptus presents a higher conversion of char, which may be associated with the higher lignin content of this fuel. Thus, variations in the chemical and physical composition, as well as in the structures and their thermal stability, justify the differences between the TG and DTG curves;
- Based on the TGA data, a kinetic analysis was performed, which relied on a two-step consecutive reaction model. The utilization of isoconversional methods proved ineffective in determining the kinetic parameters since E is not constant throughout the particle conversion. The CR method was successfully applied to determine the kinetic parameters to overcome this limitation;
- The devolatilization conversion was best characterized by the reaction kinetics model, using a second-order model, and the char oxidation was successfully described by a diffusion-controlled mechanism. The oxygen diffusion to the char affects the combustion process and should be considered the main mechanism of thermal conversion of biomass in the last stage;
- The kinetic parameters during biomass combustion are strongly dependent on the type of fuel, while the effect of the heating rate was less evident, and not all fuels presented a consistent trend with the heating rate. According to the results, during the main conversion stage, devolatilization, the branch part of the fuels presented a lower energy of activation, while eucalyptus was the most reactive fuel.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Latin symbols | |
| A | Pre-exponential factor, s−1 |
| B | Burnout performance, - |
| C | Comprehensive index, %/min2T3 |
| E | Activation energy, J/mol |
| I | Ignition performance index, %/min3 |
| k | Rate constant, s−1 |
| m | Mass, kg |
| R | Universal Gas Contant, J/mol·K/Weight loss, % |
| t | Time, s |
| T | Temperature, °C |
| V | Volatile matter release performance, %/T3 |
| Greek symbols | |
| Extent of reaction, - | |
| Reaction model, - | |
| Integral form of the reaction model, - | |
| Heating rate, K/min | |
| Subscripts and superscripts | |
| Initial | |
| Instantaneous | |
| Burnout | |
| List of Acronyms | |
| AB | Acacia Branch |
| AT | Acacia Trunk |
| CR | Coats-Redfern |
| DTG | Derivative thermogravimetry |
| EB | Eucalyptus Branch |
| EBA | Eucalyptus Bark |
| ET | Eucalyptus Trunk |
| FR | Friedman method |
| ICTAC | International Confederation for Thermal Analysis and Calorimetry |
| KAS | Kissinger-Akahira-Sunose linear integral method |
| OB | Olive Branch |
| PB | Pine Branch |
| PT | Pine Trunk |
| OFW | Ozawa-Flynn-Wall linear integral method |
Appendix A
| Fuel | β (°C/min) | Temperatures | Mass Loss | Time | Indexes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ET | 5 | 241.78 | 423.96 | 309.02 | 416.42 | 416.42 | 21.20 | 0.58 | 59.41 | 33.64 | 0.155 | 55.28 | 90.01 | 4.26 × 10−3 | 2.64 × 10−6 | 1.70 | 4.97 × 10−7 |
| 10 | 239.78 | 436.98 | 318.90 | 423.89 | 423.89 | 23.01 | 1.17 | 61.80 | 32.25 | 0.145 | 41.44 | 60.03 | 9.25 × 10−3 | 2.87 × 10−6 | 1.88 | 1.07 × 10−6 | |
| 20 | 238.42 | 448.47 | 328.18 | 423.93 | 328.18 | 24.93 | 2.14 | 56.98 | 30.92 | 0.184 | 35.75 | 45.59 | 1.53 × 10−2 | 3.33 × 10−6 | 2.19 | 2.09 × 10−6 | |
| 100 | 237.36 | 561.91 | 350.87 | 451.07 | 350.87 | 122.71 | 9.98 | 62.45 | 27.48 | 0.481 | 29.95 | 32.93 | 1.24 × 10−1 | 1.47 × 10−5 | 3.24 | 3.87 × 10−5 | |
| EB | 5 | 239.47 | 476.82 | 313.68 | 435.70 | 313.68 | 2.90 | 0.57 | 48.66 | 41.82 | 0.448 | 54.68 | 102.18 | 5.19 × 10−4 | 3.16 × 10−7 | 1.95 | 5.99 × 10−8 |
| 10 | 220.59 | 503.16 | 321.57 | 446.47 | 446.47 | 5.64 | 1.10 | 42.97 | 45.59 | 0.453 | 40.65 | 68.84 | 2.02 × 10−3 | 6.36 × 10−7 | 2.26 | 2.53 × 10−7 | |
| 20 | 207.92 | 530.03 | 336.67 | 451.63 | 451.63 | 14.57 | 2.15 | 42.48 | 45.30 | 0.391 | 33.71 | 49.72 | 8.69 × 10−3 | 1.81 × 10−6 | 2.80 | 1.37 × 10−6 | |
| 100 | 152.94 | 638.04 | 342.43 | 457.31 | 342.43 | 63.39 | 9.94 | 51.04 | 38.28 | 0.296 | 29.11 | 33.57 | 6.49 × 10−2 | 1.05 × 10−5 | 4.22 | 4.22 × 10−5 | |
| EBA | 5 | 240.55 | 432.91 | 315.18 | 428.77 | 315.18 | 6.21 | 0.57 | 51.62 | 39.23 | 0.490 | 55.06 | 93.41 | 1.21 × 10−3 | 7.21 × 10−7 | 1.69 | 1.41 × 10−7 |
| 10 | 234.19 | 429.65 | 326.22 | 419.17 | 419.17 | 36.34 | 1.12 | 55.07 | 34.86 | 0.318 | 40.90 | 59.40 | 1.50 × 10−2 | 5.12 × 10−6 | 2.10 | 1.72 × 10−6 | |
| 20 | 233.37 | 428.77 | 335.65 | 408.17 | 408.17 | 35.47 | 2.05 | 51.71 | 31.66 | 0.536 | 35.54 | 44.62 | 2.24 × 10−2 | 6.25 × 10−6 | 2.69 | 3.11 × 10−6 | |
| 100 | 148.60 | 547.86 | 356.69 | 726.63 | 356.69 | 89.77 | 9.52 | 53.41 | 32.40 | 0.914 | 27.95 | 31.54 | 1.02 × 10−1 | 4.58 × 10−6 | 1.08 | 7.06 × 10−5 | |
| PT | 5 | 251.92 | 445.11 | 316.35 | 436.46 | 316.35 | 7.14 | 0.55 | 51.85 | 36.78 | 0.007 | 57.25 | 95.76 | 1.30 × 10−3 | 7.46 × 10−7 | 1.61 | 1.39 × 10−7 |
| 10 | 247.39 | 450.12 | 328.17 | 440.06 | 440.06 | 24.05 | 1.09 | 54.60 | 33.21 | 0.102 | 43.28 | 62.54 | 8.89 × 10−3 | 2.65 × 10−6 | 1.81 | 9.52 × 10−7 | |
| 20 | 242.06 | 484.53 | 339.58 | 444.82 | 339.58 | 27.88 | 2.19 | 57.14 | 32.38 | 0.175 | 35.15 | 47.19 | 1.68 × 10−2 | 3.22 × 10−6 | 2.30 | 2.16 × 10−6 | |
| 100 | 240.67 | 579.16 | 374.80 | 505.04 | 374.80 | 111.47 | 9.98 | 61.99 | 27.83 | 0.192 | 29.62 | 32.74 | 1.15 × 10−1 | 9.49 × 10−6 | 2.60 | 3.32 × 10−5 | |
| PB | 5 | 232.17 | 443.93 | 298.05 | 433.13 | 298.05 | 3.46 | 0.54 | 47.61 | 38.96 | 0.24 | 54.09 | 96.29 | 6.64 × 10−4 | 3.70 × 10−7 | 1.57 | 7.60 × 10−8 |
| 10 | 206.23 | 461.45 | 307.79 | 423.42 | 423.42 | 22.61 | 1.08 | 47.94 | 38.07 | 0.36 | 37.99 | 63.45 | 9.38 × 10−3 | 3.08 × 10−6 | 2.21 | 1.06 × 10−6 | |
| 20 | 199.27 | 494.73 | 315.32 | 430.09 | 430.09 | 23.03 | 2.11 | 48.77 | 37.45 | 0.27 | 32.70 | 47.49 | 1.48 × 10−2 | 3.19 × 10−6 | 2.57 | 1.69 × 10−6 | |
| 100 | 170.05 | 589.39 | 323.58 | 411.21 | 323.58 | 111.16 | 10.31 | 54.54 | 38.18 | 0.31 | 28.72 | 32.57 | 1.19 × 10−1 | 2.31 × 10−5 | 4.79 | 1.75 × 10−5 | |
| AT | 5 | 238.62 | 421.24 | 308.75 | 404.83 | 308.75 | 5.08 | 0.57 | 54.83 | 36.22 | 0.56 | 54.46 | 90.78 | 1.03 × 10−3 | 7.18 × 10−7 | 1.90 | 1.20 × 10−7 |
| 10 | 225.80 | 424.92 | 316.04 | 411.38 | 411.38 | 20.68 | 1.13 | 54.07 | 36.95 | 0.25 | 40.46 | 59.00 | 8.66 × 10−3 | 3.04 × 10−6 | 2.09 | 1.08 × 10−6 | |
| 20 | 217.41 | 417.51 | 312.98 | 389.47 | 312.98 | 58.08 | 2.11 | 51.09 | 36.14 | 0.15 | 34.93 | 44.18 | 3.76 × 10−2 | 1.12 × 10−5 | 2.62 | 6.22 × 10−6 | |
| 100 | 196.59 | 539.53 | 321.96 | 457.54 | 321.96 | 146.91 | 10.50 | 58.49 | 36.33 | 0.10 | 29.23 | 32.35 | 1.55 × 10−1 | 1.71 × 10−5 | 2.53 | 7.40 × 10−5 | |
| AB | 5 | 236.71 | 471.81 | 304.35 | 417.05 | 304.35 | 2.71 | 0.57 | 54.50 | 37.74 | 0.48 | 54.73 | 101.62 | 4.87 × 10−4 | 3.34 × 10−7 | 2.09 | 5.89 × 10−8 |
| 10 | 210.91 | 505.79 | 317.73 | 511.51 | 317.73 | 5.27 | 1.15 | 57.11 | 35.51 | 0.30 | 37.58 | 66.84 | 2.10 × 10−3 | 4.06 × 10−7 | 1.52 | 2.69 × 10−7 | |
| 20 | 200.77 | 527.77 | 321.61 | 510.10 | 321.61 | 10.67 | 2.27 | 57.17 | 35.34 | 0.32 | 33.18 | 49.21 | 6.53 × 10−3 | 8.77 × 10−7 | 1.73 | 1.14 × 10−6 | |
| 100 | 165.86 | 680.97 | 332.70 | 504.08 | 332.70 | 57.00 | 10.03 | 56.09 | 34.19 | 0.37 | 28.63 | 33.38 | 5.96 × 10−2 | 6.03 × 10−6 | 3.01 | 3.05 × 10−5 | |
| OB | 5 | 240.99 | 437.76 | 313.61 | 429.19 | 313.61 | 6.67 | 0.57 | 58.18 | 32.75 | 0.02 | 55.33 | 94.43 | 1.28 × 10−3 | 7.63 × 10−7 | 1.70 | 1.49 × 10−7 |
| 10 | 229.04 | 445.48 | 327.79 | 438.42 | 438.42 | 24.56 | 1.15 | 60.08 | 32.11 | 0.11 | 40.25 | 61.21 | 9.97 × 10−3 | 2.96 × 10−6 | 1.96 | 1.20 × 10−6 | |
| 20 | 224.65 | 451.47 | 336.07 | 428.49 | 428.49 | 30.05 | 2.24 | 60.34 | 31.13 | 0.18 | 34.45 | 44.99 | 1.94 × 10−2 | 4.31 × 10−6 | 2.45 | 2.96 × 10−6 | |
| 100 | 218.13 | 558.84 | 354.60 | 433.67 | 354.60 | 122.13 | 10.50 | 67.48 | 27.25 | 0.19 | 28.70 | 31.78 | 1.34 × 10−1 | 2.00 × 10−5 | 4.31 | 4.82 × 10−5 | |
| Fuel | Reaction and Mechanism | β (°C/min) | Equation | R2 | E (kJ/mol) | A (min−1) |
|---|---|---|---|---|---|---|
| ET | Devolatilization/2nd order | 5 | 0.992 | 101.30 | 2.34 × 108 | |
| 10 | 0.991 | 101.25 | 3.08 × 108 | |||
| 20 | 0.990 | 100.49 | 3.37 × 108 | |||
| Char oxidation/1D diffusion | 5 | 0.908 | 15.84 | 0.26 | ||
| 10 | 0.906 | 16.61 | 0.58 | |||
| 20 | 0.973 | 17.57 | 1.37 | |||
| EB | Devolatilization/2nd order | 5 | 0.997 | 51.77 | 2.07 × 103 | |
| 10 | 0.997 | 49.49 | 1.96 × 103 | |||
| 20 | 0.996 | 47.92 | 2.18 × 103 | |||
| Char oxidation/1D diffusion | 5 | 0.982 | 29.08 | 3.08 | ||
| 10 | 0.985 | 31.63 | 8.67 | |||
| 20 | 0.994 | 31.23 | 13.24 | |||
| EBA | Devolatilization/2nd order | 5 | 0.998 | 85.65 | 4.89 × 106 | |
| 10 | 0.999 | 86.20 | 7.85 × 106 | |||
| 20 | 0.999 | 82.92 | 5.72 × 106 | |||
| Char oxidation/2D diffusion | 5 | 0.985 | 35.48 | 5.15 | ||
| 10 | 0.994 | 30.97 | 10.16 | |||
| 20 | 0.985 | 35.48 | 47.61 | |||
| PT | Devolatilization/2nd order | 5 | 0.994 | 98.23 | 6.81 × 107 | |
| 10 | 0.996 | 97.67 | 7.80 × 107 | |||
| 20 | 0.995 | 95.72 | 6.66 × 107 | |||
| Char oxidation/1D diffusion | 5 | 0.968 | 21.53 | 0.84 | ||
| 10 | 0.947 | 24.18 | 2.76 | |||
| 20 | 0.971 | 20.08 | 2.02 | |||
| PB | Devolatilization/2nd order | 5 | 0.997 | 62.34 | 3.84 × 104 | |
| 10 | 0.997 | 63.93 | 8.87 × 104 | |||
| 20 | 0.997 | 62.67 | 1.00 × 105 | |||
| Char oxidation/1D diffusion | 5 | 0.998 | 20.05 | 0.62 | ||
| 10 | 0.996 | 22.11 | 1.82 | |||
| 20 | 0.991 | 24.69 | 5.58 | |||
| AT | Devolatilization/2nd order | 5 | 0.996 | 85.99 | 7.46 × 106 | |
| 10 | 0.996 | 85.79 | 9.96 × 106 | |||
| 20 | 0.996 | 85.61 | 1.35 × 107 | |||
| Char oxidation/1D diffusion | 5 | 0.989 | 19.87 | 0.67 | ||
| 10 | 0.990 | 24.21 | 3.25 | |||
| 20 | 0.968 | 29.23 | 17.19 | |||
| AB | Devolatilization/2nd order | 5 | 0.999 | 54.05 | 3.87 × 103 | |
| 10 | 0.998 | 52.17 | 3.83 × 103 | |||
| AB | Devolatilization/2nd order | 20 | 0.996 | 49.45 | 3.34 × 103 | |
| Char oxidation/1D diffusion | 5 | 0.994 | 22.18 | 0.78 | ||
| 10 | 0.997 | 24.93 | 2.33 | |||
| 20 | 0.995 | 24.55 | 3.52 | |||
| OB | Devolatilization/2nd order | 5 | 0.999 | 87.74 | 9.80 × 106 | |
| 10 | 0.999 | 87.34 | 1.19 × 107 | |||
| 20 | 0.998 | 86.76 | 1.49 × 107 | |||
| Char oxidation/1D diffusion | 5 | 0.978 | 14.97 | 0.20 | ||
| 10 | 0.906 | 15.89 | 0.46 | |||
| 20 | 0.978 | 19.99 | 2.26 |
References
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Nussbaumer, T. Combustion and Co-combustion of Biomass: Fundamentals, Technologies, and Primary Measures for Emission Reduction. Energy Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- Jones, J.M.; Lea-Langton, A.R.; Ma, L.; Pourkashanian, M.; Williams, A. Pollutants Generated by the Combustion of Solid Biomass Fuels; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Perez-Jimenez, J.A.; Serrano-Bernardo, F.; Zamorano, M. Determination and comparison of combustion kinetics parameters of agricultural biomass from olive trees. Renew. Energy. 2015, 83, 897–904. [Google Scholar] [CrossRef]
- Saeed, M.A.; Andrews, G.E.; Phylaktou, H.N.; Gibbs, B.M. Global kinetics of the rate of volatile release from biomasses in comparison to coal. Fuel 2016, 181, 347–357. [Google Scholar] [CrossRef]
- Su, Y.; Luo, Y.; Wu, W.; Zhang, Y.; Zhao, S. Characteristics of pine wood oxidative pyrolysis: Degradation behavior, carbon oxide production and heat properties. J. Anal. Appl. Pyrolysis 2012, 98, 137–143. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J.; Teixeira, S.; Soares, D.; Ferreira, M.; Teixeira, J. Thermal Conversion of Pine Wood and Kinetic Analysis under Oxidative and Non-Oxidative Environments at Low Heating Rate. Proceedings 2020, 58, 23. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Zobel, N.; Berger, A.; Behrendt, F. Smouldering of pine wood: Kinetics and reaction heats. Combust. Flame. 2012, 159, 1708–1719. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Jin, B.; Fang, M.X. Thermal degradation mechanisms of wood under inert and oxidative environments using DAEM methods. Bioresour. Technol. 2011, 102, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Magalhães, D.; Kazanç, F.; Riaza, J.; Erensoy, S.; Kabaklı, Ö.; Chalmers, H. Combustion of Turkish lignites and olive residue: Experiments and kinetic modelling. Fuel 2017, 203, 868–876. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Basics and Applications of Solid-State Kinetics: A Pharmaceutical Perspective. J. Pharm. Sci. 2006, 95, 472–498. [Google Scholar] [CrossRef]
- Rico, J.J.; Pérez-Orozco, R.; Vilas, D.P.; Porteiro, J. TG/DSC and kinetic parametrization of the combustion of agricultural and forestry residues. Biomass Bioenergy 2022, 162, 106485. [Google Scholar] [CrossRef]
- Xu, X.; Pan, R.; Chen, R. Combustion Characteristics, Kinetics, and Thermodynamics of Pine Wood Through Thermogravimetric Analysis. Appl. Biochem. Biotechnol. 2021, 193, 1427–1446. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Xu, X.; Zhang, D.; Hao, R. Combustion characteristics, kinetics and thermodynamics of Pinus Sylvestris pine needle via non-isothermal thermogravimetry coupled with model-free and model-fitting methods. Case Stud. Therm. Eng. 2020, 22, 100756. [Google Scholar] [CrossRef]
- Fu, S.; Chen, H.; Yang, J.; Yang, Z. Kinetics of thermal pyrolysis of Eucalyptus bark by using thermogravimetric-Fourier transform infrared spectrometry technique. J. Therm. Anal. Calorim. 2019, 139, 3527–3535. [Google Scholar] [CrossRef]
- Vega, L.Y.; López, L.; Valdés, C.F.; Chejne, F. Assessment of energy potential of wood industry wastes through thermochemical conversions. Waste Manag. 2019, 87, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ma, X.; Fang, S.; Yu, Z.; Lin, Y. Thermogravimetric analysis of the co-combustion of eucalyptus residues and paper mill sludge. Appl. Therm. Eng. 2016, 106, 938–943. [Google Scholar] [CrossRef]
- Silva, J.P.; Teixeira, S.; Grilo, É.; Peters, B.; Teixeira, J.C. Analysis and monitoring of the combustion performance in a biomass power plant. Clean. Eng. Technol. 2021, 5, 100334. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renew. Sustain. Energy Rev. 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Evaluation of the potential for energy recovery from olive oil industry waste: Thermochemical conversion technologies as fuel improvement methods. Fuel 2020, 279, 118536. [Google Scholar] [CrossRef]
- Silva, J.P.; Teixeira, S.; Teixeira, J.C. Characterization of the physicochemical and thermal properties of different forest residues. Biomass Bioenergy 2023, 175, 106870. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J.; Teixeira, S.; Soares, D.; Ferreira, M.; Teixeira, J. Influence of Operating Conditions on the Thermal Behavior and Kinetics of Pine Wood Particles using Thermogravimetric Analysis. Energies 2020, 13, 2756. [Google Scholar] [CrossRef]
- Bahng, M.-K.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R. Current technologies for analysis of biomass thermochemical processing: A review. Anal. Chim. Acta. 2009, 651, 117–138. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487. [Google Scholar] [CrossRef]
- Kissinger, H. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. (Sci. Technol.) 1971, 16, 22–31. [Google Scholar]
- Starink, M. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Siddiqi, H.; Bal, M.; Kumari, U.; Meikap, B.C. In-depth physiochemical characterization and detailed thermo-kinetic study of biomass wastes to analyze its energy potential. Renew. Energy 2019, 148, 756–771. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. Kinetic Analysis of Biomass Pyrolysis. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–83. [Google Scholar] [CrossRef]
- Yu, D.; Chen, M.; Wei, Y.; Niu, S.; Xue, F. An assessment on co-combustion characteristics of Chinese lignite and eucalyptus bark with TG–MS technique. Powder Technol. 2016, 294, 463–471. [Google Scholar] [CrossRef]
- Conesa, J.A.; Marcilla, A.; Caballero, J.A.; Font, R. Comments on the validity and utility of the different methods for kinetic analysis of thermogravimetric data. J. Anal. Appl. Pyrolysis 2001, 58–59, 617–633. [Google Scholar] [CrossRef]
- Mishra, G.; Kumar, J.; Bhaskar, T. Kinetic studies on the pyrolysis of pinewood. Bioresour. Technol. 2015, 182, 282–288. [Google Scholar] [CrossRef]
- Starink, M.J. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S. Combustion behaviour of biomass fuels and their blends with lignite. Thermochim. Acta 2011, 526, 192–199. [Google Scholar] [CrossRef]
- Álvarez, A.; Pizarro, C.; García, R.; Bueno, J.L.; Lavín, A.G. Determination of kinetic parameters for biomass combustion. Bioresour. Technol. 2016, 216, 36–43. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Q.; Wang, X.; Xiao, B.; Liu, S. Pyrolysis behaviors and kinetic studies on Eucalyptus residues using thermogravimetric analysis. Energy Convers. Manag. 2015, 105, 251–259. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Villaseñor, J.; Sánchez, P.; Valverde, J.L. Thermogravimetric–mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef]
- Fang, X.; Jia, L.; Yin, L. A weighted average global process model based on two−stage kinetic scheme for biomass combustion. Biomass Bioenergy 2013, 48, 43–50. [Google Scholar] [CrossRef]
- Gil, M.V.; Casal, D.; Pevida, C.; Pis, J.J.; Rubiera, F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Bridgwater, A.V.; Fang, M.X. Kinetic study on thermal decomposition of woods in oxidative environment. Fuel 2009, 88, 1024–1030. [Google Scholar] [CrossRef]
- Yorulmaz, S.Y.; Atimtay, A.T. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process. Technol. 2009, 90, 939–946. [Google Scholar] [CrossRef]

| Property | Parameter | Acacia | Eucalyptus | Pine | Olive | |||
|---|---|---|---|---|---|---|---|---|
| Trunk | Branches | Bark | Trunk * | Branches | Trunk * | Branches | ||
| Proximate Analysis (%, db) | Moisture | 5.0 | 6.2 | 3.4 | 2.1 | 6.7 | 11.5 | 11.2 |
| Volatile matter | 81.8 | 77.8 | 89.6 | 88.9 | 82.9 | 83.7 | 81.5 | |
| Ashes | 1.1 | 0.7 | 4.2 | 1.0 | 3.2 | 0.8 | 1.8 | |
| Fixed Carbon | 17.1 | 21.5 | 6.2 | 10.1 | 13.9 | 15.5 | 16.7 | |
| Ultimate Analysis (%, db) | Carbon | 45.40 | 52.40 | 43.10 | 47.20 | 55.90 | 47.30 | 49.40 |
| Hydrogen | 6.88 | 7.38 | 6.46 | 7.03 | 7.55 | 6.40 | 6.95 | |
| Nitrogen | 0.77 | 3.21 | 0.25 | 0.11 | 1.44 | 0.13 | 0.24 | |
| Sulfur | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.99 | 0.88 | |
| Oxygen | 46.94 | 36.97 | 50.18 | 45.65 | 35.10 | 45.18 | 42.53 | |
| Air–Fuel Ratio (stoichiometric—gair/gsample) | 5.54 | 6.94 | 5.00 | 5.85 | 7.48 | 5.69 | 6.23 | |
| Calorific Value (db) | HHV (MJ/kg) | 17.14 | 20.41 | 15.20 | 17.56 | 22.30 | 19.10 | 17.71 |
| LHV (MJ/kg) | 15.66 | 18.81 | 13.81 | 16.05 | 20.67 | 18.60 | 16.22 | |
| Method | Equation | Eα Determination (Slope of the Plots) | Advantages and Disadvantages |
|---|---|---|---|
| FR | Simple and accurate; numerically unstable and sensitive to data noise | ||
| OFW | Oversimplified temperature integral | ||
| KAS | More accurate than OFW; oversimplified temperature integral | ||
| Starink | More accurate temperature integral approximation | ||
| CR | Forcible fitting to reaction mechanism; single values of E are obtained |
| Property | Parameter | Description |
|---|---|---|
| Temperature | Ignition temperature (°C). Corresponds to the beginning of the weight loss and is defined as the temperature at which the rate of weight loss reaches 1%/min after the initial moisture loss peak in the DTG profile. | |
| Burnout temperature (°C). It is identified when the last peak comes to the end and is the temperature at which the sample is completely oxidized. It is taken as the point immediately before the reaction ceases when the rate of weight loss is down to 1%/min. | ||
| Peak temperature in devolatilization (°C). It is the point at which the maximum reaction rate occurs during devolatilization. | ||
| Peak temperature in char combustion (°C). It is the point at which the maximum reaction rate occurs during char combustion. | ||
| Temperature at the maximum weight loss (°C). It is the point at which the maximum reaction rate occurs. | ||
| Mass Loss | Average weight loss rate, wt.%/min | |
| Maximum weight loss rate, wt.%/min | ||
| Weight loss during devolatilization, wt.% | ||
| Weight loss during char combustion, wt.% | ||
| Residual weight, mg | ||
| Time | Ignition time, min. | |
| Burnout time, min. | ||
| Indexes | It indicates the ignition performance. This means how fast or slow the fuel is ignited. wt.%/min3 | |
| Reflects the volatile matter release performance. wt.%/min.T3 | ||
| Expresses the burnout performance. It is inversely proportional to how fast the char burn. (-) | ||
| Presents the comprehensive characteristics. The higher the value, the more significantly the samples are burned. wt.%/min2.T3 |
| ET | PT | AT | |
|---|---|---|---|
| OFW | |||
| Devolatilization | 172.01 | 161.41 | 177.00 |
| Char Combustion | 248.78 | 211.84 | 258.46 |
| KAS | |||
| Devolatilization | 171.27 | 160.40 | 176.68 |
| Char Combustion | 250.55 | 219.37 | 260.69 |
| FR | |||
| Devolatilization | 106.67 | 87.64 | 103.09 |
| Char Combustion | 148.02 | 129.16 | 236.85 |
| Starink | |||
| Devolatilization | 171.13 | 166.35 | 176.92 |
| Char Combustion | 248.69 | 215.53 | 260.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.P.; Teixeira, S.; Teixeira, J.C. Thermogravimetric Assessment and Kinetic Analysis of Forestry Residues Combustion. Energies 2025, 18, 3299. https://doi.org/10.3390/en18133299
Silva JP, Teixeira S, Teixeira JC. Thermogravimetric Assessment and Kinetic Analysis of Forestry Residues Combustion. Energies. 2025; 18(13):3299. https://doi.org/10.3390/en18133299
Chicago/Turabian StyleSilva, João Pedro, Senhorinha Teixeira, and José Carlos Teixeira. 2025. "Thermogravimetric Assessment and Kinetic Analysis of Forestry Residues Combustion" Energies 18, no. 13: 3299. https://doi.org/10.3390/en18133299
APA StyleSilva, J. P., Teixeira, S., & Teixeira, J. C. (2025). Thermogravimetric Assessment and Kinetic Analysis of Forestry Residues Combustion. Energies, 18(13), 3299. https://doi.org/10.3390/en18133299








