Performance Enhancement of a-C:Cr Thin Films Deposited on 316L Stainless Steel as Bipolar Plates via a Thin Ti Layer by Mid-Frequency Magnetron Sputtering for PEMFC Application
Abstract
1. Introduction
2. Experiments
2.1. Films Deposition
2.2. Thin Film Characterization
2.3. Corrosion Resistance Measurements
2.4. Interfacial Contact Resistance Measurements
2.5. Contact Angle Measurement
3. Results
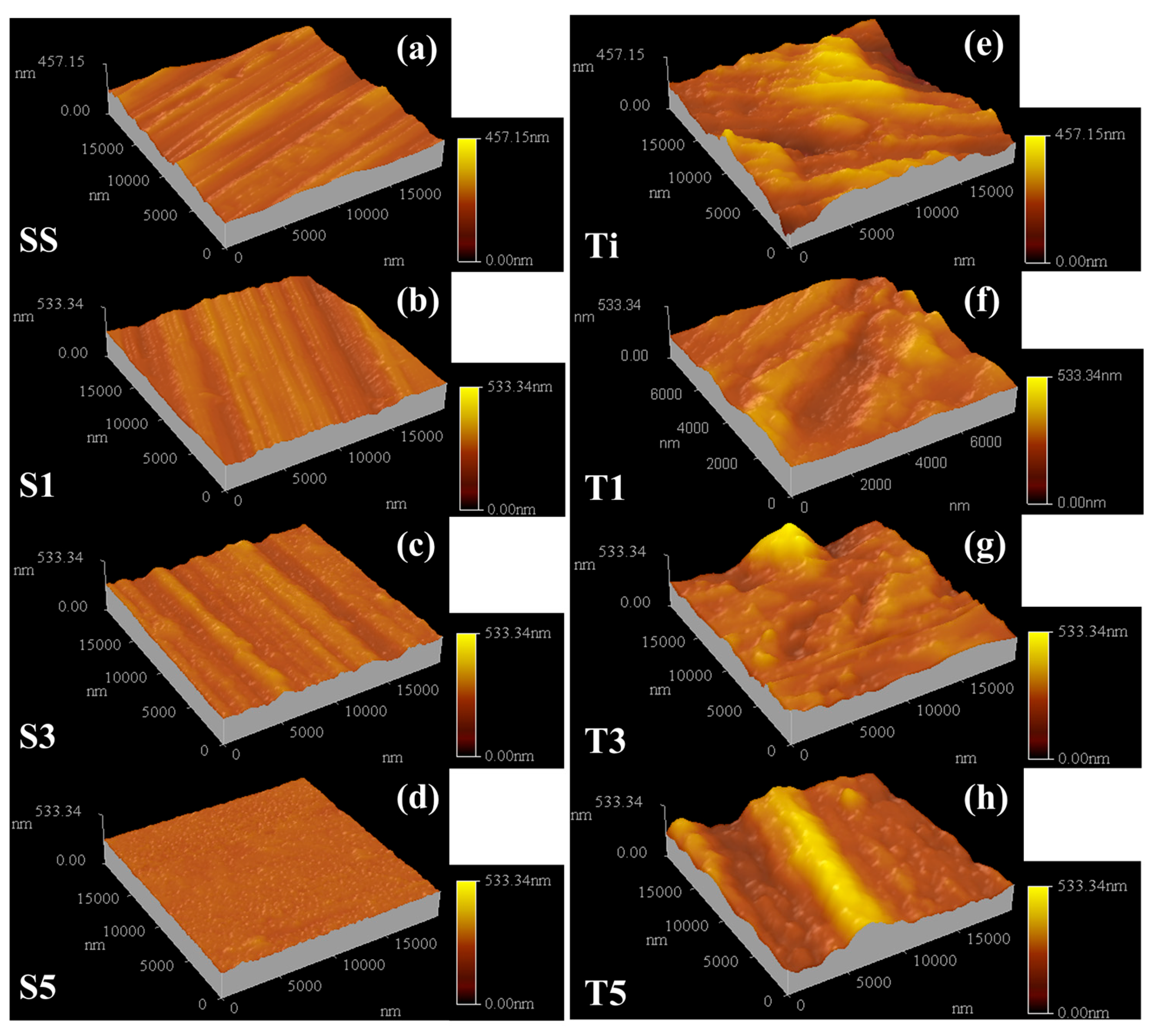
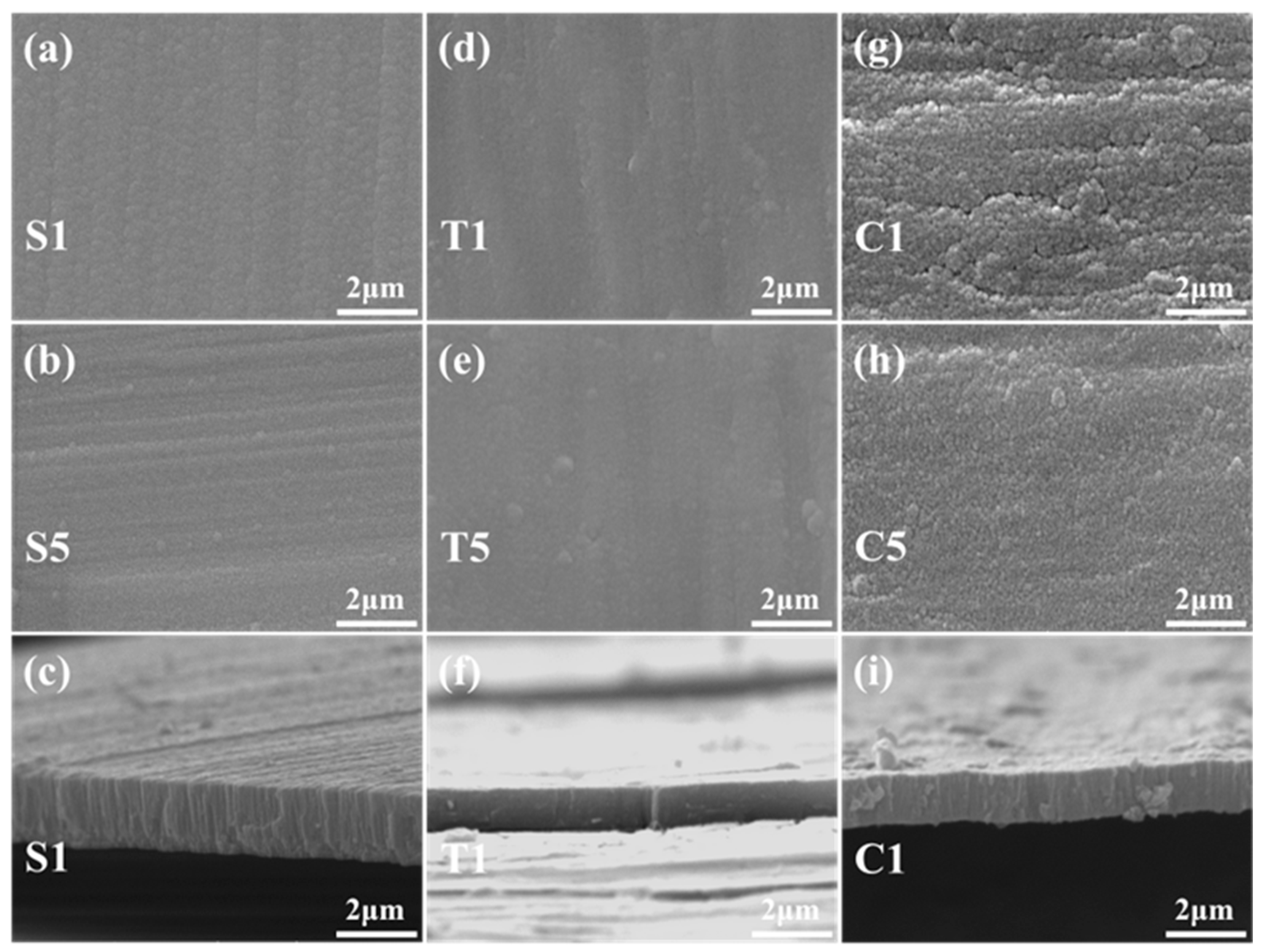
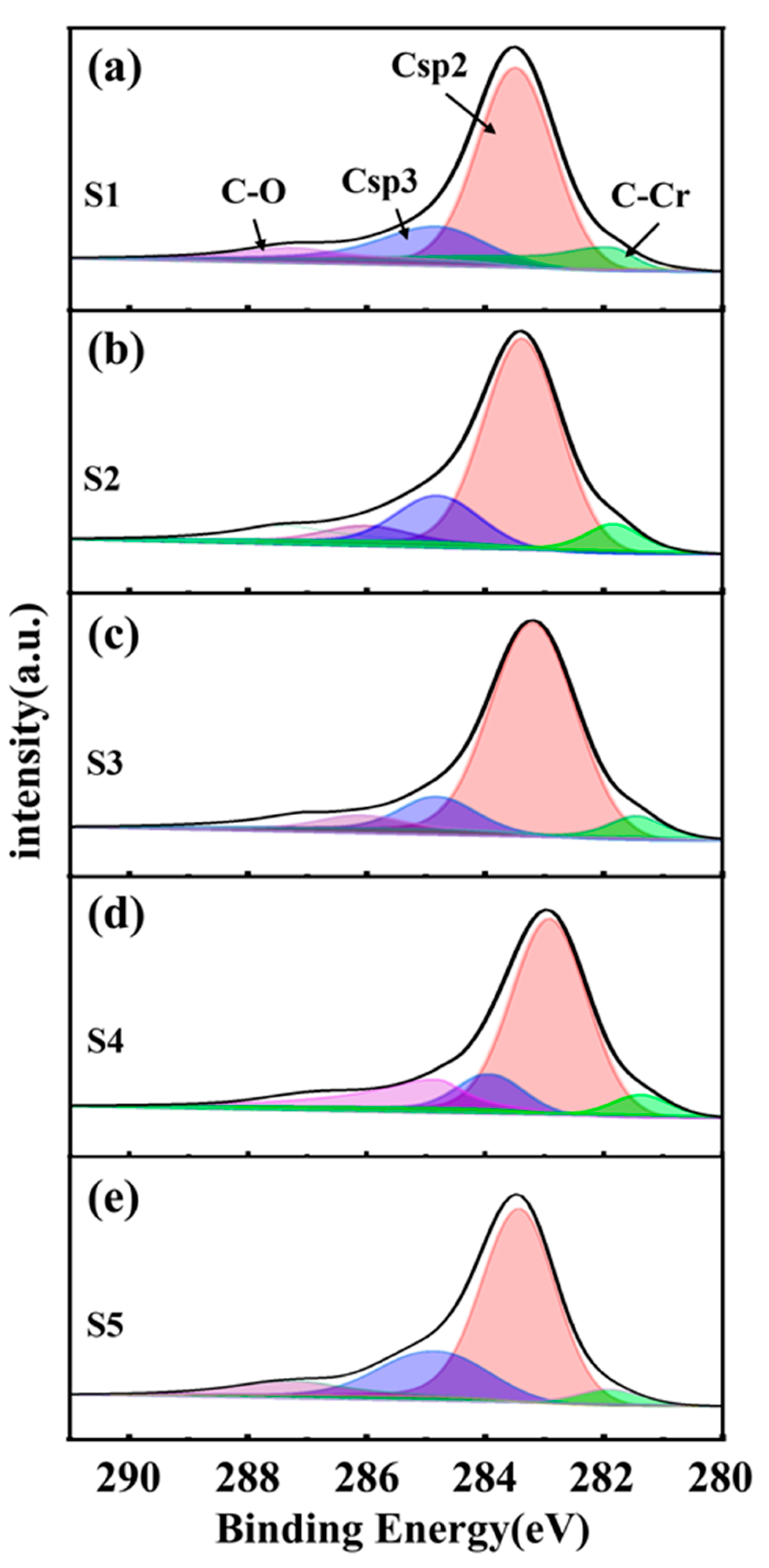
3.1. The Microstructure and Phase Composition of the Film
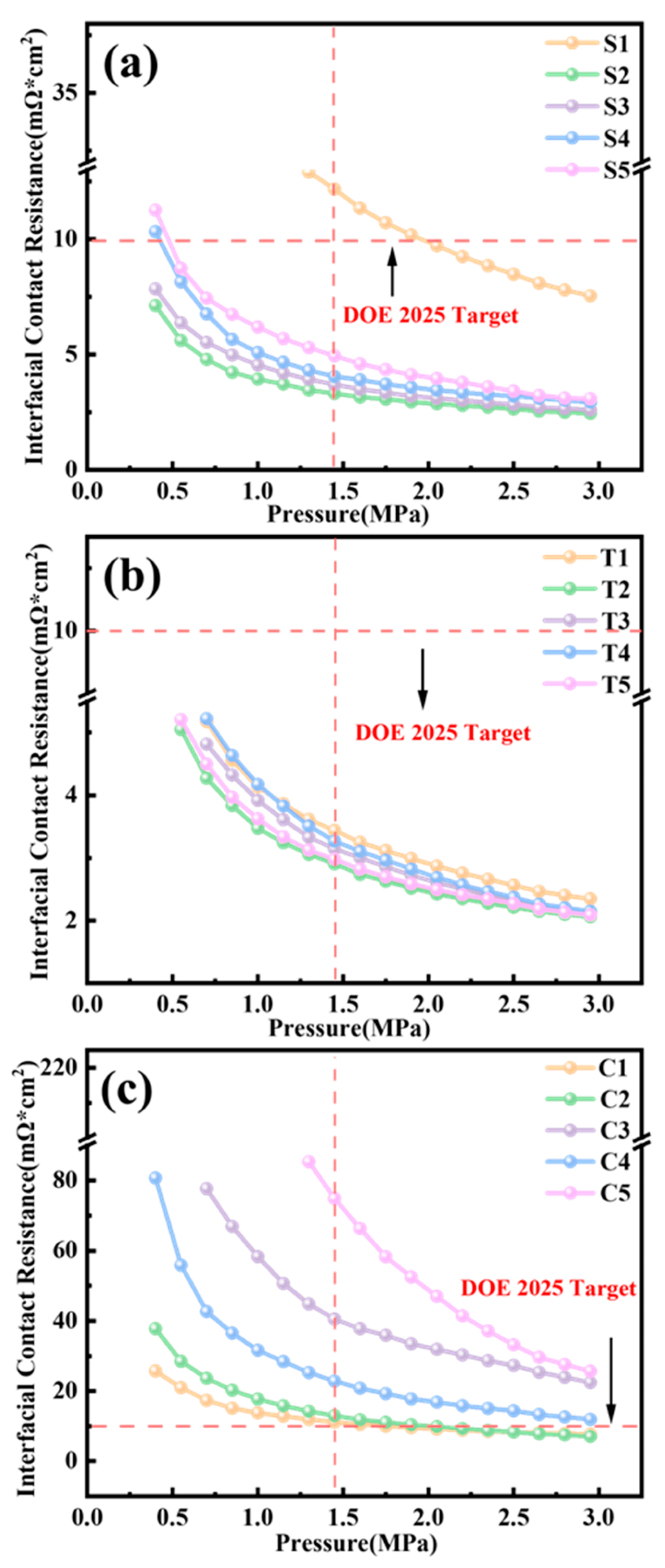
3.2. ICR Evaluation
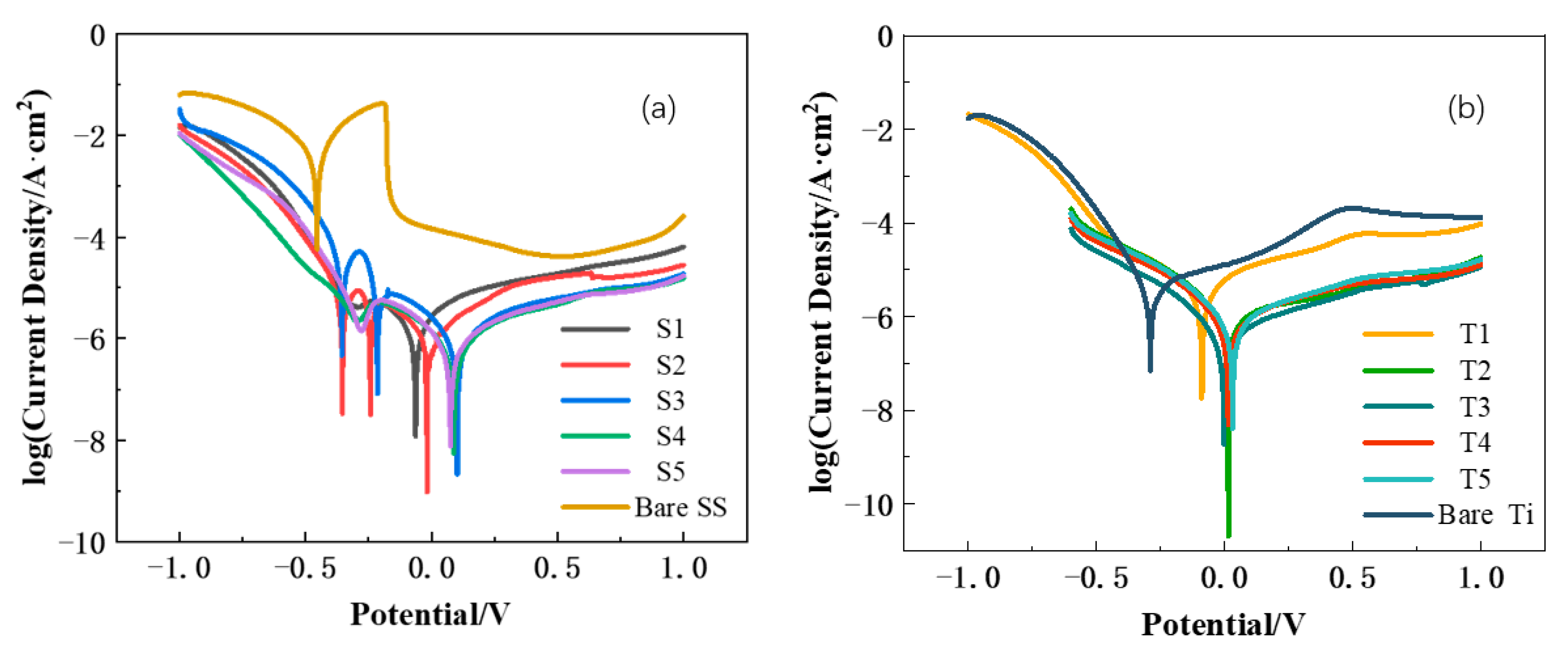
3.3. Corrosion Resistance Evaluation
3.4. Hydrophobicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Peng, L.; Yi, P.; Lai, X. Design and manufacturing of stainless steel bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2014, 39, 21127–21153. [Google Scholar] [CrossRef]
- Tu, Z.; Zhang, H.; Luo, Z.; Liu, J.; Wan, Z.; Pan, M. Evaluation of 5 kW proton exchange membrane fuel cell stack operated at 95 degrees C under ambient pressure. J. Power Sources 2013, 222, 277–281. [Google Scholar] [CrossRef]
- Yu, Y.; Tu, Z.; Zhang, H.; Zhan, Z.; Pan, M. Comparison of degradation behaviors for open-ended and closed proton exchange membrane fuel cells during startup and shutdown cycles. J. Power Sources 2011, 196, 5077–5083. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Y. Research Progress on Corrosion Behavior and Surface Protection of Stainless Steel Bipolar Plate of Proton Exchange Membrane Fuel Cell. Surf. Technol. 2021, 50, 192–200. [Google Scholar]
- Tang, Y.; Yuan, W.; Pan, M.; Wan, Z. Feasibility study of porous copper fiber sintered felt: A novel porous flow field in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 9661–9677. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Lee, J.-B.; Oh, I.H. Electrochemical characteristics and interfacial contact resistance of multi-layered Ti/TiN coating for metallic bipolar-plate of polymer electrolyte membrane fuel cells. Met. Mater. Int. 2014, 20, 629–639. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.B.; Yang, L.; Li, J.X.; Yang, L. Study on the preparation and properties of novolac epoxy/graphite composite bipolar plate for PEMFC. Int. J. Hydrogen Energy 2010, 35, 3105–3109. [Google Scholar]
- Huang, J.H.; Baird, D.G.; McGrath, J.E. Development of fuel cell bipolar plates from graphite filled wet-lay thermoplastic composite materials. J. Power Sources 2005, 150, 110–119. [Google Scholar] [CrossRef]
- Jin, J.; Liu, H.; Zheng, D.; Zhu, Z. Effects of Mo content on the interfacial contact resistance and corrosion properties of CrN coatings on SS316L as bipolar plates in simulated PEMFCs environment. Int. J. Hydrogen Energy 2018, 43, 10048–10060. [Google Scholar] [CrossRef]
- Bondarenko, A.V.; Islamov, S.R.; Ignatyev, K.V.; Mardashov, D.V. Laboratory studies of polymer compositions for well-kill under increased fracturing. Perm J. Pet. Min. Eng. 2020, 20, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ricketts, M.; Hirano, S. Ex situ evaluation of nanometer range gold coating on stainless steel substrate for automotive polymer electrolyte membrane fuel cell bipolar plate. J. Power Sources 2010, 195, 1401–1407. [Google Scholar] [CrossRef]
- Yi, P.; Zhu, L.J.; Dong, C.F.; Xiao, K. Corrosion and interfacial contact resistance of 316L stainless steel coated with magnetron sputtered ZrN and TiN in the simulated cathodic environment of a proton-exchange membrane fuel cell. Surf. Coat. Technol. 2019, 363, 198–202. [Google Scholar] [CrossRef]
- Li, J.L.; Xu, Z.L.; Li, Y.J.; Ma, X.Z.; Mo, J.M.; Weng, L.Y.; Liu, C.Y. Intergranular passivation of the TiC coating for enhancing corrosion resistance and surface conductivity in stainless-steel bipolar plates. J. Mater. Sci. 2021, 56, 8689–8703. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, S.H.; Lu, Z.X.; Wang, L.S.; Li, W.H. Preparation and performances of electrically conductive Nb-doped TiO2 coatings for 316 stainless steel bipolar plates of proton-exchange membrane fuel cells. Corros. Sci. 2018, 142, 249–257. [Google Scholar] [CrossRef]
- Wu, M.G.; Lu, C.D.; Hong, T.; Chen, G.H.; Wen, D.H. Chromium interlayer amorphous carbon film for 304 stainless steel bipolar plate of proton exchange membrane fuel cell. Surf. Coat. Technol. 2016, 307, 374–381. [Google Scholar]
- Akula, S.; Kalaiselvi, P.; Sahu, A.K.; Chellammal, S. Electrodeposition of conductive PAMT/PPY bilayer composite coatings on 316L stainless steel plate for PEMFC application. Int. J. Hydrogen Energy 2021, 46, 17909–17921. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Sun, H.L.; Liu, D.L.; An, Q.Z.; Cai, X.; Chu, P.K. Conductive amorphous carbon-coated 316L stainless steel as bipolar plates in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 6771–6777. [Google Scholar] [CrossRef]
- Che, J.; Yi, P.Y.; Peng, L.F.; Lai, X.M. Impact of pressure on carbon films by PECVD toward high deposition rates and high stability as metallic bipolar plate for PEMFCs. Int. J. Hydrogen Energy 2020, 45, 16277–16286. [Google Scholar] [CrossRef]
- DOE/EE-2114; Fuel Cell System Cost–2025. U.S. Department of Energy: Washington, DC, USA, 2022.
- Yang, S.; Teer, D.G. Investigation of sputtered carbon and carbon/chromium multi-layered coatings. Surf. Coat. Technol. 2000, 131, 412–416. [Google Scholar] [CrossRef]
- Wei, C.H.; Yen, J.Y. Effect of film thickness and interlayer on the adhesion strength of diamond like carbon films on different substrates. Diam. Relat. Mater. 2007, 16, 1325–1330. [Google Scholar] [CrossRef]
- Bi, F.F.; Li, X.B.; Yi, P.Y.; Hou, K.; Peng, L.F.; Lai, X.M. Characteristics of amorphous carbon films to resist high potential impact in PEMFCs bipolar plates for automotive application. Int. J. Hydrogen Energy 2017, 42, 14279–14289. [Google Scholar] [CrossRef]
- Li, X.B.; Hou, K.; Qiu, D.K.; Yi, P.Y.; Lai, X.M. A first principles and experimental study on the influence of nitrogen doping on the performance of amorphous carbon films for proton exchange membrane fuel cells. Carbon 2020, 167, 219–229. [Google Scholar] [CrossRef]
- Zhang, D.; Yi, P.Y.; Peng, L.F.; Lai, X.M.; Pu, J.B. Amorphous carbon films doped with silver and chromium to achieve ultra-low interfacial electrical resistance and long-term durability in the application of proton exchange membrane fuel cells. Carbon 2019, 145, 333–344. [Google Scholar] [CrossRef]
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Experimental Research of the Possibility of Applying the Hartmann–Sprenger Effect to Regulate the Pressure of Natural Gas in Non-Stationary Conditions. Processes 2025, 13, 1189. [Google Scholar] [CrossRef]
- Davies, D.P.; Adcock, P.L.; Turpin, M.; Rowen, S.J. Stainless steel as a bipolar plate material for solid polymer fuel cells. J. Power Sources 2000, 86, 237–242. [Google Scholar] [CrossRef]
- Wang, H.L.; Sweikart, M.A.; Turner, J.A. Stainless steel as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Hou, K.; Yi, P.; Li, X.; Peng, L.; Lai, X. The effect of Cr doped in amorphous carbon films on electrical conductivity: Characterization and mechanism. Int. J. Hydrogen Energy 2021, 46, 30841–30852. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Z.; Fu, Z.; Yue, W.; Yu, X.; Wang, C. Influence of individual Cr-C layer thickness on structural and tribological properties of multilayered Cr-C/a-C:Cr thin films. Surf. Coat. Technol. 2010, 204, 3319–3325. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Kok, Y.N.; Ehiasarian, A.P.; Haasch, R.; Wen, J.G.; Petrov, I. Phase separation and formation of the self-organised layered nanostructure in C/Cr coatings in conditions of high ion irradiation. Surf. Coat. Technol. 2005, 200, 1572–1579. [Google Scholar] [CrossRef]
- Detroye, M.; Reniers, F.; Buess-Herman, C.; Vereecken, J. AES-XPS study of chromium carbides and chromium iron carbides. Appl. Surf. Sci. 1999, 144–145, 78–82. [Google Scholar] [CrossRef]
- Elam, F.M.; Hsia, F.C.; van Vliet, S.; Bliem, R.; Yang, L.; Weber, B.; Franklin, S.E. The influence of corrosion on diamond-like carbon topography and friction at the nanoscale. Carbon 2021, 179, 590–599. [Google Scholar] [CrossRef]
- Tunmee, S.; Photongkam, P.; Euaruksakul, C.; Takamatsu, H.; Zhou, X.; Wongpanya, P.; Komatsu, K.; Kanda, K.; Ito, H.; Saitoh, H. Investigation of pitting corrosion of diamond-like carbon films using synchrotron-based spectromicroscopy. J. Appl. Phys. 2016, 120, 195303. [Google Scholar] [CrossRef]
- Groudeva-Zotova, S.; Vitchev, R.G.; Blanpain, B. Phase composition of Cr-C thin films deposited by a double magnetron sputtering system. Surf. Interface Anal. 2000, 30, 544–548. [Google Scholar] [CrossRef]
- Mi, B.; Wang, Q.; Qi, T.; Qin, Z.; Chen, Z.; Wang, H. Performance and structure of Ti-doped amorphous carbon/CrN/Ti multilayer coating deposited on 316L stainless steel for use as bipolar plate in proton exchange membrane fuel cell. J. Alloys Compd. 2023, 943, 169080. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Zhang, B.; Lv, J.; Zhang, J. Nano-Cr2N dominated films with high conductivity and strong corrosion resistance for Ti bipolar plates. Mater. Des. 2022, 224, 111305. [Google Scholar] [CrossRef]
- GB/T 20042.5-2023; Proton Exchange Membrane Fuel Cell—Part 5: Test Methods for Bipolar Plates. China Electrical Equipment Industry Association: Beijing, China, 1 October 2023.
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]





| Property | Target |
|---|---|
| Interface contact resistance (1.4 MPa) | <10 (mΩ·cm2) |
| Corrosion current density | <1 (μA·cm−2) |
| Cost of production | <2 ($·KW−1) |
| Sample | T1 | T2 | T3 | T4 | T5 |
| C(sp2)/at% | 0.72 | 0.8 | 0.7 | 0.73 | 0.78 |
| C(sp3)/at% | 0.11 | 0.11 | 0.12 | 0.13 | 0.11 |
| C-Cr/at% | 0.1 | 0.04 | 0.13 | 0.09 | 0.06 |
| C-O/at% | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 |
| C(sp2)/C(sp3) | 6.45 | 7.34 | 6.03 | 5.63 | 6.93 |
| Sample | S1 | S2 | S3 | S4 | S5 |
| C(sp2)/at% | 0.75 | 0.66 | 0.71 | 0.7 | 0.71 |
| C(sp3)/at% | 0.1 | 0.17 | 0.11 | 0.12 | 0.16 |
| C-Cr/at% | 0.07 | 0.08 | 0.1 | 0.1 | 0.05 |
| C-O/at% | 0.08 | 0.09 | 0.08 | 0.08 | 0.08 |
| C(sp2)/C(sp3) | 7.49 | 3.87 | 6.62 | 5.97 | 4.57 |
| Sample | SS | S1 | S2 | S3 | S4 | S5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Icorr (μA/cm2) | 5738 | 2.27 | 2.14 | 0.94 | 0.69 | 0.73 | 0.65 | 0.72 | ||
| Ecorr (mV) | −455 | −438 | −451 | −446 | −452 | −448 | −456 | −448 | ||
| Sample | Ti | T1 | T2 | T3 | T4 | T5 | ||||
| Icorr (μA/cm2) | 4.06 | 0.76 | 1.34 | 0.62 | 0.73 | 0.65 | 2.00 | 4.62 | ||
| Ecorr (mV) | −290 | 14 | 43 | 36 | 41 | 38 | 66 | 137 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, S.; Wang, S.; Ma, M.; Chen, M.; Yang, J.; Yang, C.; Li, W. Performance Enhancement of a-C:Cr Thin Films Deposited on 316L Stainless Steel as Bipolar Plates via a Thin Ti Layer by Mid-Frequency Magnetron Sputtering for PEMFC Application. Energies 2025, 18, 3270. https://doi.org/10.3390/en18133270
Zhao Y, Li S, Wang S, Ma M, Chen M, Yang J, Yang C, Li W. Performance Enhancement of a-C:Cr Thin Films Deposited on 316L Stainless Steel as Bipolar Plates via a Thin Ti Layer by Mid-Frequency Magnetron Sputtering for PEMFC Application. Energies. 2025; 18(13):3270. https://doi.org/10.3390/en18133270
Chicago/Turabian StyleZhao, Yuxing, Song Li, Saiqiang Wang, Ming Ma, Ming Chen, Jiao Yang, Chunlei Yang, and Weimin Li. 2025. "Performance Enhancement of a-C:Cr Thin Films Deposited on 316L Stainless Steel as Bipolar Plates via a Thin Ti Layer by Mid-Frequency Magnetron Sputtering for PEMFC Application" Energies 18, no. 13: 3270. https://doi.org/10.3390/en18133270
APA StyleZhao, Y., Li, S., Wang, S., Ma, M., Chen, M., Yang, J., Yang, C., & Li, W. (2025). Performance Enhancement of a-C:Cr Thin Films Deposited on 316L Stainless Steel as Bipolar Plates via a Thin Ti Layer by Mid-Frequency Magnetron Sputtering for PEMFC Application. Energies, 18(13), 3270. https://doi.org/10.3390/en18133270







